Abstract
Objective
Excessive pelvic floor muscle activity has been suggested as a source of pain in chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS). Our objective was to determine whether men with CP/CPPS have changes in neural drive that impair their ability to relax pelvic floor muscles.
Methods
We recruited 90 men (42 with CP/CPPS and 48 in the control group [without a history of pelvic pain]). All completed the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI). We quantified the ability to relax by comparing resting pelvic floor muscle activity under 2 conditions: a “rest-only” condition, in which participants were instructed to simply relax, and a “rest-between-contraction” condition, in which participants were instructed to rest for several seconds between voluntary pelvic floor muscle contractions. We used multivariate mixed-effects models to examine differences between the groups (men with CP/CPPS and men in the control group) as well as the effect of 6 symptoms captured by the NIH-CPSI: pain related to location (perineum, testicles, penis, suprapubic region) and activity (urination, ejaculation).
Results
Men with CP/CPPS were significantly different from men in the control group; men with CP/CPPS had higher resting activity in the rest-between-contraction condition than in the rest-only condition, whereas men in the control group had similar resting activities in both conditions. This effect was strongest in men who reported ejaculation-related pain, which was 70% of the CP/CPPS group.
Conclusion
Men without a history of pelvic pain were able to relax their pelvic floor muscles back to baseline after performing voluntary pelvic floor muscle contractions. In contrast, men with CP/CPPS, particularly those with ejaculation-related pain, had an impaired ability to relax their pelvic floor muscles.
Impact
This study may support the investigation of more personalized physical therapist approaches for CP/CPPS that enhance the ability to relax pelvic floor muscles as a mechanism for pain reduction.
Keywords: Chronic Disease, Chronic Pain, Electromyography, Muscle Contraction, Pelvic Floor, Pelvic Floor Disorders
Introduction
Although originally viewed as a disorder of prostate infection and inflammation, chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is currently defined as chronic idiopathic pelvic pain or discomfort in males, commonly in the perineum, suprapubic region, penis, or testicles, which is often exacerbated by ejaculation or urination.1,2 CP/CPPS is poorly understood and treatments are mostly empirical and unsatisfactory, requiring a better understanding of the etiology and clinically relevant research measures in patient subgroups.3 Perspectives on diagnosis, treatment, and prognosis have proposed a shift in the diagnostic and therapeutic focus from a single entity of disease toward associated symptoms of CP/CPPS and multimodal treatment approaches.4 Thus, CP/CPPS is now seen as a more complex disorder, involving neuromuscular,5–7 autonomic,8 and brain-level systems.9,10
Here we focus on neuromuscular dysfunction and CP/CPPS pain symptoms to potentially inform physical therapy. Early treatment trials of pelvic floor physical therapy for CP/CPPS were promising and supported the involvement of pelvic floor muscles (PFM) in CP/CPPS pathophysiology. In the largest study to date, 138 men with CP/CPPS were treated for at least 1 month with manual therapy to stretch PFM and training to relax PFM. Although many individuals in this study responded well to therapy, 41% did not respond according to the study definition of 25% or greater improvement in pain symptoms.11 These results suggest the need for more mechanistic studies of PFM in men with CP/CPPS to potentially subgroup patients better prior to therapy.
It is important to identify patients with CP/CPPS and neuromuscular dysfunction that is manifested as impairments in the regulation of PFM activity. This distinction would likely enable the identification of patients most likely to respond to physical therapy,6 because impairment can associate with CP/CPPS pain symptoms captured by the validated and widely used National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI).12,13 Thus in the future, more objectively identifying the degree of impairment in PFM control in men who have been diagnosed with CP/CPPS should provide better guidance for physical therapists.
We have previously provided a potential mechanism of impaired regulation of PFM activity in CP/CPPS: we have shown that the supplementary motor area can inhibit resting PFM activity and have demonstrated diminished resting function of supplementary motor area in men with CP/CPPS.10,14 Here our goal was to examine PFM activity directly to determine whether impairment in relaxing PFM is an important component of CP/CPPS.
Methods
Overview of Study Design
We recruited 90 men in 2 cohorts (CP/CPPS and control), and quantified the ability to relax PFM by comparing recorded rectal electromyographic (EMG) resting activity under 2 conditions: a “rest-only” condition, in which participants were instructed to simply relax, and a “rest-between-contraction” condition, in which participants were instructed to rest for several seconds between voluntary PFM contractions.
Setting
This study took place between June 2017 and March 2020 at the University of Southern California. The University of Southern California Institutional Review Board approved all procedures. All aspects of the study conformed to the principles described in the Declaration of Helsinki. Data collection was completed in a single day with each participant. All participants provided informed consent.
Participants and Study Size
Men aged 18 years or older were eligible to participate. We established our inclusion criteria based on previous studies of physical therapy in CP/CPPS as well as more general phenotyping studies of chronic pelvic pain.1,15,16 Inclusion criteria for men with CP/CPPS included a clinical diagnosis of CP/CPPS and pelvic pain symptoms present the majority of the time in any 3 of the last 6 months. Inclusion criteria for men in the control group included no clinical diagnosis of CP/CPPS. A participant was excluded if he had contraindications for the use of a rectal surface EMG sensor (active infection in the genital or anal region, within 6 weeks after surgery, pacemaker, inability to use the sensor, or induction of severe pain) or reported the following: positive urine culture and active treatment for bacterial prostatitis; severe, debilitating, or urgent medical condition (other than chronic pelvic pain); active urethral or ureteral calculi; urethral diverticulum; history of pelvic radiation therapy; tuberculous cystitis; bladder cancer; carcinoma in situ; prostate or urethral cancer; or unilateral testicular pain without other pelvic symptoms. A priori considerations suggested that a minimum of 25 participants in each cohort were needed to achieve an effect size of 0.83 on the basis of the difference between cohort means of resting PFM activity. Recruitment efforts included community advertisement and contacting physical therapists and urologists for referrals. Men with CP/CPPS were recruited from clinics in the Los Angeles, California, metropolitan area.
Variables
Symptom Questionnaire
The NIH-CPSI was developed to measure symptoms and quality of life impact of CP/CPPS and provides a valid outcome measure for men with CP/CPPS.12,13 Before measuring muscle activity, each participant completed the NIH-CPSI 13 items, which are scored in 3 discrete domains: pain (the Pain or Discomfort domain), urinary symptoms (the Urination domain), and quality-of-life impact (the Impact of Symptoms domain and the Quality of Life domain). The collection of the complete NIH-CPSI was intended to better characterize the participants of this study (Tab. 1) in addition to exploring how impairments in relaxing PFM relate to the presence of pain-specific symptoms that are the full set of 6 symptoms captured by the NIH-CPSI pain domain: pain related to location (perineum, testicles, penis, suprapubic region) and activity (urination, ejaculation).
Table 1.
Demographic Characteristicsa
| Characteristic | Men in the Control Group | Men With CP/CPPS | P |
|---|---|---|---|
| No. of participants | 48 | 42 | |
| Age, y | .1956 | ||
| Mean (SD) | 34.9 (13.3) | 38.4 (12) | |
| Range | 22.9–63.6 | 21.5–70.5 | |
| Race, % of participants | |||
| North American Indian/Native American | 4.2 | 2.4 | .6378 |
| Asian/Asian American | 8.3 | 2.4 | .2187 |
| Black/African American | 10.4 | 7.1 | .5861 |
| Native Hawaiian/Pacific Islander | 0 | 0 | |
| White | 62.5 | 73.8 | .2521 |
| Other | 14.6 | 7.1 | .2625 |
| Unknown | 0 | 7.2 | |
| Ethnicity, % of participants | |||
| Hispanic/Latino | 29.2 | 19.0 | .2651 |
| Not Hispanic/Latino | 70.8 | 78.6 | .4011 |
| Unknown | 0 | 2.4 | |
| CP/CPPS symptom duration, y | |||
| Mean (SD) | 7.5 (7.1) | ||
| Range | 0.6–32.8 | ||
| NIH-CPSI score | |||
| Pain or discomfort (possible score = 0–21) | <.0001 | ||
| Mean (SD) | 0.4 (1.4) | 11.0 (3.9) | |
| Range | 0–8 | 2–19 | |
| Urination (possible score = 0–10) | <.0001 | ||
| Mean (SD) | 0.8 (1.3) | 4.6 (2.9) | |
| Range | 0–6 | 0–10 | |
| Impact of symptoms and quality of life (possiblescore = 0–12) | <.0001 | ||
| Mean (SD) | 0.6 (1.1) | 8.5 (2.5) | |
| Range | 0–5 | 1–12 |
a CP/CPPS = chronic prostatitis/chronic pelvic pain syndrome; NIH-CPSI = National Institutes of Health Chronic Prostatitis Symptom Index.
Resting PFM Activity
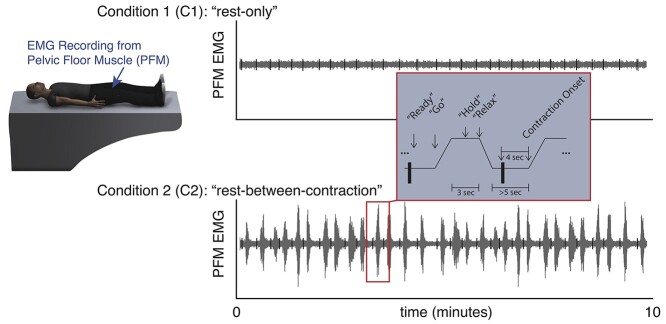
During the rest-only condition, participants were instructed to keep their heads as still as possible, relax, and not to go to sleep for 10 minutes. During the rest-between-contraction condition, participants were cued by an audio tone to contract their PFM as if they were stopping the flow of urine and then to relax, as described in our previous work.17,18 Before data collection, participants were trained on how to contract their PFM following the same verbal instruction that was preselected to engage the whole pelvic floor.19 All participants were instructed to do 30 contractions, with the intention to analyze up to 20 contractions to exclude possible effects of learning during early contractions and fatigue during late contractions. Each contraction was cued to increase over 1 second, to hold for 3 seconds, and to relax over 1 second. Contractions were cued at random times with a minimum of 5 seconds of relaxation between 2 consecutive contractions. Both conditions are shown schematically in Figure 1.
Figure 1.
Electromyographic (EMG) muscle activity to quantify the ability to relax the pelvic floor muscles (PFM). EMG recordings were made from the PFM. During condition 1 (C1) (“rest-only”), participants were instructed to rest quietly without going to sleep. During condition 2 (C2) (“rest-between-contraction”), participants repeatedly contracted and relaxed their PFM when cued by verbal instructions read by a computerized voice. Contraction onset times in the rest-between-contraction condition were calculated automatically from the EMG signal, and a series of 80-millisecond segments (black vertical bars) were examined 4 seconds prior to contraction onset during a period of time in which the participants had been instructed to relax. For consistency, the same segment timing was used to analyze both the rest-only condition and the rest-between-contraction condition.
EMG acquisition and preprocessing were based on our previous studies of PFM control.14,17,18,20 Briefly, we recorded surface EMG data from the pelvic floor with a medical-grade rectal EMG sensor (Pathway Rectal EMG Sensor; The Prometheus Group, Dover, NH, USA). The rectal EMG sensor was 50 mm long and had a stopper that ensures that 1 end of the sensor rests stably at the anal verge. The rectal EMG sensor likely recorded an aggregate signal from the PFM that included the anal sphincter, anterior and posterior levator ani, bulbospongiosus, and ischiocavernosus muscles.19,21 The EMG preamplifier filters (DELSYS, Boston, MA, USA) had a bandwidth of 20 to 450 Hz, with gains of 10,000 for the PFM and a sampling rate of 2000 Hz. All EMG tone signals were preprocessed by filtering with a fourth-order Butterworth band-pass filter with cutoffs of 16 and 470 Hz.
EMG analysis was based on common methods of EMG processing22,23 and our recent study of modulating resting PFM activity with repetitive transcranial magnetic stimulation delivered to a pelvis-specific region in the supplementary motor area—a region that we have implicated in CP/CPPS10—in which we measured the effect of repetitive transcranial magnetic stimulation on short, stable, and automatic nonvolitional EMG segments.14 Briefly, we extracted segments 80 milliseconds in duration from the preprocessed PFM EMG signal and calculated their root-mean-square (RMS) values. The timing of these samples was based on the onsets of PFM contraction in the rest-between-contraction condition, determined by automated methods described previously.18,24 We allowed the 80-millisecond windows to start 4 seconds before the onset of the voluntary PFM contractions to ensure that these time windows were not part of the voluntary contractions (Fig. 1). The RMS value of each segment was then normalized to the participant’s own maximum voluntary contraction (see next paragraph and Suppl. Fig. 1) and recorded as a data point in a time series. To create equal-length time series in all participants and allow for signal stability, we truncated each time series to contain 16 data points with common indexes among all participants (5th–20th) (Suppl. Fig. 2); we dropped data associated with the first 4 contractions because of a possible learning effect, and we dropped data associated with the last 10 contractions because some participants did not perform all 30 contractions (mean = 29; median = 30; range = 20–31 contractions).
Immediately before the repeated voluntary contractions were performed in the rest-between-contraction condition, all participants were asked to contract their PFM as strongly as possible so that we could estimate their maximum voluntary contraction. We manually identified the beginning and end of the PFM maximum contraction window in the EMG signal. Within this window, nonoverlapping 80-millisecond segments were extracted every 100 milliseconds, and the RMS value of each segment was calculated. The maximum voluntary contraction was estimated as the mean of the RMS values between the 10th and 40th segments to allow activity to rise to a maximum level and stabilize (Suppl. Fig. 1).
Furthermore, we examined cohort differences when the EMG outcome variable was assessed by measuring the peak-to-peak amplitude. Briefly, we applied the same steps described above for the RMS method, except for calculating the peak-to-peak amplitudes of the 80-millisecond segments instead of calculating the RMS values. This was to further validate the RMS method and the peak-to-peak amplitude method that we have used in our previous research,14 especially because analyzing resting muscle activity is not very common. Supplementary Figures 1 to 3 show comparisons of the 2 methods and their results.
Predictors of Resting PFM Activity
To build the statistical model of resting PFM activity, the magnitude of each resting EMG data point described above was associated with a unique participant identifier, their cohort (CP/CPPS or control), the condition (rest-only or rest-between-contraction), and responses to the 6 “yes/no” symptom questions from the NIH-CPSI described above.
Controlled Variables
We controlled for participant age. We aimed to match the age of the CP/CPPS and control cohorts; however, we entered age into the multivariate regression models to analyze cohort effects independent of age and to explore possible contributions of age to resting PFM activity because CP/CPPS can affect men at any age and aging can affect motor control.25,26
Statistical Methods
Previous work indicated that the NIH-CPSI, particularly the Pain or Discomfort domain, discriminates well between men with CP/CPPS and men without CP/CPPS12; thus, we used the total score of the Pain or Discomfort domain to confirm that the studied CP/CPPS and control cohorts were distinct on the basis of symptoms, because the CP/CPPS diagnosis has the limitation of being symptom based. We implemented a receiver operating characteristic analysis to estimate the performance of a logistic regression model in which the recorded total score of the NIH-CPSI Pain or Discomfort domain was the main predictor of the participant’s assigned cohort.
We treated the primary outcome, resting PFM activity, as a multi–data point repeated-measures variable. All data points were transformed using a natural log scale to reduce data skewness and minimize the influence of extreme values (Suppl. Fig. 2). Data skewness in resting muscle activity and its log transformation have been previously reported and implemented.5 We treated all predictors as categorical variables and centered the controlled variables around their means. Finally, inferences about impaired relaxation were obtained by examining the statistical significance of the percent difference in PFM resting activity in comparisons of PFM resting activity during the rest-between-contraction condition and PFM resting activity during the rest-only condition in men with CP/CPPS and men without CP/CPPS.
Regression models (SAS Studio 3.8; SAS Institute Inc, Cary, NC, USA) were used as follows. First, to investigate the possibility of impaired ability to relax PFM in men with CP/CPPS, we assessed whether there was a cohort–condition interaction that would imply differences between the CP/CPPS and control cohorts. A mixed-effects model of resting PFM activity was made on the basis of the interaction of cohort and condition, while controlling for age and a random effect of participant identifier (ID) in Wilkinson-Rogers notation: PFM activity ~1 + cohort + condition + (cohort : condition) + age + (1 | ID). This model was used to fit 2880 resting PFM data points (16 per condition × 2 conditions × 90 participants). Second, to test whether between-participant variance of resting PFM activity is a phenomenon that occurs in both cohorts, in each of the 4 cohort-condition subsets (control cohort during the rest-only condition; control cohort during the rest-between-contraction condition; CP/CPPS cohort during the rest-only condition; CP/CPPS cohort during the rest-between-contraction condition) the intraclass correlation coefficient (ICC) was calculated using the following model: PFM activity ~1 + age + (1 | ID). Finally, to investigate the possibility of an association between impaired ability to relax PFM and CP/CPPS pain symptoms, we assessed whether there was a condition–symptom interaction for any of the 6 CP/CPPS pain symptoms assessed by the NIH-CPSI Pain or Discomfort domain. In the subset of 42 men with CP/CPPS, the interaction between experimental condition and pain symptoms was quantified as follows: PFM activity ~1 + condition + symptom + (condition : symptom) + age + (1 | ID). This model was used to fit 1344 resting PFM data points (16 per condition × 2 conditions × 42 participants). This model was tested for each of the 6 CP/CPPS symptom variables of interest. Bonferroni correction was used to account for multiple comparisons.
Role of the Funding Source
The funders played no role in the role, design, conduct, or reporting of this study.
Results
Participants
We assessed 165 prospective participants: 75 were not studied (16 did not meet the inclusion criteria and 59 declined to participate), and 90 participants completed the study (42 men with CP/CPPS and 48 men in the control group). The use of the rectal sensor did not induce severe pain in any of the 90 participants. At the group level, pelvic pain did not increase after the insertion of the rectal sensor (P > .05 in the CP/CPPS cohort as well as in the control cohort). Description and characteristics of the studied participants are presented in Table 1. The CP/CPPS and control cohorts were not significantly different with regard to age, race, and ethnicity (P > .05) (Tab. 1). Thus, we did not remove any participant from the analyses. Finally, the recorded NIH-CPSI scores, in particular, the total score of the Pain or Discomfort domain, suggested that the recruited CP/CPPS and control cohorts had representative characteristics as previously described.10,27 In each of the 3 NIH-CPSI domains, on average, the CP/CPPS cohort scored significantly higher than the control cohort (P < .0001) (Tab. 1). Finally, we estimated a receiver operating characteristic area under the curve of 0.9928, indicating the high performance of the pain-based cohort classifier and indicating that we recruited CP/CPPS and control cohorts that were very different in their responses to the 6 pain questions that made up the NIH-CPSI Pain or Discomfort domain.
Outcome Data and Main Results
We verified that the observation of between-participant variance of resting PFM activity was a phenomenon that occurred in both cohorts. Between-participant variance was greater than within-participant variance of resting PFM activity in each of the 4 subsets: control cohort during the rest-only condition (ICC = 0.8215); control cohort during the rest-between-contraction condition (ICC = 0.8171); CP/CPPS cohort during the rest-only condition (ICC = 0.8877); and CP/CPPS cohort during the rest-between-contraction condition (ICC = 0.8902).
Cohort Differences in PFM Resting Activity
In the CP/CPPS cohort, PFM resting activity during the rest-between-contraction condition was significantly higher than that during the rest-only condition: the percent difference in PFM resting activity was +12.9% (percent difference estimate = +12.8738%; 95% CI = 8.6624%–17.2483%; P < .0001). In contrast, in the control cohort, there was no significant difference between the conditions: the percent difference in PFM resting activity was −0.3% (percent difference estimate = −0.3314%; 95% CI = −3.8147% to 3.2780%; P = .8551). Directly comparing the 2 cohorts confirmed a statistically significant difference in PFM resting activity between men with CP/CPPS and men without CP/CPPS (P < .0001). Figure 2 and Table 2 show population geometric mean resting PFM activity presented as the percent maximum voluntary contraction for each cohort and during each rest condition. We also found that both cohorts had similar PFM resting activities during either rest condition. The percent difference in PFM resting activity between the CP/CPPS cohort and the control cohort during the rest-only condition was as follows: percent difference estimate = −22.0966% (95% CI = −43.7880% to 7.9653%; P = .1339); and during the rest-between-contraction condition was as follows: percent difference estimate = −11.5736% (95% CI = −35.8440% to 21.8782%; P = .4527). This evidence of impaired ability to relax PFM in men with CP/CPPS was observed regardless of the EMG method used (Suppl. Fig. 3).
Figure 2.
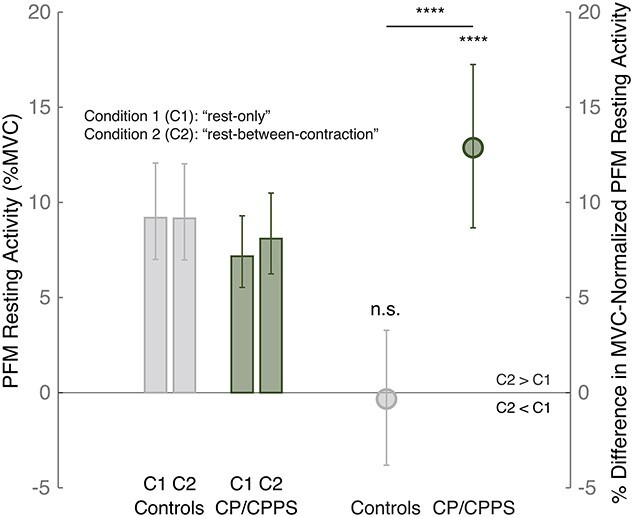
Evidence of impaired ability to relax the pelvic floor muscles (PFM) in men with chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS). Left: PFM resting activity (estimate of PFM resting activity as the percent maximum voluntary contraction [%MVC] and 95% CI) in controls and men with CP/CPPS during each rest condition (condition 1 [C1] [“rest-only”] and condition 2 [C2] [“rest-between-contraction”]). Right: Percent difference in MVC-normalized PFM resting activity in comparisons of activity during C2 and activity during C1 (estimate of percent difference in MVC-normalized PFM resting activity and 95% CI, along with the P value) in controls and men with CP/CPPS. ****P < .0001; n.s. = not significant.
Table 2.
PFM Resting Activity Differencea
| Parameter | N | C1 | C2 | % Difference in PFM Resting Activity for C2 vs C1 (95% CI) | P | ||
|---|---|---|---|---|---|---|---|
| Mean (% MVC) (95% CI) | P | Mean (% MVC) (95% CI) | P | ||||
| Control cohort | 48 | 9.1924 (7.0017 to 12.069) | <.0001 | 9.1620 (6.9787 to 12.0293) | <.0001 | −0.3314 (−3.8147 to 3.278) | .8551 |
| CP/CPPS cohort | 42 | 7.1695 (5.5295 to 9.2952) | <.0001 | 8.0923 (6.2414 to 10.4919) | <.0001 | 12.8738 (8.6624 to 17.2483) | <.0001 |
| Difference (CP/CPPS less control) | <.0001 | ||||||
| PFM resting activity difference regrouped by the presence or absence of different symptoms in 42 participants with CP/CPPS | |||||||
| Pain or discomfort during or after ejaculation? | |||||||
| No | 13 | 5.2574 (3.4191 to 8.0835) | <.0001 | 5.2015 (3.3831 to 7.9982) | <.0001 | −1.0613 (−7.4143 to 5.7276) | .7526 |
| Yes | 29 | 6.8569 (4.8196 to 9.7562) | <.0001 | 8.2103 (5.7707 to 11.6815) | <.0001 | 19.7337 (14.5300 to 25.1738) | <.0001 |
| Difference (yes less no) | <.0001b | ||||||
| Pain or discomfort in the perineum? | |||||||
| No | 11 | 9.4154 (5.5986 to 15.8329) | <.0001 | 9.3469 (5.5578 to 15.7178) | <.0001 | −0.7273 (−7.65 to 6.7143) | .8432 |
| Yes | 31 | 5.6399 (4.1097 to 7.7403) | <.0001 | 6.6624 (4.8548 to 9.1435) | <.0001 | 18.1282 (13.1494 to 23.326) | <.0001 |
| Difference (yes less no) | <.0001b | ||||||
| Pain or discomfort in the tip of the penis? | |||||||
| No | 18 | 6.7278 (4.2866 to 10.5603) | <.0001 | 7.1684 (4.5672 to 11.2515) | <.0001 | 6.5474 (0.67201 to 12.7657) | .0286 |
| Yes | 24 | 5.9119 (4.1807 to 8.3606) | <.0001 | 6.9677 (4.9272 to 9.8535) | <.0001 | 17.8568 (12.2078 to 23.7901) | <.0001 |
| Difference (yes less no) | .0084481b | ||||||
| Pain or discomfort in the pubic or bladder area? | |||||||
| No | 14 | 6.7526 (4.3021 to 10.5984) | <.0001 | 8.0183 (5.029 to 12.7838) | <.0001 | 18.7440 (11.3373 to 26.6435) | <.0001 |
| Yes | 28 | 6.0066 (4.2736 to 8.4431) | <.0001 | 6.6099 (4.7028 to 9.2911) | <.0001 | 10.0440 (5.146 to 15.1702) | <.0001 |
| Difference (yes less no) | .058638 | ||||||
| Pain or burning during urination? | |||||||
| No | 28 | 6.2923 (4.1544 to 9.5298) | <.0001 | 6.9116 (4.5634 to 10.4681) | <.0001 | 9.8428 (4.9537 to 14.9596) | .0001 |
| Yes | 14 | 6.0073 (4.0197 to 8.9782) | <.0001 | 7.1595 (4.7903 to 10.6995) | <.0001 | 19.1842 (11.7522 to 27.1104) | <.0001 |
| Difference (yes less no) | .042612 | ||||||
| Pain or discomfort in the testicles? | |||||||
| No | 18 | 6.7779 (4.477 to 10.2619) | <.0001 | 7.4347 (4.9109 to 11.2564) | <.0001 | 9.6891 (3.6283 to 16.1044) | .0015 |
| Yes | 24 | 5.8173 (4.0463 to 8.3628) | <.0001 | 6.7083 (4.666 to 9.6436) | <.0001 | 15.3153 (9.7774 to 21.1326) | <.0001 |
| Difference (yes less no) | .19201 | ||||||
a C1 = condition 1 (PFM resting activity during “rest-only”); C2 = condition 2 (PFM resting activity during “rest-between-contraction”); CP/CPPS = chronic prostatitis/chronic pelvic pain syndrome; MVC = maximum voluntary contraction; N = number of participants; PFM = pelvic floor muscles.
b Significant Bonferroni-corrected P value.
CP/CPPS Pain Symptoms and PFM Resting Activity
We found that 3 of the 6 CP/CPPS symptoms that we analyzed were significantly associated with PFM resting activity increases. The increase in mean PFM resting activity during the rest-between-contraction condition was the highest when the participants in the CP/CPPS cohort reported pain or discomfort during or after ejaculation; 29 of 42 men (~70%) with CP/CPPS reported ejaculatory pain. In participants reporting ejaculatory pain, the percent difference in PFM resting activity was +19.7% (percent difference estimate = +19.7337%; 95% CI = 14.5300% to 25.1738%; P < .0001). In contrast, for participants not reporting ejaculatory pain, the percent difference in PFM resting activity was −1.1% and was not significantly different from 0 (percent difference estimate = −1.0613%; 95% CI = −7.4143% to 5.7276%; P = .7526). We also identified possible increases in PFM resting activity during the rest-between-contraction condition when participants reported pain or discomfort in the perineum compared with those who did not, or when participants reported pain or discomfort in the tip of the penis compared with those who did not. We did not observe significant differences in PFM resting activity between the rest-only condition and the rest-between-contraction condition on the basis of the participants’ responses to the presence of pubic or bladder pain, testicle pain, or pain related to urination (Fig. 3; Tab. 2).
Figure 3.
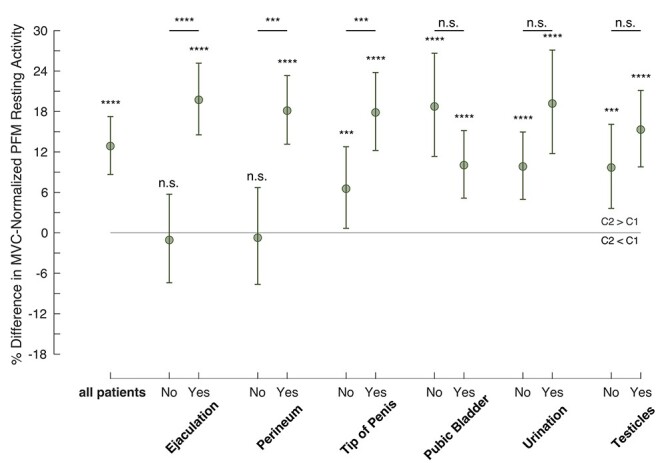
Evidence of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) symptom-specific associations with the impaired ability to relax the pelvic floor muscles (PFM). Data show the percent difference in maximum voluntary contraction (MVC)–normalized PFM resting activity in comparisons of activity during condition 2 (C2) (“rest-between-contraction”) and activity during condition 1 (C1) (“rest-only”) (estimate of percent difference in MVC-normalized PFM resting activity and 95% CI, along with the P value) in men with CP/CPPS, regrouped by reporting the presence (Yes) or absence (No) of different symptoms. ***P < .001; ****P < .0001; n.s. = not significant.
Discussion
In this largest cross-sectional case–control study of PFM resting activity in CP/CPPS to date, we provide evidence of the following. Men without CP/CPPS maintained a consistent amount of PFM resting activity regardless of whether the PFM was recently contracted. However, men with CP/CPPS had an increase in PFM resting activity that appeared to be related to repeated PFM contractions, suggesting an impaired ability of the central nervous system to relax PFM in men with CP/CPPS. This impaired ability to relax PFM was strongest in men who had CP/CPPS and who reported pain related to ejaculation.
Our study contributes to a growing literature on pelvic floor dysfunction in CP/CPPS and how it contributes to CP/CPPS symptoms.5–7,11,15,28 Our work provides evidence that the PFM in men with CP/CPPS may receive a combination of excessive neural excitation or insufficient inhibition. On the basis of our work, men with CP/CPPS that express pain related to ejaculation, the perineum, or the tip of the penis are more likely to have elevated resting PFM activity; these may constitute a symptom cluster related to different PFM, such as the ischiocavernosus and bulbospongiosus muscles (which are involved in ejaculation), the bulbospongiosus muscle (superficial muscle in the perineum), and the anterior levator ani muscle (which has been shown to refer pain to the tip of the penis).11,29,30 This conclusion is limited by collecting an aggregate signal from the pelvic floor without the possibility of detecting the individual contribution of each PFM. Technological advancement—as in the use of high-density surface electromyography—combined with comparing different verbal instructions for pelvic floor contractions can overcome this limitation.19,31 Results from our recent brain neuromodulation study in a small sample of participants with chronic pelvic pain suggested a possible imbalance between excitatory and inhibitory cortical control of PFM resting activity.14 It has been reported that several disorders involving neuromuscular dysfunction are also associated with reduced inhibitory control and inability to reduce neuromuscular drive, including spinal cord injury, dystonia, stroke, and Parkinson disease.32–35
Similar to previous work, in this work, we also do not provide evidence that men with CP/CPPS have increased PFM resting activity when it is measured during full rest. The only previous study comparing PFM resting activity between men with and without CP/CPPS similarly did not report significant group differences, though PFM resting activity during full rest may be a factor in a more complex multivariate prediction model of patient status.5 However, the value of cross-participant comparisons of PFM resting activity during full rest was questioned by subsequent work which showed very poor within-participant reliability of absolute PFM EMG activity measures across multiple sessions.36 Thus, in light of this prior work and our current study, we recommend within-participant designs that use electromyography to compare PFM activities in different states rather than across-participant designs that may be susceptible to variability because of unpredictable anatomic differences that vary the sensitivity of the EMG sensor to neuromuscular drive. Studies using ultrasound measurements of PFM structure have also pointed toward resting baseline differences between men with and without CP/CPPS7; thus future studies using both electromyography and ultrasound in the same CP/CPPS population may be useful to distinguish between PFM differences due to neural sources and those due to musculoskeletal sources.
Increased neural drive to the PFM may arise naturally as a result of central sensitization to ongoing nociceptive input at the spinal level,37 but our previous work has suggested extensive changes in PFM control circuitry even up to the supplementary motor area region of the brain.10,38 Increases in sympathetic output in men with CP/CPPS have also been observed.39,40 It is possible that increased PFM activity in this syndrome arises from more systemic arousal and response to perceived threat, coupled with a vicious cycle if the resulting disinhibition of PFM activity acts as a sensitizing stimulus causing pain and discomfort in the pelvic region.41,42 The success of early pelvic floor physical therapy studies for CP/CPPS support this interpretation, but more work is needed to define the mechanism of CP/CPPS with greater precision and identify patient subtypes.
Our study has implications for clinical care as well as new clinical trials. Pelvic floor physical therapy for CP/CPPS has 2 distinct components: therapist-guided PFM relaxation training and therapist-performed manual stretching of PFM. Manual stretching of PFM in CP/CPPS often involves the intrarectal digital stretching/compression of pelvic muscle groups by the physical therapist. Although manual stretching may be an effective means to reduce neural drive to muscle mediated by muscle afferents and spinal inhibitory reflexes,29 it may be less effective if the increased neural drive to the PFM in CP/CPPS is coming from another source. The pelvic floor is a region of the body that is very vulnerable, and we have shown that sensory input in the pelvic region may be prioritized for threat assessment by specific neural connections at the level of the brain.43 Clearly, care should be taken by physical therapists to only apply pelvic floor manual therapy when necessary. In light of our current work, a more targeted approach to manual physical therapy may involve progression: starting with pelvic muscle relaxation training in men reporting pain related to ejaculation, the perineum, or the penis tip and moving to manual therapy if symptom resolution is not adequate. Currently, an approach that applies relaxation training and manual therapy simultaneously is more commonly described in the pelvic floor physical therapy literature.11,15,44 Because of the psychosocial burden of CP/CPPS symptoms,45 the effectiveness of biopsychosocial approaches that add a cognitive behavioral therapy component to traditional physical therapy components is recently gaining much evidence and should be considered by physical therapists.4,28,46–48 Our work also provides the groundwork for future clinical trials that can test if different components of pelvic floor physical therapy can be targeted at specific CP/CPPS subgroups and whether patients would benefit from a progressive rather than simultaneous approach.
Supplementary Material
Contributor Information
Moheb S Yani, Division of Biokinesiology and Physical Therapy, University of Southern California, Los Angeles, California, USA.
Sandrah P Eckel, Division of Biostatistics, Department of Preventive Medicine, University of Southern California, Los Angeles, California, USA.
Daniel J Kirages, Division of Biokinesiology and Physical Therapy, University of Southern California, Los Angeles, California, USA.
Larissa V Rodriguez, Department of Urology, University of Southern California, Los Angeles, California, USA.
Daniel M Corcos, Department of Physical Therapy & Human Movement Sciences, Northwestern University, Chicago, Illinois, USA.
Jason J Kutch, Division of Biokinesiology and Physical Therapy, University of Southern California, Los Angeles, California, USA.
Author Contributions
Concept/idea/research design: M.S. Yani, D.J. Kirages, J.J. Kutch
Writing: M.S. Yani, S.P. Eckel, D.J. Kirages, D.M. Corcos, J.J. Kutch
Data collection: M.S. Yani
Data analysis: M.S. Yani, S.P. Eckel, D.M. Corcos, J.J. Kutch
Project management: M.S. Yani, J.J. Kutch
Fund procurement: D.M. Corcos, J.J. Kutch
Providing participants: D.J. Kirages
Providing facilities/equipment: D.J. Kirages, J.J. Kutch
Consultation (including review of manuscript before submitting): S.P. Eckel, D.J. Kirages, L.V. Rodriguez, D.M. Corcos
Funding
This study was funded by grants from the National Institutes of Health, National Institute of Diabetes and Digestive Kidney Diseases (R01DK110669, R01DK121724).
Ethics Approval
The University of Southern California Institutional Review Board approved this study.
Disclosures and Presentations
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
A portion of this study was presented as an oral presentation at the American Physical Therapy Association’s Combined Sections Meeting, February 2–5, 2022, San Antonio, Texas.
References
- 1. Landis JR, Williams DA, Lucia MS, et al. The MAPP research network: design, patient characterization and operations. BMC Urol. 2014;14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krieger JN, Ross SO, Deutsch L, Riley DE. The NIH consensus concept of chronic prostatitis/chronic pelvic pain syndrome compared with traditional concepts of nonbacterial prostatitis and prostatodynia. Curr Urol Rep. 2002;3:301–306. [DOI] [PubMed] [Google Scholar]
- 3. Clemens JQ, Kutch JJ, Mayer EA, et al. The multidisciplinary approach to the study of chronic pelvic pain (MAPP) research network: design and implementation of the symptom patterns study (SPS). Neurourol Urodyn. 2020;39:1803–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang J, Liang C, Shang X, Li H. Chronic prostatitis/chronic pelvic pain syndrome: a disease or symptom? Current perspectives on diagnosis, treatment, and prognosis. Am J Mens Health. 2020;14:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hetrick DC, Glazer H, Liu Y-W, Turner JA, Frest M, Berger RE. Pelvic floor electromyography in men with chronic pelvic pain syndrome: a case-control study. Neurourol Urodyn. 2006;25:46–49. [DOI] [PubMed] [Google Scholar]
- 6. Cornel EB, van Haarst EP, Schaarsberg RWMB-G, Geels J. The effect of biofeedback physical therapy in men with chronic pelvic pain syndrome type III. Eur Urol. 2005;47:607–611. [DOI] [PubMed] [Google Scholar]
- 7. Davis SN, Morin M, Binik YM, Khalife S, Carrier S. Use of pelvic floor ultrasound to assess pelvic floor muscle function in urological chronic pelvic pain syndrome in men. J Sex Med. 2011;8:3173–3180. [DOI] [PubMed] [Google Scholar]
- 8. Cho DS, Choi JB, Kim YS, et al. Heart rate variability in assessment of autonomic dysfunction in patients with chronic prostatitis/chronic pelvic pain syndrome. Urology. 2011;78:1369–1372. [DOI] [PubMed] [Google Scholar]
- 9. Farmer MA, Chanda ML, Parks EL, Baliki MN, Apkarian AV, Schaeffer AJ. Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2011;186:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kutch JJ, Yani MS, Asavasopon S, et al. Altered resting state neuromotor connectivity in men with chronic prostatitis/chronic pelvic pain syndrome: a MAPP research network neuroimaging study. Neuroimage Clin. 2015;8:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson RU, Wise D, Sawyer T, Chan C. Integration of myofascial trigger point release and paradoxical relaxation training treatment of chronic pelvic pain in men. J Urol. 2005;174:155–160. [DOI] [PubMed] [Google Scholar]
- 12. Litwin MS. A review of the development and validation of the National Institutes of Health chronic prostatitis symptom index. Urology. 2002;60:14–18. [DOI] [PubMed] [Google Scholar]
- 13. Litwin MS, McNaughton-Collins M, Fowler FJ, et al. The National Institutes of Health chronic prostatitis symptom index: development and validation of a new outcome measure. J Urol. 1999;162:369–375. [DOI] [PubMed] [Google Scholar]
- 14. Yani MS, Fenske SJ, Rodriguez LV, Kutch JJ. Motor cortical neuromodulation of pelvic floor muscle tone: potential implications for the treatment of urologic conditions. Neurourol Urodyn. 2019;38:1517–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fitzgerald MP, Anderson RU, Potts J, et al. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J Urol. 2013;189:S75–S85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pontari MA, Ruggieri MR. Mechanisms in prostatitis/chronic pelvic pain syndrome. J Urol. 2008;179:S61–S67. [DOI] [PubMed] [Google Scholar]
- 17. Yani MS, Wondolowski JH, Eckel SP, et al. Distributed representation of pelvic floor muscles in human motor cortex. Sci Rep. 2018;8:7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Asavasopon S, Rana M, Kirages DJ, et al. Cortical activation associated with muscle synergies of the human male pelvic floor. J Neurosci. 2014;34:13811–13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stafford RE, Ashton-Miller JA, Constantinou C, Coughlin G, Lutton NJ, Hodges PW. Pattern of activation of pelvic floor muscles in men differs with verbal instructions. Neurourol Urodyn. 2016;35:457–463. [DOI] [PubMed] [Google Scholar]
- 20. Rana M, Yani MS, Asavasopon S, Fisher BE, Kutch JJ. Brain connectivity associated with muscle synergies in humans. J Neurosci. 2015;35:14708–14716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Merletti R, Bottin A, Cescon C, et al. Multichannel surface EMG for the non-invasive assessment of the anal sphincter muscle. Digestion. 2004;69:112–122. [DOI] [PubMed] [Google Scholar]
- 22. Besomi M, Hodges PW, Clancy EA, et al. Consensus for experimental design in electromyography (CEDE) project: amplitude normalization matrix. J Electromyogr Kinesiol. 2020;53:102438. [DOI] [PubMed] [Google Scholar]
- 23. Merletti R. Standards for reporting EMG data. J Electromyogr Kinesiol. 1999;9:3–4. [Google Scholar]
- 24. Hodges PW, Sapsford R, Pengel LHM. Postural and respiratory functions of the pelvic floor muscles. Neurourol Urodyn. 2007;26:362–371. [DOI] [PubMed] [Google Scholar]
- 25. Seidler RD, Bernard JA, Burutolu TB, et al. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34:721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pontari MA. Chronic prostatitis/chronic pelvic pain syndrome in elderly men: toward better understanding and treatment. Drugs Aging. 2003;20:1111–1125. [DOI] [PubMed] [Google Scholar]
- 27. Propert KJ, Litwin MS, Wang Y, et al. Responsiveness of the National Institutes of Health chronic prostatitis symptom index (NIH-CPSI). Qual Life Res. 2006;15:299–305. [DOI] [PubMed] [Google Scholar]
- 28. Doiron RC, Shoskes DA, Nickel JC. Male CP/CPPS: where do we stand? World J Urol. 2019;37:1015–1022. [DOI] [PubMed] [Google Scholar]
- 29. Weiss JM. Pelvic floor myofascial trigger points: manual therapy for interstitial cystitis and the urgency-frequency syndrome. J Urol. 2001;166:2226–2231. [DOI] [PubMed] [Google Scholar]
- 30. Hubbard DR, Berkoff GM. Myofascial trigger points show spontaneous needle EMG activity. Spine. 1993;18:1803–1807. [DOI] [PubMed] [Google Scholar]
- 31. Dias N, Zhang C, Spitznagle T, Lai HH, Zhang Y. High-density surface electromyography assessment of pelvic floor dysfunction in women with interstitial cystitis/bladder pain syndrome. J Urol. 2020;204:1275–1283. [DOI] [PubMed] [Google Scholar]
- 32. Murray KC, Stephens MJ, Rank M, D’Amico J, Gorassini MA, Bennett DJ. Polysynaptic excitatory postsynaptic potentials that trigger spasms after spinal cord injury in rats are inhibited by 5-HT1B and 5-HT1F receptors. J Neurophysiol. 2011;106:925–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hallett M. Neurophysiology of dystonia: the role of inhibition. Neurobiol Dis. 2011;42:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pinto CB, Saleh Velez FG, Lopes F, et al. SSRI and motor recovery in stroke: reestablishment of inhibitory neural network tonus. Front Neurosci. 2017;11:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Corcos DM, Chen CM, Quinn NP, McAuley J, Rothwell JC. Strength in Parkinson’s disease: relationship to rate of force generation and clinical status. Ann Neurol. 1996;39:79–88. [DOI] [PubMed] [Google Scholar]
- 36. Auchincloss CC, McLean L. The reliability of surface EMG recorded from the pelvic floor muscles. J Neurosci Methods. 2009;182:85–96. [DOI] [PubMed] [Google Scholar]
- 37. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Clemens JQ, Mullins C, Ackerman AL, et al. Urologic chronic pelvic pain syndrome: insights from the MAPP research network. Nat Rev Urol. 2019;16:187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yilmaz U, Liu Y-W, Berger RE, Yang CC. Autonomic nervous system changes in men with chronic pelvic pain syndrome. J Urol. 2007;177:2170–2174. [DOI] [PubMed] [Google Scholar]
- 40. Kleinhans NM, Yang CC, Strachan ED, Buchwald DS, Maravilla KR. Alterations in connectivity on functional magnetic resonance imaging with provocation of lower urinary tract symptoms: a MAPP research network feasibility study of urological chronic pelvic pain syndromes. J Urol. 2016;195:639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fenske SJ, Bierer D, Chelimsky G, et al. Sensitivity of functional connectivity to periaqueductal gray localization, with implications for identifying disease-related changes in chronic visceral pain: a MAPP research network neuroimaging study. Neuroimage Clin. 2020, 28:102443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vanegas H, Schaible H-G. Descending control of persistent pain: inhibitory or facilitatory? Brain Res Rev. 2004;46:295–309. [DOI] [PubMed] [Google Scholar]
- 43. Hegarty AK, Yani MS, Albishi A, Michener LA, Kutch JJ. Salience network functional connectivity is spatially heterogeneous across sensorimotor cortex in healthy humans. NeuroImage. 2020;221:117177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Anderson RU, Wise D, Nathanson BH. Chronic prostatitis and/or chronic pelvic pain as a psychoneuromuscular disorder—a meta-analysis. Urology. 2018;120:23–29. [DOI] [PubMed] [Google Scholar]
- 45. Brünahl C, Dybowski C, Albrecht R, et al. Mental disorders in patients with chronic pelvic pain syndrome (CPPS). J Psychosom Res. 2017;98:19–26. [DOI] [PubMed] [Google Scholar]
- 46. Ariza-Mateos MJ, Cabrera-Martos I, Ortiz-Rubio A, Torres-Sánchez I, Rodríguez-Torres J, Valenza MC. Effects of a patient-centered graded exposure intervention added to manual therapy for women with chronic pelvic pain: a randomized controlled trial. Arch Phys Med Rehabil. 2019;100:9–16. [DOI] [PubMed] [Google Scholar]
- 47. Coronado RA, Brintz CE, McKernan LC, et al. Psychologically informed physical therapy for musculoskeletal pain: current approaches, implications, and future directions from recent randomized trials. Pain Rep. 2020;5:e847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tripp DA, Curtis Nickel J, Katz L. A feasibility trial of a cognitive-behavioural symptom management program for chronic pelvic pain for men with refractory chronic prostatitis/chronic pelvic pain syndrome. Can Urol Assoc J. 2011;5:328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.