Abstract
Late biomaterial-centered infection is a major complication associated with the use of biomaterial implants. In this study biomaterials that had been implanted subcutaneously in rats were hematogenously challenged with bacteria 4 weeks after implantation. Bacteria were spread either by intravenous injection or by stimulation of bacterial translocation. It was found that none of the biomaterials was infected by hematogenous spread, whereas 5% of the implants were infected by perioperative contamination. We conclude that late hematogenous infection of subcutaneous biomaterials does not occur in the rat. For humans as well, there are growing doubts whether implants actually become infected through hematogenous routes; it is thought that late infections may be caused by delayed appearance of perioperatively introduced bacteria.
A severe complication associated with the use of biomaterial implants is failure due to infection. About half of all biomaterial-centered infections occur months to years after deep tissue implantation. Controversy exists concerning the origin of the infecting microorganisms in these late infections. Either bacteria spread hematogenously from endogenous foci or they are inserted during implantation and stay clinically unnoticed for a long time; the latter are referred to as delayed infections (1).
Most hematogenous infections are believed to arise from infected skin lesions producing relapsing bacteremia (2). This is supported by the fact that in most (56%) infections where hematogenous spreading is suspected, staphylococci, which are part of the normal skin flora, are involved. Dental or other surgical interventions, bacteriuria, intestinal surgery, and pneumonia have also been proposed as possible causes of hematogenous spreading of bacteria. Another possible mechanism for hematogenous spreading from the intestinal tract is bacterial translocation (BT) (24), i.e., the escape of mainly gram-negative rods through the intestinal wall (19).
BT can be promoted by nutritional factors, such as total parenteral nutrition, fluid elemental nutrition, protein malnutrition (8), and vitamin A deficiency (25), hemorrhagic shock, extensive thermal injury, or endotoxins (10). Interestingly, intraperitoneal implants also promote BT (13, 18).
In animal studies on biomaterial-centered infections, human-derived bacteria are frequently used. In humans, however, biomaterial-centered infections are caused in most cases by the body's own commensal microflora, toward which the immune system is more tolerant than it is to foreign flora (3, 9). Since tolerated microorganisms probably survive longer in the circulatory system, it can be expected that their chances of causing biomaterial-centered infections are greater than those of nonimmunotolerated microorganisms.
The aim of this study was to determine whether hematogenous spreading of bacteria, after healing of the implantation wound, infects subcutaneous (s.c.) implants in rats. To this end, rats were intravenously (i.v.) injected either with Staphylococcus aureus, Staphylococcus epidermidis, or Pseudomonas aeruginosa or with their own total fecal flora 4 weeks after implantation of a biomaterial. To investigate the possibility of infection with translocating intestinal bacteria, BT was promoted either by special diets or by an intraperitoneal implant.
MATERIALS AND METHODS
Rats.
Forty-eight male, 12-week-old, specific-pathogen-free albino Oxford rats weighing 220 to 260 g were used. The animals were housed in a standard temperature-controlled environment (22°C) in Macrolon cages and were kept on a 12-h light-dark cycle. The rats were fed normal rat chow, unless otherwise stated, and had sterile tap water supplied ad libitum. Animals were allowed to acclimatize to our laboratory conditions for 2 weeks before the experiments. All animals received humane care in compliance with the principles of the National Institutes of Health (18a) and the Dutch Law on Experimental Animal Care.
Bacteria.
Human-derived S. aureus ATCC 12600 and S. epidermidis HBH2 102 were cultured in tryptone soy broth (Oxoid, Basingstoke, United Kingdom) in phosphate-buffered saline, and human-derived P. aeruginosa AK1 was cultured in nutrient broth (Oxoid) in phosphate-buffered saline. First, a strain was streaked and grown overnight at 37°C from a frozen stock on a blood agar plate. A colony was used to inoculate 5 ml of growth medium, which was incubated at 37°C in ambient air for 24 h and used to inoculate a second culture (150 ml) that was grown for 18 h. The bacteria from the second culture were harvested by centrifugation (for 5 min, at 5,000 × g for staphylococci and 10,000 × g for P. aeruginosa) and washed twice with sterile Millipore-Q water. Subsequently, the bacteria were resuspended in sterile 0.9% NaCl solution, and S. epidermidis was sonicated on ice to disrupt aggregates.
Gut bacteria were harvested from fresh feces of the rats. The feces were suspended in 10 ml of anaerobic 0.9% NaCl solution. The suspension was centrifuged for 2 min at 250 × g to remove larger particles. Supernatants were centrifuged at 10,000 × g for 20 min to spin down the bacteria. Finally, the pellets were suspended in 10 ml of 0.9% NaCl. The fecal flora was cultured on specific agars and demonstrated to contain anaerobic bacteria (70%), Escherichia coli (20%), lactobacilli (7%), and streptococci (3%).
Biomaterials.
Disks (diameter, 8 mm; thickness, 0.5 mm) without sharp edges were made of commercially available silicone rubber (SR), polyethylene (PE), polypropylene (PP), poly(tetrafluoroethylene) (PTFE), poly(ethylene terephthalate) (PET), poly(methyl methacrylate) (PMMA), polyurethane (PU) (Pellethane 2363-75D), or glass. The disks were cleaned in a 2% RBS 25 detergent solution under simultaneous sonication, thoroughly rinsed in demineralized water, sterilized in 70% ethanol, and finally washed with sterile Millipore-Q water.
Implantation.
Each rat received only four subcutaneous biomaterial disks, since space was limited. After induction of anesthesia by inhalation of N2O–O2 (at a 3:2 ratio) and halothane, the backs of the rats were shaved and disinfected with 0.5% chlorhexidine in 70% ethanol. Four 1-cm incisions were made, two on either side of the middle line, at least 3 cm apart. Subcutaneous pockets at least 2 cm deep were created. The four different implants were inserted as deeply as possible. The incision was then closed with degradable suture material. The surgical instruments used were disinfected after each surgical action.
i.v. inoculation experiment.
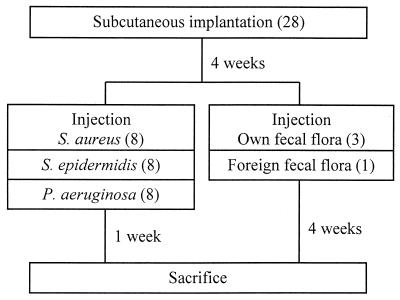
Figure 1 shows the experimental design used for i.v. inoculation. Half of the 28 rats received one disk each of SR, PTFE, PP, and PE, while the other half received one disk each of PU, PET, PMMA, and glass. After 4 weeks, 0.5 ml of a bacterial suspension was injected into the tail vein. In each biomaterial group 12 rats were each injected with a different one of the following 12 bacterial suspensions: 3 × 107, 1 × 108, 3 × 108, or 1 ×109 CFU of S. aureus or S. epidermidis/ml or 1 × 108, 3 × 108, 1 × 109, or 3 × 109 CFU of P. aeruginosa/ml. Three other rats received suspensions with 3 × 108, 1 × 109, or 3 × 109 CFU of their own fecal flora/ml, while the fecal flora of the last rat (at 3 × 109 CFU/ml) was also injected into one other rat, which thus received foreign fecal flora.
FIG. 1.
Design of i.v. injection experiments, involving a total of 28 rats, which were subsequently divided into five groups according to the infecting organism. The number of rats in each group is given in parentheses.
Stimulated BT experiment.
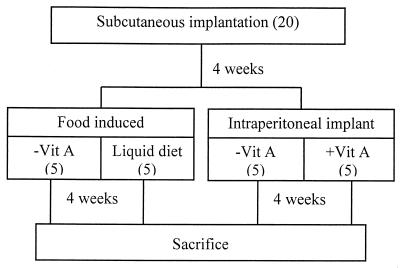
Figure 2 shows the experimental design used to stimulate BT. The rats in this experiment all received one disk each of SR, PTFE, PET, and PMMA. Five rats were fed normal rat chow, while the other 15 were fed vitamin A-free rat chow (Hope Farms, Woerden, The Netherlands), starting directly after s.c. implantation, since vitamin A deficiency has been reported to occur 4 weeks after the onset of the diet (25). Four weeks after implantation, the diet of five vitamin A-deficient rats was changed to a total liquid elemental nutrition formula (Nutrison powder; Nutricia, Zoetermeer, The Netherlands), which contained vitamin A, made according to the manufacturer's instructions with sterile demineralized water in sterile drinking bottles. Also at 4 weeks after s.c. implantation, a proteograft patch (dimensions, 3.3 by 3.3 cm; similar to Dacron velour material; Braun, Oss, The Netherlands) was intraperitoneally implanted in five vitamin A-deficient rats and in the five rats on normal rat chow. To this end the rats were anesthetized with N2O–O2 (at a 3:2 ratio) and halothane, and their anterior sides were shaved and disinfected. A 5-cm incision was made in the skin longitudinally over the middle line. Then a 4-cm incision was made in the abdominal wall. The implant was inserted close to the gut, and care was taken that it would not irritate the bladder or the liver. The abdominal wall was closed, and the skin was closed separately. The rats received painkillers (Temgesic at 0.1 mg/kg of body weight/day) postoperatively for 1 week.
FIG. 2.
Design of BT experiments, involving a total of 20 rats, divided into four groups: rats on a vitamin A-deficient diet (−Vit A), rats on a liquid diet, and rats with an intraperitoneal implant with or without vitamin A deficiency. The number of rats in each group is given in parentheses.
Harvesting.
After induction of anesthesia with N2O–O2 (at a 3:2 ratio) and halothane, the backs of the rats were shaved and disinfected. The s.c. implants were explanted and stored in 5 ml of sterile reduced transport fluid (RTF). Swabs of the pockets were taken and streaked onto blood agar. Subsequently, the anterior side of each rat was shaved and disinfected. The abdominal cavity and chest were opened through a midline incision, and 0.1 ml of ventricular blood, a swab of the inside of the abdominal wall, the intraperitoneal implant when appropriate, a halved kidney, the halved spleen, and a section of the liver were streaked onto blood agar plates. In the BT experiment a section of the lungs was also taken and streaked onto blood agar, and the mesenteric lymph nodes (MLN) were harvested and homogenized in 5 ml of RTF. The rats were killed by a cut in the heart. The MLN suspension and the biomaterials in RTF were sonicated on ice for 5 min to remove the attached bacteria and then cultured on blood agar. The blood agar plates were incubated aerobically at 37°C. The s.c. implants from the rats injected with fecal microflora and from the rats in the BT experiment, including the intraperitoneal implant, were also cultured anaerobically. The MLN were cultured anaerobically as well. The plated samples were considered infected if more than 2 of the same colonies were found on the agar plate (corresponding to more than 100 CFU/biomaterial disk). Bacteria harvested were characterized by colony morphology and Gram staining.
RESULTS
i.v. inoculation experiment.
The two rats that had received the highest dose of S. aureus and the rat receiving the highest dose of its own fecal flora were killed within 4 days because of severe illness and excluded from the study. The rat receiving foreign fecal flora died after 17 days due to sepsis but was not excluded from the study.
Table 1 shows the numbers of positive organs and biomaterial cultures, together with the numbers of CFU isolated from the disks. Out of the 100 disks implanted in the i.v. injection model, 92 showed no infections. Moreover, most of the infected biomaterial disks revealed bacterial strains different from those used for injection. These were staphylococci on both PE disks, while P. aeruginosa was used for injection; two staphylococcal strains on one PU disk with a colony morphology on agars different from that of the injected staphylococcal strain; and a staphylococcal and a streptococcal strain on the other PU disk, as also determined from colony morphology on specific agars. The SR disk was infected with two different strains of gram-positive rods, as established after Gram staining and microscopic examination.
TABLE 1.
Biomaterial-centered infections in the i.v. injection and BT models
| Treatment or condition | Positive organ(s) (no. of positive organs/ total no. of animals) | Positive biomaterial disk(s)
|
|
|---|---|---|---|
| Type (no. of positive disks/ total no. of animals) | 103 CFU | ||
| i.v. injection model | |||
| S. epidermidis HBH2 102 | Kidney (8/8) | PUa (1/4) | 8.2 |
| S. aureus ATCC 12600 | Kidney (8/8) | PUa (1/4); SRa (1/4) | 1.4; 29 |
| P. aeruginosa AK1 | None | PEa (2/4) | 100 |
| Own fecal flora | None | None | |
| Foreign fecal flora | All organs | SR (1/1); PP (1/1); PE (1/1) | 15; 2.5; 1.0 |
| BT model | |||
| Vitamin A deficiency | None | None | |
| Liquid diet | None | SRb (1/5); PMMAb (1/5) | 0.45; 100 |
| Intraperitoneal implant and vitamin A deficiency | MLN (3/5); kidney (1/5); peritoneum (1/5); intraperitoneal implant (1/5) | None | |
| Intraperitoneal implant | MLN (4/5); kidney (1/5); liver (1/5); spleen (1/5); blood (1/5); peritoneum (1/5) | PETb (1/5); PTFEb (1/5) | 4.5; 10 |
Colony form did not correspond to that of the injected strain.
Colony form and Gram staining did not correspond to those of translocated bacteria.
Stimulated BT experiment.
Results for the BT experiment are also shown in Table 1. BT to the MLN and some organs was observed only in the intraperitoneal-implant group and was found to be due to gram-negative bacilli (E. coli) and gram-positive branched rods (Actinomyces spp.). Out of the 80 implanted disks involved in the BT model, 76 showed no infections. Moreover, the translocating species were not found on the infected biomaterial disks. A PET disk showed two different strains of staphylococci, while the other infected disks revealed one staphylococcal strain.
DISCUSSION
In this study, the susceptibility of s.c. implanted biomaterials to late hematogenous infection was determined in rats 4 weeks after biomaterial implantation. Two routes of hematogenous spreading of bacteria were used: either single i.v. injection of bacteria as a model for transient bacteremia or promoted BT. None of the biomaterial disks in the nonseptic rats became infected by i.v.-injected bacterial strains, but five biomaterial disks revealed bacteria probably originating from perioperative contamination. The four infected biomaterial disks in the stimulated BT group showed exclusively staphylococcal strains, suggesting that translocation from the intestinal tract was not the source of infection, since staphylococci are numerous on the skin but scarce in the gut flora (21). Furthermore, no staphylococci were found in the MLN, indicating absence of staphylococcal translocation. Most probably, these bacteria too originated from perioperative contamination. Perioperative contamination is likely to occur in animal experiments because usually no ultraclean operating rooms are used and antibiotic prophylaxis is not common (4, 7). All infected biomaterials, were relatively hydrophobic, while glass, a hydrophilic material, was not involved in any biomaterial-centered infection. This corresponds to earlier in vitro findings that surface growth of staphylococci is slow on glass compared with that on other materials (11).
To our knowledge, the use of late hematogenous infection models with s.c. implanted biomaterials has not been reported in rats before. In mice (16), i.v. injection of 107 S. aureus organisms did not yield infection of s.c. implanted biomaterials at 1 month after implantation, while a higher dose, 108 S. aureus organisms, killed the mice. However, Blomgren and Lindgren (6) successfully induced hematogenous infections in 40% of rabbits 6 to 8 weeks after total joint replacement by i.v. injection of approximately 109 S. aureus organisms (note that this is a 10- to 100-fold-higher dose than that used in our study). Hematogenous infection directly after implantation yielded infection in 80% of the animals (5). Southwood et al. (23) also showed a similar decrease in the hematogenous infection rate in rabbits, from 40% immediately after surgery to 10% at 3 weeks after implantation. Vascular grafts in dogs were infected by i.v. challenge with 108 S. aureus organisms 3 to 6 months after implantation, yielding infections in 10 to 80% of the animals, depending on the type of graft (17). Interestingly, a similar study with rats revealed that the infection rate of caval vein grafts was reduced from 100% to zero during 2 weeks of implantation as a result of increased endothelialization, while the infection rate of aorta grafts was still 100% after these 2 weeks (26).
Evidently, hematogenous biomaterial-centered infections can be induced directly after implantation but are much more difficult to achieve after prolonged implantation times. Essentially, whether biomaterial-centered infections occur is determined by a race for the surface (12) between infecting microorganisms and host cells. When infecting organisms arrive on a biomaterial surface long after implantation, the race is won in most cases by host cells and the biomaterial surface is out of reach for adhering organisms. Yet many late biomaterial-centered infections in humans, most notably those associated with orthopedic implants, are said to be hematogenous in origin (1, 2, 4, 15), although it is also suggested that infection by the hematogenous route will occur only in immunocompromised patients. For example, 40 to 100% of all late hematogenous orthopedic implant infections are found in patients with rheumatoid arthritis (20) using immunomodulating drugs. Late infections associated with dental procedures have been reported mostly for diseased patients with drug- or irradiation-induced immunosuppression, insulin-dependent diabetes mellitus, or hemophilia (14). At this point it must be noted that in clinical practice infection is often assumed to be of hematogenous origin without any attempt to obtain proof, for instance, by culturing blood or joint fluids (1). As many strains, including S. aureus and gram-negative rods, can survive intracellularly in epithelial and scar tissues, thereby circumventing the host's immune system for prolonged periods (22), it is suggested more and more that many biomaterial-centered infections assumed to be of hematogenous origin actually result from delayed appearance of perioperatively introduced bacteria. These suggestions are in line with the results of this study, demonstrating that it is virtually impossible, despite the high injection dose used, to create a biomaterial-centered infection in rats by the hematogenous route. Of course, differences between the immune systems of rats and humans may be of crucial importance here, since rats are notoriously resistant to bacterial infections.
REFERENCES
- 1.Ahlberg A, Carlsson A S, Lindberg L. Hematogenous infection in total joint replacement. Clin Orthop. 1978;137:69–75. [PubMed] [Google Scholar]
- 2.Bengtson S, Blomgren G, Knutson K, Wigren A, Lidgren L. Hematogenous infection after knee arthroplasty. Acta Orthop Scand. 1987;58:529–534. doi: 10.3109/17453678709146393. [DOI] [PubMed] [Google Scholar]
- 3.Berg R D, Savage D C. Immune responses of specific pathogen-free and gnotobiotic mice to antigens of indigenous and nonindigenous microorganisms. Infect Immun. 1975;11:320–329. doi: 10.1128/iai.11.2.320-329.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackburn W D, Alarcon G S. Prosthetic joint infections. A role for prophylaxis. Arthritis Rheum. 1991;34:110–117. doi: 10.1002/art.1780340118. [DOI] [PubMed] [Google Scholar]
- 5.Blomgren G, Lindgren U. The susceptibility of total joint replacement to hematogenous infection in the early postoperative period: an experimental study in the rabbit. Clin Orthop. 1980;151:308–312. [PubMed] [Google Scholar]
- 6.Blomgren G, Lindgren U. Late hematogenous infection in total joint replacement: studies of gentamicin and bone cement in the rabbit. Clin Orthop. 1981;155:244–248. [PubMed] [Google Scholar]
- 7.Dankert J, Hogt A H, Feijen J. Biomedical polymers: bacterial adhesion, colonization and infection. Crit Rev Biocompat. 1986;2:219–301. [Google Scholar]
- 8.Deitch E A. Bacterial translocation: the influence of dietary variables. Gut. 1994;35:S23–S27. doi: 10.1136/gut.35.1_suppl.s23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duchmann R, Schmitt E, Knolle P, Meyer zum Buschenfelde K H, Neurath M. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur J Immunol. 1996;26:934–938. doi: 10.1002/eji.1830260432. [DOI] [PubMed] [Google Scholar]
- 10.Edmiston C E, Condon R E. Bacterial translocation. Surg Gynecol Obstet. 1991;173:73–83. [PubMed] [Google Scholar]
- 11.Gottenbos B, van der Mei H C, Busscher H J. Initial adhesion and surface growth of Staphylococcus epidermidis and Pseudomonas aeruginosa on biomedical polymers. J Biomed Mater Res. 2000;50:208–214. doi: 10.1002/(sici)1097-4636(200005)50:2<208::aid-jbm16>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 12.Gristina A G. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237:1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 13.Guo W, Andersson R, Ljungh A, Wang X D, Bengmark S. Enteric bacterial translocation after intraperitoneal implantation of rubber drain pieces. Scand J Gastroenterol. 1993;28:393–400. doi: 10.3109/00365529309098238. [DOI] [PubMed] [Google Scholar]
- 14.LaPorte D M, Waldman B J, Mont M A, Hungerford D S. Infections associated with dental procedures in total hip arthroplasty. J Bone Joint Surg Br. 1999;81:56–59. doi: 10.1302/0301-620x.81b1.8608. [DOI] [PubMed] [Google Scholar]
- 15.Maniloff G, Greenwald R, Laskin R, Singer C. Delayed postbacteremic prosthetic joint infection. Clin Orthop. 1987;223:194–197. [PubMed] [Google Scholar]
- 16.Merritt K, Shafer J W, Brown S A. Implant site infection rates with porous and dense materials. J Biomed Mater Res. 1979;13:101–108. doi: 10.1002/jbm.820130111. [DOI] [PubMed] [Google Scholar]
- 17.Moore W S, Malone J M, Keown K. Prosthetic arterial graft material. Influence on neointimal healing and bacteremic infectibility. Arch Surg. 1980;115:1379–1383. doi: 10.1001/archsurg.1980.01380110111017. [DOI] [PubMed] [Google Scholar]
- 18.Mora E M, Cardona M A, Simmons R L. Enteric bacteria and ingested inert particles translocate to intraperitoneal prosthetic materials. Arch Surg. 1991;126:157–163. doi: 10.1001/archsurg.1991.01410260041006. [DOI] [PubMed] [Google Scholar]
- 18a.National Institutes of Health. Principles of laboratory animal care. National Institutes of Health publication no. 86-23. Bethesda, Md: National Institutes of Health; 1985. [Google Scholar]
- 19.O'Boyle C J, MacFie J, Mitchell C J, Johnstone D, Sagar P M, Sedman P C. Microbiology of bacterial translocation in humans. Gut. 1998;42:29–35. doi: 10.1136/gut.42.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poss R, Thornhill T S, Ewald F C, Thomas W H, Batte N J, Sledge C B. Factors influencing the incidence and outcome of infection following total joint arthroplasty. Clin Orthop. 1984;182:117–126. [PubMed] [Google Scholar]
- 21.Printzen G. Relevance, pathogenicity and virulence of microorganisms in implant related infections. Injury. 1996;27(Suppl. 3):SC9–SC15. doi: 10.1016/0020-1383(96)89026-7. [DOI] [PubMed] [Google Scholar]
- 22.Smith R P, Baltch A L, Franke M A, Michelsen P B, Bopp L H. Levofloxacin penetrates human monocytes and enhances intracellular killing of Staphylococcus aureus and Pseudomonas aeruginosa. J Antimicrob Chemother. 2000;45:483–488. doi: 10.1093/jac/45.4.483. [DOI] [PubMed] [Google Scholar]
- 23.Southwood R T, Rice J L, McDonald P J, Hakendorf P H, Rozenbilds M A. Infection in experimental hip arthroplasties. J Bone Joint Surg Br. 1985;67:229–231. doi: 10.1302/0301-620X.67B2.3980532. [DOI] [PubMed] [Google Scholar]
- 24.Van-Leeuwen P A, Boermeester M A, Houdijk A P, Ferwerda C C, Cuesta M A, Meyer S, Wesdorp R I. Clinical significance of translocation. Gut. 1994;35:S28–S34. doi: 10.1136/gut.35.1_suppl.s28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiedermann U, Hanson L A, Bremell T, Kahu H, Dahlgren U I. Increased translocation of Escherichia coli and development of arthritis in vitamin A-deficient rats. Infect Immun. 1995;63:3062–3068. doi: 10.1128/iai.63.8.3062-3068.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zdanowski Z, Hallberg E, Schalen C, Ribbe E. Reduced susceptibility of polytetrafluoroethylene vascular prostheses to colonization by Staphylococcus aureus following in situ endothelialization. Artif Organs. 1994;18:448–453. doi: 10.1111/j.1525-1594.1994.tb02231.x. [DOI] [PubMed] [Google Scholar]