Abstract
Background & aim
Ulcerative colitis (UC) is a chronic recurrent inflammatory disease of the large intestine and rectum that oxidative stress and severe inflammation are the main features of this disease. Previous studies have shown that separate consumption of basil and gum arabic can reduce inflammation and oxidative stress. The aim of the study was evaluating the effect of treatment with basil seeds given together with gum arabic on healing, inflammation and oxidative stress in the course of experimental colitis in rats.
Experimental procedure
A total number of 50 male rats were used, randomly assigned to five groups of 10 rats each. Colitis was induced in rats by enemas with 4% solution od acetic acid. Four days after induction of colitis, rats were treated for next 4 days with saline or combination of basil seeds plus gum arabic (1 mg/kg) or sulfasalazine (100 mg/g) rectally. The experiment was terminated after last dose of treatment. Rats without induction of colitis were used as a sham group.
Results
Acetic acid-induced colitis increased the macroscopic and histopathological damage scores of the colon as well as colon levels of MDA(Malondialdehyde), MPO(Myeloperoxidase), TNFα(Tissue necrosis factor α), IL6 (Interleukin 6)and IL17(Interleukin 17) and decreased SOD(Superoxide Dismutase), GPx (Glutathione Peroxidase) and IL10 (Interleukin 10) levels compared with the control group(P < 0.001). Treatment with basil and gum arabic reduced macroscopic and histopathological damage scores (P < 0.01) of the colon, MDA, MPO, TNFα, IL6(P < 0.001) and IL17 (P < 0.01) levels of the colon and increased SOD, GPx and IL10 levels compared to the colitis group (P < 0.01).
Conclusion
Rectal administration of combination of basil seeds plus gum arabic after induction of colitis, exhibits antioxidant and anti-inflammatory effects, and accelerates the healing of the colon in experimental colitis evoked by acetic acid.
Keywords: Basil seeds, Gum Arabic, Acetic acid-induced ulcerative colitis, Ulcerative colitis, Ocimum basilicum, Acacia Senegal
Graphical abstract
1. Introduction
Ulcerative colitis (UC) is a debilitating, recurrent chronic inflammatory bowel disorder (IBD) that afflicts large population throughout the world. UC results into formation of inflammatory lesions in mucosal and sub-mucosal layers of colon.1 Epidemiological studies indicate a very different prevalence of UC based on the geographical location of life and ethnic or racial background of the individual, and are currently one of the most common causes of gastrointestinal disorders in developed countries, which is more common in urban communities.2 The United States, the United Kingdom, Norway and Sweden have the highest rates of colitis.3 In Iran, the incidence and prevalence of IBD types such as UC are increasing.4 Inflammation and ulcers of the mucous membrane in patients with ulcerative colitis can lead to symptoms such as abdominal pain, diarrhea, and bleeding. Medications effectively improve UC to no small extent, but the high cost, side effects, and treatment limitations are each significant and negligible. Drugs that have been used for treatment so far are immunosuppressants of corticosteroids or anti-TNF-α antibodies.5 All of these therapies interfere with cellular oxidative stress and cytokine production. But even the best and most common corticosteroid drugs, including prednisone, still have side effects.6 Unfortunately, anti-TNF-α antibodies have been associated with the spread of breast cancer in women with colitis.7 Other side effects of taking drugs such as sulfasalazine 5-aminosalicylic acid include stomach ulcers, muscle weakness, hyperglycemia and many other side effects.8 Today, there is a great tendency to use natural substances and medicinal plants due to the lack of side effects of herbal medicines and various useful compounds in plants. The use of these compounds has been recommended by the World Health Organization.9 Medicinal plants and natural substances have been used to treat human diseases for many years. The thousands of years of medicine and pharmacy history, which has provided valuable information on herbal medicine, confirms this.9 Gum arabic is a polysaccharide complex that emanates from the trunk of an acacia tree and is native to Africa.10,11 Gum arabic polysaccharides are structurally branched and have high molecular weight. Gum arabic has antimicrobial and antibacterial properties and significantly reduces blood sugar, insulin resistance and blood lipids.12,13 Gum arabic has several effects on gastrointestinal diseases, such as healing effects on gastric ulcers14 and improvement of inflammatory diseases and an intestinal ulcer.15 Wound healing properties are due to the wound healing properties of gum arabic and its antimicrobial and antioxidant effects.16 Gum arabic has protective effects on liver cells by reducing nitric oxide production and reducing oxidative damage to liver cells.17 The positive effects of this gum on functional diseases of the gastrointestinal tract have also been studied. Basil (O. bacillicum) is a native plant of Iran and belongs to the Lamiaceae family.18 This plant is widely produced and consumed in Iran. Basil seed is one of the commonly used plants as essential fibers source with antioxidant activity.19 Basil contains two proteins, Chitosan and Chitin, which are cellulose-like polysaccharides and have a protective effect against oxidant damage.18 Basil seed has anti-inflammatory, anti-asthma, anti-arthritic, antioxidant, anti-depressant, hypoglycemic, antibacterial, antimicrobial properties and is effective in preventing cancer.20,21 It has a therapeutic effect in inflammatory bowel diseases by inhibiting nitric oxide production and having prebiotic, antimicrobial and antioxidant properties.15 The traditional product of gum Arabic and basil seeds is one of the combined drugs used in traditional Persian medicine to treat diarrhea and intestinal inflammation. Given the above, our aim in this study was to investigate Combining Basil seeds and Gum Arabic (CBGA) on the healing process of UC damage caused by acetic acid injection in rats.
2. Material and method
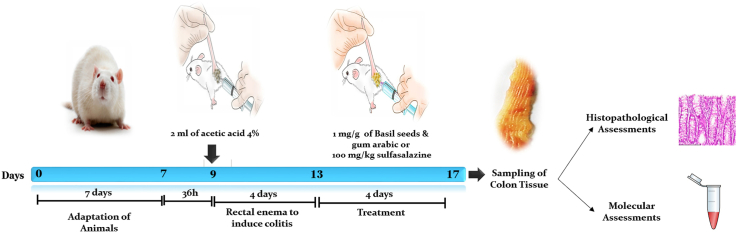
Animal care and grouping. In this study, 50 male wistar rats weighing 180–250 are used. The animals are kept in normal conditions in the medical school of Kerman Medical University of sciences with a temperature of 25 C and a dark period of 12 h of light and a standard diet. Water and food are freely available to them, and the animals are randomly divided into five groups (n = 10 in each group). (1) Sham (animals without induction of colitis); (2) colitis (animals starved for 36 h then received 2 ml acetic acid 4%; (3) colitis + saline (Colitis animals treated rectally with saline after induction of colitis); (4) colitis + BS&GA (Colitis animals treated rectally with saline after induction of colitis treated rectally with basil seeds and gum Arabic at dose of 1 mg/g rectally after induction of colitis); (5) colitis + sulfasalazine (animals treated rectally with sulfasalazine at dose of 100 mg/kg rectally after induction of colitis).
| Groups name | Groups Definition | Dose | Number of prescriptions | Day of Intervention | Prescription method |
|---|---|---|---|---|---|
| Sham | Intact animals without induction of colitis | – | – | – | Without prescription |
| colitis | animals starved for 36 h then received 2 ml acetic acid | 4% | 1 | 0 | Rectally |
| colitis + saline | animals with colitis received 2 ml saline | 0.9% | 4 | 5–9 | Rectally |
| colitis + BS&GA | animals with colitis received basil seeds and gum Arabic | 1 mg/g | 4 | 5–9 | Rectally |
| colitis + sulfasalazine | animals with colitis received sulfasalazine | 100 mg/kg | 4 | 5–9 | Rectally |
Colitis induction. To induce colitis, rats, after being starved for 36 h with free access to water, then under mild anesthesia with ether, 2 ml of 4% acetic acid(Merck, Germany) was injected into the animal's colon through a plastic tube with an inner diameter of 2.5 mm and a length of 8 cm, then the animals are kept in solitary wired cages for 24 h.22 Wounds in animals usually occur after the first hour after the operation and are completed within 3–5 days. On the fifth day after acetic acid injection, treatment was performed for four days.23,24
Preparation and prescription of drugs. According to Persian medicine sources, this herbal product is prepared in equal proportions (weight-weight proportion) from gum Arabic (Acacia Senegal) powder and roasted basil seed (Ocimum basilicum) powder. Roasted basil seeds are ground and passed through a 40-mesh sieve. Gum arabic powder was purchased from the German Merck factory. Basil seeds powder is mixed in equal proportions with gum arabic powder and is used at a dose of 1 mg/g of animal weight with distilled water (suspension form). We prepared the mixture fresh on the day of administration. In fact, we administered it fresh immediately after preparation. This substance is administered rectally after completion (the fifth day after the operation), it is given to the animal every day at 8 a.m. This process continues for about four days.23,24
Scoring Disease activity index (DAI). Animals were examined with three significant symptoms: weight loss, diarrhea, and rectal bleeding. A scoring system was used to evaluate the mentioned indicators. To assess body weight loss and diarrhea of animals during the study period, the scoring system (0: <1%, 1: 1–5%, 2: 5–10%, 3: 10–15%, and 4:> 15%) and (0: normal, 2: loose stools, and 4: diarrhea) respectively were used.To assess the presence or absence of occult blood in the stool. Using benzidine test and scoring system (0: negative, 2: positive, and 4: gross bleeding) was used, and then DAI was calculated by the following equation.
Determination of the ulcer area and ulcer index (UI). Colon surface lesions were observed and photographed using a magnifying glass lens (10%), then images were measured using Image J software. To evaluate the ulcer index (UI) was determined using the following equation.
Hematocrit Measuring. A blood sample was taken in a heparin-coated capillary tube eight days after acetic acid injection and a hematocrit measuring device then measured it.
Macroscopic evaluations. For macroscopic assessment evaluation of colon, On the same day, rats were sacrificed under deep anesthesia. The abdomen was opened through a midline incision, and 8 cm from the colon is separated from the anal at a distance of 3 cm and cut lengthwise, then washed with normal cold saline, and its wet weight is calculated.25,26 The weight to length ratio of the colon (g/cm) was calculated then other longitudinal intestinal sections were then frozen immediately to evaluate biochemical variables in liquid nitrogen. For macroscopic assessment, it was scored as follows: 1: mucosal erythema only; 2: Mild mucosal edema, minor bleeding or small abrasions. 3: Moderate edema, bleeding, erosion or sores. 4: Severe wound, erosion, swelling or tissue necrosis.
Histopathological evaluations. Histopathological methods evaluated some disease indicators. Colon samples were fixed in formalin solution (10%). Then, tissues were sectioned, deparaffinized and stained with hematoxylin and eosin (H&E). Histopathological evaluation of colon was done as described previously and by a pathologist blinded to experimental groups. We inflammation extent, inflammation severity and crypt damage scored Colon tissue changes as (0), normal (1), mild (2), moderate (3), and severe (4). Total colitis index was the summation of inflammation extent, inflammation severity and crypt damage scores.27
2.1. Oxidative stress evaluation
Superoxide Dismutase. The amount of superoxide dismutase activity was measured indirectly using the calorimetric method based on the ability of SOD to inhibit the autooxidation of Pyrogallol. For this purpose, 50 mg of colon tissue was homogenized in 250 μl of lysing buffer. The homogeneity was then centrifuged for 5 min at 12,000 rpm at 4 °C, and the supernatant was used to measure the amount of SOD activity according to the relevant kit instructions.
Malondialdehyde. Colon tissue samples were homogenized using 1.5% potassium chloride buffer and then centrifuged at 4 °C for 10 min. Malondialdehyde (MDA) level, as an index of lipid peroxidation, was estimated by the concentration of thiobarbituric acid reactive substances (TBARS) (behboud tahghigh kerman co, Iran).28
Glutathione Peroxidase. Glutathione peroxidase (Gpx) determined using their relative Randox assay kits, according to the manufacturer's protocols (Nadford, navandsalamat. Co, Iran). The measurement of glutathione peroxidase was based on the ability of glutathione peroxidase to oxidize glutathione (GSH) to oxidize glutathione (GSSH). GSSH (part of the reactions that regenerate cumene hydroperoxide). Glutathione reductase converts GSSH to GSH using nicotinamide dinucleotide phosphate (NADPH). The decrease in NADPH, measured at 340 nm, was an indicator of glutathione peroxidase activity.29
Inflammation Evaluation. Colon tissue was homogenized in PBS buffer and inflammatory and anti-inflammatory cytokines IL-6, TNF-α, IL17 and IL-10 were conducted using enzyme-linked immunosorbent assay (ELISA) kits based on the manufacturer's instructions was measured (karmania pars gen co, Iran).30
Myeloperoxidase Activity. After preparing the lung tissue, the amount of myeloperoxidase was measured with a special kit (Nadford, navandsalamat. Co, Iran) for measuring the amount of myeloperoxidase. Tissue myeloperoxidase content was used to quantify neutrophil accumulation in the colon. The colon samples were homogenized in a phosphate buffer containing 0.5% hexadecyltrimethylammonium bromide. The samples were then assayed for the ability to decompose H202 in the presence of o-dianhydride dihydrochloride by changing the adsorption at 460 nm within 1 min.
Statistical analysis. Data were presented as mean ± SEM, N = 10. The studied groups' differences were tested using one-way analysis of variance (ANOVA) followed by a post hoc test (Tukey). P values < 0.05 were considered statistically significant. Statistical tests were performed with SPSS (IBM SPSS Statistics for Windows, version 23.0.).
3. Result
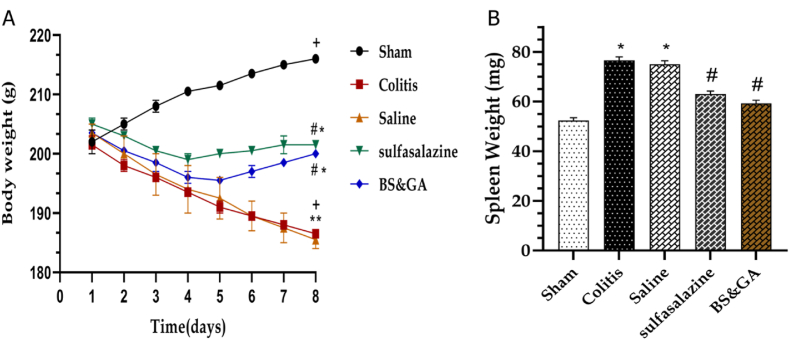
The effects of rectal administration of CBGA on body and spleen weight changes. In general, rats' body weight decreased after acetic acid instillation, and this decrease was much more severe in colitis and saline groups (P < 0.001) than pos (P < 0.05) and drug (P < 0.01) groups (Fig. 1A). As one of the common manifestations of acetic acid-induced UC, enlargement of the spleen was evaluated by the weight. We found that the spleen weight of rats with colitis increased compared to the control group (P < 0.05), while treatment with sulfasalazine (P < 0.05) and basil seeds and gum arabic significantly (P < 0.05) reduced the spleen weight of rats (Fig. 1B).
Fig. 1.
Effects of rectal administration of combination of basil seeds and gum Arabic on A: body weight and B: spleen weight changes. Data are shown as Mean ± SEM (n = 10 per group). Sham: Intact animals without colitis, Colitis: animals received 2 ml acetic acid 4%, Saline: animals with colitis received 2 ml saline, Sulfasalazine: animals with colitis received sulfasalazine at dose 100 mg/kg, BS&GA: combination of Basil Seeds and Gum Arabic at dose 1 mg/g ∗P < 0.05 vs sham group. #P < 0.05 vs colitis and saline groups. + P < 0.05 vs day 1.
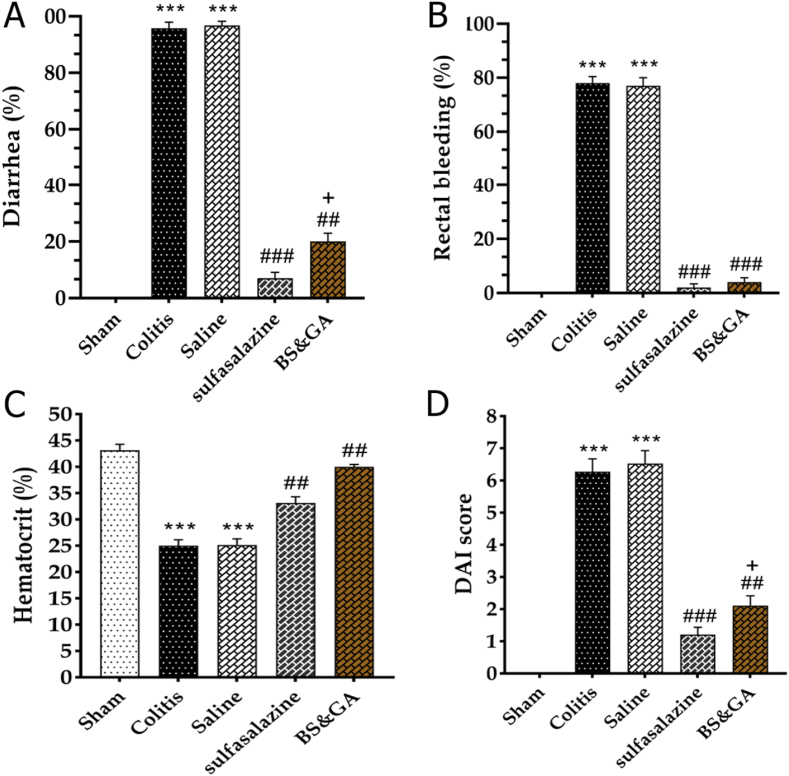
The effects of rectal administration of CBGA on Diarrhea, rectal bleeding, hematocrit changes, and DAI score. After intra-rectal administration of acetic acid in the macroscopic examination, all rats had diarrhea with bloody stools and abdominal distension, and severe colitis with extensive epithelial necrosis. But the severity of colitis and epithelial necrosis was lower in the pos and drug groups. In the colitis and the saline groups compared to the sham group, a significant increase in diarrhea was observed (P < 0.001), and treatment with CBGA and sulfasalazine reduced the mentioned index compared to the colitis and saline groups (P < 0.001) which this decrease in Pos group was also significant compared to the drug group (P < 0.05) (Fig. 2A). As previously mentioned, acetic acid leads to bleeding or hemorrhage in the large intestine, characterized by a decrease in hematocrit in the colitis group compared to the Sham group. The rate of decline in hematocrit in the pos (P < 0.001) and drug groups (P < 0.01) was lower (Fig. 2B and C). Also, DAI showed a significant increase in colitis and saline groups than the sham group (P < 0.001). Treatment with a combination of basil and gum arabic caused a considerable decrease in DAI score than colitis and saline groups (P < 0.01). The positive control group also caused a significant reduction in DAI (P < 0.001), which was greater than the Drug group (P < 0.05) (Fig. 2D).
Fig. 2.
Effects of rectal administration of combination of basil and gum arabic on A: diarrhea, B: rectal bleeding, C: hematocrit changes and D: DAI score. Data are shown as Mean ± SEM (n = 10 per group). Sham: Intact animals without colitis, Colitis: animals received 2 ml acetic acid 4%, Saline: animals with colitis received 2 ml saline, Sulfasalazine: animals with colitis received sulfasalazine at dose 100 mg/kg, BS&GA: combination of Basil Seeds and Gum Arabic at dose 1 mg/g ∗∗∗P < 0.001 vs sham group, ##P < 0.01 and ###P < 0.001 vs colitis and saline groups, + P < 0.05 vs positive control group.
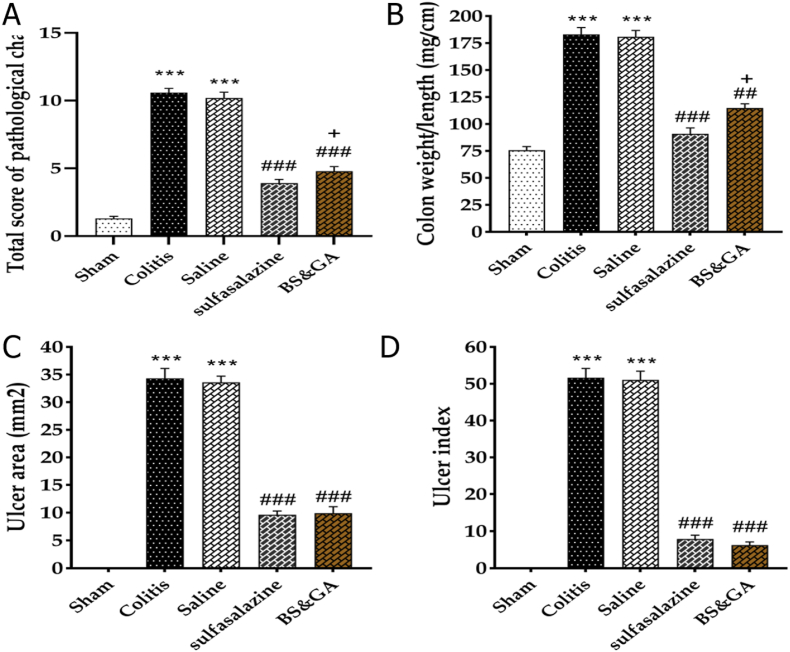
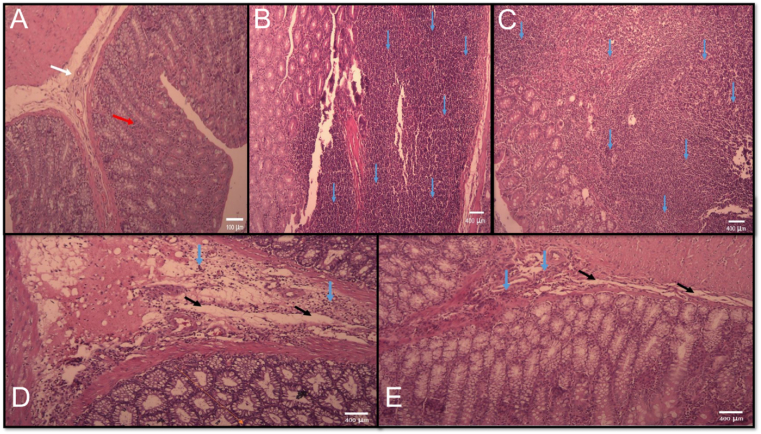
The effects of rectal administration of CBGA on histopathological changes, weight/length ratio, ulcer area, and UI. Compared to healthy rats, we observed cell infiltration, epithelial destruction, hyperemia, and necrosis in sick rats. In the colitis and the saline groups compared to the sham group, a significant increase in the total score of histopathological changes, weight/length ratio of the colon, ulcer area, and ulcer index were observed (P < 0.001). Treatment with sulfasalazine reduced the mentioned indices compared to the colitis and saline groups (P < 0.001). Treatment with a CBGA, in all the above indices in comparison with colitis and saline groups, Significant reduction was observed (P < 0.01, P < 0.01, P < 0.001, P < 0.001) respectively), which was also significant in the total score of histopathological changes, weight/length ratio of the colon compared to the drug group (P < 0.05) (Fig. 3A–D and Fig. 4A–E).
Fig. 3.
Effects of rectal administration of combination of basil and gum arabic on A: histopathological changes, B: colon weight/length ratio, C: ulcer area and D: ulcer index. Data are shown as Mean ± SEM (n = 10 per group). Sham: Intact animals without colitis, Colitis: animals received 2 ml acetic acid 4%, Saline: animals with colitis received 2 ml saline, Sulfasalazine: animals with colitis received sulfasalazine at dose 100 mg/kg, BS&GA: combination of Basil Seeds and Gum Arabic at dose 1 mg/g ∗∗∗P < 0.001 vs sham group, ##P < 0.01 and ###P < 0.001 vs colitis and saline groups, + P < 0.05 vs positive control group.
Fig. 4.
Microscopic presentation of acetic acid–induced colitis in rats stained by hematoxylin and eosin (light microscopy, 40 X): (A) normal colon without any treatments, mucus layer, and crypts are normal and leucocyte infiltration is absent; (B) colitis in control group without any treatments, mucosal and submucosal inflammation, as well as crypt damage and leucocyte infiltration are evident; (C) colitis in control group treated with saline, mucosal and submucosal inflammation, as well as crypt damage and leucocyte infiltration are evident dexamethasone-treated colitis; (D) Sulfasalazine treated colitis. (E) Combination of basil seeds and gum Arabic treated colitis. White arrow: sub mucosa, Red arrow: normal mucusa layer, Black arrow: edema, Blue arrow: inflammatory cell infiltration.
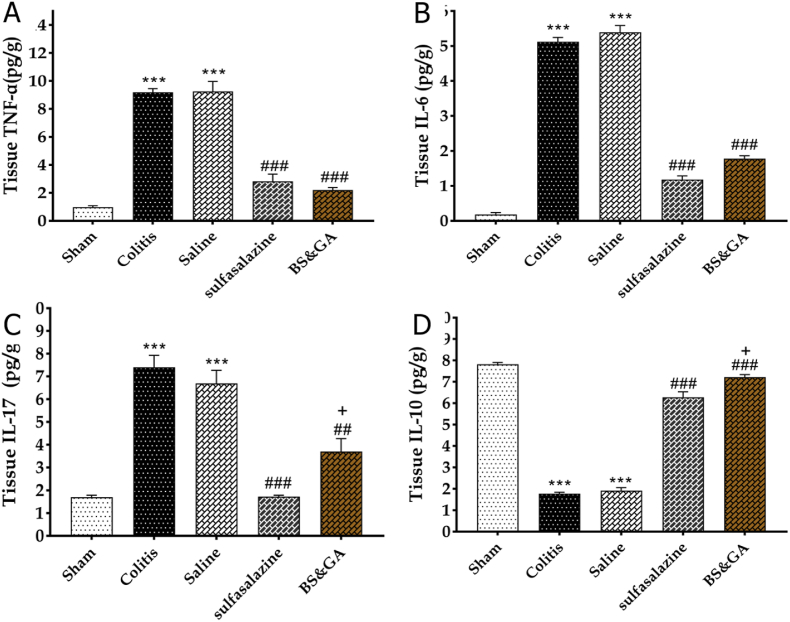
The effects of rectal administration of Gum arabic and Basil on inflammatory factors in colitis rats. Our results showed that colitis induction increased tissue concentration of TNF-α, IL-6, and IL-17, respectively (P < 0.001, P < 0.001, and P < 0.001) and decreased IL-10 tissue concentration (P < 0.001) compared to the sham group. Our observations revealed that rectally administration of the combination of basil and Gum arabic in the drug group reduced tissue concentration of TNF-α, IL-6, and IL-17 respectively (P < 0.001, P < 0.001, and P < 0.01) and increased IL-10 tissue concentration (P < 0.001) compared to the colitis group. Also, our data disclosed that IL-17 concentration in the drug group was more than Pos group significantly (P < 0.05), Which indicates the more significant effect of sulfasalazine with this factor. Our findings reported that IL-10 concentration in the drug group was more than the Pos group significantly (P < 0.05). This means that Gum arabic and basil were more effective in relation to this factor (Fig. 5A–D).
Fig. 5.
Effects of rectal administration of Arabic gum and Basil on inflammatory A: TNF-α, B: IL-6, C: IL-17 and anti-inflammatory D: IL-10 factors in colitis rats. Data are shown as Mean ± SEM (n = 10 per group). Sham: Intact animals without colitis, Colitis: animals received 2 ml acetic acid 4%, Saline: animals with colitis received 2 ml saline, Sulfasalazine: animals with colitis received sulfasalazine at dose 100 mg/kg, BS&GA: combination of Basil Seeds and Gum Arabic at dose 1 mg/g ∗∗∗P < 0.001 vs sham group, ##P < 0.01 and ###P < 0.001 vs colitis and saline groups, + P < 0.05 vs positive control group.
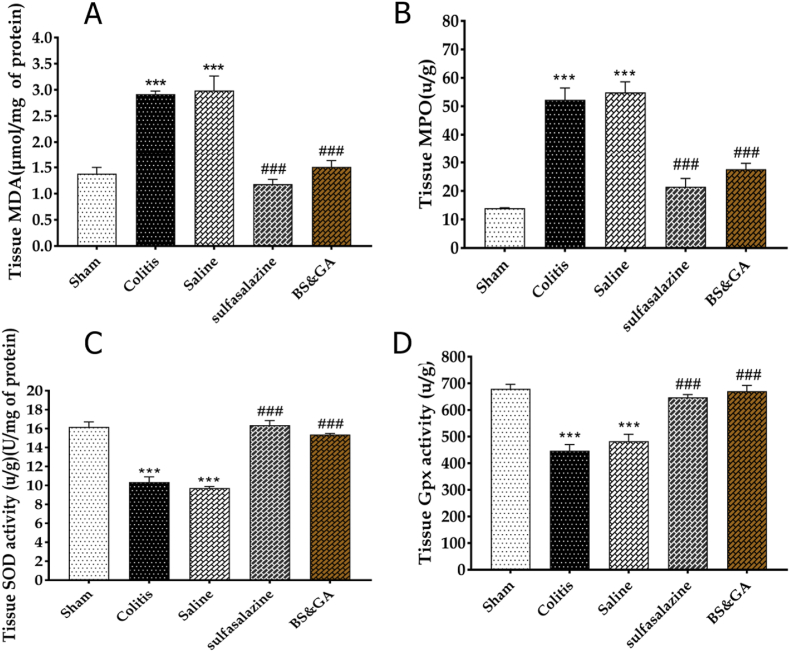
The effects of rectal administration of gum Arabic and Basil on oxidative factors in colitis rats. Our results showed that colitis induction increased tissue concentration of MDA and myeloperoxidase respectively (P < 0.01, P < 0.001) and decreased SOD and Gpx tissue concentration, respectively (P < 0.001, P < 0.001) compared to the sham group. Our observations revealed that rectally administration of the combination of basil and gum arabic in the drug group reduced tissue concentration of MDA and myeloperoxidase, respectively (P < 0.001, P < 0.001) and increased SOD and Gpx tissue concentration (P < 0.001, P < 0.001) compared to the colitis group (Fig. 6A–D).
Fig. 6.
Effects of rectal administration of gum arabic and Basil on oxidative factors in colitis rats. A: MDA, B: MPO, C: SOD, D: Gpx. Data are shown as Mean ± SEM (n = 10 per group). Sham: Intact animals without colitis, Colitis: animals received 2 ml acetic acid 4%, Saline: animals with colitis received 2 ml saline, Sulfasalazine: animals with colitis received sulfasalazine at dose 100 mg/kg, BS&GA: combination of Basil Seeds and Gum Arabic at dose 1 mg/g ∗∗∗P < 0.001 vs sham group, ###P < 0.001 vs colitis and saline groups.
4. Discussion
In this study, the anti-colitis, anti-inflammatory, and antioxidant effects of basil and gum arabic in acetic acid-induced ulcerative colitis in rats were evaluated. Significant weight loss and hematocrit and a significant increase in diarrhea, rectal bleeding, DAI, Ulcer area, Ulcer index, colonic weight/length ratios, histopathological indices, inflammatory and oxidative factors were observed after acetic acid administration. Also, treatment with a combination of basil and gum arabic prevented weight loss and hematocrit, and on the other hand, caused a significant increase in other indicators mentioned above. The results showed, to some extent, the accuracy of our study hypothesis.
In this study, we used acetic acid to induce colitis. There are several models of induction of ulcerative colitis in animals such as Dextran sulfate sodium-induced colitis, Oxazolone colitis, Salmonella-induced colitis, Adherent–invasive E. coli, Acetic acid-induced colitis and transgenic models.31 Intrarectal administration of diluted acetic acid provides an alternative method to create chemical injury to the mucosal epithelium that induces a transient phenotype mimicking UC.31 Inflammatory cells, especially neutrophils, play an important role in the development of acetic acid colitis. The role of corticosteroids in the development of this model of colitis has also been mentioned.32 Inflammation, oxidative stress and histopathological changes are among the consequences of colitis. Numerous studies have shown that inflammatory cytokines are increase in colitis and anti-inflammatory cytokines are reduced in this disease. Tahan et al. demonstrated that pro-inflammatory cytokines including IL 1β, TNFα and IL6 increased due to ulcerative colitis in rats.33 In another study, Hagar et al. showed growing colonic TNFα in colitis animals.34 It has also been shown that NFkB is elevated in the animal model of colitis, and El-Akabawy et al. in this study revealed elevation of TNFα, IL1β, IFNγ and IL6 owing to colitis in rats.35 Along with increased levels of TNFα, IL 6 and IL1β, a decrease in IL10 as an anti-inflammatory cytokine is observable in colitis animals.27 Hanif palla et al. showed that IL17 concentration increased following by colitis induction.36 Bastaki et al. demonstrated the high level of IL23 in colitis rats.37 Our results in this study showed that the levels of inflammatory cytokines (TNFα, IL6 and IL17) increased after the induction of colitis and the level of IL10 as an anti-inflammatory cytokine decreased, which almost agrees with most studies. Numerous studies have shown a direct relationship between oxidative stress and colitis. In this disease, the balance between oxidant and antioxidant factors is disturbed. Cagin et al. showed that colitis increases MDA, TOS and OSI as an oxidant indices and also decreases SOD, GPx, GSH and TAC as an anti-oxidant factors in rats.38 Another research reported the reduction of GSH and elevation of MDA due to colitis.37 A diminished level of SOD, CAT and TAC resulted from colitis induction in rats in colares et al. investigation.39 Maghool et al. revealed the reduction of SOD, GPx and total thiol and increased of MDA in colitis animals.40 Our data showed that MDA in colitis group was higher than control group and SOD and GPx in colitis group was lower than control group. Our results are in line with most papers.
Myeloperoxidase (MPO) is a marker for neutrophil infiltration which indicates the progression of acute inflammatory process.41 Neutrophil accumulation in the inflamed intestinal mucosa is a prominent feature in ulcerative colitis. The granules of neutrophil granulocytes contain a number of enzymes, for example myeloperoxidase which are important in the combat against bacteria. These granule enzymes, some of which are proteolytic can be released upon stimulation, together with cytotoxic oxygen metabolites. Therefore, activated neutrophils may contribute to tissue damage at sites of inflammation.42 Myeloperoxidase activity was assayed in two animal models of inflammation: acetic acid induced colitis in rats and Clostridium difficile enterotoxin induced enteritis in hamsters. In both models, the activity of myeloperoxidase solubilized from the inflamed tissue was directly proportional to the number of neutrophils seen in histologic sections.41,43 Most studies have shown that myeloperoxidase increases in colitis.44, 45, 46, 47Our findings also showed that myeloperoxidase was higher in colitis group compared to control group which agrees with most studies. Histopathological changes and tissue remodeling of colon tissue are very common in colitis. These histopathological changes and ulcers caused by colitis can be presented with indices such as Ulcer area and ulcer index, and also tissue inflammation is clearly visible. Many investigations showed that tissue remodeling and ulcer progression indices such as UI and UA happening in colitis animals.48, 49, 50, 51 Our results also showed that extensive histopathological changes and ulcers occurred in the colon of colitis animals. So that, UI and UA indices in the colitis group increased compared to the control group.
Basil (O. basilicum L.), is an important medicinal plant and culinary herb, belongs to the Lamiaceae family, which grows in tropical and sub-tropical climates.52,53 The most important pharmacological uses of basil are anti-cancer activity, radioprotective activity, anti-microbial activity, anti-inflammatory effects, immunomodulatory activity, anti-stress activity, anti-diabetic activity, anti-pyretic activity, anti-arthritic activity, anti-oxidant activity, as a prophylactic agent.52,53 Gastric antiulcer activity of basil showed by singh et al.54 Eftekhar et al. demonstrated the Immunomodulatory and anti-inflammatory effects of hydro-ethanolic extract of O. basilicum leaves and its protective effects on lung pathological changes in an ovalbumin-induced rat model of asthma.55 Another study showed the protective role of O. basilicum L. against aspirin-induced gastric ulcer in mice via reducing serum levels of IL 4, IL 6, TNFα and PGE2.56 Saeidi et al., in 2018 evaluated the histhopathological effects of O. basilicum on acetic acid induced colitis in rats. They reported the anti-inflammatory, anti-ulcerative effects of this plant and also they showed that O. basilicum in this animals could reduce myeloperoxidase activity.57 The main differences between our study and mentioned study are the evaluation of inflammatory cytokines and oxidative stress factors, as well as the simultaneous administration of basil and gum arabic.Also, Rashidian et al. disclosed the Protective Effect of O. basilicum Essential Oil Against Acetic Acid–Induced Colitis in Rats through reducing myeloperoxidase activity and inflammation.58Therefore, in general, the anti-inflammatory and antioxidative effects of basil are well established.59
Gum arabic is an edible, dried, gummy exudate from the stems and branches of Acacia senegal and A. seyal that is rich in non-viscous soluble fiber.60 Studies have reported a variety of effects from gum arabic, including antioxidant effects, regulation kidney function, blood sugar, liver function, and fat metabolism.61 In the gastrointestinal tract, it has been shown that gum arabic can affect the intestinal absorption and secretion of various substances.62 Wapnir et al. showed that gum arabic can modulate intestinal nuclear factor NF-kappaB.62 Badreldin H et al. demonstrated that gum arabic has anti-inflammatory and anti-oxidative effects in chronic renal failure.63 It is also shown that gum arabic modifies anti-inflammatory cytokine in mice fed with high fat diet induced obesity.64 Another research reported that gum arabic reduces inflammation, oxidative, and nitrosative stress in the gastrointestinal tract of mice with chronic kidney disease.65
The results of this study also confirmed the anti-inflammatory and antioxidant effects of basil and gum arabic. Our findings showed that combination treatment of basil and gum arabic in colitis rats can significantly improve the inflammatory, oxidative and histopathological complications of colitis.
Blood flow plays an important role in protection of normal gastric mucosa and in the protection and healing of damaged mucosa. Blood flow contributes to protection by supplying the mucosa with oxygen and HCO-3, and by removing H+ and toxic agents diffusing from the lumen into the mucosa. Low mucosal blood flow predisposes to injury, whereas high blood flow protects against injurious agents. Superficial mucosal damage is followed by increased blood flow which supports the healing process and prevents superficial lesions from developing into deep ones. The hypermic response increases the supply of HCO-3 to the mucosa and increases the resistance of the injured mucosa against back diffusing H+ and aggressive drugs.66,67
It seems that the anti-inflammatory effects of basil can be due to the effect on the accumulation or activity of macrophages and leukocytes.68,69 Modulation of cyclooxygenase 2 expression in neutrophils can also be another anti-inflammatory mechanism of basil.69 Selvakkumar et al. revealed that The crude methanolic extract of the plant O. basilicum thus exerts anti-inflammatory properties in human PBMC through down regulation of certain key pro-inflammatory cytokines and mediators under LPS induced condition.70 Other anti-inflammatory mechanisms of basil include reduced response of the acute phase of bone marrow, decreased phagocyte activity and inhibitory effect on NO synthesis.70 Basil oil was found to be an effective anti-oxidant in several in vitro assays including DPPH radical, ABTS radical, hydrogen peroxide, hydroxyl radical, nitrite and nitric oxide scavenging assay. Basil oil also exhibited ex vivo antioxidant activity in LPS-stimulated macrophages due to an inhibition of iNOS and NOX gene expressions. Thus, basil oil had radical scavenging activity and could potentially be used as a safe effective source of natural anti-oxidants in therapy against oxidative damages associated with some inflammatory conditions.71 The action of gum arabic as an antioxidant has led to the publication of a series of articles by the same group claiming protective effect of gum arabic against experimental gentamicin and cisplatin nephrotoxicity,72,73 doxorubicin cardiotoxicity74 in rats, and acetaminophen hepatotoxicity75 in mice. Some mechanisms of gum arabic antioxidative action are including diminishing lipid peroxidation and increasing glutathione.76 One of the most important anti-inflammatory mechanism of gum arabic is modulating the activity of the NFkB pathway.76 Since GA is not metabolized in the small intestine and is subject to bacterial attack only in the colon, a variety of possible interactions with specialized cells of the intestinal epithelium are possible, in addition to the physical action of GA on luminal contents. Interference by GA with the triggering action of commensal microorganisms in the lower part of the small intestine could, furthermore, alter local transcription of genes for pro- and anti-inflammatory cytokines.77
Further studies are needed to evaluate the mechanisms of effect of basil and gum arabic on colitis. The evaluation of different cytokine receptors, the assessment of gene and mRNA expression of various cytokines, and the study of some inflammatory pathways such as the NFkB pathway are among our goals in future studies.
Acetic acid-induced colitis is an experimental model of ulcerative colitis. However, the acetic acid-induced colitis is a result of local exposure of healthy mucosa to corrosive activity of acetic acid. In contrast, clinical ulcerative colitis is a chronic inflammatory disease resulting from an inappropriate immune response, in genetically susceptible individuals, to microbial antigens of commensal microorganisms. For this reason, obtained results suggest that rectal administration of combination of basil seeds plus gum arabic may be exhibit the therapeutic effect in clinical ulcerative colitis, but usefulness of this method has to be verified in clinical trials.
5. Conclusion
These findings suggest that protective effect of Basil and gum arabic in the experimental model of colitis could be through an antioxidant and anti-inflammatory mechanisms.
Declaration of competing interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Gupta R.A., Motiwala Meha N, Mahajan Ujawala N, Sabre Sapna G, et al. Protective effect of Sesbania grandiflora on acetic acid induced ulcerative colitis in mice by inhibition of TNF-α and IL-6. J Ethnopharmacol. 2018;219:222–232. doi: 10.1016/j.jep.2018.02.043. [DOI] [PubMed] [Google Scholar]
- 2.Shivananda S., Lennard-Jones J, Logan R, et al. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD) Gut. 1996;39(5):690–697. doi: 10.1136/gut.39.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouma G., Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3(7):521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 4.Malekzadeh M.M., Vahedi H, Mehdipour K, et al. Emerging epidemic of inflammatory bowel disease in a middle income country: a nation-wide study from Iran. Arch Iran Med. 2016;19(1):2–15. [PubMed] [Google Scholar]
- 5.Baumgart D.C. The diagnosis and treatment of Crohn's disease and ulcerative colitis. Dtsch Arztebl Int. 2009;106(8):123–133. doi: 10.3238/arztebl.2009.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piechota-Polanczyk A., Fichna J. The role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. N Schmied Arch Pharmacol. 2014;387(7):605–620. doi: 10.1007/s00210-014-0985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musa R.B., Usha L, Hibbeln J, et al. TNF inhibitors to treat ulcerative colitis in a metastatic breast cancer patient: a case report and literature review. World J Gastroenterol: WJG. 2014;20(19):5912. doi: 10.3748/wjg.v20.i19.5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saxena A., et al. Dietary agents and phytochemicals in the prevention and treatment of experimental ulcerative colitis. Journal of traditional and complementary medicine. 2014;4(4):203–217. doi: 10.4103/2225-4110.139111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore N., et al. News from Tartary: an ethnopharmacological approach to drug and therapeutic discovery. Br J Clin Pharmacol. 2017;83(1):33–37. doi: 10.1111/bcp.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Islam A., et al. A review of recent developments on the regulatory, structural and functional aspects of gum Arabic. Food Hydrocolloids. 1997;11(4):493–505. [Google Scholar]
- 11.Randall R., Phillips G., Williams P. The role of the proteinaceous component on the emulsifying properties of gum Arabic. Food Hydrocolloids. 1988;2(2):131–140. [Google Scholar]
- 12.Hegazy G.A., Alnoury A.M., Gad H.G. The role of Acacia Arabica extract as an antidiabetic, antihyperlipidemic, and antioxidant in streptozotocin-induced diabetic rats. Saudi Med J. 2013;34(7):727–733. [PubMed] [Google Scholar]
- 13.Bhatnagar M., et al. Hemostatic, antibacterial biopolymers from Acacia arabica (Lam.) Willd. and Moringa oleifera (Lam.) as potential wound dressing materials. Indian J Exp Biol. 2013;51(10):804–810. [PubMed] [Google Scholar]
- 14.Rahimi R., Abbasabadi Z., Abdollahi M. An evidence-based review on medicinal plants used for the treatment of peptic ulcer in traditional Iranian medicine. Int J Pharmacol. 2013;9(2):108–124. [Google Scholar]
- 15.Rahimi R., Shams-Ardekani M.R., Abdollahi M. A review of the efficacy of traditional Iranian medicine for inflammatory bowel disease. World J Gastroenterol: WJG. 2010;16(36):4504. doi: 10.3748/wjg.v16.i36.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatnagar M., et al. 2013. Hemostatic, Antibacterial Biopolymers from Acacia Arabica (Lam.) Willd. And Moringa Oleifera (Lam.) as Potential Wound Dressing Materials. [PubMed] [Google Scholar]
- 17.Hassan A., et al. Antimicrobial activity of some plant extracts having hepatoprotective effects. J Med Plants Res. 2009;3(1) 020-023. [Google Scholar]
- 18.Pirbalouti A.G., et al. Exogenous application of chitosan on biochemical and physiological characteristics, phenolic content and antioxidant activity of two species of basil (Ocimum ciliatum and Ocimum basilicum) under reduced irrigation. Scientia horticulturae. 2017;217:114–122. [Google Scholar]
- 19.Naji-Tabasi S., et al. New studies on basil (Ocimum bacilicum L.) seed gum: Part I–Fractionation, physicochemical and surface activity characterization. Food Hydrocolloids. 2016;52:350–358. [Google Scholar]
- 20.Haque N., et al. Management of type 2 diabetes mellitus by lifestyle, diet and medicinal plants. Pakistan J Biol Sci: PJBS. 2011;14(1):13–24. doi: 10.3923/pjbs.2011.13.24. [DOI] [PubMed] [Google Scholar]
- 21.Omoregbe R., Ikuebe O., Ihimire I. Antimicrobial activity of some medicinal plants extracts on Escherichia coli, Salmonella paratyphi and Shigella dysenteriae. Afr J Med Med Sci. 1996;25(4):373–375. [PubMed] [Google Scholar]
- 22.Masoumi A.Y., et al. 2010. Effect of Matricaria Recutita L. Aqueous Extract on Acetic Acid-Induced Ulcerative Colitis in Adult Male Rats. [Google Scholar]
- 23.Ran Z.H., Chen C., Xiao S.D. Epigallocatechin-3-gallate ameliorates rats colitis induced by acetic acid. Biomed Pharmacother. 2008;62(3):189–196. doi: 10.1016/j.biopha.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Okabe S., Amagase K. An overview of acetic acid ulcer models—the history and state of the art of peptic ulcer research—. Biol Pharm Bull. 2005;28(8):1321–1341. doi: 10.1248/bpb.28.1321. [DOI] [PubMed] [Google Scholar]
- 25.Mascolo N., Izzo A., G, Maiello F.M., Di Carlo G., Capasso F. Acetic acid-induced colitis in normal and essential fatty acid deficient rats. J Pharmacol Exp Therapeut. 1995;272(1):469–475. [PubMed] [Google Scholar]
- 26.Paiva L., et al. Protective effect of Copaifera langsdorffii oleo-resin against acetic acid-induced colitis in rats. J Ethnopharmacol. 2004;93(1):51–56. doi: 10.1016/j.jep.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 27.Wang G., et al. Protective effect of methane-rich saline on acetic acid-induced ulcerative colitis via blocking the TLR4/NF-κB/MAPK pathway and promoting IL-10/JAK1/STAT3-mediated anti-inflammatory response. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/7850324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bejeshk M.A., et al. The hydroalcoholic extract of nasturtium officinale reduces lung inflammation and oxidative stress in an ovalbumin-induced rat model of asthma. Evid base Compl Alternative Med. 2022:2022. doi: 10.1155/2022/5319237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajizadeh M.A., et al. Anti-inflammatory and anti-oxidative effects of myrtenol in the rats with allergic asthma. Iran J Pharm Res (IJPR): IJPR. 2019;18(3):1488. doi: 10.22037/ijpr.2019.1100749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bejeshk M., et al. Anti-inflammatory and anti-remodeling effects of myrtenol in the lungs of asthmatic rats: histopathological and biochemical findings. Allergol Immunopathol. 2019;47(2):185–193. doi: 10.1016/j.aller.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Low D., Nguyen D.D., Mizoguchi E. Animal models of ulcerative colitis and their application in drug research. Drug Des Dev Ther. 2013;7:1341. doi: 10.2147/DDDT.S40107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanei M.H., et al. Inflammatory cells' role in acetic acid-induced colitis. Adv Biomed Res. 2014;3 doi: 10.4103/2277-9175.140666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tahan G., et al. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid–induced ulcerative colitis in rats. Can J Surg. 2011;54(5):333. doi: 10.1503/cjs.013610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hagar H.H., et al. Ameliorative effect of pyrrolidinedithiocarbamate on acetic acid-induced colitis in rats. Eur J Pharmacol. 2007;554(1):69–77. doi: 10.1016/j.ejphar.2006.09.066. [DOI] [PubMed] [Google Scholar]
- 35.El-Akabawy G., El-Sherif N.M. Zeaxanthin exerts protective effects on acetic acid-induced colitis in rats via modulation of pro-inflammatory cytokines and oxidative stress. Biomed Pharmacother. 2019;111:841–851. doi: 10.1016/j.biopha.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Palla A.H., et al. Flaxseed extract exhibits mucosal protective effect in acetic acid induced colitis in mice by modulating cytokines, antioxidant and antiinflammatory mechanisms. Int Immunopharm. 2016;38:153–166. doi: 10.1016/j.intimp.2016.04.043. [DOI] [PubMed] [Google Scholar]
- 37.Bastaki S.M., et al. Menthol inhibits oxidative stress and inflammation in acetic acid-induced colitis in rat colonic mucosa. Am J Tourism Res. 2018;10(12):4210. [PMC free article] [PubMed] [Google Scholar]
- 38.Cagin Y.F., et al. Effects of dexpanthenol on acetic acid-induced colitis in rats. Exp Ther Med. 2016;12(5):2958–2964. doi: 10.3892/etm.2016.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colares J.R., et al. Effect of lecithin on oxidative stress in an experimental model of rats colitis induced by acetic acid. J Coloproctol (Rio de Janeiro) 2016;36(2):97–103. [Google Scholar]
- 40.Keshavarzi Z., et al. Protective effects of walnut extract against oxidative damage in acetic acid-induced experimental colitis rats. Physiol Pharmacol. 2019;23(1):51–58. [Google Scholar]
- 41.Krawisz J., Sharon P., Stenson W. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity: assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87(6):1344–1350. [PubMed] [Google Scholar]
- 42.Masoodi I., et al. vol. 9. GMS German Medical Science; 2011. (Biomarkers in the Management of Ulcerative Colitis: A Brief Review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weissmann G., Smolen J.E., Korchak H.M. Release of inflammatory mediators from stimulated neutrophils. N Engl J Med. 1980;303(1):27–34. doi: 10.1056/NEJM198007033030109. [DOI] [PubMed] [Google Scholar]
- 44.Ghasemi-Pirbaluti M., et al. The effect of theophylline on acetic acid induced ulcerative colitis in rats. Biomed Pharmacother. 2017;90:153–159. doi: 10.1016/j.biopha.2017.03.038. [DOI] [PubMed] [Google Scholar]
- 45.Kurutas E.B., et al. Effects of antioxidant therapy on leukocyte myeloperoxidase and Cu/Zn-superoxide dismutase and plasma malondialdehyde levels in experimental colitis. Mediat Inflamm. 2005;2005(6):390–394. doi: 10.1155/MI.2005.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadraei H., et al. Anti-inflammatory effect of apigenin and hydroalcoholic extract of Dracocephalum kotschyi on acetic acid-induced colitis in rats. Res pharmaceut sci. 2017;12(4):322. doi: 10.4103/1735-5362.212050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tao J.-H., et al. Polysaccharides from Chrysanthemum morifolium Ramat ameliorate colitis rats by modulating the intestinal microbiota community. Oncotarget. 2017;8(46) doi: 10.18632/oncotarget.20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Awaad A.S., et al. Anti-ulcerogenic and anti-ulcerative colitis (UC) activities of seven amines derivatives. Saudi Pharmaceut J. 2017;25(8):1125–1129. doi: 10.1016/j.jsps.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahdavi N.-S., Talebi A., Minaiyan M. Ameliorative effect of galantamine on acetic acid-induced colitis in rats. Res pharmaceut sci. 2019;14(5):391. doi: 10.4103/1735-5362.268199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soliman G.A., et al. Protective effects of two Astragalus species on ulcerative colitis in rats. Trop J Pharmaceut Res. 2016;15(10):2155–2163. [Google Scholar]
- 51.Soliman N., et al. The possible ameliorative effect of simvastatin versus sulfasalazine on acetic acid induced ulcerative colitis in adult rats. Chem Biol Interact. 2019;298:57–65. doi: 10.1016/j.cbi.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Dube S., Upadhyay P., Tripathi S. Antifungal, physicochemical, and insect-repelling activity of the essential oil of Ocimum basilicum. Can J Bot. 1989;67(7):2085–2087. [Google Scholar]
- 53.Shahrajabian M.H., Sun W., Cheng Q. Chemical components and pharmacological benefits of Basil (Ocimum Basilicum): a review. Int J Food Prop. 2020;23(1):1961–1970. [Google Scholar]
- 54.Singh S., Majumdar D. Evaluation of the gastric antiulcer activity of fixed oil of Ocimum sanctum (Holy Basil) J Ethnopharmacol. 1999;65(1):13–19. doi: 10.1016/s0378-8741(98)00142-1. [DOI] [PubMed] [Google Scholar]
- 55.Eftekhar N., et al. Immunomodulatory and anti-inflammatory effects of hydro-ethanolic extract of Ocimum basilicum leaves and its effect on lung pathological changes in an ovalbumin-induced rat model of asthma. BMC Compl Alternative Med. 2019;19(1):1–11. doi: 10.1186/s12906-019-2765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abd El-Ghffar E.A., et al. The protective role of Ocimum basilicum L.(Basil) against aspirin-induced gastric ulcer in mice: impact on oxidative stress, inflammation, motor deficits and anxiety-like behavior. Food Funct. 2018;9(8):4457–4468. doi: 10.1039/c8fo00538a. [DOI] [PubMed] [Google Scholar]
- 57.Saeidi F., Sajjadi S.E., Minaiyan M. Antiinflammatory effect of Ocimum Basilicum Linn. seeds hydroalcoholic extract and mucilage on acetic acid-induced colitis in rats. J Rep Pharm Sci. 2018;7:295–305. [Google Scholar]
- 58.Rashidian A., et al. Protective effect of Ocimum basilicum essential oil against acetic acid–induced colitis in rats. J evid-based complementary alternative med. 2016;21(4):NP36–NP42. doi: 10.1177/2156587215616550. [DOI] [PubMed] [Google Scholar]
- 59.Osei Akoto C., Acheampong Akwasi, Duah Boakye Yaw, Naazo Abdulai A., Adomah Derrick H., et al. Anti-inflammatory, antioxidant, and anthelmintic activities of Ocimum basilicum (Sweet Basil) fruits. J Chem. 2020:2020. [Google Scholar]
- 60.Williams P.A., Phillips G. Woodhead; Cambridge: 2000. Handbook of Hydrocolloids. [Google Scholar]
- 61.Ali B.H., Ziada A., Blunden G. Biological effects of gum Arabic: a review of some recent research. Food Chem Toxicol. 2009;47(1):1–8. doi: 10.1016/j.fct.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 62.Wapnir R.A., Teichberg S. Regulation mechanisms of intestinal secretion: implications in nutrient absorption. J Nutr Biochem. 2002;13(4):190–199. doi: 10.1016/s0955-2863(02)00181-x. [DOI] [PubMed] [Google Scholar]
- 63.Ali B.H., et al. Effect of gum Arabic on oxidative stress and inflammation in adenine–induced chronic renal failure in rats. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0055242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmed A.A., Adam Essa ME, Mollica Adriano, et al. Gum Arabic modifies anti-inflammatory cytokine in mice fed with high fat diet induced obesity. Bioactive carbohydrates dietary fibre. 2021;25 [Google Scholar]
- 65.Ali B.H., Al Za’abi Mohammed, Al Suleimani Yousuf, et al. Naunyn-Schmiedeberg’s archives of pharmacology; 2020. Gum Arabic Reduces Inflammation, Oxidative, and Nitrosative Stress in the Gastrointestinal Tract of Mice with Chronic Kidney Disease; pp. 1–10. [DOI] [PubMed] [Google Scholar]
- 66.Kawano S., Tsuji S. Role of mucosal blood flow: a conceptional review in gastric mucosal injury and protection. J Gastroenterol Hepatol. 2000;15:1–6. doi: 10.1046/j.1440-1746.2000.02142.x. [DOI] [PubMed] [Google Scholar]
- 67.Sørbye H., Svanes K. The role of blood flow in gastric mucosal defence, damage and healing. Dig Dis. 1994;12(5):305–317. doi: 10.1159/000171465. [DOI] [PubMed] [Google Scholar]
- 68.Güez C.M., de Souza Raul Oliveira, Fischer Paula, et al. Evaluation of basil extract (Ocimum basilicum L.) on oxidative, anti-genotoxic and anti-inflammatory effects in human leukocytes cell cultures exposed to challenging agents. Braz J Pharmaceut Sci. 2017;53(1) [Google Scholar]
- 69.Takeuchi H., Takahashi-Muto Chie, Nagase Mana, et al. Anti-inflammatory effects of extracts of sweet basil (ocimum basilicum L.) on a Co-culture of 3T3-L1 adipocytes and RAW264. 7 macrophages. J Oleo Sci. 2020 doi: 10.5650/jos.ess19321. [DOI] [PubMed] [Google Scholar]
- 70.Benedec D., Elena P‘rv Alina, Oniga Ilioara, Anca Toiu, BrÓndu∫a Tiperciuc, et al. Effects of Ocimum basilicum L. extract on experimental acute inflammation. Rev Med-Chir Soc Med Nat Iasi. 2007;111(4):1065–1069. [PubMed] [Google Scholar]
- 71.Kavoosi G., Amirghofran Z. Chemical composition, radical scavenging and anti-oxidant capacity of Ocimum basilicum essential oil. J Essent Oil Res. 2017;29(2):189–199. [Google Scholar]
- 72.Al-Majed A.A., Abd-Allah Adel R. A, Al-Rikabi Ammar C, Al-Shabanah Othman A., Mostafa Adel M., et al. Effect of oral administration of Arabic gum on cisplatin-induced nephrotoxicity in rats. J Biochem Mol Toxicol. 2003;17(3):146–153. doi: 10.1002/jbt.10072. [DOI] [PubMed] [Google Scholar]
- 73.Al-Majed A.A., MOSTAFA ADEL M, AL-RIKABI AMMAR C, AL-SHABANAH OTHMAN A, et al. Protective effects of oral Arabic gum administration on gentamicin-induced nephrotoxicity in rats. Pharmacol Res. 2002;46(5):445–451. doi: 10.1016/s1043661802001251. [DOI] [PubMed] [Google Scholar]
- 74.Abd-Allah A.R., Al-Majed Abdulhakeem A, M. Mostafa Adel, et al. Protective effect of Arabic gum against cardiotoxicity induced by doxorubicin in mice: a possible mechanism of protection. J Biochem Mol Toxicol. 2002;16(5):254–259. doi: 10.1002/jbt.10046. [DOI] [PubMed] [Google Scholar]
- 75.Gamal el-din A.M., M Mostafa Adel, Al-Shabanah Othman A, Al-Bekairi Abdullah, Nagi Mahmoud N, et al. Protective effect of Arabic gum against acetaminophen-induced hepatotoxicity in mice. Pharmacol Res. 2003;48(6):631–635. doi: 10.1016/s1043-6618(03)00226-3. [DOI] [PubMed] [Google Scholar]
- 76.Ali B., Alqarawi A., Ahmed I. Does treatment with gum Arabic affect experimental chronic renal failure in rats? Fund Clin Pharmacol. 2004;18(3):327–329. doi: 10.1111/j.1472-8206.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 77.Phillips G.O. Acacia gum (Gum Arabic): a nutritional fibre; metabolism and calorific value. Food Addit Contam. 1998;15(3):251–264. doi: 10.1080/02652039809374639. [DOI] [PubMed] [Google Scholar]