Abstract
Background and aim
Garlic essential oil (GEO) isolated from Garlic (Allium sativum L.) exerts biological activities in disease prevention, particularly in metabolic and liver diseases, and is used for a dietary therapy for centuries. However, due to the side effects associated with the excessive consumption of GEO, there is a need to evaluate the safety of the GEO.
Experimental procedure
Ames test using five Salmonella typhimurium strains (TA98, TA100, TA102, TA1535, and TA1537) and Chinese hamster ovary (CHO–K1) cells with or without metabolic activation (S9 system), and mammalian erythrocyte micronucleus test were used to assess the genotoxicity and clastogenic effects of GEO. A repeated dose of GEO (15, 25, and 50 mg/kg body weight, p.o.) were administrated to ICR mice for 28 days to ascertain the subacute toxicity of GEO.
Results and conclusions
The results of the Ames test with or without S9 system indicated that GEO did not induce mutagenicity nor have clastogenic effects in CHO–K1 cells with or without S9 activation. Furthermore, GEO did not affect the ratio of immature to total erythrocytes or the number of micronuclei in immature erythrocytes of ICR mice after 24 and 48 h. In a 28-day oral toxicity assessment, GEO (15, 25, and 50 mg/kg body weight, p.o.)-fed ICR mice exhibited normal behaviors, mortality, body weight, daily intake, hematology, clinical biochemistry, and organ weight. GEO shows no genotoxicity, and the no-observed-adverse-effect level (NOAEL) for GEO is considered to be greater than 50 mg/kg bw/day orally for 28 days in mice.
Keywords: Allium sativum L., Garlic essential oil, Safety evaluation, Genotoxicity, Subacute toxicity, NOAEL
Graphical abstract
Highlights
-
•
Garlic essential oil (GEO) did not induce mutagenicity in Ames test.
-
•
GEO has no clastogenic effects in CHO–K1 cells.
-
•
GEO did not affect micronuclei formation or erythropoiesis in a micronucleus test.
-
•
GEO did not induce toxic effects in a 28-day oral toxicity study.
-
•
The NOAEL of GEO is greater than 50 mg/kg bw/day for both male and female mice.
Abbreviations
- ALB
albumin
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- ALKP
alkaline phosphatase
- BASO
basophils
- BUN
blood urea nitrogen
- CHO
cholesterol
- CREA
creatinine
- Cl
chloride
- Ca
calcium
- DAS
diallyl sulfide
- DADS
diallyl disulfide
- DATS
diallyl trisulfide
- DMSO
dimethyl sulfoxide
- EO
eosinophils
- GC
gas chromatograph
- GEO
garlic essential oil
- GLU
glucose
- γ-GT
gamma-glutamyl transferase
- H&E
hematoxylin and eosin
- HGB
hemoglobin
- HCT
hematocrit
- IC50
half maximal inhibitory concentration
- IE
immature erythrocytes
- K
potassium
- LD50
median lethal dose
- LYMPH
lymphocytes
- MCV
mean corpuscular volume
- MCH
mean corpuscular hemoglobin
- MCHC
mean corpuscular hemoglobin concentration
- MNIE
micronucleated immature erythrocytes
- MONO
monocytes
- NEUT
neutrophils
- NOAEL
no-observed-adverse-effect level
- Na
sodium
- OECD
Organization for Economic Cooperation and Development
- P
phosphorus
- PLT
platelets
- RBC
red blood cells
- RDA
recommended dietary allowance
- SAMC
S-allylmercaptocysteine
- SMC
S-methylcysteine
- SD
standard deviation
- SAC
S-allylcysteine
- TG
triglyceride
- TP
total protein
- T-BIL
total bilirubin
- WBC
white blood cell
1. Introduction
Garlic (Allium sativum L.) has been consumed as both a food and medicine for thousands of years.1 A growing body of research has focused on the benefits of various organosulfur compounds (OSCs) in garlic. The OSCs can be classified into two categories, water-soluble and oil-soluble OSCs. The major water-soluble OSCs include S-allylcysteine (SAC), S-allylmercaptocysteine (SAMC), S-methylcysteine (SMC), and allicin. The oil-soluble OSCs include mostly diallyl sulfide (DAS), diallyl disulfide (DADS), diallyl trisulfide (DATS), and allyl methyl trisulfide.2,3 The biological benefits of A. sativum are reported to possess many bioactive functions, including antioxidant,4 antihyperlipidemic,5,6 antiobesity7 and hepatoprotective effects.8
Garlic essential oil (GEO), prepared from crushed garlic by steam distillation, is the most common method for obtaining organosulfur compounds.4 Among OSCs, DAS, DADS, and DATS have been identified as the three major components of GEO.3,8 Previous studies have revealed that GEO exerts beneficial effects on both ethanol-induced fatty liver and high-fat diet-induced liver injury by activating the antioxidant and detoxification system.8,9 Moreover, the administration of GEO (0.25 mg/kg body weight (bw) per day) for 6 months has been shown to downregulate serum cholesterol and triglyceride levels in patients diagnosed with coronary heart disease and hyperlipidemia.10 Based on these findings, GEO has great potential for use as a dietary supplement or functional food to mitigate the symptoms of metabolic and liver diseases.
However, despite both A. sativum and GEO being listed as generally recognized as safe (GRAS) by the U.S. Food and Drug Administration (FDA), several adverse effects, including diarrhea, gastrointestinal abnormalities, skin rash, nausea, and anemia, have been reported when consumed excessively.3,11, 12, 13 In terms of toxicity in rodents, the oral administration of GEO at a dose of 10 mg/100 g bw in rats after a 24 h fasting was found to be lethal and combined with acute pulmonary edema.14 Furthermore, hepatic injury was also observed in rats treated with fresh garlic extract added to drinking water (200 g/L) for 10 days.14 Raw garlic juice (0.5 mL) has also been reported to cause damage to the epithelial mucosal membrane after 2 h in rats; ulcers, shrinkage, and bleeding in the epithelial mucosa were also detected after 24 h of exposure.2 The median lethal dose (LD50) of aqueous extract of garlic and allicin in mice was 173.78 mL/kg bw and 204.17 μL/kg bw, respectively.15 Despite these findings, the toxic effects of A. sativum, GEO, and related products have rarely been reported in clinical studies, even at high dosages. Although several toxicological studies have concluded that garlic extract is safe and nontoxic,16, 17, 18 a comprehensive study focusing on the safety evaluation of GEO is lacking.
Since GEO has great potential for use as a functional food, and most of the functional foods are typically applied in the long term for health promotion or disease prevention, there is a need to evaluate the safety of GEO and define its no-observed-adverse-effect level (NOAEL) for potential precluding side effects. In the present study, we aimed to assess the genotoxicity and subacute toxicity of GEO using in vitro and in vivo genotoxicity assays and a 28-day subacute oral toxicity study in mice.
2. Materials and methods
2.1. Preparation of GEO
Garlic (A. sativum) was obtained from the Yunlin County Farmers’ Association (Yunlin, Taiwan). GEO extraction was performed as described in our previous study.8 Briefly, garlic was stirred with 2 vol of distilled water and extracted by steam distillation for 4 h. Volatile extracts were then dehydrated to obtain GEO and stored at −20 °C until use. The yield of GEO from fresh garlic was 0.30 ± 0.05% (w/w), calculated according to the mean fresh weight of garlic used in each extraction procedure. The concentrations of three major components, namely DAS, DADS, and DATS in GEO were determined based on calibration curves constructed using the respective reference standards by a gas chromatograph (GC, Thermo Scientific Focus GC) equipped with an AI 3000 II autosampler, a flame ionization detector, and a Stabilwax (Crossbond Carbowax-PEG, Restek) capillary column (60 m × 0.32 mm, 1.0 μm thick film). The GC settings and analyses were performed as described in our previous work.8 DAS (purity 97%), DADS (purity 80%) and DATS (purity 98%) were purchased from Sigma-Aldrich (St Louis, MO, USA).
2.2. Preparation of metabolic activation system (S9)
The procedure to obtain rat liver enzymes was performed as described previously, with some modifications.19,20 Briefly, five days before sacrifice, 3-methylcho-lanthrene was diluted in corn oil and i.p. injected into 8-weeks-old male Sprague-Dawley rats (20 mg/kg bw/day) for three consecutive days. Rats could consume food and water ad libitum until 12 h before euthanasia, when food, but not water, was removed. On the fifth day of induction, the rats were euthanized with CO2 and immediately decapitated. The livers were isolated, washed with 0.15 M KCl, minced with sterile scissors, and homogenized using a Teflon pestle. Homogenates were centrifuged for 10 min at 9000×g. The supernatant (S9 fraction) was collected and stored at −80 °C. An S9 mix containing the S9 fraction and cofactors (glucose-6-phosphate, β-nicotinamide adenine dinucleotide phosphate, MgCl2, KCl, and sodium phosphate buffer) was prepared immediately before performing the metabolic activation test.
2.3. Animal housing and dose design
Male and female ICR mice (five-week-old) were purchased from the Animal Center of the College of Medicine, National Taiwan University (Taipei, Taiwan). All mice were housed under controlled temperature (23 ± 2 °C), humidity (50–70% RH), and a 12 h/12 h light/dark cycle in the animal facility. Food and water were provided ad libitum. After one week of acclimatization, the mice were used for the in vivo mammalian erythrocyte micronucleus test and a 28-day oral toxicity study. In both studies, the dosages of GEO followed the required health food regulations from the Taiwan Food and Drug Administration (TFDA) based on the concept of margin of safety (MOS).21 According to a previous report, the recommended dietary allowance (RDA) in humans of GEO is 0.25 mg/kg bw/day.10 To determine the no-observed-adverse-effect level (NOAEL) of GEO, the 60-, 100-, and 200-fold exposures to human RDA were tested, corresponding to 15, 25, and 50 mg/kg bw, respectively, in mice. All animal procedures and dose design were approved by the Institutional Animal Care and Use Committee (IACUC) of the National Taiwan University (IACUC approval no. NTU-101-EL-124).
2.4. Genotoxicity
The bacterial reverse mutation assay, in vitro mammalian chromosome aberration test, and in vivo micronucleus test were performed to evaluate the genotoxicity of GEO. These experiments followed the guidelines of the Organization for Economic Cooperation and Development (OECD) and the TFDA.
2.4.1. Bacterial reverse mutation assay (Ames test)
To identify substances that cause point mutations involving substitution, addition, or deletion of DNA base pair(s) in amino-acid-requiring strains of Salmonella typhimurium, a bacterial reverse mutation test was used.22 The histidine auxotroph mutant strains of S. Typhimurium, namely TA98, TA100, TA102, 1535 and TA1537, were obtained from the Bioresource Collection and Research Center, Food Industry Research and Development Institute (Hsinchu, Taiwan). To determine the mutagenicity of GEO, the plate incorporation method with the presence or absence of a metabolic activation system (S9) was performed as described previously23 and in accordance with the test guidelines of the OECD 471.24 The testing concentrations of GEO were 0.05, 0.1, 0.125, 0.25, 0.5, and 1 mg/plate, which followed the TFDA guidelines for safety assessment of health food. Briefly, 0.1 mL of each test S. Typhimurium culture (109 cells/mL), 2 mL of soft agar (0.75% agar and 0.5% NaCl, 48 ± 2 °C), 0.2 mL of 0.5 mM histidine/biotin, 0.5 mL of S9 mix (as appropriate), and the test substance were mixed in a tube and immediately poured into a minimal agar plate (1.5% agar, 2% glucose, and Vogel-Bonner medium E). Compounds with known mutagenic activity are used for positive control for each tester strain: 4-nitro-o-phenylenediamine (0.5 μg/plate) for TA98; sodium azide (1 μg/plate) for TA100 and TA1535; mitomycin c (0.5 μg/plate) for TA102; 9-aminoacridine (0.5 μg/plate) for TA1537. For metabolic activation system (S9), Benzo[a]pyrene (1.0 μg/plate) is used for TA98 and TA102; 2-aminoanthracene (4 μg/plate) is used for TA100, TA1535 and TA1537. The Dimethyl sulfoxide (DMSO) is used for negative control. After the agar solidified, the plates were incubated for 48–72 h at 37 °C in the dark, and revertant colonies were counted using a microscope.
2.4.2. In vitro mammalian chromosomal aberrations test
To identify substances that cause structural chromosomal aberrations in cultured mammalian cells, we conducted an in vitro mammalian chromosomal aberration test.25 Chinese hamster ovary cells (CHO–K1) were obtained from the Bioresource Collection and Research Center, Food Industry Research and Development Institute (Hsinchu, Taiwan). Chromosomal abnormalities induced by GEO in the presence or absence of S9 were determined according to the OECD 473 guidelines.26 The concentrations of GEO to be tested adhered the TFDA guidelines for the safety assessment of health food. Since the half maximal inhibitory concentration (IC50) of GEO in CHO–K1 cells was 15 μg/mL (unpublished results), the concentrations were set at 1, 1.5, 2.5, 5, and 10 μg/mL. Briefly, CHO–K1 cells (4 × 105 cells/mL) were cultured in 6-cm dishes with complete medium of Ham F-12. The complete medium also served as a diluent for GEO and the negative control. Two of the known mutagens, mitomycin C (0.07 μg/mL) and cyclophosphamide (10 μg/mL), were used as positive controls. GEO (or appropriate controls) was introduced to the cells under the following conditions. For short-term treatment, GEO (or control) was incubated with or without S9 mix for 3 h and then changed to complete medium for a further 17 h. For continuous treatment, GEO (or control) was incubated without S9 mix for 20 h. After the aforementioned treatments, cells were treated with colchicine (0.2 μg/mL) for 2 h to induce metaphase arrest. Cells were harvested, spread onto glass slides, and stained with Giemsa solution (5%). Chromosome aberrations were observed under 1000 × magnification in 100 well-spread metaphases per concentration, with controls equally divided among the duplicates. The percentage of structural chromosomal abnormalities, including chromatid and chromosome types, was determined. Aberrations include gaps, breaks, dicentrics, rings, multiple aberrations, and acentric fragments.
2.4.3. In vivo mammalian erythrocyte micronucleus test
The in vivo mammalian micronucleus test was used to screen for genotoxic substances by determining micronuclei formation, that is, lagging chromosome fragments or whole chromosomes. To ascertain the genotoxicity of GEO, we performed a micronucleus test using mice in accordance with the OECD 474 guidelines.27 Briefly, 50 male ICR mice (six weeks old) were divided into five groups (n = 10): negative control (olive oil, 0.1 mL by oral administration), positive control (mitomycin C, 2 mg/kg bw by i.p. injection), and GEO (single oral dose at 15, 25, or 50 mg/kg bw) groups. GEO was mixed and diluted with 0.1 mL olive oil before oral administration to the mice. At 24 h and 48 h post-treatment, blood samples (4 μL) were collected from the orbital sinus, smeared onto slides, and stained with acridine orange (1 mg/mL). Micronucleated reticulocytes were observed using fluorescence microscopy. The number of micronucleated immature erythrocytes (MNIE, based on 2000 erythrocytes/animal) and immature erythrocytes (IE, based on 1000 erythrocytes/animal) were recorded.
2.5. 28-day oral toxicity study
To assess the subacute toxicity of GEO in animals, a repeated dose 28-day oral toxicity study was conducted in mice in accordance with OECD 407 guidelines.28 Briefly, male and female ICR mice (six weeks old) were randomly divided into four groups (n = 10/sex/group). The treatment groups were orally administered a low-, medium-, or high-dose GEO (15, 25, and 50 mg/kg bw/day, respectively) for 28 consecutive days. GEO was mixed and diluted with 0.1 mL of olive oil before administration. Mice in the negative control group were orally administered 0.1 mL of olive oil. The mice were observed once a day during the experimental period for their general appearance, such as behavioral changes, daily activities, rough hair coat, porphyrin discharges in eyes and nose.29 Food and water intake and body weight were recorded twice per week. At the end of the experiment (day 29), the mice underwent blood collection and laparotomy under anesthesia. Then, the major organs, such as the brain, heart, liver, spleen, lung, kidney, adrenal, thymus, testis (male), epididymis (male), ovary (female), and uterus (female), were isolated, weighed, and recorded. The relative organ weight was determined based on the terminal body weight.
2.5.1. Haematological and serum biochemical analysis
Blood samples were collected from the abdominal aorta and transferred to ethylenediaminetetraacetic acid tubes for haematological analysis. Hematological parameters, including red blood cells (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelets (PLT), white blood cell (WBC), neutrophils (NEUT), lymphocytes (LYMPH), monocytes (MONO), eosinophils (EO), and basophils (BASO), were measured. Blood samples were centrifuged at 3000×g for 30 min to obtain serum for biochemical analysis. Biochemical parameters, including cholesterol (CHO), triglyceride (TG), blood urea nitrogen (BUN), creatinine (CREA), total protein (TP), total bilirubin (T-BIL), albumin (ALB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALKP), glucose (GLU), γ-glutamyltransferase (γ-GT), calcium (Ca), phosphorus (P), sodium (Na), potassium (K), and chloride (Cl), were analyzed.
2.5.2. Histopathological analysis
Organs were fixed in 10% neutral buffered formalin for one week, embedded in paraffin, and sliced into 5-μm sections for histopathological examination. The sectioned samples were stained with hematoxylin and eosin (H&E). A blinded histological assessment of the sectioned samples under a microscope with digital camera was performed by a veterinary pathologist at the Graduate Institute of Veterinary Pathobiology of the National Chung Hsing University, Taiwan. The histopathological images were enlarged 400 times and the scale bars were included by ImageJ.
2.6. Statistical analysis
All data are presented as the mean ± standard deviation (SD). Statistical comparisons among experimental groups were analyzed by one-way analysis of variance and Tukey's multiple comparison test (genotoxic and clastogenic evaluations) or Dunnett's post-hoc test (28-day subacute toxicity study) using the SPSS software program. Differences were considered significant at P < 0.05.
3. Results and discussion
3.1. Chemical composition of GEO
The three major components of GEO, DAS, DADS, and DATS were analyzed with GC. As shown in the GEO chromatogram (Supplementary Fig. 1), the concentrations of DAS, DADS, and DATS were 6.7%, 40.7%, and 21.6%, respectively, which accounted for 69.0% of the total oil. The chemical composition of GEO was similar to our previous work.8 Also, the result of GEO profile is in consistent with previous studies, demonstrating that DADS is the major organosulfur compound in GEO.30,31
3.2. GEO did not cause genotoxicity or clastogenicity in vitro or in vivo
To evaluate the mutagenesis of GEO, we conducted a bacterial reverse mutation test using five S. typhimurium strains (TA98, TA100, TA102, TA1535, and TA1537) in the presence or absence of a metabolically activated condition (S9 system). According to the initial antibacterial test, we found significant toxicity to all strains when the concentration was set at 1 mg/plate (unpublished data). Hence, we tested GEO at concentrations of 0.05, 0.1, 0.125, 0.25, and 0.5 mg/plate. This finding is consistent with previous studies, which also demonstrate that garlic and its pure compound, allicin act as bactericides.32,33 As shown in Table 1, we found that GEO at all concentrations did not significantly increase the number of revertant colonies for any strain employed either in the presence or absence of metabolic activation (S9) as compared to the negative control group. However, the average number of revertants in all five strains showed a significant increase in the positive control group. The average number of the revertants in the positive group were 3- to 4-fold greater than those that found in the negative control group, which could be indicated as genotoxicity.34,35 This indicates that all the data obtained from this test are valid. Also, GEO at all concentrations decrease the number of revertant colonies for any strain employed either in the presence or absence of metabolic activation (S9) as compared to the positive control group. Collectively, these results indicated that GEO did not cause a reverse mutation in S. typhimurium.
Table 1.
Mutagenicity assay of GEO on Salmonella typhimurium TA98, TA100, TA102, TA1535, and TA1537 in the presence or absence of metabolic activation.
| Treatment | Revertant colonies |
||||
|---|---|---|---|---|---|
| TA98 | TA100 | TA102 | TA1535 | TA1537 | |
| Without S9 metabolic activation | |||||
| Negative controla | 51 ± 5.3 | 216 ± 5.0 | 299 ± 7.2 | 29 ± 2.6 | 13 ± 2.6 |
| Positive controlb | 132 ± 5.6∗∗ | 606 ± 7.0∗∗ | 761 ± 7.2∗∗ | 133 ± 5.1∗∗ | 53 ± 4.5∗∗ |
| GEO (mg/plate) | |||||
| 0.05 | 56 ± 5.3## | 219 ± 3.5## | 294 ± 6.7## | 28 ± 1.5## | 12 ± 2.5## |
| 0.1 | 47 ± 2.5## | 211 ± 8.5## | 310 ± 5.0## | 30 ± 1.5## | 13 ± 1.5## |
| 0.125 | 55 ± 4.0## | 220 ± 5.5## | 280 ± 12.1## | 29 ± 3.2## | 12 ± 1.2## |
| 0.25 | 51 ± 7.2## | 206 ± 7.6## | 290 ± 2.5## | 28 ± 2.1## | 11 ± 3.1## |
| 0.5 |
58 ± 4.5## |
205 ± 5.1## |
301 ± 4.7## |
30 ± 1.0## |
12 ± 1.7## |
| With S9 metabolic activation | |||||
| Negative controla | 57 ± 2.5 | 224 ± 8.7 | 293 ± 12.2 | 30 ± 1.0 | 10 ± 2.1 |
| Positive controlc | 165 ± 3.2∗∗ | 715 ± 4.5∗∗ | 935 ± 4.7∗∗ | 106 ± 5.1∗∗ | 45 ± 4.5∗∗ |
| GEO (mg/plate) | |||||
| 0.05 | 52 ± 3.0## | 209 ± 13.4## | 292 ± 4.5## | 29 ± 1.5## | 7 ± 0.6## |
| 0.1 | 51 ± 5.5## | 226 ± 6.1## | 306 ± 5.0## | 32 ± 2.1## | 9 ± 0.6## |
| 0.125 | 54 ± 4.6## | 229 ± 5.0## | 299 ± .4.0## | 33 ± 2.1## | 11 ± 1.2## |
| 0.25 | 52 ± 2.3## | 213 ± 10.1## | 293 ± 5.7## | 31 ± 2.1## | 9 ± 0.6## |
| 0.5 | 49 ± 5.3## | 218 ± 11.0## | 302 ± 3.1## | 31 ± 2.5## | 11 ± 2.1## |
Data are presented as the mean ± SD (n = 3 plates/group). Statistical analysis was performed using one-way ANOVA followed by Tukey's post-hoc multiple comparison test. ∗∗, P < 0.01 denotes significant differences compared to negative control group. ##, P < 0.01 denotes significant differences compared to positive control group.
Dimethyl sulfoxide (DMSO).
4-nitro-o-phenylenediamine (0.5 μg/plate) for TA98; sodium azide (1 μg/plate) for TA100 and TA1535; mitomycin C (0.5 μg/plate) for TA102; 9-aminoacridine (0.5 μg/plate) for TA1537.
Benzo[a]pyrene (1.0 μg/plate) for TA98 and TA102; 2-aminoanthracene (4 μg/plate) for TA100, TA1535, and TA1537.
We further investigated the clastogenic effect of GEO using a chromosomal aberration test in mammalian CHO–K1 cells in the presence or absence of S9.25 According to our initial toxicity assessment, the IC50 of GEO in CHO–K1 cells was 15 μg/ml (unpublished data), and we then tested GEO at concentrations of 1, 1.5, 2.5, 5, and 10 μg/mL in CHO–K1 cells. As shown in Table 2, chromosomal aberrations were observed in approximately 4% of the cells in the negative control group. We found the frequencies of chromosomal aberrations in our negative control group and GEO treatments were in consistence to a previous report, which were around 2–5% and were considered as no clastogenic effect.35 Further, incidences of aberrant cells in the negative control group are normally similar between treatment regimens.36 GEO at concentrations of 1, 1.5, 2.5, 5, and 10 μg/mL did not induce any obvious aberrations in chromosomes in any of the treatment groups (without S9 for 3 h or 20 h; with S9 for 3 h) when compared to the negative control group. In our case, the overall percentage of aberrations in GEO was 3–5%, which was similar to that in the negative control group. Conversely, approximately 15% of cells in the positive control group showed noticeable aberrations in chromosomes and chromatids, including gaps, breaks, dicentrics, rings, multiple aberrations, and acentric fragments. This increase in the percentage of chromosomal aberrations in the positive group was markedly higher than that in the negative control group, confirming the clastogenicity of compounds used in this test.37 In summary, these results suggested that GEO did not induce chromosomal aberrations in cultured mammalian CHO–K1 cells.
Table 2.
Chromosomal aberrations test of GEO-treated Chinese hamster ovary (CHO–K1) cells.
| Treatment | No. of chromosome aberrations in 100 cellsd |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| G | B | D | R | g | b | MA | AF | Aberrationse (%) | |
| 3 h without S9 metabolic activation | |||||||||
| Negative controla | 1 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 5 |
| Positive controlb | 1 | 4 | 0 | 0 | 3 | 4 | 2 | 1 | 15 |
| GEO (μg/mL) | |||||||||
| 1 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 3 |
| 1.5 | 1 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 4 |
| 2.5 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 5 |
| 5 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 4 |
| 10 |
1 |
1 |
0 |
0 |
0 |
2 |
1 |
0 |
5 |
| 20 h without S9 metabolic activation | |||||||||
| Negative controla | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 4 |
| Positive controlb | 1 | 5 | 0 | 1 | 5 | 3 | 1 | 0 | 17 |
| GEO (μg/mL) | |||||||||
| 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 3 |
| 1.5 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 4 |
| 2.5 | 0 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 5 |
| 5 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 3 |
| 10 |
0 |
0 |
1 |
0 |
1 |
0 |
2 |
0 |
4 |
| 3 h with S9 metabolic activation | |||||||||
| Negative controla | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 4 |
| Positive controlc | 1 | 4 | 0 | 1 | 2 | 2 | 3 | 1 | 14 |
| GEO (μg/mL) | |||||||||
| 1 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 5 |
| 1.5 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 5 |
| 2.5 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 4 |
| 5 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 4 |
| 10 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 3 |
Dimethyl sulfoxide (DMSO).
Mitomycin C (0.07 μg/mL).
Cyclophosphamide (10 μg/mL).
G, chromosome gap; B, chromosome break; D, dicentric chromosome; R, ring; g, chromatid gap; b, chromatid break; MA, multiple aberration; AF, acentric fragment.
Chromosome and chromatid gaps were recorded.
In 1999, Spielmann et al. found that IC50 in in vitro cytotoxicity was able to predict the LD50 in in vivo oral toxicity study, and suggested to use the predicted LD50 as the starting dose.38 A recent toxicological study further claimed that in vitro genotoxicity test may also exhibited a similar impact and applicability.39 We calculated the predicted LD50 of GEO via the IC50 (15 μg/ml) obtained from CHO–K1 cells, and the result was 13.7 mg/kg bw. In addition to the theoretical calculation, the RDA in humans of GEO is 0.25 mg/kg bw/day based on a previous report.10 In order to determine the NOAEL of GEO in animals, we set 60-, 100-, and 200-fold exposures to human RDA, which correspond to 15, 25, and 50 mg/kg bw, respectively, in mice. Interestingly, these two different methods both support that 15 mg/kg bw can be applied to the in vivo oral toxicity study as a starting dose. We therefore used 15, 25, and 50 mg/kg bw GEO to conduct safety evaluation in the following in vivo toxicological studies.
We evaluated the potential toxicological effects of GEO on peripheral blood micronuclei using an in vivo mouse model. As shown in Table 3, mice administered a single dose of GEO at 15, 25, or 50 mg/kg bw exhibited no significant differences in the amount of MNIE and IE compared to the negative control group at either 24 or 48 h. A significant difference in the amount of MNIE and IE was observed in the positive control group (mitomycin C) at either 24 or 48 h post-treatment (P < 0.01). This is consistent with a previous study, which revealed a higher incidence of micronuclei in peripheral blood erythrocytes 48 h after mitomycin C treatment in rodents.40 In addition, the amount of immature erythrocytes in the total erythrocyte population is regarded as an indicator of cytotoxicity or erythropoiesis in evaluating a given substance.41 We found a significant increases in MNIE in the positive groups at both 24 and 48 h, demonstrating the validity of mitomycin C. In contrast, the proportion of immature erythrocytes and the incidence of micronuclei in the peripheral blood cells of mice were not changed by GEO administration as compared to the negative control group. Further, significant differences in the amount of MNIE and IE in all GEO treatments were observed when compared to the positive control group at both 24 and 48 h. These results indicated that GEO did not affect micronuclei formation or erythropoiesis in an in vivo mammalian erythrocyte micronucleus test. Overall, our results suggested that GEO did not cause genotoxicity or clastogenicity, which are consistent to previous reports.42,43
Table 3.
Micronucleus assay for the effect of GEO on peripheral blood cells of mice.
| Treatment | 24 h |
48 h |
||
|---|---|---|---|---|
| MNIEc | IEd | MNIEc | IEd | |
| Negative controla | 4.30 ± 0.95 | 25.28 ± 5.67 | 6.20 ± 1.14 | 20.88 ± 5.49 |
| Positive controlb | 15.10 ± 1.29∗∗ | 14.2 ± 4.70∗∗ | 25.60 ± 2.37∗∗ | 11.32 ± 3.15∗∗ |
| 15 mg/kg | 5.20 ± 1.48## | 26.9 ± 6.02## | 7.20 ± 1.14## | 19.5 ± 6.30# |
| 25 mg/kg | 5.10 ± 1.52## | 26.4 ± 8.67## | 6.70 ± 1.34## | 18.36 ± 5.85# |
| 50 mg/kg | 5.40 ± 1.43## | 24.58 ± 8.99# | 6.90 ± 0.99## | 19.68 ± 5.99# |
Data are presented as the mean ± SD (n = 10 mice/group). Statistical analysis was performed using one-way ANOVA followed by Tukey's post-hoc multiple comparison test. ∗∗, P < 0.01 denotes significant differences compared to negative control group. #, P < 0.05, ##, P < 0.01 denote significant differences compared to positive control group.
Olive oil.
Mitomycin C (2 mg/kg bw, i.p.).
Number of micronucleated immature erythrocytes (MNIE, based on 2000 erythrocytes/animal).
Number of immature erythrocytes (IE, based on 1000 erythrocytes/animal).
3.3. GEO did not induce toxic effects in a 28-day oral toxicity study
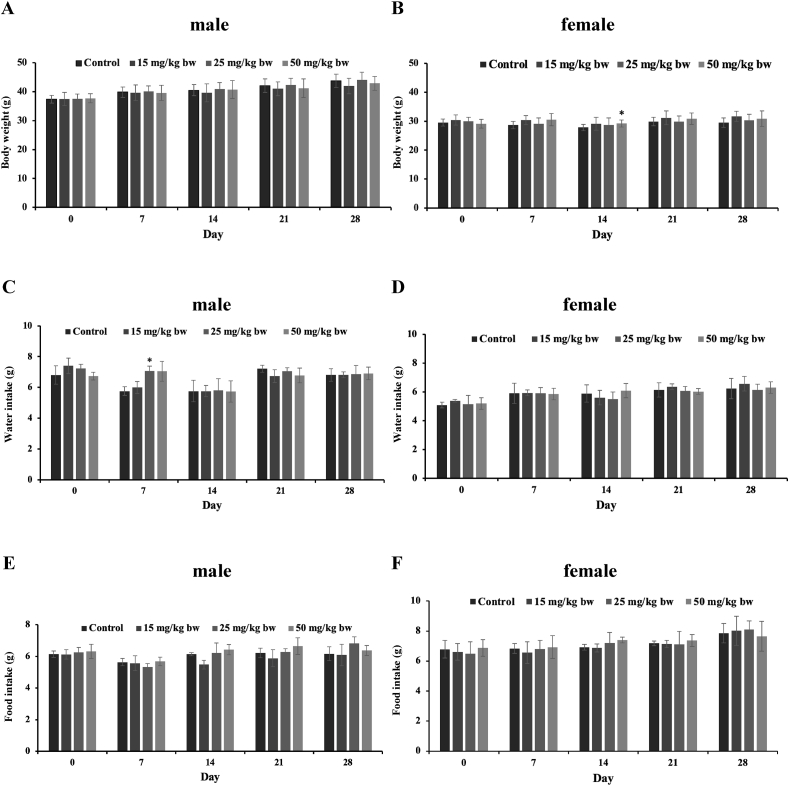
Next, we conducted an oral toxicity study to assess the potential toxic effects in a subacute manner (28-day). No ophthalmological abnormalities or deaths were observed during the course of the study. Changes in body weight were not significantly different between all treatment groups and controls in either sex or time, except for day 14 (Fig. 1A and B). We noticed a significant increase in body weight of female mice in the high dose (50 mg/kg bw) of GEO group as compared to those in the control group on day 14 (Fig. 1B); however, this higher body weight was not observed after 14 days. A significant increase in water intake was observed in male mice belonging to the medium dose (25 mg/kg) of the GEO group on day 7 (Fig. 1C). Similarly, this increase was transient and appeared not to be related to GEO treatment since it no longer existed after day 7 (Fig. 1C and D). There was no obvious change in food intake in either male or female mice during the course of the study (Fig. 1E and F). Collectively, the overall results suggested that oral GEO treatment for 28 days did not cause significant changes in body weight or daily intake in mice.
Fig. 1.
Effects of GEO on body weight, water intake and food intake of ICR mice treated in a 28-day subacute toxicity study. Body weight, daily water intake (g/day) and daily food intake (g/day) at the end day of each week in male (A, C, E) and female (B, D, F) mice orally administered sterile distilled water (control) or GEO (15, 25, and 50 mg/kg bw) daily for 28 days was presented. Data are presented as the mean ± SD (n = 10 mice/group), followed by one-way analysis of variance (ANOVA) and Dunnett's post-hoc test. ∗, P < 0.05 denotes significant differences compared to control group.
At the end of the experiment, all animals were euthanized and subjected to hematology, serum biochemistry, and histopathology. As shown in Supplementary Table 1, treatment with GEO at any dose for 28 days did not alter hematological parameters. The serum biochemistry of GEO in mice is summarized in Supplementary Table 2. We found the significant increases in the blood glucose levels in male mice treated with medium and high doses (25 and 50 mg/kg bw) of GEO compared to those in the control group (P < 0.05). The changes by medium and high doses of GEO were +15.8% and +14.7%, respectively. Hyperglycemia induces several physiological and pathological alterations, such as elevation in oxidative stress, formation of advanced glycation end-products, damages on pancreatic beta cells, and insulin resistance, which all exacerbate toxic effects on cells.44 Therefore, the blood glucose is often monitored in toxicological studies. The baseline levels and the fluctuation of blood glucose in mice were similar to those in a previous report.45 The increase in blood glucose in GEO-treated mice may be attributed to the nature of glucose sensitivity in the ICR strain,46 which was observed in a previous study.20 Since the garlic product has been reported to have beneficial effects on blood glucose,47 GEO and its active components are less likely to cause hyperglycemia. The higher blood glucose levels found in GEO-treated mice may also come from the strong odor of the GEO. The odor either activates the alarm system to release corticosterone which antagonizes the insulin action,48 or triggers the endocrine system to increase the circulating glucose by different metabolism between animals and humans.49 Nonetheless, no changes in serum biochemistry were observed in a dose-response manner. Hence, we concluded that there is insufficient evidence of toxicity in hematology or serum biochemistry found after oral treatment with GEO for 28 days.
3.4. GEO did not induce macropathological or histopathological lesions
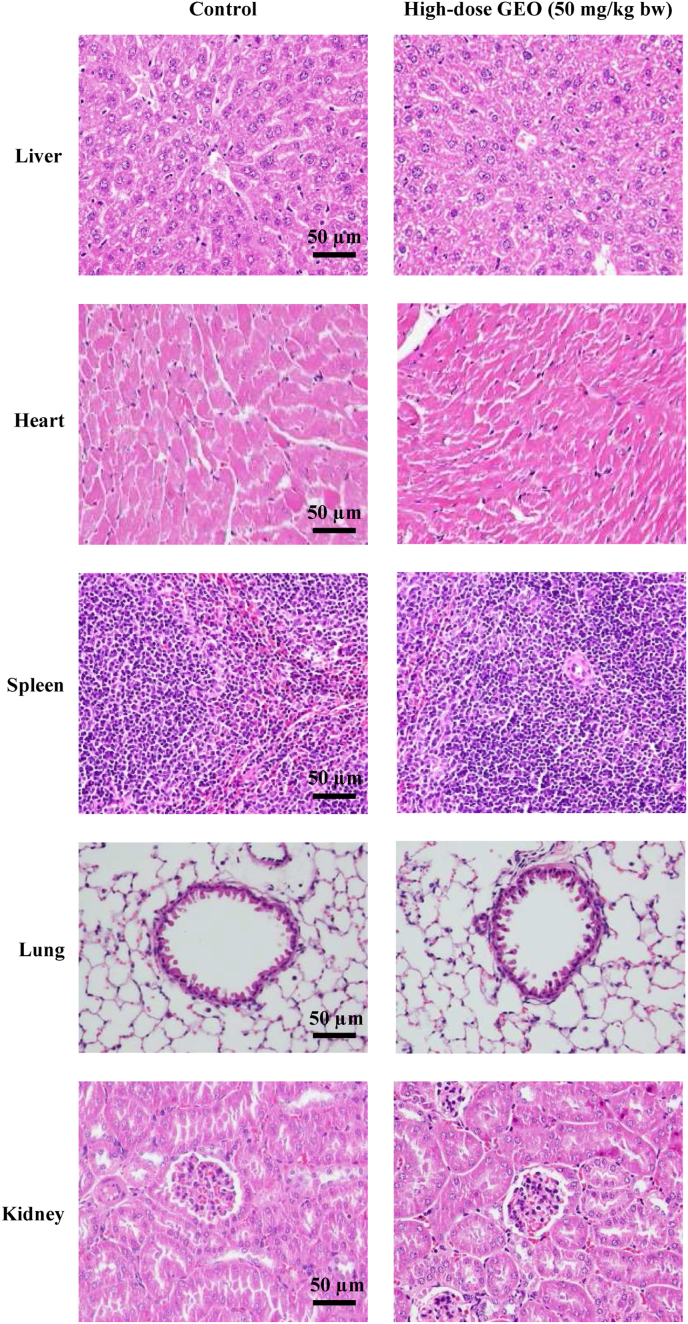
The relative organ weights isolated from the animals are shown in Supplementary Table 3. No significant differences in any relative organ weights were found in either male or female mice treated with GEO for 28 days. Representative histological images of vital organs of mice in the control and high dose (50 mg/kg bw) of GEO are shown in Fig. 2. No obvious histopathological findings were detected in the brain, heart, liver, spleen, lung, kidney, stomach, or intestine in either male or female mice after a 28-day consecutive treatment with a high dose (50 mg/kg bw) of GEO. These assessments concluded that there were no substance-induced pathological lesions in mice treated with GEO for 28 days.
Fig. 2.
Histopathological examination of organs from GEO-treated ICR mice in a 28-day subacute toxicity study. Representative hematoxylin and eosin (H&E) staining images from male control (A) and high-dose GEO (50 mg/kg bw) groups (B). These images are enlarged as 400-fold. No substance-induced pathological lesions were detected in the vital organs of either male or female mice. Scale bar: 50 μm.
4. Conclusion
In the present study, we demonstrated for the first time that GEO is non-genotoxic, based on findings from a bacterial reverse mutation test, chromosome aberration test, and micronucleus test. No obvious oral toxicity was found in mice treated with 15, 25, and 50 mg/kg bw/day GEO for 28 days. Our findings suggest that GEO is safe at a dose of 50 mg/kg bw/day in mice, corresponding to a 200-fold exposure of the human equivalent dose. We also concluded that the NOAEL of GEO can be considered to be greater than 50 mg/kg bw/day for both male and female mice. These findings support the prospective applications of GEO for the development functional foods or dietary supplements in human subjects in future clinical research.
Funding body information
This study was supported in part by grants from the Ministry of Science and Technology (MOST 102-2628-B-002-010-MY2 and 107-2320-B-002-022-MY3), Taiwan. The funding sources had no role in the study design, data collection and interpretation, or the decision to submit the work for publication.
Author contributions
Yu-En Lin: Writing-original draft, Writing-review & editing, Data curation, Validation, Visualization. Meng-Hsuan Lin: Formal analysis, Investigation. Ti-Yen Yeh: Methodology, Formal analysis, Investigation. Yi-Syuan Lai: Formal analysis, Investigation. Kuan-Hung Lu: Supervision. Huai-Syuan Huang: Writing-review & editing. Fu-Chuo Peng: Methodology, Supervision, Writing-review & editing. Shing-Hwa Liu: Supervision, Writing-review & editing. Lee-Yan Sheen: Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
We thank Prof. Jiunn-Wang Liao for the histopathological analysis.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2022.05.001.
Contributor Information
Shing-Hwa Liu, Email: shinghwaliu@ntu.edu.tw.
Lee-Yan Sheen, Email: lysheen@ntu.edu.tw.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Rivlin R.S. Historical perspective on the use of garlic. J Nutr. 2001;131(3s):951–954. doi: 10.1093/jn/131.3.951S. [DOI] [PubMed] [Google Scholar]
- 2.Amagase H., Petesch B.L., Matsuura H., Kasuga S., Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001;131(3s):955–962. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 3.Amagase H. Clarifying the real bioactive constituents of garlic. J Nutr. 2006;136(3 Suppl):716S–725S. doi: 10.1093/jn/136.3.716S. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee S.K., Mukherjee P.K., Maulik S.K. Garlic as an antioxidant: the good, the bad and the ugly. Phytother Res. 2003;17(2):97–106. doi: 10.1002/ptr.1281. [DOI] [PubMed] [Google Scholar]
- 5.Jalal R., Bagheri S.M., Moghimi A., Rasuli M.B. Hypoglycemic effect of aqueous shallot and garlic extracts in rats with fructose-induced insulin resistance. J Clin Biochem Nutr. 2007;41(3):218–223. doi: 10.3164/jcbn.2007031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panyod S., Wu W.K., Lu K.H., et al. Allicin modifies the composition and function of the gut microbiota in alcoholic hepatic steatosis mice. J Agric Food Chem. 2020;68(10):3088–3098. doi: 10.1021/acs.jafc.9b07555. [DOI] [PubMed] [Google Scholar]
- 7.Lee M.S., Kim I.H., Kim C.T., Kim Y. Reduction of body weight by dietary garlic is associated with an increase in uncoupling protein mRNA expression and activation of AMP-activated protein kinase in diet-induced obese mice. J Nutr. 2011;141(11):1947–1953. doi: 10.3945/jn.111.146050. [DOI] [PubMed] [Google Scholar]
- 8.Lai Y.S., Chen W.C., Ho C.T., et al. Garlic essential oil protects against obesity-triggered nonalcoholic fatty liver disease through modulation of lipid metabolism and oxidative stress. J Agric Food Chem. 2014;62(25):5897–5906. doi: 10.1021/jf500803c. [DOI] [PubMed] [Google Scholar]
- 9.Zeng T., Zhang C.L., Song F.Y., Zhao X.L., Xie K.Q. Garlic oil alleviated ethanol-induced fat accumulation via modulation of SREBP-1, PPAR-alpha, and CYP2E1. Food Chem Toxicol. 2012;50(3–4):485–491. doi: 10.1016/j.fct.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Bordia A., Verma S.K., Srivastava K.C. Effect of garlic on blood lipids in patients with coronary heart disease. Am J Clin Nutr. 1981;34(10):2100–2103. doi: 10.1093/ajcn/34.10.2100. [DOI] [PubMed] [Google Scholar]
- 11.Caporaso N., Smith S.M., Eng R.H. Antifungal activity in human urine and serum after ingestion of garlic (Allium sativum) Antimicrob Agents Chemother. 1983;23(5):700–702. doi: 10.1128/aac.23.5.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakagawa S., Masamoto K., Sumiyoshi H., Kunihiro K., Fuwa T. Effect of raw and extracted-aged garlic juice on growth of young rats and their organs after peroral administration [author's transl.] J Toxicol Sci. 1980;5(1):91–112. doi: 10.2131/jts.5.91. [DOI] [PubMed] [Google Scholar]
- 13.Tattelman E. Health effects of garlic. Am Fam Physician. 2005;72(1):103–106. [PubMed] [Google Scholar]
- 14.Joseph P.K., Rao K.R., Sundaresh C.S. Toxic effects of garlic extract and garlic oil in rats. Indian J Exp Biol. 1989;27(11):977–979. [PubMed] [Google Scholar]
- 15.Chowdhury A.K., Ahsan M., Islam S.N., Ahmed Z.U. Efficacy of aqueous extract of garlic & allicin in experimental shigellosis in rabbits. Indian J Med Res. 1991;93:33–36. [PubMed] [Google Scholar]
- 16.Nakagawa S., Masamoto K., Sumiyoshi H., Harada H. Acute toxicity test of garlic extract. J Toxicol Sci. 1984;9(1):57–60. doi: 10.2131/jts.9.57. [DOI] [PubMed] [Google Scholar]
- 17.Sumiyoshi H., Kanezawa A., Masamoto K., et al. Chronic toxicity test of garlic extract in rats. J Toxicol Sci. 1984;9(1):61–75. doi: 10.2131/jts.9.61. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida S., Hirao Y., Nakagawa S. Mutagenicity and cytotoxicity tests of garlic. J Toxicol Sci. 1984;9(1):77–86. doi: 10.2131/jts.9.77. [DOI] [PubMed] [Google Scholar]
- 19.Ames B.N., Durston W.E., Yamasaki E., Lee F.D. Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci U S A. 1973;70(8):2281–2285. doi: 10.1073/pnas.70.8.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu K.H., Ou G.L., Chang H.P., Chen W.C., Liu S.H., Sheen L.Y. Safety evaluation of water extract of Gastrodia elata Blume: genotoxicity and 28-day oral toxicity studies. Regul Toxicol Pharmacol. 2020;114 doi: 10.1016/j.yrtph.2020.104657. [DOI] [PubMed] [Google Scholar]
- 21.Wang H.W. The review system of safety and efficacy of health foods in Taiwan [in Chinese] Regen Med. 2019;103:13–21. [Google Scholar]
- 22.Ames B.N., Mccann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- 23.Ames B.N., Lee F.D., Durston W.E. An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc Natl Acad Sci U S A. 1973;70(3):782–786. doi: 10.1073/pnas.70.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.OECD . OECD Publishing; Paris: 1997. Guideline for the Testing of Chemicals. Test No. 471: Bacterial Reverse Mutation Test. [Google Scholar]
- 25.Galloway S.M., Armstrong M.J., Reuben C., et al. Chromosome aberrations and sister chromatid exchanges in Chinese hamster ovary cells: evaluations of 108 chemicals. Environ Mol Mutagen. 1987;10(Suppl 10):1–175. doi: 10.1002/em.2850100502. [DOI] [PubMed] [Google Scholar]
- 26.OECD . Vitro Mammalian Chromosome Aberration Test. OECD Publishing; Paris: 1997. Guideline for the testing of chemicals. Test No. 473. [Google Scholar]
- 27.OECD . OECD Publishing; Paris: 1997. Guideline for the Testing of Chemicals. Test No. 474: Mammalian Erythrocyte Micronucleus Test. [Google Scholar]
- 28.OECD . OECD Publishing; Paris: 2008. Guideline for the Testing of Chemicals. Test No. 407: Repeated Dose 28-Day Oral Toxicity Study in Rodents. [Google Scholar]
- 29.ARRP . Orange, Animal Welfare Unit, NSW Department of Primary Industries; 2012. Guidelines for the Housing of Mice in Scientific Institutions. Guideline Sec. 3-7: Monitoring of Mice. [Google Scholar]
- 30.Banerjee S.K., Maulik S.K. Effect of garlic on cardiovascular disorders: a review. Nutr J. 2002;1(4) doi: 10.1186/1475-2891-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheen L.Y., Chen H.W., Kung Y.L., Liu C.T., Lii C.K. Effects of garlic oil and its organosulfur compounds on the activities of hepatic drug-metabolizing and antioxidant enzymes in rats fed high- and low-fat diets. Nutr Cancer. 1999;35(2):160–166. doi: 10.1207/S15327914NC352_10. [DOI] [PubMed] [Google Scholar]
- 32.Farbman K.S., Barnett E.D., Bolduc G.R., Klein J.O. Antibacterial activity of garlic and onions: a historical perspective. Pediatr Infect Dis J. 1993;12(7):613–614. doi: 10.1097/00006454-199307000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Iwalokun B.A., Ogunledun A., Ogbolu D.O., Bamiro S.B., Jimi-Omojola J. In vitro antimicrobial properties of aqueous garlic extract against multidrug-resistant bacteria and Candida species from Nigeria. J Med Food. 2004;7(3):327–333. doi: 10.1089/jmf.2004.7.327. [DOI] [PubMed] [Google Scholar]
- 34.Mortelmans K., Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat Res. 2000;455(1−2):29–60. doi: 10.1016/s0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y.T., Lue P.Y., Chen P.W., Chueh P.J., Tsai F.J., Liao J.W. Comparison of genotoxicity and pulmonary toxicity study of modified SiO2 nanomaterials. Appl Sci. 2021;11(24) [Google Scholar]
- 36.Registre M., Proudlock R. In: Genetic Toxicology Testing, A Laboratory Manual. Proudlock R., editor. Academic Press, Elsevier Inc; Boone, NC: 2016. The in vitro chromosome aberration test; pp. 207–267. [Google Scholar]
- 37.Matsuoka A., Hayashi M., Ishidate M., Jr. Chromosomal aberration tests on 29 chemicals combined with S9 mix in vitro. Mutat Res. 1979;66(3):277–290. doi: 10.1016/0165-1218(79)90089-2. [DOI] [PubMed] [Google Scholar]
- 38.Spielmann H., Genschow E., Liebsch M., Halle W. Determination of the starting dose for acute oral toxicity (LD50) testing in the up and down procedure (UDP) from cytotoxicity data. Altern Lab Anim. 1999;27(6):957–966. doi: 10.1177/026119299902700609. [DOI] [PubMed] [Google Scholar]
- 39.Soeteman-Hernández L.G., Fellows M.D., Johnson G.E., Slob W. Correlation of in vivo versus in vitro benchmark doses (BMDs) derived from micronucleus test data: a proof of concept study. Toxicol Sci. 2015;148(2):355–367. doi: 10.1093/toxsci/kfv189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krishna G., Hayashi M. In vivo rodent micronucleus assay: protocol, conduct and data interpretation. Mutat Res. 2000;455(1−2):155–166. doi: 10.1016/s0027-5107(00)00117-2. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki Y., Nagae Y., Li J., et al. The micronucleus test and erythropoiesis. Effects of erythropoietin and a mutagen on the ratio of polychromatic to normochromatic erythrocytes (P/N ratio) Mutagenesis. 1989;4(6):420–424. doi: 10.1093/mutage/4.6.420. [DOI] [PubMed] [Google Scholar]
- 42.Abraham S.K., Kesavan P.C. Genotoxicity of garlic, turmeric and asafoetida in mice. Mutat Res. 1984;136(1):85–88. doi: 10.1016/0165-1218(84)90138-1. [DOI] [PubMed] [Google Scholar]
- 43.Kalantari H., Larki A., Latifi S.M. The genotoxicity study of garlic and pasipy herbal drops by peripheral blood micronucleus test. Acta Physiol Hung. 2007;94(3):261–266. doi: 10.1556/APhysiol.94.2007.3.10. [DOI] [PubMed] [Google Scholar]
- 44.Giri B., Dey S., Das T., Sarkar M., Banerjee J., Dash S.K. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: an update on glucose toxicity. Biomed Pharmacother. 2018;107:306–328. doi: 10.1016/j.biopha.2018.07.157. [DOI] [PubMed] [Google Scholar]
- 45.Shin H.J., Cho Y.M., Shin H.J., et al. Comparison of commonly used ICR stocks and the characterization of Korl:ICR. Lab Anim Res. 2017;33(1):8–14. doi: 10.5625/lar.2017.33.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimizu R., Sakazaki F., Okuno T., Nakamuro K., Ueno H. Difference in glucose intolerance between C57BL/6J and ICR strain mice with streptozotocin/nicotinamide-induced diabetes. Biomed Res. 2012;33(1):63–66. doi: 10.2220/biomedres.33.63. [DOI] [PubMed] [Google Scholar]
- 47.Miki S., Inokuma K., Takashima M., et al. Aged garlic extract suppresses the increase of plasma glycated albumin level and enhances the AMP-activated protein kinase in adipose tissue in TSOD mice. Mol Nutr Food Res. 2017;61 doi: 10.1002/mnfr.201600797. [DOI] [PubMed] [Google Scholar]
- 48.Hirasawa Y., Shirasu M., Okamoto M., Touhara K. Subjective unpleasantness of malodors induces a stress response. Psychoneuroendocrinology. 2019;106:206–215. doi: 10.1016/j.psyneuen.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 49.Lushchak O.V., Carlsson M.A., Nässel D.R. Food odors trigger an endocrine response that affects food ingestion and metabolism. Cell Mol Life Sci. 2015;72(16):3143–3155. doi: 10.1007/s00018-015-1884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.