Abstract
A hallmark of the media publicity surrounding COVID-19 has been the message that land change causes zoonotic diseases to spill over from wild animals to humans. The secondary peer-reviewed literature sends a similar message. However, as indicated in the primary peer-reviewed literature, the complexity of interacting variables involved in zoonotic disease spillover makes it unlikely for such a claim to be universally applicable. The secondary peer-reviewed literature and the mainstream media also differ markedly from the primary peer-reviewed literature in their lack of nuance in messaging about the relationship between land change and spillover risk. We advocate accurate, nuanced messaging for the sake of the local communities at greatest risk from zoonotic disease, for the sake of scientific credibility, and so that proportionate attention may be given to other possible drivers of spillover risk.
Keywords: COVID-19, degradation, habitat fragmentation, land use, science communication
Although the source of SARS-CoV-2 remains uncertain, the most widely publicized theory of its origin has been zoonotic spillover from a wild animal or animals at a Wuhan wet market (World Health Organization 2021a). This theory has, unsurprisingly, directed attention toward the potential risks to human health posed by direct contact with wildlife (Halbwax 2020). Between 40% and 50% of emerging infectious diseases in human beings are believed to have come from wild animals, feral animals, or captive or farmed wildlife (Jones et al. 2008, Billinis 2013, Haider et al. 2020), including 71.5% of viruses known to infect humans (Olival et al. 2017) and possibly all seven of the human-infecting coronaviruses (Ye et al. 2020).
For less obvious reasons COVID-19 and emerging infectious diseases more generally have been linked with the way humans degrade or destroy nature. A headline in TheNew York Times Magazine posed the question “What do COVID-19, Ebola, Lyme, and AIDS have in common?,” answering confidently with “They jumped to humans from animals after we started destroying habitats and ruining ecosystems” (Jaber 2020). CNN reported experts as having said that “rampant deforestation will only uncork more novel viruses” (Weir 2020). A Guardian headline announced, “Pandemics result from destruction of nature, say UN and WHO” (Carrington 2020). Considering the world's astounding diversity of ecosystems, pathogens, vectors, and forms of land change and the almost infinite combinations of these variables, one might expect some variety in the direction of messages on this topic and a little more nuance. However, all 37 news webpages that we sampled (see the supplemental material for our methodology) associated land change (a catch-all phrase that we use here for the many forms of land-use change, land cover change and habitat destruction) with increased spillover risk. We also sampled 95 webpages of organizations, from the World Health Organization (2021b) to the World Bank (Estavão and Kemper 2021). All but one of them conveyed a similar one-sided message.
The validity, accuracy, and implications of messaging that links land change to spillover risk are the focus of the present article. We are not arguing for a different consensus on the relationship between land change and spillover risk. Rather, we are cautioning against the widespread implication that a consensus exists. Our appeal is aimed at scientists, journalists, and anyone else communicating scientific knowledge.
Why question messaging that links land change to disease spillover?
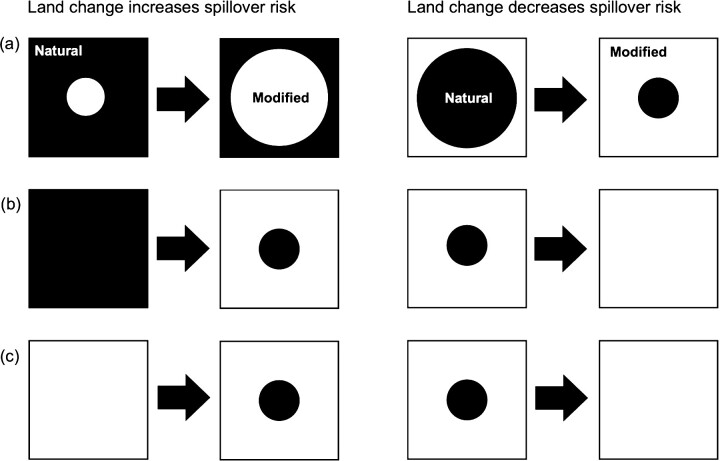
To begin with, the term land change includes various discrete states and processes that may have divergent implications for spillover risk. The form of land change that is perhaps most convincingly and commonly implicated in spillover risk is habitat fragmentation. Fragmentation typically increases the interface between natural and modified habitats (Beasley et al. 2013, Allen and Wesner 2016, Kleinschroth and Healey 2017, Borremans et al. 2019, Brock et al. 2019), which potentially increases human contact with wildlife and their pathogens (Hosseini et al. 2017, Faust et al. 2018). But this does not account for the fact that an inward expansion of modified habitat could result in decreased interface between natural and modified habitat (figure 1a). Along similar lines, if a fragment of natural habitat is the sole source of a zoonotic disease, then the complete destruction of that fragment could eliminate the risk of spillover altogether (figure 1b). Furthermore, “fragments” of nature can also be created—for example, by establishing a natural park within a city, which is regarded as being beneficial to human health (Elmqvist et al. 2015). The fragmentation rhetoric, in contrast, implies that creating such fragments would increase spillover risk, and their removal, by extension, would decrease that risk (figure 1c).
Figure 1.
Three simplified hypothetical sets of scenarios in which land change may either increase or decrease zoonotic disease spillover risk. Each assumes that the natural area (black) is the source of spillover, and is devoid of human presence, whereas humans inhabit the modified habitat (white). In panel (a), increasing the size of modified habitat increases edge, and therefore spillover risk, if habitat modification expands outward, but decreases edge and spillover risk if it expands inward. In panel (b), reduction of natural area to fragments introduces an interface, and therefore a risk of spillover. However, if land change continues so that the fragment disappears altogether, there is once again no interface and therefore no spillover risk. In panel (c), restoration or creation of a fragment of natural area amid modified habitat could increase spillover risk, according to theory on habitat fragmentation. As in panel (b), removal of the fragment theoretically removes that risk.
When it comes to the process, as opposed to the pattern, of land change, spillover risk is challenging to model because of the complexities of wildlife host and pathogen dynamics (Alexander et al. 2012). But some differences in risk are intuitive. Burning vegetation, for example, involves minimal human–wildlife contact and decreases the abundance of some pathogens (Albery et al. 2021), although smoke may help to spread others (Kobziar and Thompson 2020). Logging, on the other hand, more predictably exposes people to wildlife and their pathogens and for longer (Faust et al. 2018).
Whatever the process or the pattern of land change, it is thought that some adaptable and mobile wildlife, such as rodents, may respond by resettling in adjacent human habitat, taking their pathogens with them (Altizer et al. 2011, Hernández-Camacho et al. 2012, Ferreira-Junior et al. 2018, Mendoza et al. 2020, Santini 2021). It is, however, not certain whether their relocation is a response to land change or simply a preference for accessible human habitat. Indeed, the literature on the relationship between land change and spillover risk seems sometimes to conflate proximity to nature per se, with proximity to the “newly exposed” nature that land change brings about.
These scenarios are partly just thought experiments, but they illustrate how difficult it can be to assess the risk of zoonotic spillover without knowing the amount, spatial pattern, and type of land change, not to mention zoonotic disease prevalence in wildlife, and incidences from which spillover could take place. Even when pathogen prevalence increases in wildlife populations, we still lack data and insights into the concomitant change in risk of exposure and transmission to human populations in the modified environment. Unsurprisingly, therefore, some authors acknowledge that the relationship between land change and spillover risk remains poorly understood (Sehgal 2010, Cumming et al. 2015, Suzán et al. 2015, Mastel et al. 2018, Stark et al. 2019, Davey and Selvey 2020, White and Razgour 2020, Plowright et al. 2021, Reaser et al. 2021). The complexity of pathogen responses to land change cannot be reduced to simple one-size-fits-all proclamations.
Reviewing the rhetoric
Most primary research on the relationship between land change and spillover risk reports increased spillover risk with land change (Plowright et al. 2008, Beasley et al. 2013, Vanwormer et al. 2013, Scinachi et al. 2017, Santos and Almeida 2018). However, other authors report mixed results (Young et al. 2017, Afelt et al. 2018, Maaz et al. 2018, Young et al. 2021) and even decreased risk (Kowalewski et al. 2011, Shapiro et al. 2020, Riquelme et al. 2021). In some cases, the drainage of marshland (Jacups et al. 2011) and the removal of vegetation (Ducheyne et al. 2009), for example, have protected local communities from zoonotic disease.
We sampled 145 peer-reviewed papers with abstracts that made statements about the relationship between land change and spillover risk. Only 43 papers reported on the authors’ own findings on this relationship (primary research, including empirical studies and models), 23 of which (53%) associated land change with increased spillover risk. This proportion is roughly consistent with the findings of a systematic literature review by Gottdenker and colleagues (2014), in which 57% of studies documented increased pathogen transmission, whereas 43% observed decreased pathogen transmission, variable and complex pathogen responses, or no detectable changes. Other factors thought to influence rates of pathogen spillover (e.g., the loss of biodiversity, which is intertwined with land change) also turn out to be complex and heterogeneous in both the strength and the direction of the relationship (Wood et al. 2014, Rohr et al. 2020). In all of these cases, nonsignificant results (i.e., no relationship between land change and spillover risk) are likely to be underreported, as is expressed in the file drawer problem (Sterling 1959, Csada et al. 1996, Wood 2020, West and Bergstrom 2021).
In contrast to the variability evidenced in primary papers, we found that, of the 102 secondary papers (peer-reviewed review articles and commentaries) we sampled, 78% associated land change with increased spillover risk. This apparent overstating of the evidence was even more pronounced among the webpages we sampled, only one of which did not associate land change with increased spillover risk.
Messaging also differed in terms of nuance. Although 79% of primary papers acknowledged uncertainty in the way they communicated their findings about the relationship between land change and spillover risk, this figure was 53% for secondary papers and 31% for webpages. Although 51% of primary papers indicated that the relationship was not necessarily causal, this figure was 10% for secondary papers and 4% for webpages. Although 74% of primary papers specified the pathogens responsible, this figure was 30% for secondary papers and 17% for webpages. Although 60% of primary papers specified the geographical location to which the relationship between land change and spillover risk applied down to the ecosystem or local scale, this figure was 3% for both secondary papers and webpages.
Peer-reviewed research can be expected to be more specific and nuanced than mainstream messaging, as was indicated in the primary papers we sampled. However, by some of the measures mentioned above, the specificity and nuance in secondary peer-reviewed papers was more akin to mainstream sources than to the primary research on which it is ostensibly based. Furthermore accuracy matters regardless of whether a message is communicated to a scientific or a general audience. Giving the impression that a phenomenon is universally applicable, when the empirical research suggests otherwise, is inaccurate and potentially misleading in any context.
Implications of simplistic messaging
Media attention to COVID-19 has been used as an opportunity to advocate the value of nature as a defense against future pandemics. But there are considerable risks in drawing oversimplified and generic conclusions from complex and nuanced phenomena. Gregg and colleagues (2021) pointed out how well-intentioned rhetoric could inadvertently send a message precisely opposite to the message intended: implying that nature is a threat to humanity because of the thousands of viruses lurking unseen within it. We propose three additional arguments for accuracy and nuance.
First, policy or management decisions that are based on simplistic messaging risk neglecting local context. Policymakers and other decision-makers must understand that context if they are to make appropriate decisions for their communities. Solutions required at the local level will vary considerably with location and ecosystem, across species of pathogen, vector, and host, and across different forms of land change. Restoration or conservation of the local environment is not guaranteed to decrease spillover risk. If vectors or reservoir hosts happen to be dependent on certain habitats (see, e.g., Bradley and Altizer 2007), the conservation of those habitats near human settlement might make spillover more likely. Honest appraisals and mitigation of spillover risk are required in order to prioritize public health, although they may narrow down the options for simultaneously achieving conservation objectives.
Second, when messaging turns out to be false or inaccurate, it can erode credibility. Every conservation message has the potential to contribute positively or negatively to the reputation of conservation and even of science more broadly, depending largely on the care with which it attempts to approximate the truth. In a survey of attitudes on public trust in science, Kreps and Kriner (2020, p. 1) noted that “careful science communication is critical to maintaining public support for science-based policies as the scientific consensus shifts over time.” They argued that this risk is magnified by the public attention garnered by COVID-19. If the disease turns out to have no links with land change, then strongly worded headlines about protecting nature for the sake of public health are less likely to be taken seriously next time around. Examples of such consequences include public mistrust in scientific information on climate change and vaccines (Rowland et al. 2022) and on nutrition (Nagler 2013, Garza et al. 2019).
Third, cases in which messaging implies that land change is the sole reason for spillover can detract from other important spillover risks. Especially on the webpages we sampled, it was not rare for messaging to give the impression that spillover risk is entirely dependent on land change. This could divert attention from other factors that may increase zoonotic disease spillover risk, such as the wildlife trade (Karesh et al. 2005), wildlife farming (Magouras et al. 2020 and the references therein), global travel (Baker et al. 2022), climate change (Carlson et al. 2022), socioeconomics (Power et al. 2022), and transmission to researchers working on zoonotic diseases (National Research Council 2012).
Recommendations
Science communication, whether in peer-reviewed journals or the mainstream media, is meant to make scientific knowledge understandable. When it comes to topics as complex as the relationship between land change and zoonotic disease spillover, we contend that science communicators are more likely to succeed at that task when messaging is accurate and carefully nuanced. To that end we offer the following recommendations, using the relationship between land change and spillover risk as an example:
Messaging should specify context and explanatory variables such as the relevant ecosystem, scale, pathogen, vector, host, form of land change, confounders, and effect measure modifiers. The strength of effect linking land change to zoonotic spillover risk within that context should also be communicated when possible. A message is true only within the specific context for which evidence exists and, if that context is not stated, then the message is not being communicated accurately. When generalization is unavoidable it should, at least, be acknowledged that a generalized conclusion is being communicated.
Defined and consistent terminology—for example, to specify type of land change—can facilitate clearer communication both within science and to the broader public (Herrando-Pérez et al. 2014, Fraser et al. 2015, Peacor et al. 2020). Undefined and inconsistent terminology in some of our sampled papers and webpages made them difficult to compare with one another—an observation shared by others (Gottdenker et al. 2014).
Messaging that describes the mechanisms that underlie phenomena is more likely to be accurate, to facilitate understanding, and to allow practitioners to identify leverage points for intervention. For example, a brief explanation of the mechanism underlying the effect of land change on spillover risk in a particular context may tell the reader more about the conditions under which that effect can be expected in future. Although science communicators cannot be expected to provide all of the relevant detail, we noted a lack of any explanation—or even a mention—of the mechanisms linking land change to spillover risk in some of the less nuanced messaging that we sampled.
Finally, the simple acknowledgement of uncertainty and exceptions can be enough to capture nuance, even in pithy social media posts.
Having said this, recipients of science communication would do well to remember that science communicators are human beings too. The motivations behind messaging vary: Interest groups might be tempted to cherry-pick science that supports their cause, media outlets are rewarded by numbers of copies or clicks, and, with the advent of indices such as the Altmetric Attention Score, scientists may be motivated to extend the reach of their work.
Conclusions
The last time the world experienced anything like COVID-19 was when the Great Influenza pandemic struck in 1918. At that time, it was still common for messaging to rely on carrier pigeons and dispatch riders. It predated the first radio news program by 2 years, and the first trans-Atlantic telephone call was still almost a decade away. A century later, with the click of a button, a message can instantly reach millions. The present article was written out of concern for the potential consequences of simplistic messaging at that speed and scale—including messaging about one of the most significant events of our lifetime. It was written in the hopes of encouraging more nuanced discourse, both within and beyond the peer-reviewed literature. If the goal of science communication is to improve understanding, it must strike a balance: sufficient simplicity to be grasped by as broad an audience as possible but sufficient nuance to capture the complexity of an issue and contribute meaningfully to the discussion around it, especially when it goes viral.
Supplementary Material
Author Biographical
André D. Mader (mader@iges.or.jp) is a programme director at the Institute for Global Environmental Strategies, in Hayama, Kanagawa, Japan. Neil A. Waters (nawaters3@gmail.com) is a postgraduate student in the Division of Environmental Studies at the University of Tokyo, in Kashiwa, in Chiba Prefecture, Japan. Erin C. Kawazu (kawazu@iges.or.jp) is a program coordinator at the Institute for Global Environmental Strategies, in Hayama, Kanagawa, Japan. Michelle Marvier (mmarvier@scu.edu) is a professor in the Department of Environmental Studies and Sciences at Santa Clara University, in Santa Clara, California, in the United States. Noémie Monnin (noemiebmonnin@gmail.com) is a postgraduate student in environment and sustainable development at University College London, in London, England, in the United Kingdom. Daniel J. Salkeld (dan.salkeld@colostate.edu) is a research scientist in the Department of Biology at Colorado State University, in Fort Collins, Colorado, in the United States.
Contributor Information
André D Mader, Institute for Global Environmental Strategies, Hayama, Kanagawa, Japan.
Neil A Waters, University of Tokyo, Kashiwa, Chiba Prefecture, Japan.
Erin C Kawazu, Institute for Global Environmental Strategies, Hayama, Kanagawa, Japan.
Michelle Marvier, Santa Clara University, Santa Clara, California, United States.
Noémie Monnin, University College London, London, England, United Kingdom.
Daniel J Salkeld, Colorado State University, Fort Collins, Colorado, United States.
References cited
- Afelt A, Lacroix A, Zawadzka-Pawlewska U, Pokojski W, Buchy P, Frutos R.. 2018. Distribution of bat-borne viruses and environment patterns. Infection, Genetics and Evolution 58: 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albery GF, Turilli I, Joseph MB, Foley J, Frere CH, Bansal S.. 2021. From flames to inflammation: How wildfires affect patterns of wildlife disease. Fire Ecology 17: 23. [Google Scholar]
- Alexander KA, Lewis BL, Marathe M, Eubank S, Blackburn JK.. 2012. Modeling of wildlife-associated zoonoses: Applications and caveats. Vector-Borne and Zoonotic Diseases 12: 1005–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DC, Wesner JS.. 2016. Synthesis: Comparing effects of resource and consumer fluxes into recipient food webs using meta-analysis. Ecology 97: 594–604. [PubMed] [Google Scholar]
- Altizer S, Bartel R, Han BA. 2011. Animal migration and infectious disease risk. Science 331: 296–302. [DOI] [PubMed] [Google Scholar]
- Baker RE, et al. 2022. Infectious disease in an era of global change. Nature Reviews Microbiology 20: 193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley JC, Eagan TS, Page LK, Hennessy CA, Rhodes OE.. 2013. Baylisascaris procyonis infection in white-footed mice: Predicting patterns of infection from landscape habitat attributes. Journal of Parasitology 99: 743–747. [DOI] [PubMed] [Google Scholar]
- Billinis C. 2013. Wildlife diseases that pose a risk to small ruminants and their farmers. Small Ruminant Research 110: 67–70. [Google Scholar]
- Borremans B, Faust C, Manlove KR, Sokolow SH, Lloyd-Smith JO.. 2019. Cross-species pathogen spillover across ecosystem boundaries: Mechanisms and theory. Philosophical Transactions of the Royal Society B 374: 20180344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley CA, Altizer S.. Urbanization and the ecology of wildlife diseases. 2007. Trends in Ecology and Evolution 22: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock PM, Fornace KM, Grigg MJ, Anstey NM, William T, Cox J, Drakeley CJ, Ferguson HM, Kao RR.. 2019. Predictive analysis across spatial scales links zoonotic malaria to deforestation. Proceedings of the Royal Society B 286: 20182351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson CJ, Albery GF, Merow C, Trisos CH, Zipfel CM, Eskew EA, Olival KJ, Ross N, Bansal S. 2022. Climate change increases cross-species viral transmission risk. Nature 607:555–562. 10.1038/s41586-022-04788-w. [DOI] [PubMed] [Google Scholar]
- Carrington D. 2020. Pandemics result from destruction of nature, say UN and WHO. Guardian (17 June 2020).www.theguardian.com/world/2020/jun/17/pandemics-destruction-nature-un-who-legislation-trade-green-recovery [Google Scholar]
- Csada RD, James PC, Espie RH.. 1996. The “file drawer problem” of non-significant results: Does it apply to biological research? Oikos 76: 591–593. [Google Scholar]
- Cumming GS, Abolnik C, Caron A, Gaidet N, Grewar J, Hellard E, Henry DAW, Reynolds C.. 2015. A social–ecological approach to landscape epidemiology: Geographic variation and avian influenza. Landscape Ecology 30: 963–985. [Google Scholar]
- Davey TM, Selvey LA.. 2020. Relationship between land use/land-use change and human health in Australia: A scoping study. International Journal of Environmental Research and Public Health 17: 8992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducheyne E, Mweempwa C, De Pus C, Vernieuwe H, De Deken R, Hendrickx G, Van den Bossche P.. 2009. The impact of habitat fragmentation on tsetse abundance on the plateau of eastern Zambia. Preventive Veterinary Medicine 91:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist T, Setälä H, Handel SN, van der Ploeg S, Aronson J, Blignaut JN, Gómez-Baggethun E, Nowak DJ, Kronenberg J, de Groot R.. 2015. Benefits of restoring ecosystem services in urban areas. Current Opinion in Environmental Sustainability 14: 101–108. [Google Scholar]
- Estavão and Kemper . 2021. How fiscal policy can help save forests. World Bank Blogs (29 March 2021). https://blogs.worldbank.org/climatechange/how-fiscal-policy-can-help-save-forests. [Google Scholar]
- Faust CL, McCallum HI, Bloomfield LSP, Gottdenker NL, Gillespie TR, Torney CJ, Dobson AP, Plowright RK.. 2018. Pathogen spillover during land conversion. Ecology Letters. 21: 471–483. [DOI] [PubMed] [Google Scholar]
- Ferreira-Junior FC, De Angeli Dutra D, Silveira P, Pacheco RC, Witter R, De Souza Ramos DG, Pacheco MA, Escalante AA, Braga EM.. 2018. A new pathogen spillover from domestic to wild animals: Plasmodiumjuxtanucleare infects free-living passerines in Brazil. Parasitology 145: 1949–1958. [DOI] [PubMed] [Google Scholar]
- Fraser H, Garrard GE, Rumpff L, Hauser CE, McCarthy MA.. 2015. Consequences of inconsistently classifying woodland birds. Frontiers in Ecology and Evolution 3: 83. [Google Scholar]
- Garza C, Stover PJ, Ohlhorst SD, Field MS, Steinbrook R, Rowe S, Woteki C, Campbell E.. 2019. Best practices in nutrition science to earn and keep the public's trust. American Journal of Clinical Nutrition 109: 225–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottdenker N, Streicker D, Faust C, Carroll C.. 2014. Anthropogenic land use change and infectious diseases: A review of the evidence. Ecohealth 11: 619–632. [DOI] [PubMed] [Google Scholar]
- Gregg EA, Kusmanoff AM, Garrard GE, Kidd LR, Bekessy SA.. 2021. Biodiversity conservation cannot afford COVID-19 communication bungles. Trends in Ecology and Evolution 36: 879–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider N, et al. 2020. COVID-19—Zoonosis or emerging infectious disease? Frontiers in Public Health 8: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbwax M. 2020. Addressing the illegal wildlife trade in the European Union as a public health issue to draw decision makers attention. Biological Conservation 251: 108798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Camacho N, Jones RW, Pineda-López RF, López-González CA.. 2012. Mexican wild and domestic canids: A potential risk of zoonosis? A review. Pages 229–238 in Boeri F, Chung JA, eds. Nematodes: Morphology, Functions and Management Strategies. Nova Science. [Google Scholar]
- Herrando-Pérez S, BW Brook, Bradshaw CJA.. 2014. Ecology needs a convention of nomenclature. BioScience 64: 311–321. [Google Scholar]
- Hosseini PR, et al. 2017. Does the impact of biodiversity differ between emerging and endemic pathogens? The need to separate the concepts of hazard and risk. Philosophical Transactions of the Royal Society B 2017: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber F. 2020. How humanity unleashed a flood of new diseases. New York Times Magazine (17 June 2020). www.nytimes.com/2020/06/17/magazine/animal-disease-covid.html [Google Scholar]
- Jacups S, Kurucz N, Whitters R, Whelan P.. 2011. Habitat modification for mosquito control in the Ilparpa Swamp, Northern Territory, Australia. Journal of Vector Ecology 36:292–299. [DOI] [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P.. 2008. Global trends in emerging infectious diseases. Nature 451: 990–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karesh WB, Cook RA, Bennett EL, Newcomb J.. 2005. Wildlife trade and global disease emergence. Emerging Infectious Diseases 11: 1000–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschroth F, Healey JR.. 2017. Impacts of logging roads on tropical forests. Biotropica 49: 620–635. [Google Scholar]
- Kobziar LN, Thompson GR.. 2020. Wildfire smoke, a potential infectious agent. Science 370: 1408–1410. [DOI] [PubMed] [Google Scholar]
- Kowalewski MM, Salzer JS, Deutsch JC, Raño M, Kuhlenschmidt MS, Gillespie TR.. 2011. Black and gold howler monkeys (Alouatta caraya) as sentinels of ecosystem health: Patterns of zoonotic protozoa infection relative to degree of human-primate contact. American Journal of Primatology 73: 75–83. [DOI] [PubMed] [Google Scholar]
- Kreps SE, Kriner DL.. 2020. Model uncertainty, political contestation, and public trust in science: Evidence from the COVID-19 pandemic. Science Advances 6: abd4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaz D, Krücken J, Blümke J, Richter D, McKay-Demeler J, Matuschka F-R, Hartmann S, Von Samson-Himmelstjerna G. 2018. Factors associated with diversity, quantity and zoonotic potential of ectoparasites on urban mice and voles. PLOS ONE 13: e0199385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magouras I, BrookesVJ Jori F, Martin A, Pfeiffer DU, Dürr S.. 2020. Emerging zoonotic diseases: Should we rethink the animal–human interface? Frontiers in Veterinary Science 7: 582743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastel M, Bussalleu A, Paz-Soldán VA, Salmón-Mulanovich G, Valdés-Velásquez A, Hartinger SM.. 2018. Critical linkages between land use change and human health in the Amazon region: A scoping review. PLOS ONE 13: e0196414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza H, Rubio AV, García-Peña GE, Suzán G, Simonetti JA.. 2020. Does land-use change increase the abundance of zoonotic reservoirs? Rodents say yes. European Journal of Wildlife Research 66: 6. [Google Scholar]
- Nagler RH. 2013. Adverse outcomes associated with media exposure to contradictory nutrition messages. Journal of Health Communication 19: 24–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . 2012. Biosecurity Challenges of the Global Expansion of High-Containment Biological Laboratories: Summary of a Workshop. National Academies Press. [PubMed] [Google Scholar]
- Olival KJ, Hosseini PR, Zambrana-Torrelio C, Ross N, Bogich TL, Daszak P.. 2017. Host and viral traits predict zoonotic spillover from mammals. Nature 546: 646–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacor SD, Barton BT, Kimbro DL, Sih A, Sheriff MJ.. 2020. A framework and standardized terminology to facilitate the study of predation-risk effects. Ecology 101: e03152. [DOI] [PubMed] [Google Scholar]
- Plowright RK, Field HE, Smith C, Divljan A, Palmer C, Tabor G, Daszak P, Foley JE.. 2008. Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus). Proceedings of the Royal Society B 275: 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright RK, Reaser JK, Locke H, Woodley SJ, Patz JA, Becker DJ, Oppler G, Hudson PJ, Tabor GM.. 2021. Land use-induced spillover: A call to action to safeguard environmental, animal, and human health. Lancet Planetary Health 5: e237–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power GM, et al. 2022. Socioeconomic risk markers of arthropod-borne virus (arbovirus) infections: A systematic literature review and meta-analysis. BMJ Global Health 7: e007735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaser JK, et al. 2021. Land use-induced spillover: Priority actions for protected and conserved area managers. Parks 27: 161–178. [Google Scholar]
- Riquelme M, Salgado R, Simonetti JA, Landaeta-Aqueveque C, Fredes F, Rubio AV.. 2021. Intestinal helminths in wild rodents from native forest and exotic pine plantations (Pinusradiata) in central Chile. Animals 11: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Civitello DJ, Halliday FW, Hudson PJ, Lafferty KD, Wood CL, Mordecai EA.. 2020. Towards common ground in the biodiversity–disease debate. Nature Ecology and Evolution 4: 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland J, Estevens J, Krzewińska A, Warwas I, Delicado A. 2022. Trust and mistrust in sources of scientific information on climate change and vaccines: Insights from Portugal and Poland. Science Education 9: 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini M. 2021. The land use–food–coronavirus nexus. Nature Food 2: 390–391. [DOI] [PubMed] [Google Scholar]
- Santos AS, Almeida AN.. 2018. The impact of deforestation on malaria infections in the Brazilian Amazon. Ecological Economics 154: 247–256. [Google Scholar]
- Scinachi CA, Takeda GACG, Mucci LF, Pinter A.. 2017. Association of the occurrence of Brazilian spotted fever and Atlantic rain forest fragmentation in the São Paulo metropolitan region. Acta Tropica 166: 225–233. [DOI] [PubMed] [Google Scholar]
- Sehgal RNM. Deforestation and avian infectious diseases. 2010. Journal of Experimental Biology 213: 955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro JT, Sovie AR, Faller CR, Monadjem A, Fletcher RJ, McCleery RA.. 2020. Ebola spillover correlates with bat diversity. European Journal of Wildlife Research 66: 12. [Google Scholar]
- Stark DJ, Fornace KM, Brock PM, Abidin TR, Gilhooly L, Jalius C, Goossens B, Drakeley CJ, Salgado-Lynn M.. 2019. Long-Tailed macaque response to deforestation in a Plasmodiumknowlesi-endemic area. Ecohealth 16: 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling TD. 1959. Publication decisions and their possible effects on inferences drawn from tests of Significance—or vice versa. Journal of the American Statistical Association 54: 30–34. [Google Scholar]
- Suzán G, et al. 2015. Metacommunity and phylogenetic structure determine wildlife and zoonotic infectious disease patterns in time and space. Ecology and Evolution 5: 865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanwormer E, Conrad PA, Miller MA, Melli AC, Carpenter TE, Mazet JAK.. 2013. Toxoplasma gondii, source to sea: Higher contribution of domestic felids to terrestrial parasite loading despite lower infection prevalence. Ecohealth 10: 277–289. [DOI] [PubMed] [Google Scholar]
- Weir B. 2020. Coronavirus and deforestation rip through Brazil's people and the world's lungs. CNN World (July 19 2020). https://edition.cnn.com/2020/07/19/americas/brazil-coronavirus-amazon-deforestation-bolsonaro-weir/index.html [Google Scholar]
- West JD, Bergstrom CT.. 2021. Misinformation in and about science. Proceedings of the National Academy of Sciences 118: e1912444117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RJ, Razgour O.. 2020. Emerging zoonotic diseases originating in mammals: A systematic review of effects of anthropogenic land-use change. Mammal Review 50: 336–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CL, Lafferty KD, Deleo G, Young HS, Hudson PJ, Kuris AM.. 2014. Does biodiversity protect humans against infectious disease? Ecology 95: 817–832. [DOI] [PubMed] [Google Scholar]
- Wood KA. 2020. Negative results provide valuable evidence for conservation. Perspectives in Ecology and Conservation 18: 235–237. [Google Scholar]
- World Health Organization . 2021a. WHO-convened Global Study of Origins of SARS-CoV-2: China Part. World Health Organization. [Google Scholar]
- World Health Organization . 2021b. Zoonoses. World Health Organization. www.who.int/news-room/fact-sheets/detail/zoonoses. [Google Scholar]
- Ye ZW, Yuan S, Yuen KS, Fung SY, Chan CP, Jin DY. 2020. Zoonotic origins of human coronaviruses. International Journal of Biological Sciences 16: 1686–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HS, et al. 2017. Interacting effects of land use and climate on rodent-borne pathogens in central Kenya. Philosophical Transactions of the Royal Society B 372: 20160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KI, Buenemann M, Vasilakis N, Perera D, Hanley KA.. 2021. Shifts in mosquito diversity and abundance along a gradient from oil palm plantations to conterminous forests in Borneo. Ecosphere 12: e03463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.