Abstract
Objectives:
Mounting evidence indicates that vascular risk factors (VRFs) are elevated in HIV and play a significant role in the development and persistence of HIV-associated neurocognitive disorder. Given the increased longevity of people living with HIV (PLWH), there is a great need to better elucidate vascular contributions to neurocognitive impairment in HIV. This systematic review and meta-analysis examine relationships between traditional VRFs, cardiovascular disease (CVD), and cognition in PLWH in the combination antiretroviral therapy era.
Methods:
For the systematic review, 44 studies met inclusion criteria and included data from 14,376 PLWH and 6,043 HIV-seronegative controls. To better quantify the contribution of VRFs to cognitive impairment in HIV, a robust variance estimation meta-analysis (N = 11 studies) was performed and included data from 2139 PLWH.
Results:
In the systematic review, cross-sectional and longitudinal studies supported relationships between VRFs, cognitive dysfunction, and decline, particularly in the domains of attention/processing speed, executive functioning, and fine motor skills. The meta-analysis demonstrated VRFs were associated with increased odds of global neurocognitive impairment (odds ratio [OR ]= 2.059, p = .010), which remained significant after adjustment for clinical HIV variables (p = .017). Analyses of individual VRFs demonstrated type 2 diabetes (p = .004), hyperlipidemia (p = .043), current smoking (p = .037), and previous CVD (p = .0005) were significantly associated with global neurocognitive impairment.
Conclusions:
VRFs and CVD are associated with worse cognitive performance and decline, and neurocognitive impairment in PLWH. Future studies are needed to examine these relationships in older adults with HIV, and investigate how race/ethnicity, gender, medical comorbidities, and psychosocial factors contribute to VRF-associated cognitive dysfunction in HIV.
Keywords: Neurocognitive disorders, Cognitive aging, Vascular diseases, Cardiovascular diseases, Highly active antiretroviral therapy, Diabetes mellitus
INTRODUCTION
Combination antiretroviral therapy (cART) has dramatically improved the landscape of HIV-associated mortality and morbidity. In the United States, HIV-associated dementia has become rare, nevertheless, milder forms of HIV-associated neurocognitive disorder (HAND) persist despite viral suppression (Heaton et al., 2010). The persistence of neurocognitive impairment (NCI) and central nervous system (CNS) dysfunction in people living with HIV (PLWH) in the cART era is important as these impairments affect the quality of life and daily functioning, and may impact cognitive aging (Heaton et al., 2004; Thames, Arentoft, Rivera-Mindt, & Hinkin, 2013). Critically, the rates of vascular risk factors (VRFs) and vascular disease are elevated in HIV, which likely substantially contribute to cognitive dysfunction. In the general population, VRFs tend to increase with age and are consistently associated with cognitive dysfunction and dementia (Jefferson et al., 2015). It is estimated that 45% of PLWH in the United States are at least 50-years-old (Centers for Disease Control and Prevention [CDC], 2020). It is unclear if long-term survivors of HIV who present with HAND and VRFs are at a higher risk for neurodegenerative disease, thus, it is important to investigate VRFs as a mechanism for NCI (Cysique & Brew, 2019).
Vascular Risk in HIV
Many studies have documented higher rates of cardiovascular disease (CVD) and cerebrovascular disease among PLWH (Demir et al., 2018; Singer, Valdes-Sueiras, Commins, Yong, & Carlson, 2013). Similarly, HIV infection has been associated with subclinical CVD and elevated rates of VRFs such as dyslipidemia, type 2 diabetes mellitus (T2DM), hypertension, and abdominal obesity (Grinspoon & Carr, 2005; Hanna et al., 2015; Seaberg et al., 2010; Triant, 2013). Though HIV was once associated with wasting, the rates of obesity in HIV populations are now similar to the general population (Crum-Cianflone, Tejidor, Medina, Barahona, & Ganesan, 2008).
Etiology of VRFs and CVD in HIV
Possible mechanisms for the elevated rates of VRFs and CVD include side effects of cART, HIV-specific factors, chronic inflammation, and comorbidities. While cART treatment has prolonged life of PLWH, cART is associated with several cardiometabolic side effects, and increased risk of myocardial infarction (Nou, Lo, & Grinspoon, 2016; Onen et al., 2010). The virus itself is associated with inflammatory changes, and can damage the vascular endothelium and increase arterial stiffness through the modification of aortic wall vascular smooth muscle cell behavior and extracellular matrix composition (Rider et al., 2014). Studies of untreated and cART-naïve HIV populations support the evidence of increased vascular dysfunction (Hsue et al., 2009; Palella & Phair, 2011). Further, there is a strong consensus that immune activation contributes to CVD pathogenesis in HIV (Longenecker, Sullivan, & Baker, 2016). Studies of untreated HIV infection support relationships between immune activation, inflammation, and CVD in HIV (Lang et al., 2012; Lo et al., 2010; Longenecker et al., 2016; Stein & Hsue, 2012). Even with viral suppression and cART, immune activation persists, albeit at a lower level, contributing to chronic low-grade inflammation. Markers of monocyte activation and inflammation are typically lowered by cART, but are still higher in comparison to HIV-seronegative individuals (Nou, Lo, & Grinspoon, 2016). Finally, PLWH may also be at an elevated risk of cardiovascular complications due to poor health behaviors and comorbidities. Rates of cigarette smoking are also increased among PLWH (Soliman et al., 2015). Coinfections and higher rates of substance use/abuse might also augment CVD risk; Hepatitis C, cytomegalovirus, and substance abuse have been associated with cardiovascular dysfunction (Triant, 2013).
Purpose of Review
The elevated rates of VRFs and CVD in HIV are concerning as it may put PLWH at an increased risk for cognitive dysfunction and decline. VRFs are thought to detrimentally affect cognition through direct effects on the vascular system and indirect effects on brain structure and perfusion. Insult to the cardiovascular system can negatively impact cognition through several mechanisms, including ischemic brain damage characterized by white matter lesions, atrophy, and reductions in cerebral blood flow (CBF; Figure 1). In the last 10–15 years, a growing number of studies have examined the contributions of commonly studied VRFs in the relationship between HIV and cognitive function; however, to date, no one has systematically reviewed the literature on this topic. The purpose of the present paper is to better elucidate VRF contributions to cognition among PLWH in the cART era. The paper will provide a qualitative review of the literature on relationships between VRFs, subclinical CVD, and cognition, and a quantitative meta-analysis of VRF contributions to NCI in HIV.
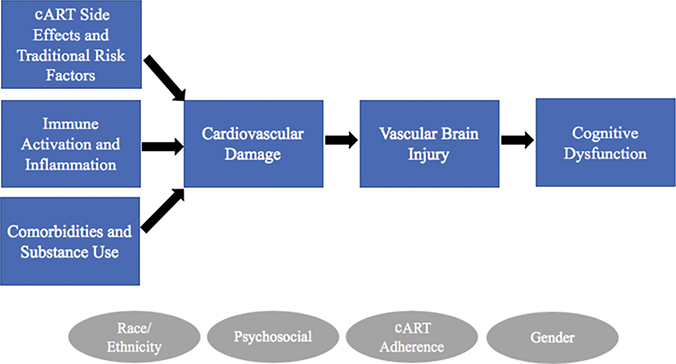
Fig. 1.
Conceptual diagram of vascular contributions to neurocognitive dysfunction in HIV. Diagram illustrates hypothesized relationships between HIV infection, cardiovascular damage, and cognitive dysfunction. Increased prevalence of subclinical CVD (e.g. arterial stiffness, atherosclerosis) and CVD in HIV has multiple etiologies including cART side effects and traditional risk factors (e.g. smoking, type 2 diabetes mellitus, hypertension, dyslipidemia, obesity), immune activation and associated inflammation, comorbidities (e.g. Hepatitis C, cytomegalovirus) and substance use (e.g. cocaine, methamphetamine). Cardiovascular damage leads to brain injury through vascular mechanisms including ischemia (e.g. cerebral small vessel disease, white matter hyperintensities), and reduced blood flow to the brain, which in turn leads to neurocognitive dysfunction. Circles represent additional factors that may influence these relationships.
METHOD
Articles published between January 2000 and June 2020 were identified through searches in SCOPUS, PubMed, and Google Scholar. Search terms included “HIV” or “AIDS”, and “cardiovascular” or cerebrovascular” or “vascular” or “obese” or “obesity” or “diabetes” or “metabolic” or “insulin” or “waist” or “body mass index (BMI)” or “atherosclerosis”, and “cognitive” or “cognition” or “neurocognitive” or “neuropsychological.” Articles were additionally filtered by language (English only), document type (Article only), year of publication (since year 2000), and species if available (Human only). The SCOPUS search produced 390 records, and 2 additional records were identified through Google Scholar and PubMed for a total of 392 records.
Study Selection
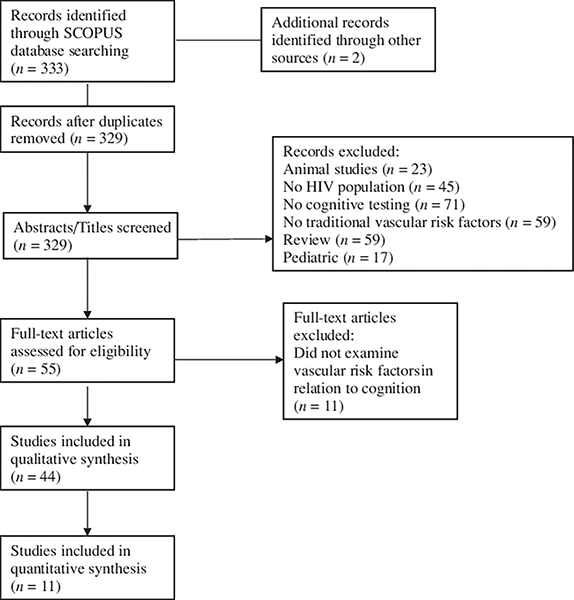
The selected abstracts were reviewed using the following inclusion criteria: human participants; adult population; measurement and/or self-report of traditional VRFs or subclinical CVD; the assessment of neurocognitive functioning using neuropsychological tests; the examination of relationship between VRFs and neurocognition. A total of 44 studies satisfied the selection criteria (Figure 2).
Fig. 2.
PRISMA flow chart of study selection.
For the meta-analytic review, studies were subjected to the following additional selection criteria: data reported on cART usage (e.g. percent on cART), cognition measured by a comprehensive neuropsychological test battery (i.e. >2 cognitive tests and no cognitive screening measures), VRFs quantified with clinically guided cutoffs (i.e. hypertension defined by blood pressure readings and/or prescribed antihypertensives), VRFs based on quantitative measurement or treatment (i.e. self-report not permitted), the assessment of global NCI using normative data, and presence of cross-sectional neurocognitive analyses assessing relationships between VRFs and NCI. A total of 11 studies met criteria and were included in the meta-analysis (Figure 2). Of these studies, over 50% of the sample reported cART use.
Data Extraction and Management
Systematic review
For selected articles, ECM and KT extracted data for qualitative synthesis, including participant demographics, study design, sample size, cART status, HIV variables, and summarized the main findings. Study demographics, methodologies, and results are summarized in Supplementary Material Table 1.
Meta-analysis
For selected articles, ECM and KT independently extracted the following data: total sample size, number of participants per VRF group included in cognitive analyses (i.e. number of participants with T2DM), age (mean and standard deviation or median and interquartile range), race (e.g. percent White), sex, nadir CD4 count, cART use, undetectable viral load (i.e. percent with viral load <50 copies/ml), AIDS history, and country where study was performed. For the majority of studies (N = 8), study reported odds ratios (ORs) were extracted and additional information (e.g. OR confidence interval and sample size) needed to compute variance. For the rest of studies (N = 3; Gomez et al. 2017; Saloner et al., 2019; Yu et al. 2019), ORs were computed through an effect size calculator using other statistics provided in addition to sample sizes (e.g. Cohen’s d, chi-square, and binary proportions with cognitive impairment) (Wilson, n.d.). Once converted, all ORs and confidence intervals underwent log transformation for analysis to ensure symmetry (Borenstein, Hedges, Higgins, & Rothstein, 2010). Standard errors and variances were then calculated using the log-transformed values of the upper and lower OR CI using formulas (Higgins, Li, & Deeks, 2019). Following meta-analysis, log ORs were converted to OR for the ease of interpretation (Borenstein et al., 2010). In longitudinal studies, only baseline data were used as the primary outcome of interest was current cognitive impairment and not decline or future cognitive impairment.
To evaluate the effects of VRFs on cognition, each VRF was assigned to one of the following for subgroup analyses: current smoking, obesity (defined by BMI or waist-to-hip ratio), T2DM (defined by blood glucose, and/or T2DM diagnosis, and/or T2DM treatment), hypertension (defined by blood pressure and/or hypertension treatment), hyperlipidemia (defined by total cholesterol, low-density lipoprotein (LDL) cholesterol, and/or triglycerides, and/or the use of lipid-lowering medication), and the previous history of CVD or event (e.g. myocardial infarction, coronary artery disease, congestive heart failure, peripheral vasculopathy, and angina pectoris). VRFs that had a subgroup sample size of N = 1 or those that could not be classified within the listed subgroups were not included.
Authors also sought to investigate relationship between VRFs and individual cognitive domain performance. Given concern for bias due to limited sample size (N = 3 studies), heterogeneity of studies, and the lack of data for all domains (e.g. learning and memory), domain-specific analyses were not performed. Nevertheless, we summarize the results from these studies in our systematic review.
Meta-Analytic Approach
Meta-analysis was conducted on all studies that included a comparison group that allowed us to examine the unique effect of the VRF in question (e.g. hypertension instead of continuous blood pressure) on NCI. First, a meta-analytic model exploring the overall effect of VRFs on NCI was estimated. Many studies evaluated multiple VRF types (e.g. hypertension and T2DM) within the same study. Notably, effect sizes from the same study and sample are statistically dependent and thus violate the assumption of independence and thus are typically a challenge for conventional meta-analytic approaches (Borenstein et al., 2010). As such, a robust variance estimation (RVE) meta-analytic approach was conducted which allows for a hierarchical approach to within and between study effects’ sizes and thus allows for multiple indicators from a single study (Hedges, Tipton, & Johnson, 2010; Tanner-Smith, Tipton, & Polanin, 2016).
An intercept only hierarchical RVE model was estimated as it adjusts for nonindependence between effect sizes and provides a better estimate of the effect compared to traditional weighting models (Tanner-Smith et al., 2016). A secondary RVE meta-analysis was estimated with the addition of covariates to determine the overall effect of VRFs on NCI after accounting for important HIV variables that included nadir CD4, AIDS diagnosis, and HIV duration. All covariates were mean centered to reduce multicollinearity.
Given the variability present in study selection (e.g. differential definitions of cognitive impairment, sample size, location of sample, and heterogeneous definitions of VRF classifications), a random effects model was run to assess the overall magnitude of effect of VRFs on NCI. This model was run primarily to obtain overall heterogeneity statistics for the RVE meta-analytic model.
Subgroup analyses were performed to examine the unique effects of VRFs and NCI via multiple random effects models. Random effects modeling was chosen a priori as this allows for the consideration of additional studies that were unable to be included (e.g. due to publication bias, language barriers, and “gray literature”) and potential study-level effects that may vary between studies. All models were run using R statistical software (R Core Team, 2014). RVE meta-analyses were estimated using robumeta package and the “robu” function (Fisher, Tipton, & Zhipeng, 2017). Random effects models were run using meta for package using the “rma” function (Viechtbauer, 2010).
Publication Bias
In order to determine the level of publication bias present in this current study, a number of quantitative estimates were employed. First, visual inspection of the funnel plot (Borenstein et al., 2010) was conducted to assess for asymmetry. Next, rank correlation test for publication bias was used to estimate Kendall’s tau (Begg & Mazumdar, 1994). Finally, in order to test the robust nature of our estimates, Rosenthal’s Fail-safe N was also calculated (Borenstein et al., 2010; Rosenthal, 1979).
RESULTS
Systematic Review Findings
Type 2 diabetes mellitus
Several cross-sectional analyses demonstrated relationships between T2DM, prediabetes, insulin resistance, and poorer global cognition, and particularly worse performance on tasks assessing memory, attention/psychomotor speed, executive functioning, and fine motor skills (Dufouil et al., 2015; Fabbiani et al., 2013; Gomez, Power, Gill, & Fujiwara, 2017; McCutchan et al., 2012; Saloner et al., 2019; Schouten et al., 2016; Valcour et al., 2006, 2012; Yu et al., 2019). However, one study examining relationships between VRFs and HAND status did not find any relationships with T2DM or any VRF examined (Ciccarelli et al., 2019). The relationship between T2DM and HIV-associated dementia was particularly pronounced in late middle-aged and older adults (Valcour et al., 2005).
Longitudinal studies reported relationships between dysglycemia and cognitive decline. Dufouil et al. showed T2DM was independently related to accelerated decline on attention, executive functioning, and memory tests. Poorly managed T2DM was associated with decline on tasks assessing psychomotor speed/attention, and executive function in both HIV-status groups (Yang et al., 2018). Baseline hyperinsulinemia, impaired insulin sensitivity, and insulin resistance were each associated with cognitive decline in PLWH without a known history of T2DM (Khuder et al., 2019).
Few studies examined interactions between HIV and T2DM on neurocognitive performance. The Hawaii Aging with HIV cohort reported HIV and T2DM were independent predictors of global cognitive function, but no interactive relationship was found (Nakamoto et al., 2011). In contrast, the Women’s Interagency HIV Study (WIHS) cohort reported an interaction between HIV and insulin resistance on working memory and attention/processing speed (Valcour et al., 2015). Previously, the same cohort found HIV status did not modify the relationship between insulin resistance and an attention/processing speed task using an abbreviated battery (Valcour et al., 2012).
Hypertension and dyslipidemia
Two studies demonstrated relationships between hypertension and cognitive dysfunction in middle-aged adults with HIV (Ding, Lin, Shen, et al., 2017; Wright et al., 2010). Studies among late middle-aged and older adults similarly reported relationships between elevated blood pressure and cognitive dysfunction (Montoya et al., 2017; Nakamoto et al., 2011). Similar findings were reported for both PLWH and HIV-seronegative controls in these cohorts. Another study did not find any association between hypertension and global cognition (Sanford et al., 2019).
Two cross-sectional studies cited relationships between dyslipidemia, lipodystrophy, and worse cognitive functioning or HAND status (Gomez et al., 2017; Wright et al., 2010). The Multicenter AIDS Cohort Study (MACS) reported hypercholesterolemia was associated with cognitive decline in domains of attention and working memory in both HIV-infected and uninfected men, but men living with HIV exhibited faster cognitive decline which was attenuated with statin use (Mukerji et al., 2016). Similarly, another cohort reported baseline dyslipidemia was marginally associated with cognitive impairment at follow-up, and was a risk factor for memory impairment (Ciccarelli et al., 2015).
Obesity and adiposity
In the CNS HIV Antiretroviral Therapy Effects Research (CHARTER) sample, greater waist circumference and lower BMI were associated with NCI (McCutchan et al., 2012). The CHARTER sample later reported waist circumference was associated with global deficit score in abdominally obese individuals, and waist circumference affected global cognition directly and indirectly via inflammatory marker IL-6 (Sattler et al., 2015). Similarly, the AGEhIV cohort reported elevated waist-to-hip ratio was associated with reduced cognitive performance (Su et al., 2016). In a cohort of largely cART-naïve participants, both underweight and overweight/obese individuals had greater rates of cognitive impairment (Jumare et al., 2019). An Italian cohort reported higher baseline BMI was associated with memory decline over two years (Ciccarelli et al., 2015). However, some studies reported null findings between obesity, BMI, and waist circumference and cognition (Fabbiani et al., 2013; Sanford et al., 2019). Similarly, the male MACS study did not find an association between regional adipose tissue and neurocognition for either HIV-status group (Lake et al., 2015).
In contrast, some studies reported adiposity was somewhat protective for cognitive function. WIHS did not consistently find relationships between adiposity and worse cognition in middle-aged women living with HIV. Results showed that BMI-defined obesity, elevated waist circumference, and higher waist-to-hip ratio were mostly associated with better performance on processing speed and executive functioning tasks in PLWH (Gustafson et al., 2013). Similarly, another study reported that higher BMI was associated with better global cognitive performance among cART-naïve individuals with HIV (Wright et al., 2015). In the MACS cohort, adiposity was associated with worse motor function in PLWH in cross-sectional analyses, though adiposity appeared protective against decline in motor skills over follow-up (Rubin et al., 2019).
Tobacco smoking
A few cross-sectional studies demonstrated tobacco use was associated with worse cognitive function or impairment, which was observed in tasks assessing motor function, psychomotor/processing speed, and global cognition (Ding, Lin, Shen, et al., 2017; Fabbiani et al., 2013; Nakamoto et al., 2011). In contrast, the SMART cohort reported smoking was associated with worse timed gait, but not with NCI (Wright et al., 2010).
Cumulative VRF burden
Similar to HIV-seronegative populations, studies reported the presence of multiple VRFs or cerebrovascular disease was associated with cognitive impairment (Elicer, Byrd, Clark, Morgello, & Robinson-Papp, 2018; Fabbiani et al., 2013). The Strategic Timing of Antiretroviral Treatment trial found Framingham risk score and T2DM were independently associated with worse neuropsychological performance (Wright et al., 2015). Another study showed the presence of at least one VRF was associated with worse processing speed performance. Participants with untreated vascular risk performed worse than those with treated VRFs on multiple domains including processing speed, learning/memory, and executive functioning (Foley et al., 2010). Finally, the presence of at least one VRF was associated with information processing speed in younger, but not older adults with HIV (Patel et al., 2013).
Subclinical CVD and atherosclerosis
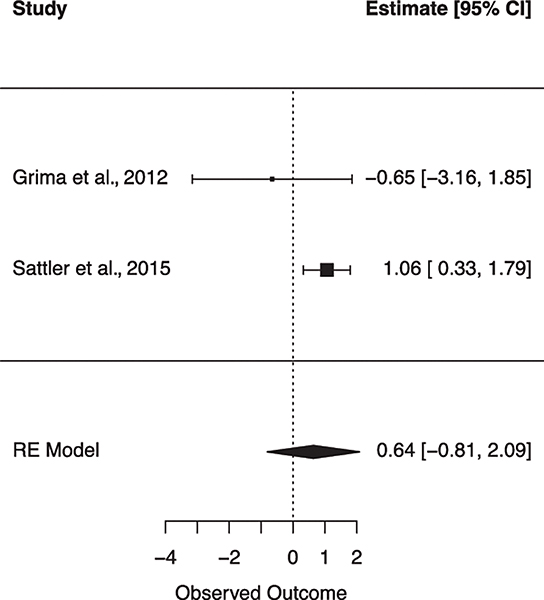
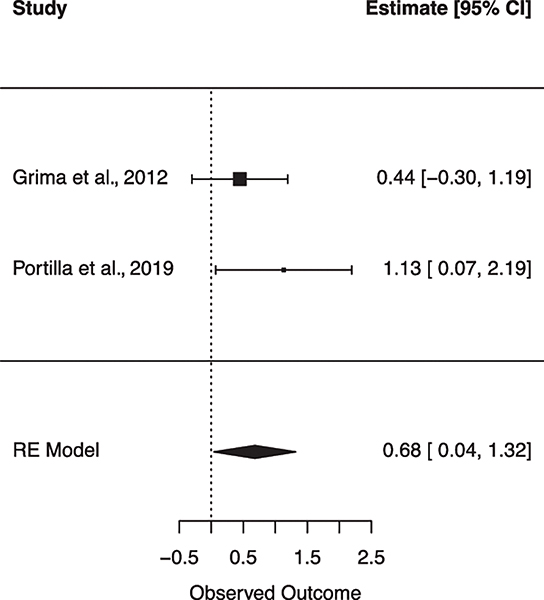
Several studies reported relationships between subclinical CVD and cognitive performance. In the MACS sample, carotid intima media thickness (cIMT) was associated with worse processing speed (Becker et al., 2009). In the WIHS, carotid lesions and cIMT were related to worse performance on an executive functioning task, but not processing speed (Crystal et al., 2011). A longitudinal study showed abnormal cIMT was associated with baseline cognitive impairment, and with cognitive and memory impairment at a 2-year follow-up (Ciccarelli et al., 2015; Fabbiani et al., 2013). The same team of investigators reported increased ophthalmic artery resistance index was independently associated with the risk for cognitive impairment, specifically in domains of attention/executive functioning, and psychomotor speed (Grima et al., 2012). Another group showed subclinical atherosclerosis was associated with worse-delayed recall in participants without any history of hypertension and/or T2DM (Portilla et al., 2019). In contrast to the findings reported above, one study did not find an association between cIMT and cognition (Yaldizli et al., 2006).
WIHS reported greater carotid stiffness was associated with cognitive decline over 10 years on attention/processing speed, executive functioning, and psychomotor speed tasks regardless of HIV status. Though women with HIV evidenced greater stiffness, HIV status did not modify the relationship between baseline carotid stiffness and cognitive decline (Huck et al., 2018). Another study reported pulse pressure, a surrogate marker of carotid stiffness, had a quadratic relationship with cognition such that lowest and highest pulse pressure was associated with worse fine motor function, processing speed, and executive functioning (Montoya et al., 2017).
Neuroimaging, VRFs, and cognition
Studies reported greater white matter hyperintensities (WMH) load was associated with worse global cognition (Sanford et al., 2019; Su et al., 2016), and worse executive functioning, and psychomotor speed (Watson et al., 2017). Increased WMH load was associated with prior immune deficiency in one study (Su et al., 2016), but was not related to any HIV clinical markers in another (Sanford et al., 2019). HIV status did not modify relationship between WMH and cognition in these studies. In Su et al., HIV-related differences in cognition were attenuated when adding WMH to model suggesting WMH may mediate relationship between HIV and cognitive dysfunction. Interestingly, the appearance, location, and the pattern of WMH in men with HIV looked similar to controls and PLWH with vascular cognitive impairment, suggesting that WMHs in HIV may reflect cerebral small vessel disease (Pantoni, 2010).
A functional neuroimaging study reported elevated waist circumference and triglycerides, and history of AIDS, was associated with a decreased CBF among PLWH. However, there was no association between cognitive scores and CBF (Su et al., 2017). A MACS structural neuroimaging study reported VRFs, including T2DM and hypertension, were not related to brain volume, which may have been due to low amount of risk factors present in the sample (Becker et al., 2012). In another cohort of late middle-aged PLWH, glucose dysfunction was associated with white matter microstructural abnormalities in the hippocampus and caudate. However, these abnormalities were not associated with cognitive functioning (Nakamoto et al., 2012).
HIV-by-VRF interactions
Only two studies reported interactions between HIV status and VRFs, although there is reason to suspect interactive effects. HIV-associated brain injury may lower the threshold of cognitive reserve thereby amplifying the negative effects of VRFs on cognition (Mukerji et al., 2016; Satz et al., 1993; Valcour et al., 2015). Valcour et al. reported there was an interaction between HIV status and insulin resistance on attention and processing speed tasks where PLWH performed worse, and insulin resistance interacted with nadir CD4 count on attention/processing speed. Mukerji et al. showed an interaction between HIV status and cholesterol levels such that PLWH with elevated cholesterol declined at a faster rate than HIV-seronegative controls. In contrast, other studies reviewed here either did not test for or did not find evidence of an interaction between HIV and VRFs on cognition (Nakamoto et al., 2011; Watson et al., 2017).
Meta-Analytic Findings
Overall effects
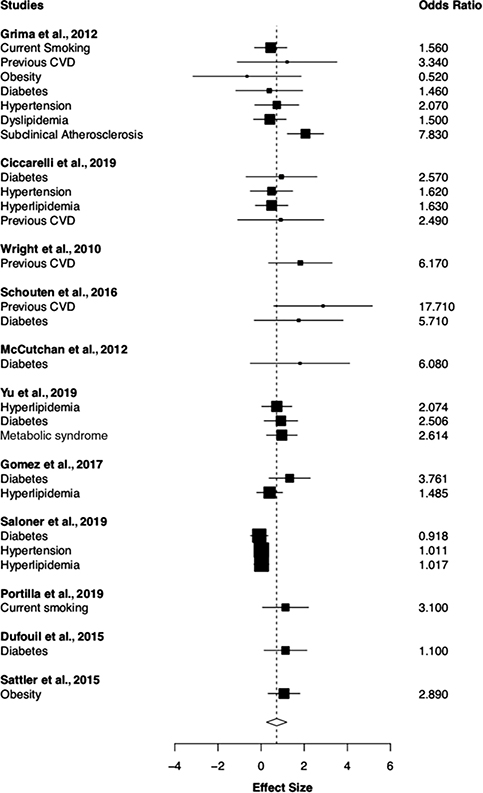
The results for the RVE models including covariates are shown in Table 1. Eleven studies reported 26 VRF effect sizes for NCI with a total sample size of 2139 participants. The individual effect sizes can be found in the forest plot shown in Figure 3. The I2 estimate of variance was suggestive of moderate heterogeneity of effect sizes (I2 = 59.28%), suggesting moderator or subgroup analyses may help to explain additional variance (Higgins, Thompson, Deeks, & Altman, 2003). The first RVE model estimated the overall effect of VRFs on NCI. Results indicate VRFs were associated with increased odds of NCI (OR = 2.059, p = .010). Next, we included covariates of AIDS diagnosis, HIV duration, and nadir CD4 count. None of the covariates were statistically significant with respect to our outcome variable of NCI. Inclusion of covariates did not change the magnitude of the overall effect (OR = 2.15, p = .017); however, the degrees of freedom were less than 4, suggesting the results should be interpreted with caution due to potential power limitations for the adjusted model (Tanner-Smith et al., 2016).
Table 1.
Overall effect of vascular risk factors on global neurocognitive impairment
| Variable | I2 (%) | k | # of effect sizes | OR | log(OR) | p | 95% CI | df |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Unadjusted intercept | 59.28 | 11 | 26 | 2.059** | 0.722 | .009 | [1.288–3.287] | 5.65 |
| Adjusted intercept | - | 9 | 24 | 2.136* | 0.759 | .017 | [1.351–3.989] | 2.30 |
| AIDS (proportion) | - | - | - | 2.239 | 0.806 | .611 | [0.005–1009.59] | 1.88 |
| Nadir CD4 (cells/microliter) | - | - | - | 1.008 | 0.008 | .335 | [0.984–1.033] | 2.58 |
| HIV duration (mean/median) | - | - | - | 0.971 | −0.029 | .669 | [0.005–1009.59] | 2.51 |
Note: The unadjusted intercept value is the overall effect size. Adjusted intercept refers to overall effect size after accounting for listed covariates. Models with degrees of freedom <4 are not trustworthy. All models were run separately and estimated using robust variance estimation meta-analysis. Abbreviations: k = number of outcomes included in the categoryOR = odds ratio; CI = confidence interval of the OR; df = degree of freedom; AIDS = acquired immune-deficiency syndrome; HIV duration = human immunodeficiency virus years since diagnosis.
p < .01.
p < .05.
Fig. 3.
Flow chart detailing study selection procedures. Forest plot for RVE meta-analysis examining the overall effect of all vascular risk factors on global cognitive impairment. Effect size = log(OR).
Subgroup analyses results
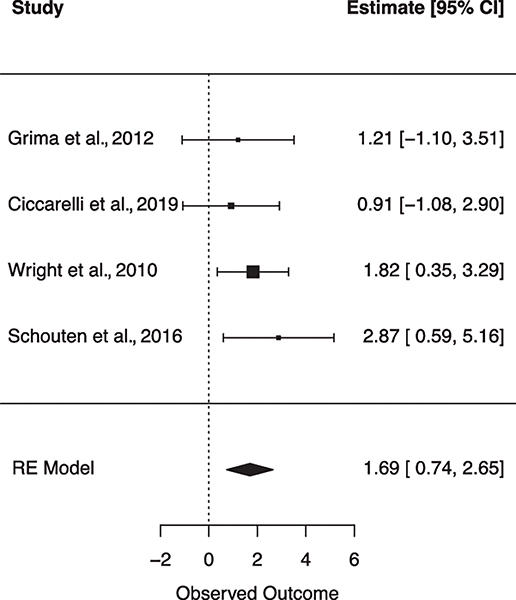
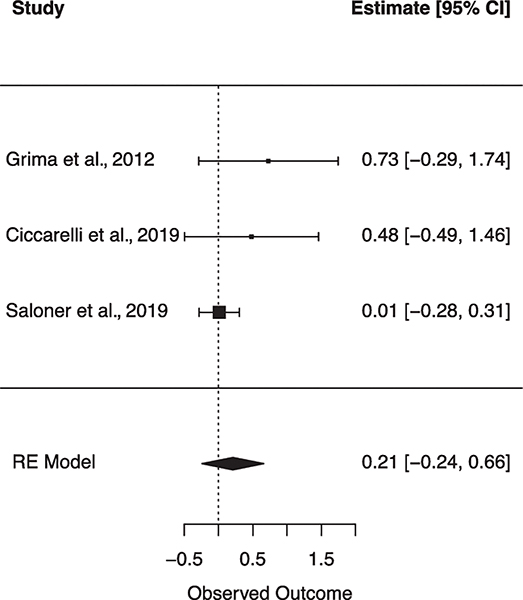
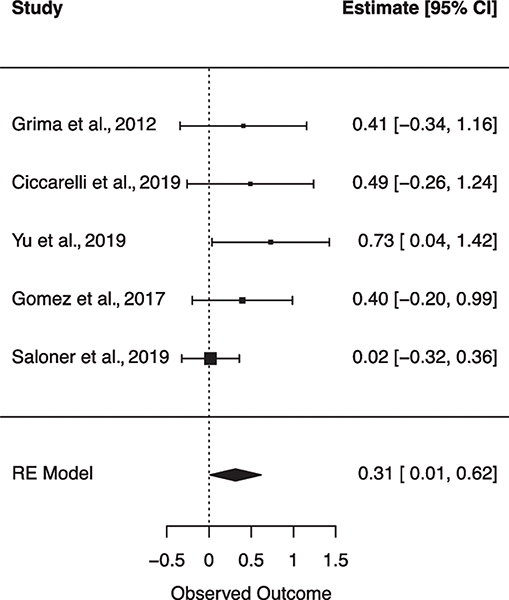
Six subgroup random effects meta-analyses were run to explore the unique relationships between individual VRFs on NCI (Table 2). Figures 4–9 show forest plots for each subgroup VRF. First, we examined the effect of T2DM on NCI (N = 8 studies). The I2 suggests there was moderate heterogeneity between effect sizes. Results from this model indicated that for PLWH, having T2DM incurs a 2.238 increase in odds for NCI. Next, the effect of hyperlipidemia on NCI was estimated (N = 5 studies). The I2 suggests there was minimal heterogeneity between effect sizes. Results from this model revealed that for PLWH, having hyperlipidemia increased odds for NCI by 1.369. Next, the effect of current smoking was examined (N = 2 studies). The I2 suggests there was minimal heterogeneity between effect sizes. Results from this model revealed current smoking is associated with a 1.973 increase in odds for NCI. finally, the effect of previous CVD was examined (N = 4 studies). The I2 suggests there was a very minimal heterogeneity between effect sizes. Results from this model revealed a history of previous CVD increased the odds of NCI by 5.423. There were no significant associations found for hypertension (N = 2 studies) or obesity (N = 2 studies) on NCI.
Table 2.
Subgroup meta-analyses of individual vascular risk factors on global neurocognitive impairment
| Vascular risk factor | k | I2 (%) | OR | log(OR) | p | 95% CI | Q(df) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Diabetes | 8 | 51.66 | 2.238** | 0.806 | .004 | [0.262–1.349] | 16.493* (7) |
| Hypertension | 3 | 28.36 | 1.233 | 0.209 | .363 | [0.785–1.938] | 2.407 (2) |
| Hyperlipidemia | 5 | 26.35 | 1.369* | 0.314 | .043 | [1.011–1.854] | 4.415 (4) |
| Obesity | 2 | 39.74 | 1.894 | 0.639 | .387 | [0.445–8.062] | 1.659 (7) |
| Current smoking | 2 | 7.03 | 1.973* | 0.679 | .037 | [1.042–3.737] | 1.076 (1) |
| Previous CVD | 4 | 0.00 | 5.423*** | 1.691 | <.001 | [2.086–14.095] | 1.819 (3) |
Note: All models were run separately and estimated using random effects meta-analysis. Abbreviations: k = number of outcomes included in the category; OR = odds ratio; CI = confidence interval of the OR; Q-statistic = null hypothesis of homogeneity, larger values represent more heterogeneity across effect sizes; CVD = cardiovascular disease.
p < .001.
p < .01.
p < .05.
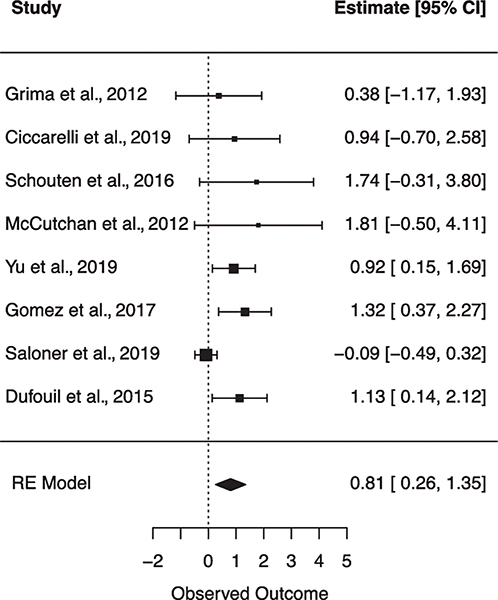
Fig. 4.
Forest plot for random effects meta-analysis examining the effect of type 2 diabetes mellitus on global cognitive impairment. Observed outcome = log(OR).
Fig. 9.
Forest plot for random effects meta-analysis examining the effect of previous cardiovascular disease on global cognitive impairment. Observed outcome = log(OR).
Risk of Publication Bias
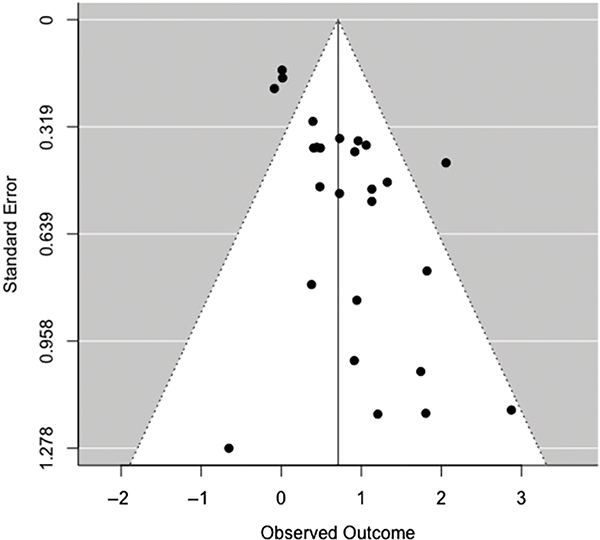
For the omnibus effect of VRFs on NCI, Rosenthal’s Fail-safe N was 560, indicating the number of missing studies required to nullify the effect (Rosenthal, 1979). Begg and Mazumdar’s (1994) rank correlation (τ = 0.225, p = .113) does not indicate significant bias. However, limited power due to small sample sizes may influence this measure, and thus, nonsignificant findings cannot be fully attributable to an absence of bias in this context. Further, examination of the funnel plot (Figure 10) demonstrates asymmetry, which suggests potential for minimal-to-modest publication bias within our sample (Duval & Tweedie, 2000a, 2000b).
Fig. 10.
Funnel plot of random-effects meta-analysis examining the overall effect of VRFs on global cognitive impairment.
DISCUSSION
The present review demonstrated that VRFs, subclinical CVD, and history of CVD are associated with cognition in PLWH similar to uninfected populations.
T2DM, Prediabetes, and Insulin Resistance
In summary, 15 studies reported associations between T2DM, prediabetes, or insulin resistance and cognitive dysfunction, particularly in the domains of attention/processing speed, psychomotor speed, executive functioning, working memory, and memory. These effects were independent of other traditional VRFs and HIV-specific factors presenting strong evidence that T2DM, as well as prediabetes and insulin resistance, adversely affects cognitive function in PLWH. Consistent with literature among uninfected populations, poorly managed T2DM was also related to worse cognition (Yang et al., 2018). Based on limited evidence for interactive effects, it is possible that HIV infection and T2DM may pose an additive rather than synergistic effect on cognition (Nakamoto et al., 2011).
Future studies of T2DM and related conditions and cognition in HIV will benefit from longitudinal studies, and appropriate control groups as differences in cognition can be influenced by psychosocial variables including socioeconomic status (SES), education quality, race/ethnicity, and diet.
Hypertension
Somewhat surprisingly, few studies cited hypertension as a correlate of cognitive dysfunction in HIV (Ding, Lin, Liu, et al., 2017; Montoya et al., 2017; Nakamoto et al., 2011; Wright et al., 2010). It is possible that the prevalence of hypertension was low given that the participants were largely in middle to late middle age, and may have been too young to observe commonly cited relationships between hypertension, cognitive dysfunction, and dementia seen in in older adults (Kivipelto et al., 2001; Raz, Rodrigue, & Acker, 2003; Taki et al., 2004). Hypertension is not as highly cited as a side effect of cART compared to other cardiometabolic factors (Nou et al., 2016). Stratification by age in future studies may help to elucidate whether hypertension plays a larger role in cognitive impairment in older adults.
Dyslipidemia
Similar to hypertension, the relationship between dyslipidemia and cognitive function was not as highly cited as T2DM. However, of the studies that examined dyslipidemia, there were documented relationships between dyslipidemia and cognitive functioning in PLWH (Ciccarelli et al., 2015; Gomez et al., 2017; Mukerji et al., 2016; Wright et al., 2010). This is in contrast to studies of HIV-seronegative adults, whereby the relationship between dyslipidemia and cognitive dysfunction has been less consistent (van den Berg, Kloppenborg, Kessels, Kappelle, & Biessels, 2009). These findings highlight that dyslipidemia in patients with HIV is different from the general population, due to the fact that HIV treatment not only may induce dyslipidemia but also may interact with lipid-lowering medication (Husain & Ahmed, 2015). HIV-related dyslipidemia is increasingly recognized as a problem in patients on cART, particularly those on PI-based regimens (Calza, Manfredi, & Chiodo, 2004).
Thus, it is possible that dyslipidemia may be a stronger predictor of cognitive function among PLWH, and that cholesterol-driven cognitive decline may increase with age. The inclusion of younger adults in this literature may obscure the relationship between dyslipidemia and cognitive dysfunction.
Tobacco smoking
Though only three studies reported relationships between tobacco smoking and cognition, the majority of these studies reported smoking was associated with slowed motor speed (Fabbiani et al., 2013; Nakamoto et al., 2011; Wright et al., 2010). Consistent with these findings, cigarette smoking in HIV-seronegative adult populations has also been negatively associated with several neurocognitive domains, including processing speed and fine motor dexterity (Durazzo, Meyerhoff, & Nixon, 2012). These results are consistent with imaging findings that cigarette smokers have less striatal dopamine transporter availability (Yang et al., 2008). Given the elevated rates of smoking in HIV, smoking should also be considered when assessing HIV-related impairment in motor function.
Obesity and adiposity
This review demonstrated complex relationships between obesity, BMI, and fat distribution in HIV. Some results were consistent with hypotheses (e.g. visceral fat negatively associated with cognition) (Ciccarelli et al., 2015; Okafor et al., 2017; Sattler et al., 2015; Schouten et al., 2016), while others found the opposite (i.e. obesity associated with better cognition) (Gustafson et al., 2013; Lake et al., 2015; Wright et al., 2015). There are several possible reasons for these conflicting findings. BMI has been shown to have complex, nonlinear relationships with cognition in HIV-seronegative populations; both lower than and higher than normal BMIs have been associated with worse cognition (Gustafson, 2008). Further complicating this picture, while higher BMI in midlife has been found to be detrimental, higher BMI may be protective in later life thus differences in cohort demographics may explain some inconsistencies (Fitzpatrick et al., 2009). In the studies reviewed here, studies differed in how they defined and quantified adiposity. Additionally, HIV-associated lipodystrophy may distort traditional relationships between visceral fat and cognition. Lake et al. demonstrated higher adiponectin levels were unexpectedly associated with worse cognition in PLWH, and adiponectin was not correlated with visceral adipose tissue. Further, inflammatory markers were not correlated with visceral adipose tissue among men with HIV, but the expected relationship was reported in controls. Though little can be drawn from just one study, the results suggest that there may be adiponectin dysfunction that could obscure expected relationships. Additional research is needed to determine if adiposity has similar relationships with cognitive function across the lifespan among HIV-seronegative populations.
Cumulative VRF Burden
Increased vascular risk burden was consistently associated with worse cognition similar to HIV-seronegative populations (Fabbiani et al., 2013; Foley et al., 2010; Kaffashian et al., 2011; Unverzagt et al., 2011; Wright et al., 2015). Though these findings are intuitive, it is important to recognize the detrimental effects of multiple risk factors given multimorbidity in HIV.
Subclinical CVD and Atherosclerosis
Overall, there was a consistent evidence that subclinical CVD was associated with cognitive function in HIV (Becker et al., 2009; Ciccarelli et al., 2015; Crystal et al., 2011; Fabbiani et al., 2013; Grima et al., 2012; Huck et al., 2018; Montoya et al., 2017). These results suggest that atherosclerosis and arterial stiffening may play an important role in the development of neurocognitive dysfunction in the context of HIV. Despite associations between HIV and cIMT and vascular stiffness, none of the studies reported an interaction between HIV status and subclinical CVD measures on cognition.
Neuroimaging, VRFs, and Cognition
All neuroimaging studies reviewed showed an evidence of brain injury or abnormalities in PLWH. Most studies also showed that brain measures were associated with cognitive functioning and/or VRFs. Studies of WMH were particularly strong as they related WMH load to both VRFs and cognition, suggesting WMHs may be a mechanism driving relationships between VRFs and cognition (Su et al., 2016; Watson et al., 2017). While some abnormalities shown in HIV (e.g. increased WMH and reduced CBF) likely have a vascular origin, it is important to note that HIV infection can enter the CNS and may cause irreversible damage to the brain, particularly the white matter, where damage could have occurred at the peak of immune deficiency and viral toxicity prior to cART treatment. Further, even without detectable plasma viral replication, there is an evidence that the CNS can serve as a reservoir of viral replication. Given similar patterns of WMHs in subcortical regions observed in HIV and vascular disease, it is important that neuroimaging studies in the context of HIV look for other indicators of vascular disease to distinguish whether white matter abnormalities are vascular in origin, or related to HIV-specific processes such as legacy effect or continued replication in the CNS.
Meta-Analysis
The meta-analytic results largely confirmed findings in the systematic literature review; VRFs were related to NCI in HIV, which was driven by several VRFs including T2DM, hyperlipidemia, current smoking, and previous CVD. Interestingly, hyperlipidemia was a significant risk factor for NCI, and may be more relevant to cognitive health among PLWH compared to uninfected populations as discussed above. Importantly, the relationship between VRFs and impairment remained significant after accounting for HIV clinical variables suggesting that VRFs independently contribute to impairment. Though this meta-analysis had a relatively small sample size, it is the first paper to quantitatively document independent VRF contributions to cognitive impairment among PLWH, supporting the importance of VRFs as a contributor to HAND. As research continues in this field, future research should attempt to quantify VRF contributions to specific domain impairments.
Opportunities for Future Research
An important limitation of the current literature is the lack of HIV-seronegative control groups. A large proportion of studies in this review (N = 21 studies, 47.7%) did not include control groups. Further, of those that did include control groups, several studies did not provide data needed (e.g. demographic information) on both their HIV-infected and HIV-seronegative groups to determine whether groups were similar on variables related to cognition. Well-matched control populations will be critical in elucidating whether PLWH with VRFs are at an increased risk for cognitive dysfunction or dementia. This will prove important in understanding the etiology of HAND and implications for cognitive aging. Controls should be matched, to the extent possible, on past and current SES factors (e.g. education quality, income, and access to healthcare), drug abuse, comorbidities, and psychiatric issues. However, even with well-matched controls, there are certain factors that will confound this question. One of the most important confounds is the survival effect. PLWH with more advanced illness (e.g. AIDS) may have died prematurely due to the lack of access to treatment or comorbid conditions. It is also important to recognize generational differences. Many older adults who acquired HIV decades ago did not get treatment immediately, and were treated with antiretroviral drug classes known to cause cardiometabolic side effects. In contrast, newly diagnosed HIV patients are treated with cART immediately, and may never take medications known to cause metabolic syndrome. Thus, the question of whether HIV poses a synergistic or interactive effect on cognition will likely depend on when people acquired HIV and their HIV treatment history.
Related to the paucity of control groups, few studies reported investigating interactions between HIV status and VRFs on cognition. This is important to further explore as HIV status and vascular risk both independently affect cognition, and there may be synergistic effects. Also, the effects of VRFs on cognition may vary by HIV severity. PLWH who are classified as having asymptomatic NCI or no impairment may present similarly to controls with respect to the effects of VRFs on cognition. PLWH with mild to moderate forms of cognitive impairment may show more severe impairments in the context of VRFs due to lower cognitive reserve. As previously discussed, VRFs, particularly T2DM and multiple VRFs, are strongly tied to increased risk for dementia in older adults. Thus, it is critical to assess whether older adults with HIV and vascular risk are at a greater risk for dementia compared to HIV-seronegative peers with similar VRF profiles.
Race/ethnicity was not a focus in any studies reviewed. In the United States, PLWH are disproportionately African–American; 44% of PLWH are African–American, though they comprise only 12% of the population (CDC, 2017). A few studies in North America were predominantly non-Hispanic white (N = 6, 13.6%), and thus, may be unrepresentative of the larger HIV population. Additionally, there were several studies that did not provide information about race/ethnicity, particularly European cohort studies (N = 10, 22.7%), where participants were likely predominantly non-Hispanic white. Atherosclerotic disease outcomes and the prevalence of VRFs differ between ethnic groups (Gijsberts et al., 2015). In the Veterans Aging Cohort Study, researchers reported that race/ethnicity modified the relationship between HIV-specific risks for negative health outcomes in HIV and cognition (Marquine et al., 2018). Future studies are needed to examine how race/ethnicity modifies relationships between VRFs and cognition in HIV as it could inform treatment or identify racial disparities.
Socio-environmental factors play an important role in the development of vascular and cognitive risks. Many of the studies reviewed were careful to consider psychiatric comorbidities (e.g. substance use and depression) and educational factors such as educational attainment or literacy. Education is a particularly important contributor to cognition and strongly related to SES. Unfortunately, it was difficult to compare study populations on level of education. A large study of treated PLWH showed 25% of patients had less than a high school education, and 19% of patients had a college degree or more (Cunningham et al., 2005). In contrast, some cohorts in this review were more highly educated. For example, MACS reported 45% of their HIV-infected sample had a college degree, and the UCSF cohort reported a mean of 16.4 years of education (e.g. Bachelor’s degree) for their HIV-infected sample raising issues for generalizability (Becker et al., 2009; Watson et al., 2017). Additionally, years of education is not the best measure of premorbid functioning due to differences in education quality and geographic variation; it would be helpful if studies included tests assessing premorbid intellectual functioning or estimated intellectual quotient. Further, poverty and other indicators of SES and exposure to trauma were less attended to across studies. PLWH are disproportionately affected by poverty, limited resources, violence, and trauma which are related to worse cognition (Tedaldi, Minniti, & Fischer, 2015). Stress is strongly tied to these social issues and impacts both vascular health and cognition (Dimsdale, 2008). Both acute and chronic stress are associated with increased pro-inflammatory markers which, over time, cause cellular damage in the periphery and CNS via oxidative stress and microglia activation (Valdez, Rubin, & Neigh, 2016). Adding measures of stress, whether through biological markers (e.g. cortisol) or questionnaires, may further elucidate relationships between VRFs and cognition in HIV.
Sex differences are another gap in the literature that warrants attention. An examination of sex/gender showed that a large number of studies (N = 21, 47.7%) were predominantly male (e.g. >75%). While HIV is more common in males in developed countries such as the United States (CDC, 2018), the dearth of female participants in these studies is troubling. HIV-associated cardiovascular risk may be at least as high in women (Triant, Lee, Hadigan, & Grinspoon, 2007; Womack et al., 2014). Further, the D:A:D study highlighted decreased rates of cardiovascular interventions in women with HIV highlighting a potential gender disparity in VRF treatment (Hatleberg et al., 2014). If women living with HIV are indeed at a higher risk for vascular disease, then it is important that studies include more women, and investigate how this may predispose this population to greater risk of dementia. Further, it is important to evaluate if sex modifies relationships between VRFs and cognition in HIV.
Future work may also clarify if relationships between vascular dysfunction and cognition in HIV are modified by age. Participant age generally ranged from middle-age to older age, which may have washed out age-specific effects. Since vascular risk increases with age, older adults with HIV may be especially vulnerable to vascular-associated cognitive dysfunction.
Investigation of VRF treatment in HIV as it relates to cognition is also warranted. Few studies differentiated between treated versus untreated VRFs. However, the few studies that did account for VRF pharmacological treatment or management showed that participants with untreated or poorly managed VRFs performed worse on cognitive tasks, while treatment attenuated relationships between VRFs and cognition (Foley et al., 2010; Mukerji et al., 2016; Yang et al., 2018). These findings are consistent with literature in HIV-seronegative adults (Duron & Hanon, 2010; McIntosh & Nation, 2019).
Another important limitation of these studies is information related to HIV severity and treatment. Important HIV-related factors such as nadir and current CD4, viral load, cART adherence, and cART medications were inconsistently reported, yet each of these variables has been shown to be related to cognitive functioning and/or vascular risk. Furthermore, research in this area might consider the role of clinical AIDS as a modifier of the relationship between VRFs and cognition. Though there is a likely survivor bias as discussed above, it would be interesting to examine if clinical AIDS moderates the relationship between vascular risk and cognitive impairment; PLWH who were diagnosed with clinical AIDS may be more susceptible to vascular-related cognitive deficits. Future studies in this literature should include these clinical characteristics in order to properly characterize the role of VRFs and CVD on cognition in HIV.
Conceptual Model
The etiology of cardiovascular damage in HIV infection is complex and multifactorial with suspected contributions from cART side effects and traditional risk factors (e.g. smoking, diet, and sedentary lifestyle), chronic immune activation and inflammation, and medical comorbidities (e.g. hepatitis C, and cytomegalovirus) and substance abuse (e.g. stimulants). Although this review focused on more commonly studied risk factors, multiple risk factors in HIV-infected populations likely contribute to the observed premature atherosclerosis and arterial stiffness. As in HIV-seronegative populations, cardiovascular damage may inflict vascular brain injury through ischemic injury (e.g. cerebral small vessel disease and WMHs) and reduced hypoperfusion leading to neurocognitive dysfunction (Figure 1). Relationships between cardiovascular damage and neurocognitive dysfunction in PLWH may be influenced by several factors including race, gender, psychosocial risk factors (e.g. stress and healthcare disparities), and cART adherence. Future research on vascular contributions to HAND should explore independent and interactive effects of these additional variables on direct and indirect relationships between cardiovascular risk and neurocognitive dysfunction.
CONCLUSIONS
In summary, this systematic review and meta-analysis showed commonly studied VRFs and subclinical CVD are associated with neurocognitive dysfunction and impairment among PLWH. Despite better metabolic profiles in PLWH taking newer cART medications, elevated rates of VRFs and CVD in HIV will likely remain problematic due to chronic immune activation and inflammation in the context of low viremia. Research shows that HIV infection and VRFs independently negatively affect cardiovascular health and cognition, but it is presently unknown whether HIV and VRFs have an additive or synergistic effect on cognition. This question is particularly important as the HIV population ages, and thus, should be a focus of future research. Additionally, future studies should address how race/ethnicity, sex, medical comorbidities, drug use, SES, and psychosocial factors moderate relationships between CVD risk and cognition in this unique population.
Supplementary Material
Fig. 5.
Forest plot for random effects meta-analysis examining the effect of hypertension on global cognitive impairment. Observed outcome = log(OR).
Fig. 6.
Forest plot for random effects meta-analysis examining the effect of hyperlipidemia on global cognitive impairment. Observed outcome = log(OR).
Fig. 7.
Forest plot for random effects meta-analysis examining the effect of obesity on global cognitive impairment. Observed outcome = log(OR).
Fig. 8.
Forest plot for random effects meta-analysis examining the effect of current smoking on global cognitive impairment. Observed outcome = log(OR).
ACKNOWLEDGEMENTS
The authors thank Dr. Nicholas A. Gage of the University of Florida for his statistical consultation for meta-analysis.
Footnotes
CONFLICT OF INTEREST
The authors have nothing to disclose.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/S1355617720001022
REFERENCES
- Becker JT, Kingsley L, Mullen J, Cohen B, Martin E, Miller EN, . . . Multictr ACS (2009). Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology, 73(16), 1292–1299. doi: 10.1212/WNL.0b013e3181bd10e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Maruca V, Kingsley LA, Sanders JM, Alger JR, Barker PB, . . . Selnes O (2012). Factors affecting brain structure in men with HIV disease in the post-HAART era. Neuroradiology, 54(2), 113–121. doi: 10.1007/s00234-011-0854-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg CB & Mazumdar M (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics, 50(4), 1088–1101. [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JP, & Rothstein HR (2010). A basic introduction to fixed-effect and random-effects models for meta-analysis. Research Synthesis Methods, 1(2), 97–111. doi: 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- Calza L, Manfredi R, & Chiodo F (2004). Dyslipidaemia associated with antiretroviral therapy in HIV-infected patients. The Journal of Antimicrobial Chemotherapy, 53(1), 10–14. doi: 10.1093/jac/dkh013 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2020, May). HIV Surveillance Report 2018 (Updated); vol. 31. Retrieved from http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html [Google Scholar]
- Ciccarelli N, Baldonero E, Milanini B, Fabbiani M, Cauda R, Di Giambenedetto S, & Silveri MC (2019). Cognitive impairment and cardiovascular disease related to alexithymia in a well-controlled HIV-infected population. Le Infezioni in Medicina, 27(3), 274–282. [PubMed] [Google Scholar]
- Ciccarelli N, Grima P, Fabbiani M, Baldonero E, Borghetti A, Milanini B, . . . Di Giambenedetto S (2015). Baseline CD4(+) T-cell count and cardiovascular risk factors predict the evolution of cognitive performance during 2-year follow-up in HIV-infected patients. Antiviral Therapy, 20(4), 433–440. doi: 10.3851/imp2925 [DOI] [PubMed] [Google Scholar]
- Crum-Cianflone N, Tejidor R, Medina S, Barahona I, & Ganesan A (2008). Obesity among HIV Patients: The Latest Epidemic. AIDS Patient Care STDS, 22(12), 925–930. doi: 10.1089/apc.2008.0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal HA, Weedon J, Holman S, Manly J, Valcour V, Cohen M, . . . Kaplan RC (2011). Associations of cardiovascular variables and HAART with cognition in middle-aged HIV-infected and uninfected women. Journal of Neurovirology, 17(5), 469–476. doi: 10.1007/s13365-011-0052-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham WE, Hays RD, Duan N, Andersen R, Nakazono TT, Bozette SA, & Shapiro ME (2005). The effect of socioeconomic status on the survival of people receiving care for HIV infection in the United States. Journal of Health Care for the Poor and Underserved, 16(4), 655–676. doi: 10.1353/hpu.2005.0093 [DOI] [PubMed] [Google Scholar]
- Cysique LA & Brew BJ (2019). Vascular cognitive impairment and HIV-associated neurocognitive disorder: A new paradigm. Journal of NeuroVirology. doi: 10.1007/s13365-018-0706-5 [DOI] [PubMed] [Google Scholar]
- Demir OM, Candilio L, Fuster D, Muga R, Barbaro G, Colombo A, & Azzalini L (2018). Cardiovascular disease burden among human immunodeficiency virus-infected individuals. International Journal of Cardiology, 265, 195–203. doi: 10.1016/j.ijcard.2018.03.137 [DOI] [PubMed] [Google Scholar]
- Dimsdale JE (2008). Psychological stress and cardiovascular disease. Journal of the American College of Cardiology, 51(13), 1237–1246. doi: 10.1016/j.jacc.2007.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YY, Lin HJ, Liu X, Zhang YC, Wong FY, Sun YV, . . . He N (2017). Hypertension in HIV-infected adults compared with similar but uninfected adults in China: Body mass index-dependent effects of nadir CD4 count. Aids Research and Human Retroviruses, 33(11), 1117–1125. doi: 10.1089/aid.2017.0008 [DOI] [PubMed] [Google Scholar]
- Ding YY, Lin HJ, Shen WW, Wu QH, Gao MY, & He N (2017). Interaction effects between HIV and aging on selective neurocognitive impairment. Journal of Neuroimmune Pharmacology, 12(4), 661–669. doi: 10.1007/s11481-017-9748-3 [DOI] [PubMed] [Google Scholar]
- Dufouil C, Richert L, Thiebaut R, Bruyand M, Amieva H, Dauchy FA, . . . Chêne G (2015). Diabetes and cognitive decline in a French cohort of patients infected with HIV-1. Neurology, 85(12), 1065–1073. doi: 10.1212/wnl.0000000000001815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, & Nixon SJ (2012). A comprehensive assessment of neurocognition in middle-aged chronic cigarette smokers. Drug and Alcohol Dependence, 122(1–2), 105–111. doi: 10.1016/j.drugalcdep.2011.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duron E & Hanon O (2010). Antihypertensive treatments, cognitive decline, and dementia. Journal of Alzheimer’s Disease, 20(3), 903–914. doi: 10.3233/jad-2010-091552 [DOI] [PubMed] [Google Scholar]
- Duval S & Tweedie R (2000a). A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. Journal of the American Statistical Association, 95(449), 89–98. [Google Scholar]
- Duval S & Tweedie R (2000b). Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics, 56(2), 455–463. doi: 10.1111/j.0006-341x.2000.00455.x [DOI] [PubMed] [Google Scholar]
- Elicer IM, Byrd D, Clark US, Morgello S, & Robinson-Papp J (2018). Motor function declines over time in human immunodeficiency virus and is associated with cerebrovascular disease, while HIV-associated neurocognitive disorder remains stable. Journal of NeuroVirology, 24(4), 514–522. doi: 10.1007/s13365-018-0640-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbiani M, Ciccarelli N, Tana M, Farina S, Baldonero E, Di Cristo V, . . . Di Giambenedetto S (2013). Cardiovascular risk factors and carotid intima-media thickness are associated with lower cognitive performance in HIV-infected patients. HIV Medicine, 14(3), 136–144. doi: 10.1111/j.1468-1293.2012.01044.x [DOI] [PubMed] [Google Scholar]
- Fisher Z, Tipton E, & Zhipeng H (2017). Robumeta: Robust variance meta-regression (Version 2.0) [R package]. Retrieved from https://CRAN.R-project.org/package=robumeta
- Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O’Meara ES, Longstreth WT, & Luchsinger JA (2009). Midlife and late-life obesity and the risk of dementia: Cardiovascular health study. Archives of Neurology, 66(3), 336–342. doi: 10.1001/archneurol.2008.582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J, Ettenhofer M, Wright MJ, Siddiqi I, Choi M, Thames AD, . . . Hinkin CH (2010). Neurocognitive functioning in HIV-1 infection: Effects of cerebrovascular risk factors and age. Clinical Neuropsychologist, 24(2), 265–285. doi: 10.1080/13854040903482830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijsberts CM, Groenewegen KA, Hoefer IE, Eijkemans MJC, Asselbergs FW, Anderson TJ, . . . den Ruijter HM (2015). Race/ethnic differences in the associations of the Framingham risk factors with carotid IMT and cardiovascular events. PLoS One, 10(7). doi: 10.1371/journal.pone.0132321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez D, Power C, Gill MJ, & Fujiwara E (2017). Determinants of risk-taking in HIV-associated neurocognitive disorders. Neuropsychology, 31(7), 798–810. doi: 10.1037/neu0000366 [DOI] [PubMed] [Google Scholar]
- Grima P, Fabbiani M, Ciccarelli N, Tana M, Farina S, Colafigli M, . . . Di Giambenedetto S (2012). Increased ophthalmic artery resistance index is associated with cognitive impairment in HIV-infected patients. Journal of Infection, 65(5), 439–446. doi: 10.1016/j.jinf.2012.07.005 [DOI] [PubMed] [Google Scholar]
- Grinspoon S & Carr A (2005). Cardiovascular risk and body-fat abnormalities in HIV-infected adults. The New England Journal of Medicine, 352(1), 48–62. doi: 10.1056/NEJMra041811 [DOI] [PubMed] [Google Scholar]
- Gustafson D (2008). A life course of adiposity and dementia. European Journal of Pharmacology, 585(1), 163–175. doi: 10.1016/j.ejphar.2008.01.052 [DOI] [PubMed] [Google Scholar]
- Gustafson D, Mielke MM, Tien PC, Valcour V, Cohen M, Anastos K, . . . Crystal HA (2013). Anthropometric measures and cognition in middle-aged HIV-infected and uninfected women. The Women’s Interagency HIV Study. Journal of Neurovirology, 19(6), 574–585. doi: 10.1007/s13365-013-0219-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna DB, Post WS, Deal JA, Hodis HN, Jacobson LP, Mack WJ, . . . Kingsley LA (2015). HIV infection is associated with progression of subclinical carotid atherosclerosis. Clinical Infectious Diseases, 61(4), 640–650. doi: 10.1093/cid/civ325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatleberg CI, Ryom L, El-Sadr W, Mocroft A, Reiss P, de Wit S, . . . Sabin C (2014). Gender differences in HIV-positive persons in use of cardiovascular disease-related interventions: D:A:D study. Journal of the International AIDS Society, 17(4 Suppl 3), 19516. doi: 10.7448/ias.17.4.19516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, . . . Grant I (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology, 75(23), 2087–2096. doi: 10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, . . . Grant I (2004). The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society, 10(3), 317–331. doi: 10.1017/s1355617704102130 [DOI] [PubMed] [Google Scholar]
- Hedges LV, Tipton E, & Johnson MC (2010). Robust variance estimation in meta-regression with dependent effect size estimates. Research Synthesis Methods, 1(1), 39–65. doi: 10.1002/jrsm.5 [DOI] [PubMed] [Google Scholar]
- Higgins JP, Li T, & Deeks JJ (2019). Chapter 6: Choosing effect measures and computing estimates of effect. In Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, & Welch VA (Eds.), Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, & Altman DG (2003). Measuring inconsistency in meta-analyses. BMJ, 327(7414), 557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsue PY, Hunt PW, Schnell A, Kalapus SC, Hoh R, Ganz P, . . . Deeks SG (2009). Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS, 23(9), 1059–1067. doi: 10.1097/QAD.0b013e32832b514b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck DM, Hanna DB, Rubin LH, Maki P, Valcour V, Springer G, . . . Kizer JR (2018). Carotid artery stiffness and cognitive decline among women with or at risk for HIV infection. Journal of Acquired Immune Deficiency Syndromes, 78(3), 338–347. doi: 10.1097/qai.0000000000001685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain NEO & Ahmed MH (2015). Managing dyslipidemia in HIV/AIDS patients: Challenges and solutions. HIV/AIDS (Auckland, NZ), 7, 1–10. doi: 10.2147/hiv.s46028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AL, Hohman TJ, Liu D, Haj-Hassan S, Gifford KA, Benson EM, . . . Ruberg FL (2015). Adverse vascular risk is related to cognitive decline in older adults. Journal of Alzheimer’s Disease, 44(4), 1361–1373. doi: 10.3233/jad-141812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumare J, El-Kamary SS, Magder L, Hungerford L, Umlauf A, Franklin D, . . . McCutchan JA (2019). Body mass index and cognitive function among HIV-1 infected individuals in China, India and Nigeria. JAIDS – Journal of Acquired Immune Deficiency Syndromes, 80(2), e30–e35. doi: 10.1097/qai.0000000000001906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaffashian S, Dugravot A, Nabi H, Batty GD, Brunner E, Kivimaki M, & Singh-Manoux A (2011). Predictive utility of the Framingham general cardiovascular disease risk profile for cognitive function: Evidence from the Whitehall II study. European Heart Journal, 32(18), 2326–2332. doi: 10.1093/eurheartj/ehr133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuder SS, Chen S, Letendre S, Marcotte T, Grant I, Franklin D, . . . Haughey NJ (2019). Impaired insulin sensitivity is associated with worsening cognition in HIV-infected patients. Neurology, 92(12), e1344–e1353. doi: 10.1212/wnl.0000000000007125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, . . . Nissinen A (2001). Midlife vascular risk factors and Alzheimer’s disease in later life: Longitudinal, population based study. BMJ, 322(7300), 1447–1451. doi: 10.1136/bmj.322.7300.1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake JE, Vo QT, Jacobson LP, Sacktor N, Miller EN, Post WS, . . . Brown TT (2015). Adiponectin and interleukin-6, but not adipose tissue, are associated with worse neurocognitive function in HIV-infected men. Antiviral Therapy, 20(2), 235–244. doi: 10.3851/imp2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S, Mary-Krause M, Simon A, Partisani M, Gilquin J, Cotte L, . . . Costagliola D (2012). HIV replication and immune status are independent predictors of the risk of myocardial infarction in HIV-infected individuals. Clinical Infectious Diseases, 55(4), 600–607. doi: 10.1093/cid/cis489 [DOI] [PubMed] [Google Scholar]
- Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, . . . Grinspoon SK (2010). Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS, 24(2), 243–253. doi: 10.1097/QAD.0b013e328333ea9e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker CT, Sullivan C, & Baker JV (2016). Immune activation and cardiovascular disease in chronic HIV infection. Current Opinion in HIV and AIDS, 11(2), 216–225. doi: 10.1097/coh.0000000000000227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquine MJ, Heaton A, Johnson N, Rivera-Mindt M, Cherner M, Bloss C, . . . Heaton RK (2018). Differences in neurocognitive impairment among HIV-infected Latinos in the United States. Journal of the International Neuropsychological Society, 24(2), 163–175. doi: 10.1017/s1355617717000832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan JA, Marquie-Beck JA, FitzSimons CA, Letendre SL, Ellis RJ, Heaton RK, . . . Grp C (2012). Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology, 78(7), 485–492. doi: 10.1212/WNL.0b013e3182478d64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh EC & Nation DA (2019). Importance of treatment status in links between type 2 diabetes and Alzheimer’s disease. Diabetes Care, 42(5), 972–979. doi: 10.2337/dc18-1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya JL, Iudicello J, Fazeli PL, Hong SZ, Potter M, Ellis RJ, . . . the HIV Neurobehavioral Research Program (HNRP) Group (2017). Elevated markers of vascular remodeling and arterial stiffness are associated with neurocognitive function in older HIV plus adults on suppressive antiretroviral therapy. JAIDS – Journal of Acquired Immune Deficiency Syndromes, 74(2), 134–141. doi: 10.1097/qai.0000000000001230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerji SS, Locascio JJ, Misra V, Lorenz DR, Holman A, Dutta A, . . . Gabuzda D (2016). Lipid profiles and APOE4 allele impact midlife cognitive decline in HIV-infected men on antiretroviral therapy. Clinical Infectious Diseases, 63(8), 1130–1139. doi: 10.1093/cid/ciw495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto BK, Jahanshad N, McMurtray A, Kallianpur KJ, Chow DC, Valcour VG, . . . Shikuma CM (2012). Cerebrovascular risk factors and brain microstructural abnormalities on diffusion tensor images in HIV-infected individuals. Journal of Neurovirology, 18(4), 303–312. doi: 10.1007/s13365-012-0106-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto BK, Valcour V, Kallianpur K, Liang CY, McMurtray A, Chow D, . . . Shikuma CM (2011). Impact of cerebrovascular disease on cognitive function in HIV-infected patients. JAIDS – Journal of Acquired Immune Deficiency Syndromes, 57(3), e66–e68. doi: 10.1097/QAI.0b013e31821ff8bd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nou E, Lo J, & Grinspoon SK (2016). Inflammation, immune activation, and cardiovascular disease in HIV. AIDS, 30(10), 1495–1509. doi: 10.1097/qad.0000000000001109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okafor CN, Kelso NE, Bryant V, Burrell LE, Miguez MJ, Gongvatana A, . . . Cohen RA (2017). Body mass index, inflammatory biomarkers and neurocognitive impairment in HIV-in fected persons. Psychology Health & Medicine, 22(3), 289–302. doi: 10.1080/13548506.2016.1199887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onen NF, Overton ET, Seyfried W, Stumm ER, Snell M, Mondy K, & Tebas P (2010). Aging and HIV infection: A comparison between older HIV-infected persons and the general population. HIV Clinical Trials, 11(2), 100–109. doi: 10.1310/hct1102-100 [DOI] [PubMed] [Google Scholar]
- Palella FJ & Phair JP (2011). Cardiovascular disease in HIV infection. Current Opinion in HIV and AIDS, 6(4), 266–271. doi: 10.1097/COH.0b013e328347876c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L (2010). Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. The Lancet Neurology, 9(7), 689–701. doi: 10.1016/s1474-4422(10)70104-6 [DOI] [PubMed] [Google Scholar]
- Patel SM, Thames AD, Arbid N, Panos SE, Castellon S, & Hinkin CH (2013). The aggregate effects of multiple comorbid risk factors on cognition among HIV-infected individuals. Journal of Clinical and Experimental Neuropsychology, 35(4), 421–434. doi: 10.1080/13803395.2013.783000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portilla I, Reus S, León R, van-der Hofstadt C, Sánchez J, López N, . . . Portilla J (2019). Neurocognitive impairment in well-controlled HIV-infected patients: A cross-sectional study. AIDS Research and Human Retroviruses, 35(7). doi: 10.1089/AID.2018.0279 [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, & Acker JD (2003). Hypertension and the brain: Vulnerability of the prefrontal regions and executive functions. Behavioral Neuroscience, 117(6), 1169–1180. doi: 10.1037/0735-7044.117.6.1169 [DOI] [PubMed] [Google Scholar]
- Rider OJ, Asaad M, Ntusi N, Wainwright E, Clutton G, Hancock G, . . . Holloway CJ (2014). HIV is an independent predictor of aortic stiffness. Journal of Cardiovascular Magnetic Resonance, 16(1), 57. doi: 10.1186/s12968-014-0057-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R (1979). The file drawer problem and tolerance for null results. Psychological Bulletin, 86(3), 638–641. doi: 10.1037/0033-2909.86.3.638 [DOI] [Google Scholar]
- Rubin LH, Gustafson D, Hawkins KL, Zhang L, Jacobson LP, Becker JT, . . . Erlandson KM (2019). Midlife adiposity predicts cognitive decline in the prospective Multicenter AIDS Cohort Study. Neurology, 93(3). doi: 10.1212/WNL.0000000000007779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner R, Campbell LM, Serrano V, Montoya JL, Pasipanodya E, Paolillo EW, . . . Moore DJ (2019). Neurocognitive SuperAging in older adults living with HIV: Demographic, neuromedical and everyday functioning correlates. Journal of the International Neuropsychological Society, 25(5), 507–519. doi: 10.1017/s1355617719000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford R, Strain J, Dadar M, Maranzano J, Bonnet A, Mayo NE, . . . Collins DL (2019). HIV infection and cerebral small vessel disease are independently associated with brain atrophy and cognitive impairment. AIDS (London, England), 33(7). doi: 10.1097/QAD.0000000000002193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler FR, He JX, Letendre S, Wilson C, Sanders C, Heaton R, . . . the CHARTER Group (2015). Abdominal obesity contributes to neurocognitive impairment in HIV-infected patients with increased inflammation and immune activation. JAIDS – Journal of Acquired Immune Deficiency Syndromes, 68(3), 281–288. doi: 10.1097/qai.0000000000000458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satz P, Morgenstern H, Miller EN, Selnes OA, McArthur JC, Cohen BA, . . . D’Elia LF (1993). Low education as a possible risk factor for cognitive abnormalities in HIV-1: Findings from the multicenter AIDS Cohort Study (MACS). JAIDS – Journal of Acquired Immune Deficiency Syndromes, 6(5), 503–511. [PubMed] [Google Scholar]
- Schouten J, Su T, Wit FW, Kootstra NA, Caan MWA, Geurtsen GJ, . . . AGEhIV Study Group (2016). Determinants of reduced cognitive performance in HIV-1-infected middle-aged men on combination antiretroviral therapy. AIDS, 30(7), 1027–1038. doi: 10.1097/qad.0000000000001017 [DOI] [PubMed] [Google Scholar]
- Seaberg EC, Benning L, Sharrett AR, Lazar JM, Hodis HN, Mack WJ, . . . Kaplan RC (2010). Association between human immunodeficiency virus infection and stiffness of the common carotid artery. Stroke, 41(10), 2163–2170. doi: 10.1161/strokeaha.110.583856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer EJ, Valdes-Sueiras M, Commins DL, Yong W, & Carlson M (2013). HIV stroke risk: Evidence and implications. Therapeutic Advances in Chronic Disease, 4(2), 61–70. doi: 10.1177/2040622312471840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman EZ, Sharma S, Arasteh K, Wohl D, Achhra A, Tambussi G, . . . Phillips A (2015). Baseline cardiovascular risk in the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Medicine, 16(Suppl 1), 46–54. doi: 10.1111/hiv.12233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij V, Umlauf A, Chung SA, Cochran ML, Soontornniyomkij B, Gouaux B, . . . Achim CL (2014). HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS, 28(9), 1297–1306. doi: 10.1097/qad.0000000000000262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JH & Hsue PY (2012). Inflammation, immune activation, and CVD risk in individuals with HIV infection. JAMA, 308(4), 405–406. doi: 10.1001/jama.2012.8488 [DOI] [PubMed] [Google Scholar]
- Su T, Mutsaerts H, Caan MWA, Wit F, Schouten J, Geurtsen GJ, . . . AGEhIV Study Group (2017). Cerebral blood flow and cognitive function in HI-infected men with sustained suppressed viremia on combination antiretroviral therapy. AIDS, 31(6), 847–856. doi: 10.1097/qad.0000000000001414 [DOI] [PubMed] [Google Scholar]
- Su T, Wit F, Caan MWA, Schouten J, Prins M, Geurtsen GJ, . . . AGEhIV Study Group (2016). White matter hyperintensities in relation to cognition in HIV-infected men with sustained suppressed viral load on combination antiretroviral therapy. AIDS, 30(15), 2329–2339. doi: 10.1097/qad.0000000000001133 [DOI] [PubMed] [Google Scholar]
- Taki Y, Goto R, Evans A, Zijdenbos A, Neelin P, Lerch J, . . . Fukuda H (2004). Voxel-based morphometry of human brain with age and cerebrovascular risk factors. Neurobiology of Aging, 25(4), 455–463. doi: 10.1016/j.neurobiolaging.2003.09.002 [DOI] [PubMed] [Google Scholar]
- Tanner-Smith EE, Tipton E, & Polanin JR (2016). Handling complex meta-analytic data structures using robust variance estimates: A tutorial in R. Journal of Developmental and Life-Course Criminology, 2(1), 85–112. doi: 10.1007/s40865-016-0026-5 [DOI] [Google Scholar]
- R Core Team (2014). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Retrieved from http://www.R-project.org/ [Google Scholar]
- Tedaldi EM, Minniti NL, & Fischer T (2015). HIV-associated neurocognitive disorders: The relationship of HIV infection with physical and social comorbidities. BioMed Research International, 2015, 641913. doi: 10.1155/2015/641913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Arentoft A, Rivera-Mindt M, & Hinkin CH (2013). Functional disability in medication management and driving among individuals with HIV: A 1-year follow-up study. Journal of Clinical and Experimental Neuropsychology, 35(1), 49–58. doi: 10.1080/13803395.2012.747596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triant VA (2013). Cardiovascular disease and HIV infection. Current >HIV/AIDS Reports, 10(3), 199–206. doi: 10.1007/s11904-013-0168-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triant VA, Lee H, Hadigan C, & Grinspoon SK (2007). Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. The Journal of Clinical Endocrinology and Metabolism, 92(7), 2506–2512. doi: 10.1210/jc.2006-2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unverzagt FW, McClure LA, Wadley VG, Jenny NS, Go RC, Cushman M, . . . Howard G (2011). Vascular risk factors and cognitive impairment in a stroke-free cohort. Neurology, 77(19), 1729–1736. doi: 10.1212/WNL.0b013e318236ef23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Maki P, Bacchetti P, Anastos K, Crystal H, Young M, . . . Tien PC (2012). Insulin resistance and cognition among HIV-infected and HIV-uninfected adult women: The Women’s Interagency HIV Study. AIDS Research and Human Retroviruses, 28(5), 447–453. doi: 10.1089/aid.2011.0159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Rubin LH, Tien P, Anastos K, Young M, Mack W, . . . Maki PM (2015). Human immunodeficiency virus (HIV) modulates the associations between insulin resistance and cognition in the current combination antiretroviral therapy (cART) era: A study of the Women’s Interagency HIV Study (WIHS). Journal of Neurovirology, 21(4), 415–421. doi: 10.1007/s13365-015-0330-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Sacktor NC, Paul RH, Watters MR, Selnes OA, Shiramizu BT, . . . Shikuma CM (2006). Insulin resistance is associated with cognition among HIV-1-infected patients – The Hawaii aging with HIV cohort. JAIDS – Journal of Acquired Immune Deficiency Syndromes, 43(4), 405–410. doi: 10.1097/01.qai.0000243119.67529.f5 [DOI] [PubMed] [Google Scholar]
- Valcour V, Shikuma CM, Shiramizu BT, Williams AE, Watters MR, Poff PW, . . . Sacktor NC (2005). Diabetes, insulin resistance, and dementia among HIV-1-infected patients. JAIDS – Journal of Acquired Immune Deficiency Syndromes, 38(1), 31–36. doi: 10.1097/00126334-200501010-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez AN, Rubin LH, & Neigh GN (2016). Untangling the Gordian knot of HIV, stress, and cognitive impairment. Neurobiology of Stress, 4, 44–54. doi: 10.1016/j.ynstr.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg E, Kloppenborg RP, Kessels RP, Kappelle LJ, & Biessels GJ (2009). Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: A systematic comparison of their impact on cognition. Biochimica et Biophysica Acta, 1792(5), 470–481. doi: 10.1016/j.bbadis.2008.09.004 [DOI] [PubMed] [Google Scholar]
- Viechtbauer W (2010). Conducting meta-analyses in R with the metafor package. Journal of Statistical Software, 36, 1–48. [Google Scholar]
- Watson C, Busovaca E, Foley JM, Allen IE, Schwarz CG, Jahanshad N, . . . Valcour VG (2017). White matter hyperintensities correlate to cognition and fiber tract integrity in older adults with HIV. Journal of Neurovirology, 23(3), 422–429. doi: 10.1007/s13365-016-0509-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DB (n.d.). Practical meta-analysis effect size calculator [Online calculator]. Retrieved from https://campbellcollaboration.org/research-resources/effect-size-calculator.html
- Womack JA, Chang CC, So-Armah KA, Alcorn C, Baker JV, Brown ST, . . . Freiberg MS (2014). HIV infection and cardiovascular disease in women. Journal of the American Heart Association, 3(5), e001035. doi: 10.1161/jaha.114.001035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright EJ, Grund B, Cysique LA, Robertson KR, Brew BJ, Collins G, . . . Int Network Strategic, I. (2015). Factors associated with neurocognitive test performance at baseline: A substudy of the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Medicine, 16, 97–108. doi: 10.1111/hiv.12238 [DOI] [PubMed] [Google Scholar]
- Wright EJ, Grund B, Robertson K, Brew BJ, Roediger M, Bain MP, . . . Price RW (2010). Cardiovascular risk factors associated with lower baseline cognitive performance in HIV-positive persons. Neurology, 75(10), 864–873. doi: 10.1212/WNL.0b013e3181f11bd8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaldizli O, Kastrup O, Obermann M, Esser S, Wilhelm H, Ley C, . . . Maschke M (2006). Carotid intima-media thickness in HIV-Infected individuals: Relationship of premature atherosclerosis to neuropsychological deficits? European Neurology, 55(3), 166–171. doi: 10.1159/000093576 [DOI] [PubMed] [Google Scholar]
- Yang J, Jacobson LP, Becker JT, Levine A, Martin EM, Munro CA, . . . Brown TT (2018). Impact of glycemic status on longitudinal cognitive performance in men with and without HIV infection. AIDS, 32(13), 1849–1860. doi: 10.1097/qad.0000000000001842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YK, Yao WJ, Yeh TL, Lee IH, Chen PS, Lu RB, & Chiu NT (2008). Decreased dopamine transporter availability in male smokers – a dual isotope SPECT study. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 32(1). doi: 10.1016/j.pnpbp.2007.08.018 [DOI] [PubMed] [Google Scholar]
- Yu B, Pasipanodya E, Montoya JL, Moore RC, Gianella S, McCutchan A, . . . Marquine MJ (2019). Metabolic syndrome and neurocognitive deficits in HIV infection. JAIDS – Journal of Acquired Immune Deficiency Syndromes, 81(1). doi: 10.1097/QAI.0000000000001964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen T, Brouillette MJ, Fellows LK, Ellis RJ, Letendre S, Heaton R, . . . CHARTER group (2017). Personalized risk index for neurocognitive decline among people with well-controlled HIV infection. JAIDS – Journal of Acquired Immune Deficiency Syndromes, 76(1), 48–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.