Abstract
Chronic thromboembolic pulmonary hypertension (CTEPH) is the result of incompletely resolved pulmonary emboli (PE) that lead to chronic right heart failure. The two mechanical treatment options are pulmonary thromboendarterectomy (PTE) and balloon pulmonary angioplasty (BPA). There are no formal criteria for BPA patient selection and treatment decisions vary according to a center's experience with BPA and PTE. We performed a retrospective review of consecutive patients treated with PTE and BPA at UCSD from March 2015 to 2021. Clinical and hemodynamic data were collected. Patients were categorized according to the rationale for BPA. One hundred fifty three patients underwent 643 BPA sessions, and 1104 patients underwent PTE. Patients selected for PTE had worse baseline hemodynamics with mean pulmonary artery pressure 41.1 ± 11.7 versus 34.6 ± 11.2 mmHg, p < 0.001. 59% of patients selected for BPA had surgically inaccessible disease, 21% had residual CTEPH after PTE, 10% had a discordance between disease burden and symptoms/hemodynamics, 7% had comorbidities that prevented PTE and 3% refused PTE surgery. 28% of patients who underwent PTE had exclusively level III or IV disease based on surgical specimen. There were no BPA procedure‐related mortalities and minor pulmonary vascular complication rates during BPA were 9.2%. The most common reason for BPA selection was surgically inaccessible disease followed by residual CTEPH after PTE. Almost one third of patients who underwent PTE had exclusively distal disease by surgical criteria and might have been directed to BPA at a less experienced surgical center.
Keywords: chronic thromboembolic pulmonary hypertension, CTEPH, balloon pulmonary angioplasty, BPA, pulmonary thromboendarterectomy
INTRODUCTION
Chronic thromboembolic pulmonary hypertension (CTEPH) is the result of incompletely resolved pulmonary emboli (PE) that organize to obstruct or limit flow through pulmonary arteries. 1 Chronic obstructions and an associated remodeling of the pulmonary vascular bed lead to progressive dyspnea, worsening exercise tolerance, pulmonary hypertension (PH), increased right ventricular workload, and if untreated, right heart failure and death.
The initial evaluation and management of CTEPH are targeted at identifying patients who can undergo mechanical treatment to remove chronic obstructive material, restore perfusion, reduce right ventricular workload and reduce pulmonary artery pressures. Pulmonary thromboendarterectomy (PTE) surgery 2 is the gold standard treatment for CTEPH and when performed at experienced centers, is safe and effective even in patients with severe PH, distal disease, and significant medical comorbidities. 3 Over the past decade, the percutaneous approach of balloon pulmonary angioplasty (BPA) has emerged as an additional method of treating CTEPH. Current guidelines recommend BPA for patients who are not candidates for PTE after evaluation at experienced CTEPH centers or for patients with residual PH after PTE. 4 , 5
There are no formal criteria for BPA patient selection and so the treatment decisions vary according to a given center's experience with both BPA and PTE. It is a subjective process that includes center‐specific factors, such as surgical experience and volume, as well as patient‐specific factors, such as distal location of chronic clot, severity of hemodynamic impairment, and medical comorbidities. University of California San Diego (UCSD), is a referral center for PTE and performs a high volume of PTE surgeries even for patients with severely impaired hemodynamics, advanced age, distal disease, and medical comorbidities. In 2015, UCSD initiated a BPA program to complement the surgical program and offer patients the full range of CTEPH treatment options. This report describes experience with BPA over the 6 years from initiation from March 2015 to 2021 at a high‐volume PTE center.
METHODS
We performed a retrospective review of consecutive patients treated with BPA at UCSD from the inception of our BPA program in March 2015 to 2021. Patients were evaluated and selected according to our standard multidisciplinary approach. 6 All patients were first considered for PTE surgery and once determined inoperable were selected for BPA. Evaluation included testing to confirm the diagnosis of CTEPH with ventilation–perfusion scintigraphy (V/Q), computed tomography angiography (CTA) and/or nonselective digital subtraction pulmonary angiography (DSA). Disease severity was established with New York Heart Association (NYHA) functional class assessment, six‐minute walk distance (6MWD), echocardiography (ECHO), N‐terminal pro‐brain natriuretic peptide (NT‐pro BNP), and right heart catheterization hemodynamics. Patients were categorized according to the rationale for BPA selection. Reason for BPA included:
-
1.
Persistent, symptomatic CTEPH after PTE surgery (and not candidates for repeat PTE).
-
2.
Deemed by multidisciplinary CTEPH team to have surgically inaccessible disease.
-
3.
Hemodynamic impairment or symptoms disproportionate to the degree of visible disease on imaging.
-
4.
Technically operable but with significant comorbidities that prohibited PTE surgery.
-
5.
Technically operable but refused PTE surgery.
Although some patients fit into more than one of the above categories, they were grouped according to their single greatest contributor for BPA selection.
A retrospective review of patients who underwent PTE at UCSD during the same period from March 2015 to 2021 was also performed. Data on baseline characteristics as well as surgical level of the removed endarterectomy specimens were collected.
Statistical analyses were conducted with RStudio software. Categorical variables were presented as number with percentage, and continuous variables as mean ± standard deviation or as median with range. We performed Pearson's Chi‐squared test on complications and BPA medical therapies with a p value < 0.05 considered statistically significant. To assess the efficacy of BPA and the differences between BPA and PTE cohorts, hemodynamics means were tested with Student's t‐test and a two‐tailed p value < 0.05 considered to indicate statistical significance.
PH‐TARGETED THERAPY DURING BPA
Patients were maintained on existing background PH‐targeted therapies while undergoing BPA. In cases of severely impaired hemodynamics, PH‐targeted treatment was initiated in advance of BPA to improve hemodynamics and mitigate BPA‐related complications. Throughout the course of BPA treatment PH‐targeted therapies were added and/or removed based on the clinical judgment of the treating physicians at UCSD in conjunction with their referring treatment team.
BPA PROCEDURE
A right heart catheterization with measurement of right atrial pressure, pulmonary artery pressures, pulmonary artery wedge pressure, cardiac output/index (CO/CI) by thermodilution and indirect Fick methods, and pulmonary vascular resistance (PVR) was performed at the start of each BPA session.
All BPA procedures were performed as described previously with a telescoping technique of a 9‐Fr short sheath through which a 6‐Fr long sheath and 6‐Fr guide catheter were placed. 7 Patients were administered heparin to maintain an activated clotting time (ACT) between 200 and 250 s. The preferred guidewire for the procedure was a low tip load, 0.014″ non‐hydrophilic coated wire. The BPA intervention was limited to a single lung per session, targeting as many segments as possible. Targets were selected based on the location of defects on V/Q and selective angiography. Lower lobe segments were preferentially targeted compared to upper lobe segments. Balloons were sized angiographically and ranged from 1 to 6 mm in diameter. Subsequent BPA procedures were performed in intervals of 1–3 months to allow time for a vascular remodeling and hemodynamic changes to occur. Treatment was performed on all lesion types 8 ; however, large, complete occlusions, without clear distal runoff were approached only after low‐risk territories were treated. For complete occlusions, wire escalation and microcatheter support were often required.
The number of BPA sessions ranged from 1 to 10 per patient. The decision to end BPA treatment was not standardized as there are no accepted guidelines applicable to all BPA cases. The decision was made on a case‐by‐case basis after weighing the residual lesions amenable to BPA, residual hemodynamic burden, residual symptom burden, and residual use of PH‐targeted therapy.
The last invasive hemodynamic measurement was derived at the time of the final BPA session performed at UCSD. As a result, the last recorded invasive hemodynamics are obtained before the final BPA session and do not capture the hemodynamic benefit of the final session(s). Similarly, NYHA functional class assessments, NT‐pro BNP, 6MWD, and PH‐targeted therapies were documented immediately before the final BPA session and may not reflect the impact of the final session.
RESULTS
Baseline characteristics
From March 2015 to 2021, a total of 153 patients underwent 643 BPA sessions at UCSD. During the same period, 1104 patients underwent PTE (Table 1). The mean age for the BPA cohort was 59.6 years (range 22–91) versus 54.1 years (range 15–87, p < 0.01) in the PTE group. There were more females than males in the BPA cohort, while the PTE group was more evenly split by sex.
Table 1.
Baseline characteristics of patients who underwent BPA and PTE at UCSD from March 2015 to 2021
| BPA baseline (n = 153) | PTE baseline (n = 1104) | p Value | |
|---|---|---|---|
| Age (years); mean (range) | 59.6 (22–91) | 54.1 (15–87) | p < 0.001 |
| sPAP (mmHg); mean ± SD | 57.9 ± 20.2 | 70.3 ± 20.3 | p < 0.001 |
| dPAP (mm Hg); mean ± SD | 20.7 ± 6.9 | 25 ± 8.7 | p < 0.001 |
| mPAP (mmHg); mean ± SD | 34.6 ± 11.2 | 41.1 ± 11.7 | p < 0.001 |
| CO (L/min); mean ± SD | 5.5 ± 1.4 | 4.8 ± 1.4 | p < 0.001 |
| CI (L/min/m2); mean ± SD | 2.8 ± 0.6 | 2.4 ± 0.6 | p < 0.001 |
| PVR (dynes s cm−5); mean ± SD | 353.3 ± 223.2 | 564.7 ± 347.9 | p < 0.001 |
| 6MWD (m); mean ± SD | 407.9 ± 144.2 | ||
| NT‐proBNP (pg/ml); mean ± SD | 514.7 ± 971.5 | ||
| Sex; n (% of total) | |||
| Male | 67 (44%) | 578 (52%) | |
| Female | 86 (56%) | 526 (48%) | p = 0.047 |
| Any PH Meds; n (% of total) | 120 (78%) | 599 (54%) | p < 0.001 |
|
Number of PH medications; n (% of total) |
|||
| 0 | 33 (22%) | ||
| 1 | 72 (47%) | ||
| 2 | 33 (22%) | ||
| 3 | 15 (10%) | ||
|
NYHA functional class; n (% of total) |
|||
| 1 | 5 (3%) | 11 (1%) | |
| 2 | 40 (26%) | 224 (20%) | |
| 3 | 102 (67%) | 792 (72%) | |
| 4 | 6 (4%) | 77 (7%) | |
| Prior splenectomy; n (% of total) | 29 (19%) | 43 (4%) | p < 0.001 |
| Intravascular device; n (% of total) | 7 (5%) | 55 (5%) |
Abbreviations: BPA, balloon pulmonary angioplasty; CI, cardiac index; CO, cardiac output; dPAP, diastolic pulmonary artery pressure; mPAP, mean pulmonary artery pressure; NT‐pro BNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; PH, pulmonary hypertension; PTE, pulmonary thromboendarterectomy; PVR, pulmonary vascular resistance; sPAP, systolic pulmonary artery pressure; UCSD, University of California San Diego; 6MWD, six‐minute walk distance.
Overall, patients who were selected for PTE had worse baseline hemodynamics with mean pulmonary artery pressure (mPAP) 41.1 ± 11.7 versus 34.6 ± 11.2 mmHg, p < 0.001, PVR 565 ± 346 versus 353 ± 223 dynes s cm−5, p < 0.001 and cardiac index of 2.4 ± 0.6 versus 2.8 ± 0.6 L/min/m2, p < 0.001. Despite the differences in hemodynamics between the PTE and BPA cohorts, the distribution of functional class between groups was similar with the majority in both groups NYHA functional class III (67%–72%). The use of PH‐targeted therapies was more common in patients who underwent BPA (78% vs. 54%, p < 0.001). The number of patients in the BPA cohort on 1, 2, and 3 PH therapies at the time of their first BPA were 72 (47%), 33 (22%), and 15 (10%), respectively.
Patients with a prior history of splenectomy and thromboembolic events associated with intravascular devices often have more distal location of chronic clots that can make PTE surgery more challenging. While more patients in the BPA cohort had a history of splenectomy (19% vs. 4%, p < 0.001), the number of patients with intravascular devices was similar between groups, 5%.
Patient selection for BPA is most often driven by perceived peripheral (e.g. subsegmental) location of chronic thromboembolic material. At UCSD however, PTE is routinely performed on patients with segmental and subsegmental disease. According to standard surgical practice at UCSD, the endarterectomy specimens were assigned a surgical classification according to the location of the chronic thromboembolic material. Of the 1104 patients who underwent PTE, 1100 had surgical level recorded, 310 using the older Jamieson Type (before 2017), and 790 using UCSD Level Classification. Of the 310 patients, 90 (29%) had bilateral Jamieson Type 3 disease (segmental) and 225 of 790 (28.5%) using the UCSD Level had bilateral level 3 (segmental) and level 4 (subsegmental) disease. Overall, there were 315 out of 1100 (28.6%) patients treated with PTE who exclusively had segmental or subsegmental level disease.
Rationale for BPA
The decision to recommend BPA was made after multidisciplinary review. Patients often had multiple factors driving the decision to select BPA, however for this analysis, patients were categorized according to the single most significant factor. The majority (n = 90; 59%) of patients were selected for BPA due to peripheral appearance of chronic thromboembolic material. For these patients, it was felt that thromboendarterectomy was less likely to clear the peripheral disease (Figure 1). This was determined based on a combination of functional perfusion imaging (CT and V/Q) and anatomic imaging (CTA, DSA). Only 5 (3%) patients had technically operable disease, were offered PTE surgery but declined and underwent BPA instead. While this was an acceptable treatment option for these patients, not all patients who decline PTE are good BPA candidates. Patients with large pouch occlusions and bulky proximal disease can be particularly challenging for BPA.
Figure 1.
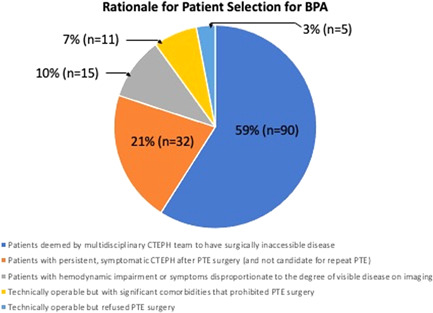
Rationale for patient selection for BPA. BPA, balloon pulmonary angioplasty; CTEPH, chronic thromboembolic pulmonary hypertension; PTE, pulmonary thromboendarterectomy.
Patients that develop recurrent thromboembolism after PTE or undergo an incomplete endarterectomy during their first PTE, may be considered candidates for a second PTE operation. However, most patients with residual PH after PTE are not eligible for redo PTE due to the distal location of the residual chronic thromboembolic material. Thirty‐two (21%) patients treated with BPA had residual PH after PTE surgery. Thirty of these patients had prior PTE performed at UCSD and 2 had prior PTE performed at other CTEPH surgical centers in the United States. No patients had BPA sooner than 6 months after PTE to allow for adequate surgical recovery. Thirteen patients had BPA less than 2 years after PTE, 8 patients had BPA 2–5 years after PTE, 5 patients had BPA 5–10 years after PTE, and 6 patients had BPA > 10 years after PTE.
Only 11 (7%) patients treated with BPA had surgically accessible clot but had medical comorbidities that prevented the possibility of PTE. The mean age in this group was 74 years (range 57–85 years) and older than the overall BPA cohort. Medical comorbidities that prevented PTE included metastatic breast cancer, cerebral meningioma, lung cancer with history of lung resection, myelodysplastic syndrome with transfusion‐dependent anemia, cerebellar movement disorder, and several patients who were deemed too frail to undergo PTE.
Fifteen (10%) patients were selected for BPA because of hemodynamic impairment or symptoms disproportionate to the degree of chronic thromboembolic material. These patients typically fell into 1 of 2 patterns. The first were patients with severe PH and limited thromboembolic burden. In these patients, BPA was chosen because it was unclear to what degree PTE would offer hemodynamic improvement. In the second pattern, patients had symptoms that were disproportionately severe compared to their thromboembolic burden and PH. Typically, these patients had mild PH and BPA was selected because it was unclear to what degree PTE would lead to an improvement in symptoms.
BPA outcomes
We performed an analysis of the effect of BPA on hemodynamics, 6MWD, NT‐pro BNP, NYHA functional class, and the use of PH‐targeted therapies. For the outcome analysis, we included 97 patients who completed BPA treatment and had completed at least three BPA sessions (Figure 2). This group of completed patients was selected because invasive hemodynamics obtained during the third BPA session, reflect the hemodynamic impact of BPA sessions 1 and 2. Patients who completed BPA but underwent fewer than three sessions were excluded because no follow‐up hemodynamic data were available for analysis. Of the 56 patients excluded from the hemodynamic outcome analysis, 23 had not yet completed BPA treatment. Thirty three of the 56 patients completed BPA but were excluded from the analysis because they had completed fewer than three BPA sessions; 21 of the 33 completed BPA after two sessions and 12 completed BPA after just one session.
Figure 2.
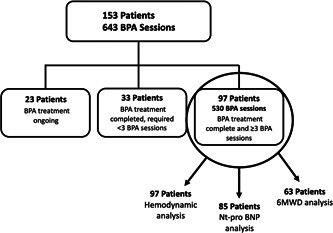
Patient selection for outcome analysis
The 97 patients who were included for outcome analysis underwent at a total of 530 sessions with a mean of 5.5 ± 1.78 (range 3–10) sessions per patient (Table 2). The mean interval between the first and final BPA sessions was 308 days. Hemodynamic improvement was observed with reductions in mPAP from 37 ± 10.9 to 31.4 ± 9.2 mmHg, p < 0.01 and PVR from 398.6 ± 221.4 to 304.2 ± 177.5 dynes s cm−5, p < 0.01. There was an improvement in NYHA functional class following treatment with BPA. Before BPA 4% (n = 4) were NYHA class IV and 68% (n = 66) were functional class III. Following BPA no patient remained NYHA class IV and 30% (n = 29) remained NYHA class III (p < 0.01). The percentage of patients who were NYHA functional class I also increased from 1%v(n = 1) to 15% (15) after treatment (p < 0.01).
Table 2.
BPA Outcomes for 97 patients who completed BPA treatment and had at least three BPA sessions
| Before BPA (n = 97) | Post BPA (n = 97, 530 BPA sessions) | p Value | |
|---|---|---|---|
| sPAP (mmHg); mean ± SD | 62 ± 19.9 | 52.2 ± 17.2 | <0.001 |
| dPAP (mmHg); mean ± SD | 22 ± 6.7 | 18.3 ± 5.7 | <0.001 |
| pre mPAP (mmHg); mean ± SD | 37 ± 10.9 | 31.4 ± 9.2 | <0.001 |
| CO (L/min); mean ± SD | 5.4 ± 1.3 | 5.4 ± 1.2 | 0.9627 |
| CI (L/min/m2); mean ± SD | 2.8 ± 0.6 | 2.8 ± 0.5 | 0.8674 |
| PVR (dynes s cm−5); mean ± SD | 398.6 ± 221.4 | 304.2 ± 177.5 | <0.01 |
| NYHA functional class; n (% of total) | |||
| 1 | 1 (1%) | 15 (15%) | <0.001 |
| 2 | 26 (27%) | 53 (55%) | <0.001 |
| 3 | 66 (68%) | 29 (30%) | <0.001 |
| 4 | 4 (4%) | 0 (0%) | |
| Number of PH medications; n (% of total) | |||
| 0 | 15 (15%) | 24 (25%) | 0.12 |
| 1 | 44 (45%) | 38 (39%) | 0.38 |
| 2 | 26 (27%) | 27 (28%) | 0.87 |
| 3 | 12 (12%) | 8 (8%) | 0.34 |
| 6MWD (m); mean ± SD (n = 63) | 416.5 ± 135.1 | 453.3 ± 132.2 | <0.01 |
| NT‐proBNP (pg/ml); mean ± SD (n = 85) | 535.9 ± 878.3 | 361.6 ± 737.2 | 0.013 |
Abbreviations: BPA, balloon pulmonary angioplasty; CI, cardiac index; CO, cardiac output; dPAP, diastolic pulmonary artery pressure; mPAP, mean pulmonary artery pressure; NT‐pro BNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; sPAP, systolic pulmonary artery pressure; 6MWD, six‐minute walk distance.
Eighty five of the 97 patients had NT‐pro BNP values for analysis. NT‐pro BNP improved from 535.9 ± 878.3 to 361.6 ± 737.2 pg/ml (p = 0.13); 63 of the 97 patients had 6MWD available for analysis. In these patients 6MWD improved from 416.5 ± 135.1 to 453.3 ± 132.2 m (p < 0.01) an improvement of 36.8 m. Despite improvements in functional class, 6MWD, and NT‐pro BNP, there was no significant change in the use of PH‐targeted therapy.
Twenty of the 97 patients (21%) who completed BPA treatment had a prior history of splenectomy and 16 of the 97 (16%) had prior PTE surgery. Baseline hemodynamics, functional status, and outcomes were not significantly different in these subgroups.
Complications
Over 6 years and 643 BPA sessions there was no procedure‐related mortality. There were 65 procedure‐related complications resulting in a per‐procedure complication rate of 10.4%. Three (<0.05%) complications were related to the vascular access site and one patient developed a minor allergic reaction to contrast. There were 59 (9.2% per‐procedure) minor pulmonary vascular complications during BPA. Minor vascular injuries were frequently associated with a new cough and/or hemoptysis during the BPA procedure. Minor vascular complications were managed according to the severity of the injury and severity of hemodynamics. Management was typically conservative, including strategies such as close observation, reversal of anticoagulation, or occasionally temporary balloon occlusion. None of the minor vascular injuries required embolization or covered stenting. Minor vascular injury episodes typically resolved within 24 h and full anticoagulation was safely resumed. Only one major episode of pulmonary hemorrhage occurred following a BPA session that required intubation, mechanical ventilation, extracorporeal membrane oxygenation, and surgical resection of the injured lung segment. The patient survived this event and was able to be discharged home.
There was no significant difference in the complication rates of patients who had a prior history of splenectomy (9% vs. 14% p = 0.11) or prior PTE (10% vs. 8%, p = 0.5). As observed in other BPA series, patients with less severe hemodynamic impairment had a lower complication rate. In our series, patients with a mPAP < 25 mmHg at baseline (n = 33) had a complication rate of 4% versus 11% (p = 0.03) in patients with a mPAP ≥25 mmHg.
DISCUSSION
BPA is now an accepted and essential part of the CTEPH treatment algorithm. 2 , 9 , 10 It has been proven safe and effective across multiple centers worldwide. 11 , 12 , 13 , 14 , 15 BPA has moved beyond questions of safety and efficacy, and we are now faced with the challenge of selecting patients who are most appropriate for BPA.
This decision is most problematic at the edges of patient selection where either there is legitimate equipoise whether surgical resection is possible, or uncertainty exists if small vessel PH is the primary disease process. As surgical techniques for distal endarterectomy have improved and new surgical approaches such as minimally invasive PTE become options, these decisions have only grown more complex. 16 Similarly, the expanding role of medical therapy in the treatment of CTEPH adds an additional layer of complexity on the patient selection process and treatment timing. 17 , 18 , 19 , 20
In the 6 years that BPA has been performed at UCSD, almost a third (28.6%) of the patients who underwent PTE had exclusively distal disease (Jamieson III or UCSD levels III and IV). This is representative of our patient selection strategy at UCSD where PTE surgery remains the gold standard treatment for CTEPH, even for patients with perceived segmental and subsegmental disease. This also means that at centers with less experience with distal endarterectomy, almost a third of the patients who were able to undergo PTE at UCSD might have been directed to BPA or medical therapy instead. This emphasizes our belief, in accordance with recommendations from multiple guidelines, that evaluation at an experienced PTE center is essential in selecting the appropriate treatment. Importantly, PTE can be performed safely and effectively in patients with exclusively distal disease as shown by de Perrot and colleagues 21 who demonstrated similar mortality (2.8% vs 2.4%: p = 0.8) comparing 401 consecutive patients at a single center with Jamieson III versus I and II. One‐year survival was also similar (7.7% vs. 5.5%; p = 0.4) but patients with Jamieson III were more likely to require additional treatment with PH‐targeted medical therapy of BPA.
The majority (59%) of patients who underwent BPA at UCSD were deemed to have surgically inaccessible disease after review by our multidisciplinary team and this is expected to remain the largest factor in patient selection for BPA moving forward. Imaging modalities play a key role in the patient selection process and as imaging continues to improve, we hope that our accuracy improves in parallel. 22
Patients with persistent, symptomatic CTEPH after PTE surgery, not candidates for repeat PTE (21%) represent a growing cohort of patients treated with BPA. This is due in large part to the increased awareness of BPA as a treatment option and a growing number of patients living with residual PH after PTE surgery. As demonstrated at our center and as has been reported at other centers, 23 , 24 we believe BPA can be successfully performed in patients who have previously undergone PTE surgery, with a safety and efficacy profile, similar to patients who are naïve to PTE surgery.
PH‐targeted medical therapy was more widely used in our BPA cohort when compared to patients who underwent PTE (78% vs. 54%). Preoperative PH‐targeted therapy in patients who undergo PTE has been associated with delay in referral time without clear benefit. 25 On the other hand, data from the RACE trial 26 demonstrated that pretreatment with riociguat was associated with a lower BPA complication rate (14% vs. 42% of patients). This observation is in accordance with our treatment strategy at UCSD where patients with severe hemodynamic impairment are first treated with PH‐targeted medical therapy before BPA. As more data becomes available, it is our expectation that PH‐targeted medical therapy should be used adjunctively with BPA to achieve optimal results.
Hemodynamic outcomes from BPA at our center are less robust than those observed at Japanese centers and in the more recent RACE trial. 26 , 27 They more closely mirror BPA outcomes of case series at other European CTEPH centers where BPA has resulted in mPAP reductions of 18%–30%, significant decrements in pulmonary vascular resistance (PVR), and improvements in exercise capacity. 11 , 12 , 13 , 14 We observed similar outcomes with a 23% reduction in PVR from 398.6 ± 221.4 to 304.2 ± 177.5 dynes s cm−5, p < 0.01. We also observed significant improvements in NYHA's functional status. Before BPA, only 28% of patients were NYHA class I or II. Following BPA, 70% of patients were NYHA class I or II and no patient remained NYHA class IV. 6MWD also improved 36.8 m (416.5 ± 135.1 to 453.3 ± 132.2 m; p < 0.01). Importantly, we achieved these outcomes with no procedure‐related mortality and a minor complication rate of 9.2%.
Compared with data from the recent RACE trial and MR BPA trial we demonstrated a significant, but less robust improvement in hemodynamics. This observation is most likely a result of patient selection. Baseline PVR for the 97 patients included for hemodynamic analysis at our center was lower, 398.6 ± 221.4 versus 767.2 ± 251.2 dynes s cm−5 in the RACE trial and 645 ± 266.7 in the MR BPA trial. 27 Additionally, 41 of the 97 patients at our center had a baseline PVR < 320 dynes s cm−5 and 16 patients had previously undergone PTE surgery. Interpretation of the hemodynamic results is also limited by the absence of invasive hemodynamic assessment to capture the final BPA session(s).
Along with successful BPA outcomes, we also recognize a subset of patients who, despite multiple sessions of BPA, only achieve modest hemodynamic and clinical improvement. 28 These patients typically have “sclerotic” appearing vessels and appear to have defects not amenable to BPA (Figure 3). Despite adequate ballooning of the “sclerotic” vessels, little improvement in distal flow, capillary blush, or venous drainage can be achieved. This group, albeit hard to identify before BPA, may benefit from escalation and optimization of medical therapy rather than repeated BPA attempts.
Figure 3.
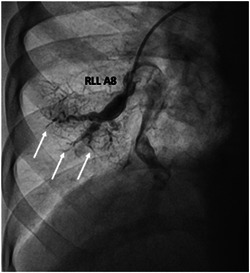
Right Lower Lobe (A8) with guide and wire engaged in patent proximal portion. “Sclerotic” distal vessels indicated with white arrows.
With more than 6 years of experience, BPA has become an essential component of the CTEPH treatment strategy at UCSD. In accordance with established guidelines, BPA is still reserved for patients who are inoperable, have residual PH after PTE, and select patients who refuse PTE surgery. We have been able to incorporate BPA safely and effectively in our patient population which is likely different than other BPA centers without the treatment option of segmental/subsegmental endarterectomy. We believe that just like PTE surgery, patients who undergo BPA will have the best outcomes at experienced, high‐volume BPA centers.
AUTHOR CONTRIBUTIONS
David S. Poch: Primary Author; manuscript preparation. Ehtisham Mahmud: Editing; revisions. Mitul Patel: Editing; revisions. Demosthenes Papamatheakis: Editing; revisions. Timothy Fernandes: Editing; revisions. Kim Kerr: Editing; revisions. Jenny Yang: Editing; revisions. Victor Pretorius: Editing; revisions. Michael M. Madani: Editing; revisions. Nick H. Kim: Editing; revisions.
CONFLICTS OF INTEREST
David S. Poch—Principal Investigator Janssen, Merck; Demosthenes Papamatheakis—Speaker: Janssen; Timothy Fernandes—Consulting: Bayer; Kim Kerr—Research Support: Bayer; Michael Madani—Consultant: Bayer, Johnson and Johnson, and Wexler Surgical; Nick H. Kim—Consultant: Bayer, Janssen, Merck, United Therapeutics, CTEPH.com Speaker: Bayer, Janssen. Other authors: None.
ETHICS STATEMENT
The ethics statement is not available.
Poch DS, Mahmud E, Patel M, Papamatheakis D, Fernandes T, Kerr K, Yang J, Pretorius V, Madani MM, Kim NH. Patient selection for balloon pulmonary angioplasty: six‐year results from a high volume PTE surgical center. Pulm Circ. 2022;12:e12148. 10.1002/pul2.12148
REFERENCES
- 1. Simonneau G, Torbicki A, Dorfmüller P, Kim N. The pathophysiology of chronic thromboembolic pulmonary hypertension. European Respir Rev. 2017;26:160112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim NH, Delcroix M, Jais X, Madani MM, Matsubara H, Mayer E, Ogo T, Tapson VF, Ghofrani HA, Jenkins DP. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;53:1801915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. D'armini AM, Morsolini M, Mattiucci G, Grazioli V, Pin M, Valentini A, Silvaggio G, Klersy C, Dore R. Pulmonary endarterectomy for distal chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg. 2014;148:1005–11. 1012.e1‐2; discussion 1011‐2. [DOI] [PubMed] [Google Scholar]
- 4. Pepke‐Zaba J, Delcroix M, Lang I, Mayer E, Jansa P, Ambroz D, Treacy C, D'Armini AM, Morsolini M, Snijder R, Bresser P, Torbicki A, Kristensen B, Lewczuk J, Simkova I, Barberà JA, de Perrot M, Hoeper MM, Gaine S, Speich R, Gomez‐Sanchez MA, Kovacs G, Hamid AM, Jaïs X, Simonneau G. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124:1973–81. [DOI] [PubMed] [Google Scholar]
- 5. Inami T, Kataoka M, Kikuchi H, Goda A, Satoh T. Balloon pulmonary angioplasty for symptomatic chronic thromboembolic disease without pulmonary hypertension at rest. Int J Cardiol. 2019;289:116–8. [DOI] [PubMed] [Google Scholar]
- 6. Mahmud E, Madani MM, Kim NH, Poch D, Ang L, Behnamfar O, Patel MP, Auger WR. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2018;71:2468–86. [DOI] [PubMed] [Google Scholar]
- 7. Mahmud E, Behnamfar O, Ang L, Patel MP, Poch D, Kim NH. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Interventional Cardiol Clin. 2018;7:103–17. [DOI] [PubMed] [Google Scholar]
- 8. Fukuda T, Ogo T, Nakanishi N, Ueda J, Sanda Y, Morita Y, Sugiyama M, Fukui S, Tsuji A, Naito H. Evaluation of organized thrombus in distal pulmonary arteries in patients with chronic thromboembolic pulmonary hypertension using cone‐beam computed tomography. Jpn J Radiol. 2016;34:423–31. 10.1007/s11604-016-0538-2 [DOI] [PubMed] [Google Scholar]
- 9. Galiè N, Humbert M, Vachiery J‐L, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document Group . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 10. Wilkens H, Konstantinides S, Lang IM, Bunck AC, Gerges M, Gerhardt F, Grgic A, Grohé C, Guth S, Held M, Hinrichs JB, Hoeper MM, Klepetko W, Kramm T, Krüger U, Lankeit M, Meyer BC, Olsson KM, Schäfers HJ, Schmidt M, Seyfarth HJ, Ulrich S, Wiedenroth CB, Mayer E. Chronic thromboembolic pulmonary hypertension (CTEPH): updated recommendations from the Cologne Consensus Conference 2018. Int J Cardiol. 2018;272:69–78. [DOI] [PubMed] [Google Scholar]
- 11. Hoole SP, Coghlan JG, Cannon JE, Taboada D, Toshner M, Sheares K, Fletcher AJ, Martinez G, Ruggiero A, Screaton N, Jenkins D, Pepke‐Zaba J. Balloon pulmonary angioplasty for inoperable chronic thromboembolic pulmonary hypertension: the UK experience. Open Hear. 2020;7:e001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olsson KM, Wiedenroth CB, Kamp J‐C, Breithecker A, Fuge J, Krombach GA, Haas M, Hamm C, Kramm T, Guth S, Ghofrani HA, Hinrichs JB, Cebotari S, Meyer K, Hoeper MM, Mayer E, Liebetrau C, Meyer BC. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: the initial German experience. Eur Respir J. 2017;49:1602409. [DOI] [PubMed] [Google Scholar]
- 13. Brenot P, Jaïs X, Taniguchi Y, Garcia Alonso C, Gerardin B, Mussot S, Mercier O, Fabre D, Parent F, Jevnikar M, Montani D, Savale L, Sitbon O, Fadel E, Humbert M, Simonneau G. French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;53:1802095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ogawa A, Satoh T, Fukuda T, Sugimura K, Fukumoto Y, Emoto N, Yamada N, Yao A, Ando M, Ogino H, Tanabe N, Tsujino I, Hanaoka M, Minatoya K, Ito H, Matsubara H. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: results of a multicenter registry. Circ Cardiovasc Qual Outcomes. 2017;10:e004029. [DOI] [PubMed] [Google Scholar]
- 15. van Thor MCJ, Lely RJ, Braams NJ, Ten Klooster L, Beijk M, Heijmen RH, van den Heuvel D, Rensing B, Snijder RJ, Vonk Noordegraaf A, Nossent EJ, Meijboom LJ, Symersky P, Mager JJ, Bogaard HJ, Post MC. Safety and efficacy of balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension in the Netherlands. Neth Hear J Mon J Neth Soc Cardiol Neth Hear Found. 2020;28:81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Madani MM. Pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension: state‐of‐the‐art 2020. Pulm Circ. 2021;11:20458940211007372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ghofrani HA, Simonneau G, D'armini AM, Fedullo P, Howard LS, Jaïs X, Jenkins DP, Jing ZC, Madani MM, Martin N, Mayer E, Papadakis K, Richard D, Kim NH, MERIT study investigators . Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT‐1): results from the multicentre, phase 2, randomised, double‐blind, placebo‐controlled study. Lancet Respir Med. 2017;5:785–94. 10.1016/s2213-2600(17)30305-3 [DOI] [PubMed] [Google Scholar]
- 18. Simonneau G, D'armini AM, Ghofrani HA, Grimminger F, Hoeper MM, Jansa P, Kim NH, Wang C, Wilkins MR, Fritsch A, Davie N, Colorado P, Mayer E. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension: a long‐term extension study (CHEST‐2). Eur Respir J. 2014;45:1293–302. [DOI] [PubMed] [Google Scholar]
- 19. Ghofrani HA, D'armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, Simonneau G, Wilkins MR, Fritsch A, Neuser D, Weimann G, Wang C, CHEST‐ Study G. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369:319–29. [DOI] [PubMed] [Google Scholar]
- 20. Jais X, Brenot P, Bouvaist H, Canuet M, Chabanne C, Chaouat A, Cottin V, Degroote P, Favrolt N, Horeau‐Langlard D, Jevnikar M, Magro P, Montani D, Parent F, Pison C, Prévot G, Renard S, Savale L, Sitbon O, Trésorier R, Tromeur C, Piedvache C, Fadel E, Humbert M, Simonneau G. BPA and riociguat for the management of inoperable CTEPH: results of the extension study following the RACE randomized controlled trial (RCT). D3 D003 Come Together – Clin Adv Pulm Hypertens Lessons Best Abstr. 2021:A1182. [Google Scholar]
- 21. de Perrot M, Donahoe L, McRae K, Thenganatt J, Moric J, Chan J, McInnis M, Jumaa K, Tan KT, Mafeld S, Granton J, Canadian CTEPH Working Group . Outcome after pulmonary endarterectomy for segmental chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg. 2022;164:696–707. 10.1016/j.jtcvs.2021.10.078 [DOI] [PubMed] [Google Scholar]
- 22. Kligerman S, Hsiao A. Optimizing the diagnosis and assessment of chronic thromboembolic pulmonary hypertension with advancing imaging modalities. Pulm Circ. 2021;11:20458940211007376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shimura N, Kataoka M, Inami T, Yanagisawa R, Ishiguro H, Kawakami T, Higuchi Y, Ando M, Fukuda K, Yoshino H, Satoh T. Additional percutaneous transluminal pulmonary angioplasty for residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol. 2015;183:138–42. [DOI] [PubMed] [Google Scholar]
- 24. Araszkiewicz A, Darocha S, Pietrasik A, Pietura R, Jankiewicz S, Banaszkiewicz M, Sławek‐Szmyt S, Biederman A, Mularek‐Kubzdela T, Lesiak M, Torbicki A, Kurzyna M. Balloon pulmonary angioplasty for the treatment of residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol. 2019;278:232–7. [DOI] [PubMed] [Google Scholar]
- 25. Jensen KW, Kerr KM, Fedullo PF, Kim NH, Test VJ, Ben‐Yehuda O, Auger WR. Pulmonary hypertensive medical therapy in chronic thromboembolic pulmonary hypertension before pulmonary thromboendarterectomy. Circulation. 2009;120:1248–54. [DOI] [PubMed] [Google Scholar]
- 26. Jaïs X, Brenot P, Bouvaist H, Jevnikar M, Canuet M, Chabanne C, Chaouat A, Cottin V, De Groote P, Favrolt N, Horeau‐Langlard D, Magro P, Savale L, Prévot G, Renard S, Sitbon O, Parent F, Trésorier R, Tromeur C, Piedvache C, Grimaldi L, Fadel E, Montani D, Humbert M, Simonneau G. Balloon pulmonary angioplasty versus riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension (RACE): a multicentre, phase 3, open‐label, randomised controlled trial and ancillary follow‐up study. Lancet Respir Med. 2022;10:961–71. 10.1016/s2213-2600(22)00214-4 [DOI] [PubMed] [Google Scholar]
- 27. Kawakami T, Matsubara H, Shinke T, Abe K, Kohsaka S, Hosokawa K, Taniguchi Y, Shimokawahara H, Yamada Y, Kataoka M, Ogawa A, Murata M, Jinzaki M, Hirata K, Tsutsui H, Sato Y, Fukuda K. Balloon pulmonary angioplasty versus riociguat in inoperable chronic thromboembolic pulmonary hypertension (MR BPA): an open‐label, randomised controlled trial. Lancet Respir Med. 2022;10:949–60. 10.1016/s2213-2600(22)00171-0 [DOI] [PubMed] [Google Scholar]
- 28. Tsuji A, Ogo T, Ueda J, Fukui S, Morita Y, Fukuda T, Nakanishi N, Ogawa H, Yasuda S. Predictors of residual pulmonary hypertension after balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Int J Cardiol. 2017;226:118–20. [DOI] [PubMed] [Google Scholar]


