Abstract
Human herpesvirus 8 (HHV-8), or Kaposi's sarcoma-associated herpesvirus, is a gammaherpesvirus first detected in Kaposi's sarcoma tumor cells and subsequently in primary effusion lymphoma (PEL) tumor cells and peripheral blood mononuclear cells from PEL patients. PEL has been recognized as an individual nosologic entity based on its distinctive features and consistent association with HHV-8 infection. PEL is an unusual form of body cavity-based B-cell lymphoma (BCBL). It occurs predominantly in human immunodeficiency virus (HIV)-positive patients but occasionally also in elderly HIV-negative patients. We describe a case of PEL, with ascites, bilateral pleural effusions, and a small axillary lymphadenopathy, in a 72-year-old HIV-negative man. PCR performed on a lymph node specimen and in liquid effusion was positive for HHV-8 and negative for Epstein-Barr virus. The immunophenotype of the neoplastic cells was B CD19+ CD20+ CD22+ with coexpression of CD10 and CD23 and with clonal kappa light chain rearrangement. The patient was treated with Rituximab, a chimeric (human-mouse) anti-CD20 monoclonal antibody. Thirteen months later, the patient continued in clinical remission. This is the first report of an HHV-8-associated BCBL in an HIV-negative patient in Argentina.
In 1994 Chang et al. (10) identified a new herpesvirus sequence in human immunodeficiency virus (HIV)-positive Kaposi's sarcoma dermopathy patients, named Kaposi's sarcoma-associated herpesvirus, or human herpesvirus type 8 (HHV-8). Later reports associated HHV-8 with a nonmalignant disease, Castleman's disease (19, 36), and with body cavity-based lymphoma (BCBL), also called primary effusion lymphoma (PEL) (7, 18, 31, 33). Since 1989 (15) most malignant effusion lymphomas reported have occurred in HIV-positive males (7, 31). PEL is a B-cell neoplasm characterized by infection of the tumor clone with HHV-8 and by liquid-filled body spaces without significant adenopathy. Although other lymphomas may develop cavity effusions, PEL is the only HHV-8-associated body cavity effusion lymphoma (11, 37). Recently, several PEL cases have been reported for HIV-negative individuals (5, 6, 9, 32, 34). PEL cells are usually coinfected with HHV-8 and Epstein-Barr virus (EBV) (7, 8, 31). However, there are cases of PEL cells infected with HHV-8 only (6, 9, 33). Because PEL is a malignant lymphoma, the treatment used for the past 15 years has been the standard treatment for non-Hodgkin lymphoma (NHL): cyclophosphamide, hydroxydoxorubicin, oncovin or vincristine, and prednisone (CHOP) in cyclic administration (22). If relapse or resistance to CHOP treatment occurs in cases of NHL, monoclonal-antibody therapy may be used (12). Satisfactory remissions of low-grade NHL have been obtained with monoclonal-antibody therapy (12, 13). There is no standard polychemotherapy for BCBL or PEL because of its very low incidence. Rituximab is a chimeric (human-mouse) monoclonal antibody that binds to the transmembrane antigen of the CD20+ B cell, inducing apoptosis and complement-mediated cytotoxicity (17). In this work we report, for the first time in Argentina, a rare case of an HHV-8-associated BCBL with a B-cell phenotype in an HIV-negative male, in clinical remission after anti-CD20 treatment.
CASE REPORT
A 72-year-old man was referred to the Hematology Service at the Santojanni Hospital for investigation of pericardial and bilateral pleural effusions, plus ascites and chronic itching. Two years earlier he had presented with a lymphoproliferative disease, and biopsy of a 13-mm-diameter lymph node specimen showed a B CD19+ CD20+ CD22+ immunophenotype with coexpression of CD10 and CD23 and with clonal kappa light chain rearrangement. After eight cycles of CHOP chemotherapy he was in clinical remission for 16 months, but prurigo remained. On examination, the patient was mildly dyspneic, with ascites and massive bilateral effusions, requiring several drainages. Lesions from scratching could be seen, but neither hepatosplenomegaly nor significant adenopathy was present. Laboratory tests showed eosinophilia (16%), a hemoglobin level of 115 g/liter, a white blood cell count of 5.7 × 109/liter, and a platelet count of 350 × 109/liter. Levels of markers for lymphoma evolution were increased as follows: lactic dehydrogenase, 740 IU (from 460); β2-microglobulin, 55 g/liter (from a range of 11 to 30). Results of additional studies, including serum protein electrophoresis and routine serum biochemistry (glucose, urea, albumin, cholesterol, glutamic-pyruvic transaminase, glutamic-oxalacetic transaminase, alkaline phosphatase, and creatinine), were normal. Results of enzyme-linked immunosorbent assay serology for HIV, HTLV 1 and 2, hepatitis B virus surface antigen, and hepatitis C virus were negative.
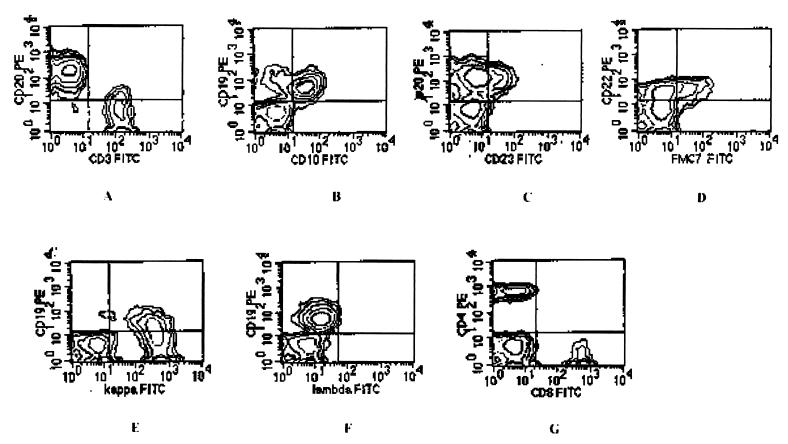
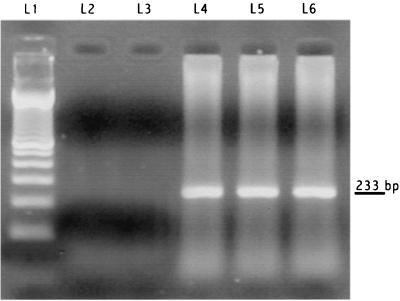
A chest computed-tomography scan showed bilateral pleural effusions; a computed-tomography scan of the abdomen revealed ascites with no hepatosplenomegaly and retroperitoneal adenopathies with diameters of less than 1.5 mm. A new lymph axillary node biopsy specimen was studied, and cytopathology was found, as was the case 2 years earlier. The immunophenotypic profile was 61% B lymphocytes and 34% T lymphocytes (Fig. 1A); the B-cell population expressed CD19 (Fig. 1B), CD20 (Fig 1C), and CD22 (Fig 1D), with coexpression of CD10 and CD23 antigens (Fig. 1B and C) and kappa light chain restriction (Fig. 1E). The T-cell population consisted of 21% CD4+ cells and 12% CD8+ cells (Fig. 1G). Bone marrow was not infiltrated by lymphoma cells. PCR was positive for HHV-8 (Fig. 2) and negative for EBV in both a lymph node biopsy specimen and liquid effusion. The patient underwent a four-cycle, 1-month Rituximab anti-CD20 treatment at the recommended dosage (26, 29). One month after the end of treatment, all effusions disappeared; itching and eosinophilia were also resolved. Seven months later, while in clinical remission, the patient was serologically tested by indirect immunofluorescence assay for HHV-8 (serum titer, 1/10) and EBV (serum titer, 1/40); nested PCR of peripheral blood mononuclear cells was positive for both HHV-8 and EBV. Thirteen months later, the patient continued in clinical remission.
FIG. 1.
Contour plots showing flow cytometry profile. (A) CD20+ B-cell population (21%) and CD3+ T-cell population (34%); (B) coexpression of CD19 and CD10; (C) coexpression of CD20 and CD23; (D) CD22 and FMC7 expression; (E) kappa light chain restriction; (F) normal expression of lambda chain; (G) T-cell population (21% CD4–12% CD8). FITC, fluorescein isothiocyanate; PE, phycoerythrin.
FIG. 2.
PCR for HHV-8 (233 bp). Lanes: L1, molecular weight marker; L2, negative control (Raji cells positive for EBV and negative for HHV-8); L3, H2O; L4, lymph node DNA; L5, pleural effusion DNA; L6, positive control (BCBL-1 cells [National Institutes of Health AIDS Research Program] positive for HHV-8 and negative for EBV).
MATERIALS AND METHODS
Cytological studies were performed on formalin fixed, paraffin-embedded lymph node specimens for hematoxylin-eosin staining. The immunophenotypic profile was determined by FACSscan flow cytometry (Becton Dickinson Immunocytometry Systems) with fluorescein isothiocyanate- and phycoerythrin-conjugated monoclonal antibodies (Becton Dickinson) on the specimen obtained by aspiration biopsy.
PCR for HHV-8 was carried out on DNA extracted from 250 μl of fresh pleural effusion and from a paraffin-embedded lymph node specimen. PCR amplified the 233 bp of the KS 330 Bam region described by Chang et al. (10). PCR for EBV amplified the repeat segment in the BamH-IW region, as previously described (35). Nested PCR for the same fragment of HHV-8 was carried out in peripheral lymphocytes as described elsewhere (25).
Ambulatory treatment with Rituximab was given at the recommended dose of 375 mg/m2 of body surface in weekly infusions for 4 weeks (26, 27, 29) Sixty minutes before the treatment, a vial of acetaminophen was administered. Vital signs were monitored. The initial infusion of 50 mg/h was raised to 100 mg/h for 6 h in the 1st week. Subsequent administrations were reduced to 5 h.
Neither intolerance nor side effects were recorded.
RESULTS AND DISCUSSION
PEL was first described in 1989 by Knowles et al. (24), who observed major lymphomatous effusions in body cavities, usually growing in the absence of an identifiable tumor mass. Other investigators subsequently confirmed this observation (23, 38). In 1995, Cesarman et al. (7) reported the presence of HHV-8 in AIDS-related BCBL. Then the term PEL was suggested for AIDS-related and non-AIDS-related BCBLs that are characterized by effusions in body cavities without lymphadenopathy, tumor masses, or bone marrow involvement and are typically associated with HHV-8 (2, 3, 6, 9, 31). The main manifestations of lymphoma in the case reported in this paper were pleural effusions and ascites. The volume and locations of these effusions prompted us to review the case and to look for HHV-8 in effusions and lymph node specimens. Reports describing early lymph node involvement of HHV-8 DNA in an HIV-negative PEL patient (1), small intra-abdominal solid tumors detected by imaging in a few cases (30), and HHV-8 DNA in fluid effusions (7, 21, 31) support the possibility that HHV-8 may play a role in PEL pathogenesis. Some cases of PEL in HIV-negative patients have been reported previously (1, 5, 9, 14, 21, 32). The average age of these patients was 72 years, coincident with the age of our patient. Although other investigators have reported that the main features of PEL are large, anaplastic CD19− CD20− cells (9, 16, 23, 31, 38), there are CD20+ (33) and CD19/20+ PELs (6, 9, 20), and some cases with an intermediate lymphocytic cytomorphology have been described (9). Moreover, on the basis of immunoglobulin VH gene mutational analysis, it has been suggested that PEL may not be restricted to one stage of B-cell differentiation but may represent transformation of B cells at different stages of ontogeny (28). In an animal model, mice injected with lymphoma cells from a PEL patient developed small intra-abdominal tumors (4). It has also been proposed that the clinical spectrum of manifestations at presentation of PEL may be wider than initially described (1). On the basis of the reports cited and the clinical and molecular results obtained, the diagnosis for this patient was PEL. Because of its very low incidence, there is no standard polychemotherapy for BCBL or PEL. Most PEL patients do not respond to CHOP therapy (1). Recently, a PEL with a B-cell phenotype successfully treated with prednisone was reported (20), but the patient died 15 months after PEL diagnosis. There are reports of satisfactory remissions in low-grade NHL (12, 13) and in bone marrow transplant purging following treatment with anti-B-cell monoclonal antibodies. Rituximab is a chimeric (human-mouse) monoclonal antibody that binds to the CD20 B cell's transmembrane antigen, inducing apoptosis and complement-dependent cytotoxicity (17). We chose Rituximab therapy for this case based on previous CHOP treatment failure and the CD20+ immunophenotype.
We report here a case of a patient with an HHV-8-associated BCBL, with a B-cell phenotype, treated with an anti-CD20 monoclonal antibody. In view of the poor results of present schemes, monoclonal therapy may be another modality of treatment for PEL patients.
REFERENCES
- 1.Ariad S, Benharroch D, Lupu L, Davidovici B, Dupin N, Boshoff C. Early peripheral lymph node involvement of human herpesvirus 8-associated, body cavity-based lymphoma in a human immunodeficiency virus-negative patient. Arch Pathol Lab Med. 2000;124:753–755. doi: 10.5858/2000-124-0753-EPLNIO. [DOI] [PubMed] [Google Scholar]
- 2.Ascoli V, Scalzo C C, Danese C, Vacca K, Pistilli A, Lo Coco F. Human herpesvirus 8-associated primary effusion lymphoma of the pleural cavity in HIV-negative elderly men. Eur Respir J. 1999;14:1231–1234. doi: 10.1183/09031936.99.14512319. [DOI] [PubMed] [Google Scholar]
- 3.Asou H, Said J, Yang R, Munker R, Park D J, Kamada N, Koeffler H P. Mechanisms of growth control of Kaposi's sarcoma-associated herpesvirus-associated primary effusion lymphoma cells. Blood. 1998;91:2475–2481. [PubMed] [Google Scholar]
- 4.Boshoff C, Gao S J, Healy L E, Matthews S, Thomas A J, Coignet L, Warnke R A, Strauchen J A, Matutes E, Kamel O W, Moore P S, Weiss R A, Chang Y. Establishing a KSHV+ cell line (BCP-1) from peripheral blood and characterizing its growth in Nod/SCID mice. Blood. 1998;91:1671–1679. [PubMed] [Google Scholar]
- 5.Boshoff C, Whitby D, Hatziioannou T, Fisher C, van der Walt J, Hatzakis A, Weiss R, Schulz T. Kaposi's sarcoma-associated herpesvirus in HIV-negative Kaposi's sarcoma. Lancet. 1995;345:1043–1044. doi: 10.1016/s0140-6736(95)90780-7. [DOI] [PubMed] [Google Scholar]
- 6.Carbone A, Gloghini A, Vaccher E, Zagonel V, Pastore C, Dalla Palma P, Branz F, Saglio G, Volpe R, Tirelli U, Gaidano G. Kaposi's sarcoma-associated herpesvirus DNA sequences in AIDS-related and AIDS-unrelated lymphomatous effusions. Br J Haematol. 1996;94:533–543. doi: 10.1046/j.1365-2141.1996.d01-1826.x. [DOI] [PubMed] [Google Scholar]
- 7.Cesarman E, Chang Y, Moore P, Said J, Knowles D. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 8.Cesarman E, Moore P S, Rao P H, Inghirami G, Knowles D M, Chang Y. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi's sarcoma-associated herpesvirus-like (KSHV) sequences. Blood. 1995;86:2708–2714. [PubMed] [Google Scholar]
- 9.Cesarman E, Nador R G, Aozasa K, Delsol G, Said J W, Knowles D M. Kaposi's sarcoma-associated herpesvirus in non-AIDS-related lymphomas occurring in body cavities. Am J Pathol. 1996;149:53–57. [PMC free article] [PubMed] [Google Scholar]
- 10.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 11.Cobo F, Montserrat E, Campo E. Linfoma primario de cavidades: una nueva entidad clínicopatológica. Med Clin. 1997;109:712–714. [PubMed] [Google Scholar]
- 12.Czuzman M, Grillo-López A J, White C. Rituximab/CHOP chemoimmunotherapy in patients with low-grade lymphoma: progression-free survival after 3 years' median follow-up. Blood. 1999;94(Suppl. 1):99A. [Google Scholar]
- 13.Czuzman M S, Grillo-López A J, White C A, Saleh M, Gordon L, LoBuglio A F, Jonas C, Klippenstein D, Dallaire B, Varns C. Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol. 1999;17:268–276. doi: 10.1200/JCO.1999.17.1.268. [DOI] [PubMed] [Google Scholar]
- 14.Dotti G, Fiocchi R, Motta T, Facchinetti B, Chiodini B, Borleri G, Gavazzeni G, Barbui T, Rambaldi A. Primary effusion lymphoma after heart transplantation: a new entity associated with human herpesvirus-8. Leukemia. 1999;13:664–670. doi: 10.1038/sj.leu.2401390. [DOI] [PubMed] [Google Scholar]
- 15.Feiner H D, et al. Frequent null cell status of lymphomas in the pleura in HIV+ patients. Lab Investig. 1989;60:28A. [Google Scholar]
- 16.Green I, Espíritu E, Ladanyi M, Chaponda R, Wieczorek R, Gallo L, Feiner H. Primary lymphomatous effusions in AIDS: a morphological, immunophenotypic, and molecular study. Mod Pathol. 1995;8:39–45. [PubMed] [Google Scholar]
- 17.Harjunpaa A, Junnikkala S, Meri S. Rituximab (anti-CD20) therapy of B-cell lymphomas: direct complement killing is superior to cellular effector mechanisms. Scand J Immunol. 2000;51:634–641. doi: 10.1046/j.1365-3083.2000.00745.x. [DOI] [PubMed] [Google Scholar]
- 18.Harris N L, Jaffe E S, Stein H, Banks P M, Chan J K, Cleary M L, Delsol G, De Wolf-Peeters C, Falini B, Gatter K C. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 19.Humphrey R W, Davis D A, Newcomb F M, Yarchoan R. Human herpesvirus 8 (HHV-8) in the pathogenesis of Kaposi's sarcoma and other diseases. Leukemia Lymphoma. 1998;28:255–264. doi: 10.3109/10428199809092681. [DOI] [PubMed] [Google Scholar]
- 20.Iwahashi M, Iida S, Sako S, Inoue S, Kikuchi H, Otsuka E, Nasu M. Primary effusion lymphoma with B-cell phenotype. Am J Hematol. 2000;64:317–318. doi: 10.1002/1096-8652(200008)64:4<317::aid-ajh15>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 21.Jones D, Ballestas M E, Kaye K M, Gulizia J M, Winters G L, Fletcher J, Scadden D T, Aster J C. Primary-effusion lymphoma and Kaposi's sarcoma in a cardiac-transplant recipient. N Engl J Med. 1998;339:444–449. doi: 10.1056/NEJM199808133390705. [DOI] [PubMed] [Google Scholar]
- 22.Jones S E, et al. Superiority of adriamycin-containing combination chemotherapy in the treatment of diffuse lymphoma: a Southwest Oncology Group study. Cancer. 1979;43:417–425. doi: 10.1002/1097-0142(197902)43:2<417::aid-cncr2820430203>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 23.Karcher D S, Dawkins F, Garret C T, Schulof R S. Body cavity-based non-Hodgkin's lymphoma (NHL) in HIV-infected patients: B-cell lymphoma with unusual clinical, immunophenotypic, and genotypic features. Lab Investig. 1992;66:80A. [Google Scholar]
- 24.Knowles D, Inghirami G, Ubriaco A, Dalla Favera R. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792–799. [PubMed] [Google Scholar]
- 25.Lock M, Griffiths P, Emery V. Development of a quantitative competitive polymerase chain reaction for human herpesvirus 8. J Virol Methods. 1997;64:19–26. doi: 10.1016/s0166-0934(96)02139-8. [DOI] [PubMed] [Google Scholar]
- 26.Maloney D G, Liles T M, Czerwinski D K, Waldichuk C, Rosenberg J, Grillo-López A, Levy R. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84:2457–2461. [PubMed] [Google Scholar]
- 27.Maloney D, Press O. Newer treatments for non-Hodgkin's lymphoma: monoclonal antibodies. Oncology. 1998;12(Suppl. 8):63–76. [PubMed] [Google Scholar]
- 28.Matolcsy A, Nador R, Cesarman E, Knowles D. Immunoglobulin VH gene mutational analysis suggests that primary effusion lymphomas derive from different stages of B-cell maturation. Am J Pathol. 1998;153:1609–1614. doi: 10.1016/S0002-9440(10)65749-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLaughlin P, Grillo López A, Czuzman M. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 30.Morassut S, Vaccher E, Balestreri L, Gloghini A, Gaidano G, Volpe R, Tirelli U, Carbone A. HIV-associated human herpesvirus 8-positive primary lymphomatous effusions: radiologic findings in six patients. Radiology. 1997;205:459–463. doi: 10.1148/radiology.205.2.9356629. [DOI] [PubMed] [Google Scholar]
- 31.Nador R, Cesarman E, Chadburn A, Dawson D B, Ansari M Q, Said J, Knowles D M. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpesvirus. Blood. 1996;88:645–656. [PubMed] [Google Scholar]
- 32.Nador R, Cesarman E, Knowles D, Said J W. Herpes-like DNA sequences in a body cavity-based lymphoma in an HIV-negative patient. N Engl J Med. 1995;333:943. doi: 10.1056/NEJM199510053331417. [DOI] [PubMed] [Google Scholar]
- 33.Said J, Chien K, Takeuchi S, Tasaka T, Asou H, de Vos S, Cesarman E, Knowles D, Koeffler H P. Kaposi's sarcoma-associated herpesvirus (KSHV or HHV-8) in primary effusion lymphoma: ultrastructural demonstration of herpesvirus in lymphoma cells. Blood. 1996;87:4937–4943. [PubMed] [Google Scholar]
- 34.Said J, Tasaki T, Takeuchi S, Asou H, de Vos S, Cesarman E, Knowles D M, Koeffler H P. Primary effusion lymphoma in women: report of two cases of Kaposi's sarcoma herpesvirus-associated effusion-based lymphoma in human immunodeficiency virus-negative women. Blood. 1996;88:3124–3128. [PubMed] [Google Scholar]
- 35.Saito I, Servenius B, Compton T, Fox R. Detection of Epstein-Barr virus DNA by polymerase chain reaction in blood and tissue biopsies from patients with Sjogren's syndrome. J Exp Med. 1989;169:2191–2198. doi: 10.1084/jem.169.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teruya-Feldstein J, Zauber P, Setsuda J, Berman E, Sorbara L, Raffeld M, Tosato G, Jaffe E. Expression of human herpesvirus-8 oncogene and cytokine homologues in an HIV-seronegative patient with multicentric Castleman's disease and primary effusion lymphoma. Lab Investig. 1998;78:1637–1642. [PubMed] [Google Scholar]
- 37.Uphoff C, Carbone A, Gaidano G, Drexler H. HHV-8 infection is specific for cell lines derived from primary effusion (body cavity-based) lymphomas. Leukemia. 1998;12:1806–1809. doi: 10.1038/sj.leu.2401194. [DOI] [PubMed] [Google Scholar]
- 38.Walts A E, Shintaku I P, Said J W. Diagnosis of malignant lymphoma in effusion from patients with AIDS by gene rearrangement. Am J Clin Pathol. 1990;94:170–175. doi: 10.1093/ajcp/94.2.170. [DOI] [PubMed] [Google Scholar]