Abstract
We have investigated the clonality of β-chain T-cell receptor (TCR) transcripts from the cerebrospinal fluid (CSF) and peripheral blood from a 7-year old child who developed a multiphasic disseminated encephalomyelitis following an infection with hepatitis A virus. We amplified β-chain TCR transcripts by nonpalindromic adaptor (NPA)-PCR–Vβ-specific PCR. TCR transcripts from only five Vβ families (Vβ13, Vβ3, Vβ17, Vβ8, and Vβ20) were detected in CSF. The amplified products were combined, cloned, and sequenced. Sequence analysis revealed in the CSF substantial proportions of identical β-chain of TCR transcripts, demonstrating oligoclonal populations of T cells. Seventeen of 35 (48%) transcripts were 100% identical, demonstrating a major Vβ13.3 Dβ2.1 Jβ1.3 clonal expansion. Six of 35 (17%) transcripts were also 100% identical, revealing a second Vβ13 clonal expansion (Vβ13.1 Dβ2.1 Jβ1.2). Clonal expansions were also found within the Vβ3 family (transcript Vβ3.1 Dβ2.1 Jβ1.5 accounted for 5 of 35 transcripts [14%]) and within the Vβ20 family (transcript Vβ20.1 Dβ1.1 Jβ2.4 accounted for 3 of 35 transcripts [8%]). These results demonstrate the presence of T-cell oligoclonal expansions in the CSF of this patient following infection with hepatitis A virus. Analysis of the CDR3 motifs revealed that two of the clonally expanded T-cell clones exhibited substantial homology to myelin basic protein-reactive T-cell clones. In contrast, all Vβ TCR families were expressed in peripheral blood lymphocytes. Oligoclonal expansions of T cells were not detected in the peripheral blood of this patient. It remains to be determined whether these clonally expanded T cells are specific for hepatitis A viral antigen(s) or host central nervous system antigen(s) and whether molecular mimicry between hepatitis A viral protein and a host protein is responsible for demyelinating disease in this patient.
Multiple sclerosis (MS) is an inflammatory, demyelinating disease of the central nervous system (CNS) (2, 32, 66–68). The etiology and pathogenesis of the disease and its relationship to other less common demyelinating disorders, such as acute disseminated encephalomyelitis (ADEM), remain poorly understood (4, 59, 76). The natural history of MS and its geographic distribution suggest that it may be due to a virus contracted during childhood by susceptible individuals. There is a general agreement that MS is an autoimmune disease of the CNS mediated by T cells (21, 45, 73, 74, 80, 82). How the virus may trigger the autoimmune response to neuroantigen(s) or other self-antigen(s) is not fully understood. Analysis of T-cell responses in patients with long-standing MS revealed that these T cells recognized certain host myelin proteins, such as myelin basic protein (MBP), proteolipid protein (PLP), and myelin-associated glycoprotein (MAG) (17, 27, 39, 50, 52, 53, 72, 77). These T cells may have been generated long after the onset of the disease. However, the specificity, either viral or host, of the T cells that trigger the disease and are present at the onset of the disease needs to be elucidated. Analysis of T cells at the earliest possible time likely holds the key to our understanding the immunopathogenesis of MS. In this context, the relationship (if any) between MS and ADEM (also known as perivenous encephalomyelitis) becomes important in terms of the immunopathogenesis of MS. There are considerable similarities between ADEM and experimental allergic encephalomyelitis (EAE), which is traditionally considered to be a relevant animal model for MS (4, 59, 76).
With the objective of identifying antigen-driven clonally expanded populations of T cells, we amplified, cloned, and sequenced β-chain T-cell receptor (TCR) transcripts from the cerebrospinal fluid (CSF) and the peripheral blood of a 7-year-old female who developed multiphasic demyelinating disease shortly after infection with hepatitis A virus. Sequence analysis revealed substantial proportions of identical β-chain TCR transcripts in the CSF, suggesting the presence of oligoclonal T cells. The patient exhibited two episodes of neurological symptoms 5 months apart. The first episode occurred 5 days after hepatitis A virus infection, which was serologically confirmed, and deteriorated to coma and quadriplegia. Hepatitis A virus transcripts were present in the CSF and the peripheral blood of this patient during the first neurological episode. Anti-hepatitis A virus total antibody and immunoglobulin M (IgM) antibody were detected in the serum of this patient during the first neurological episode. Magnetic resonance imaging (MRI) studies demonstrated diffuse white matter changes consistent with demyelinating disease. Five months after the first episode, the patient developed a second neurological episode, characterized by aphasia and optic neuritis.
The clinical symptoms and signs, and the CSF and MRI findings observed in this patient indicate the presence of multiple, metachronous, predominantly white matter demyelinating lesions widely spread through neuraxis. These findings resemble either the multiphasic variant of ADEM or a transitional form of demyelinating disease bearing features of ADEM and MS (32, 59, 62, 76). Extrahepatic manifestations of the infection with hepatitis A virus are rare. However, a number of reports have previously suggested an association of hepatitis A virus infection with neurological symptoms (i.e., encephalitis, transverse myelitis, cervical myelopathy, or optic neuritis) (6, 10, 15; P. J. Hughes, I. K. Saadeh, J. P. Cox, and L. S. Illis, Letter, Lancet 342:302, 1993). To our knowledge, this is the first case of infection with hepatitis A virus that preceded the onset of demyelinating disease of the CNS, in which a clonal expansion of T cells has been demonstrated.
(This work was presented at the 5th Symposium on the Neurovirology and Neuroimmunology of Schizophrenia and Bipolar Disorders, Bethesda, Md., 4 to 6 November 1999.)
CASE REPORT
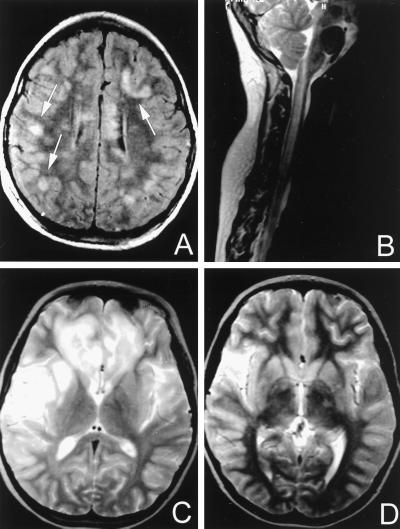
The patient was a 7-year-old female who had two episodes of diffuse and focal neurologic deficits separated by 5 months. The first episode presented with fever, headache, tiredness, vomiting, jaundice, pruritus, and mild, tender hepatomegaly. Five days later, she developed lower extremity weakness and progressive lethargy. Her condition deteriorated to coma and flaccid quadriplegia over 72 h. Liver function tests revealed elevated aspartate aminotransferase (AST; 100 U/liter) alanine aminotransferase (ALT; 333 U/liter), and bilirubin (total bilirubin, 3.2 mg/dl; direct bilirubin, 2.8 mg/dl). Anti-hepatitis A virus total antibody and IgM antibody were found in the serum. No clinical or laboratory evidence of hepatic coma was found (normal blood ammonia level). Serum antibody titers for Mycoplasma pneumoniae, Epstein-Barr virus, cytomegalovirus, and Borrelia burgdorferi were all negative. Human herpes virus 6 (HHV-6) antibody titers were not measured. CSF studies revealed a lymphocytic pleocytosis (197 lymphocytes), normal glucose (65 mg/dl), elevated protein (62 mg/dl), and elevated IgA (2 mg/dl), IgG (28 mg/dl), and IgM (2.4 mg/dl) levels. CSF did not contain herpes simplex virus, as determined by PCR. Routine oligoclonal banding studies were negative. CSF cultures were sterile. Hepatitis A virus RNA was present in both serum and CSF as determined by reverse transcription-PCR (RT-PCR) and sequencing. The diagnosis of hepatitis A virus infection was also supported by epidemiological data. The patient traveled to her hometown in Peru 3 weeks prior to the initiation of the clinical symptoms. She had gone to the municipal swimming pool, and, retrospectively, it was found that other people in town who used the pool had developed symptoms compatible with the diagnosis of hepatitis A virus infection. MRI studies were carried out and demonstrated diffuse white matter changes in the brain, involving the cerebrum and the brain stem, as well as in the spinal cord (Fig. 1). These white matter changes were consistent with demyelinating disease. The patient received intravenous (i.v.) methylprednisolone, plasmapheresis, and i.v. Ig therapy. Her clinical status improved over 4 months to near baseline.
FIG. 1.
(A) First demyelinating episode. T1-weighted MRI of axial section 5 days after the onset of neurological symptoms. Multiple, multifocal, hyperintense lesions of various sizes are disseminated throughout the cerebral white matter. There is a distributional predilection of these lesions for the subcortical (convolutional) white matter at the corticomedullary junction (arrows). A smaller hyperintense focal area is also present in the left periventricular white matter. (B) First demyelinating episode. T2-weighted MRI of a parasagittal section of the spinal cord. Diffuse spinal cord swelling associated with confluent, ill-defined, high-signal intra-axial lesions. (C and D) Second demyelinating episode. T2-weighted MRI of axial sections 3 days after the onset of neurological symptoms. Diffuse heterogeneous areas of abnormal signal are seen in the frontal and temporal lobes bilaterally. Contiguous high-signal lesions are particularly predominant in the left opercular, insular, and superior temporal white matter and the mesial frontal convolutional white matter bilaterally (C). In addition, heterogeneous high-signal abnormalities are detected in the thalamic region bilaterally (from right to left) (D).
Five months after initial presentation, the patient exhibited a second neurological episode, characterized by acute onset aphasia and bilateral visual loss secondary to retrobulbar optic neuritis. Levels of AST, ALT, and bilirubin in blood were normal. CSF studies demonstrated a normal cell count (5 lymphocytes) and glucose level (69 mg/dl), with mildly elevated protein (44 mg/dl). The MRI revealed diffuse heterogeneous areas of abnormal signal on T2-weighted images in the frontal and temporal lobes bilaterally that were also enhanced with gadolinium, again consistent with demyelinating disease. The patient received i.v. methylprednisolone for 3 days followed by oral prednisone with subsequent clinical improvement and resolution of the MRI abnormalities.
MATERIALS AND METHODS
Amplification of hepatitis A virus RNA.
The VP3-VP1 junction (positions 2132 to 2451) of the hepatitis A virus genome was amplified from serum and spinal fluid specimens by RT-PCR, as previously described (34). The sensitivity, specificity, and validation of the RT-PCR hepatitis A virus VP3-VP1 junction test is described elsewhere (9).
Nucleic acid sequencing of amplified hepatitis A virus. PCR products of amplified hepatitis A virus were purified with a Qiaquick PCR purification kit (Qiagen, Inc., Valencia, Calif.). Sequencing reactions were performed with a prism dye terminator cycle sequencing kit (Applied Biosystems, Foster City, Calif.) and an automated sequencer (ABI model 373 or 377; Applied Biosystems) following the instructions of the manufacturer. Preliminary sequence analysis was performed with ABI software, and further analysis was performed with GCG (Genetics Computer Group, Madison, Wis.) software (18).
CSF and PBMCs.
Cells in the CSF of this patient were collected by centrifugation at 400 × g for 15 min at 4°C and immediately used for RNA isolation.
Peripheral blood mononuclear cells (PBMCs) from this patient or normal donors were isolated from heparinized peripheral blood by centrifugation on a Ficoll-Hypaque density cushion, following established methods. PBMCs were collected from the interface and were washed twice before preparation of RNA. These studies have been approved by the Institutional Review Board of Temple University Hospital.
Preparation of RNA from mononuclear cells.
Total RNA was prepared either from mononuclear cells isolated from CSF or from PBMCs by the guanidinium thiocyanate phenol-chloroform single-step extraction method, following the procedure recommended by the manufacturer (Stratagene, La Jolla, Calif.) as described previously (58). Phenol extraction was performed at least twice on all specimens. To check the purity of the isolated RNA, the rRNA 28S and 18S bands were visualized after agarose gel electrophoresis.
Synthesis of cDNA.
Total RNA isolated from PBMCs (5 μg) or from the CSF was used for double-stranded cDNA synthesis, as previously described (12, 13, 43, 61). The first strand was synthesized (in a 20-μl reaction volume) by using Superscript RTase (Gibco-BRL) and either a NotI-oligo(dT)15 primer or a NotI-human constant region β-chain (NotI-hCβ) primer (Table 1) and incubated at 42°C for 1 h. The second-strand cDNA was synthesized in a reaction volume of 160 μl by adding directly to the first-strand reaction mixture 5 U of Escherichia coli DNA ligase, 40 U of E. coli DNA polymerase, 1.5 U of RNAse H, 0.19 mM deoxynucleoside triphosphates (dNTP), and 3.8 μM dithiothreitol in second strand buffer and incubating for 2 h at 16°C. Ten units of T4 polymerase was added, and the mixture was incubated for 45 min at 16°C. The double-stranded cDNA was extracted with equal volume of phenol-chloroform (1:1) precipitated with 0.5 volume of NH4OAc (4 M) and 2.5 volumes of 100% ethanol, was washed once with 70% ethanol and resuspended in 10 μl of sterile water.
TABLE 1.
Human Vβ family primers used for amplification
| Primer | Sequence |
|---|---|
| NPA-PCR | |
| 5′ end | 5′-AATTCGAACCCCTTCGAGAATGCT-3′ |
| 3′ end (hCβ3) | 5′-CAGGCAGTATCTGGAGTCATTGA-3′ |
| Vβ-specific amplification | |
| 5′ end | |
| Vβ1 | CCGCACAACAGTTCCCTGACTTGC |
| Vβ2 | GGCCACATACGAGCAAGGCGTCGA |
| Vβ3 | GTCTCTAGAGAGAAGAAGGAGCGC |
| Vβ4 | TTCCCATCAGCCGCCCAAACCTAA |
| Vβ5 | ATACTTCAGTGAGACACAGAGA |
| Vβ6 | TCTCAGGTGTGATCCAAATTCGGG |
| Vβ7 | CCTGAATGCCCCAACAGCTCTCTC |
| Vβ8 | CCATGATGCGGGGACTGGAGTTGC |
| Vβ9 | TTCCCTGGAGCTTGGTGACTCTGC |
| Vβ10 | CCACGGAGTCAGGGGACACAGCAC |
| Vβ11 | TGCCAGGCCCTCACATACCTCTCA |
| Vβ12 | TGTCACCAGACTGGGAACCACCAC |
| Vβ13 | CACTGCGGTGTACCCAGGATATGA |
| Vβ14 | GGGCTCGGCTTAAGGCAGACCTAC |
| Vβ15 | CAGGCACAGGCTAAATTCTCCCTG |
| Vβ16 | GCCTGCAGAACTGGAGGATTCTGG |
| Vβ17 | TCCTCTCACTGTGACATCGGCCA |
| Vβ18 | CTGCTGAATTTCCCAAAGAGGGCC |
| Vβ19 | TCTCAATGCCCCAAGAACGCACCC |
| Vβ20 | TGCCCCAGAATCTCTCAGCCTCCA |
| Vβ21 | GATTCACAGTTGCCTAAGGA |
| Vβ22 | AAGTGATCTTGCGCTGTGTCCCCA |
| Vβ23 | GCAGGGTCCAGGTCAGGACCCCCA |
| Vβ24 | CCCAGTTTGGAAAGCCAGTGACCC |
| 3′ end (hCβ2) | 5′-ACCAGCACTCAGCTCCACGTGGTC-3′ |
Adaptor ligation and NotI digestion.
A nonpalindromic double-stranded adaptor (Table 1) comprised of the 5′-AATTCGAACCCCTTCGAGAATGCT-3′ nucleotide and its complementary 5′ end nucleotide 5′-pCGCATTCTCGAAGGGGTTCG-3′ were ligated onto the 5′ and 3′ blunt ends of the cDNA with 1.4 U of T4 DNA ligase (Gibco-BRL) and incubated overnight at 14°C. This adaptor is a modification of the one that we have described previously (12, 13, 43, 61). The adaptor was removed from the 3′ end of the cDNA by digestion for 3 h with 7.5 U of NotI restriction nuclease (GIBCO-BRL) in a 50-μl reaction volume. The NotI nuclease-digested cDNA was purified with a G-50 spin column, by centrifugation for 5 min at 1,100 × g, according to the procedure recommended by the manufacturer (5 Prime to 3 Prime, Boulder, Colo.).
Amplification by NPA-PCR–Vβ-specific PCR. (i) First cycle of amplification by NPA-PCR.
Nonpalindromic adaptor-PCR (NPA-PCR) was carried out essentially as described previously (12, 13, 43, 61) with minor modifications. NotI-digested cDNA (1/5 to 1/10 volume), purified by centrifugation with G-50 spin columns, was denatured by heating at 94°C for 3 min (in a reaction volume of 100 μl) and amplified by 35 cycles of PCR with Taq polymerase (Promega, Madison, Wis.) at 94°C for 1 min, 45°C for 1 min, and a final extension at 72°C for 10 min. The NPA 5′-AATTCGAACCCCTTCGAGAATGCT-3′ was used as the 5′ amplification primer. A human Cβ oligonucleotide, designated hCβ3 (5′-CAGGCAGTATCTGGAGTCATTGA-3′) (Table 1), was used as the 3′ amplification primer, and it is located 5′ to the hCβ primer used for cDNA synthesis (nested design). The hCβ3 primer was located in the Cβ region starting at nucleotide 208. The amplified transcripts were purified with a G-50 spin column.
(ii) Second cycle of amplification by individual Vβ-specific PCR.
A second cycle of amplification was carried out using as a template β-chain TCR cDNA (5 to 10 μl) amplified by NPA-PCR, as described in the previous paragraph. Oligonucleotides from each of 24 Vβ families (Vβ1 to Vβ24) (22) were used as a 5′ end amplification primer in 24 separate amplification reactions (Table 1). A human Cβ primer designated hcβ2 (5′-ACCAGCACTCAGCTCCACGTGGTC-3′) was used as a 3′ amplification primer, and it was located 5′ of the hcβ3 primer used for the first amplification (nested design). This oligonucleotide was located in the Cβ region starting at nucleotide 113. The reaction mixture (50 μl) was denatured by heating at 94°C for 3 min and amplified by 35 cycles of PCR with Taq polymerase (Promega), at 94°C for 1 min, 55°C for 1 min, 72°C for 1 min, and a final extension at 72°C for 10 min.
Cloning of TCR transcripts.
Ten microliters from each of the products of each Vβ-specific PCR amplification was run on 1% agarose gel. The DNA bands corresponding to similar molecular weights were excised, purified by Geneclean kit (Bio 101, Vista, Calif.) as recommended by the manufacturer, and mixed, and 2 μl of the mixture was ligated with 50 ng of vector pCR2.1 (Invitrogen, San Diego, Calif.) with 2 U of T4 ligase (Gibco-BRL) in a total volume of 10 μl. DH5α competent cells (Gibco-BRL) were transformed, and white colonies were picked up.
Sequencing of TCR transcripts.
Plasmids were isolated with the Perfect Prep kit according to the procedure provided by the manufacturer (5 Prime to 3 Prime). Sequencing was carried out by the dideoxy chain termination method with Sequenase 2.0 (U.S. Biochemicals, Cleveland, Ohio).
The maximum theoretical number of potentially unique β-chain TCR transcripts has been estimated (7) to be approximately 1012. Theoretically, the probability of randomly finding two identical copies of a single β-chain TCR transcript within a given independent sample population is negligible. During transformation of DH5α competent cells, the plasmid-cell mix was subjected to heat shock (at 42°C for 45 s) followed by incubation on ice for 2 min and growth for 1 h in SOC medium (29) at 37°C before plating. Under ideal conditions (log phase), E. coli has a doubling time of 20 min, which would result in two doublings after 60 min (29). After heat shock, though, the cells do not immediately enter log phase. However, the unlikely possibility that a few of the transformed cells may double before plating does exist. Therefore, identical TCR sequences from two different colonies (a doublet) may indicate a clonal expansion or could be the result of a singly transfected E. coli cell that doubled before plating. Therefore, two identical copies of a single β-chain TCR transcript may or may not represent a clonal expansion. The odds of a triplet (three identical transcripts) being due to this heat shock artifact are negligible.
The NPA-PCR–Vβ-specific PCR–mixed ligation–mixed transformation–mixed cloning method that we employed here has been designed to determine whether there are clonal expansions of T cells in the CSF rather than to quantitate with accuracy these clonal expansions. Equal volumes of each of the Vβ-specific PCR amplification products are mixed after purification, and before ligation, transformation, and cloning. Comparison of this method to the NPA-PCR–Vβ-specific PCR–separate ligation–separate transformation–separate cloning method (which is considerably more laborious) resulted in the identification of the same clonal expansions, which were found to be present in similar proportions (data not shown). However, two important assumptions affect the ability of PCR amplification methods to permit quantitation: (i) all amplification reactions must be in the linear phase, and (ii) the efficiencies of the amplification reactions must be equal for all primers. Therefore, the method employed here can provide a general approximation of the relative frequency of the clonally expanded TCR transcripts, and in that sense is rather a semiquantitative than a quantitative method.
Computer analysis of DNA sequences.
The nucleic acid and the deduced amino acid sequence obtained were compared to those in the GenBank/EMBL/SWISS PROT databases by using FASTA, BLAST, and PSI-BLAST software (3). There is no information on the maximum number of CDR3 amino acid differences that permit substantial CDR3 homology. Differences of two conservative amino acids and one nonconservative amino acid were chosen arbitrarily in this study as the maximum number of differences allowed between CDR3 motifs from different T-cell clones in order to define substantial CDR3 homology.
RESULTS
Hepatitis A virus transcripts were detected by RT-PCR in both serum and CSF of this patient during the first neurological episode. The PCR product obtained from the serum and the CSF were sequenced to confirm the specificity of the amplified product. Sequences obtained from the serum and the CSF were identical and belong to genotype 1a. Anti-hepatitis A virus total antibody and IgM antibody were found in the serum of this patient during the first neurological episode.
To investigate the clonality of β-chain TCR transcripts from the CSF of this patient, we amplified β-chain TCR transcripts from CSF lymphocytes by the NPA-PCR–Vβ-specific PCR method described in Materials and Methods. CSF was obtained during the second neurological episode of this patient. β-Chain TCR transcripts from only 5 (Vβ13, Vβ3, Vβ17, Vβ8, and Vβ20) out of 24 Vβ families were detected in the CSF. The amplified products were combined, cloned, and sequenced as described in Materials and Methods. Sequence analysis revealed substantial proportions of identical β-chain TCR transcripts in the CSF of this patient (Table 2), demonstrating the presence of oligoclonal populations of T cells. Seventeen of 35 transcripts (48%) were 100% identical, demonstrating a major Vβ13.3 Dβ2.1 Jβ1.3 clonal expansion (clone 1; CDR3 [CASSLEVYSGNT]). Six of 35 transcripts (17%) were also 100% identical, revealing a second Vβ13 clonal expansion (clone 2; Vβ13.1 Dβ2.1 Jβ1.2; CDR3: CASSYRGDGYT). Furthermore, clonal expansions were found within the Vβ3 family (clone 3; Vβ3.1 Dβ2.1 Jβ1.5; 5 out of 35 [14%] transcripts were identical; CDR3: LCASSSVGQPQH) and within the Vβ20 family (clone 7; Vβ20.1 Dβ1.1 Jβ2.4; 3 out of 35 [8%] transcripts were identical; CDR3: YLCAWGRSGTANIQY).
TABLE 2.
CSF from patient AMS-HA contains oligoclonal T cells (sequence analysis revealed substantial proportions of identical β-chain TCR transcripts)a
| Clone | Variable | N-Diverse-N | Joining | β-Chain transcript |
|---|---|---|---|---|
| C A S S | L E V Y | S G N T | Vβ13.3 Dβ2.1 Jβ1.3 | |
| 1 | TGTGCCAGCTCCCT | GGAAGTCTAC | TCTGGAAACACC | (17 of 35; 48%) |
| C A S S | Y R G | D G Y T | Vβ13.1 Dβ2.1 Jβ1.2 | |
| 2 | TGTGCCAGCAGTTA | CAGGGGTG | ATGGCTACACC | (6 of 35; 17%) |
| L C A S | S S V G | Q P Q H | Vβ3.1 Dβ2.1 Jβ1.5 | |
| 3 | CTCTGTGCCAGCAG | TTCGGTGGGG | CAGCCCCAGCAT | (5 of 35; 14%) |
| L C A S | S S P N T K R D R L | A Y E Q | Vβ3.1 Dβ1.1 Jβ2.7 | |
| 4 | CTCTGTGCCAGCAG | CTCACCTAATACCAAACGGGACAGATTGG | CCTACGAGCAG | (1 of 35; 3%) |
| L C A S | S I T Y L | F N Q P Q | Vβ17.1 Dβ2.1 Jβ1.5 | |
| 5 | CTCTGTGCCAGTAG | TATCACGTACTTGTT | CAATCAGCCCCAG | (2 of 35; 6%) |
| C A S S | S A | N Y G Y | Vβ8.1 Dβ2.1 Jβ1.2 | |
| 6 | TGTGCCAGCAGTTC | TGCT | AACTATGGCTAC | (1 of 35; 3%) |
| L C A W | G R S G T A | N I Q Y | Vβ20.1 Dβ1.1 Jβ2.4 | |
| 7 | CTCTGTGCCTGGGG | GAGAAGCGGGACAGCT | AACATTCAGTAC | (3 of 35; 8%) |
In contrast to these findings with CSF lymphocytes, similar analysis revealed that all Vβ TCR families were expressed in PBMCs from this patient. Sequence analysis after PCR amplification and cloning revealed that PBMC β-chain transcripts from this patient were unique when compared to each other (with the exception of clones Vβ5.1 Dβ2.1 Jβ2.7 and Vβ13.7 Dβ1.1 Jβ1.1, which appear twice in the peripheral blood but not in the CSF), suggesting polyclonal populations of T cells (Table 3). As discussed in Materials and Methods, the appearance of a transcript only in duplicate may not necessarily indicate the presence of a clonal expansion. This duplicate transcript may appear because of E. coli division during 1 h of the cloning process (described above). Similarly, sequence analysis of β-chain TCR transcripts from the CSF of a healthy subject without any neurological diseases revealed unique TCR transcripts when compared to each other, typical of polyclonal populations of T cells (data not shown).
TABLE 3.
β-Chain TCR transcripts from the peripheral blood of patient AMS-HA
 |
Comparison of the nucleic acid and deduced amino acid sequences of these β-chain TCR transcripts to those in the GenBank/EMBL/SWISS PROT databases revealed that these sequences were typical of β-chain TCR, have not been previously identified and therefore are novel.
Analysis of the CDR3 motifs of the clonally expanded sequences (Table 2) by using the gapped BLAST and PSI BLAST protein database search programs (3) revealed that clone 1 (Vβ13.3 Dβ2.1 Jβ1.3; CDR3: CASSLEVYSGNT) exhibited substantial CDR3 homology to a T-cell clone derived from connective tissue disease, reactive with small nuclear ribonucleoprotein (snRNP), AAB42075; CDR3: CASSLRRFSGNT). The two T-cell clones were different in the CDR3 only by two conservative amino acids and one nonconservative amino acid. These differences have been chosen arbitrarily in this study as the maximum number of differences allowed between CDR3 motifs from different T-cell clones in order to have substantial CDR3 homology.
Clones 2 and 3 (Table 2) exhibited substantial CDR3 homology to myelin basic protein (MBP)-reactive T-cell clones. Clone 2 (Vβ13.1 Dβ2.1 Jβ1.2; CD3: CASSYRGDGYT) was homologous to clone AAD15105 (CDR3: SSYQGMGYTL) (79). Clone 3 (Vβ3.1 Dβ2.1 Jβ1.5; CDR3: CASSSVGQPQH) was homologous to clone AAB31432 (CDR3: CASSIPGQPGHFG) (83). Clone 3 (Vβ3.1 Dβ2.1 Jβ1.5; CDR3: CASSSVGQPQH) also exhibited substantial CDR3 homology to clone AAD15172 (CDR3: CASSSTGGQPQH) of unknown specificity, identified in human thymocytes (37).
Clone 5 (Vβ17.1 Dβ2.1 Jβ1.2; CDR3: CASSITYL) exhibited CDR3 homology to human TCR clone AAB03951 (CDR3: CASSITVV) of unknown specificity, identified in the brain of a patient with chronic encephalitis of Rasmussen (42). Clone 6 (Vβ8.1 Dβ2.1 Jβ1.2; CDR3: CASSSANY) exhibited substantial CDR3 homology to three different human TCR clones of unknown specificity: (i) AAD29450 (CDR3: CASEESANY), (ii) SO3496 (CDR3: CASSQATDY) (38), and (iii) AAF24066 (CDR3: CASSPTVNY). Clone 7 (Vβ20.1 Dβ1.1 Jβ2.4; CDR3: LCAWGRSGTANIQY) exhibited substantial CDR3 homology to two different human TCR clones of unknown specificity: (i) AAC97292 (CDR3: LCAWGTSGTS) and AAC97254 (CDR3: LCAWSGTS).
DISCUSSION
We have demonstrated the presence of oligoclonal expansions of T cells in the CSF of a patient with demyelinating MS-like disease, consistent with multiphasic disseminated encephalomyelitis, which followed an initial hepatitis A virus infection. Out of 24 Vβ families of TCR examined, only 5 Vβ families were detected in the CSF of this patient after relapse. A major clonal expansion has been detected within the Vβ13 family where 17 of 35 (48%) of Vβ13 TCR transcripts were identical (Vβ13.3 Dβ2.1 Jβ1.3). Another Vβ13 TCR clonal expansion has also been detected (Vβ13.1 Dβ2.1 Jβ1.2; 6 of 35 [17%] identical transcripts). Clonal expansions were also found within the Vβ3 and Vβ20 families. In contrast, all Vβ TCR families were expressed in PBMCs from this patient and were polyclonal. Clonal expansions of T cells have been identified in the CSF of certain patients with chronic progressive MS (27, 39, 44, 81). In contrast, patients with encephalitis of Rasmussen (42), a patient with subacute sclerosing panencephalitis, a patient with herpes zoster meningoencephalitis, and a patient with atypical MS, which was fatal within 8 months, did not exhibit oligoclonal T cells in the CSF (27).
Analysis of the CDR3 motifs obtained in this study revealed that two of the clonally expanded T-cell clones (clones 2 and 3) (Table 2) exhibited substantial homology to MBP-reactive T-cell clones. These findings are in contrast with those obtained with TCR transcripts from brain plaques from a patient with acute MS, which did not exhibit any homology to CDR3 motifs specific for MBP (E. L. Oleszak, J. Pappas, C. A. Slachta, W. L. Lin, L. I. Sakkas, and C. D. Platsoucas, unpublished data). These results suggest that perhaps different T-cell responses may be involved in brain plaques and in CSF of patients with acute MS. Studies with additional patients are needed to examine this possibility. Little information is available in the literature on the homology of clonally expanded T-cell clones in the CSF and of MBP-specific T-cell clones (1, 39, 44, 81). Allegretta et al. (1) reported CDR3 homology and common Vβ usage between clonally expanded T cells from the brain and CSF of patients with MS and T cells mediating EAE.
Hepatitis A virus transcripts were identified by RT-PCR and sequencing of the VP3-VP1 junction (position 2132 to 2451) of the hepatitis A virus genome in the serum and the CSF of this patient during the first neurological episode. Anti-hepatitis A virus IgM antibody and total antibody were found in the serum of this patient during the first neurological episode. There is epidemiologic evidence supporting the diagnosis of hepatitis A virus infection. Hepatitis A virus is a common cause of food-borne disease (hepatitis) and does not manifest often as neurological syndrome (26, 41, 48). However, hepatitis A virus is a picornavirus, and these viruses are known to infect the CNS (49).
The clinical symptoms and signs, including the presence of quadriparesis, combined with the serum and CSF biochemical findings, the MRI changes, and the absence of fulminant hepatic failure, firmly rule out hepatic encephalopathy in the setting of hepatitis A virus infection. Importantly, the structural and anatomical changes seen by MRI were distinctly multifocal, albeit confluent, and were accompanied by perilesional as opposed to diffuse cytotoxic edema and/or secondary sequelae (hemorrhages and/or hypoxia), typifying hepatic encephalopathy.
Extrahepatic manifestations of hepatitis A virus infections are rare. However, meningoencephalitis, transverse myelitis, Guillain-Barré syndrome, and optic neuritis have been all described as complications of hepatitis A virus infections, quite often without clinically obvious liver diseases (6–8, 10, 11, 15, 16, 28, 33, 46, 51, 78; Hughes et al., Letter, 1993; J. L. Parajna, M. Riera, and M. J. Garcia, Letter, Med. Clin. 97:279, 1991). Hepatitis A infection has been also associated with exacerbation of MS symptoms in patients with quiescent MS (60).
There are a number of reports describing MS in children. However, the incidence of MS in children before the age of 10 is low and has been estimated as 1.5 to 7 per 1,000 (20, 47, 64). Poser examined 812 patients with MS, and only one of them had an onset before age 10 (65). Furthermore, childhood MS may present different neuropathological picture from adult MS (14, 19, 23, 25, 30, 64, 70). Poser (63) has reported large, tumor-like, inflammatory demyelinating lesions in the area of the centrum semiovale in children with MS. Similar large, tumor-like brain lesions have been described by Kepes in his study of 31 patients, including children (36). Although large, focal tumor-like lesions are rather characteristic of ADEM, such lesions have been seen also in adult patients with MS (24, 48, 84). There are instances in which the clinical, imaging, and pathological distinction between ADEM and MS in children is difficult if not impossible to ascertain (32, 36, 59, 62, 76). Absent a pathological evaluation, the case at hand is best characterized as a recurrent disseminated encephalomyelitis (involving the cerebrum, brain stem, and spinal cord) with MRI futures compatible with demyelinating-type lesions. Some of these lesions were quite large, probably the result of confluent expansion bordering the cortical mantle. The lesions were distributed extensively throughout the hemispheric white matter but had a distinctive predilection for the subcortical convolutional white matter (corticomedullary junction). That said, deep periventricular white matter hyperintense signals on T1-weighted images and focal areas of thalamic enhancement on T2-weighted images were also present (Fig. 1). This distributional pattern by anatomical imaging, in the absence of oligoclonal bands, and the apparent recovery of the patient would favor the diagnosis of either a multiphasic variant of ADEM or a transitional form between ADEM and childhood MS (32). On the other hand, a number of investigators pointed out recently that discrimination between ADEM and MS is difficult, and ADEM may very well be a part of the MS spectrum (31, 35, 69).
The presence of substantial proportions of β-chain TCR transcripts in the CSF of this patient suggests the presence of oligoclonal T cells in the CSF. These T cells have very likely expanded in the CSF in response to particular antigens, viral (hepatitis A) or host. Alternatively, it is possible that the clonaly expanded T cells identified in this study have been generated in a process known as epitope spreading (40, 71, 77), in response to CNS self-antigens, which may have been released during the first neurological episode because of viral and immunologic damage resulting in cell death. These perhaps previously sequestered CNS self-antigens may be presented now by antigen-presenting cells of the host. Because of the injury during the first neurological episode, tolerance against these self-epitopes may be broken and an immune response is generated. It is also possible that the clonally expanded T cells in the CSF may recognize as viral host determinants cross-reacting with the virus due to molecular mimicry. Molecular mimicry is defined as the sharing of common antigenic epitopes between microorganisms (bacteria, viruses, etc.) and host proteins (21, 54–57, 75), and it is responsible for the development of several autoimmune diseases. It has been reported that a hepatitis B virus polymerase peptide, which shares six consecutive amino acids with the encephalitogenic site of rabbit MBP, is able to induce EAE (21). Although functional studies with hepatitis A virus peptides have not been reported, it is important to note that VP3 structural protein of hepatitis A virus shares with MBP seven common tripeptides (5). Therefore, molecular mimicry between hepatitis A virus antigens and host proteins may be responsible for demyelinating disease.
In conclusion, it remains to be established whether the clonally expanded T cells in the CSF of a patient with hepatitis A virus infection recognize hepatitis A peptides or are specific for host antigen(s) of neuronal and/or glial origin. This case may allow us to determine how a ubiquitous virus can trigger demyelinating disease.
ACKNOWLEDGMENTS
We express our most sincere thanks to Harold S. Margolis and Omana Naiman, Hepatitis Branch, National Center for Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Ga., for evaluating our patient's serum and CSF for hepatitis A virus transcripts.
This work was supported in part by a grant from the National Multiple Sclerosis Society and grant T32 AI07101 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Allegretta M, Albertini R J, Howell M D, Smith L R, Martin R, McFarland H F, Sriram S, Brostoff S, Steinman L. Homologies between T-cell receptor junctional sequences unique to multiple sclerosis and T cells mediating experimental allergic encephalomyelitis. J Clin Investig. 1994;94:105–109. doi: 10.1172/JCI117295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen I, Brankin B. Pathogenesis of multiple sclerosis—the immune diathesis and the role of viruses. J Neuropathol Exp Neurol. 1993;52:95–105. doi: 10.1097/00005072-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvord E C, Rose L M, Richards T L. Chronic experimental allergic encephalomyelitis as a model of multiple sclerosis. In: Martenson R E, editor. Myelin—biology and chemistry. Boca Raton, Fla: CRC Press; 1992. pp. 849–891. [Google Scholar]
- 5.Aw S E. Autoimmune disease—pathogenesis through molecular mimicry at the tripeptide level. Ann Acad Med Singap. 1986;15:546–554. [PubMed] [Google Scholar]
- 6.Beeri R, Golan G, Newman D, Steiner I, Mevorach D, Brezis M. Transverse myelitis heralding hepatitis A. J Clin Gastroenterol. 1995;20:262–263. doi: 10.1097/00004836-199504000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Boehm T, Rabbitts T H. The human T-cell receptor genes are targets for chromosomal abnormalities in T-cell tumors. FASEB J. 1989;3:2344–2359. doi: 10.1096/fasebj.3.12.2676678. [DOI] [PubMed] [Google Scholar]
- 8.Bosch V V, Dowling P C, Cook S D. Hepatitis A virus immunoglobulin M antibody in acute neurological disease. Ann Neurol. 1983;14:685–687. doi: 10.1002/ana.410140613. [DOI] [PubMed] [Google Scholar]
- 9.Bower W A, Nainan O V, Han X, Margolis H. Duration of viremia in hepatitis A virus infection. J Infect Dis. 2000;182:12–17. doi: 10.1086/315701. [DOI] [PubMed] [Google Scholar]
- 10.Breningstall G N, Belani K K. Acute transverse myelitis and brainstem encephalitis associated with hepatitis A infection. Pediatr Neurol. 1995;12:169–171. doi: 10.1016/0887-8994(94)00153-s. [DOI] [PubMed] [Google Scholar]
- 11.Bromberg K, Newhall D N, Peter G. Hepatitis A and meningoencephalitis. JAMA. 1982;247:815. [PubMed] [Google Scholar]
- 12.Chen P F, Freedman R S, Chernajovsky Y, Platsoucas C D. Amplification of immunoglobulin transcripts by the non-palindromic adaptor polymerase chain reaction (NPA-PCR). Nucleotide sequence analysis of two human monoclonal antibodies recognizing two cell surface antigens expressed in ovarian, cervix, breast, colon and other carcinomas. Hum Antib Hybrid. 1994;5:131–142. [PubMed] [Google Scholar]
- 13.Chen P F, Platsoucas C D. Development of the non-palindromic adaptor polymerase chain reaction (NPA-PCR) for the amplification of alpha- and beta-chain T-cell receptor cDNAs. Scand J Immunol. 1992;35:539–549. doi: 10.1111/j.1365-3083.1992.tb03253.x. [DOI] [PubMed] [Google Scholar]
- 14.Cole G F, Auchterlonie L A, Best P V. Very early onset multiple sclerosis. Dev Med Child Neurol. 1995;37:667–672. doi: 10.1111/j.1469-8749.1995.tb15011.x. [DOI] [PubMed] [Google Scholar]
- 15.Davis L E, Brown J E, Robertson B H, Khanna B, Polish L B. Hepatitis A post-viral encephalitis. Acta Neurol Scand. 1993;87:67–69. doi: 10.1111/j.1600-0404.1993.tb04078.x. [DOI] [PubMed] [Google Scholar]
- 16.Debray D. Serious hepatitis A in a child. Arch Pediatr. 1999;6:183s–185s. doi: 10.1016/s0929-693x(99)80406-0. [DOI] [PubMed] [Google Scholar]
- 17.de Rosbo N K, Ben-Nun A. T-cell responses to myelin antigens in multiple sclerosis: relevance of the predominant autoimmune reactivity to myelin oligodendrocyte glycoprotein. J Autoimmun. 1998;11:287–299. doi: 10.1006/jaut.1998.0202. [DOI] [PubMed] [Google Scholar]
- 18.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the Vax. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebner F, Millner M M, Justich E. Multiple sclerosis in children: value of serial MR studies to monitor patients. Am J Neuroradiol. 1990;11:1023–1027. [PMC free article] [PubMed] [Google Scholar]
- 20.Eraksoy M. Multiple sclerosis in children: a review. In: Siva A, Kesselring J, Thompson A, editors. Frontiers in multiple sclerosis. Malden, Mass: Blackwell Science; 1999. pp. 67–73. [Google Scholar]
- 21.Fujinami R S, Oldstone M B. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science. 1985;230:1043–1045. doi: 10.1126/science.2414848. [DOI] [PubMed] [Google Scholar]
- 22.Genevee C, Diu A, Nierat J, Caignard A, Dietrich P Y, Ferradini L, Roman-Roman S, Triebel F, Hercend T. An experimentally validated panel of subfamily-specific oligonucleotide primers (V alpha 1-w29/V beta 1-w24) for the study of human T cell receptor variable V gene segment usage by polymerase chain reaction. Eur J Immunol. 1992;22:1261–1269. doi: 10.1002/eji.1830220522. [DOI] [PubMed] [Google Scholar]
- 23.Ghezzi A, Deplano V, Faroni J, Grasso M G, Liguori M, Marrosu G, Pozzilli C, Simone I L, Zaffaroni M. Multiple sclerosis in childhood: clinical features of 149 cases. Mult Scler. 1997;3:43–46. doi: 10.1177/135245859700300105. [DOI] [PubMed] [Google Scholar]
- 24.Giang D W, Poduri K R, Eskin T A, Ketonen L M, Friedman P A, Wang D D, Herndon R M. Multiple sclerosis masquerading as a mass lesion. Neuroradiology. 1992;34:150–154. doi: 10.1007/BF00588163. [DOI] [PubMed] [Google Scholar]
- 25.Guilhoto L M, Osorio C A, Machado L R, de Castro C P, Manreza M L, Callegaro D, Kok F, Diament A. Pediatric multiple sclerosis report of 14 cases. Brain Dev. 1995;17:9–12. doi: 10.1016/0387-7604(94)00091-b. [DOI] [PubMed] [Google Scholar]
- 26.Gust I D. Epidemiological patterns of hepatitis A in different parts of the world. Vaccine. 1992;10:S56–S58. doi: 10.1016/0264-410x(92)90544-t. [DOI] [PubMed] [Google Scholar]
- 27.Hafler D A, Duby A D, Lee S J, Benjamin D, Seidman J G, Weiner H L. Oligoclonal T lymphocytes in the cerebrospinal fluid of patients with multiple sclerosis. J Exp Med. 1988;167:1313–1322. doi: 10.1084/jem.167.4.1313. . (Erratum, 168:459.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammond G W, MacDougall B K, Plummer F, Sekla L H. Encephalitis during the prodromal stage of acute hepatitis A. Can Med Assoc J. 1982;126:269–270. [PMC free article] [PubMed] [Google Scholar]
- 29.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 30.Hanefeld F, Bauer H J, Christen H J, Kruse B, Bruhn H, Frahm J. Multiple sclerosis in childhood: report of 15 cases. Brain Dev. 1991;13:410–416. doi: 10.1016/s0387-7604(12)80038-6. [DOI] [PubMed] [Google Scholar]
- 31.Hartung H P, Grossman R I. ADEM. Distinct disease or part of the MS spectrum? Neurology. 2001;56:1297. doi: 10.1212/wnl.56.10.1257. [DOI] [PubMed] [Google Scholar]
- 32.Hickey W F. The pathology of multiple sclerosis: a historical perspective. J Neuroimmunol. 1999;98:37–44. doi: 10.1016/s0165-5728(99)00079-x. [DOI] [PubMed] [Google Scholar]
- 33.Hodges J R. Hepatitis A and meningo-encephalitis. J Neurol. 1987;234:364. doi: 10.1007/BF00314299. [DOI] [PubMed] [Google Scholar]
- 34.Hutin Y J F, Pool V, Cramer E H, Nainan O V, Weth J, Williams I T, Goldstein S T, Gensheimer K F, Bell B P, Shapiro C N, Alter M J, Margolis H S. A multistate, foodborne outbreak of hepatitis A. N Engl J Med. 1999;340:595–602. doi: 10.1056/NEJM199902253400802. [DOI] [PubMed] [Google Scholar]
- 35.Hynson J L, Kornberg A J, Coleman L T, Shield L, Harvey A S, Kean M J. Clinical and neuroradiologic features of acute disseminated encephalomyelitis in children. Neurology. 2001;56:1308–1312. doi: 10.1212/wnl.56.10.1308. [DOI] [PubMed] [Google Scholar]
- 36.Kepes J J. Large focal tumor-like demyelinating lesions of the brain: intermediate entity between multiple sclerosis and acute disseminated encephalomyelitis? A study of 31 patients. Ann Neurol. 1993;33:18–27. doi: 10.1002/ana.410330105. [DOI] [PubMed] [Google Scholar]
- 37.Kimura N, Toyonaga B, Yoshikai Y, Ou R F, Mak T W. Sequences and repertoire of the human T-cell receptor alpha and beta chain variable region genes in thymocytes. Eur J Immunol. 1987;17:375–383. doi: 10.1002/eji.1830170312. [DOI] [PubMed] [Google Scholar]
- 38.Kimura N, Toyonaga B, Yoshikai Y, Triebel F, Debre P, Minden M D, Man T W. Sequences and diversity of human T cell receptor beta chain variable region genes. J Exp Med. 1986;164:739–750. doi: 10.1084/jem.164.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee S J, Wucherpfennig K W, Brod S A, Benjamin D, Weiner H L, Hafler D A. Common T-cell receptor V beta usage in oligoclonal T lymphocytes derived from cerebrospinal fluid and blood of patients with multiple sclerosis. Ann Neurol. 1991;29:33–40. doi: 10.1002/ana.410290109. [DOI] [PubMed] [Google Scholar]
- 40.Lehmann P V, Forsthuber T, Miller A, Sercarz E E. Spreading of T cell autoimmunity to cryptic determinants of autoantigen. Nature. 1992;358:155. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 41.Lemon S M. Type A viral hepatitis: epidemiology, diagnosis, and prevention. Clin Chem. 1997;43:1494–1499. [PubMed] [Google Scholar]
- 42.Li Y, Uccelli A, Laxer K D, Jeong M C, Vinters H V, Tourtellotte W W, Hauser S L, Oksenberg J R. Local-clonal expansion of infiltrating T lymphocytes in chronic encephalitis of Rasmussen. J Immunol. 1997;158:1428–1437. [PubMed] [Google Scholar]
- 43.Lin W L, Kuzmak J, Pappas J, Peng G, Chernajovsky Y, Platsoucas C D, Oleszak E L. Amplification of T-cell receptor alpha- and beta-chain transcripts from mouse spleen lymphocytes by the nonpalindromic adaptor-polymerase chain reaction. Hematopathol Mol Hematol. 1998;11:73–88. [PubMed] [Google Scholar]
- 44.Lozeron P, Chabas D, Duprey B, Lyon-Caen O, Liblau R. T-cell receptor Vbeta5 and Vbeta17 clonal diversity in cerebrospinal fluid and peripheral blood lymphocytes of multiple sclerosis patients. Mult Scler. 1998;4:154–161. doi: 10.1177/135245859800400313. [DOI] [PubMed] [Google Scholar]
- 45.Martin R, McFarland H F. Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis. Crit Rev Clin Lab Sci. 1995;32:121–182. doi: 10.3109/10408369509084683. [DOI] [PubMed] [Google Scholar]
- 46.Matsushima K, Niwa K, Fujita H, Yamamoto M, Shinohara Y. Acute hepatitis A (HA) presenting findings of meningoencephalitis. Rinsho Shinkeigaku. 1992;32:441–443. [PubMed] [Google Scholar]
- 47.Matthews W. Clinical aspects. In: McAlpine D, Matthews W B, editors. McAlpine's multiple sclerosis. 2nd ed. New York, N.Y: Churchill Livingstone; 1991. pp. 41–51. [Google Scholar]
- 48.Melnick J. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 655–712. [Google Scholar]
- 49.Melnick J L. History and epidemiology of hepatitis A virus. J Infect Dis. 1995;171(Suppl. 1):S2–S8. doi: 10.1093/infdis/171.supplement_1.s2. [DOI] [PubMed] [Google Scholar]
- 50.Musette P, Bequet D, Delarbre C, Gachelin G, Kourilsky P, Dormont D. Expansion of a recurrent V beta 5.3+ T-cell population in newly diagnosed and untreated HLA-DR2 multiple sclerosis patients. Proc Natl Acad Sci USA. 1996;93:12461–12466. doi: 10.1073/pnas.93.22.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishimura M, Shigemoto R, Matsubayashi K, Mimori Y, Kameyama M. Meningoencephalitis during the pre-icteric phase of hepatitis A—a case report. Rinsho Shinkeigaku. 1987;27:1441–1444. [PubMed] [Google Scholar]
- 52.Oksenberg J R, Panzara M A, Begovich A B, Mitchell D, Erlich H A, Murray R S, Shimonkevitz R, Sherritt M, Rothbard J, Bernard C C, et al. Selection for T-cell receptor V beta-D beta-J beta gene rearrangements with specificity for a myelin basic protein peptide in brain lesions of multiple sclerosis. Nature. 1993;362:68–70. doi: 10.1038/362068a0. [DOI] [PubMed] [Google Scholar]
- 53.Oksenberg J R, Stuart S, Begovich A B, Bell R B, Erlich H A, Steinman L, Bernard C C. Limited heterogeneity of rearranged T-cell receptor V alpha transcripts in brains of multiple sclerosis patients. Nature. 1990;345:344–346. doi: 10.1038/345344a0. . (Erratum, 353:94, 1991.) [DOI] [PubMed] [Google Scholar]
- 54.Oldstone M B. Virus-induced autoimmunity: molecular mimicry as a route to autoimmune disease. J Autoimmun. 1989;2(Suppl.):187–194. doi: 10.1016/0896-8411(89)90130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oleszak E L, Perlman S, Leibowitz J L. MHV S peplomer protein expressed by a recombinant vaccinia virus vector exhibits IgGFc-receptor activity. Virology. 1992;186:122–132. doi: 10.1016/0042-6822(92)90066-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oleszak E L. Molecular mimicry between Fc receptors and viral antigens. Arch Immunol Ther Exp. 1994;42:83–88. [PubMed] [Google Scholar]
- 57.Oleszak E L, Kuzmak J, Hogue B, Parr R, Collisson E W, Rodkey L S, Leibowitz J L. Molecular mimicry between Fc receptor and S peplomer protein of mouse hepatitis virus, bovine corona virus, and transmissible gastroenteritis virus. Hybridoma. 1995;14:1–8. doi: 10.1089/hyb.1995.14.1. [DOI] [PubMed] [Google Scholar]
- 58.Oleszak E L, Katsetos C D, Kuzmak J, Varadhachary A. Inducible nitric oxide synthase in Theiler's murine encephalomyelitis virus infection. J Virol. 1997;71:3228–3235. doi: 10.1128/jvi.71.4.3228-3235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Orrell R W. Grand rounds—Hammersmith Hospitals. Distinguishing acute disseminated encephalomyelitis from multiple sclerosis. Br Med J. 1996;313:802–804. doi: 10.1136/bmj.313.7060.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Owen R L, Dau P C, Johnson K P, Spitler L E. Immunologic mechanisms in multiple sclerosis. Exacerbation by type A hepatitis and skin test antigens. JAMA. 1980;244:2307–2309. doi: 10.1001/jama.244.20.2307. [DOI] [PubMed] [Google Scholar]
- 61.Platsoucas C, Chen P, Oleszak E. Analysis of T-cell receptor and immunoglobulin transcripts by the nonpalindromic adaptor polymerase chain reaction. Methods Mol Genet. 1994;1994:65–90. [Google Scholar]
- 62.Polman C. Monophasic isolated inflammatory demyelinating syndromes. In: Siva A, Kesselring J, Thompson A, editors. Frontiers in multiple sclerosis. Malden, Mass: Blackwell Science; 1999. pp. 15–18. [Google Scholar]
- 63.Poser C. Myelinoclastic diffuse sclerosis. In: Vinken P J, Bruyn G W, Klawans H L, editors. Handbook of clinical neurology. New York, N.Y: Elsevier Science Publishers; 1985. pp. 419–428. [Google Scholar]
- 64.Poser C M, Paty D W, Scheinberg L, McDonald W I, Davis F A, Ebers G C, Johnson K P, Sibley W A, Silberberg D H, Tourtellotte W W. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 65.Poser S. Multiple sclerosis: an analysis of 812 cases by means of electronic data processing. Berlin, Germany: Springer-Verlag; 1978. [PubMed] [Google Scholar]
- 66.Prineas J. The neuropathology of multiple sclerosis. In: Vinken P J, Bruyn G W, Klawans H L, editors. Handbook of clinical neurology. New York, N.Y: Elsevier Science Publishers; 1985. pp. 213–257. [Google Scholar]
- 67.Raine C. Demyelinating diseases. In: Davis R L, Robertson D M, editors. Textbook of neuropathology. 3rd ed. Baltimore, Md: Williams & Wilkins; 1997. pp. 627–714. [Google Scholar]
- 68.Sadovnick A D, Ebers G C. Epidemiology of multiple sclerosis: a critical overview. Can J Neurol Sci. 1993;20:17–29. doi: 10.1017/s0317167100047351. [DOI] [PubMed] [Google Scholar]
- 69.Schwarz S, Mohr A, Knauth M, Wildemann B, Storch-Hagenlocher B. Acute disseminated encephalomyelitis. A follow-up study of 40 adult patients. Neurology. 2001;56:1313–1318. doi: 10.1212/wnl.56.10.1313. [DOI] [PubMed] [Google Scholar]
- 70.Selcen D, Anlar B, Renda Y. Multiple sclerosis in childhood: report of 16 cases. Eur Neurol. 1996;36:79–84. doi: 10.1159/000117213. [DOI] [PubMed] [Google Scholar]
- 71.Sercarz E E, Lehmann P V, Ametani A, Benichou G, Miller A, Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annu Rev Immunol. 1993;11:729. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- 72.Shimonkevitz R, Murray R, Kotzin B. Characterization of T-cell receptor V beta usage in the brain of a subject with multiple sclerosis. Ann N Y Acad Sci. 1995;756:305–306. doi: 10.1111/j.1749-6632.1995.tb44527.x. [DOI] [PubMed] [Google Scholar]
- 73.Sobel R A. The pathology of multiple sclerosis. Neurol Clin. 1995;13:1–21. [PubMed] [Google Scholar]
- 74.Sommer N, Martin R. The role of T cells and cytokines in the pathogensis of multiple sclerosis. In: Thompson A, Polman C, Hohlfeld R, editors. Multiple sclerosis: clinical challenges and controversies. London, United Kingdom: Martin Dunitz; 1997. pp. 87–108. [Google Scholar]
- 75.Srinivasappa J, Saegusa J, Prabhakar B S, Gentry M K, Buchmeier M J, Wiktor T J, Koprowski H, Oldstone M B A, Notkins A L. Molecular mimicry: frequency of reactivity of monoclonal antiviral antibodies with normal tissues. J Virol. 1986;57:397–401. doi: 10.1128/jvi.57.1.397-401.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tselis A, Lisak R. Acute disseminated encephalomyelitis. In: Antel J P, Birnbaum G, Hartung H-P, editors. Clinical neuroimmunology. Malden, Mass: Blackwell Science; 1998. pp. 116–147. [Google Scholar]
- 77.Tuohy V K, Yu M, Yin L, Kawczak J A, Johnson J M, Mathisen P M, Weinstock-Guttman B, Kinkel R P. The epitope spreading cascade during progression of experimental autoimmune encephalomyelitis and multiple sclerosis. Immunol Rev. 1998;164:93–100. doi: 10.1111/j.1600-065x.1998.tb01211.x. [DOI] [PubMed] [Google Scholar]
- 78.van de Geijn E J, Tukkie R, van Philips L A, Punt H. Bilateral optic neuritis with branch retinal artery occlusion associated with vaccination. Doc Ophthalmol. 1994;86:403–408. doi: 10.1007/BF01204599. [DOI] [PubMed] [Google Scholar]
- 79.Vandevyver C, Mertens N, van den Elsen P, Medaer R, Raus J, Zhang J. Clonal expansion of myelin basic protein-reactive T cells in patients with multiple sclerosis: restricted T cell receptor V gene rearrangements and CDR3 sequence. Eur J Immunol. 1995;25:958–968. doi: 10.1002/eji.1830250416. [DOI] [PubMed] [Google Scholar]
- 80.Waksman B H, Reynolds W E. Multiple sclerosis as a disease of immune regulation. Proc Soc Exp Biol Med. 1984;175:282–294. doi: 10.3181/00379727-175-41798. [DOI] [PubMed] [Google Scholar]
- 81.Wilson D B, Golding A B, Smith R A, Dafashy T, Nelson J, Smith L, Carlo D J, Brostoff S W, Gold D P. Results of a phase I clinical trial of a T-cell receptor peptide vaccine in patients with multiple sclerosis. I. Analysis of T-cell receptor utilization in CSF cell populations. J Neuroimmunol. 1997;76:15–28. doi: 10.1016/s0165-5728(97)00028-3. [DOI] [PubMed] [Google Scholar]
- 82.Wucherpfennig K W, Strominger J L. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wucherpfennig K W, Zhang J, Witek C, Matsui M, Modabber Y, Ota K, Hafler D A. Clonal expansion and persistence of human T cells specific for an immunodominant MBP peptide. J Immunol. 1994;152:5581–5592. [PubMed] [Google Scholar]
- 84.Youl B D, Kermode A G, Thompson A J, Revesz T, Scaravilli F, Barnard R O, Kirkham F J, Kendall B E, Kingsley D, Moseley I F, et al. Destructive lesions in demyelinating disease. J Neurol Neurosurg Psychiatry. 1991;54:288–292. doi: 10.1136/jnnp.54.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]