Abstract
Background
Thromboinflammation plays a central role in severe COVID‐19. The kallikrein pathway activates both inflammatory pathways and contact‐mediated coagulation. We investigated if modulation of the thromboinflammatory response improves outcomes in hospitalized COVID‐19 patients.
Methods
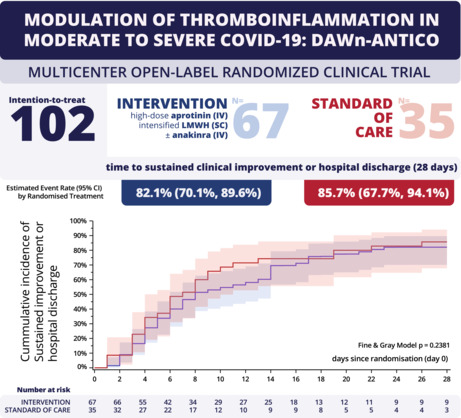
In this multicenter open‐label randomized clinical trial (EudraCT 2020‐001739‐28), patients hospitalized with COVID‐19 were 1:2 randomized to receive standard of care (SOC) or SOC plus study intervention. The intervention consisted of aprotinin (2,000,000 IE IV four times daily) combined with low molecular weight heparin (LMWH; SC 50 IU/kg twice daily on the ward, 75 IU/kg twice daily in intensive care). Additionally, patients with predefined hyperinflammation received the interleukin‐1 receptor antagonist anakinra (100 mg IV four times daily). The primary outcome was time to a sustained 2‐point improvement on the 7‐point World Health Organization ordinal scale for clinical status, or discharge.
Findings
Between 24 June 2020 and 1 February 2021, 105 patients were randomized, and 102 patients were included in the full analysis set (intervention N = 67 vs. SOC N = 35). Twenty‐five patients from the intervention group (37%) received anakinra. The intervention did not affect the primary outcome (HR 0.77 [CI 0.50‐1.19], p = 0.24) or mortality (intervention n = 3 [4.6%] vs. SOC n = 2 [5.7%], HR 0.82 [CI 0.14‐4.94], p = 0.83). There was one treatment‐related adverse event in the intervention group (hematuria, 1.49%). There was one thrombotic event in the intervention group (1.49%) and one in the SOC group (2.86%), but no major bleeding.
Conclusions
In hospitalized COVID‐19 patients, modulation of thromboinflammation with high‐dose aprotinin and LMWH with or without anakinra did not improve outcome in patients with moderate to severe COVID‐19.
Keywords: anakinra, aprotinin, COVID‐19, heparin, inflammation, low‐molecular‐weight, thrombosis
Essentials.
Severe COVID‐19 is associated with a high incidence of blood clots and inflammation.
This trial studied a multi‐target strategy to prevent blood clots and inflammation.
This strategy did not improve clinical outcome compared to the standard treatment.
Overall mortality was low with both the experimental and standard treatment.
1. BACKGROUND
COVID‐19 continues to dominate global health. In patients who develop severe disease, a thromboinflammatory response follows the initial phase of viral replication. Indeed, markers of inflammation and hypercoagulation are associated with disease severity and outcome. 1 , 2 We therefore designed a multitarget multistep intervention to study whether modulation of this excessive thromboinflammatory response is feasible and improves outcome in patients with severe COVID‐19. 3 The study intervention consists of aprotinin to suppress the kallikrein‐kinin system and higher dose low molecular weight heparin (LMWH). Additionally, anakinra was added to inhibit the interleukin‐1 (IL‐1) pathway in patients with signs of hyperinflammation.
The kallikrein‐kinin system might drive the pathogenesis of thrombo‐inflammation in COVID‐19 because it induces inflammation and activation of coagulation through the contact pathway. 4 , 5 , 6 , 7 Specific inhibitors of the kallikrein‐bradykinin‐system are not readily available. However, the nonspecific serine protease‐inhibitor aprotinin strongly suppresses kallikrein activity. Being a nonspecific serine protease inhibitor, aprotinin also could inhibit the protease activity of transmembrane serine protease 2 (TMPRSS2). This human protease cleaves the spike protein between the S1 and S2 subunit, but thereby facilitates SARS‐CoV‐2 cell entry. Indeed, preliminary research showed that by inhibiting TMPRSS2, aprotinin inhibits SARS‐CoV‐2 entry and replication in vitro. 8 , 9 , 10 LMWH, on the other hand, inhibits coagulation factors Xa and IIa and reduces thrombotic events in patients with severe COVID‐19. 11 , 12 The optimal dose of thromboprophylaxis has been the focus of various dedicated clinical trials. This clinical trial, however, is not designed to determine the optimal dose of thromboprophylaxis but instead investigates the concept of multitargeted thromboinflammatory modulation (aprotinin and LMWH). In patients with hyperinflammation, the recombinant interleukin‐1 receptor antagonist anakinra was added to suppress the hyperinflammatory state induced by the overproduction of inflammatory cytokines in COVID‐19. Anakinra has been successfully investigated in other pathologies with hyperinflammatory properties and is, therefore, an available compound to suppress the IL‐1–IL‐6 pathway.
2. METHODS
2.1. Study design and oversight
This open‐label multicenter randomized clinical trial was performed in three Belgian hospitals. The study's design has been published and the full protocol is in Appendix S2. 3 The Ethics Committee an institutional review board at each participating center approved the protocol. The clinical study was registered as EudraCT 2020‐001739‐28. The steering committee and Data and Safety Monitoring Board (DSMB) were responsible for the oversight of the trial. A safety and feasibility review was planned after the pilot phase (November 2020). After this prespecified interim analysis of day 6 D‐dimer in the first 50 patients, the DSMB recommended to continue the study but ordered a midway safety and futility analysis after randomizing 105 patients (i.e., 50%). After this futility analysis, on 31 March 2021, the DSMB advised terminating the study because of a conditional power of <0.1% to reach the primary endpoint with the planned sample size.
2.2. Patients
Male or nonpregnant female adult patients aged 18 years or older at the time of enrollment with confirmed diagnosis of SARS‐CoV‐2 infection were eligible for participation. The main exclusion criteria included known thromboembolic disease, recent myocardial infarction, creatine clearance <20 ml/min or renal replacement therapy, active bleeding or increased bleeding risk, platelet count < 30,000/μl, indication for therapeutic anticoagulation, heart failure, and suspicion of latent tuberculosis or severe bacterial infection before randomization or start of anakinra. Known thromboembolic disease also includes new venous thromboembolism diagnosed before enrollment. Patients were not routinely screened through computed tomography pulmonary angiogram or venous ultrasound to exclude asymptomatic venous thromboembolism. The full list of exclusion criteria is available as an online supplement and in the published study protocol paper. 3 All included patients provided written informed consent.
2.3. Randomization and masking
Briefly, eligible and consenting patients were randomized to receive standard of care (SOC) or study intervention, according to a 2:1 allocation scheme stratified by study site, using randomly selected block sizes of 6 or 9. Randomization was done using a centralized web‐based randomization application. In this open‐label study, patients, clinicians, and study personnel were aware of the assigned treatment. The trial statistician was not given access to the full database and was not aware of the allocated treatments until database lock.
2.4. Intervention and procedures
Standard of care included LMWH thromboprophylaxis per hospital protocol (Table S1). 13 , 14 As illustrated in Figure 1, the study intervention consisted of aprotinin (2,000,000 IE four times per day for 4 days) combined with weight‐adjusted LMWH (enoxaparin or nadroparin, 50 IU/kg twice daily in the ward, 75 IU/kg twice daily in intensive care unit [ICU]; for 14 days and reduced to once daily when creatinine clearance dropped below 30 ml/min/1.73 m2). Additionally, patients developing hyperinflammation during the first 14 days after randomization were treated with anakinra unless contraindicated (100 mg IV four times per day for up to 7 days). Hyperinflammation was defined as lymphocytopenia (<1000 cells/ml) with two of the following: (1) ferritin > 800 ng/ml, (2) LDH > 400 U/L, or (3) D‐dimer > 1000 ng/ml. During the first 14 days after randomization, there was daily follow‐up until discharge. Additional follow‐up was planned on days 15 and 28. A follow‐up visit was provided 5 to 7 weeks after discharge.
FIGURE 1.
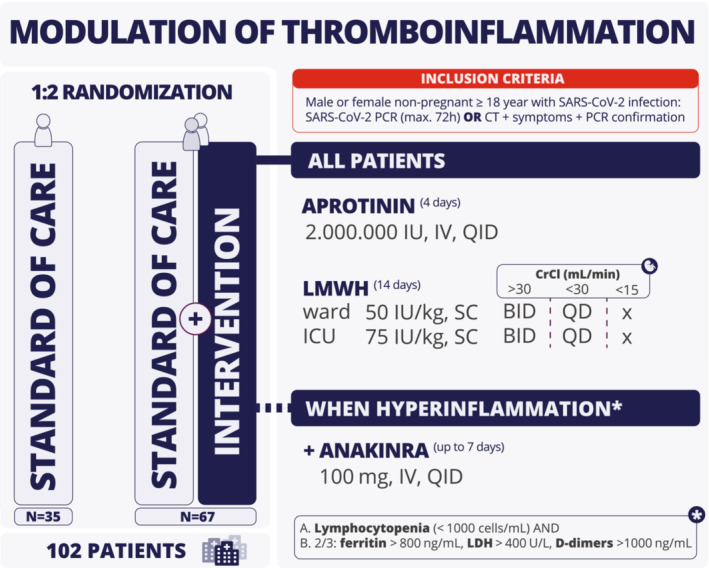
Study overview. BID, twice daily; CrCl, creatinine clearance; CT, computed tomography; ICU, intensive care unit; IU, international units; IV, intravenous; LMWH, low molecular weight heparin; PCR, polymerase chain reaction; QD, once daily; QID, four times a day; SC, subcutaneous.
2.5. Trial outcomes
The primary clinical outcome was time to discharge or sustained 2‐point improvement in the World Health Organization 7‐point ordinal scale for clinical status or hospital discharge. A 2‐point improvement was defined as an improvement of >2 points compared with the highest value recorded on day 0 or 1 and sustained for at least 3 days. Clinical status was recorded daily until discharge and on days 15 and 28 and follow‐up visit 5 to 7 weeks after discharge. Secondary outcomes included all‐cause mortality on days 15 and 28, incidence and duration of supplemental oxygen and mechanical ventilation up to day 28, duration of hospital and ICU stay, and thromboinflammatory parameters at predefined timepoints. Details on laboratory assays for outcome parameter D‐dimer (cutoff 500 μg/L fibrinogen equivalent units; Werfen and Stago) and C‐reactive protein (Roche and Abbott) are summarized in Table S2. Safety outcomes included adverse events, thrombotic events (venous thromboembolism and others) and major bleeding as defined by the International Society on Thrombosis and Haemostasis. 15 A detailed description of secondary and safety end points is available as an online supplement or in the published study protocol. 3
2.6. Statistical analysis
sLearning from early randomized trials, we assumed a 40% improvement rate on day 15 in the control group for our power calculations. Based on the log‐rank test, with a two‐sided significance level of 5% and 80% statistical power and using a 2:1 randomization ratio in favor of the intervention, we estimated that a total sample size of 196 patients would suffice to detect an absolute improvement of 20% (60% in the intervention group). We proposed a pragmatic sample size of 210 patients considering early dropouts. A detailed description of the analysis is provided in the Statistical Analysis Plan, which was finalized and filed before database lock (Appendix S3). A summary is provided here. Analysis sets were finalized prior to database lock when the investigators were unaware of the study results. The Full Analysis Set (FAS) included all randomized patients, except one patient with chronic alcoholism and three patients who withdrew consent to use any data immediately after randomization and before treatment administration. The Per Protocol Set included all FAS patients, except for patients randomized to intervention who had fewer than 3 days of aprotinin dosing, who had hyperinflammation but received no anakinra, or who received anakinra but had no hyperinflammation. The primary analysis set of interest was the FAS, but all efficacy analyses were repeated on the Per Protocol Set as a sensitivity analysis.
Missing clinical status data were accounted for by means of multiple imputation, using a total of 100 imputations. Treatment effects for all end points were estimated by an appropriate measure and presented with 95% confidence intervals and were adjusted for study site and period (before and after start of second peak of COVID hospitalizations [7 September 2021]). The primary end point was compared using competing‐risk methodology, using cumulative incidence functions to estimate event rates and a Fine & Gray regression model to obtain cause‐specific hazard ratios. Daily clinical status was analyzed using a proportional odds logistic regression to estimate the common odds ratio. All‐cause mortality and survival without mechanical ventilation up to 30 days were assessed using a Cox regression to obtain hazard ratios. Incidence rates were estimated using Kaplan–Meier methodology. Time to hospital discharge, incidence and duration of supplemental oxygen, mechanical ventilation, and ICU stay were analyzed using the same methodology as for the primary end point. Cumulative clinical status scores were analyzed using a general linear model on the log‐transformed scores to obtain a treatment ratio of geometric means between the treatment groups.
Prespecified subgroup analyzes were performed for the primary end point, considering the following subgroups: age (according to observed median), study period, admission to ICU at hospital admission, presence of hyperinflammation at baseline, clinical status on day 0 (≥5 vs. <5).
All tests were two‐sided and assessed at a significance level of 5%. No correction was made for multiple secondary endpoints. All analyses were performed using SAS software version 9.4 for Windows 10.
2.7. Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
3. RESULTS
3.1. Baseline demographics
Between 24 June 2020, and 1 February 2021, 105 patients were randomized at three hospitals in Belgium. Thirty‐six patients were randomized to SOC and 69 to intervention, of whom 25 patients (37%) were additionally treated with anakinra. After excluding one patient in the SOC group and two patients in the intervention group, 102 patients were analyzed in the FAS (Figures 1 and 2). The mean age was 58 years (SD 13) and 75% of patients were male. There was no significant difference in ethnicity between both groups; 86% of patients was Caucasian (details in Table S3). Except for chronic systemic corticosteroid therapy, there were no baseline differences in demographics, medical history, vital signs at admission, and medical treatment or mechanical support started on admission between SOC and intervention (Table 1). Patients had elevated D‐dimer (median 665 μg/L, IQR 460–1090 μg/L) and C‐reactive protein (median 65 mg/L, IQR 41–112 mg/L) at admission, confirming baseline activation of coagulation and inflammatory pathways. At admission, criteria of hyperinflammation were met in 29% of cases and D‐dimer were elevated in 29% of cases.
FIGURE 2.

Study profile. Low molecular weight heparin, LWMH (CONSORT flowchart also in Appendix S1).
TABLE 1.
Patient characteristics
| Statistic | Randomized treatment | |||
|---|---|---|---|---|
| Intervention | Standard of care | p value | ||
| Baseline characteristics | N | 67 | 35 | |
| Age, y | [n] Mean (SD) | [67] 58 (13) | [35] 59 (14) | 0.75 |
| Male | n/N (%) | 51/67 (76.1%) | 25/35 (71.4%) | 0.61 |
| Caucasian | n/N (%) | 56/66 (84.85%) | 31/35 (88.57%) | 0.91 |
| Body weight, kg | [n] Mean (SD) | [65] 92 (22) | [34] 91 (19) | 0.79 |
| Medical history | ||||
| Diabetes mellitus | n/N (%) | 13/67 (19.4%) | 10/35 (28.6%) | 0.46 |
| Arterial hypertension | n/N (%) | 34/67 (50.8%) | 16/35 (45.7%) | 0.66 |
| Smoking status | ||||
| Active | n/N (%) | 3/67 (4.5%) | 2/35 (5.7%) | 0.16 |
| Former | n/N (%) | 9/67 (13.4%) | 10/35 (28.6%) | |
| Never | n/N (%) | 55/67 (82.1%) | 23/35 (65.7%) | |
| COPD | n/N (%) | 3/67 (4.5%) | 2/35 (5.7%) | 0.57 |
| Asthma | n/N (%) | 11/67 (16.4%) | 4/35 (11.4%) | 0.45 |
| Heart failure | n/N (%) | 2/67 (3.0%) | 0/35 (0%) | 0.25 |
| Ischemic heart disease | n/N (%) | 5/67 (7.5%) | 1/35 (2.9%) | 0.37 |
| Moderate or severe liver disease | n/N (%) | 0/67 (0%) | 0/35 (0%) | 0.30 |
| Chronic kidney disease | n/N (%) | 4/67 (6.0%) | 0/35 (0%) | 0.19 |
| Active cancer | n/N (%) | 2/67 (3.0%) | 0/35 (0%) | 0.30 |
| Previous medications | ||||
| Antiplatelet agent | n/N (%) | 7/67 (10.5%) | 4/35 (11.4%) | 0.88 |
| Anticoagulation | n/N (%) | 5/67 (7.5%) | 1/35 (2.9%) | 0.59 |
| Statins | n/N (%) | 14/67 (20.9%) | 6/35 (17.1%) | 0.82 |
| Chronic systemic corticosteroid therapy | n/N (%) | 0/67 (0%) | 6/35 (17.1%) | 0.001 |
| Other immune‐suppressing therapy | n/N (%) | 1/67 (1.5%) | 2/35 (5.7%) | 0.38 |
| Antibiotics | n/N (%) | 7/67 (10.5%) | 4/35 (11.4%) | 0.88 |
| Laboratory data at admission | ||||
| Hemoglobin, g/dl | [n] Mean (SD) | [65] 14 (2) | [34] 14 (1) | 0.32 |
| WBC, 109/L | [n] Mean (SD) | [65] 7 (3) | [34] 7 (2) | 0.56 |
| Platelet count, 109/L | [n] Mean (SD) | [65] 231 (96) | [33] 231 (102) | 1.00 |
| CRP, mg/L | [n] Median (Q1; Q3) | [66] 64 (43; 104) | [35] 73 (31; 114) | 0.79 |
| D‐dimer, μg/L | [n] Median (Q1; Q3) | [60] 735 (465; 1230) | [31] 570 (454; 877) | 0.16 |
| Serum creatinine [mg/dl] | [n] Mean (SD) | [65] 1 (0) | [34] 1 (0) | 0.64 |
| High‐sensitivity troponin T, μg/ml | [n] Median (Q1; Q3) | [28] 0 (0; 0) | [14] 0 (0; 0) | 0.77 |
| NT‐proBNP, ng/L | [n] Median (Q1; Q3) | [14] 144 (74; 766) | [7] 298 (99; 717) | 0.65 |
| Hyperinflammation | ||||
| No | n/N (%) | 37/57 (64.9%) | 27/33 (81.8%) | 0.09 |
| Yes | n/N (%) | 20/57 (35.1%) | 6/33 (18.2%) | |
| D‐dimer >1000 μg/L at baseline | ||||
| No | n/N (%) | 40/60 (66.7%) | 25/31 (80.7%) | 0.22 |
| Yes | n/N (%) | 20/60 (33.3%) | 6/31 (19.4%) | |
| In‐hospital support | N | 67 | 35 | |
| Oxygen | n/N (%) | 64/67 (95.5%) | 33/35 (94.3%) | 1.00 |
| High‐flow oxygen | n/N (%) | 33/63 (52.4%) | 13/33 (39.4%) | 0.28 |
| Noninvasive ventilation | n/N (%) | 13/63 (20.6%) | 7/33 (21.2%) | 1.00 |
| Invasive ventilation | n/N (%) | 16/63 (25.4%) | 4/33 (12.1%) | 0.19 |
| Prone ventilation | n/N (%) | 14/63 (22.2%) | 4/33 (12.1%) | 0.28 |
| ECMO | n/N (%) | 1/67 (1.5%) | 2/35 (5.7%) | 0.27 |
| Inhaled nitric oxide | n/N (%) | 3/67 (4.5%) | 2/35 (5.7%) | 1.00 |
| Dialysis | n/N (%) | 1/67 (1.5%) | 0/35 (0.0%) | 1.00 |
| In‐hospital medical treatment | N | 67 | 35 | |
| Hydroxychloroquine | n/N (%) | 0/67 (0.0%) | 0/35 (0.0%) | |
| Favipiravir | n/N (%) | 0/67 (0.0%) | 0/35 (0.0%) | |
| Remdesivir | n/N (%) | 5/67 (7.5%) | 7/35 (20.0%) | 0.10 |
| Lopinavir/ritonavir | n/N (%) | 0/67 (0.0%) | 0/35 (0.0%) | |
| Other antivirals | n/N (%) | 1/67 (1.5%) | 1/35 (2.9%) | 1.00 |
| Tocilizumab | n/N (%) | 1/67 (1.5%) | 0/35 (0.0%) | 1.00 |
| Antibiotics | n/N (%) | 36/67 (53.7%) | 22/35 (62.9%) | 0.41 |
| Antifungal treatment | n/N (%) | 5/67 (7.5%) | 4/35 (11.4%) | 0.49 |
| Systemic corticosteroids | n/N (%) | 54/67 (80.6%) | 26/35 (74.3%) | 0.46 |
Abbreviations: COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; ECMO, extracorporeal membrane oxygenation; WBC, white blood cell.
3.2. Efficacy outcomes
There was no effect of the intervention on the primary outcome of time to sustained clinical improvement or hospital discharge (Figure 3A). Additionally, there were no significant differences between intervention and SOC in secondary clinical outcomes (Table 2A and Figure 3B). During hospitalization, one of three patients were admitted to the ICU (29% SOC vs. 42% intervention; HR 1.56 [CI 0.79‐3.06], p = 0.20) and 20% of patients needed invasive ventilation (11% SOC vs. 24% intervention; HR 2.35 [CI 0.79‐6.96], p = 0.12). Table 2B shows the evolution of secondary biochemical outcomes. Of note, after a significant reduction of D‐dimer levels at day 3 (estimated treatment ratio 0.43 [CI 0.30‐0.61], p < 0.01), D‐dimer levels rose again at days 6 and 15 without a significant difference between the two groups. There was no sustained effect on the biochemical outcomes D‐dimer and C‐reactive protein (Table 2B).
FIGURE 3.
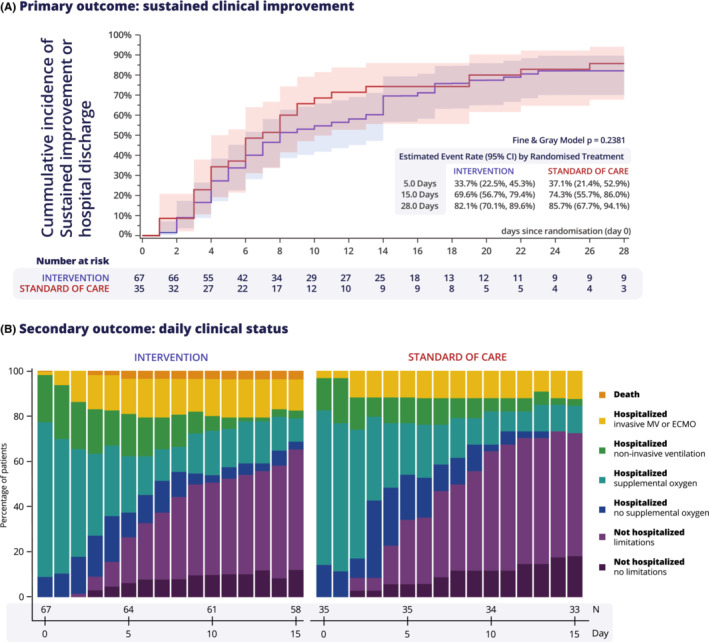
Outcomes. (A) primary outcome of time to sustained 2‐point improvement on 7‐point World Health Organization scale or hospital discharge. (B) Secondary outcome of daily clinical status. ECMO, extracorporeal membrane oxygenation; MV, mechanical ventilation.
TABLE 2.
Secondary outcomes
| Secondary outcome up to day 28 | Statistic | Estimate (95% CI) | Treatment effect | Estimate (95% CI) | |
|---|---|---|---|---|---|
| Intervention (N = 67) | SOC (N = 35) | ||||
| A. Clinical secondary outcome | |||||
| All‐cause mortality | [n] KM [%] | [3] 4.6 (1.5; 13.7) | [2] 5.7 (1.5; 21.0) | Hazard ratio | 0.82 (0.14‐4.94) |
| Mechanical ventilation | [n] CIF [%] | [16] 24.0 (14.5; 34.9) | [4] 11.4 (3.5; 24.4) | Subdist. HR | 2.35 (0.79‐6.96) |
| ICU admission | [n] CIF [%] | [28] 42.2 (30.1; 53.8) | [10] 28.6 (14.7; 44.1) | Subdist. HR | 1.56 (0.79‐3.06) |
| Supplemental oxygen | [n] CIF [%] | [61] 91.0 (80.9; 95.9) | [31] 88.6 (72.0; 95.6) | Subdist. HR | 1.02 (0.88‐1.18) |
| Hospital discharge | [n] CIF [%] | [52] 80.9 (68.5; 88.8) | [31] 88.6 (70.2; 95.9) | Subdist. HR | 0.72 (0.47‐1.09) |
| Limitations on daily activities on day 28 | n/N (%) | 34/47 (72) | 19/30 (63) | ||
| B. Biochemical secondary outcome | |||||
| C‐reactive protein, mg/L | |||||
| Admission | Geometric mean | 63.6 (51.3; 78.7) | 66.79 (45.4; 98.2) | Treatment ratio | |
| Day 3 | Geometric mean | 27.8 (20.3; 38.1) | 27.45 (16.0; 47.0) | Treatment ratio | 1.08 (0.61‐1.92) |
| Day 6 | Geometric mean | 18.2 (12.3; 26.8) | 14.7 (7.9; 27.4) | Treatment ratio | 1.26 (0.63‐2.52) |
| Day 15 | Geometric mean | 7.9 (3.8; 16.3) | 7.1 (3.4; 15.0) | Treatment ratio | 1.13 (0.40‐3.25) |
| D‐dimer, μg/L | |||||
| Admission | Geometric mean | 832.7 (701.7; 988.0) | 686.0 (537.8; 875.0) | Treatment ratio | |
| Day 3 | Geometric mean | 432.3 (366.0; 510.6) | 909.9 (652.7; 1268.5) | Treatment ratio | 0.43 (0.30‐0.61) |
| Day 6 | Geometric mean | 792.3 (641.4; 978.8) | 1015.6 (699.5; 1474.4) | Treatment ratio | 0.70 (0.48‐1.01) |
| Day 15 | Geometric mean | 980.6 (727.3; 1322.1) | 1478.3 (884.9; 2469.7) | Treatment ratio | 0.60 (0.32‐1.09) |
Note: Hazard ratios were obtained using a Cox regression including factors for randomized treatment, study period, and site. Subdistribution hazard ratios were obtained using a Fine & Gray regression model (accounting for competing risk) including factors for randomized treatment, study period and site. Ratios of geometric means between treatments were obtained using a general linear model including the baseline value as a covariate and factors for randomized treatment, study period and site, after log‐transformation of the data; estimated means and treatment differences obtained using the model were back‐transformed using the exponential function.
Abbreviations: CI, confidence interval; 95% CI calculated using log(−log)‐transformation; CIF, incidence estimated using Cumulative Incidence Function accounting for competing risk; HR, hazard ratio; ICU, intensive care unit; KM, incidence estimated using Kaplan–Meier methodology; SOC, standard of care; Subdist. HR, subdistribution hazard ratio.
3.3. Safety outcomes
There was one thrombotic event in the SOC group (not further specified) and one in the intervention group (pulmonary embolism). There was no major bleeding in either study groups. There was no difference in study‐related adverse events (Table 3).
TABLE 3.
Thrombosis, major bleeding, and treatment related serious adverse event
| Statistic | Actual treatment | |||
|---|---|---|---|---|
| Intervention | Standard of care | Total | ||
| Total number of subjects | N | 67 | 35 | 102 |
| Thrombosis | n/N (%) | 1/67 (1.5%) | 1/35 (2.9%) | 2/102 (2.0%) |
| Pulmonary embolism | n/N (%) | 1/67 (1.5%) | 0/35 (0%) | 1/102 (1.0%) |
| Deep vein thrombosis | n/N (%) | 0/67 (0%) | 0/35 (0%) | 0/102 (0%) |
| Other thrombotic events | n/N (%) | 0/67 (0%) | 1/35 (2.9%) | 1/102 (1.0%) |
| Major bleeding | n/N (%) | 0/67 (0%) | 0/35 (0%) | 0/102 (0%) |
| Treatment‐related serious adverse event | ||||
| Hematuria | n/N (%) | 1 (1.5%) | 0 (0.0%) | 1 (1.0%) |
Note: Major bleeds are any in‐hospital bleeds that require a blood transfusion.
4. DISCUSSION
In hospitalized patients with moderate to severe COVID‐19, a multitarget strategy to modulate thromboinflammation with high‐dose IV aprotinin, intensified weight‐ and severity‐dosed LMWH with or without anakinra, did not show signs of safety issues, but did not improve outcome. Overall mortality was low.
Some aspects of our trial require comment. First, after analysis of 102 randomized patients, early termination was recommended by the DSMB because futility, as stated in the Methods. Second, despite the disease severity of the study population with considerable baseline activation of coagulation and inflammation, overall mortality was low in both SOC and intervention. Sustained clinical improvement by 2 points on the 7‐point World Health Organization ordinal scale was obtained in 74% of control patients at day 15, which is more than the anticipated 40% used in the power calculation; this may reflect improved care for COVID‐19 patients. It therefore becomes increasingly difficult for clinical trials to demonstrate significant improvement of care with new treatment strategies. Third, despite randomization, there are some differences in baseline demographics. Note that patients in the intervention group had numerically but not significantly elevated D‐dimer and hyperinflammation, potentially reflecting a more severely ill intervention group or the higher portion of chronic steroid use at baseline. Despite these trends, clinical outcome did not differ. Fourth, in the intervention group, more intravenous fluids were administrated, which might result in cardiopulmonary deterioration in certain precarious patients. This could explain the (nonsignificant) trend of the intervention curves starting around day 4, when looking at daily clinical status and respiratory support. Fifth, after introducing an intensified COVID‐19 thromboprophylaxis, incidence of venous thromboembolism was low. 14 This antithrombotic, but possibly also anti‐inflammatory, effect of LMWH might have helped suppress thromboinflammation. Nevertheless, we observed a significant decrease of D‐dimer at day 3, probably reflecting an aprotinin effect.
4.1. Aprotinin
Other studies have targeted the thromboinflammatory response. Regarding aprotinin, a noncomparative clinical trial investigated the therapeutic in vivo antiviral effects of aprotinin in COVID‐19. 16 In addition to SOC and thromboprophylaxis with 40 mg enoxaparin, patients without (noninvasive) mechanical ventilation were included in three cohorts with (1) low‐dose IV aprotinin (106 KIU) plus hydroxychloroquine, (2) nasal aprotinin (625 KIU four times a day) plus hydroxychloroquine, or (3) low‐dose IV aprotinin (106 KIU) plus avifavir, with the latter being the most effective in the combined primary outcome of normalization of polymerase chain reaction, D‐dimer and C‐reactive protein. The difference in primary outcome could reflect an avifavir effect compared with hydroxychloroquine in the other cohorts. In a phase III randomized trial with 60 patients with mild COVID‐19, nebulized aprotinin seemed to decrease admission time compared with placebo. 17
4.2. Low molecular weight heparin
COVID‐19 is associated with a high incidence of subclinical and symptomatic venous thromboembolism in hospitalized patients despite prophylactic doses of LMWH. 18 Thrombosis and coagulation markers are associated with worse clinical outcomes and intermediate or therapeutic doses of LMWH have been suggested early in the pandemic to improve outcome. 1 , 2 , 11 , 12 Although many (randomized) studies have focused on identifying the optimal dose of LMWH, in‐hospital thromboprophylaxis is still highly debatable. The LMWH dose used to treat the patients in the SOC group was based on the guidance published by the Belgian Society on Thrombosis and Haemostasis. 13 Ultimately, the dose of LMWH in the intervention group is higher, although not therapeutic, compared with the dosages in the guidance document to counteract the antifibrinolytic effect of aprotinin. This study was, therefore, not designed to determine the optimal dose of LMWH in COVID‐19 thromboprophylaxis. However, even in our SOC group (with prophylactic to intermediate‐dosed anticoagulation), the overall mortality is lower compared with the intervention group in both the critically and noncritically ill cohorts of the REMAP‐CAP trials (with therapeutic‐dosed anticoagulation) for example, debating the need of therapeutic dosed thromboprophylaxis in COVID‐19. 19 , 20
4.3. Anakinra
Anakinra showed promising results in specific subpopulations, 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 but in our study, anakinra had no significant effect on clinical or biochemical outcomes in a multitarget approach. However, our trial was designed to evaluate a strategy of modulation of thromboinflammation as a whole and thus lacks the power to evaluate the effect of individual components of the intervention. Based on the studies discussed previously, we believe that anakinra is indeed best investigated early in subgroups with hyperinflammation and biomarkers for clinical progression in COVID‐19.
4.4. Limitations
Our multicenter randomized clinical trial provides insights into a unique multistep multitarget approach in targeting thromboinflammation in COVID‐19. Some limitations are worth mentioning. Because of the state of emergency during the first waves of the pandemic, we opted for an open‐label study design to allow for new treatment options as SOC in the rapidly evolving landscape. Rapidly evolving COVID‐19 care was also noticeable in the primary outcome that was obtained in 74% of control patients at day 15, which is more than the anticipated 40% used in the power calculation. Because of the better‐than‐expected outcome, the power calculation was overly optimistic. Additionally, the study faced early termination because of futility. However, neither the primary nor any relevant clinical or biochemical secondary end point showed any trend toward a benefit of the intervention, making a type II error unlikely. In COVID‐19 treatment trials with improvement and time to improvement as outcome, both cause‐specific and subdistribution hazards are possible approaches to analyze these outcomes. 29 After peer review, we also performed a Cox regression with deaths censored at the time of death. The resulting cause‐specific hazard ratio was 0.782 (CI 0.496‐1.234), p = 0.29. As expected, because of the very low number of deaths and the equal death rates between treatment groups, the cause‐specific hazard ratio is very close to the subdistribution hazard ratio and does not affect our conclusion.
5. CONCLUSION
In hospitalized patients with moderate to severe COVID‐19, a strategy to modulate thromboinflammation with high‐dose aprotinin and LMWH with or without anakinra was associated with an overall low mortality, but did not improve clinical or biochemical outcome compared with standard of care.
AUTHOR CONTRIBUTIONS
T.V., P.V., L.L., J.W., J.G., C.W., C.V., and S.R. conceptualized the study and methodology. T.V. and P.V. acquired funding. T.V., P.V., M.M.E., M.T., T.F., D.M., D.R., and L.H. supervised research activity. M.M.E., Q.V.T., A.B., I.G., A.O., C.D., C.P.M., V.S., J.W., J.G., C.W., C.V., S.R., A.W., P.M., G.V.d.B., D.D., M.T., T.F., D.M., D.R., L.H., I.D., S.T., and B.D.T. all contributed to resources. A.O., C.D., M.M.E., T.V., and P.V. contributed to project administration. A.Be. performed statistical analysis. M.M.E., T.V., and A.Be. visualized the data. M.M.E., T.V., and P.V. drafted the manuscript, and all other authors reviewed the drafted manuscript.
RELATIONSHIP DISCLOSURE
J.G. reports grants from Research Foundation ‐ Flanders, during the conduct of the study. S.R. reports grants from Nordic Pharma, personal fees from Nordic Pharma, during the conduct of the study. T.M. reports grants from Research Foundation Flanders, during the conduct of the study. P.V. reports grants from Nordic Pharma, during the conduct of the study. T.V. reports grants from Research Foundation Flanders (FWO), grants from KU Leuven/UZ Leuven COVID research fund, grants from Nordic Pharma, during the conduct of the study. All other authors report no conflicts of interest within the submitted work.
ETHICAL STATEMENT
Based on the Declaration of Helsinki, the local Ethical Committee (S63979) approved the study protocol and all the participants provided informed consent.
Supporting information
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
ACKNOWLEDGMENTS
This study was funded by Life Sciences Research Partners (LSRP), Research Foundation Flanders (FWO) project G0G4720N and the COVID‐19 fund of the KU Leuven. We are grateful to all patients, their family members, and all health care workers who help to perform clinical studies in extraordinary and challenging times during this COVID‐19 pandemic, and by doing so contribute to fighting SARS‐CoV‐2. We would like to express our gratitude to all collaborators, the clinical trial center of UZ Leuven and all participating centers with their study teams for their collaboration and efforts. A list with all collaborators of the DAWn consortium is provided as in Appendix S2. We want to thank the following DAWn collaborators personally: Eric Van Wijngaerden, Wim Janssens, Geert Meyfroidt, Robin Vos, Timothy Devos, Paul De Munter, Johan Neyts, Lieven Dupont, Isabel Spriet, Geert Verbeke, Kathleen Claes, Wim Robberecht, Chris Van Geet, Barbara Debaveye, Helga Ceunen, Veerle Servaes, Katrien Cludts, Kristine Vanheule, Cato Jacobs, Daimy Roebroek, Paulien Dreesen, Nele Smet, Jan Dolhain, Mieke Hoppenbrouwers, Kathleen Wens, Kristel Daems, Monique D'hondt.
Engelen MM, Van Thillo Q, Betrains A, et al. Modulation of thromboinflammation in hospitalized COVID‐19 patients with aprotinin, low molecular weight heparin, and anakinra: The DAWn‐Antico study. Res Pract Thromb Haemost. 2022;6:e12826. doi: 10.1002/rth2.12826
Handling Editor: Dr Lana Antoinette Castellucci
Contributor Information
Matthias M. Engelen, Email: matthias.engelen@uzleuven.be.
DAWn Consortium Members:
Eric Van Wijngaerden, Wim Janssens, Geert Meyfroidt, Robin Vos, Timothy Devos, Paul De Munter, Johan Neyts, Lieven Dupont, Isabel Spriet, Geert Verbeke, Kathleen Claes, Wim Robberecht, Chris Van Geet, Barbara Debaveye, Helga Ceunen, Veerle Servaes, Katrien Cludts, Kristine Vanheule, Cato Jacobs, Daimy Roebroek, Paulien Dreesen, Nele Smet, Jan Dolhain, Mieke Hoppenbrouwers, Kathleen Wens, Kristel Daems, and Monique D’hondt
DATA AVAILABILITY STATEMENT
Patient level data will not be made available. The study protocol is published and available online (https://doi.org/10.1186/s13063‐020‐04878‐y). Together with the statistical analysis plan, the study protocol will also be attached to the Appendix S2.
REFERENCES
- 1. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Waite AAC, Hamilton DO, Pizzi R, Ageno W, Welters ID. Hypercoagulopathy in severe COVID‐19: implications for acute care. Thromb Haemost. 2020;120(12):1654‐1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vanassche T, Engelen MM, Van Thillo Q, et al. A randomized, open‐label, adaptive, proof‐of‐concept clinical trial of modulation of host thromboinflammatory response in patients with COVID‐19: the DAWn‐Antico study. Trials. 2020;21(1):1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carvalho PR, Sirois P, Fernandes PD. The role of kallikrein‐kinin and renin‐angiotensin systems in COVID‐19 infection. Peptides. 2021;135:170428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pampalakis G, Zingkou E, Panagiotidis C, Sotiropoulou G. Kallikreins emerge as new regulators of viral infections. Cell Mol Life Sci. 2021;78(21–22):6735‐6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meini S, Zanichelli A, Sbrojavacca R, et al. Understanding the pathophysiology of COVID‐19: could the contact system be the key? Front Immunol. 2020;11:2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martens CP, Van Mol P, Wauters J, et al. Dysregulation of the kallikrein‐kinin system in bronchoalveolar lavage fluid of patients with severe COVID‐19. EBioMedicine. 2022;83:104195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bestle D, Heindl MR, Limburg H, et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS‐CoV‐2 in human airway cells. Life Sci Alliance. 2020;3(9):e202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Solun B, Shoenfeld Y. Inhibition of metalloproteinases in therapy for severe lung injury due to COVID‐19. Med Drug Discov. 2020;7:100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Di Castelnuovo AF, Costanzo S, Iacoviello L. Heparin in COVID‐19 patients is associated with reduced in‐hospital mortality: the multicentre Italian CORIST study. Thromb Haemost. 2021;121(8):1054‐1065. [DOI] [PubMed] [Google Scholar]
- 13. Vanassche T, Orlando C, Vandenbosch K, et al. Belgian clinical guidance on anticoagulation management in hospitalised and ambulatory patients with COVID‐19. Acta Clin Belg. 2020;3:1‐6. [DOI] [PubMed] [Google Scholar]
- 14. Engelen MM, Vandenbriele C, Spalart V, et al. Thromboprophylaxis in COVID‐19: weight and severity adjusted intensified dosing. Res Pract Thromb Haemost. 2022;6(3):e12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schulman S, Kaeron C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3(4):692‐694. [DOI] [PubMed] [Google Scholar]
- 16. Ivashchenko AA, Azarova VN, Egorova AN, et al. Effect of Aprotinin and Avifavir(®) combination therapy for moderate COVID‐19 patients. Viruses. 2021;13(7):1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Redondo‐Calvo FJ, Padín JF, Muñoz‐Rodríguez JR, et al. Aprotinin treatment against SARS‐CoV‐2: a randomized phase III study to evaluate the safety and efficacy of a pan‐protease inhibitor for moderate COVID‐19. Eur J Clin Invest. 2022;52(6):e13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaptein FHJ, Stals MAM, Grootenboers M, et al. Incidence of thrombotic complications and overall survival in hospitalized patients with COVID‐19 in the second and first wave. Thromb Res. 2021;199:143‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goligher EC, Bradbury CA, McVerry BJ, et al. Therapeutic anticoagulation with heparin in critically ill patients with COVID‐19. N Engl J Med. 2021;385(9):777‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lawler PR, Goligher EC, Berger JS, et al. Therapeutic anticoagulation with heparin in noncritically ill patients with COVID‐19. N Engl J Med. 2021;385(9):790‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cavalli G, De Luca G, Campochiaro C, et al. Interleukin‐1 blockade with high‐dose anakinra in patients with COVID‐19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2(6):e325‐e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dimopoulos G, de Mast Q, Markou N, et al. Favorable anakinra responses in severe COVID‐19 patients with secondary hemophagocytic lymphohistiocytosis. Cell Host Microbe. 2020;28(1):117‐23.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID‐19: a cohort study. Lancet Rheumatol. 2020;2(7):e393‐e400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Navarro‐Millán I, Sattui SE, Lakhanpal A, Zisa D, Siegel CH, Crow MK. Use of anakinra to prevent mechanical ventilation in severe COVID‐19: a case series. Arthritis Rheumatol. 2020;72(12):1990‐1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kooistra EJ, Waalders NJB, Grondman I, et al. Anakinra treatment in critically ill COVID‐19 patients: a prospective cohort study. Crit Care. 2020;24(1):688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kyriazopoulou E, Poulakou G, Milionis H, et al. Early treatment of COVID‐19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double‐blind, randomized controlled phase 3 trial. Nat Med. 2021;27(10):1752‐1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tharaux P, Pialoux G, Pavot A, et al. Effect of anakinra versus usual care in adults in hospital with COVID‐19 and mild‐to‐moderate pneumonia (CORIMUNO‐ANA‐1): a randomised controlled trial. Lancet Respir Med. 2021;9(3):295‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kyriazopoulou E, Huet T, Cavalli G, et al. Effect of anakinra on mortality in patients with COVID‐19: a systematic review and patient‐level meta‐analysis. Lancet Rheumatol. 2021;3(10):e690‐e697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beyersmann J, Friede T, Schmoor C. Design aspects of COVID‐19 treatment trials: improving probability and time of favorable events. Biom J. 2022;64(3):440‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Data Availability Statement
Patient level data will not be made available. The study protocol is published and available online (https://doi.org/10.1186/s13063‐020‐04878‐y). Together with the statistical analysis plan, the study protocol will also be attached to the Appendix S2.


