Abstract
Objectives
Post-COVID-19 cholangiopathy (PCC) is a rare but poorly understood and serious complication of COVID-19 infection. We sought to better understand the epidemiology, mechanism of action, histology, imaging findings, and outcomes of PCC.
Methods
We searched PubMed, Cochrane Library, Embase, and Web of Science from December 2019 to December 2021. Mesh words used “post-Covid-19 cholangiopathy,” “COVID-19 liver injury,” “Covid-19 and cholangiopathy,” and “COVID-19 liver disease.” The data on epidemiology, mechanism of action, histology, imaging findings, and outcomes were collected.
Results
PCC was reported in 30 cases during the study period. The mean (standard deviation [SD]) age was 53.7 (5). Men accounted for cases (83.3%). All patients had required intensive level of care and mechanical ventilation. Mean (SD) number of days from COVID infection to severe disease or liver disease was 63.5 (38). Peak mean (SD) alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, and total bilirubin were 2014 (831.8) U/L, 1555 (2432.8) U/L, 899.72 (1238.6) U/L, and 10.32 (9.32) mg/dl, respectively. Four patients successfully underwent liver transplantation.
Conclusion
PCC is a severe and progressive complication of COVID-19 infection. More research is needed to better understand the pathophysiology and best treatment approach. Clinicians should suspect PCC in patients with cholestatic liver injury following COVID-19 infection.
Keywords: COVID-19, cholangiopathy, hepatopathy, sclerosing cholangitis
Highlights
What is known?
-
•
A strong correlation between liver injury and COVID-19 infection.
-
•
COVID-19 has an affinity to ACE-2 receptors on the hepatocytes.
-
•
Little is known about COVID-19-induced injury to cholangiocytes.
What is new here?
-
•
Post-COVID-19 cholangiopathy (PCC) is rare.
-
•
Review the demographics, presentation, and natural history of PCC.
-
•
Review of the evidence and mechanism of injury related to PCC.
-
•
Identify potential risk factors for PCC.
-
•
Clarify the evidence behind the role of liver transplantation for patients with PCC.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 virus) is a major public health concern and has been associated with substantial morbidity and mortality across the globe.1 According to the World Health Organization, SARS-CoV-2 has led to over 5 million deaths worldwide as of January 20, 2022.3 Although the most reported complications after SARS-CoV-2 are related to the cardiopulmonary system, the virus has been implicated in causing multiorgan failure and multiple postrecovery complications.2, 3, 4 Extrapulmonary complications include liver injury, kidney failure, coagulopathy, and neurological defects.5
Elevated liver enzymes are reported in approximately 20% of COVID-infected patients and can be a harbinger of outcomes.6,7 Elevated aminotransferases are associated with greater severity of COVID-19 infection, likelihood of admission, respiratory failure, and death.7 COVID-19 can also lead to cholestatic pattern of liver injury, which is especially associated with worse outcome.8 One manifestation of this cholestatic presentation is post-COVID-19 cholangiopathy (PCC).4,9,10 However, there is no consensus in diagnostic criteria for this rare secondary cholangiopathic complication of COVID-19.
The epidemiology and pathophysiology of PCC are not very well understood. In this systematic literature review, we discuss the epidemiology, clinical and laboratory presentation, evaluation, treatment, and outcomes of PCC. In this review, we also discuss proposed mechanisms of action that may contribute to the development of COVID cholangiopathy.
Methods
We conducted a systematic search of literature using PubMed, Cochrane Library, Embase, Web of Science, Google Scholar, and Google Search from December 1, 2019, to June 30, 2022. A combination of keywords was used in the medical subjects headings, including: “COVID-19,” “Cholangiopathy,” “Hepatopathy,” “Post-COVID-19 Cholangiopathy.” We screened the bibliographies and manuscripts of all the primary articles that contained all the cases. Our research was limited to articles written in English. We limited our research to case reports, case series, and letters to the editor.
Inclusion Criteria/Exclusion
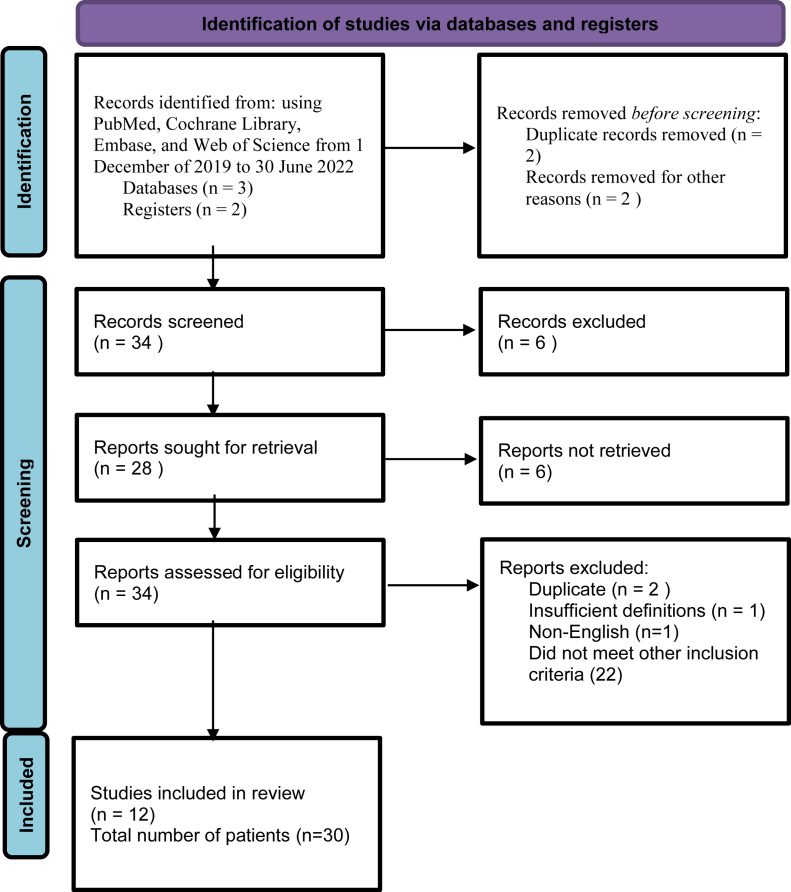
Our inclusion criteria incorporated only studies published in English. Non-English studies were excluded. Studies without clear COVID-19 polymerase chain reaction diagnosis were excluded. Our research was in line with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Figure 1). Patients with other etiologies of liver disease and possible drug-induced liver injury from a known hepatotoxic drug were excluded. Papers that did not include diagnostic modality to confirm cholangiopathy were excluded. Diagnostic modalities for PCC were defined as liver biopsy, magnetic retrograde cholangiopancreatography (MRCP), and/or endoscopic retrograde cholangiopancreatography (ERCP). A total of 106 cases were identified by our literature search. We extracted information from 28 articles (Table 1).4,9,11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 We collected data on patients’ demographics, symptom onset, liver associated values including initial values and peak values, diagnostic modalities, pathology findings, modes of treatments, and overall disease course. Simple statistics were utilized, and the data were reported.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) chart.
Table 1.
Summary of Articles/Cases of COVID-19-Related Cholangiopathy Published.
| Author (reference) | Country | Gender | Age (years) | Ethnicity | Presenting labs | Peak labs | Diagnosis | Treatment | Outcome | Months since (COVID-19 diagnosis) | Hospitalization status/Mechanical ventilation status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Roth et al.4 | USA | Male | 38 | Non-Hispanic/White | AP - 81 AST- 30 ALT- 34 TB- 0.3 |
AP- 3665; AST- 539; ALT- 456 TB- 9.8 |
MRCP, ERCP, Liver biopsy |
Hydroxychloroquine Azithromycin Tocilizumab Ampicillin Cefepime Ertapenem Vancomycin No UDCA |
Alive, no LT | 6 months | Hospitalized/Required mechanical ventilation |
| Roth et al.4 | USA | Male | 25 | Hispanic/Multiracial | AP- 80 AST- 55 ALT- 52 TB- 0.5 |
AP- 2892; AST- 4491; ALT-1573 TB- 23.9 |
MRCP, ERCP, Liver biopsy | Hydroxychloroquine Azithromycin Ivermectin Corticosteroids Tocilizumab Anakinra Convalescent plasma Remdesivir Meropenem Piperacillin-tazobactam Vancomycin no UDCA |
Alive, no LT | 5 months | Hospitalized/Required mechanical ventilation |
| Roth et al.4 | USA | Female | 40 | Hispanic/Multiracial | AP- 163 AST- 24 ALT- 20 TB-0.3 |
AP- 2784; AST- 8860; ALT- 2546 TB- 12.7 |
MRCP, Liver biopsy | Hydroxychloroquine Azithromycin Corticosteroids Anakinra Aztreonam Cefepime Ertapenem Meropenem Nitrofurantoin Piperacillin-tazobactam Vancomycin no UDCA |
Remained hospitalized | 6 months | Hospitalized/Required mechanical ventilation |
| Durazo et al.9 | USA | Male | 47 | Non-hispanic/White | AP- 90 AST- 79 ALT- 52 TB 0.3 |
AP- 1644; AST- 384; ALT- 175 TB- 19 |
MRCP, ERCP, Liver biopsy | Hydroxychloroquine Azithromycin High dose Vitamin C no UDCA |
Alive, had LT | 2 months | Hospitalized/Required mechanical ventilation |
| Rojas et al.11 | Colombia | Female | 29 | Hispanic/Multiracial | AP- 180 AST- 60 ALT-50 TB- 0.4 |
AP- 470; AST- 410 ALT- 410; TB- 19 |
MRCP, ERCP, Liver biopsy | Antibiotics (unspecified) Colchicine Dexamethasone Furosemide UDCA |
Alive, no LT | Lost to follow-up | Hospitalized/Required mechanical ventilation |
| Linnewebber et al.12 | Germany | Male | 64 | Not reported | Elevated liver enzymes | TB – 17; Others not described |
ERCP | Supportive standard COVID treatment (Not specified), UDCA. | Alive, no LT | Lost to follow-up | Hospitalized/Required mechanical ventilation |
| Linnewebber et al.12 | Germany | Male | 72 | Not reported | Elevated liver enzymes | TB – 7.5; Others not described | MRCP, ERCP | Supportive standard COVID treatment (Not specified), UDCA. | Deceased, no LT | Hospitalized/Required mechanical ventilation | |
| Faraqui et al.13 | USA | Male | 73 | Non-hispanic/White | Elevated liver enzymes | AP- 1221; AST-336; ALT- 242 TB 16.9 |
MRCP, ERCP, Liver biopsy | Azithromycin UDCA | Alive, declined LT evaluation | 7 months | Hospitalized/Required mechanical ventilation |
| Faraqui et al.13 | USA | Male | 39 | Hispanic | Elevated liver enzymes | AP- 2129; AST- 328; ALT- 242 TB 2.2 |
MRCP, ERCP, Liver biopsy | Tocilizumab Azithromycin UDCA |
Alive, no LT | 5 months | Hospitalized/Required mechanical ventilation |
| Faraqui et al.13 | USA | Male | 64 | Other | Elevated liver enzymes | AP- 2035; AST- 323; ALT- 338 TB- 16.9 |
MRCP, ERCP, Liver biopsy | Hydroxychloroquine Azithromycin UDCA |
Alive, had LT | 10 months | Hospitalized/Required mechanical ventilation |
| Faraqui et al.13 | USA | Male | 77 | Non-hispanic/White | Elevated liver enzymes | AP- 1855; AST- 711; ALT- 792 TB- 8.5 |
MRCP, ERCP, Liver biopsy | Hydroxychloroquine Azithromycin Remdesivir UDCA |
Alive, no LT | 10 months | Hospitalized/Required mechanical ventilation |
| Faraqui et al.13 | USA | Male | 46 | Non-hispanic/White | Elevated liver enzymes | AP-2366; AST-2739; ALT- 2171 TB- 2.9 |
MRCP | Hydroxychloroquine Azithromycin Tocilizumab UDCA |
Alive, no LT | 9 months | Hospitalized/Required mechanical ventilation |
| Faraqui et al.13 | USA | Male | 72 | Hispanic | Elevated liver enzymes | AP-2200; AST-1260; ALT-595 TB-16.0 |
MRCP | Hydroxychloroquine Azithromycin UDCA |
Deceased, no LT | 7 months | Hospitalized/Required mechanical ventilation |
| Faraqui et al.13 | USA | Male | 38 | Non-hispanic/White | Elevated liver enzymes | AP-1723; AST-409 ALT- 929; TB- 10.22 |
MRCP | Hydroxychloroquine Azithromycin UDCA |
Deceased, listed for LT | 9 months | Hospitalized/Required mechanical ventilation |
| Faraqui et al.13 | USA | Male | 60 | Non-hispanic/White | Elevated liver enzymes | AP -1325; AST- 30 ALT- 34; TB- 0.3 |
MRCP | Hydroxychloroquine Azithromycin UDCA |
Alive, listed for LT | 10 months | Hospitalized/Required mechanical ventilation |
| Faraqui et al.13 | USA | Male | 42 | Hispanic | Elevated liver enzymes | AP-1036; AST-576 ALT-385; TB-21.6 |
MRCP | Remdesivir Valacyclovir Foscarnet UDCA |
Deceased, no LT | 4 months | Hospitalized/Required mechanical ventilation |
| Faraqui et al.13 | USA | Male | 57 | Hispanic | Elevated liver enzymes | AP-2544; AST-332 ALT-260; TB-35 |
MRCP | Azithromycin UDCA | Deceased, no LT | 4 months | Hospitalized/Required mechanical ventilation |
| Faraqui et al.13 | USA | Male | 68 | Other | Elevated liver enzymes | AP- 2057; AST- 420; ALT- 286 TB-2.0 |
MRCP | Hydroxychloroquine UDCA |
Alive, declined LT | 10 months | Hospitalized/Required mechanical ventilation |
| Faraqui et al.13 | USA | Female | 62 | Other | Elevated liver enzymes | AP- 965; AST-7400; LT-5854 TB- 4.4 |
MRCP | Azithromycin no UDCA | Alive, no LT | 6 months | Hospitalized/Required mechanical ventilation |
| Lee et al.14 | USA | Male | 64 | Not reported | Normal Liver enzymes initially | Elevated but not reported | MRCP, ERCP, Liver biopsy | Hydroxychloroquine Azithromycin Tocilizumab Convalescent plasma No UDCA |
Alive, had LT | 8 months | Hospitalized/Required mechanical ventilation |
| Tafreshi S et al.15 | USA | Male | 38 | Not reported | AP- 81 AST- 30 ALT- 34 TB-0.3 |
AP- 3665 AST- 539 ALT- 456 TB- 9.8 |
MRCP, ERCP, Liver biopsy | Hydroxychloroquine Azithromycin Tocilizumab no UDCA |
Alive, no LT | Lost to follow up | Hospitalized/Required mechanical ventilation |
| Klindt et al.16 | Germany | Male | 47 | Not reported | AP-203 AST-83 ALT- 91 TB- 0.4 |
AP- 1700 AST- 470 ALT- 754 TB- 18 |
MRCP, Liver biopsy | Lopinvir – ritonavir Remdesivir Piperacillin-tazobactam Meropenem no UDCA |
Alive, had LT | 5 months | Hospitalized/Required mechanical ventilation |
| Kate et al.17 | UK | Male | 59 | Not reported | Reportedly normal | ALP 130 AST 83 ALT 102 Bili T 12 |
MRCP | Corticosteroids | Alive, persistent disease | 6 months | Required mechanical ventilation |
| Butikofer et al.18 | Switzerland | Male | 59 | Not reported | Normal | ALP 18 | MRCP | Hydroxychloroquine | On transplant waitlist | 7 months | Required mechanical ventilation |
| Butikofer et al.18 | Switzerland | Male | 67 | Not reported | Unknown | Peak ALP 21 x ULN | MRCP | Hydroxychloroquine | Exitus letalis | 55 days | Required mechanical ventilation |
| Butikofer et al.18 | Switzerland | Female | 54 | Note reported | Unknown | Peak ALP 18.8 ULN | MRCP | Hydroxychloroquine | Alive with persistent disease | 9 months 2 weeks | Required mechanical ventilation |
| Butikofer et al.18 | Switzerland | Male | 64 | Not reported | Unknown | Peak ALP 12.85 ULN | MRCP | Hydroxychloroquine | Exitus letalis | 14 days | Required mechanical ventilation |
| Rela et al.19 | India | Male | 50 | Not reported | Normal | Peak ALP 420 IU | Liver biopsy post OLT | Hydroxychloroquine | Status post OLT with normal liver tests | 6 months | Required mechanical ventilation |
| Franzini et al.20 | Brazil | Male | 65 | No reported | Not reported | Peak ALP 807 IU | MRCP and ERCP | Corticosteroids | Continued elevation in liver tests | 1 month | Required mechanical ventilation |
| Rojas et al.21 | Columbia | Female | 29 | Not reported | Not reported | Peak ALP 400 IU | Liver biopsy | Corticosteroids | Improvement | 3 months | Required mechanical ventilation |
AP (U/L), alkaline phosphatase, ALT (U/L), alanine aminotransferase, AST (U/L), aspartate aminotransferase, TB (mg/dl), total bilirubin, LT, liver transplant. MRCP, magnetic resonance cholangiopancreatography, ERCP, endoscopic retrograde cholangiopancreatography, UDCA, ursodeoxycholic acid; ULN, upper limit of normal.
Operational Definitions
The definitions of COVID-19 cholangiopathy and severe COVID-19 were characterized by the authors of the included studies. In regard to the definition of COVID-19 cholangiopathy, all studies demonstrated injury to the biliary system determined by endoscopy, imaging, or liver biopsy. The most frequent laboratory definition used for a diagnosis of COVID-19 cholangiopathy included: COVID-19-induced cholestasis was defined as a rise in alkaline phosphatase (ALP) by ≥ 1.5 times the upper limit of normal (ULN) with serum bilirubin (≥2 ULN); gamma glutamyl transferase (≥3 ULN); absence of active sepsis; and exclusion of other underlying causes of chronic liver disease.8, 9, 10, 11, 12, 13 The most common features of severe pulmonary COVID-19 were respiratory rate >30/minute; dyspnea and/or SpO2 < 90% on room air; need for mechanical ventilation related to COVID-19 illness; and detectable COVID-19 by polymerase chain reaction.4,9,11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21
Results
Demographics and Patient Information
We identified 30 cases of patients with PCC that matched our inclusion criteria.4,9,11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 (Table 1). Most cases described were from the United States.4,9,13, 14, 15 The mean (SD) age was 53.7 (5). Men accounted for cases (83.3%). Seven patients were non-Hispanic whites, and there were seven patients of Hispanic ethnicity. In the cohort, the most common metabolic disorders including hypertension (53.3%) and obesity (40.9%). The mean (SD) time from diagnosis of COVID-19 infection to diagnosis of PCC was 66 (36.0) days. Demographic data can be found in (Table 2). All patients required hospitalization and mechanical ventilator support. Nine patients were evaluated for liver transplant (Table 1). Four of those patients were successfully transplanted,9,14,16,19 and one expired while on the list.13
Table 2.
Demographics and Baseline Characteristics of Patients Identified in This Review.
| Variable | Total patients (N = 30) |
|---|---|
| Age (mean), Years | 53.7 ± 5 |
| Gender | |
| Female | 5 (16.7%) |
| Male | 25 (83.3%) |
| Race/ethnicity | |
| Non-Hispanic White | 7 (31.8%) |
| Hispanic | 7 (31.8%) |
| Other or unknown | 8 (36.4%) |
| Alcohol status | |
| Mild (<4 drinks/mo) | 3 (13.6%) |
| Moderate | 1 (4.55%) |
| Not reported | 1 (4.55%) |
| Comorbidities | |
| Obesity | 9 (40.9%) |
| Diabetes | 7 (31.8%) |
| Hypertension | 14 (53.3%) |
| Chronic liver disease | 0 (0%) |
| Cardiovascular disease | 2 (9%) |
| Cerebrovascular disease | 1 (4.5%) |
| Hyperlipidemia | 8 (36.4%) |
| Other | 5 (22.7%) |
| None | 7 (31.81%) |
Laboratory features
Pertinent liver associated test results are shown in Table 1. Initial presenting labs were not reported in over half of the patients.12,13,15,16 The labs were described as normal in three patients.14 Of the seven patients with reported presenting labs, the AST, alanine aminotransferase (ALT), and ALP were elevated in three, four, and three patients of the cohort, respectively. Presenting total bilirubin was normal in the seven patients. Peak mean (SD) ALP, aspartate aminotransferase, ALT, and total bilirubin were 2014 (831.8) U/L, 1555 (2432.8) U/L, 899.72 (1238.6) U/L, and 10.32 (9.32) mg/dl, respectively (Table 3).
Table 3.
Peak Relevant Laboratory Values and Scores.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21
| Numbers of cases | N = 30 |
|---|---|
| Mean peak AP (U/L), SD | 2014 ± 831.8 |
| Mean peak AST (U/L), SD | 1555 ± 2432.8 |
| Mean peak ALT (U/L), SD | 899.72 ± 1238.6 |
| Mean peak bilirubin (mg/dl), SD | 10.32 ± 9.32 |
| Mean number of days (time) from COVID infection to severe disease or liver disease | 63.5 ± 38 |
AP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase, SD, standard of deviation, SE, standard error.
Liver biopsy features
Of the 30 cases identified in this review, 14 underwent a liver biopsy as part of their evaluation.4,9,11,13, 14, 15, 16,19,21 The results are summarized in Table 4. The common histological finding was moderate portal and periportal fibrosis in eight patients.4,9,11,13,15,16 The next common histological finding in six patients was degenerative cholangiocyte injury, with prominent cholangiocyte vacuolization, regenerative change, apoptosis, and necrosis of the cholangiocyte epithelial layer of terminal bile ducts and marginal ductules.4,9,13,14 Histologic evidence of both small and large duct obstruction was described in one and three patients, respectively.13 Bile duct paucity or absence was described in four patients.13,16,19
Table 4.
Summary of Histopathological Findings.
| Authors (reference) | N | Histopathology findings |
|---|---|---|
| Roth et al.4 | 3 |
|
| ||
| ||
| ||
| Rojas et al.11 | 1 |
|
| Durazo et al.9 | 1 |
|
| Faraqui et al.13 | 12 |
|
| ||
| ||
| ||
| Lee et al.14 | 1 |
|
| Tafreshi et al.15 | 1 |
|
| ||
| Klindt et al.16 | 1 |
|
| ||
|
Cholangiography features
Twenty-nine of the thirty patients in our cohort underwent MRCP.4,9,11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 The results of the MRCP examinations are shown in Table 5. The most common finding reported in 23 patients was intrahepatic bile ducts beading with multiple short segmental strictures and intervening dilatation.4,9,13, 14, 15,17, 18, 19, 20, 21 Bile duct thickening and hyper enhancement were reported in 14 patients and peribiliary diffusion high signal reported in 13 patients.13, 14, 15, 16 Twelve patients underwent ERCP. The summary of ERCP findings are listed in Table 6. Briefly, eight patients had evidence of diffuse intrahepatic biliary strictures or cholangiopathy.9,12, 13, 14 Ten patients required extraction of stones and sludge.9,12, 13, 14 Six patients required common bile duct stent placement.12, 13, 14
Table 5.
Summary of Imaging Findings (MRCP) (N = 21).
| Authors (reference) | Number | MRCP findings |
|---|---|---|
| Roth et al.4 | 3 | Intrahepatic bile ducts beading with multiple short segmental strictures and intervening dilatation |
| Rojas et al.11 | 1 | Cystic-appearing lesion in segment VII of the liver with no biliary obstruction |
| Durazo et al.9 | 1 | Mild intrahepatic biliary ductal dilatation with multifocal strictures or beading without extrahepatic biliary dilatation |
| Faraqui et al.13 | 12 | Intrahepatic duct beading Bile duct thickening and hyper enhancement Peribiliary diffusion high signal |
| Lee et al.14 | 1 | Mild intrahepatic biliary ductal dilatation and mild patchy T2 hyper intensity within the right hemiliver |
| Tafreshi et al.15 | 1 | Normal liver morphology with diffuse mild intrahepatic biliary distension, marked beading and irregularity, as well as mild irregularity of extra hepatic common bile duct Diffuse periductal enhancement |
| Klindt et al.16 | 1 | Aggravated accentuation of intra- and extrahepatic biliary ducts |
| Linneweber et al.12 | 1 | Did not show intrahepatic cholestasis opting against SSC Showed dilatation of the common bile duct |
MRCP, magnetic retrograde cholangiography.
Table 6.
Summary of ERCP Findings (N = 12).
| Authors (reference) | Number | ERCP findings and interventions |
|---|---|---|
| Roth et al.4 | 2 | Extraction of stones and sludge. |
| Rojas et al.11 | 1 | Negative for Choledocholithiasis. |
| Durazo et al.9 | 1 | A small pigment stone retrieved Diffuse intrahepatic biliary strictures or cholangiopathy |
| Faraqui et al.13 | 4 | Case 1: 1 Plastic CBD stent placed, Multiple biliary strictures were noted in the intrahepatic ducts, Stones removal, repeat ERCP in 1 month with removal of the stent. Case 2: 2 ERCPs done, stone removal, CBD stent placement and removal, and balloon dilation of strictures in the right and left hepatic ducts without improvement. Case 3: dilation of left main hepatic duct and placement of a plastic stent. Case 4: ERCP done after a bile leak after a laparoscopic cholecystectomy. Other eight patients did not undergo ERCP due to predominance of diffuse intrahepatic biliary tract abnormalities did not seem likely to be conductive to endoscopic intervention |
| Lee et al.14 | 1 | Irregular intrahepatic radicals consistent with cholangiopathy. Loose stone material was removed from the CBD Biliary stent placed in bile duct Repeat ERCP on day 150 showed ductopenia and subtle ductal beading consistent with secondary sclerosing cholangitis |
| Tafreshi et al.15 | 1 | Tortuous and attenuated intrahepatic bile ducts with normal caliber extrahepatic ducts |
| Linnewever et al.12 | 2 | Inflammation, stricture formation and rarefication of the peripheral bile duct system consistent with SSC Choledocholithiasis Repeat ERCP three times with ductal dilation and stent implantation |
ERCP, endoscopic retrograde cholangiography.
Treatment
There was no consensus pharmacologic therapy used by all the 30 patients in our review. Thirteen patients received hydroxychloroquine and 10 remdesivir. Three patients received corticosteroids. Ursodiol was prescribed to most patients (14 of 30 patients received ursodiol). It was noted to be of low benefit.11, 12, 13 Endoscopic interventions to help with biliary drainages such as sphincterotomy, balloon dilatation, and stenting of the bile ducts relieved the cholestasis and improved liver associated laboratory values in five patients.13,14 However, endoscopic interventions did not impact LT free prognosis in patients who were evaluated for LT.9,14,16,19 Four patients underwent liver transplantation.9,14,16,19 One study did not report follow-up after liver transplantation.16 Follow-up in the remaining three patients was 1, 7, and 8 months9,14,16 reported patient continued to have normal transaminases post-transplant.
Discussion
PCC is serious progressive cholestatic liver complication that can result in liver failure requiring transplantation. This rare complication has been reported in the context of case reports across the globe. The severity and progression of the disease vary and are not very well understood. The exact mechanism for the development of PCC is not completely known. In this systematic review, we describe the clinical presentation and natural history of PCC.
Our study shows that men with comorbid conditions who require mechanical ventilation are at the highest risk of developing PCC. Specifically, most patients with PCC were men (87%), and most patients had a diagnosis of hypertension (53.3%). Table 2 lists patient demographics. The biochemical presentation varied substantially in our cohort, with few patients having normal liver tests. Peak mean (SD) ALP, aspartate aminotransferase, ALT, total bilirubin were 2014 (831.8) U/L, 1555 (2432.8) U/L, 899.72 (1238.6) U/L, and 10.32 (9.32) mg/dl, in our cohort, respectively. All patients required intensive unit level of care reflecting the severity of COVID-19 infection. There was no uniform pharmacologic treatment in our cohort. The most common therapies used for COVID-19 being hydroxychloroquine, azithromycin, and ursodeoxycholic acid. Unfortunately, no treatment has been consistently effective. Mortality occurred in 7 out of 30 patients (23.3%).12,13,18 Liver transplant evaluation and listing were completed in 27.2% in our series, but LT was performed in 16% at the time of publication (refs). Sixty-eight percent of the patients previously reported had continued elevation in transaminases and ALP post-COVID-19 recovery. Studies published after our review suggest a possible role of plasmaphereses as a bridge to transplant.21,24,26 The proposed beneficial mechanism of action for plasmaphereses is the removal of antibodies from that can be contributing to liver injury. In the study, plasma exchange was done in five patients, and two were successfully bridged to living donor liver transplantation in the unvaccinated group of the study.24 A number of studies have emerged discussing liver disease and PCC describing up to 250 cases; however, these studies did not meet our search criteria therefore are not included, which shows the elevance of the diseases by replication of the publications.21, 22, 23, 24, 25, 26
There are a number of proposed mechanisms for the development of PCC. One of mechanisms revolves around the role of ACE2 receptors in the pathogenesis of COVID-related cholangiopathy.27, 28, 29, 30, 31, 32, 33, 34, 35 Direct damage to the cholangiocytes may be related to direct viral entry because of concentration of ACE-2 receptors found on the cholangiocytes. Another proposed mechanism include ischemic injury since the liver biliary system is particularly at risk of ischemia because of its single hepatic artery blood supply. As a result, cholangiocytes are easily damaged in situations of prolonged ischemia.34,37,46 Prolonged mechanical ventilation, sepsis, and hypotension during prolonged mechanical ventilation result in decreased blood supply to the cholangiocytes causing cell death, scaring, and stricture of the bile ducts.38,39 Furthermore, another proposed mechanism is direct cholangiopathy toxic metabolic injury from viral particles and medications associated with ICU stay.4,14,43 Finally, immune-mediated cholangiocyte damage due to cytokine and immune cell storm has also been proposed for the development of PCC.40,47 It is likely that the exact mechanism of action is multifactorial, which includes ischemia, receptor-mediated ACE-2 selective viral entry to cholangiocytes, toxic metabolic due to medications and viral particles, and immune-mediated effects. Several studies have suggested that COVID-19 cholangiopathy is a result of progressive paucity of bile ducts the exact pathophysiology to explain the histologic finding of bile duct paucity is not well known.16,27,30,31 A number of mechanisms have been proposed and include ischemia, direct viral insult, drug-induced injury, autoimmune mediated, or a combination of all.11, 12, 13, 14, 15, 16,36,41,42,44,45,47
There are a number of important limitations to our review. One limitation is that changing variants of Covid-19 infection. COVID infection in the current studies likely reflects the original variant. Subsequent variants may not share the same risk of PCC as the original one. Another limitation is the evolving literature available after our inclusion study dates. Updated reviews will be necessary to assess differences in risk factors, management, and outcomes of patients with PCC. For instance, studies included in our review were published largely before immunization against COVID-19 was available. The results of recent case series by Anand et al describe a potential lower risk of liver failure in COVID-19-immunized individuals.24 Plasma exchange was done in five patients, and two were successfully bridged to living donor liver transplantation in unvaccinated group.24
PCC is a rare complication to viral infection. Men who suffered severe disease requiring intubation and mechanical ventilation with history of chronic disease including diabetes, hypertension, obesity and dyslipidemia are at the higher risk. High-risk population should be closely monitored post disease recovery for evidence of PCC.40 There appears to be a strong correlation between age, gender, mechanical ventilation, lack of immunization against COVID-19, and COVID-19 cholangiopathy; however, this correlation does not necessarily suggest causation. Unfortunately, no treatment has been consistently effective, and patient with worsening liver function should be referred to a liver transplant center and considered for liver transplantation if condition permits. Clinicians should be vigilant to identify patients with PCC. More studies are needed to determine the true prevalence and long-term outcomes of those who undergo liver transplantation and who exhibit incomplete recovery.
Credit author statement
Conception and design: BY and SS. Administrative support: SS. Provision of study materials or patients: SS. Collection and assembly of data: All authors. Data analysis and interpretation: All authors. Manuscript writing: BY, MA, DS, MA, and SS. Final approval of manuscript: All authors.
Conflicts of interest
The authors have none to declare.
Funding
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2022.10.009.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.World Health Organization WHO issues a consensus document on the epidemiology of SARS. Wkly Epidimiol Rec. 2003;78:373–375. [PubMed] [Google Scholar]
- 2.WHO director WHO Director-General’s opening remarks at the media briefing on COVID-19. World Health Organ. 2020 https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19%5F-11-march-2020 11 March 2020. [Google Scholar]
- 3.WHO Health Emergency Dashboard WHO coronavirus (COVID-19) dashboard. World Health Organ. 2020 https://covid19.who.int/ [Google Scholar]
- 4.Roth N., Kim A., Vitkoski T., et al. Post-COVID-19 cholangiopathy: a novel entity. National Library Med. 2021;116:1077–1082. doi: 10.14309/ajg.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 5.Zheng K.I., Feng G., Liu W.Y., Targher G., Byrne C.D., Zheng M.H. Extrapulmonary complications of COVID-19: a multisystem disease? J Med Virol. 2021;93:323–335. doi: 10.1002/jmv.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kullar R., Patel A.P., Saab S. Hepatic injury in patients with COVID-19. J Clin Gastroenterol. 2020;54:841–849. doi: 10.1097/MCG.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 7.Guan W., Ni Z., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernstein D., Roth N., Kim A., et al. Presentation, patterns and predictive value of baseline liver tests on outcomes in COVID-19 patients without chronic liver disease. World J Gastroenterol. 2021;27:7350–7361. doi: 10.3748/wjg.v27.i42.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durazo F., Nicholas A., Mahaffey J., et al. Post-COVID-19 cholangiopathy- A new indication of liver transplantation: a case report. Transplant Proc. 2021;53:1132–1137. doi: 10.1016/j.transproceed.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma A., Jaiswal P., Kerakhan Y., et al. Liver disease and outcomes among COVID-19 hospitalized patients–a systematic review and meta-analysis. Ann Hepatol. 2020;21:100273. doi: 10.1016/j.aohep.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rojas M., Rodriguez Y., Zapata E., et al. Cholangiopathy as part of post-COVID syndrome. J Transl Autoimmun. 2021;4:100116. doi: 10.1016/j.jtauto.2021.100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linneweber L., Mann A.B., Denk G., Kraft E., Weber S. Cholangiopathy in early rehabilitation after intensive care treatment of patients with COVID-19. Am J Gastroenterol. 2022;117:197–198. doi: 10.14309/ajg.0000000000001511. [DOI] [PubMed] [Google Scholar]
- 13.Faraqui S., Okoli F., Olsen S., et al. Cholangiopathy after severe COVID-19: clinical features and prognostic implications. Am J Gastroenterol. 2021;116:1414–1425. doi: 10.14309/ajg.0000000000001264. [DOI] [PubMed] [Google Scholar]
- 14.Lee A., Wein A.N., Doyle M.B.M., Chapman W.C. Liver transplantation for post-COVID-19 sclerosing cholangitis. BMJ Case Reports CP. 2021;14 doi: 10.1136/bcr-2021-244168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tafreshi S., Whiteside I., Levine I., D'Agostino C. A case of secondary sclerosing cholangitis due to COVID-19. Clin Imag. 2021;80:239–242. doi: 10.1016/j.clinimag.2021.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klindt C., Jensen B.E., Brandenburger T., et al. Secondary sclerosing cholangitis as a complication of severe COVID-19: a case report and review of the literature. Clin Case Rep. 2021;9 doi: 10.1002/ccr3.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards K., Allison M., Ghuman S. Secondary sclerosing cholangitis in critically ill patients: a rare disease precipitated by severe SARS-CoV-2 infection. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-237984. Published 2020 Nov 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bütikofer S., Lenggenhager D., Wendel Garcia P.D., et al. Secondary sclerosing cholangitis as cause of persistent jaundice in patients with severe COVID-19. Liver Int. 2021;41:2404–2417. doi: 10.1111/liv.14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rela M., Rajakannu M., Veerankutty F.H., Vij M., Rammohan A. First report of auxiliary liver transplantation for severe cholangiopathy after SARS-CoV-2 respiratory infection [published online ahead of print, 2022 Aug 5] Am J Transplant. 2022:10. doi: 10.1111/ajt.17165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franzini T.A.P., Guedes M.M.F., Rocha H.L.O.G., Fleury C.A., Bestetti A.M., Moura E.G.H. Cholangioscopy in a POST-COVID-19 cholangiopathy patient. Arq Gastroenterol. 2022;59:321–323. doi: 10.1590/S0004-2803.202202000-58. [DOI] [PubMed] [Google Scholar]
- 21.Rojas M., Rodríguez Y., Zapata E., Hernández J.C., Anaya J.M. Cholangiopathy as part of post-COVID syndrome. J Transl Autoimmun. 2021;4:100116. doi: 10.1016/j.jtauto.2021.100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Da B.L., Suchman K., Roth N., et al. Cholestatic liver injury in COVID-19 is a rare and distinct entity and is associated with increased mortality. J Intern Med. 2021;290:470–472. doi: 10.1111/joim.13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keta-Cov research group Electronic address: vincent.mallet@aphp.fr; Keta-Cov research group. Intravenous ketamine and progressive cholangiopathy in COVID-19 patients. J Hepatol. 2021;74:1243–1244. doi: 10.1016/j.jhep.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulkarni A.V., Khlegi A., Sekaran A., et al. Post COVID-19 cholestasis: a case series and review of literature [published online ahead of print, 2022 Jun 11] J Clin Exp Hepatol. 2022:10. doi: 10.1016/j.jceh.2022.06.004-15. patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadeghi A., Dooghaie Moghadam A., Eslami P., Pirsalehi A., Salari S., Roshandel E. Vasculopathy-related cutaneous lesions and intrahepatic cholestasis as synchronous manifestations in a COVID-19 patient; a case report. Gastroenterol Hepatol Bed Bench. 2020;13:400–404. [PMC free article] [PubMed] [Google Scholar]
- 26.Roda S., Ricciardi A., Maria Di Matteo A., et al. Post-acute coronavirus disease 2019 (COVID 19) syndrome: HLH and cholangiopathy in a lung transplant recipient. Clin Infect Pract. 2022;15:100144. doi: 10.1016/j.clinpr.2022.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamming I., Timens W., Bulthuis M., Lely A., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. The single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to Wuhan 2019-nCoV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H., Li H.B., Lyu J.R., et al. Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. Int J Infect Dis. 2020;96:19–24. doi: 10.1016/j.ijid.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou P., Yang X., Wang X., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chai X., Hu L., Zhang Y., et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. Preprint: bioRxiv. 2020:931766. [Google Scholar]
- 32.Leonhardt S., Veltzke-Schlieker W., Adler A., et al. Trigger mechanisms of secondary sclerosing cholangitis in critically ill patients. Crit Care Med. 2015;19:1–12. doi: 10.1186/s13054-015-0861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiltebrand L.B., Krejci V., Sigurdsson G.H. Effects of dopamine, dobutamine, and dopexamine on microcirculatory blood flow in the gastrointestinal tract during sepsis and anesthesia. ASA. 2004;100:1188–1197. doi: 10.1097/00000542-200405000-00022. [DOI] [PubMed] [Google Scholar]
- 34.Gomersall C.D., Joynt G.M. Epinephrine impairs splanchnic perfusion in septic shock. Crit Care Med. 1998;26:620–621. doi: 10.1097/00003246-199803000-00046. [DOI] [PubMed] [Google Scholar]
- 35.Gaudio E., Franchitto A., Pannarale L., et al. Cholangiocytes and blood supply. World J Gastroenterol. 2006;12:3546–3552. doi: 10.3748/wjg.v12.i22.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Putensen C., Wrigge H., Hering R. The effects of mechanical ventilation on the gut and abdomen. Crit Care Med. 2006;12:160–165. doi: 10.1097/01.ccx.0000216585.54502.eb. [DOI] [PubMed] [Google Scholar]
- 37.Abdelkalik M.A., Elewa G.M., Kamaly A.M., Elsharnouby N.M. Incidence and prognostic significance of intra-abdominal pressure in critically ill patients. Ain Shams J Anaesthesiol. 2014;7:107–113. [Google Scholar]
- 38.Beuers U., Hohenester S., de Buy Wenniger L.J.M., Kremer A.E., Jansen P.L., Elferink R.P.O. The biliary HCO3− umbrella: a unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. J Hepatol. 2010;52:1489–1496. doi: 10.1002/hep.23810. [DOI] [PubMed] [Google Scholar]
- 39.Utkarsh D., Loretz C., Li A.P. In vitro evaluation of hepatotoxic drugs in human hepatocytes from multiple donors: identification of P450 activity as a potential risk factor for drug-induced liver injuries. Chem Biol Interact. 2016;255:12–22. doi: 10.1016/j.cbi.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 40.Kujawski S.A. COVID-19 Investigation Team. First 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Nat Med. 2020;26:861–868. doi: 10.1038/s41591-020-0877-5. [DOI] [PubMed] [Google Scholar]
- 41.Connors J.M., Levy J.H. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemostasis. 2020;18:1559–1561. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams D.H., Hubscher S.G. Systemic viral infections and collateral damage in the liver. Am J Pathol. 2006;168:1057–1059. doi: 10.2353/ajpath.2006.051296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tartey S., Takeuchi O. Pathogen recognition and Toll-like receptor targeted therapeutics in innate immune cells. Int Rev Immunol. 2017;36:57–73. doi: 10.1080/08830185.2016.1261318. [DOI] [PubMed] [Google Scholar]
- 44.Bangash M.N., Patel J., Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529–530. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J., Fan J.G. Characteristics and mechanism of liver injury in 2019 coronavirus disease. J Clin Transl Hepatol. 2020;8:13–17. doi: 10.14218/JCTH.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKenzie J.E., Bossuyt P.M., Boutron I., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boehm E., Kronig I., Neher R.A., Eckerle I., Vetter P., Kaiser L. Geneva centre for emerging viral diseases. Novel SARS-CoV-2 variants: the pandemics within the pandemic. Clin Microbiol Infect. 2021;27:1109–1117. doi: 10.1016/j.cmi.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.