Abstract
Background & Aims:
Successful treatment of chronic hepatitis C with oral direct-acting antivirals (DAAs) leads to virological cure, however, the subsequent risk of hepatocellular carcinoma (HCC) persists. Our objective was to evaluate the cost-effectiveness of biannual surveillance for HCC in patients cured of hepatitis C and the optimal age to stop surveillance.
Methods:
We developed a microsimulation model of the natural history of HCC in individuals with hepatitis C and advanced fibrosis or cirrhosis who achieved virological cure with oral DAAs. We used published data on HCC incidence, tumor progression, real-world HCC surveillance adherence, and costs and utilities of different health states. We compared biannual HCC surveillance using ultrasound and alpha-fetoprotein for varying durations of surveillance (from 5 years to lifetime) vs. no surveillance.
Results:
In virologically cured patients with cirrhosis, the incremental cost-effectiveness ratio (ICER) of biannual surveillance remained below $150,000 per additional quality-adjusted life year (QALY) (range: $79,500-$94,800) when surveillance was stopped at age 70, irrespective of the starting age (40–65). Compared with no surveillance, surveillance detected 130 additional HCCs in ‘very early’/early stage and yielded 51 additional QALYs per 1,000 patients with cirrhosis. In virologically cured patients with advanced fibrosis, the ICER of biannual surveillance remained below $150,000/QALY (range: $124,600-$129,800) when surveillance was stopped at age 60, irrespective of the starting age (40–50). Compared with no surveillance, surveillance detected 24 additional HCCs in ‘very early’/early stage and yielded 12 additional QALYs per 1,000 patients with advanced fibrosis.
Conclusion:
Biannual surveillance for HCC in patients cured of hepatitis C is cost-effective until the age of 70 for patients with cirrhosis, and until the age of 60 for patients with stable advanced fibrosis.
Lay summary:
Individuals who are cured of hepatitis C using oral antiviral drugs remain at risk of developing liver cancer. The value of lifelong screening for liver cancer in these individuals is not known. By simulating the life course of hepatitis C cured individuals, we found that ultrasound-based biannual screening for liver cancer is cost-effective up to age 70 in those with cirrhosis and up to age 60 in those with stable advanced fibrosis.
Keywords: Liver cancer, screening, simulation modeling, health economics
Graphical abstract

Introduction
Chronic hepatitis C is the leading cause of hepatocellular carcinoma (HCC) in the United States. However, with the availability of new direct-acting antivirals (DAAs), 90–100% of patients with hepatitis C, even those who could not be treated with previously available interferon-based therapies, can now achieve a virological cure (defined as a sustained virological response or SVR).1 Although successful treatment resulting in SVR represents a “cure” from the standpoint of persistent infection, the subsequent risk of HCC persists after treatment with DAAs and remains an important contributor to liver-related morbidity and mortality in the post-DAA era.2–4
With the wide use of DAAs, the number of patients with virologically cured hepatitis C has rapidly increased from 0.5 million in 2012 to 1.5 million in 2020 in the United States.5 Similar trends are expected in other countries that have successfully treated a large number of patients with DAAs. Furthermore, the proportion of patients with advanced liver disease among this cohort has increased.6 As such, most patients with hepatitis C in clinical practice will be those who have been virologically cured with DAAs and yet have advanced liver disease, either fibrosis or cirrhosis.5 For instance, the number of individuals who could potentially benefit from HCC surveillance, i.e., those with advanced liver disease is projected to increase from 106,000 in 2012 to 649,000 in 2030.6 For this growing population, routine HCC surveillance is critical to improve early cancer detection and improve survival outcomes.7
The optimal policy for routine HCC surveillance for patients with virologically cured hepatitis C remains unclear.8,9 Despite limited evidence on the cost-effectiveness of HCC surveillance for this patient group, the American Association for the Study of Liver Diseases (AASLD) recommends biannual ultrasound-based (with or without alpha-fetoprotein) surveillance for HCC in patients with cirrhosis who achieve virological cure with DAAs,1 but does not recommend surveillance in patients with advanced fibrosis. In contrast, the European Association for the Study of the Liver (EASL) guidelines recommend biannual surveillance in patients with cirrhosis as well as bridging fibrosis.10 Furthermore, none of the guidelines specify any age at which to discontinue surveillance. Virologically cured hepatitis C patients have a higher life expectancy than patients with active hepatitis C,11 and the value of lifetime HCC surveillance in this cohort is not known.
Our study’s objective was to perform a comparative cost-effectiveness analysis to determine in which patients, and up to what age, the benefits of HCC surveillance outweigh the costs.
Materials and methods
Overview
We developed a microsimulation model (an individual-level state-transition model) that simulates the natural history of hepatitis C and HCC in patients who achieved SVR after hepatitis C treatment with DAAs. The model was used to evaluate the cost-effectiveness of biannual HCC surveillance by varying the starting age for surveillance from 40 to 70 (base age 50) and stopping age from 50 to lifetime. We incorporated data from recent studies on the risk of HCC in patients who achieved SVR with DAAs,2,12–17 real-world adherence to HCC surveillance,18 the accuracy of screening and diagnostic testing modalities,19–22 as well as HCC treatments including liver transplantation for selected patients.20,23–35 The model used a monthly time-step. Primary outcomes included life expectancy, quality-adjusted life years (QALYs), total costs, and incremental cost-effectiveness ratio (ICER) of each of the simulated surveillance strategies.
Baseline population and natural history model
For the baseline population, we created a virtual cohort of 50-year-old patients (mean age of HCV treatment), all of whom had hepatitis C that had been cured by DAA treatment. We developed different patient profiles that varied age (range 40–70), sex, and the extent of liver disease (advanced fibrosis METAVIR score F3 or compensated cirrhosis METAVIR score F4). For each patient, we simulated their remaining lifespan with and without HCC surveillance. We tracked life expectancy, development of HCC, and stage of HCC at diagnosis for each virtual patient in this simulation model.
For each virtual patient, we simulated the clinical course of cured hepatitis C, including HCC development as well as the progression of liver disease (Fig. 1). In each month, patients could develop HCC at a “very early” stage that could later progress to “early” and “intermediate/advanced” stages. We defined HCC stage based on Barcelona Clinic Liver Cancer staging system and tumor size: “very early HCC” included patients with a tumor less than 2 cm in diameter, “early HCC” for those with a single tumor less than 5 cm or up to 3 tumors all less than 3 cm, and “intermediate/advanced HCC” for the remaining stages. We assumed that patients with F3 advanced fibrosis could not develop cirrhosis once in SVR.36 In each month, patients with F4 cirrhosis could progress to decompensated cirrhosis. The rate of HCC development varied by the extent of liver disease, such that patients without HCC could develop more severe liver disease, thus increasing their risk of HCC (Table S1).
Fig. 1. Model schematic for the natural history of HCC in patients with hepatitis C who achieved an SVR on direct-acting antivirals.

Patients could develop HCC and subsequently progress through different stages of HCC. Patients with cirrhosis could also progress to decompensated cirrhosis. DC, decompensated cirrhosis; F3, advanced fibrosis; F4, cirrhosis; HCC, hepatocellular carcinoma; SVR, sustained virologic response; TACE, transarterial chemoembolization.
HCC incidence
We used recently published studies to estimate the incidence of HCC among hepatitis C patients who achieved SVR. For patients with compensated cirrhosis (F4 fibrosis stage), we used a weighted average of 6 studies that resulted in an incidence rate of 1.95 per 100 person-years (Supplement Section S1).2,12–16 For patients with advanced fibrosis (F3 stage), the HCC incidence rate was estimated from 2 studies that resulted in a weighted average of 0.58 per 100 person-years.16,17 In a sensitivity analysis, we allowed HCC risk to be higher and lower than the base case values.
HCC surveillance
For each virtual patient, we simulated ‘no surveillance’ vs. biannual HCC surveillance using both ultrasound and alpha-fetoprotein,19,22 as recommended by clinical guidelines. We simulated different HCC surveillance strategies by varying the duration of surveillance from 10 years to lifetime (in 5-year increments). For example, we simulated the following surveillance strategies for a 50-year-old patient: biannual HCC surveillance from age 50 to age 60, 65, 70, etc. If surveillance tests were normal, no further intervention was needed and the patient followed their natural history until the next surveillance test; if the surveillance tests were abnormal, the patient received a contrast-enhanced abdominal MRI to potentially confirm the diagnosis of HCC.19–21 After confirmation of HCC, patients began treatment for HCC. HCC could also be detected incidentally regardless of surveillance. We assumed that only a single MRI was required for diagnostic confirmation.37,38 We also accounted for imperfect adherence to biannual HCC surveillance as observed in practice (see Supplementary Section S2).39
HCC treatment
We considered 4 HCC treatment options: ablation, resection, liver transplantation, transarterial chemoembolization, and other palliative treatments (including selective internal radiotherapy, external beam radiotherapy, and systemic chemotherapy). Selection of treatment depended on HCC stage as well as the liver disease severity at diagnosis (Table S2). Following the guidelines, we assumed that liver transplantation was available only for patients under the age of 75 with either decompensated cirrhosis or “early stage” HCC (i.e., within Milan criteria); and decompensated patients were not eligible for resection. Treatment distributions by both HCC stage and fibrosis stage were determined from published observational studies19,40 and expert opinion (see Supplementary Section S3).
Unlike resection, ablation, and transarterial chemoembolization, which were delivered soon after the diagnosis, patients selected for liver transplantation were assumed to be on the transplant waitlist for organ availability. During each month, patients could receive a liver transplant; the probability of transplant was based on the median wait times that were provided from Organ Procurement Transplant Network data, which were dependent on the HCC tumor stage and liver stage (Table S1). However, in each month, HCC could progress such that patients were no longer eligible for transplant. In addition, if patients reached the age of 75, they would no longer be eligible for liver transplant (see Supplementary Section S4).
Costs and quality-of-life
The cost of surveillance tests, HCC treatments, liver transplantation, and general liver disease management were extracted from published sources (Table S1). All costs were converted to 2020 US dollars using the Consumer Price Index. We also assigned quality-of-life utility weights to each health state and treatment. These utility weights were adjusted by age, liver disease stage, and for the year of treatment (initial year vs. later years)23,32,41,42 (Table S1).
Model outcomes
For each simulated surveillance strategy and for each patient profile, we estimated the number of HCC cases detected by tumor stage, total QALYs, and costs. All future QALYs and costs were discounted at 3% per year. We then calculated the ICERs for each surveillance strategy. For each surveillance strategy and patient profile, we could determine if surveillance was cost-effective if the ICER remained below the willingness-to-pay threshold of $150,000 per QALY.43 To reduce first-order uncertainty, we simulated outcomes in 200 million virtual patients.
Subgroup and sensitivity analysis
We evaluated the cost-effectiveness of surveillance in several subgroups described by age, sex, diabetes status, and alcohol use.2
As a part of our sensitivity analyses, we varied the incidence of HCC following SVR across a range informed by several observational studies.2,12–17 We further evaluated the impact of uncertainty in each model input on outcomes by conducting univariate probabilistic sensitivity analysis on each model input. This was performed by randomly sampling values from each parameter’s recommended statistical distribution one at a time (Table S1). We also conducted a multivariate probabilistic sensitivity analysis on all model parameters simultaneously by sampling 4,000 sets of parameter values from the recommended statistical distributions and evaluating each parameter set with a population size of 1.5 million. The multivariate probabilistic sensitivity analysis results were presented as cost-effectiveness acceptability curves.
Results
Base case cost-effectiveness results for HCC surveillance
Table 1 shows the costs, QALYs, and ICERs of each HCC surveillance strategy. For 50-year-old patients with cirrhosis, the ICER of biannual HCC surveillance was $93,587 per QALY when the stopping age of surveillance was 70 and $223,364 per QALY when the stopping age increased to 75 (Fig. 2A, Table 1), suggesting that HCC surveillance is cost-effective until the age 70 using a willingness-to-pay threshold of $150,000. Compared with no surveillance, the cost-effective surveillance strategy – surveillance up to age 70 – provided QALY gains of 51 per 1,000 patients at an additional cost of $2,694,400. Surveillance was cost-effective until the age of 70 regardless of starting age (from 40–65), with the ICERs remaining below the $150,000-per-QALY threshold (Fig. 2A). Of note, for 65-year-old patients, the ICER of routine surveillance up to age 75 was $147,563–below the $150,000 threshold.
Table 1.
Base case cost-effectiveness results of biannual HCC surveillance in 50-year-old patients with virologically cured hepatitis C.
| Compensated cirrhosis | Advanced fibrosis | |||||
|---|---|---|---|---|---|---|
| Surveillance stopping age | QALYs | Costs ($) | ICERs ($/QALY) | QALYs | Costs ($) | ICERs ($/QALY) |
| No surveillance | 11.718 | 38,534 | – | 14.384 | 3,568 | – |
| 60 | 11.755 | 40,243 | 46,000 | 14.396 | 5,081 | 129,781 |
| 65 | 11.765 | 40,819 | 60,625 | 14.399 | 5,649 | 160,348 |
| 70 | 11.769 | 41,228 | 93,587 | 14.401 | 6,096 | 275,280 |
| 75 | 11.770 | 41,526 | 223,364 | 14.401 | 6,444 | 772,400 |
HCC, hepatocellular carcinoma; ICERs, incremental cost-effectiveness ratios; QALYs, quality-adjusted life years.
Fig. 2. Incremental cost-effectiveness ratios of biannual HCC surveillance.
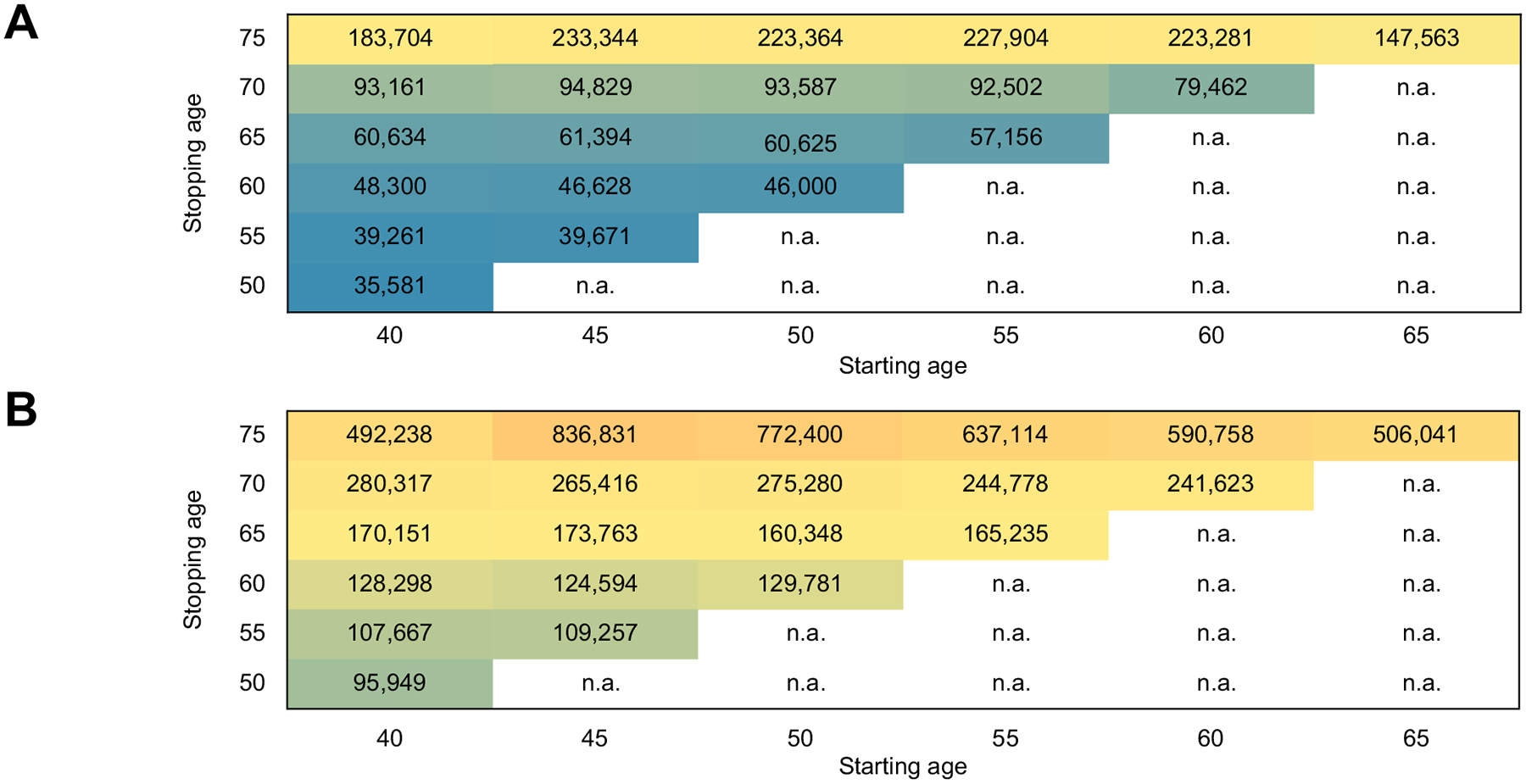
(A) In patients with cirrhosis and (B) in patients with advanced fibrosis who achieved virological cure with direct-acting antivirals. In patients with cirrhosis, HCC surveillance is cost-effective up to approximately age 70 using a willingness-to-pay threshold of $150,000 per quality-adjusted life year. In patients with advanced fibrosis, HCC surveillance is cost-effective up to age 60. HCC, hepatocellular carcinoma.
For 50-year-old patients with advanced fibrosis, the ICER of biannual HCC surveillance was $98,800 per QALY when the stopping age of surveillance was 65 and $129,781 per QALY when the stopping age increased to 70 (Fig. 2B), suggesting that HCC surveillance is cost-effective until age 65 using a willingness-to-pay threshold of $150,000. However, the ICER of surveillance was cost-effective only up to age 60 when the starting age was 40, 45, and 50 (Fig. 2A). Compared with no surveillance, the cost-effective surveillance strategy – surveillance up to age 60 – provided QALY gains of 12 per 1,000 patients at an additional cost of $1,512,900.
For 50-year-old patients with cirrhosis in the “no surveillance” arm, for every 1,000 patients, 381 HCC cases were detected either as incidental findings or through symptoms – 9 were detected in very early stage, 93 in early stage, and 279 in intermediate/advanced stages (Fig. 3). In contrast, the cost-effective surveillance strategy (surveillance every 6 months until the age of 70) detected a total of 389 HCC cases – 82 were detected in very early stage, 150 in early stage, and 157 in intermediate/advanced stages. Therefore, this surveillance strategy detected 130 additional HCCs in ‘very early’/early stage. The surveillance strategy also identified 8 additional patients with HCC who would have died from competing mortality under no surveillance. Similar trends were observed in patients with advanced fibrosis. In 50-year-old patients with advanced fibrosis, 3 cases were detected in a very early stage, 37 in early stage, and 111 in intermediate/advanced stage in the “no surveillance” arm. In contrast, the cost-effective surveillance strategy (surveillance every 6 months until the age of 60) detected 17 cases in very early stage, 47 in early stage, and 88 in intermediate/advanced stage. Therefore, this surveillance strategy detected 24 additional HCCs in ‘very early’/early stage.
Fig. 3. Number of HCC cases according to surveillance approach.

Number of HCC cases detected by tumor size in 50-year-old virologically cured patients under no surveillance vs. biannual surveillance until the age of 70 for patients with cirrhosis and until the age of 60 for patients with advanced fibrosis. CHC, chronic hepatitis C; HCC, hepatocellular carcinoma; SVR, sustained virologic response.
Effect of remaining life expectancy on surveillance
We found that biannual HCC surveillance would not be cost-effective in cirrhosis patients if their remaining life expectancy was under 16 years and not cost-effective in advanced fibrosis patients if their life expectancy was under 28 years. This finding did not change with the age of individuals.
Subgroup analysis
In 50-year-old patients with cirrhosis, biannual HCC surveillance was cost-effective until the age of 70 irrespective of alcohol use, male sex, diabetes status, and BMI (Table 2).
Table 2.
Cost-effectiveness of HCC surveillance in selected subgroups of 50-year-old patients with virologically cured hepatitis C.
| Compensated cirrhosis | |||||||
|---|---|---|---|---|---|---|---|
| HCC incidence per 100 person-years | Cost-effective until age | ICER for 20 years of surveillance | |||||
| Base case | 1.95 | 70 | 93,600 | ||||
| Diabetes | |||||||
| Yes | 2.07 | 70 | 87,800 | ||||
| No | 1.85 | 70 | 92,800 | ||||
| Alcohol use* | |||||||
| Yes | 2.15 | 70 | 86,500 | ||||
| No | 1.66 | 70 | 98,900 | ||||
| Sex | |||||||
| Male | 2.01 | 70 | 89,500 | ||||
| Body mass index | |||||||
| ≥30 kg/m2 | 2.48 | 70 | 79,100 | ||||
| <30 kg/m2 | 1.81 | 70 | 94,100 | ||||
| Male; age > 55; alcohol use: Yes | 2.36 | 70 | 83,100 | ||||
| Male; age > 55; diabetes: Yes | 2.09 | 70 | 89,700 | ||||
| Advanced fibrosis | |||||||
| Cost-effective until age | ICER for 10 years of surveillance | ||||||
| Base case | 0.58 | 60 | 129,800 | ||||
| Male age ≥55 | 1.36 | 70 | 89,300 | ||||
HCC, hepatocellular carcinoma; ICER, incremental cost-effectiveness ratio.
Alcohol use was defined based on the AUDIT-C score ≥4 for men and ≥3 for women.
In patients with advanced fibrosis, biannual HCC surveillance was found to be cost-effective for an additional 10 years (until the age of 70) for men over the age of 55.
Sensitivity analysis
We evaluated a total of 59 parameters in the univariate probability sensitivity analysis. Tables S3 and S4 show the top 10 parameters, respectively, to which the model was most sensitive in virologically cured patients with compensated cirrhosis and advanced fibrosis, respectively. For individuals with compensated cirrhosis, the cost-effective surveillance strategy was most sensitive to the likelihood of incidental detection of HCC, the progression rate from early-stage HCC to intermediate/advanced stage HCC, and overall survival following palliative care. For advanced fibrosis individuals, the cost-effective surveillance strategy was most sensitive to the likelihood of incidental detection of HCC, MRI sensitivity for very early-stage HCC, and overall survival following liver transplant.
The results of the multivariate probabilistic sensitivity analysis are presented in the cost-effectiveness acceptability curve (Fig. 4), which shows the likelihood of the cost-effectiveness of biannual HCC surveillance for different stopping ages when accounting for uncertainty in all model parameters. In patients with cirrhosis, surveillance for 25 years (i.e., until the age 75) – in contrast to age 70 in the base case – was the most cost-effective option at the willingness-to-pay threshold of $150,000. In patients with advanced fibrosis, surveillance for 20 years (i.e., until age 70) – in contrast to age 60 in the base case – was the most cost-effective strategy at the willingness-to-pay threshold of $150,000 per QALY. This suggests that the exact duration of HCC surveillance be longer than that reported in the base case results.
Fig. 4. Cost-effectiveness acceptability curves.

Cost-effectiveness acceptability curves showing the probability of cost-effectiveness for each surveillance strategy by different durations of hepatocellular carcinoma surveillance as defined by the stopping age in patients with (A) compensated cirrhosis and (B) advanced fibrosis. QALY, quality-adjusted life year.
Discussion
Patients with hepatitis C that has been cured with oral DAAs remain at risk of developing HCC. With the wide use of DAAs, the number of patients virologically cured will rapidly increase in the near future.5 In this study, we evaluated the value of routine surveillance for HCC using a decision-analytic model that simulated the natural history of HCC in hepatitis C cured individuals. We found that biannual HCC surveillance is cost-effective in patients with compensated cirrhosis until the age of 70 and in patients with advanced fibrosis until the age of 60. These findings are important because virologically cured patients have a higher life expectancy than patients with active hepatitis C, and lifelong surveillance may not be warranted in this growing cohort.
Our study’s conclusion supports AASLD and EASL’s current HCC surveillance recommendations for HCC surveillance in patients with compensated cirrhosis who achieve virological cure with DAAs. For patients with stable advanced fibrosis – for whom the AASLD and EASL surveillance recommendations differ – our results align with the EASL’s recommendation of the need for HCC surveillance. Of note, our study also identified an age cut-off at which to stop surveillance in both cohorts, which is not mentioned in either guideline.
An earlier study found that HCC surveillance is cost-effective in hepatitis C individuals who were successfully treated if they have cirrhosis.9 However, this study assumed patients would undergo HCC surveillance for the duration of their lifetime. In contrast, we opted to test various ages to start and stop surveillance, as we know hepatitis C patients after achieving cure can live longer than those who do not achieve a SVR or those with other etiologies of liver disease. Our study’s primary finding – that biannual surveillance is cost-effective until the age of 70 in individuals with compensated cirrhosis or until the age of 60 in individuals with advanced fibrosis – is important for the medical community as they work to define the optimal surveillance policy for HCC.
Surveillance may not be warranted in patients with HCC who are not eligible for curative treatments given factors such as age, comorbidity, liver disease severity, and access to transplant, among others. Therefore, we examined the benefits as well as the harms associated with surveillance in key subgroups, including those with advanced comorbidity. We found that HCC surveillance would not be deemed cost-effective if the remaining life expectancy is less than 16 years in patients with cirrhosis and less than 28 years in patients with advanced fibrosis. While our study found that HCC surveillance is cost-effective in virologically cured patients with advanced fibrosis until the age of 60, there could be subgroups of patients who have an elevated risk of HCC and could benefit from longer durations of surveillance. Therefore, future research is needed to identify risk factors and biomarkers in virologically cured patients with advanced fibrosis who could benefit from routine HCC surveillance.
Early diagnosis of HCC is critical to improved survival – patients with advanced HCC have a median survival of less than 1 year, while patients with early HCC can achieve 5-year survival rates near 70% with resection or transplantation.44 Our analysis found that surveillance could produce a substantial stage shift, in which most HCC would be diagnosed at an early stage. Compared with ‘no surveillance’ arm, surveillance per 1,000 cirrhosis patients detected 130 additional HCCs in ‘very early’/ early stage and yielded 51 additional QALYs. Similarly, surveillance per 1,000 patients with advanced fibrosis detected 24 additional HCCs in ‘very early’/early stage and yielded 12 additional QALYs.
Unlike other major cancers such as breast, prostate, and colorectal, surveillance for HCC remains underutilized in practice.45 Several recent observational studies have shown the benefits of routine HCC surveillance.7,46 Ideally, a large randomized controlled trial could compare the long-term outcomes of surveillance with a no surveillance control arm;47 however, this would be lengthy, and the use of a control arm would strain medical ethics.47,48 In this type of situation, decision-analytic models can simulate a virtual trial to generate comparative effectiveness data and inform screening policy. Simulation models are frequently used by policymakers to inform guidelines for cancer screening.49–52 Together with other recent reports,8,9 we believe our study provides helpful data to policymakers and professional societies in their evaluation of recommendations for HCC surveillance among high-risk patients.
This study has limitations. While our results remain robust for patients with compensated cirrhosis, limited data on HCC incidence in patients with advanced fibrosis warrant further research before making strong conclusions on the duration of surveillance in these individuals. Second, we assumed that patients with fibrosis successfully treated with DAAs were no longer at risk of progression to cirrhosis, despite the clinical reality that these patients may show progression in cases of alcohol consumption or weight change. Future research is warranted to estimate HCC risk and cost-effectiveness of HCC surveillance in those subgroups. We believe future research should also evaluate the cost-effectiveness of HCC surveillance tailored to dynamic risk factors that change over time. Third, we only modeled primary HCC treatment, whereas most patients with HCC receive a sequence of treatments (such as a combination of ablation and transplantation). However, our estimates of life expectancy after HCC diagnosis and primary treatment included this treatment variability. We opted to simulate surveillance in 5-year increments rather than shorter time frames because such age intervals are easier to implement in practice. Lastly, aside from liver transplantation being restricted to patients under 75, no other age or comorbidity restrictions were assumed for other treatments.
In conclusion, we found that biannual surveillance for HCC in virologically cured patients with cirrhosis is cost-effective until age 70 and in virologically cured patients with stable advanced fibrosis until the age of 60. Future studies should identify subgroups of patients without cirrhosis who remain at risk of developing HCC and could benefit from a longer duration of HCC surveillance.
Supplementary Material
Highlights.
The value of lifelong surveillance for HCC in individuals after SVR is not known.
Ultrasound-based bi-annual surveillance for HCC appears to be cost-effective up to age 75 in those with cirrhosis.
This surveillance strategy was cost effective up to age 60 in those with stable advanced fibrosis.
Compared with no surveillance, surveillance detected 86 additional HCCs in ‘very early’/early stage per 1,000 patients with cirrhosis.
Financial support
This study was supported by the American Cancer Society Research Scholar Grant RSG-17-022-01-CPPB (Chhatwal); the Office of the Assistant Secretary of Defense for Health Affairs under Award Numbers W81XWH-19-10689 (Kanwal) and W81XWH-19-10690 (Chhatwal); and the National Institutes of Health grants P30 DK 56338 (Kanwal), U01CA230997 (Kanwal), and K08 CA248473 (Peters). Dr. Kanwal is an investigator at the Veterans Administration Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413). None of the funding agencies have specific role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Conflict of interest
Dr. Peters received honoraria from Bayer, Exelixis and Agios, travel support from Halozyme, AstraZeneca and Exelixis, research support from Bayer, institutional research support from Taiho, AstraZeneca, BeiGene, Berg, Merck, all outside the submitted work. Dr. Ayer received consulting fee from Merck and served as principal scientist to Value Analytics Labs outside the submitted work. Dr. Chhatwal received consulting fee from Gilead, Merck, and Novo Nordisk, and served as principal scientist to Value Analytics Labs outside the submitted work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- DAAs
direct-acting antivirals
- EASL
European Association for the Study of the Liver
- HCC
hepatocellular carcinoma
- ICER
incremental cost-effectiveness ratio
- QALY
quality-adjusted life year
- SVR
sustained virological response
Footnotes
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2022.01.027.
Data availability statement
All data generated from the simulation model are made available in the manuscript.
References
- [1].Ursoniu S, Sahebkar A, Serban MC, Antal D, Mikhailidis DP, Cicero A, et al. Lipid-modifying effects of krill oil in humans: systematic review and meta-analysis of randomized controlled trials. Nutr Rev 2017;75(5):361–373. [DOI] [PubMed] [Google Scholar]
- [2].Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology 2017;153(4):996–1005 e1. [DOI] [PubMed] [Google Scholar]
- [3].Kanwal F, Kramer JR, Asch SM, Cao Y, Li L, El-Serag HB. Long-term risk of hepatocellular carcinoma in HCV patients treated with direct acting antiviral agents. Hepatology 2020;71(1):44–55. [DOI] [PubMed] [Google Scholar]
- [4].Maan R, Feld JJ. Risk for hepatocellular carcinoma after hepatitis C virus antiviral therapy with direct-acting antivirals: case closed? Gastroenterology 2017;153(4):890–892. [DOI] [PubMed] [Google Scholar]
- [5].Chhatwal J, Wang X, Ayer T, Kabiri M, Chung RT, Hur C, et al. Hepatitis C disease burden in the United States in the era of oral direct-acting antivirals. Hepatology 2016;64(5):1442–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen Q, Ayer T, Adee MG, Wang X, Kanwal F, Chhatwal J. Assessment of incidence of and surveillance burden for hepatocellular carcinoma among patients with hepatitis C in the era of direct-acting antiviral agents. JAMA Netw Open 2020;3(11). e2021173-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 2014;11(4):e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Singal AG, Ioannou GN. For whom is hepatocellular carcinoma surveillance after sustained virologic response cost-effective? Clin Gastroenterol Hepatol : the official Clin Pract J of the American Gastroenterological Association 2019;17(9):1732–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Farhang Zangneh H, Wong WWL, Sander B, Bell CM, Mumtaz K, Kowgier M, et al. Cost effectiveness of hepatocellular carcinoma surveillance after a sustained virologic response to therapy in patients with hepatitis C virus infection and advanced fibrosis. Clin Gastroenterol Hepatol 2019;17(9):1840–1849.e16. [DOI] [PubMed] [Google Scholar]
- [10].EASL Clinical Practice Guidelines. Management of hepatocellular carcinoma. J Hepatol 2018;69(1):182–236. [DOI] [PubMed] [Google Scholar]
- [11].Krajden M, Cook DA, Wong S, Yu A, Butt ZA, Rossi C, et al. What is killing people with hepatitis C virus infection? Analysis of a population-based cohort in Canada. Int J Drug Pol 2019;72:114–122. [DOI] [PubMed] [Google Scholar]
- [12].Calvaruso V, Cabibbo G, Cacciola I, Petta S, Madonia S, Bellia A, et al. Incidence of hepatocellular carcinoma in patients with HCV-associated cirrhosis treated with direct-acting antiviral agents. Gastroenterology 2018;155(2):411–421.e4. [DOI] [PubMed] [Google Scholar]
- [13].Carrat F, Fontaine H, Dorival C, Simony M, Diallo A, Hezode C, et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. The Lancet 2019;393(10179): 1453–1464. [DOI] [PubMed] [Google Scholar]
- [14].Li DK, Ren Y, Fierer DS, Rutledge S, Shaikh OS, Lo Re V, et al. The short-term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: an ERCHIVES study. Hepatology 2018;67(6):2244–2253. [DOI] [PubMed] [Google Scholar]
- [15].Nahon P, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology 2018;155(5):1436–1450.e6. [DOI] [PubMed] [Google Scholar]
- [16].Shiha G, Mousa N, Soliman R, Nnh Mikhail N, Adel Elbasiony M, Khattab M. Incidence of HCC in chronic hepatitis C patients with advanced hepatic fibrosis who achieved SVR following DAAs: a prospective study. J of Viral Hepat 2020;27(7):671–679. [DOI] [PubMed] [Google Scholar]
- [17].Sánchez-Azofra M, Fernández I, García-Buey ML, Domínguez-Domínguez L, Fernández-Rodríguez CM, Mancebo A, et al. Hepatocellular carcinoma risk in hepatitis C stage-3 fibrosis after sustained virological response with direct-acting antivirals. Liver Int 2021;41(12):2885–2891. [DOI] [PubMed] [Google Scholar]
- [18].Zhao C, Jin M, Le RH, Le MH, Chen VL, Jin M, et al. Poor adherence to hepatocellular carcinoma surveillance: a systematic review and meta-analysis of a complex issue. Liver Int 2018;38(3):503–514. [DOI] [PubMed] [Google Scholar]
- [19].Andersson KL, Salomon JA, Goldie SJ, Chung RT. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2008;6(12):1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Goossens N, Singal AG, King LY, Andersson KL, Fuchs BC, Besa C, et al. Cost-effectiveness of risk score-stratified hepatocellular carcinoma screening in patients with cirrhosis. Clin Transl Gastroenterol 2017;8(6):e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ronot M, Vilgrain V. Hepatocellular carcinoma: diagnostic criteria by imaging techniques. Best Pract Res Clin Gastroenterol 2014;28(5): 795–812. [DOI] [PubMed] [Google Scholar]
- [22].Singal AG, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MAM, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther 2009;30(1):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cheung YB, Thumboo J, Gao F, Ng G, Pang G, Koo WH, et al. Mapping the English and Chinese versions of the functional assessment of cancer therapy–general to the EQ-5D utility index. Value in health 2009;12(2):371–376. [DOI] [PubMed] [Google Scholar]
- [24].El-Fattah MA, Aboelmagd M, Elhamouly M. Prognostic factors of hepatocellular carcinoma survival after radiofrequency ablation: a US population-based study. United Eur Gastroenterol J 2017;5(2):227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ikai I, Arii S, Kojiro M, Ichida T, Makuuchi M, Matsuyama Y, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer 2004;101(4):796–802. [DOI] [PubMed] [Google Scholar]
- [26].Lang K, Danchenko N, Gondek K, Shah S, Thompson D. The burden of illness associated with hepatocellular carcinoma in the United States. J of Hepatol 2009;50(1):89–99. [DOI] [PubMed] [Google Scholar]
- [27].Lencioni R, Cioni D, Crocetti L, Franchini C, Della Pina C, Lera J, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology 2005;234(3):961–967. [DOI] [PubMed] [Google Scholar]
- [28].Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology 2008;47(1):82–89. [DOI] [PubMed] [Google Scholar]
- [29].Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003;37(2):429–442. [DOI] [PubMed] [Google Scholar]
- [30].Llovet JM, Ducreux M. EASL–EORTC clinical practice guidelines: management of hepatocellular carcinoma. J of Hepatol 2012;56(4):908–943. [DOI] [PubMed] [Google Scholar]
- [31].Sucandy I, Cheek S, Golas BJ, Tsung A, Geller DA, Marsh JW. Longterm survival outcomes of patients undergoing treatment with radiofrequency ablation for hepatocellular carcinoma and metastatic colorectal cancer liver tumors. HPB (Oxford) 2016;18(9):756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Toro A, Pulvirenti E, Palermo F, Di Carlo I. Health-related quality of life in patients with hepatocellular carcinoma after hepatic resection, transcatheter arterial chemoembolization, radiofrequency ablation or no treatment. Surg Oncol 2012;21(1):e23–e30. [DOI] [PubMed] [Google Scholar]
- [33].Wolfe RA, Roys EC, Merion RM. Trends in organ donation and transplantation in the United States, 1999–2008. American J of Transplant 2010;10(4p2):961–972. [DOI] [PubMed] [Google Scholar]
- [34].Yang W, Yan K, Goldberg SN, Ahmed M, Lee JC, Wu W, et al. Ten-year survival of hepatocellular carcinoma patients undergoing radiofrequency ablation as a first-line treatment. World J Gastroenterol 2016;22(10):2993–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ratcliffe J, Longworth L, Young T, Bryan S, Burroughs A, Buxton M. Assessing health-related quality of life pre–and post–liver transplantation: a prospective multicenter study. Liver Transplant 2002;8(3):263–270. [DOI] [PubMed] [Google Scholar]
- [36].Cardoso AC, Moucari R, Figueiredo-Mendes C, Ripault MP, Giuily N, Castelnau C, et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J Hepatol 2010;52(5):652–657. [DOI] [PubMed] [Google Scholar]
- [37].Coon JT, Rogers G, Hewson P, Wright D, Anderson R, Jackson S, et al. Surveillance of cirrhosis for hepatocellular carcinoma: a cost–utility analysis. Br J of Cancer 2008;98(7):1166–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Trevisani F, De Notariis S, Rapaccini G, Farinati F, Benvegnu L, Zoli M, et al. Semiannual and annual surveillance of cirrhotic patients for hepatocellular carcinoma: effects on cancer stage and patient survival (Italian experience). Am J Gastroenterol 2002;97(3):734–744. [DOI] [PubMed] [Google Scholar]
- [39].Choi DT, Kum HC, Park S, Ohsfeldt RL, Shen Y, Parikh ND, et al. Hepatocellular carcinoma screening is associated with increased survival of patients with cirrhosis. Clin Gastroenterol Hepatol 2019;17(5):976–987.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mourad A, Deuffic-Burban S, Ganne-Carrie N, Renaut-Vantroys T, Rosa I, Bouvier AM, et al. Hepatocellular carcinoma screening in patients with compensated hepatitis C virus (HCV)-related cirrhosis aware of their HCV status improves survival: a modeling approach. Hepatology 2014;59(4):1471–1481. [DOI] [PubMed] [Google Scholar]
- [41].Chhatwal J, Ferrante SA, Brass C, El Khoury AC, Burroughs M, Bacon B, et al. Cost-effectiveness of boceprevir in patients previously treated for chronic hepatitis C genotype 1 infection in the United States. Value in Health 2013;16(6):973–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making 2006;26(4):391–400. [DOI] [PubMed] [Google Scholar]
- [43].Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness – the curious resilience of the $50,000-per-QALY threshold. New Engl J of Med 2014;371(9):796–797. [DOI] [PubMed] [Google Scholar]
- [44].Artinyan A, Mailey B, Sanchez-Luege N, Khalili J, Sun CL, Bhatia S, et al. Race, ethnicity, and socioeconomic status influence the survival of patients with hepatocellular carcinoma in the United States. Cancer 2010;116(5):1367–1377. [DOI] [PubMed] [Google Scholar]
- [45].Singal AG, Tiro JA, Gupta S. Improving hepatocellular carcinoma screening: applying lessons from colorectal cancer screening. Clin Gastroenterol Hepatol 2013;11(5):472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kansagara D, Papak J, Pasha AS, O’Neil M, Freeman M, Relevo R, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med 2014;161(4):261–269. [DOI] [PubMed] [Google Scholar]
- [47].Sherman M Whither hepatocellular carcinoma screening? Hepatology 2012;56(6):2412–2414. [DOI] [PubMed] [Google Scholar]
- [48].Chirovsky D, Lich KH, Barritt ASt. Screening for hepatocellular carcinoma in chronic liver disease. Ann Intern Med 2015;162(3):238–239. [DOI] [PubMed] [Google Scholar]
- [49].Kim JJ, Burger EA, Regan C, Sy S. Screening for cervical cancer in primary care: a decision analysis for the US preventive Services task force. Jama 2018;320(7):706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].van Ravesteyn NT, Miglioretti DL, Stout NK, Lee SJ, Schechter CB, Buist DSM, et al. Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years: a comparative modeling study of risk. Ann of Intern Med 2012;156(9):609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Knudsen AB, Zauber AG, Rutter CM, Naber SK, Doria-Rose VP, Pabiniak C, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US preventive Services task force. JAMA 2016;315(23):2595–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Section 5. Modeling. U.S. Preventive Services Task Force; July 2017. Retreieved from: https://www.uspreventiveservicestaskforce.org/uspstf/procedure-manual/procedure-manual-section-5-modeling. [Accessed 28 November 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated from the simulation model are made available in the manuscript.


