Abstract
Gestational Bisphenol A (BPA) exposure leads to peripheral insulin resistance, and hepatic and skeletal muscle oxidative stress and lipotoxicity during adulthood in the female sheep offspring. To investigate transcriptional changes underlying the metabolic outcomes, coding and non-coding (nc) RNA in liver and muscle from 21-month-old control and prenatal BPA-treated (0.5mg/kg/day from days 30 to 90 of gestation; Term: 147days) female sheep were sequenced. Prenatal BPA-treatment dysregulated: expression of 194 genes (138 down, 56 up) in liver and 112 genes (32 down, 80 up) in muscle (FDR<0.05 and abs log2FC>0.5); 155 common gene pathways including mitochondrial-related genes in both tissues; 1415 gene pathways including oxidative stress and lipid biosynthetic process specifically in the liver (FDR<0.01); 192 gene pathways including RNA biosynthetic processes in muscle (FDR<0.01); 77 lncRNA (49 down, 28 up), 14 microRNAs (6 down, 8 up), 127 snoRNAs (63 down, 64 up) and 55 snRNAs (15 down, 40 up) in the liver while upregulating 6 lncRNA and dysregulating 65 snoRNAs (47 down, 18 up) in muscle (FDR<0.1, abs log2FC>0.5). Multiple ncRNA correlated with LCORL, MED17 and ZNF41 mRNA in liver but none of them in the muscle. Discriminant analysis identified (p<0.05) PECAM, RDH11, ABCA6, MIR200B, and MIR30B in liver and CAST, NOS1, FASN, MIR26B, and MIR29A in muscle as gene signatures of gestational BPA exposure. These findings provide mechanistic clues into the development and/or maintenance of the oxidative stress and lipid accumulation and potential for development of mitochondrial and fibrotic defects contributing to the prenatal BPA-induced metabolic dysfunctions.
Keywords: Endocrine disrupting chemicals, Bisphenol A, RNA sequencing, non-coding RNA, biomarkers
Introduction
Endocrine society’s scientific statement describes endocrine disrupting chemicals (EDCs) as “exogenous agents that interfere with synthesis, secretion, transport, metabolism, binding action, or elimination of natural blood-borne hormones that are present in the body and are responsible for homeostasis, reproduction, and developmental process” (Gore et al., 2015). Of concern, many EDCs are ubiquitously present in the environment and detected in pregnant women (Gore et al., 2015; Gingrich et al., 2020; Padmanabhan et al., 2021). Pregnancy levels of EDCs are associated with disruption of maternal steroid homeostasis (Sathyanarayana et al., 2017; Kolatorova et al., 2018; Banker et al., 2021), poor birth outcomes (Zhang et al., 2018b; Woods et al., 2017; Street and Bernasconi, 2020; Padmanabhan et al., 2021) and adult-onset of cardiometabolic disorders in their offspring (Heindel et.al., 2017; Sargis and Simmons, 2019). Due to the importance of steroids in fetal growth and differentiation (Solano and Arck, 2019), exposure of developing fetus to EDCs with steroidogenic action are especially of concern.
Bisphenol-A (BPA), one such EDC, has received considerable attention over the years (Vogel, 2009;-Vandenberg et al., 2009; Ma et al., 2019;-Abraham and Chakraborty, 2020; Vom Saal and Vandenberg, 2021). BPA has estrogenic, antiandrogenic, and antithyroid activities and disrupts insulin homeostasis (Lee et al., 2003; Vom Saal et al., 2012; Ahmed, 2016; Akash 2016), processes that are important for fetal growth and differentiation (Solano and Arck, 2019). BPA is found in food storage containers, water bottles, medical equipment, electronics, cosmetics, and dental sealants. Biomonitoring studies have documented the presence of BPA in greater than 90% of subjects studied (Calafat et ., 2008, Vandenberg et al., 2010,-Woodruff et al., 2011, Corrales et.al., 2015). BPA has been detected in maternal circulation, amniotic fluid, and umbilical cord samples (Padmanabhan et al., 2021), raising concerns regarding the risk posed to the developing fetus.
Complementing the human epidemiological studies demonstrating association of BPA exposure with offspring metabolic health, animal studies have provided causal evidence of developmental BPA exposure. Gestational BPA exposure have resulted in dysregulation of the offspring metabolic system, culminating in insulin resistance, diabetes, obesity, and metabolic syndrome (vom Saal et al., 2007; Akash et al., 2020; Heindel, 2019, Rubin et al., 2019). In sheep, the animal model used in this study, prenatal BPA-treatment not only induced peripheral insulin resistance and adipose tissue disruptions (Veiga-Lopez et al., 2016), but also lipotoxicity in liver and muscle (Puttabyatappa et al., 2019), contributors of insulin resistance (Yazıcı and Sezer, 2017). Furthermore, lipotoxicity in liver and muscle was accompanied by increased proinflammatory markers and oxidative stress (Puttabyatappa et al., 2019). Risks associated with steatosis include diabetes mellitus, hypertension, obesity and non-alcoholic fatty liver disease (NAFLD) (Loomba et al., 2021; Younossi, 2019). An understanding of the mechanisms contributing to steatosis would help target interventions for preventing the progression to NAFLD or diabetes, which can be accomplished thorough mapping of gene networks operational within each tissue.
With this goal in mind, the present study undertook a comprehensive transcriptional profiling of the effects of prenatal BPA exposure of female sheep in liver and muscle. The goals were to: 1) identify common and divergent gene expression and gene pathways underlying tissue-specific expression in two metabolic tissues of relevance, liver and muscle, 2) determine the disruptions in gene expression and gene networks induced by prenatal BPA-treatment, 3) explore the relationship between non-coding RNA, epigenetic regulators of gene transcription (Siouds 2021; Studniarek et al., 2021), and mRNA and 4) look for potential biomarkers of developmental impact of BPA exposure in female sheep.
Materials and Methods
Animal studies were conducted at University of Michigan Sheep Research Facility (Ann Arbor, MI) and used multiparous female Suffolk sheep. This study was conducted under Institutional Animal Care and Use Committee of the University of Michigan approved protocol and was in line with the requirements of National Research Council’s Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act. The animal husbandry, breeding, prenatal treatments, and lambing have been described elsewhere (Veiga-Lopez et al., 2016). Animals from both control and prenatal BPA-treated groups were co-inhabited under similar conditions and fed a similar maintenance diet to prevent obesity and potential phytoestrogen exposure via diet, as described earlier (Veiga-Lopez et al., 2016).
Prenatal Treatment:
Pregnant sheep were randomly assigned to control and BPA-treatment groups. Between days 30 and 90 of gestation, control animals received vehicle (corn oil) and the BPA-treated group 0.5mg/kg/day BPA (purity ≥ 99%, catalog number 239658; Aldrich Chemical Co, Milwaukee, Wisconsin) solubilized in corn oil (Veiga-Lopez et al., 2016), via daily subcutaneous injections. The average BPA level achieved in the umbilical artery with this dose was ~2.6 ng/ml (Veiga-Lopez et al., 2013), which is within the range reported in human biomonitoring studies (Gerona et al., 2013; Veiga-Lopez et al., 2015a; Lee et al., 2018). From this cohort, four control and prenatal BPA-treated female sheep, ensuring mother as the experimental unit, were randomly selected for use in the current study. The effects of prenatal BPA-treatment on insulin sensitivity, adiposity, and mediators on insulin sensitivity from the full cohort have been previously published (Veiga-Lopez et al., 2015b; Veiga-Lopez et al., 2016; Puttabyatappa et al., 2019).
Tissue Harvest:
Female offspring liver and skeletal muscle tissues were collected during the second breeding season (~21 months-of-age) following 48 hours fast. Tissues were collected during the follicular phase as the prenatal BPA-treated animals continue to cycle (Savabieasfahani et al., 2006). Estrous was synchronized with two injections of prostaglandin F2α (PGF2α, 10 mg, i.m.; Lutalyse, Pfizer Animal Health, Florham Park, NJ) administered 11 days apart. Tissues were harvested 24 hours after the second PGF2α injection during the late follicular phase. Animals were euthanized with barbiturate overdose (Fatal Plus; Vortech Pharmaceuticals, Dearborn, MI) and liver sampled from the tip of the left lobe and skeletal muscle from the vastus lateralis, snap frozen and stored at −80°C freezer until processed.
Total RNA Isolation, Library Construction, and Sequencing:
Tissues were homogenized in Trizol reagent (Life Technologies, Carlsbad, CA) and total RNA was isolated following the manufacturer’s recommendations. RNA was purified using RNeasy kit (Qiagen, Germantown, MD) with contaminating DNA removed using RNAse free DNAse. The purity and RNA integrity, was evaluated using Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA). For RNA sequencing, total RNA libraries were prepared with SMARTer universal low input RNA kit (Takara, Mountain View, CA) following ribosome depletion. Paired-end sequencing was performed on a NovaSeq (Illumina, San Diego, CA) for 150 cycles (75 base pairs on each paired end). Libraries for ncRNA (small RNA) were prepared from total RNA and sequencing libraries generated using the NEBNext smallRNA kit (New England Biolabs, Ipswich, MA). Sequencing of the libraries was accomplished on a Nextseq (Illumina, San Diego, CA) instrument. Extraction of small RNA from total RNA, sample quality evaluation, library preparation, and post-library quality control metrics were performed at the University of Michigan Advanced Genomics Core.
Total RNA Sequencing Data Processing and Quality Control:
For the analysis of data from sequencing reads, adapters from the first read of the paired end reads were removed using trimmomatic (Bolger et al., 2014) with parameters maximum mismatch count, palindrome clip threshold, and clip threshold score parameters for removing Illumina adapters: 2:30:10 settings. Additionally, in trimmomatic, leading and trailing reads with a quality control threshold below 3 and average quality control score per base below 15 while scanning using a 4bp window were dropped. FastQC (v0.11.5) (Andrews, 2010) was performed on both the raw and trimmed files to determine mean read quality scores, duplicated reads, and GC content. Data from multiple samples were summarized using MultiQC (Ewels et al., 2016) Trimmed reads from either liver or muscle samples were separately mapped to the sheep reference genome (Oar_rambouillet_v1.0) using Spliced Transcripts Alignment to a Reference (STAR) aligner (v2.6.0c) (Dobin et al., 2013) and the quality and number of reads mapped to the genome were determined using Quality of RNA-Seq Tool-Set (QoRTS, v1.3.6) software (Hartley and Mullikin, 2015). The number of reads per gene was assessed using featureCounts (v1.6.1) software (Liao et al., 2014).
ncRNA Data Processing and Quality Control:
The adapter sequence ‘AGATCGGAAGAGCACACGTCTGAACTCCAGTCAC’ was trimmed from the 5’ end of the sequencing reads and no trimming was performed at the 3’ end. Reads of low quality and <17bps in length were removed from the sequencing data using cutadapt (v3.2) software (Martin, 2011). FastQC was performed on the raw reads and trimmed reads to determine the quality of sequences and summarized using MultiQC. The trimmed reads were aligned to the sheep reference genome (Oar_rambouillet_v1.0) using STAR and the quality control metrics for the aligned reads were determined using QoRTS (v1.3.6). featureCounts was used to count the number of reads of each ncRNA. Multiple classes of ncRNA were evaluated: long noncoding (lncRNA), microRNA (miRNA), small nucleolar RNAs (snoRNA) and small nuclear RNA (snRNA). For featureCounts, annotation files subset to each class of ncRNA were created using the whole genome annotation file. Subsequent analyses were stratified by class of ncRNA.
Differential RNA Expression:
The DESeq2 (v1.24.0) package in R statistical software was used to evaluate the differential expression (Love et al., 2014). DESeq2 performs linear regressions modelling counts on a negative binomial distribution. Sample normalization was done with default settings. Differential expression was determined using both statistical significance, accounting for multiple comparisons with false discovery rate (FDR), as well as magnitude of difference between groups, using the absolute value of log2(fold change), represented by log2FC. The following comparisons were performed 1) coding genes between liver and muscle in control tissues (FDR<0.05, absolute log2FC>0.5), 2) coding genes between control and prenatal BPA-treatment (FDR<0.05, absolute log2FC>0.5) and 3) ncRNA between control and prenatal BPA-treatment (FDR<0.1, absolute log2FC>0.5). The differentially expressed RNA for each evaluation was visualized using the EnhancedVolcano package (Blighe et al., 2021).
Data Reduction and Identification of Potential Biomarkers:
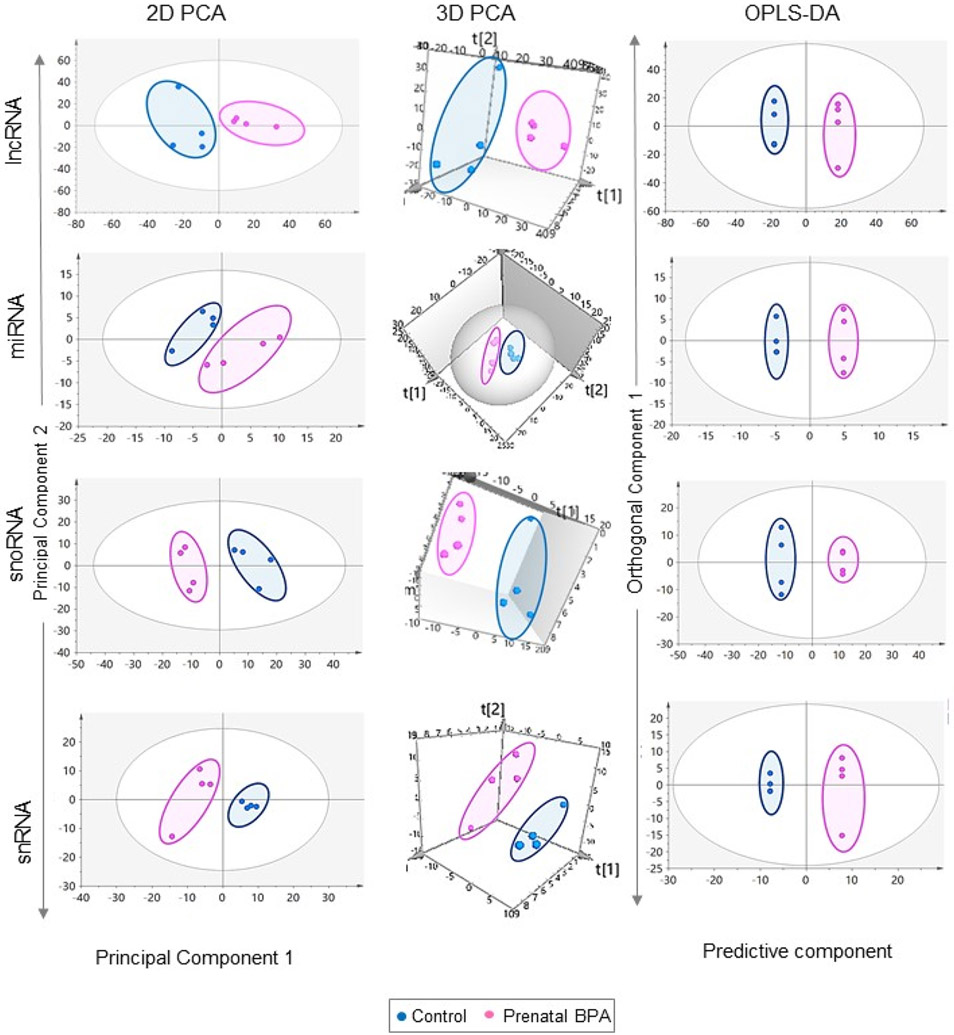
SIMCA P+ version 15 (Sartorius Stedim Data Analytics AB, Sweden) software was used for data reduction and biomarker identification. Normalized counts for coding and non-coding RNA from liver and muscle tissues were imported and multivariate modeling and unit variance (UV) scaling applied. To get an overview of the data and identify potential outliers and trends/groupings in the data unsupervised principal component analysis (PCA) was performed (Lamichhane et al., 2018) and two-dimensional and three-dimensional (3D) PCA plots were explored. To identify strong outliers, Hotelling’s T2, a multivariate generalization of Student’s t-distribution, was used to draw a tolerance ellipse around each sample cluster and any data outside the ellipses were considered outliers.
Orthogonal Projections to Latent Structures Discriminant Analysis or Orthogonal Partial Least Square Discriminant Analysis (OPLS-DA) was also used to visualize differences between the control and prenatal BPA-treated animals. As a supervised technique (Lamichhane et al., 2018) this allowed for rotation of the model such that the variations arising from treatment are the first predictive component, and variations not related to treatment explained in the orthogonal components (Trygg and Wold, 2002). For this model, the status of treatment (control and prenatal BPA) was the outcome (Y) variable, and the gene expression data was the predictor (X) variable matrix and association between these highlighted by filtering the orthogonal variation. To summarize the differences in the transcriptome profiles, OPLS-DA score plots were also generated. Each score/point on the score plot represents one observation and clear separation and groupings would indicate differences in gene expression profiles between the groups. Score plots for the first predictive component (variation in the transcriptome data related to Y) with the first orthogonal component (variation in the transcriptome data not related to Y) were developed. OPLS-DA loadings provide information about the variables responsible for these differences in profiles, variables away from the origin with smaller confidence intervals serving as better markers of the differences in the groups. To identify potential biomarkers of prenatal BPA-treatment, Variable Importance to Projection (VIP) of the predictive component and S-plots were used. For this, higher VIP values indicate higher importance to the model and responsibility for the observed separation of the two groups, and VIP value above 1 indicate important variable to the model (all coding signatures identified in this study had VIP values > 2 and all noncoding signatures had VIP values > 1.7). The S-plots were created where the predictive component loading explaining model covariance is plotted against the model correlation to identify potential biomarkers with high significance, low variability, low FDR, and higher magnitude. Additionally, S-plots and VIP predictive plots were modified for the gene expression data to add chromosome information. Furthermore, OPLS-DA model loadings and significance (calculated based on differential expression fold change) of these potential biomarker candidates were investigated to identify potential biomarkers (Saadat et al., 2021) of prenatal BPA-treatment in liver and muscle.
Gene Set Enrichment Testing:
The human orthologs of sheep genes was assigned using BioMart (Durinck et al., 2009). Differentially expressed genes were tested for enrichment in pathways using RNA-enrich (Lee et al., 2016). RNA-enrich uses a p-value cut-off free enrichment methodology. Differential expression results from each comparison (between liver and muscle from control tissues or between control and prenatal BPA) were evaluated against several databases including Biocarta pathways, EMHN metabolic pathways, Gene Ontology, KEGG pathways, Panther pathways, and transcription factor databases. An empirical cutoff of FDR<0.01 was used to filter the top enriched pathways and determine overlapping and unique pathways. The enriched pathways were visualized using heatmaps generated by heatmap.2 function in the gplots package (Galili, 2021).
Correlation between ncRNA and total RNA:
The Differential Gene Correlation Analysis (DGCA) package (McKenzie et al., 2016) was used to determine putative ncRNA-mRNA pairs. Differentially expressed ncRNA and coding genes from the same samples were matched and tested using correlation matrices. As an exploratory analysis, only the ncRNA-mRNA pairs that met the Pearson correlation coefficients with p-values <0.05 across control and BPA-treatment groups were retained and visualized.
Data and Code Availability:
The code for analysis used in this study are available online (https://github.com/bakulskilab). Data generated and used in this study are available at the Gene Expression Omnibus (GEO accession: GSE190328).
Results
Descriptive statistics:
Both coding and ncRNA in the liver and muscle had high mean read quality scores across all base pairs. Per sequence GC content ranged from 40-60% and sequence duplication levels ranged from 20-60%, as evident from quality control plots (Supplementary Figures S1 and S2).
Tissue specific differential gene expression between control liver and muscle:
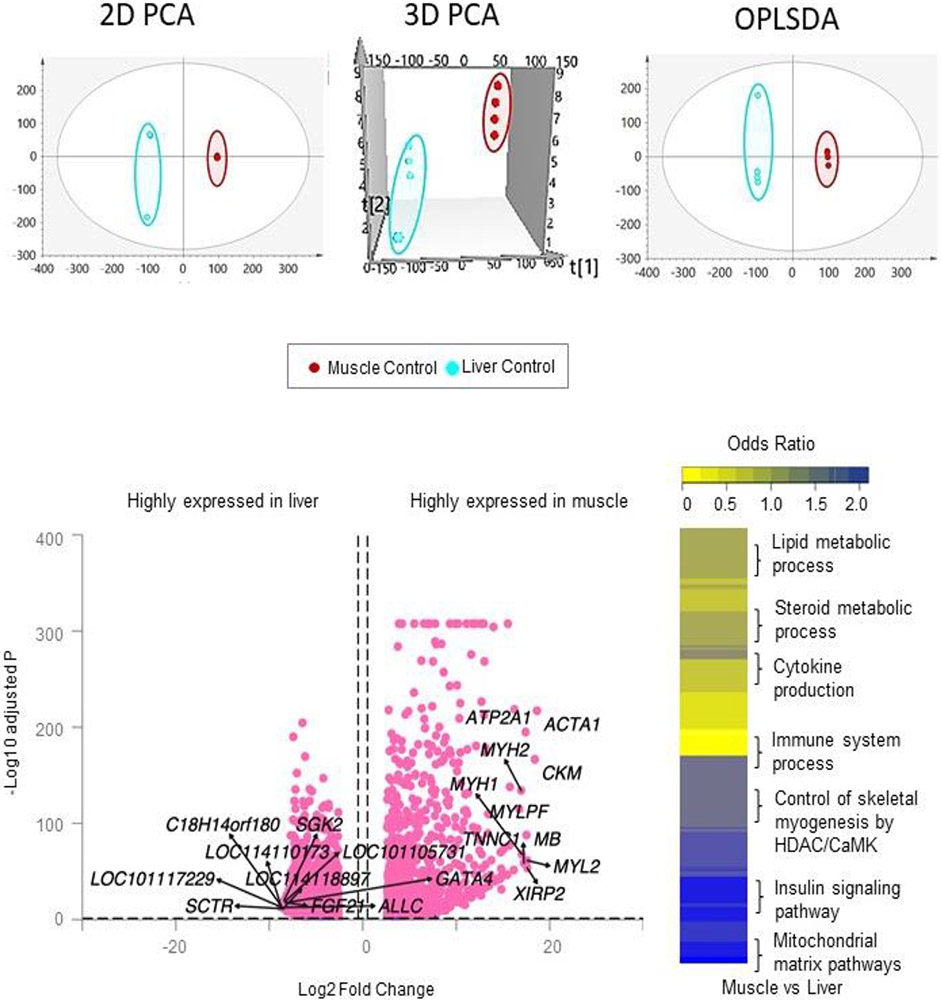
Unsupervised PCA analysis of the total RNA from control animals showed clear separation of the liver and muscle samples in 2D and 3D plots indicating differences in gene expression profiles between these two tissues (Figure 1). Supervised modeling using OPLS-DA technique substantiated this by showing clear separation of the two tissues with little improvement from the unsupervised PCA model (Figure 1). Comparison of liver and muscle coding RNA in controls showed tissue-specific expression (Figure 1; Supplemental Table S1). There were 10,438 genes differentially expressed (FDR<0.05 and abs log2FC>0.5) between liver and muscle tissues. The top 10 genes more highly expressed (FDR<0.05 and abs log2FC>0.5) in the liver and muscle of the control animals are shown in Table 1.
Figure 1. Clustering and tissue-specific expression of coding RNA in liver and muscle from control animals.
Principal Component Analysis (PCA) 2D and 3D score plots and OPLS-DA score plots of the liver and muscle samples from control animals, are shown on the top. PCA 2D and 3D plots are plotted with principal component 1 on X-axis and principal component 2 on Y-axis, and for the OPLS-DA score plot with predictive component on X-axis and first orthogonal component on Y-axis, showing separation between the liver (light blue) and muscle (dark pink) from control animals. Each point represents one animal. The Volcano plot, plotted by log2 fold change and -log10 adjusted p-values, showing differential gene expression comparing liver and muscle in control animals is shown bottom left. The pink points represent genes that have absolute log2 fold change > 0.5 and FDR < 0.05. Differentially regulated gene pathways across both liver and muscle at false discovery rate <0.01 are shown as heatmap on the bottom-right.
Table 1:
Top 10 coding RNA differentially expressed in the liver and muscle of control female sheep.
| Liver | Muscle | ||||
|---|---|---|---|---|---|
| Gene | log2FC | padj | Gene | log2FC | padj |
| LOC101117229 | −8.77 | 6.00E-14 | ACTA1 | 18.63 | 8.00E-218 |
| LOC114110173 | −8.74 | 1.00E-14 | CKM | 18.4 | 2.00E-167 |
| C18H14orf180 | −8.63 | 2.00E-13 | MYL2 | 17.59 | 5.00E-62 |
| LOC114118897 | −8.45 | 6.00E-13 | XIRP2 | 17.59 | 2.00E-54 |
| SCTR | −8.42 | 6.00E-12 | MB | 17.51 | 1.00E-88 |
| SGK2 | −8.41 | 3.00E-21 | ATP2A1 | 17.41 | 1.00E-195 |
| LOC101105731 | −8.36 | 3.00E-19 | TNNC1 | 17.23 | 2.00E-59 |
| ALLC | −8.29 | 2.00E-13 | MYH1 | 16.99 | 8.00E-69 |
| GATA4 | −8.27 | 3.00E-18 | MYH2 | 16.93 | 5.00E-135 |
| FGF21 | −8.26 | 2.00E-18 | MYLPF | 16.74 | 1.00E-115 |
Genes in either the liver or muscle from control female sheep enriched at adjusted-p value (padj) < 0.05 and absolute log2 fold change (log2FC) > 0.5 are represented. Genes highly expressed in the liver are represented as negative values and those highly expressed in the muscle are represented as positive values.
In the liver tissue, among the top 10 genes are several uncharacterized genes including LOC101117229, LOC114110173, chromosome 18 C14orf180 homolog (C18H14orf180), LOC114118897 and LOC101105731. There was also higher expression of liver fibrosis related Secretin Receptor (SCTR), gluconeogenic Serum/Glucocorticoid Regulated Kinase 2 (SGK2) (Wu et al., 2016;) and metabolic regulator Fibroblast growth factor 21 (FGF21) (Kleinert and Müller, 2020). Other genes that are highly expressed in the liver are transcription factor, GATA Binding Protein 4 (GATA4) and urolytic peroxisome protein, Allantoicase (ALLC). In contrast, the top 10 genes more highly expressed in the muscle relative to the liver included genes that form structural and functional components of the skeletal muscle such as alpha actin (ACTA1), creatine kinase, M-type (CKM), myosin light chain 2 (MYL2), Xin Actin Binding Repeat Containing 2 (XIRP2), Myoglobin (MB), ATPase Sarcoplasmic/Endoplasmic Reticulum Ca2+ Transporting 1 (ATP2A1), Troponin C1, Slow Skeletal and Cardiac Type (TNNC1), myosin heavy chain 1 (MYH1), myosin heavy chain 2 (MYH2), and myosin light chain, phosphorylatable, fast skeletal muscle (MYLPF).
Differentially expressed genes between liver and muscle in control animals were enriched in 453 gene pathways (FDR<0.01) (Figure 1; Supplemental Table S2). In the liver genes enriched in 239 gene pathways, including those related to cellular membrane components, immune response and cytokine production, lipid homeostasis, and steroid biosynthetic process. In the muscle genes enriched in 164 gene pathways, such as myofibrillar and contractile fibers, and straited muscle development related gene pathways related to structural and functional components of skeletal muscle.
Tissue-specific ncRNA expression differences between control liver and muscle:
As was the case with coding RNA, ncRNAs also showed tissue-specific expression in liver and muscle of control animals (Supplementary Table S3). The top 10 ncRNAs in each class for liver and muscle are shown in Table 2. The top 10 lncRNA, snoRNA and snRNA that were highly expressed in liver and muscle only included uncharacterized RNAs (Table 2). The top 10 microRNAs that were highly expressed in the liver included MIR200A, MIR194, MIR148A, MIR154A, MIR136, MIR411A, MIR494, MIR655, MIR30A, and MIR369. In the muscle, the highly prevalent miRNAs were MIR10B, MIR133, MIR181A-2, MIR150, MIR362, MIR125B, MIR22, MIRLET7D, MIR106B, and MIR30C.
Table 2:
Top 10 differentially expressed ncRNAs lncRNA, miRNA, snoRNA and snRNA in the liver and muscle from control female sheep.
| Highly expressed in the liver | Highly expressed in the Muscle | ||||
|---|---|---|---|---|---|
| Gene | log2FC | padj | Gene | log2FC | padj |
| lncRNA | |||||
| LOC105607466 | −6.72 | 4.0E-08 | LOC105603743 | 12.19 | 7.16E-26 |
| LOC101110918 | −5.81 | 2.2E-40 | LOC105604459 | 7.17 | 3.47E-09 |
| LOC114110111 | −4.98 | 2.0E-50 | LOC114109081 | 4.26 | 0.004582 |
| LOC114115269 | −4.92 | 5.6E-03 | LOC105606221 | 4.16 | 1.01E-18 |
| LOC105609312 | −4.69 | 2.0E-02 | LOC105611269 | 4.01 | 3.03E-12 |
| LOC105602783 | −4.27 | 5.6E-03 | LOC105616014 | 3.85 | 0.054834 |
| LOC106990145 | −4.26 | 7.0E-02 | LOC105609560 | 3.79 | 0.065616 |
| LOC114118741 | −4.17 | 7.6E-02 | LOC105612888 | 3.15 | 1.34E-06 |
| LOC114116119 | −3.88 | 5.2E-02 | LOC114117599 | 2.83 | 8.05E-13 |
| LOC105606290 | −3.75 | 3.5E-04 | LOC105604676 | 2.58 | 2.30E-13 |
| miRNA | |||||
| MIR200A | −5.22 | 1.0E-33 | MIR10B | 4.44 | 2.30E-07 |
| MIR194 | −4.91 | 4.3E-62 | MIR133 | 4.37 | 0.006168 |
| MIR148A | −4.01 | 8.2E-90 | MIR181A-2 | 4.35 | 4.16E-20 |
| MIR154A | −3.92 | 3.0E-03 | MIR150 | 3.06 | 1.40E-25 |
| MIR136 | −3.76 | 1.5E-05 | MIR362 | 2.6 | 2.09E-10 |
| MIR411A | −3.12 | 3.5E-24 | MIR125B | 2.23 | 6.18E-14 |
| MIR494 | −3.06 | 6.0E-16 | MIR22 | 2.21 | 5.79E-33 |
| MIR655 | −2.97 | 3.0E-04 | MIRLET7D | 2.15 | 1.23E-28 |
| MIR30A | −2.88 | 8.3E-57 | MIR106B | 1.84 | 1.52E-17 |
| MIR369 | −2.81 | 1.6E-05 | MIR30C | 1.8 | 2.63E-16 |
| snoRNA | |||||
| LOC114116284 | −5.91 | 5.9E-05 | LOC114117125 | 5.05 | 2.16E-04 |
| LOC114108878 | −5.17 | 2.1E-08 | LOC114111821 | 4.82 | 2.46E-17 |
| LOC114110520 | −5.14 | 2.1E-08 | LOC114117126 | 3.91 | 1.21E-02 |
| LOC114114248 | −4.92 | 2.6E-33 | LOC114117367 | 2.89 | 1.23E-11 |
| LOC114109764 | −3.96 | 8.1E-04 | LOC114114654 | 2.52 | 5.79E-04 |
| LOC114111305 | −3.75 | 1.0E-03 | LOC114118122 | 2.51 | 4.73E-04 |
| LOC114110705 | −3.63 | 2.7E-02 | LOC114113393 | 2.27 | 1.21E-04 |
| LOC114117100 | −3.59 | 6.4E-02 | LOC114114215 | 2.01 | 8.09E-04 |
| LOC114110211 | −3.57 | 6.0E-02 | LOC114110868 | 1.98 | 2.62E-02 |
| LOC114110194 | −3.43 | 1.5E-06 | LOC114117067 | 1.9 | 5.84E-06 |
| snRNA | |||||
| LOC114109241 | −4.48 | 5.5E-11 | LOC114113408 | 2.01 | 6.96E-04 |
| LOC114115462 | −4.4 | 1.1E-96 | LOC114113424 | 3.42 | 6.58E-04 |
| LOC114115947 | −4.33 | 4.8E-03 | LOC114110196 | 3.51 | 4.58E-14 |
| LOC114109230 | −4.11 | 9.1E-04 | LOC114115964 | 3.65 | 6.37E-02 |
| LOC114115954 | −2.34 | 1.0E-03 | LOC114117119 | 4.25 | 9.09E-02 |
| LOC114114213 | −2.3 | 8.2E-02 | LOC114117121 | 4.6 | 3.53E-02 |
| LOC114114282 | −1.94 | 1.1E-02 | LOC114111415 | 4.73 | 9.28E-02 |
| LOC114117117 | 4.83 | 1.89E-02 | |||
lncRNA, miRNA, snoRNA, snRNA enriched at adjusted p-value (padj) < U.1 in either the liver or muscle from control animals are represented. Genes highly expressed in the liver are represented as negative values and those highly expressed in the muscle are represented as positive values.
Prenatal BPA induced coding RNA changes in liver and muscle:
Comparison of overall patterns of gene expression by BPA in liver:
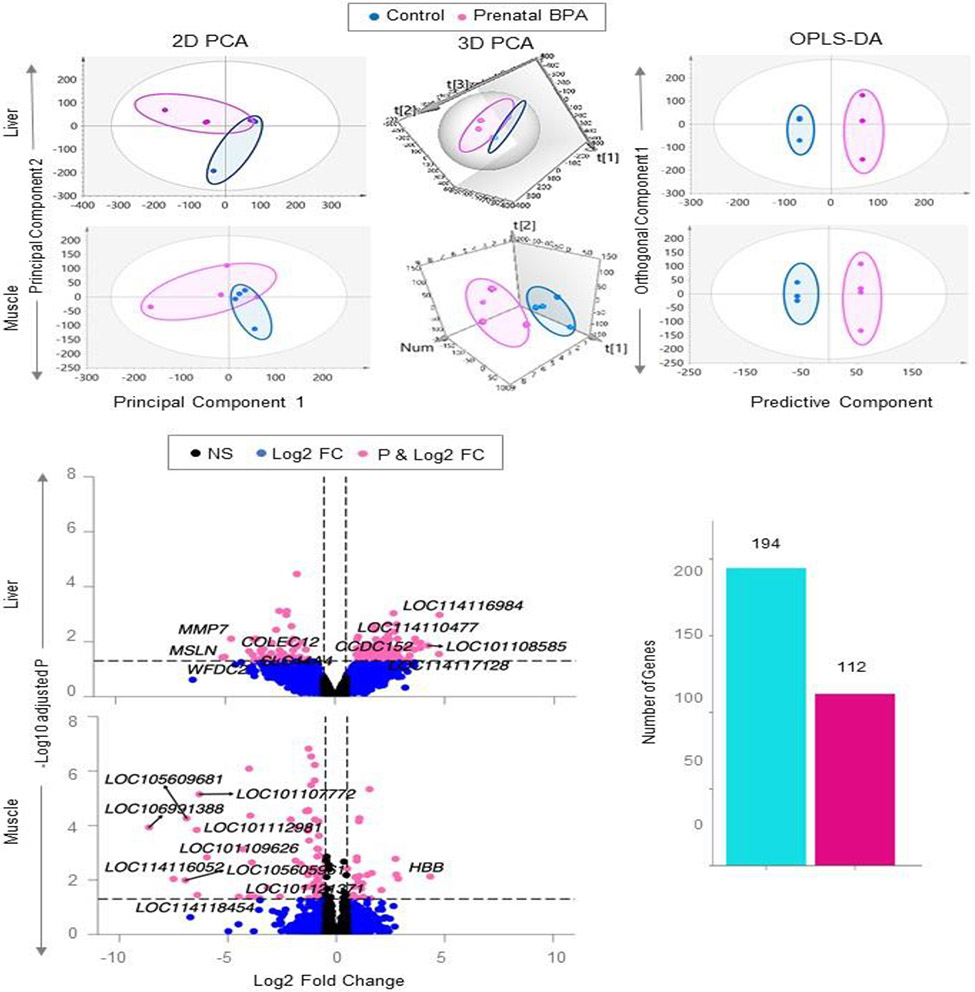
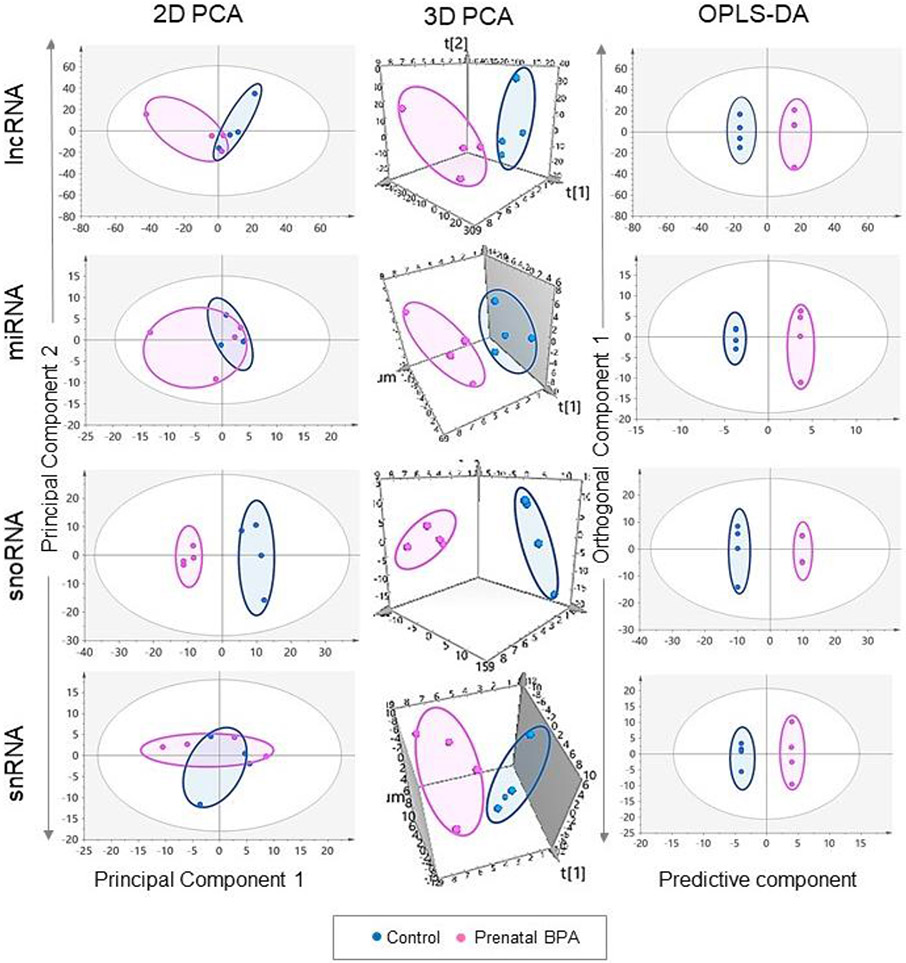
Unsupervised PCA model of liver from the control and prenatal BPA-treated sheep showed slight overlap in the 2D plot and well-defined separation in the 3D PCA plot. In the OPLS-DA model where unrelated variance was removed, the control and prenatal BPA-treated groups were well separated, pointing to differences in total RNA gene transcription profiles between the two groups (Figure 2).
Figure 2. Clustering and differential expression of coding RNA in liver and muscle from prenatal BPA-treated animals.
The 2D and 3D PCA and OPLS-DA score plots showing groupings and separation between control (blue) and prenatal BPA-treated (pink) groups in the coding RNA from liver and muscle tissue are shown on the top. PCA 2D and 3D plots are plotted with principal component 1 on X-axis and principal component 2 on Y-axis, and OPLS-DA score plot with predictive component on X-axis and first orthogonal component on Y-axis with each point representing one animal. The Volcano plot, plotted by log2 fold change in the X-axis and -log10 p-adjusted values in the Y-axis, shows prenatal BPA induced differential gene expression in the liver and muscle (bottom-left). Pink points denote the genes that have absolute log2 foldchange > 0.5 and p-adjusted values < 0.05. Black dots represent genes that did not meet p-adjusted cut-off of < 0.05 and absolute log2 fold change > 0.5, and blue dots represent genes which met meet the absolute log2 fold change > 0.5 but did not meet p-adjusted cut-off of < 0.05. The bar plots (bottom-right) represent the number of genes differentially modulated that are unique to liver and muscle.
Differential gene expression by BPA in liver:
In the liver, prenatal BPA dysregulated 194 genes (138 downregulated and 56 upregulated) at FDR<0.05 and absolute log2FC>0.5 (Figure 2, Supplementary Table S4). Among the top 10 dysregulated genes (Table 3), prenatal BPA-treatment decreased expression of extracellular matrix related genes WAP four-disulfide core domain 2 (WFDC2), mesothelin (MSLN), matrix metallopeptidase 7 (MMP7), and collectin subfamily member 12 (COLEC12), and mitochondrial transmembrane protein with role in lipid transport solute carrier family 44 member 4 (SLC44A4). In contrast, prenatal BPA-treatment increased expression of cell cycle and proliferation regulator coiled-coil domain containing 152 (CCDC152), and genes with yet to be identified role thioredoxin-like protein 1 (LOC114110477), putative olfactory receptor 3A4 (LOC101108585), and elongation factor 1-beta-like (LOC114116984).
Table 3:
Top 10 prenatal BPA modulated coding RNA in the liver and muscle.
| Liver | Muscle | ||||
|---|---|---|---|---|---|
| Gene | log2FC | padj | Gene | log2FC | padj |
| WFDC2 | −5.14 | 3.76E-02 | LOC106991388 | −8.63 | 0.00E+00 |
| MSLN | −5.03 | 3.54E-02 | LOC114116052 | −7.51 | 9.00E-03 |
| LOC114116984 | 4.77 | 1.10E-03 | LOC105605961 | −6.96 | 1.00E-02 |
| LOC114117128 | 4.74 | 2.82E-02 | LOC105609681 | −6.91 | 0.00E+00 |
| MMP7 | −4.73 | 7.80E-03 | LOC101112981 | −6.44 | 0.00E+00 |
| LOC101108585 | 4.24 | 1.39E-02 | LOC114118454 | −6.41 | 3.60E-02 |
| COLEC12 | −3.91 | 2.21E-02 | LOC101107772 | −6.31 | 0.00E+00 |
| LOC114110477 | 3.90 | 1.09E-02 | HBB | 4.33 | 7.00E-03 |
| CCDC152 | 3.89 | 1.71E-02 | LOC114116675 | −4.32 | 1.00E-03 |
| SLC44A4 | −3.74 | 3.05E-02 | LOC101104054 | −4.03 | 0.00E+00 |
Genes highly modulated in either the liver or muscle with adjusted p-value (padj) < 0.05 and absolute log2 fold change (log2FC) > 0.5 are represented. The top 10 genes sorted by absolute magnitude in each tissue are represented with their direction of effect.
Comparison of overall patterns of gene expression by BPA in muscle:
The control and prenatal BPA-treated groups were not well separated on the 2D PCA plot however when the model was tilted in the 3D PCA plot the two groups showed clear separation (Figure 2) indicating the overlap was due to 2D projection of the plot. The separation of the two groups improved in the OPLS-DA model for the muscle tissue samples (Figure 2).
Differential gene expression by BPA in muscle:
In the muscle prenatal BPA-treatment dysregulated 112 genes with 32 genes downregulated and 80 genes upregulated at FDR<0.05 and absolute log2FC>0.5 (Figure 2; Supplemental Table S5). Among the top 10 dysregulated genes there were nine downregulated and one upregulated gene as depicted in Table 3. Prenatal BPA-treatment induced downregulation of multidrug resistance-associated protein 4-like genes (LOC106991388, LOC101107772, LOC114116675, and LOC101104054), ATP-binding cassette sub-family C member 4-like (LOC105605961), uncharacterized genes (LOC114116052 and LOC105609681), high mobility group protein 20A-like (LOC101112981), and 40S ribosomal protein S3a pseudogene (LOC114118454). In contrast, the top upregulated gene was the hemoglobin subunit beta (HBB).
Comparison of the gene expression in the two tissues found prenatal BPA-induced coding RNAs were unique to either liver or muscle with no overlap between them (Figure 2).
Functional Enrichment for Genes Differentially Affected by Prenatal BPA:
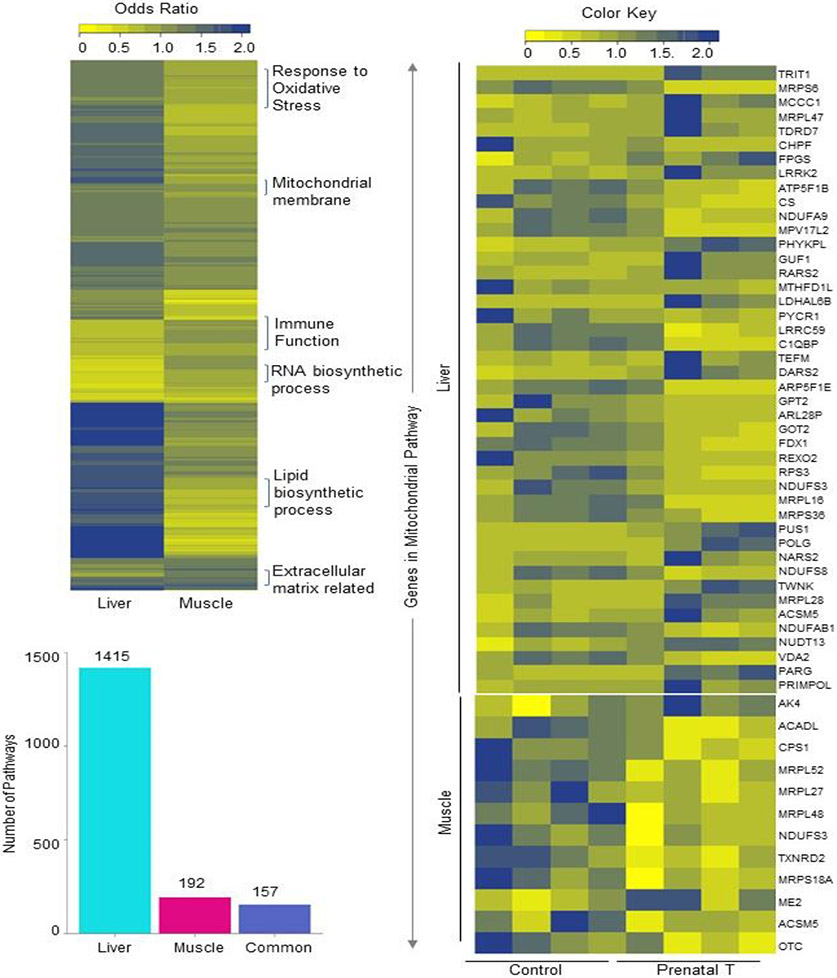
Prenatal BPA dysregulated several gene pathways in the liver and muscle of adult female sheep (Figure 3; Supplemental Table S6). Among these, 157 gene pathways were enriched both in the muscle and liver. Gene pathways belonging to the mitochondrial (Figure 3), extracellular matrix-related and oxidative phosphorylation pathways were enriched in both tissues from prenatal BPA-treated animals. However, 1415 gene pathways in the liver and 192 gene pathways in the muscle showed tissue specificity in their enrichment. In the liver, response to oxidative stress (Figure 4), lipid biosynthetic process, endoplasmic reticulum (ER), and Golgi apparatus structure and function (Supplemental Table S6) gene pathways were specifically enriched with prenatal BPA-treatment. In the muscle, RNA biosynthetic process (Figure 4), immune function, and collagen synthetic gene pathways (Supplemental Table S6) were enriched.
Fig. 3. Enrichment of gene pathways in liver and muscle and expression levels of genes in commonly dysregulated pathways by prenatal BPA-treatment.
Heat map (top left) represents the differentially regulated gene pathways in liver and muscle from prenatal BPA-treated animals enriched at FDR < 0.01. The bar plots (bottom-left) represent the number of gene pathways differentially modulated that are unique to liver and muscle. Heatmap (right) showing genes involved in the mitochondrial membrane pathway that is dysregulated in both liver (top) and muscle (bottom). The normalized counts of genes in the mitochondrial gene pathway in control and prenatal BPA-treated animals are plotted along a gradient of colors, with blue representing highest and yellow the lowest.
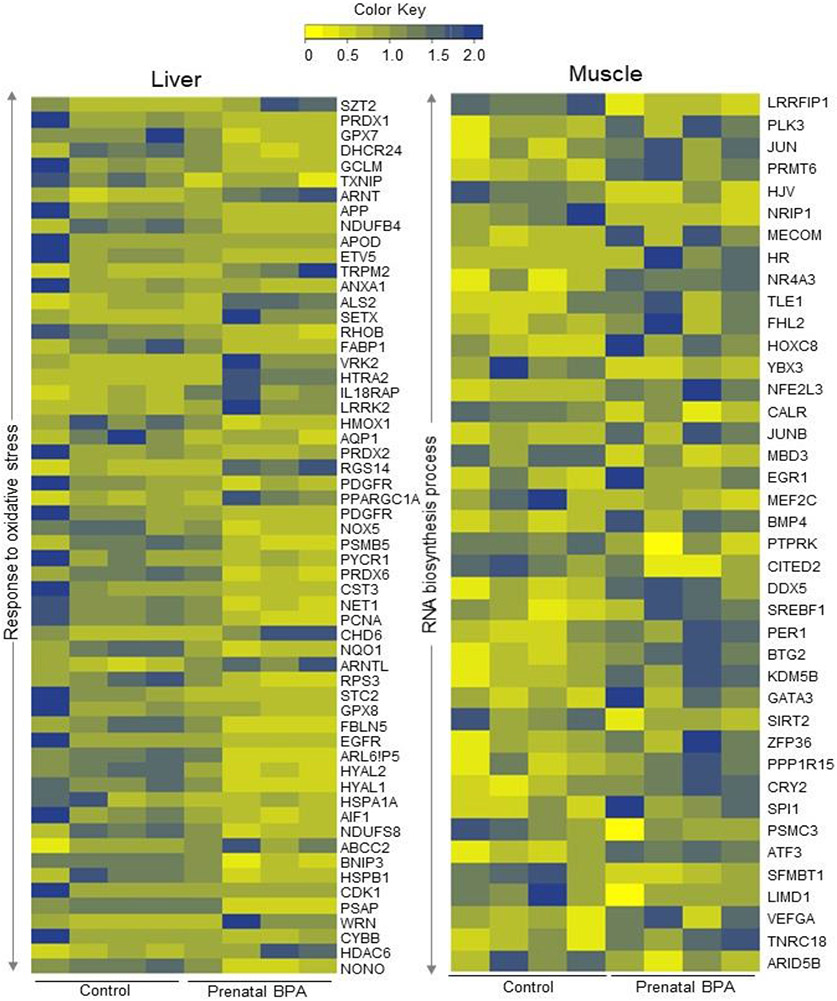
Fig. 4. Genes in dysregulated pathways enriched in tissue-specific manner in either liver or muscle:
Heatmap showing genes involved in response to oxidative stress in the liver (left) and RNA biosynthetic process in the muscle (right) that are dysregulated by prenatal BPA-treatment at FDR < 0.01. The normalized counts of genes in the respective pathways in each tissue from control and prenatal BPA-treated animals are represented as a gradient of colors with blue representing the highest and yellow representing the lowest.
Gene signatures (coding RNA) of prenatal BPA-treatment in liver and muscle:
Liver:
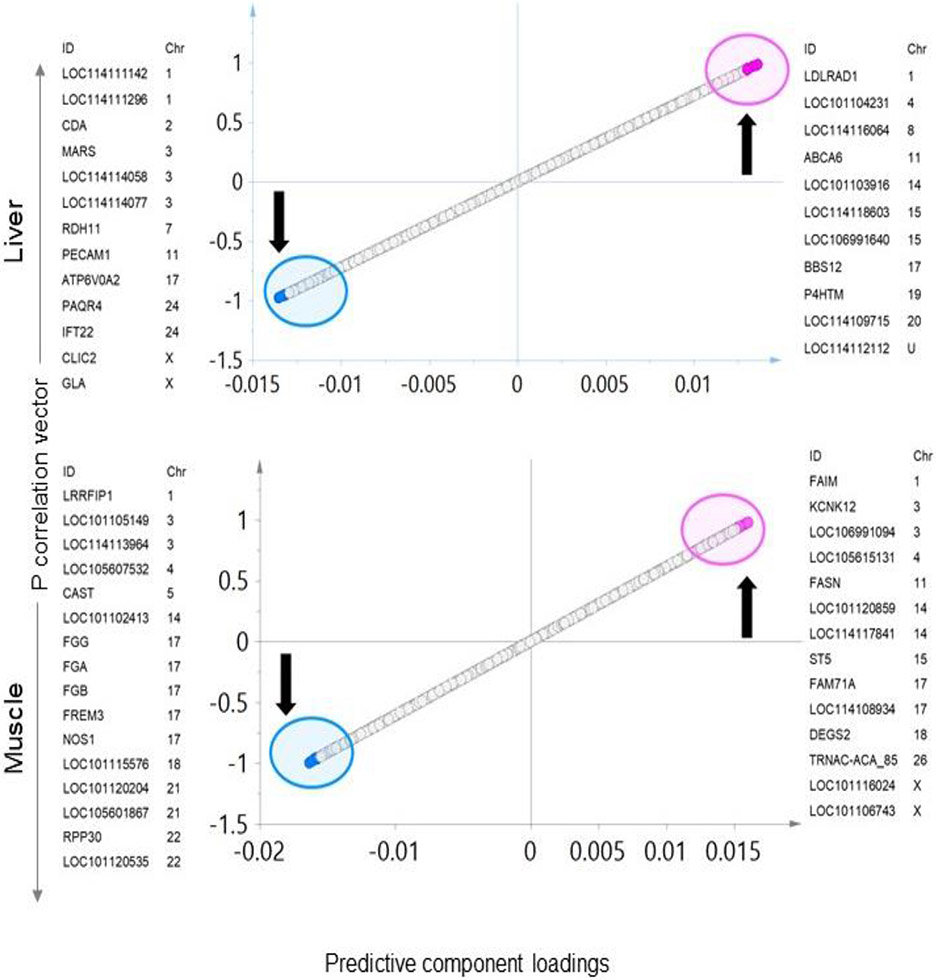
Investigation of the supervised OPLS-DA model coupled with the use of score-plots (S-plot (Figure 5) and VIP-plot (Figure 6)) and model loadings identified 13 downregulated and 11 upregulated genes in the liver of prenatal BPA-treated sheep as having the potential to serve as signatures of the prenatal BPA-treatment effect (Figure 5). Further Investigation of the significance of these genes based on high VIP values, model loading confidence interval, and fold change in differential expression revealed significant (fold change p value <0.05 without FDR adjustment) and high VIP values (all signatures had VIP value > 2) decreased expression of several protein coding genes. These genes were Cytidine Deaminase (CDA), Retinol Dehydrogenase 11 (RDH11), ATPase H+ Transporting V0 Subunit A2 (ATP6V0A2) a subunit of the vacuolar ATPase, Platelet And Endothelial Cell Adhesion Molecule 1 (PECAM1) shown to have multifunctional role in inflammation, Progestin And AdipoQ Receptor Family Member 4 (PAQR4), Intraflagellar Transport 22 (IFT22) that enables GTP binding and GTPase activity, Chloride Intracellular Channel 2 (CLIC2), Galactosidase Alpha (GLA) active in lysosomes and uncharacterized gene LOC114114077. Among the candidate genes which were significantly (fold change p value < 0.05 without FDR adjustment) were uncharacterized genes LOC101104231, LOC114116064, LOC101103916, LOC114118603, LOC106991640, LOC114109715, and LOC114112112, ATP Binding Cassette Subfamily A Member 6 (ABCA6) implicated in macrophage lipid homeostasis, Bardet-Biedl Syndrome 12 (BBS12) part of membrane trafficking complex, Prolyl 4-Hydroxylase, Transmembrane (P4HTM) with a role in hypoxia adaptation. Some of these genes, namely CDA, RDH11, CLIC2, LOC101104231, ABCA6, BBS12, LOC114109715, and LOC114112112, also showed significance based on differential expression analysis with FDR adjusted p value cutoff < 0.05. Two functionally relevant genes with anti-inflammatory roles, PECAM1 and GLA, had FDR adjusted p value = 0.06.
Fig. 5. Liver or muscle specific transcriptome signatures of prenatal BPA-treatment:
Total RNA, OPLS-DA S- Plots from liver (top) and muscle (bottom) showing potential coding signatures of BPA-treatment. The up and down arrow represents the upregulated and downregulated genes with prenatal BPA -treatment, respectively. Each point represents a coding RNA with pink and blue points representing the potential signatures of prenatal BPA-treatment that are up and downregulated, respectively.
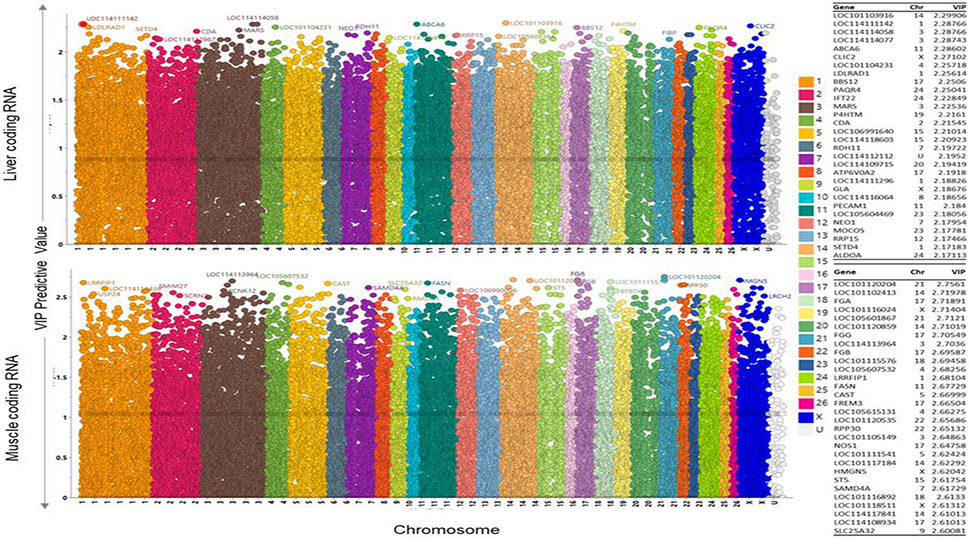
Fig. 6. Variable Important to the projection plots for coding RNA liver and muscle:
OPLS-DA Variable importance to projection (VIP) plots showing the coding RNA VIP in liver (top) and muscle (bottom). Chromosome ID on X-axis (each color representing a chromosome) and VIP values on the Y-axis. The higher VIP value indicate high importance to the model and responsible for the separation of prenatal BPA and control animals on the OPLS-DA score plot. The top 30 genes-based on VIP values with chromosome ID are listed on the right side of the plots.
Muscle:
The OPLS-DA model S-plot (Figure 5) and VIP-plot (Figure 6) and the model loadings found 16 downregulated and 14 upregulated genes as candidates to serve as potential signatures of the prenatal BPA exposure. The genes that were downregulated by prenatal BPA-treatment that achieved significance (fold change p value ≤ 0.05 without FDR adjustment) and high VIP values (all signatures had VIP value > 2) include: LRR Binding FLII Interacting Protein 1 (LRRFIP1) implicated in several biological processes and signal transduction pathways including inflammation (Takimoto, 2019), deoxyribose-phosphate aldolase (LOC101105149) with a role in stress response, myogenic factor 6 (LOC114113964) that regulates skeletal Fibrinogen Alpha Chain (FGA), Fibrinogen Beta Chain (FGB), FRAS1 Related Extracellular Matrix 3 (FREM3 -p value=0.050), Nitric Oxide Synthase 1 (NOS1), LOC101115576, Serum amyloid A protein (LOC101120204), LOC105601867, Ribonuclease P/MRP Subunit P30 (RPP30), cytochrome P450 2C23-like (LOC101120535). Upregulated candidate genes that achieved significance (fold change p value ≤ 0.05 without FDR correction) and high VIP values (all signatures had VIP pred value > 2) include: Fas apoptotic inhibitory molecule (FAIM), Potassium Two Pore Domain Channel Subfamily K Member 12 (KCNK12), Fatty Acid Synthase (FASN), LOC101120859, DENN Domain Containing 2B (ST5), Family with Sequence Similarity 71 Member A (FAM71A), Delta 4-Desaturase, Sphingolipid 2 (DEGS2), TRNAC-ACA_85, LOC101116024, LOC101106743 (p =0.05). The genes which achieved significance based on differential expression analysis after the FDR adjustment p value < 0.05 includes LOC101105149, CAST, LOC101102413, FGG, FGA, FGB, NOS1, LOC101115576, LOC101120204, and LOC105601867, FAIM, FASN, ST5, FAM71A and LOC101116024.
Prenatal BPA mediated changes in ncRNA expression in liver and muscle:
Liver:
Prenatal BPA-induced profound changes in ncRNA of the liver with dysregulation observed across all classes of ncRNA. Among the differentially expressed ncRNAs were a total of 77 lncRNA (49 downregulated, 28 upregulated), 14 miRNAs (6 down and 8 up), 127 snoRNAs (63 down and 64 up) and 55 snRNAs (15 down and 40 up) (Figure 7; Supplementary Table S7).
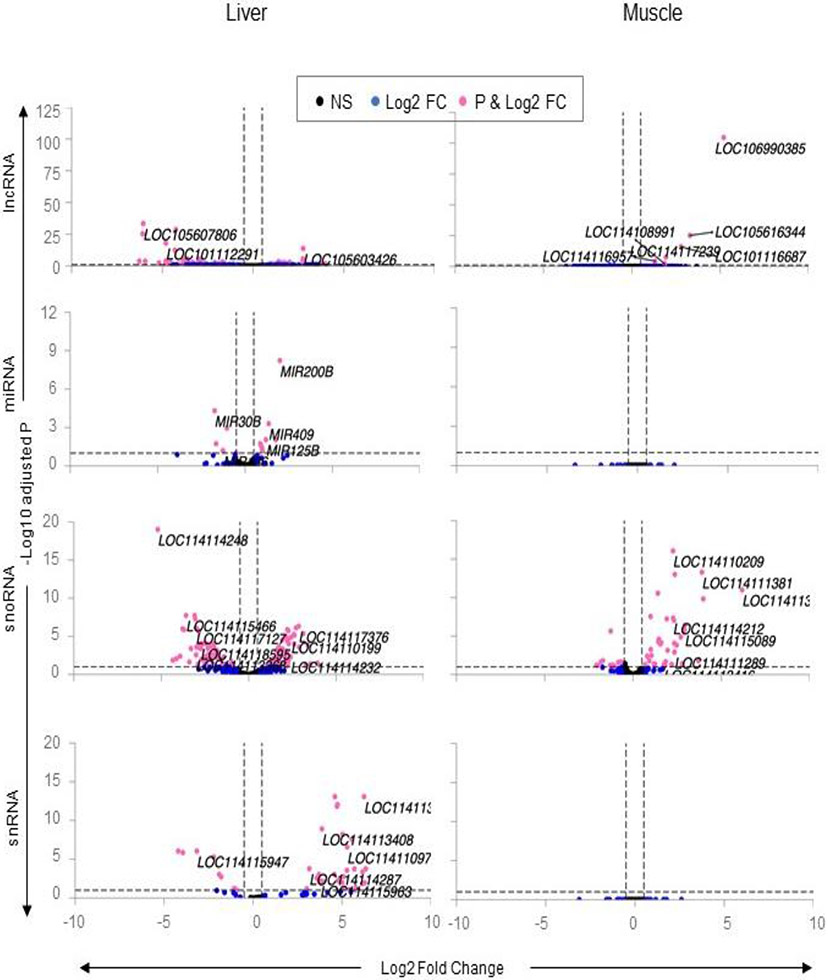
Fig. 7. Differential expression of non-coding RNA in liver and muscle from prenatal BPA-treated Animals.
The Volcano plot, plotted by log2 fold change in the X-axis and -log10 p-adjusted values in the Y-axis, shows prenatal BPA induced differential gene expression in the liver and muscle. Pink points denote the genes that have absolute log2 foldchange > 0.5 and p-adjusted values < 0.05. Black dots represent genes that did not meet p-adjusted cut-off of < 0.05 and absolute log2 fold change > 0.5, and blue dots represent genes which met meet the absolute log2 fold change > 0.5 but did not meet p-adjusted cut-off of < 0.05.
The top 10 differentially expressed lncRNAs (Table 4) included uncharacterized LOC105607806, LOC114118468, LOC101110918, LOC101112291, LOC105608205, LOC114112974, LOC101120180, and LOC105606290 that were downregulated and lncRNA LOC114110055 and LOC105603426 that were upregulated. The top 10 miRNAs (Table 4) included upregulation of MIR200B, MIR409, MIR125B, MIR543, MIR25, MIR22, and MIR191 and downregulation of MIR30B, MIR26B and MIR154A.
Table 4:
Top 10 prenatal BPA modulated ncRNAs lncRNA, miRNA, snoRNA and snRNA in the liver.
| Liver | Muscle | ||||
|---|---|---|---|---|---|
| Gene | log2FC | padj | Gene | log2FC | padj |
| lncRNA | |||||
| LOC105607806 | −6.06 | 3.88E-34 | LOC106990385 | 5.19 | 2.13E-105 |
| LOC114118468 | −4.26 | 2.28E-29 | LOC105616344 | 3.28 | 6.99E-26 |
| LOC101110918 | −6.09 | 4.95E-26 | LOC101116687 | 2.78 | 9.98E-17 |
| LOC101112291 | −4.81 | 9.20E-19 | LOC114117239 | 1.93 | 2.77E-08 |
| LOC105603426 | 2.76 | 1.64E-14 | LOC114116957 | 1.32 | 5.24E-05 |
| LOC105608205 | −4.31 | 4.19E-13 | LOC114108991 | 1.85 | 0.005 |
| LOC114110055 | 2.75 | 2.93E-06 | |||
| LOC114112974 | −2.91 | 2.93E-06 | |||
| LOC101120180 | −3.62 | 2.93E-06 | |||
| LOC105606290 | −3.86 | 8.38E-06 | |||
| miRNA | |||||
| MIR200B | 1.99 | 5.97E-09 | |||
| MIR30B | −1.74 | 4.89E-05 | |||
| MIR409 | 1.36 | 5.02E-04 | |||
| MIR26B | −1.04 | 1.11E-03 | |||
| MIR125B | 1.19 | 8.95E-03 | |||
| MIR543 | 1.76 | 8.95E-03 | |||
| MIR154A | −1.66 | 1.84E-02 | |||
| MIR25 | 0.87 | 1.84E-02 | |||
| MIR22 | 0.90 | 2.44E-02 | |||
| MIR191 | 0.97 | 4.04E-02 | |||
| snoRNA | |||||
| LOC114114248 | −5.20 | 9.73E-20 | LOC114114232 | 3.47 | 6.12E-79 |
| LOC114115466 | −3.58 | 1.85E-08 | LOC114118129 | 4.96 | 1.21E-58 |
| LOC114118597 | −3.09 | 1.85E-08 | LOC114113382 | 4.51 | 4.17E-48 |
| LOC114108878 | −3.05 | 5.34E-08 | LOC114110194 | 4.50 | 1.05E-45 |
| LOC114117376 | 2.86 | 4.89E-07 | LOC114111283 | 3.25 | 5.83E-41 |
| LOC114113389 | 2.70 | 7.80E-07 | LOC114115928 | 3.72 | 5.05E-38 |
| LOC114117127 | −3.02 | 7.80E-07 | LOC114117064 | 3.96 | 1.29E-32 |
| LOC114113367 | −3.79 | 1.03E-06 | LOC114110200 | 3.64 | 7.47E-30 |
| LOC114114229 | 2.21 | 1.32E-06 | LOC114110519 | 5.40 | 5.23E-28 |
| LOC114116533 | −3.70 | 1.33E-06 | LOC114117650 | 3.58 | 3.97E-27 |
| snRNA | |||||
| LOC114113424 | 6.24 | 8.32E-14 | |||
| LOC114110196 | 4.60 | 8.32E-14 | |||
| LOC114117112 | 4.75 | 9.20E-13 | |||
| LOC114117113 | 4.75 | 9.20E-13 | |||
| LOC114117114 | 4.75 | 9.20E-13 | |||
| LOC114117116 | 4.75 | 9.20E-13 | |||
| LOC114117115 | 4.72 | 1.52E-12 | |||
| LOC114113408 | 3.87 | 1.23E-09 | |||
| LOC114118780 | 5.03 | 6.38E-09 | |||
| LOC114108901 | 5.03 | 6.38E-09 | |||
The top 10 snoRNAs that were differentially regulated by prenatal BPA-treatment (Table 4) included three upregulated snoRNAs namely LOC114117376, LOC114113389 and LOC114114229 and seven downregulated snoRNAs including LOC114114248, LOC114115466, LOC114118597, LOC1141088878, LOC114117127, LOC114113367 and LOC114116533. In contrast, the differentially expressed snRNAs included only upregulated genes (Table 4) LOC114113424, LOC114110196, LOC114117112, LOC114117113, LOC114117114, LOC114117116, LOC114117115, LOC114113498, LOC114118780, and LOC114108901.
Muscle:
As opposed to prenatal BPA-treatment having an impact on all classes of ncRNA in the liver, prenatal BPA-treatment had an impact only at the level of lncRNA and snoRNAs in the muscle (Figure 7; Supplementary Table S8). Among lncRNA, prenatal BPA-treatment upregulated six different uncharacterized lncRNAs (Table 4; LOC106990385, LOC105616344, LOC101116687, LOC114117239, LOC114116957 and LOC114108991). On the other hand, prenatal BPA-treatment dysregulated 65 snoRNAs manifested as downregulation of 47 and upregulation of 18 snoRNAs (Figure 7; Supplemental Table S7).
Only upregulated snoRNAs were represented in the top 10 (FDR<0.1, log2FC>0.5) (Table 4). These included SNORD59 (LOC114114232), SNORD111 (LOC114118129), SNORD82 (LOC114113382), SNORD25 (LOC114110194), SNORD38 (LOC114111283), SNORD18 (LOC114115928), SNORD49 (LOC114117064), SNORD26 (LOC114110200), SNORD58 (LOC114110519), and SNORD56 (LOC114117650).
Potential ncRNA biomarkers of prenatal BPA-exposure in liver and muscle:
Liver:
There was clear separation of the control and prenatal BPA groups in all the ncRNA 2D and 3D PCA plots as well as OPLS-DA score plot documenting clear differences in the ncRNA profiles of these two groups (Figure 8).
Fig. 8.
PCA and OPLS-DA score plots for ncRNA in liver. The 2D and 3D PCA and OPLS-DA score plots for IncRNA, miRNA, snoRNA and snRNA in liver from control (blue) and prenatal BPA-treated (pink) animals. PCA 2D and 3D plots are plotted with principal component 1 on X-axis and principal component 2 oo Y-axis, and OPLS-DA score plot with predictive component on X-axis and first orthogonal component on Y-axis with each point representing one animal.
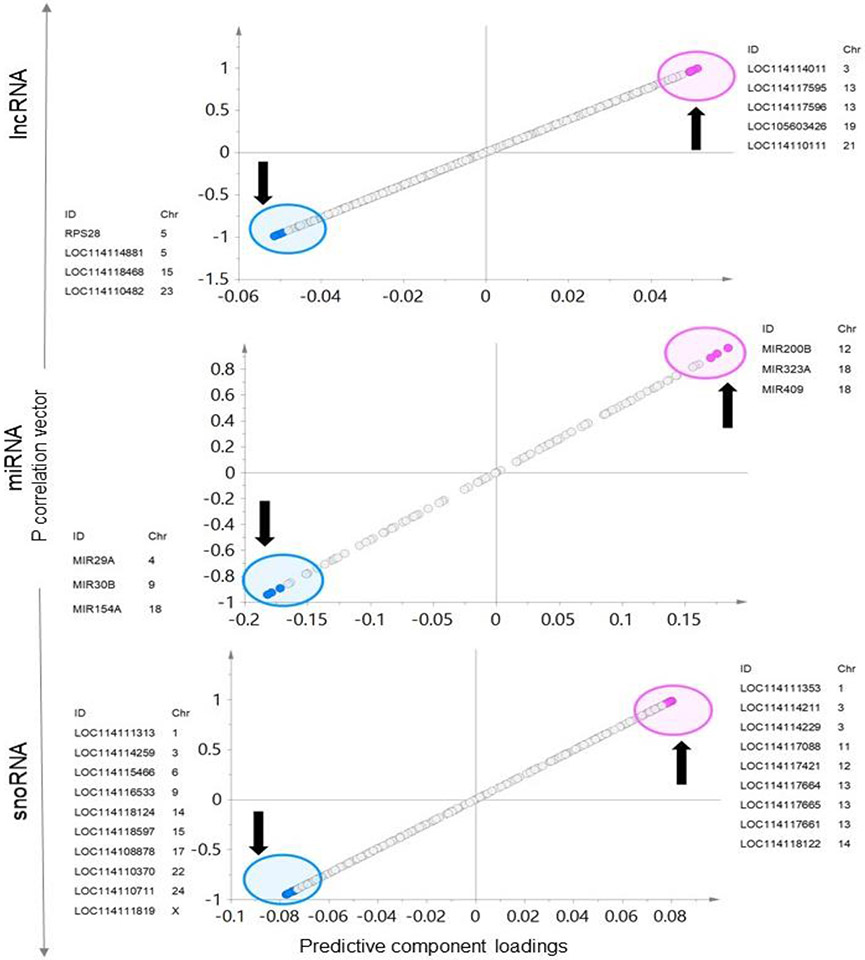
Investigation of OPLS-DA models, S-plots (Figure 9 and supplementary Figure 3), model loadings and VIP plots to identify ncRNA signatures found four lncRNA that were lower in expression and five lncRNA that were higher in expression as having the potential to serve as biomarkers of the prenatal BPA impact (S-plot - Figure 9). These include RPS28, LOC114114881, LOC114118468, LOC114110482 that were downregulated and LOC114114011, LOC114117595, LOC114117596, LOC105603426, and LOC114110111 that were upregulated. All four of the potential lncRNAs that were downregulated and two lncRNA LOC105603426 and LOC114110111 that were upregulated were significant based on both fold change unadjusted and FDR adjusted p-value cutoff of <0.05.
Fig. 9. Specific ncRNA markers of prenatal BPA exposure in liver:
The OPLS-DA S-Plots for ncRNA (lncRNA, miRNA, and snoRNA) from liver showing potential ncRNA markers of prenatal BPA-treatment. The up and down arrow represents the upregulated and downregulated genes with prenatal BPA-treatment, each point represents a non-coding RNA with pink and blue points representing the potential signatures of prenatal BPA-treatment.
With reference to miRNA, three downregulated (MIR29A, MIR30B, and MIR154A) and three upregulated (MIR200B, MIR323A, and MIR409) miRNA showed potential to serve as the signatures of prenatal BPA exposure based on OPLS-DA S-plot (Figure 9). All these six markers were significant based on unadjusted fold change p value cutoff < 0.05 and two of the miRNA that were downregulated (MIR30B and MIR154A) and two of the miRNA that were upregulated (MIR200B and MIR409) were also significant based on the fold change FDR adjusted p value < 0.05. One microRNA MIR29A had fold change FDR adjusted p value < 0.1.
Ten snoRNA that were lower in expression and nine snoRNA that were higher in expression were identified based on S-plot (Figure 9) as potential signature grouping of prenatal BPA exposure. These included LOC114111313, LOC114114259, LOC114115466, LOC114116533, LOC114118124, LOC114118597, LOC114108878, LOC114110370, LOC114110711 and LOC114111819 that were downregulated and LOC114111353, LOC114114211, LOC114114229, LOC114117088, LOC114117421, LOC114117664, LOC114117665, LOC114117661, and LOC114118122 that were upregulated. All these markers showed significance based on fold change FDR adjusted p-value < 0.05.
Based on the S-plot, five snRNA that were downregulated and 10 snRNA that were upregulated by prenatal BPA-treatment were identified as potential snRNA signatures of the prenatal BPA-treatment (Supplementary Figure 3). These markers include LOC114114660, LOC114115462, LOC114115947, LOC114117093, and LOC114117060 that were downregulated and LOC114115961, LOC114115962, LOC114117112, LOC114117113, LOC114117114, LOC114117115, LOC114117116, LOC114117117, LOC114117120, and LOC114110196 that were upregulated. Most of these markers (four downregulated and eight upregulated) showed significance based on fold change FDR adjusted p- value < 0.05. Three snRNA, one downregulated (LOC114114660) and 2 upregulated (LOC114117117 and LOC114117120) did not meet the criteria of fold change FDR adjusted p value < 0.05.
Muscle:
Clear separation of the control and prenatal BPA groups was observed in all three plots for the snoRNA (2D and 3D PCA plots and OPLS-DA score plot). While there was an overlap of the two groups on 2D PCA plots for lncRNA, miRNA and snRNA, clear separation was observed in the 3D PCA plots and the OPLS-DA score plots indicative of differences in the ncRNA profiles for the two groups (Figure 10).
Fig. 10. PCA and OPLS-DA score plots for ncRNA in muscle.
The PCA 2D and 3D and OPLS-DA score plots for lncRNA, miRNA, snoRNA and snRNA in the muscle from control (blue) and prenatal BPA-treated (pink) animals. PCA 2D and 3D plots are plotted with principal component 1 on X-axis and principal component 2 on Y-axis, and OPLS-DA score plot with predictive component on X-axis and first orthogonal component on Y-axis with each point representing one animal.
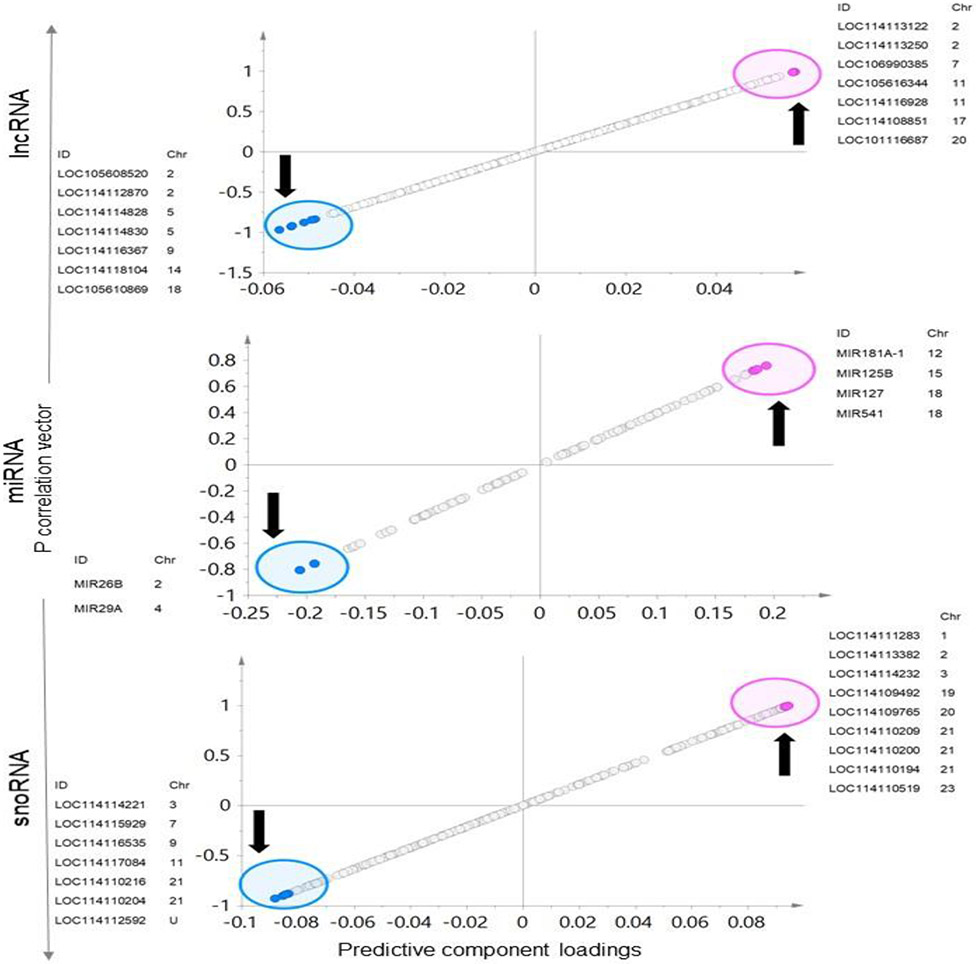
The OPLSA-DA models, S-plots (Figure 11 and supplementary Figure 3), model loadings and VIP plots used to select the potential ncRNA signatures found seven lncRNA with lower expression and seven lncRNA with higher expression as potential signatures of prenatal BPA impact (Figure 11). The markers with lower expression include LOC105608520, LOC114112870, LOC114114828, LOC114114830, LOC114116367, LOC114118104, and LOC105610869 and the markers with high expression include LOC114113122, LOC114113250, LOC106990385, LOC105616344, LOC114116928, LOC114108851, and LOC101116687. Among these LOC106990385, LOC105616344 and LOC101116687 that were upregulated were significant based on fold change FDR adjusted p value < 0.05. In contrast, five lncRNA that were downregulated while not meeting the criteria for FDR adjusted p value <0.05, but showed fold change significance p value <0.05 (not adjusted for FDR) included LOC105608520, LOC114112870, LOC114114830, LOC114116367, and LOC105610869.
Fig. 11. Specific ncRNA markers of prenatal BPA exposure in muscle:
The OPLS-DA S-Plots for ncRNA (lncRNA, miRNA, and snoRNA) from muscle showing potential ncRNA markers of prenatal BPA-treatment. The up and down arrow represents the upregulated and downregulated genes with prenatal BPA-treatment, each point represents a non-coding RNA with pink and blue points representing the potential signatures of prenatal BPA-treatment.
Based on S-plot, two miRNA (MIR26B and MIR29A) that were downregulated and four miRNA (MIR181A-1, MIR125B, MIR127 and MIR541) that were upregulated were identified as potential signatures (Figure 11) of prenatal BPA-treatment effect. Among these two miRNAs which were downregulated (MIR26B and MIR29A) and two which were upregulated (MIR125B and MIR127) showed significance at p value < 0.05 (not adjusted for FDR). None of the miRNA met the criteria of fold change FDR adjusted p value < 0.05.
Seven downregulated and nine upregulated snoRNA showed the potential to serve as snoRNA signatures of prenatal BPA-treatment (Figure 11). These markers include downregulated snoRNAs LOC114114221, LOC114115929, LOC114116535, LOC114117084, LOC114110216, LOC114110204, LOC114112592 and upregulated snoRNAs LOC114111283, LOC114113382, LOC114114232, LOC114109492, LOC114109765, LOC114110209, LOC114110200, LOC114110194, and LOC114110519. All nine snoRNA that were upregulated showed significance based on fold change FDR adjusted p value < 0.05. Among the seven snoRNA which were downregulated, no snoRNA met the criteria of FDR adjusted p value < 0.05 (five showed FDR adjusted p value < 0.1), however six showed fold change significance unadjusted p value< 0.05 only excluding one snoRNA LOC114117084.
With snRNA, only one snRNA that was downregulated and four snRNA that were upregulated were identified as potential signatures of the prenatal BPA-treatment (Supplementary Figure 3), including LOC114115964 which was downregulated and LOC114114271, LOC114117097, LOC114108888, and LOC114108889 that were upregulated. None of these snRNA met the criteria of fold change significance with FDR adjustment p value< 0.05. Only one snRNA LOC114115964 that was downregulated showed fold change significance (not adjusted for FDR) p value < 0.05.
Correlation of ncRNA with coding RNA:
Liver:
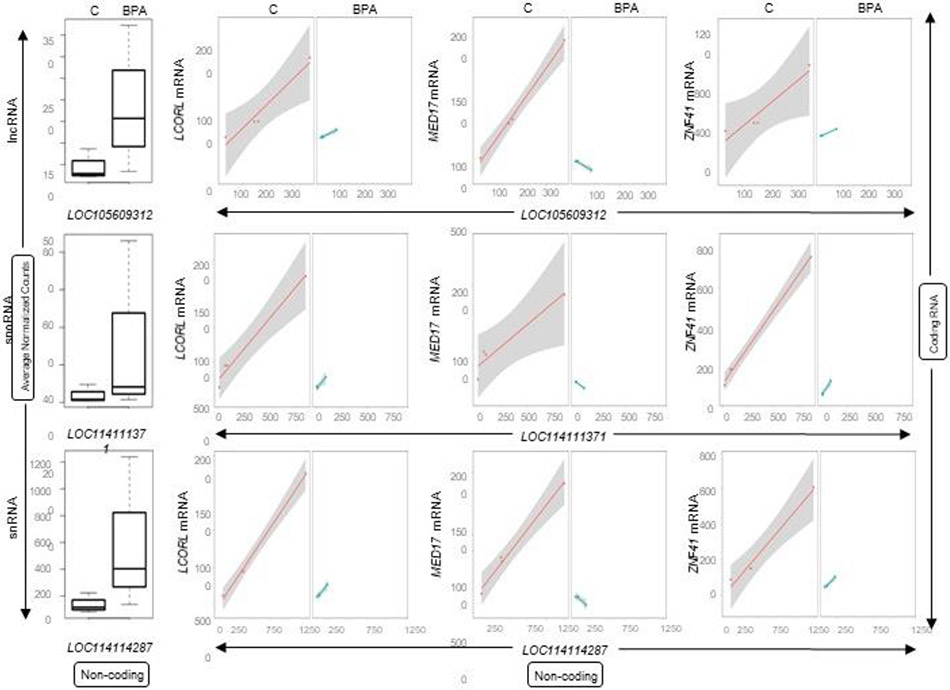
None of the 14 miRNAs in the liver modulated by prenatal BPA-treatment were correlated with levels of coding RNA. In contrast, several lncRNA, snoRNA, and snRNA differentially expressed by prenatal BPA-treatment in the liver showed significant correlation with coding RNA (Supplemental Table S9). Of the 77 prenatal BPA induced differentially expressed lncRNA, five lncRNA correlated with mRNAs (Supplemental Table S9). Among the lncRNAs and mRNA pairs in the liver, uncharacterized lncRNAs LOC105609312, LOC106991099, LOC114113174, LOC105602706, and LOC105609364 putatively correlated with 36, 28, 18, 5 and 4 mRNAs, respectively. Of the 127 differentially expressed snoRNAs, four unique snoRNAs formed 71 putative snoRNA-mRNA pairs (Supplemental Table S9). Of these snoRNAs-mRNA putative pairs, four snoRNAs LOC114111371, LOC114114259, LOC114113393, and LOC114117128 correlated with 35, 29, 5, and 2 mRNAs respectively. Likewise, the 55 snRNA differentially modulated by prenatal BPA-treatment, only snRNA LOC114114287 formed 29 putative snRNA-coding RNA pairs (Supplemental Table S9). The top 10 genes that correlated with lncRNA, snoRNA and snRNA are shown in Table 5. Among the coding RNA that commonly correlated with the ncRNAs lncRNA (LOC105609312), snoRNA (LOC114111371) and snRNA (LOC114114287) included mitochondrial protein AFG1 like ATPase (AFG1L), cell cycle regulators establishment of sister chromatid cohesion N-acetyltransferase 1 (ESCO1) and CDK5 regulatory subunit associated protein 1 like 1 (CDKAL1), and transcriptional regulators interactor of little elongation complex ELL subunit 2 (ICE2), mediator complex subunit 17 (MED17), ligand dependent nuclear receptor corepressor like (LCORL) and zinc finger protein family members (ZNF41, ZNF112, ZNF502 and ZNF577). Additional genes that correlated are ubiquitin specific peptidase 45 (USP45), NEDD4 binding protein 2 like 2 (N4BP2L2), mirror-image polydactyly 1 (MIPOL1), ribosomal protein S6 kinase A6 (RPS6KA6), centrosome and spindle pole associated protein 1 (CSPP1), prostate transmembrane protein, androgen induced 1 (PMEPA1), and progesterone immunomodulatory binding factor 1 (PIBF1). The expression plots for lncRNA (LOC105609312), snoRNA (LOC114111371) and snRNA (LOC114114287) and their correlation plots with three commonly correlated mRNAs (LCORL, MED17 and ZNF41) are represented in Figure 12.
Table 5:
ncRNAs lncRNA, snoRNA and snRNA that correlated with mRNA in liver from prenatal BPA-treated animals.
| ncRNA | Coding RNA |
Control Correlation |
Control pVal |
Prenatal BPA Correlation |
Prenatal BPA pVal |
Z Score Diff |
pVal Diff |
Classes |
|---|---|---|---|---|---|---|---|---|
| lncRNA-Coding RNA | ||||||||
| LOC106991099 | KIT | −0.96 | 0.04 | 1.00 | 1.18E-03 | 3.28 | 1.04E-03 | −/+ |
| LOC114113174 | ALDH1A2 | −0.97 | 0.03 | 1.00 | 1.52E-03 | 3.38 | 7.18E-04 | −/+ |
| LOC106991099 | ARMCX3 | −0.99 | 0.01 | 1.00 | 2.40E-03 | 3.69 | 2.28E-04 | −/+ |
| LOC114113174 | AP1M2 | −0.97 | 0.03 | 1.00 | 2.55E-03 | 3.32 | 8.94E-04 | −/+ |
| LOC105609312 | ESCO1 | 0.98 | 0.02 | 1.00 | 2.73E-03 | 0.25 | 8.06E-01 | +/+ |
| LOC106991099 | IL2RG | −0.98 | 0.02 | 1.00 | 3.57E-03 | 3.44 | 5.73E-04 | −/+ |
| LOC105609312 | ICE2 | 0.97 | 0.03 | 1.00 | 3.64E-03 | 0.38 | 7.02E-01 | +/+ |
| LOC105609364 | CABYR | 0.97 | 0.03 | 1.00 | 4.60E-03 | 0.33 | 7.43E-01 | +/+ |
| LOC114113174 | C1QTNF1 | −0.98 | 0.02 | 1.00 | 4.91E-03 | 3.50 | 4.70E-04 | −/+ |
| LOC106991099 | CST3 | −0.98 | 0.02 | 0.99 | 7.32E-03 | 3.48 | 4.95E-04 | −/+ |
| snoRNA-Coding RNA | ||||||||
| LOC114114259 | ZNF599 | 0.96 | 0.04 | 1.00 | 0.00 | 0.51 | 0.61 | +/+ |
| LOC114111371 | ZNF41 | 0.99 | 0.01 | 1.00 | 0.00 | 0.10 | 0.92 | +/+ |
| LOC114111371 | CCDC152 | 0.98 | 0.02 | 1.00 | 0.00 | 0.20 | 0.84 | +/+ |
| LOC114113393 | BHMG1 | 0.96 | 0.04 | 1.00 | 0.00 | 0.51 | 0.61 | +/+ |
| LOC114111371 | ZNF782 | −0.96 | 0.04 | 1.00 | 0.00 | 3.23 | 0.00 | −/+ |
| LOC114114259 | CLK4 | 0.98 | 0.02 | 1.00 | 0.00 | 0.16 | 0.87 | +/+ |
| LOC114114259 | LCORL | 0.99 | 0.01 | 1.00 | 0.00 | 0.07 | 0.94 | +/+ |
| LOC114114259 | WRN | 0.98 | 0.02 | 1.00 | 0.00 | 0.26 | 0.80 | +/+ |
| LOC114114259 | ESCO1 | 0.99 | 0.01 | 1.00 | 0.00 | 0.14 | 0.89 | +/+ |
| LOC114111371 | ZNF624 | 0.99 | 0.01 | 1.00 | 0.00 | 0.09 | 0.93 | +/+ |
| snRNA-Coding RNA | ||||||||
| LOC114114287 | CCDC66 | 0.96 | 0.04 | 1.00 | 0.00 | 0.45 | 0.65 | +/+ |
| LOC114114287 | ZNF112 | 0.98 | 0.02 | 1.00 | 0.00 | 0.31 | 0.75 | +/+ |
| LOC114114287 | ESCO1 | 0.98 | 0.02 | 1.00 | 0.00 | 0.31 | 0.76 | +/+ |
| LOC114114287 | N4BP2L2 | 0.98 | 0.02 | 1.00 | 0.00 | 0.17 | 0.87 | +/+ |
| LOC114114287 | MIPOL1 | 0.98 | 0.02 | 1.00 | 0.00 | 0.30 | 0.76 | +/+ |
| LOC114114287 | LCORL | 0.98 | 0.02 | 1.00 | 0.00 | 0.26 | 0.80 | +/+ |
| LOC114114287 | USP45 | 0.97 | 0.03 | 1.00 | 0.00 | 0.45 | 0.66 | +/+ |
| LOC114114287 | PIBF1 | 0.96 | 0.04 | 1.00 | 0.00 | 0.53 | 0.59 | +/+ |
| LOC114114287 | NSMCE2 | 0.95 | 0.05 | 1.00 | 0.00 | 0.57 | 0.57 | +/+ |
| LOC114114287 | MED17 | −0.96 | 0.04 | 1.00 | 0.00 | 3.25 | 0.00 | −/+ |
Fig. 12. Correlation of coding and ncRNA expression in liver from prenatal BPA-treated animals.
Box plots representing the average normalized counts for lncRNA, snoRNA or snRNAare shown on the left. The treatment groups - control (C) or prenatal BPA (BPA) - are denoted on the X-axis and the average normalized counts on the Y-axis. The line plots representing the correlation of expression between the lncRNA-mRNA, snoRNA-mRNA or snRNA-mRNA pairs in control or treatment group are shown on the right. Correlations for each pair is represented in orange line for control animals (C) and as a green line for prenatal BPA-treated animals (BPA).
Muscle:
None of the lncRNAs and snoRNAs differentially expressed with BPA-treatment in the muscle were correlated with coding RNA.
Discussion:
This study capitalizing on next generation transcriptomic profiling of the liver and muscle from ovary intact sheep shows distinct patterns of coding and non-coding RNA expression in the liver and muscle. The tissue-specific expression of coding RNAs in the liver and muscle are in line with roles for glucose and lipid metabolism in the liver and structure and function in the skeletal muscle. Similarly, prenatal BPA-treatment led to discrete changes in coding and non-coding RNA in both liver and muscle. In both tissues, genes differentially expressed by prenatal BPA-treatment were enriched in gene pathways related to mitochondrial and oxidative phosphorylation pathways, consistent with the lipotoxic phenotype evident in these tissues (Puttabyatappa et al., 2019). The changes in the genes related to oxidative stress, lipid biosynthetic process, and extracellular matrix related pathways in the liver from prenatal BPA-treated sheep were in agreement with the oxidative and lipotoxic disruptions in this tissue (Puttabyatappa et al., 2019). Tissue-specific RNA signatures of prenatal BPA-treatment identified through the predictive ability of OPLS-DA VIP-plots revealed downregulation in anti-inflammatory gene PECAM and dysregulation in lipid metabolism related genes RDH11 and ABCA6 in the liver. Similarly, signatures identified in the muscle included downregulation of ER stress and oxidative stress related genes CAST and NOS1 and upregulation of the lipogenic gene FASN. These changes are in accordance with the proinflammatory state, oxidative stress, and lipotoxicity in the liver and muscle from prenatal BPA-treated female sheep (Puttabyatappa et al., 2019). The significance of these findings in relation to the prenatal BPA-induced metabolic changes are discussed below.
Tissue-specific gene expression profile in metabolic tissues of control females
In line with previous reports in mouse, pigs, and humans (Consortium et al., 2015; Li et al., 2017; González-Prendes et al., 2019) and our report in the female sheep (Saadat et al., 2021), tissue-specific gene expression revealed by RNA sequence analysis is in line with the functional role of the liver and muscle. In the liver, genes involved in the metabolic role of glucose and lipid metabolism were highly expressed and included genes such as SGK2, a transcriptional co-activator of gluconeogenic genes (Gotoh and Negishi, 2015) and GATA4, a transcriptional factor involved in lipid and glucose metabolism (Zheng et al., 2013). In the skeletal muscle, genes related to the myocontractile function such as actin and myosin proteins were highly enriched (Squire, 2019).
Similar to observations with coding RNA, ncRNA also showed tissue-specific differential gene expression, consistent with the roles of the tissues. For example, the microRNA, MIR200A, highly expressed in the liver is involved in hepatic stellate cell activation (Yang et al., 2017), while MIR10B, which is highly expressed in the muscle, is involved in myoblast proliferation (Ge et al., 2019). Many of the coding and non-coding tissue specific signatures in control liver and muscle validate our previous findings (Saadat et al., 2021) in spite of the daily subcutaneous injections of corn oil that the current cohort of controls in this study received (control animals in previous study did not receive vehicle treatment).
Impact of prenatal BPA-treatment on gene expression profile in liver
Prenatal BPA-treatment induced oxidative stress and ectopic lipid accumulation in the liver (Puttabyatappa et al., 2019). The downregulation of SLC44A4, a member of mitochondrial solute carrier family of proteins with role in lipid transfer (Clémençon et al., 2013; Janer et al., 2016), observed in this model parallels downregulation in a diet-induced obese mouse model of hepatic steatosis (Le et al., 2021). Such changes have a negative impact on insulin sensitivity (Lonardo et al., 2005) and further, if not mitigated in a timely manner, culminate in tissue damage and eventual fibrosis (Roehlen et al., 2020). Supportive of this possibility is the BPA-programmed downregulation of WFDC2, a protease inhibitor that prevents the degradation of type I collagen (LeBleu et al., 2013), leading to collagen accumulation and development of hepatic fibrosis. On the other hand, the downregulation of MSLN, an activator of hepatic fibroblasts (Koyama et al., 2017) and MMP7, a protease involved in extracellular matrix turnover, observed in the liver from prenatal BPA-treated sheep, may reflect a compensatory response to prevent the development of hepatic fibrosis. In line with this premise, pathway analysis showed enrichment of gene pathways involved in response to oxidative stress, lipid biosynthetic processes, and extracellular matrix related genes.
As was the case with the coding RNA, the changes in ncRNA expression were reflective of the hepatic phenotype. The upregulation of MIR200B and MIR30B observed in steatotic livers from prenatal BPA-treated sheep parallel observations in rats (Dai et al., 2019) fed a high fat diet. In stark contrast, mice fed a high fat diet had elevated lipogeneic gene expression and hepatic lipid accumulation that was associated with suppression (not upregulation) of MIR200B expression (Guo et al., 2016). The upregulation of MIR30B in the liver of prenatal BPA-treated sheep may underlie dysregulation of ER gene pathways evident in this model (Supplemental Table S6). Consistent with this premise, overexpression of MIR30B induced ER stress in normal-fed rats, as opposed to its inhibition suppressing ER stress in rats fed a high fat diet (Dai et al., 2019). This study also reported that higher serum MIR30B levels were associated with hepatic steatosis in humans (Dai et al., 2019). Hepatic lipid accumulation, oxidative stress, and ER stress are linked with hepatic cellular injury and the development of fibrosis. BPA-induced upregulation of MIR200B and MIR409 and downregulation of MIR26B and MIR154A are supportive of such a possibility, in view of their reported association with hepatic fibrosis (Wu et al., 2017; Wu et al., 2019). Overall changes in coding and ncRNA evident in the present study are in accordance with the hepatic phenotype induced by prenatal BPA-exposure and should be evaluated further, along with the differentially expressed lncRNA, snoRNA, and snRNA whose roles are yet to be fully elucidated.
Impact of prenatal BPA-treatment on gene expression profile of muscle
Similar to disruptions seen in the liver, prenatal BPA-treatment induced lipotoxicity and oxidative stress in the skeletal muscle (Puttabyatappa et al., 2019). However, unlike in the liver, the majority of the differentially expressed genes identified by RNA sequencing analyses in the muscle were uncharacterized genes whose functional changes are yet to be identified. Among the genes whose functional roles are well characterized were upregulated genes NR4A1 and FASN and downregulated genes CAST and NOS1. Nuclear receptor NR4A1 is involved in glucose and lipid metabolism in metabolic tissues including skeletal muscle (Zhang et al., 2018a) and acts as a sensor of lipotoxicity in the pancreatic beta cells (Briand et al., 2012). The increased expression of this gene is therefore in accordance with the lipotoxic changes exhibited by the skeletal muscle from prenatal BPA-treated sheep. Additionally, the downregulation of CAST, a gene that ameliorates ER stress (Li et al., 2018), and NOS1, a gene involved in oxidative stress (Saraiva et al., 2005), are supportive of the association of lipotoxicity with the development of ER and oxidative stress.
Prenatal BPA-induced Gene Expression Signatures in Liver and Muscle
The traditional analysis of RNA sequencing derived transcriptomics data has the advantage of unbiased discovery of differentially expressed genes. However, the multivariate modeling technique, OPLS-DA, reduces data into fewer weighted variables through combinations of related information that helps predict the transcriptional signatures of prenatal BPA-treatment (Trygg and Wold, 2002). Gene expression signatures identified using these predictive component plots were in line with the phenotypic outcomes in the liver and muscle of prenatal BPA exposure. For example, predictive component analysis identified genes linked with the hepatic lipid accumulation such as the downregulated gene RDH11. As RDH11 is a retinaldehyde reductase that is essential for the maintenance of physiological levels of retinol in the liver (Belyaeva et al., 2018) and is also shown to be involved in the regulation of hepatic glucose and lipid metabolism (Kiefer et al., 2012). Therefore, downregulation of RDH11 may therefore impair fatty acid metabolism contributing to lipotoxic conditions (Haffar et al., 2015). Elevation of ABCA6, a lipid transport associated protein involved in macrophage lipid homeostasis (Kaminski et al., 2001), complements the RDH11 reduction. Considering macrophages play a central role in the development and progression of hepatic steatosis (Kazankov et al., 2019), elevated ABCA6 levels maybe reflective of increased macrophage activity in the liver in response to prenatal BPA-induced hepatic lipid accumulation. Similar transcriptional signatures associated with lipid metabolism dysfunction reflective of lipid accumulation in the muscle from prenatal BPA-treated sheep have also identified. These included downregulated genes CAST, a preventer of ER stress (Li et al., 2018) and NOS1, an oxidative stress related gene (Saraiva et al., 2005) and upregulation of FASN, a lipogenic gene (Menendez and Lupu, 2007). As a lipotoxic state can arise as a result of increased lipid biosynthesis, which in turn can lead to development of ER stress and an oxidative state, these coding RNAs identified through predictive component plot may serve as transcriptional signatures of metabolic dysfunction associated with gestational BPA exposure. In line with these coding RNA changes, the ncRNA signatures are also linked with hepatic and muscle lipid dysfunctions. MIR200B and MIR30B in the liver and MIR26B and MIR29A in the muscle are linked not only with dysregulation of hepatic lipid biosynthetic process and ER stress (Guo et al., 2016; Dai et al., 2019) but also with fibrosis (Wu et al., 2017; Wu et al., 2019).
Relationship between coding and non-coding RNA
Since ncRNA regulates expression of the coding RNAs through transcriptional and post-transcriptional control, the relationship between these in liver and muscle were examined. Surprisingly, none of the ncRNA in the skeletal muscle from prenatal BPA-treated female sheep were correlated with the coding RNA changes. In contrast, several ncRNAs belonging to lncRNA, snoRNA, and snRNA were correlated with coding RNA in the liver, the functional role of which remains to be elucidated. However, the coding RNAs that correlated with them provide some clue to potential role in the liver and prenatal BPA-associated metabolic dysfunctions. Coding RNA, LCORL, which correlated with multiple ncRNAs is a transcription factor with links to human and farm animal body traits (Metzger et al., 2013). Interestingly, this gene has a genetic basis in the fatty liver development in geese (Yang et al., 2020), a feature also observed in livers from prenatal BPA-treated female sheep (Puttabyatappa et al., 2019). Since lipid accumulation in the liver may result from increased fatty acid biosynthesis, a contributor could be MED17, the other coding RNA that correlated with multiple ncRNA in the liver, which is involved in transcriptional activation of lipogenic genes (Viscarra et al., 2017).
Transcriptome changes in this study vs. target analysis of BPA and disease transcriptome databases
The changes in gene expression observed in our study are not part of the genes tested in the CLARITY-BPA study as the CLARITY liver studies focused only on a selected set of genes quantified by qPCR (Greenberg, 2021). Changes in the adult skeletal muscle is also not available to make such a comparison. With respect to disease phenotype, the noncoding RNA, miR-200b, which was upregulated in the liver of prenatal BPA-treated sheep was also found to be upregulated in atleast two independent studies using mouse models of steatosis and human NASH (Tran et al., 2017; Wang et al., 2018), although downregulated in a third study (Guo et al., 2016). These differences may reflect different stages of disease progression in NAFLD. Similarly, MIR30 that is downregulated in the liver of BPA-treated sheep was reported to be downregulated in the liver tissues from 2 different human studies of steatosis (Latorre et al., 2017). These NAFLD findings of microRNA have been sufficiently reviewed (Guo et al., 2016; Latorre et al., 2017; Latorre et al., 2020; Tran et al., 2017; Wang et al., 2018; Zhang et al. 2021). Thus, in our study, which predominantly focuses on the role of non-coding RNA and their correlation with mRNA expression, the direction of effect is concordant with those in studies in NAFLD livers from humans. Since none of these studies were conducted in skeletal muscle there is not sufficient information to make similar comparisons.
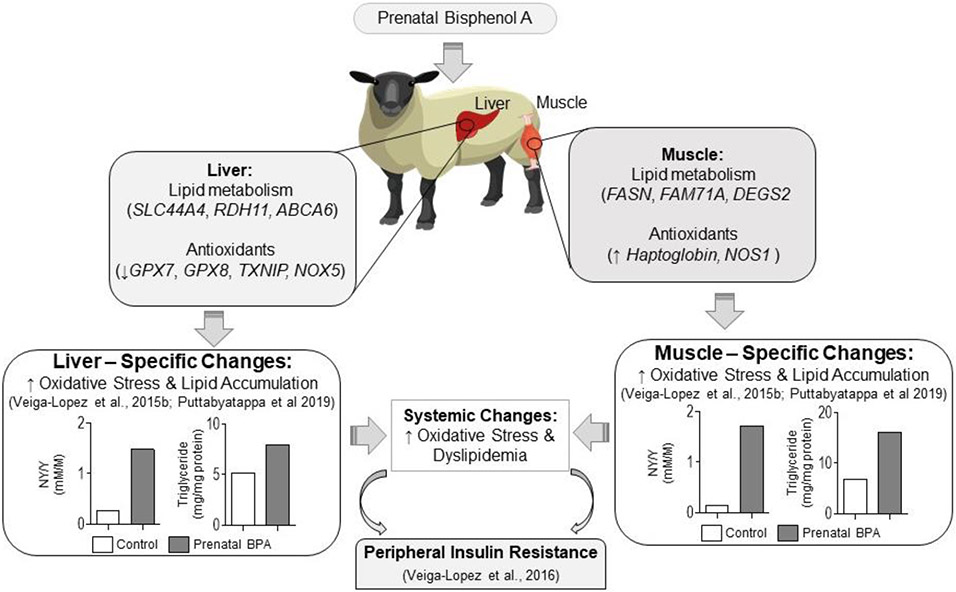
Conclusions
Our previous findings have shown that tissue-specific changes in liver and muscle (Puttabyatappa et al., 2019) may underlie prenatal BPA-induced metabolic dysfunction of peripheral insulin resistance (Veiga-Lopez et al., 2016). These tissue-specific changes in liver and muscle involve increases in oxidative stress and ectopic lipid accumulation, factors that negatively modulate insulin sensitivity (Figure 13). The findings from the RNAseq analysis from the present study, which shows dysregulation of genes / networks involved in regulation of lipid metabolism and oxidative stress are in keeping with the reported functional outcomes. The observed gene expression changes from the RNAseq study therefore offer mechanistic insights into the programming of oxidative stress and lipid accumulation that likely contribute to the fibrotic defects in the liver and skeletal muscle and ultimately the prenatal BPA-induced metabolic dysfunctions.
Fig. 13.
Summary of RNAseq findings programmed by prenatal BPA-treatment in the liver and muscle and their relationship with previous findings and potential impact on tissue-specific insulin sensitivity.
The findings of BPA-induced disruptions in gene signatures in the liver and muscle especially at levels found in pregnant women (Woodruff et al., 2011; Veiga-Lopez et al., 2015a) are of translational relevance as they are also typified in patients with NAFLD (Robinson and Shah, 2020). Evidence points to a role for BPA in promoting hepatic steatosis and/or NAFLD (Thayer et al., 2012; Lubrano et al., 2013).
A major strength of this study is the use of a precocial model of relevance to human disease state (Padmanabhan and Veiga-Lopez, 2014; Symonds et al., 2015; Gonzalez-Bulnes and Chavatte-Palmer, 2017; Fuller-Jackson and Henry, 2018). Another strength is the parallel assessment of both coding RNA and ncRNA in metabolically relevant organs. This approach allowed identification of not only gene expression signatures but also interrogation of the relation between non-coding and coding RNA. However, these findings should be interpreted while taking into account the use of whole liver and muscle tissues, which are made up of multiple cell types. In addition, while the study expands our previous phenotypic observations at animal and tissue levels to gene targets and pathways, how these identified targets impact the metabolic outcomes at the level of cellular and protein levels remains to be investigated. In conclusion, findings from this study in a precocial model of human translational relevance is the first step towards gaining mechanistic insights in the programming of metabolic dysfunctions from environmental exposure to BPA, an endocrine disruptor of public health relevance.
Supplementary Material
Highlights.
Prenatal BPA programmed mRNA/ncRNA changes of metabolic relevance in liver/muscle
Prenatal BPA dysregulated hepatic and muscle mitochondrial gene pathways
Prenatal BPA disrupted hepatic oxidative stress and lipid biosynthetic mediators.
Multiple ncRNA correlated with LCORL, MED17 and ZNF41 mRNA in liver
Prenatal BPA exposure signatures include liver and muscle lipid metabolism genes.
Acknowledgements
We thank Mr. Douglas Doop and Gary McCalla for their valuable assistance in breeding, lambing, and animal care; Dr. Almudena Veiga-Lopez, Dr. Bachir Abi Salloum, Mr. Evan Beckett, Mrs. Carol Herkimer and students supported through the Undergraduate Research Opportunity Program (University of Michigan) for the help provided with administration of treatments and tissue harvest.
Research reported in this publication was supported by National Institute of Environmental Health Sciences R01 ES016541, R01 ES 030374, and P30 ES017885. MP was supported via Ruth L. Kirschstein Institutional Training Grant T32 ES007062. NS is supported via National Institute of Diabetes and Digestive and Kidney Diseases Institutional Training Grant T32 DK071212
Footnotes
Disclosure statement: Authors have nothing to disclose.
Declaration of competing interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham A, Chakraborty P, 2020. A review on sources and health impacts of bisphenol A. Rev Environ Health 35, 201–210. [DOI] [PubMed] [Google Scholar]
- Ahmed RG, 2016. Maternal bisphenol A alters fetal endocrine system: Thyroid adipokine dysfunction. Food Chem Toxicol 95, 168–174. [DOI] [PubMed] [Google Scholar]
- Akash MSH, Sabir S, Rehman K, 2020. Bisphenol A-induced metabolic disorders: From exposure to mechanism of action. Environ Toxicol Pharmacol 77, 103373. [DOI] [PubMed] [Google Scholar]
- Andrews S, 2010. FastQC: A quality control tool for high throughput sequence data. https://github.com/s-andrews/FastQC. Accessed Dec 14th 2021. [Google Scholar]
- Banker M, Puttabyatappa M, O'Day P, Goodrich JM, Kelley AS, Domino SE, Smith YR, Dolinoy DC, Song PXK, Auchus RJ, Padmanabhan V, 2021. Association of Maternal-Neonatal Steroids With Early Pregnancy Endocrine Disrupting Chemicals and Pregnancy Outcomes. J Clin Endocrinol Metab 106, 665–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyaeva OV, Wu L, Shmarakov I, Nelson PS, Kedishvili NY, 2018. Retinol dehydrogenase 11 is essential for the maintenance of retinol homeostasis in liver and testis in mice. Journal of Biological Chemistry 293, 6996–7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blighe K, Rana S, Lewis M, 2021. EnhancedVolcano: Publication-ready volcano plots with enhanced colouring and labeling. https://github.com/kevinblighe/EnhancedVolcano. Accessed Dec 14th 2021. [Google Scholar]
- Bolger AM, Lohse M, Usadel B, 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand O, Helleboid-Chapman A, Ploton M, Hennuyer N, Carpentier R, Pattou F, Vandewalle B, Moerman E, Gmyr V, Kerr-Conte J, 2012. The nuclear orphan receptor Nur77 is a lipotoxicity sensor regulating glucose-induced insulin secretion in pancreatic β-cells. Molecular Endocrinology 26, 399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL, 2008. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 116, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clémençon B, Babot M, Trézéguet V, 2013. The mitochondrial ADP/ATP carrier (SLC25 family): pathological implications of its dysfunction. Molecular aspects of medicine 34, 485–493. [DOI] [PubMed] [Google Scholar]
- Consortium G, Ardlie KG, Deluca DS, Segrè AV, Sullivan TJ, Young TR, Gelfand ET, Trowbridge CA, Maller JB, Tukiainen T, 2015. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 348, 648–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenye T, 2021. Do results obtained with RNA-sequencing require independent verification? Biofilm. 2021 Jan 13;3:100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales J, Kristofco LA, Steele WB, Yates BS, Breed CS, Williams ES, Brooks BW, 2015. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose Response 13(3):1559325815598308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai LL, Li SD, Ma YC, Tang JR, Lv JY, Zhang YQ, Miao YL, Ma YQ, Li CM, Chu YY, 2019. MicroRNA-30b regulates insulin sensitivity by targeting SERCA2b in non-alcoholic fatty liver disease. Liver International 39, 1504–1513. [DOI] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR, 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durinck S, Spellman PT, Birney E, Huber W, 2009. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat Protoc 4, 1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewels P, Magnusson M, Lundin S, Käller M, 2016. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32, 3047–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Jackson JP, Henry BA, 2018. Adipose and skeletal muscle thermogenesis: studies from large animals. J Endocrinol 237, R99–R115. [DOI] [PubMed] [Google Scholar]
- Galili T, 2021. gplots package. https://github.com/talgalili/gplots. Accessed Dec 14th 2021. [Google Scholar]
- Ge G, Yang D, Tan Y, Chen Y, Jiang D, Jiang A, Li Q, Liu Y, Zhong Z, Li X, 2019. miR-10b-5p regulates C2C12 myoblasts proliferation and differentiation. Bioscience, biotechnology, and biochemistry 83, 291–299. [DOI] [PubMed] [Google Scholar]
- Gerona RR, Woodruff TJ, Dickenson CA, Pan J, Schwartz JM, Sen S, Friesen MW, Fujimoto VY, Hunt PA, 2013. Bisphenol-A (BPA), BPA glucuronide, and BPA sulfate in midgestation umbilical cord serum in a northern and central California population. Environ Sci Technol 47, 12477–12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich J, Ticiani E, Veiga-Lopez A, 2020. Placenta Disrupted: Endocrine Disrupting Chemicals and Pregnancy. Trends Endocrinol Metab 31, 508–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Bulnes A, Chavatte-Palmer P, 2017. Contribution of Large Animals to Translational Research on Prenatal Programming of Obesity and Associated Diseases. Curr Pharm Biotechnol 18, 541–551. [DOI] [PubMed] [Google Scholar]
- González-Prendes R, Mármol-Sánchez E, Quintanilla R, Castelló A, Zidi A, Ramayo-Caldas Y, Cardoso TF, Manunza A, Cánovas Á, Amills M, 2019. About the existence of common determinants of gene expression in the porcine liver and skeletal muscle. BMC genomics 20, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT, 2015. EDC-2: The Endocrine Society's Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev, er20151010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh S, Negishi M, 2015. Statin-activated nuclear receptor PXR promotes SGK2 dephosphorylation by scaffolding PP2C to induce hepatic gluconeogenesis. Scientific reports 5, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg A, 2021. CLARITY-BPA: Diabetes/Pancreas/Liver (Greenberg) DOI: 10.22427/NTP-DATA-018-00007-0001-000-7. [DOI] [Google Scholar]
- Guo J, Fang W, Sun L, Lu Y, Dou L, Huang X, Sun M, Pang C, Qu J, Liu G, 2016. Reduced miR-200b and miR-200c expression contributes to abnormal hepatic lipid accumulation by stimulating JUN expression and activating the transcription of srebp1. Oncotarget 7, 36207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffar T, Bérubé-Simard F, Bousette N, 2015. Impaired fatty acid oxidation as a cause for lipotoxicity in cardiomyocytes. Biochemical and biophysical research communications 468, 73–78. [DOI] [PubMed] [Google Scholar]
- Hartley SW, Mullikin JC, 2015. QoRTs: a comprehensive toolset for quality control and data processing of RNA-Seq experiments. BMC Bioinformatics 16, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, 2019. The developmental basis of disease: Update on environmental exposures and animal models. Basic Clin Pharmacol Toxicol 125 Suppl 3, 5–13. [DOI] [PubMed] [Google Scholar]
- Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis R, Vandenberg LN, Vom Saal F, 2017. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol 68, 3–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TR, 2009. 'Validation' in genome-scale research. J Biol, 8(1), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janer A, Prudent J, Paupe V, Fahiminiya S, Majewski J, Sgarioto N, Des Rosiers C, Forest A, Lin ZY, Gingras AC, 2016. SLC 25A46 is required for mitochondrial lipid homeostasis and cristae maintenance and is responsible for Leigh syndrome. EMBO molecular medicine 8, 1019–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski WE, Wenzel JJ, Piehler A, Langmann T, Schmitz G, 2001. ABCA6, a novel a subclass ABC transporter. Biochemical and biophysical research communications 285, 1295–1301. [DOI] [PubMed] [Google Scholar]
- Kazankov K, Jørgensen SMD, Thomsen KL, Møller HJ, Vilstrup H, George J, Schuppan D, Grønbæk H, 2019. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nature reviews Gastroenterology & hepatology 16, 145–159. [DOI] [PubMed] [Google Scholar]
- Kiefer FW, Orasanu G, Nallamshetty S, Brown JD, Wang H, Luger P, Qi NR, Burant CF, Duester G, Plutzky J, 2012. Retinaldehyde dehydrogenase 1 coordinates hepatic gluconeogenesis and lipid metabolism. Endocrinology 153, 3089–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinert M, Müller TD, 2020. A New FGF21 Analog for the Treatment of Fatty Liver Disease. Diabetes 69, 1605–1607. [DOI] [PubMed] [Google Scholar]
- Kolatorova L, Vitku J, Vavrous A, Hampl R, Adamcova K, Simkova M, Parizek A, Starka L, Duskova M, 2018. Phthalate metabolites in maternal and cord plasma and their relations to other selected endocrine disruptors and steroids. Physiol Res 67, S473–S487. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Wang P, Liang S, Iwaisako K, Liu X, Xu J, Zhang M, Sun M, Cong M, Karin D, 2017. Mesothelin/mucin 16 signaling in activated portal fibroblasts regulates cholestatic liver fibrosis. The Journal of clinical investigation 127, 1254–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane S, Sen P, Dickens AM, Hyötyläinen T, Orešić M, 2018. An overview of metabolomics data analysis: current tools and future perspectives. Comprehensive analytical chemistry 82, 387–413. [Google Scholar]
- Latorre J, Moreno-Navarrete JM, Mercader JM, Sabater M, Rovira Ò, Gironès J, Ortega FJ, 2017. Decreased lipid metabolism but increased FA biosynthesis are coupled with changes in liver microRNAs in obese subjects with NAFLD. Int J Obes (Lond), 41(4), 620–630. [DOI] [PubMed] [Google Scholar]
- Latorre J, Ortega FJ, Liñares-Pose L, Moreno-Navarrete JM, Lluch A, Comas F, … Fernández-Real JM, 2020. Compounds that modulate AMPK activity and hepatic steatosis impact the biosynthesis of microRNAs required to maintain lipid homeostasis in hepatocytes. EBioMedicine, 53, 102697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J, Fu Y, Han Q, Wei X, Ji H, Chen Y, Wang Q, Pi P, Li J, Lin X, 2021. Restoration of mRNA Expression of Solute Carrier Proteins in Liver of Diet-Induced Obese Mice by Metformin. Frontiers in endocrinology 12, 720784. doi: 10.3389/fendo.2021.720784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBleu VS, Teng Y, T O'Connell J, Charytan D, Müller GA, Müller CA, Sugimoto H, Kalluri R, 2013. Identification of human epididymis protein-4 as a fibroblast-derived mediator of fibrosis. Nature medicine 19, 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Patil S, Sartor MA, 2016. RNA-Enrich: a cut-off free functional enrichment testing method for RNA-seq with improved detection power. Bioinformatics 32, 1100–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Chattopadhyay S, Gong EY, Ahn RS, Lee K, 2003. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol Sci 75, 40–46. [DOI] [PubMed] [Google Scholar]
- Lee J, Choi K, Park J, Moon HB, Choi G, Lee JJ, Suh E, Kim HJ, Eun SH, Kim GH, Cho GJ, Kim SK, Kim S, Kim SY, Eom S, Choi S, Kim YD, 2018. Bisphenol A distribution in serum, urine, placenta, breast milk, and umbilical cord serum in a birth panel of mother-neonate pairs. Sci Total Environ 626, 1494–1501. [DOI] [PubMed] [Google Scholar]
- Li B, Qing T, Zhu J, Wen Z, Yu Y, Fukumura R, Zheng Y, Gondo Y, Shi L, 2017. A comprehensive mouse transcriptomic BodyMap across 17 tissues by RNA-seq. Scientific reports 7, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Ma J, Li J-B, Lacefield JC, Jones DL, Peng T-Q, Wei M, 2018. Over-expression of calpastatin attenuates myocardial injury following myocardial infarction by inhibiting endoplasmic reticulum stress. Journal of thoracic disease 10, 5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W, 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930. [DOI] [PubMed] [Google Scholar]
- Lonardo A, Lombardini S, Ricchi M, Scaglioni F, Loria P, 2005. Hepatic steatosis and insulin resistance. Alimentary pharmacology & therapeutics 22, 64–70. [DOI] [PubMed] [Google Scholar]
- Loomba R, Friedman SL, Shulman GI, 2021. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 184, 2537–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S, 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubrano C, Genovesi G, Specchia P, Costantini D, Mariani S, Petrangeli E, Lenzi A, Gnessi L, 2013. Obesity and metabolic comorbidities: environmental diseases? Oxid Med Cell Longev 2013, 640673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Liu H, Wu J, Yuan L, Wang Y, Du X, Wang R, Marwa PW, Petlulu P, Chen X, Zhang H, 2019. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ Res 176, 108575. [DOI] [PubMed] [Google Scholar]
- Martin M, 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal, 17, 10–12. 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- McKenzie AT, Katsyv I, Song WM, Wang M, Zhang B, 2016. DGCA: A comprehensive R package for Differential Gene Correlation Analysis. BMC Syst Biol 10, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez JA, Lupu R, 2007. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nature Reviews Cancer 7, 763–777. [DOI] [PubMed] [Google Scholar]
- Metzger J, Schrimpf R, Philipp U, Distl O, 2013. Expression levels of LCORL are associated with body size in horses. PloS one 8, e56497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Song W, Puttabyatappa M, 2021. Praegnatio Perturbatio-Impact of Endocrine-Disrupting Chemicals. Endocr Rev 42, 295–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A, 2014. Reproduction Symposium: developmental programming of reproductive and metabolic health. J Anim Sci 92, 3199–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttabyatappa M, Martin JD, Andriessen V, Stevenson M, Zeng L, Pennathur S, Padmanabhan V, 2019. Developmental programming: Changes in mediators of insulin sensitivity in prenatal bisphenol A-treated female sheep. Reprod Toxicol 85, 110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai MF, Tycksen ED, Sandell LJ, & Brophy RH, 2018. Advantages of RNA-seq compared to RNA microarrays for transcriptome profiling of anterior cruciate ligament tears. J Orthop Res, 36(1), 484–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Van Vleet TR, Ciurlionis R, Buck WR, Mittelstadt SW, Blomme EAG, & Liguori MJ, 2018. Comparison of RNA-Seq and Microarray Gene Expression Platforms for the Toxicogenomic Evaluation of Liver From Short-Term Rat Toxicity Studies. Front Genet, 9, 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KE, Shah VH, 2020. Pathogenesis and pathways: nonalcoholic fatty liver disease & alcoholic liver disease. Transl Gastroenterol Hepatol 5, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehlen N, Crouchet E, Baumert TF, 2020. Liver fibrosis: mechanistic concepts and therapeutic perspectives. Cells 9, 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, Schaeberle CM, Soto AM, 2019. The Case for BPA as an Obesogen: Contributors to the Controversy. Front Endocrinol (Lausanne) 10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat N, Puttabyatappa M, Elangovan VR, Dou J, Ciarelli JN, Thompson RC, Bakulski KM, Padmanabhan V, 2021. Developmental Programming: Prenatal Testosterone Excess on Liver and Muscle Coding and Non-Coding RNA in Female Sheep. Endocrinology 163, 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva RM, Minhas KM, Raju SV, Barouch LA, Pitz E, Schuleri KH, Vandegaer K, Li D, Hare JM, 2005. Deficiency of neuronal nitric oxide synthase increases mortality and cardiac remodeling after myocardial infarction: role of nitroso-redox equilibrium. Circulation 112, 3415–3422. [DOI] [PubMed] [Google Scholar]
- Sargis RM, Simmons RA, 2019. Environmental neglect: endocrine disruptors as underappreciated but potentially modifiable diabetes risk factors. Diabetologia 62, 1811–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Butts S, Wang C, Barrett E, Nguyen R, Schwartz SM, Haaland W, Swan SH, Team T, 2017. Early Prenatal Phthalate Exposure, Sex Steroid Hormones, and Birth Outcomes. J Clin Endocrinol Metab 102, 1870–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savabieasfahani M, Kannan K, Astapova O, Evans NP, Padmanabhan V, 2006. Developmental programming: differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function. Endocrinology 147, 5956–5966. [DOI] [PubMed] [Google Scholar]
- Sioud M, 2021. RNA Interference: Story and Mechanisms. Methods Mol Biol 2282. 1–15. [DOI] [PubMed] [Google Scholar]
- Solano ME, Arck PC, 2019. Steroids, Pregnancy and Fetal Development. Front Immunol 10, 3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire J, 2019. The actin-myosin interaction in muscle: Background and overview. International journal of molecular sciences 20, 5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street ME, Bernasconi S, 2020. Endocrine-Disrupting Chemicals in Human Fetal Growth. Int J Mol Sci 21:1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studniarek C, Egloff S, Murphy S, 2021. Noncoding RNAs Set the Stage for RNA Polymerase II Transcription. Trends Genet 37,:279–291. [DOI] [PubMed] [Google Scholar]
- Symonds ME, Pope M, Budge H, 2015. The Ontogeny of Brown Adipose Tissue. Annu Rev Nutr 35, 295–320. [DOI] [PubMed] [Google Scholar]
- Takimoto M, 2019. Multidisciplinary roles of LRRFIP1/GCF2 in human biological systems and diseases. Cells 8, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer KA, Heindel JJ, Bucher JR, Gallo MA, 2012. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect 120, 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran M, Lee SM, Shin DJ, & Wang L, 2017. Loss of miR-141/200c ameliorates hepatic steatosis and inflammation by reprogramming multiple signaling pathways in NASH. JCI Insight, 2(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trygg J, Wold S, 2002. Orthogonal projections to latent structures (O-PLS). Journal of Chemometrics: A Journal of the Chemometrics Society 16, 119–128. [Google Scholar]
- Vandenberg LN. Chahoud I. Heindel JJ. Padmanabhan V. Paumgartten FJ. Schoenfelder G, 2010. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 18, 1055–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM, Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption., 2009. Endocr Rev 30, 75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Kannan K, Liao C, Ye W, Domino SE, Padmanabhan V, 2015a. Gender-Specific Effects on Gestational Length and Birth Weight by Early Pregnancy BPA Exposure. J Clin Endocrinol Metab 100, E1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Luense LJ, Christenson LK, Padmanabhan V, 2013. Developmental programming: gestational bisphenol-A treatment alters trajectory of fetal ovarian gene expression. Endocrinology 154, 1873–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Moeller J, Sreedharan R, Singer K, Lumeng C, Ye W, Pease A, Padmanabhan V, 2016. Developmental programming: interaction between prenatal BPA exposure and postnatal adiposity on metabolic variables in female sheep. Am J Physiol Endocrinol Metab 310, E238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Pennathur S, Kannan K, Patisaul HB, Dolinoy DC, Zeng L, Padmanabhan V, 2015b. Impact of gestational bisphenol A on oxidative stress and free fatty acids: Human association and interspecies animal testing studies. Endocrinology 156, 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscarra JA, Wang Y, Hong I-H, Sul HS, 2017. Transcriptional activation of lipogenesis by insulin requires phosphorylation of MED17 by CK2. Science signaling 10, eaai8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel SA, 2009. The politics of plastics: the making and unmaking of bisphenol a "safety". Am J Public Health 99 Suppl 3, S559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, Farabollini F, Guillette LJ Jr., Hauser R, Heindel JJ, Ho SM, Hunt PA, Iguchi T, Jobling S, Kanno J, Keri RA, Knudsen KE, Laufer H, LeBlanc GA, Marcus M, McLachlan JA, Myers JP, Nadal A, Newbold RR, Olea N, Prins GS, Richter CA, Rubin BS, Sonnenschein C, Soto AM, Talsness CE, Vandenbergh JG, Vandenberg LN, Walser-Kuntz DR, Watson CS, Welshons WV, Wetherill Y, Zoeller RT, 2007. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol 24, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vom Saal FS, Nagel SC, Coe BL, Angle BM, Taylor JA, 2012. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol Cell Endocrinol 354, 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vom Saal FS, Vandenberg LN, 2021. Update on the Health Effects of Bisphenol A: Overwhelming Evidence of Harm. Endocrinology 162, bqaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JM, Qiu Y, Yang Z, Kim H, Qian Q, Sun Q, .Zhang K, 2018. IRE1α prevents hepatic steatosis by processing and promoting the degradation of select microRNAs. Sci Signal, 11(530). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods MM, Lanphear BP, Braun JM, Lawre McCandles L.Cs., 2017. Gestational exposure to endocrine disrupting chemicals in relation to infant birth weight: a Bayesian analysis of the HOME Study. Environ Health 16, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM, 2011. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environ Health Perspect 119, 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Song X, Ling Y, Zhou J, Tao Z, Shen Y, 2019. Comprehensive bioinformatics analysis of critical lncRNAs, mRNAs and miRNAs in non-alcoholic fatty liver disease. Molecular medicine reports 19, 2649–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Meng F, Invernizzi P, Bernuzzi F, Venter J, Standeford H, Onori P, Marzioni M, Alvaro D, Franchitto A, 2016. The secretin/secretin receptor axis modulates liver fibrosis through changes in transforming growth factor-β1 biliary secretion in mice. Hepatology 64, 865–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Meng F, Zhou T, Han Y, Kennedy L, Venter J, Francis H, DeMorrow S, Onori P, Invernizzi P, 2017. Prolonged darkness reduces liver fibrosis in a mouse model of primary sclerosing cholangitis by miR-200b down-regulation. The FASEB Journal 31, 4305–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J-J, Tao H, Liu L-P, Hu W, Deng Z-Y, Li J, 2017. miR-200a controls hepatic stellate cell activation and fibrosis via SIRT1/Notch1 signal pathway. Inflammation Research 66, 341–352. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang H, Li G, Liu Y, Wang C, He D, 2020. Exploring the genetic basis of fatty liver development in geese. Scientific reports 10, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazıcı D, Sezer H, 2017. Insulin Resistance, Obesity and Lipotoxicity. Adv Exp Med Biol 960, 277–304. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, 2019. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol 70, 531–544. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang Q, Liu W, Liu F, Ji A, Li Y, 2018a. The orphan nuclear receptor 4A1: a potential new therapeutic target for metabolic diseases. Journal of diabetes research 2018, 9363461. 10.1155/2018/9363461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Gao H, Mao LJ, Tao XY, Ge X, Huang K, Zhu P, Hao JH, Wang QN, Xu YY, Jin ZX, Sheng J, Xu YQ, Yan SQ, Tao XG, Tao FB, 2018b. Effects of the phthalate exposure during three gestation periods on birth weight and their gender differences: A birth cohort study in China. Sci Total Environ 613-614, 1573–1578. [DOI] [PubMed] [Google Scholar]
- Zheng R, Rebolledo-Jaramillo B, Zong Y, Wang L, Russo P, Hancock W, Stanger BZ, Hardison RC, Blobel GA, 2013. Function of GATA factors in the adult mouse liver. PloS one 8, e83723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Moon R, Thorne JL, & Moore JB, 2021. NAFLD and vitamin D: Evidence for intersection of microRNA-regulated pathways. Nutr Res Rev, 1–20. [DOI] [PubMed] [Google Scholar]
- Zhao S, Fung-Leung WP, Bittner A, Ngo K, & Liu X, 2014. Comparison of RNA-Seq and microarray in transcriptome profiling of activated T cells. PLoS One, 9(1), e78644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The code for analysis used in this study are available online (https://github.com/bakulskilab). Data generated and used in this study are available at the Gene Expression Omnibus (GEO accession: GSE190328).