Abstract
Introduction
The efficacy of molnupiravir (MLN) on Omicron sublineages is limited. We investigated the effectiveness of MLN in older adults diagnosed with Omicron BA.2.
Methods
Data of elderly COVID-19 patients (over 60 years) admitted to Chinghai Hospital (Shanghai, China) from 26 March to 31 May 2022 were reviewed. Study outcomes were a composite of undetectable viral load (VL) and disease progression [all-cause mortality, initiation of oxygen supply through high-flow device or invasive mechanical ventilation (IMV), or intensive care unit (ICU) admission] and their individual outcomes.
Results
A total of 42 elderly patients were enrolled: 26 of them received MLN, 17 (40.5%) were males, the median age was 84 years, and 12 were fully vaccinated (31.0%). Among these elderly COVID-19 patients, five (11.90%) experienced obvious dyspnea or were transferred to ICU [three MLN users (11.5%) versus two non-MLN users (12.5%)]. Compared with no MLN use, MLN use was associated with rapid undetectable VL. At day 10, MLN users achieved significantly greater undetectable VL than non-MLN users. Adjusted analysis showed that elderly patients who received MLN were 7.584 times more likely to achieve undetectable VL at day 10 than non-MLN users. Overall, elderly patients experienced a median hospital stay of 13 days. Compared with patients receiving standard care (SC), the median hospital stay of MLN users was reduced by 2.5 days.
Conclusion
Early initiation of MLN in elderly COVID-19 was associated with fast undetectable VL and short hospital stay.
Keywords: Antimicrobial, Antiviral, Covid-19, Elderly patient, Exacerbation, Molnupiravir, SARS-CoV-2
Key Summary Points
| Why carry out the study? |
| As the probability of serious COVID-19 disease is higher in people aged ≥ 60 years, it is urgent to carry out research on the antiviral drugs for elderly patients with COVID-19. |
| The efficacy and safety of molnupiravir in a real-world setting against the Omicron variant is still lacking, especially for hospitalized elderly patients. |
| What was learned from the study? |
| We conducted a real-world, single-center, retrospective, observational study to evaluate if molnupiravir would be safe and decrease the time to undetectable viral load (VL) in nasopharyngeal swabs, as well as improve disease progression. |
| We found that early initiation of molnupiravir within 5 days of a first positive test or symptom onset in elderly COVID-19 patients was associated with fast viral clearance. |
| The efficacy of molnupiravir in fully vaccinated elderly patients requires further evaluation. |
Introduction
Coronavirus disease 2019 (COVID-19), triggered by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has devastating impacts on human health and economic development. By 3 June 2022, the number of confirmed COVID-19 cases reported to the World Health Organization (WHO) had reached 528,816,317, including 6,294,969 deaths (https://covid19.who.int). COVID-19 manifests from fully asymptomatic to severe disease and death, and the risk for severe illness increases with age [1]. During the COVID-19 pandemic wave in Shanghai from 26 February to 25 April 2022, 190 deaths were recorded, with an average age of 82.52 years, among which 180 were over 60 years (accounting for 94.7%). During the 2022 COVID-19 outbreak in Hong Kong, 95.9% of deaths occurred in people ≥ 60 years old [2].
The Omicron variant (B.1.1.529) and its sublineages BA.1 and BA.2 have become the predominant variant across the globe. This variant has caused an increase in vaccine breakthrough infections and widespread escape from existing neutralizing antibodies due to high mutations in its spike protein, which is the target of most COVID-19 vaccines and therapeutic antibodies [3–8]. Previously published in vitro data have shown that antiviral agents such as remdesivir, molnupiravir (MLN), or nirmatralvir/ritonavir, which target the highly conserved protein of SARS-CoV-2 [RNA-dependent RNA polymerase (RdRp) or the conserved viral main protease], consistently retains in vitro data activity against the BA.1 and BA.2 sublineages [9–11]. However, in vivo data evaluation of the efficacy of these agents against the new variant is limited. Reports focusing on MLN activity against the currently circulating Omicron variant have primarily used data from the Hong Kong pandemic between January and May 2022. They found that MLN use is associated with a significantly low risk of all-cause mortality compared with non use, whereas hospitalizations are comparable to controls [8, 11, 12]. Data on the efficacy of MLN on hospitalized COVID-19 elderly adults infected with the Omicron variant are still lacking.
The present real-world study focused on evaluating the clinical and virological outcomes associated with MLN use in elderly COVID-19 patients during a community epidemic dominated by the Omicron BA.2 variant in Shanghai, China.
Methods
Study Design, Participants, and Data Collection
This single-center, retrospective, observational study was approved by the local ethical committee (CHEC2022-111). This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Written consent was not required because anonymized data were used and because of the extraordinary nature of the COVID-19 pandemic.
Elderly hospitalized COVID-19 patients with Omicron BA.2 diagnosis were considered for this study if they were admitted to Changhai Hospital (Shanghai, China) between 26 March 2022 and 31 May 2022. There were no other lineages detected in our hospital population during this period. Eligible patients were required to be over 60 years old with SARS-CoV-2 infection confirmed by real-time PCR. The exclusion criteria were as follows: (1) patient was diagnosed as COVID-19 recurrence by PCR test, (2) patient had ever received convalescent plasma for COVID-19 and monoclonal antibodies to SARS-CoV-2 prior to MLN treatment or before admission, (3) patient had ever taken antiviral drugs against SARS-CoV-2 other than MLN, (4) patient was enrolled in other investigational drugs for COVID-19 treatment, and (5) hospital electronic information system data were missing.
Clinical electronic medical records were reviewed for all patients who tested positive for SARS-CoV-2 RNA. Basic demographic and clinical data were collected, including age, sex, weight, height, oxygen saturation (with or without oxygen supply), medical history and basic medications, vital signs (temperature, pulse, respiratory rate, and blood pressure), and symptom onset (fever, fatigue, cough, sputum, dyspnea, sore throat, or diarrhea). We also determined the interval between symptom onset and first positive SARS-CoV-2 RNA test (nasopharyngeal swab), as well as the treatment strategy (antiviral agent, standard care, glucocorticoid, anticoagulation therapy, and tocilizumab) and clinical outcome [recovery, initiative of oxygen supply through high-flow device, invasive mechanical ventilation (IMV), or registered death]. Laboratory (blood, biochemical, inflammation, and coagulation) tests and pulmonary computed tomography (CT) scans were conducted. Pulmonary CT showed ground-glass opacity, consolidation, lymphadenopathy, and pleural effusion, which were confirmed to be due to SARS-CoV-2 infection. Results of SARS-CoV-2 RNA RT-PCR test (nasopharyngeal swab) for monitoring viral-replication level were collected. These data were stored independently and limited to this analysis.
Treatment Exposure and Follow-Up Period
MLN has only been available in our center since 20 April 2022. Prior to this, all admitted patients received only standard care (SC) recommended by the Chinese COVID-19 prevention and treatment program, including nonsteroidal antiinflammatory drugs, paracetamol (if body temperature ≥ 38 °C), and cough mixture (licorice mixture, a traditional Chinese medicine). Subsequently, all the elderly patients were prescribed with MLN except one patient who had a definite history of food and multiple drug allergies. MLN therapy was initiated within 5 days of symptom onset or first positive nucleic acid test, whichever was earlier. Patients receiving MLN (Lot number 0,000,033,094, provided by National Engineering Research Center for the Emergency Drug, Beijing, China) 800 mg twice daily for 5 days were defined as MLN users. Patients with only SC during the observation period were defined as SC users.
All patients were hospitalized for precaution, and were observed from hospital admission date until registered death, the occurrence of outcome events, or the end of the observation period (30 days from admission date), whichever came first.
Outcomes
Clinical outcomes were a composite of viral shedding in nasopharyngeal swabs and disease progression [all-cause mortality, initiation of oxygen supply through high-flow device or IMV, or intensive care unit (ICU) admission] and the individual outcomes. SARS-CoV-2 viral load (VL) in nasopharyngeal swabs on alternate days since hospital admission was quantified by a commercially available RT-PCR test [Novel Coronavirus (2019-nCoV) Real Time RT-PCR Kit, cat. no. RR-0479–02, Liferiver Bio-Tech(China) Corp. Shanghai]. PCR-positive throat swab samples from the patients were used to isolate SARS-CoV-2 virus via Vero-E6 cell. The virus isolation was sequenced and submitted to GenBank (ON965380; ON965371; ON965362; ON965361). A cycle threshold (Ct) value ≥ 35 for both ORF1ab and N genes was considered as negative. Two consecutive negative tests at least 24 h apart were defined as a negative nucleic acid test or undetectable VL. The undetectable VL time was defined as the first positive nucleic acid test date to the first negative test date (two consecutive times).
Statistical Analysis
The VL test result was expressed as Log2 (Ct value). Continuous data are expressed as mean ± standard deviation(SD) or median [interquartile range(IQR)], and the Mann–Whitney U test was used for univariate comparisons between two groups. Categorical variables were expressed as number (percentage) and tested by chi-square or Fisher’s exact tests. Cumulative incidences of virological outcomes were estimated and plotted by the Kaplan–Meier method with right censoring. The logistic regression model was used to identify predictors of early viral shedding. P-value < 0.05 was considered statistically significant. All analyses were performed using SPSS software (version 21.0.0; Chicago, IL, USA). The graph was made with GraphPad Prism 9 (San Diego, CA, USA).
Results
Patients’ Basic Characteristics
A total of 42 elderly COVID-19 patients were enrolled into this study: 26 of them were MLN users, 17 were male, the median age was 84 (69.5–91.25) years, and they had a median body mass index (BMI) of 24.32 kg/m2. A high proportion of these elderly patients had preexisting medical comorbidities, of which the top three were hypertension (71.4%), diabetes (19.0%), and coronary heart disease (26.2%). About 35.7% of these patients had multiple underlying medical conditions, and 40.5% has an age-adjusted Charlson Index over 6. Vaccination rates were relatively low (31.0%), especially in patients over 80 years old (9.1%) (Table 1).
Table 1.
Demographic and baseline clinical characteristics
| Characteristics | Therapy strategy categories | |||
|---|---|---|---|---|
| Total (n = 42) | Molnupiravir (n = 26) | SC (n = 16) | P-value | |
| Age, median (IQR), years | 84 (69.5,91.25) | 84.0 (71.0, 92.0) | 84.0 (66.0,88.0) | 0.476 |
| Male, n (%) | 17 (40.5) | 12 (46.2) | 5 (31.3) | 0.339 |
| Overweight, n (%) | 23 (54.8) | 17 (65.4) | 6 (37.5) | 0.078 |
| Medical history, n (%) | ||||
| Hypertension | 30 (71.4) | 20 (76.9) | 10 (62.5) | 0.514 |
| Diabetes | 8 (19.0) | 6 (23.1) | 2 (12.5) | 0.658 |
| Coronary heart disease | 11 (26.2) | 7 (26.9) | 4 (25) | 1.000 |
| COPD | 4 (9.5) | 3 (11.5) | 1 (6.3) | 0.979 |
| CKD | 5 (11.9) | 2 (7.7) | 3 (18.8) | 0.559 |
| Cancer | 1 (2.38) | 0 (0) | 1 (6.25) | – |
| Alzheimer’s disease | 4 (7.1) | 3 (11.5) | 1 (6.3) | 0.979 |
| Liver cirrhosis | 3 (7.1) | 1 (3.8) | 2 (12.5) | 0.659 |
| > 1 comorbidities | 15 (35.7) | 7 (26.9) | 8 (50.0) | 0.130 |
| Charlson Index (≥ 6) | 17 (40.5) | 11 (42.3) | 6 (37.5) | 0.758 |
| Vaccination status, n (%) | ||||
| Unvaccinated | 29 (69.0) | 16 (61.5) | 13 (81.3) | 0.318 |
| aFully vaccinated | 12 (31.0) | 9 (34.6) | 3 (18.8) | 0.451 |
| Initial SARS-CoV-2 RNA level (Log2 Ct value) | ||||
| ORF1ab, mean ± SD | 4.16 ± 0.27 | 4.14 ± 0.29 | 4.18 ± 0.25 | 0.621 |
| N, mean ± SD | 4.06 ± 0.24 | 4.01 ± 0.25 | 4.16 ± 0.19 | 0.053 |
| Administration time since the first positive test | ||||
| Median (IQR), days | 2 (2–4.0) | 2 (1–3.25) | 2.5 (2.0–6.75) | 0.019 |
| ≤ 5 days, n (%) | 37 (89.0) | 23 (88.5) | 14 (87.5) | 0.271 |
SC standard care, SD standard deviation, IQR interquartile range, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, COPD chronic obstructive pulmonary disease, CKD chronic kidney disease
P-value refers to the comparison between molnupiravir and SC group
P < 0.05 means significant difference
aFully vaccinated patients were defined as those with at least two doses of Comirnaty or three doses of CoronaVac
The median administration time since the first RNA test positive or symptom onset was 2 days, and the initial VL at admission was high with a low mean Ct value (ORF1ab 4.16 Log2 and N 4.06 Log2). The majority (88.5%) of MLN users were treated within 5 days of the first positive test for SARS-CoV-2, with a median of 2 days. All MLN users finished the intended 5-day therapy. No adverse events were documented for patients receiving MLN. Moreover, all patients received symptom-remission treatment according to clinical manifestations and laboratory examinations, including nonsteroidal antiinflammatory drugs and cough mixtures.
The patients were stratified into two groups according to whether they had been prescribed MLN. Good comparability was found between the two groups in terms of demographic variables, including age, sex, and BMI, as well as medical history, including vaccination history (Table 1).
Clinical Characteristics of Elderly COVID-19 Patients with Omicron BA.2 Infection
A total of five (11.90%) patients experienced in-hospital disease exacerbation, manifested as pronounced dyspnea and oxygen saturation on room air below 93%, of whom three patients received MLN. Four of these five patients required oxygen through a high-flow oxygen device. One required IMV and was admitted to the ICU due to COVID-19. No difference in the disease-progression rate was found between MLN users and non-users (11.5% versus 12.5%; P = 1.00) (Table 2).
Table 2.
Clinical features and outcomes of patients by antiviral strategy
| Variables | Therapy strategy categories | |||
|---|---|---|---|---|
| Total (N = 42) | MLN (N = 26) | SC (N = 16) | P-value | |
| Clinical type, n (%) | ||||
| Mild | 20 (47.6) | 12 (46.2) | 8 (50.0) | 0.808 |
| Moderate | 17 (40.5) | 11 (42.3) | 6 (37.5) | 0.758 |
| Severe/critical | 5 (11.9) | 3 (11.5) | 2 (12.5) | 1.000 |
| Symptoms, n (%) | ||||
| Fever | 23 (54.8) | 17 (65.4) | 6 (37.5) | 0.078 |
| Cough | 12 (28.6) | 5 (19.2) | 7 (43.8) | 0.175 |
| Sore throat | 11 (26.2) | 7 (26.9) | 4 (25%) | 1.000 |
| Sputum production | 21 (50.0) | 16 (61.5) | 5 (31.3) | 0.057 |
| Dyspnea | 5 (11.9) | 4 (14.8) | 1 (6.7) | 0.776 |
| Fatigue | 14 (33.3) | 11 (42.3) | 3 (18.8) | 0.116 |
| Others | 17 (40.5) | 13 (50.0) | 4 (25.0) | 0.109 |
| > 1 symptom | 36 (85.71) | 25 (96.17) | 11 (68.75) | 0.044 |
| In-hospital disease exacerbation, n (%) | ||||
| In-hospital death | 0 (0) | 0 (0) | 0 (0) | – |
| Oxygen through high-flow device or IMV | 4 (9.52) | 2 (7.69) | 2 (12.5) | 1.000 |
| ICU transfer | 1 (2.38) | 1 (3.85) | 0 (0) | 1.000 |
| Virological outcomes | ||||
| Viral shedding time (days), median (IQR) | 13 (9–15) | 11 (8–14.5) | 14 (12.25–16.5) | 0.101 |
| D5 undetectable VL, n (%) | 3 (7.1) | 3 (11.5) | 0 (0) | 0.428 |
| D7 undetectable VL, n (%) | 7 (16.7) | 6 (23.1) | 1 (6.3) | 0.320 |
| D10 undetectable VL, n (%) | 14 (33.3) | 12 (46.2) | 2 (12.5) | 0.025 |
| D14 undetectable VL, n (%) | 29 (69) | 19 (73.1) | 10 (62.5) | 0.428 |
| D21 undetectable VL, n (%) | 39 (92.9) | 24 (92.3) | 15 (93.8) | 1.000 |
| Clinical outcomes | ||||
| Hospital stay (days), median (IQR) | 13 (9–16) | 11.5 (8.5–15.25) | 14 (12.25–16.75) | 0.135 |
| 28-day all-cause death rate, n (%) | 0 (0) | 0 (0) | 0 (0) | – |
MLN molnupiravir, SC standard care, IMV invasive mechanic ventilation, ICU intensive care unit, VL viral load, IQR interquartile range, D5 day 5, D7 day 7, D10 day 10, D14 day 14
P-value refers to the comparison between the molnupiravir and SC groups
P < 0.05 means significant difference
Overall, elderly COVID-19 patients experienced a long median hospital stay of 13 days. Most had at least one symptom associated with viral infection (95.24%), and 85.71% patients experienced multiple symptoms, with the most frequently reported symptoms being fever (54.8%), cough and sputum production (50.0%), and fatigue (33.3%) (Table 2). In terms of pulmonary CT scan, all patients with moderate clinical process had at least one appearance of viral infection-related manifestations, such as ground-glass opacity, consolidation, lymphadenopathy, and pleural effusion.
Clinical Outcomes
All of these elderly patients recovered and were discharged from the hospital, with a median hospital stay of 13 days. Compared with SC users, the median hospital stay time of the MLN users was reduced by 2.5 days. However, no significant difference was found in the in-hospital disease exacerbation rate between MLN users and non-users (Table 2). However, for those over 85 years old, a Charlson Comorbidity Index ≥ 6 and a lymphocyte count less than 1 × 109/L was associated with disease exacerbation (Table 3).
Table 3.
Subgroup analysis of the differences in the rate of progression to severe disease
| No oxygen n (%) |
Oxygen through high-flow device or IMV, or ICU transfer, n (%) | P-value | |
|---|---|---|---|
| Age | |||
| ≥ 85 years (N = 20) | 15 (75) | 5 (25.0) | 0.043 |
| < 85 years (N = 22) | 22 (100) | 0 (0) | |
| Gender | |||
| Female (N = 25) | 23 (92.0) | 2 (8.0) | 0.644 |
| Male (N = 17) | 14 (82.4) | 3 (17.6) | |
| BMI, kg/m2 | |||
| ≥ 24 (N = 23) | 20 (87.0) | 3 (13.0) | 1.00 |
| < 24 (N = 19) | 17 (89.5) | 2 (10.5) | |
| COPD | |||
| Yes (N = 4) | 2 (50) | 2 (50) | 0.097 |
| No (N = 38) | 35 (92.1) | 3 (7.9) | |
| Charlson Index | |||
| ≥ 6 (N = 11) | 6 (54.4) | 5 (45.5) | 0.001 |
| < 6 (N = 31) | 31 (100) | 0 (0) | |
| Therapy strategy | |||
| MLN use (N = 26) | 23 (88.5) | 3 (11.5) | 1.000 |
| SC only (N = 16) | 14 (87.5) | 2 (12.5) | |
| Lymphocyte count (× 109/L) | |||
| < 1.0 (N = 13) | 9 (69.2) | 4 (30.8) | 0.044 |
| ≥ 1.0 (N = 29) | 28 (96.6) | 1 (3.4) | |
| CRP (mg/L) | |||
| ≥ 10 (N = 12) | 9 (75) | 3 (25) | 0.258 |
| < 10 (N = 30) | 28 (93.3) | 2 (6.7) | |
| IL-6 (pg/ml) | |||
| ≥ 10 (N = 23) | 18 (78.3) | 5 (21.7) | 0.092 |
| < 10 (N = 19) | 19 (100) | 0 | |
| D-dimer level (mg/L) | |||
| ≥ 0.5 (N = 22) | 17 (77.3) | 5 (22.7) | 0.073 |
| < 0.5 (N = 20) | 20 (100) | 0 | |
IMV invasive mechanic ventilation, ICU intensive care unit, BMI body mass index, COPD chronic obstructive pulmonary disease, MLN molnupiravir, SC standard care, CRP C-reactive protein, IL-6 interleukin 6
P < 0.05 means significant difference
Virological Outcomes
The median SARS-CoV-2 RNA shedding time of elderly COVID-19 patients was 13 days (IQR 9–15 days) from therapy time. The rate of undetectable VL in MLN users at days 5, 7, and 10 were 3/26 (11.56%), 6/26 (23.1%), and 12/26 (46.2%), respectively. Compared with non-users, MLN users achieved a significantly higher undetectable VL rate at day 10 (46.2% versus 12.5%, respectively; P = 0.025) (Table 2; Fig. 1). Multivariate adjusted analysis showed that elderly patients who received early MLN treatment were 7.584 times more likely to achieve undetectable VL at day 10 than non-users (Table 4).
Fig. 1.
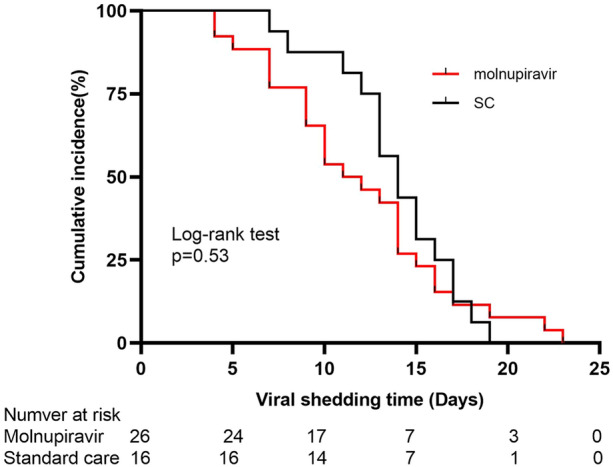
Cumulative incidence of lower viral load (Ct ≥ 35) for molnupiravir users and standard care users. SC standard care, Ct cycle threshold
Table 4.
Day 10 undetectable viral load predictors (logistic analysis)
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Male | 1.35 | 0.359–5.078 | 0.657 | |||
| Age < 80 years | 4.50 | 1.117–18.132 | 0.034 | 6.949 | 1.295–37.295 | 0.024 |
| BMI < 24 kg/m2 | 0.205 | 0.047–0.898 | 0.036 | 0.237 | 0.043–1.303 | 0.098 |
| Fully vaccinated | 2.03 | 0.493–8.408 | 0.325 | |||
| Charlson Comorbidity Index < 6 | 2.842 | 0.522–15.465 | 0.227 | |||
| COPD | 0.462 | 0.058–3.679 | 0.465 | |||
| Lymphocyte count ≥ 1.0 × 109/L | 1.184 | 0.291–4.825 | 0.814 | |||
| D-dimer < 0.5 mg/L | 2.782 | 0.735–10.524 | 0.132 | |||
| CRP ≥ 10 mg/L | 1.737 | 0.386–7.807 | 0.472 | |||
| Interleukin-6 ≥ 10 pg/ml | 2.061 | 0.56–7.577 | 0.276 | |||
| Molnupiravir therapy | 6.00 | 1.129–31.880 | 0.036 | 7.584 | 1.094–52.562 | 0.04 |
OR odds ratio, CI confidence interval, BMI body mass index, COPD chronic obstructive pulmonary disease, CRP C-reactive protein
P < 0.05 means significant difference
Discussion
In this real-world retrospective observational study, the clinical features of elderly patients infected with SARS-CoV-2 Omicron variant BA.2 and the efficacy of MLN in these patients were described and evaluated. We found that elderly COVID-19 patients with Omicron BA.2 experienced a relatively long hospital stay, and early initiation of MLN at a median of 2 days from the first positive test or symptom onset was associated with fast VL shedding by 3 days compared with not using any oral antivirals. Overall, our experience confirmed the role of early MLN therapy and remission therapy in SARS-CoV-2 VL shedding and reducing the morbidity and mortality of COVID-19 in elderly patients.
In theory, the high mutations in the spike protein and RdRp of the Omicron variant enable it to reduce the potency of existing RdRp inhibitors, such as MLN, along with successfully evading neutralizing antibodies [1, 6, 10]. Laboratory studies have demonstrated that the inhibitory concentration (IC)50 of the Omicron strain for RdRp inhibitors is similar to that of early strains (i.e., IC50 values for remdesivir and MLN differing by factors of 1.2 and 0.8, respectively) [9–11]. Real-world studies among community-dwelling COVID-19 in both in- and outpatients during the Hong Kong pandemic dominated by the Omicron variant have shown that early initiation of MLN is associated with lower risks of disease progression and all-cause mortality, in addition to achieving low VL faster than non-use [8, 11]. However, in patients not fully vaccinated and elderly patients (> 65 years old), the effectiveness of MLN is not consistent [11]. In the current study, the patients were a group of hospitalized elderly COVID-19 patients amid a pandemic wave of Omicron BA.2 variant in Shanghai, China. We obtained similar results in that the early initiation of MLN was associated with fast shedding of VL compared with non use, but MLN was not associated with low risk of disease progression. The inferior efficacy in elderly patients may be closely related to the higher rate of patients who were not fully vaccinated and their hypoimmune state. These results suggested that, although MLN retained its efficacy toward the Omicron variant, it may not be the optimum choice in some special cases, such as patients aged > 65 years and who are not fully vaccinated. Additionally, current information on MLN efficacy in elderly COVID-19 patients with the Omicron variant was targeted at non-fully vaccinated elderly people, so the efficacy of MLN on fully vaccinated elderly patients requires further evaluation.
Although our research fills some gaps in the application of MLN, several limitations remain. First, our small dataset, retrospective approach, and single-center nature limited our ability to fully characterize the efficacy difference between subgroups of patients with different vaccination statuses and age. Evaluating the difference in severity of preexisting comorbidities among elderly patients was also impossible. In addition, owing to the small sample size and overall low number of events, the possibility of performing a powerful multivariable analysis to detect a difference between treatment strategy groups is limited. Second, we reported a limited follow-up time, and long-term outcomes related to COVID-19 diseases (e.g., long sequelae beyond 30 days) were not captured in the present study. Finally, our study was also subject to selection bias because the treatment options for patients were contingent to our center's treatment strategy. In the early stage of the pandemic, antiviral agents and certain treatments (e.g., nirmatrelvir and ritonavir) were unavailable in our center. Consequently, we cannot make suggestions on the optimization of oral antiviral agents for elderly patients.
Conclusions
Our experience suggests that, during a pandemic wave of the Omicron variant BA.2, elderly COVID-19 patients experienced a difficult clinical process. Early initiation of MLN within 5 days of the first positive test or symptom onset among hospitalized elderly patients was associated with rapidly achieving low VL and shorter hospital stay.
Acknowledgements
We would like to extend our thanks to all study participants for their involvement in the study.
Funding
This study was supported by the Key research project of COVID-19 by Changhai hospital of Naval Medical University (reference number: COVID-ZD-006, COVID-ZD-011) (Xuesong Liang) and grants from the National Key Research and Development Project (2021YFC2300704) (Wu ZHONG). The journal's Rapid Service fee was funded by National Key Research and Development Project (2021YFC2300704).
Author Contributions
Yayun Liu recruited the patients and collected specimens, edited the clinical data and performed the data analysis; Lingling Ge edited the clinical data of enrolled patients; Shiyong Fan drafted the manuscript; Aijing Xu recruited the patients and treated the patients; Wang, Xu Dong, Mingxiao Xu and Wenhan Fan treated the patients; Wu Zhong conceived and designed the study; Xuesong Liang conceived and designed the study; checked the data analysis and drafted the manuscript; All authors have read and approved the final manuscript.
Disclosures
Yayun Liu, Lingling Ge, Shiyong Fan, Aijing Xu, Xinyu Wang, XuDong, Mingxiao Xu, Wenhan Fan, Wu Zhong and Xuesong Liang declare that they have no competing interests.
Compliance with Ethics Guidelines
This study was approved by the Ethics Committee of Changhai Hospital. (CHEC2022-111).Written informed consent was exempted from all the study participants. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Data Availability
All data and analysis results are included in this article. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. All authors have read and approved the final manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yayun Liu, Lingling Ge, and Shiyong Fan are co-first authors.
Contributor Information
Yayun Liu, Email: 1042956281@qq.com.
Lingling Ge, Email: 18351977696@163.com.
Shiyong Fan, Email: fansy@bmi.ac.cn.
Aijing Xu, Email: xuaijing86@163.com.
Xinyu Wang, Email: 617943178@qq.com.
Xu Dong, Email: yxdongxu@126.com.
Mingxiao Xu, Email: 452055289@qq.com.
Wenhan Fan, Email: redmaples2005@163.com.
Wu Zhong, Email: zhongwu@bmi.ac.cn.
Xuesong Liang, Email: liangxuesong2000@163.com.
References
- 1.Islam F, Dhawan M, Nafady MH, et al. Understanding the omicron variant (B.1.1.529) of SARS-CoV-2: mutational impacts, concerns, and the possible solutions. Ann Med Surg (Lond). 2022;78:103737. doi: 10.1016/j.amsu.2022.103737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith DJ, Hakim AJ, Leung GM, et al. COVID-19 mortality and vaccine coverage—Hong Kong Special Administrative Region China, January 6, 2022–March 21, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(15):545–548. doi: 10.15585/mmwr.mm7115e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dejnirattisai W, Huo J, Zhou D, et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185(3):467–84e15. doi: 10.1016/j.cell.2021.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo Y, Han J, Zhang Y, et al. SARS-CoV-2 omicron variant: epidemiological features, biological characteristics, and clinical significance. Front Immunol. 2022;13:877101. doi: 10.3389/fimmu.2022.877101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohsin M, Mahmud S. Omicron SARS-CoV-2 variant of concern: a review on its transmissibility, immune evasion, reinfection, and severity. Medicine (Baltimore) 2022;101(19):e29165. doi: 10.1097/MD.0000000000029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao L, Li Y, Yang S, et al. The adenosine analog prodrug ATV006 is orally bioavailable and has preclinical efficacy against parental SARS-CoV-2 and variants. Sci Transl Med. 2022 doi: 10.1126/scitranslmed.abm7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir among COVID-19 inpatients during Hong Kong’s Omicron BA.2 wave: an observational study. medRxiv. 2022 doi: 10.1101/2022.05.19.22275291. [DOI] [Google Scholar]
- 9.Vangeel L, Chiu W, De Jonghe S, et al. Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 omicron and other variants of concern. Antiviral Res. 2022;198:105252. doi: 10.1016/j.antiviral.2022.105252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takashita E, Kinoshita N, Yamayoshi S, et al. Efficacy of antibodies and antiviral drugs against Covid-19 Omicron variant. N Engl J Med. 2022;386(10):995–998. doi: 10.1056/NEJMc2119407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir against mortality, hospitalization, and in-hospital outcomes among community-dwelling, ambulatory COVID-19 patients during the BA.2.2 wave in Hong Kong: an observational study. medRxiv. 2022 doi: 10.1101/2022.05.26.22275631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386(15):1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and analysis results are included in this article. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. All authors have read and approved the final manuscript.


