Abstract
Neurogranin (Nrgn) is a neural protein that is enriched in the cerebral cortex and is involved in synaptic plasticity via its interaction with calmodulin. Recently we reported its expression in the brain of the adult zebrafish (Alba-González et al. J Comp Neurol 530:1569–1587, 2022). In this study we analyze the development of Nrgn-like immunoreactivity (Nrgn-like-ir) in the brain and sensory structures of zebrafish embryos and larvae, using whole mounts and sections. First Nrgn-like positive neurons appeared by 2 day post-fertilization (dpf) in restricted areas of the brain, mostly in the pallium, epiphysis and hindbrain. Nrgn-like populations increased noticeably by 3 dpf, reaching an adult-like pattern in 6 dpf. Most Nrgn-like positive neurons were observed in the olfactory organ, retina (most ganglion cells, some amacrine and bipolar cells), pallium, lateral hypothalamus, thalamus, optic tectum, torus semicircularis, octavolateralis area, and viscerosensory column. Immunoreactivity was also observed in axonal tracts originating in Nrgn-like neuronal populations, namely, the projection of Nrgn-like immunopositive primary olfactory fibers to olfactory glomeruli, that of Nrgn-like positive pallial cells to the hypothalamus, the Nrgn-like-ir optic nerve to the pretectum and optic tectum, the Nrgn-like immunolabeled lateral hypothalamus to the contralateral region via the horizontal commissure, the octavolateralis area to the midbrain via the lateral lemniscus, and the viscerosensory column to the dorsal isthmus via the secondary gustatory tract. The late expression of Nrgn in zebrafish neurons is probably related to functional maturation of higher brain centers, as reported in the mammalian telencephalon. The analysis of Nrgn expression in the zebrafish brain suggests that it may be a useful marker for specific neuronal circuitries.
Keywords: Neurogranin, RC3, Immunohistochemistry, Brain development, Teleost, Danio rerio
Introduction
Neurogranin (Nrgn; also known as p17, RC3 and BICKS) is a small neural protein (Baudier et al. 1989, 1991; Watson et al. 1990; Coggins et al. 1993; Huang et al. 1993) that seems to regulate synaptic plasticity through its interaction with calmodulin and other proteins (Li et al. 2020; Zhong and Gerges 2020). Initially purified from bovine forebrain (named p17; Baudier et al. 1989, 1991), it was also identified as a cortex-enriched mRNA in rat brain (rat cortex-enriched cDNA clone 3 or RC3; Watson et al. 1990; see also Deloulme et al. 1991). Together with neuromodulin (GAP-43), PEP-19 (purkinje cell protein 4, pcp-4) and Igloo (Neel and Young, 1994; Gerendasy and Sutcliffe 1997; Gerendasy 1999), neurogranin is part of the so-called “calpacitin” family.
Neurogranin seems to regulate synaptic plasticity by favoring long-term potentiation (LTP) over long-term depression (LTD) (Fedorov et al. 1995; Ramakers et al. 1995, 1997; Chen et al. 1997; Pak et al. 2000; Huang et al. 2004; Lee 2006; Zhabotinsky et al. 2006; Zhong et al. 2009, 2011; Zhong and Gerges 2020). Neurogranin interacts with calmodulin through its highly conserved IQ domain (Baudier et al. 1989, 1991; Deloulme et al. 1991; Prichard et al. 1999), which also contains a specific site for protein kinase C (PKC) phosphorylation (Baudier et al. 1989, 1991; Watson et al. 1990; Deloulme et al. 1991; Huang et al. 1993; Paudel et al. 1993; Gerendasy et al. 1994) and interaction with phosphatidic acid (Domínguez-González et al. 2007). When Ca2+ levels reach a certain threshold inside the cell, Nrgn is phosphorylated and releases calmodulin, which can then interact with other proteins (Baudier et al. 1989, 1991; Watson et al. 1990; Deloulme et al. 1991; Gerendasy et al. 1994; Gerendasy and Sutcliffe 1997; Lee 2006; Li et al. 2020). The phosphorylated form of Nrgn may also have down-stream targets to be fully determined yet, such as the calmodulin dependent nitric oxide synthase (Martzen and Slemmon 1995) or G-protein coupled second messengers (Cohen et al. 1993; Watson et al. 1996).
Given Nrgn function in synaptogenesis and synaptic plasticity, it is not surprising that it has been related to various human neurological diseases and disorders, which include Alzheimer disease (Chang et al. 1997; Hellwig et al. 2015; Bereczki et al. 2016; Casaletto et al. 2017; Lista and Hampel 2017; Kvartsberg et al. 2019), Parkinson and Parkinsonian disorders (Koob et al. 2014; Selnes et al. 2017), schizophrenia (Giegling et al. 2010; Van Winkel et al. 2010; Gurung and Prata 2015; Wen et al. 2016; Zhang et al. 2019; Jin et al. 2019) and Huntington’s disease (DiFiglia 1990). In fact, Nrgn is used as a CSF biomarker for synapsis loss in Alzheimer disease (Lashley et al. 2018; Blennow and Zetterberg 2018a, b), and could be a marker for other diseases and pathological states (Yang et al. 2015; Bereczki et al. 2017). In addition, sleep deprivation has shown to decrease Nrgn levels (Rhyner et al. 1990; Neuner-Jehle et al. 1995), which again could indicate a role of Nrgn in synaptic plasticity.
It is also very likely that Nrgn is crucial in the development of certain areas and circuits of the brain. Neurogranin genomic region contains regulatory elements for retinoic acid and steroid hormone receptors (Iñiguez et al. 1994; Enderlin et al. 1997; Husson et al. 2003, 2004; Féart et al. 2005; Buaud et al. 2010), as well as binding domains for different transcription factors (Iñiguez et al. 1994; Martínez de Arrieta et al. 1997; Sato et al. 1995). Several studies have suggested a cell specific regulation of Nrgn expression by thyroid hormones (Muñoz et al. 1991; Iñiguez et al. 1992, 1993, 1996), likely through thyroid responsive elements within the Nrgn first intron (Martínez de Arrieta et al. 1999; Morte et al. 1999).
Despite most likely having key roles during brain development, only a couple of studies have analyzed Nrgn distribution during development in the rat (Gerendasy et al. 1994; Álvarez-Bolado et al. 1996) and the mouse olfactory bulb (Gribaudo et al. 2012). In the adult, Nrgn brain distribution was studied in the rat (Represa et al. 1990; Watson et al. 1990, 1992; Neuner-Jehle et al. 1996; Houben et al. 2000; Singec et al. 2003), mouse (Singec et al. 2003), three species of monkey (Cercopithecus aetiops by Singec et al. 2003, and Macaca fascicularis and M. nemestrina by Guadaño-Ferraz et al. 2005), adult zebra finches (Clayton et al. 2009) and recently in the adult zebrafish (Alba-González et al. 2022). In adult zebrafish, we previously showed by Western blot of brain protein extracts the presence of three Nrgn-immunoreactive peptide bands with MW corresponding to those of peptides in mouse brain extracts, validating this antibody for zebrafish brain studies (Alba-González et al. 2022). These three proteins are coded in zebrafish by two paralog neurogranin genes, nrgna and nrgnb, but distinction of cells expressing one or other of these was not studied and thus the immunoreactivity was named as neurogranin-like. The study of Nrgn-like expression along development (present results) in comparison with those of the adult stage (Alba-González et al. 2022) provides new neuroanatomical data for a more precise topological location of nuclei and tracts in early postembryonic stages in zebrafish. In addition, given the growing use of zebrafish as a model in neurobiology and the availability of tools in this species (Key and Devine 2003; Friedrich et al. 2010; Wyatt et al. 2015; Adams and Kafaligonul 2018; Vanwalleghem et al. 2018; Bao et al. 2019; Zakowski 2020), we believe our study also sets the basis for future work using zebrafish to tackle the Nrgn roles in health and disease.
Materials and methods
Animal maintenance and embryo collection
Wild-type zebrafish adults (Danio rerio) were kept in aquaria under standard conditions of 14/10 h light/dark periods, 28.0 ± 1.0 °C, pH 7.0 ± 1.0. Water quality was monitored weekly and kept within recommended parameters (0–50 mg/L nitrate, < 1 mg/L nitrite, and < 0.2 mg/L ammonium) (see Aleström et al. 2019). Adults were fed with a mixture of decapsuled Artemia salina and commercial dry flake food twice a day.
For obtaining embryos and larvae, adults were transferred to mating tanks in a 2:1 ratio (female: male). The next morning, fertilized eggs were collected in Petri dishes and maintained at 28.0 ± 1.0 °C in an incubator until their use.
Neurogranin immunocytochemistry
Samples analyzed
Various embryonic and larval stages were analyzed, these included 1 day post-fertilization (dpf), 2 dpf, 3 dpf, 5 dpf, 5.5 dpf, 6 dpf, 16 dpf [L1 stage following Singleman and Holtzman 2014] and 21 dpf [advanced L1, Singleman and Holtzman 2014] stages.
Embryos and larvae were euthanized by tricaine methanesulfonate (MS222; Sigma, St. Louis, MO) overdose and fixed by immersion in 4% paraformaldehyde (PFA) in 0.1 M pH 7.4 phosphate buffer (PB) at room temperature. After being rinsed in saline PB (PBS), fish were transferred to PBS and kept at 4 °C until use.
Whole-mount immunocytochemistry
The protocol used for whole-mount immunocytochemistry in embryos and larvae was that described by Turner et al. (2014). In brief, embryos and larvae were dehydrated in 50% methanol and stored in 100% methanol at – 20 °C for at least 30 min. Samples were then rehydrated, washed 3 times (10 min each) in 0.5% Triton-X-100 in 0.1 M PBS (PBST; pH 7.4) and permeabilized with Proteinase K (Sigma-Aldrich, P2308). Then, to prevent non-specific antibody binding sites, fish were incubated with a blocking solution of 10% normal goat serum (NGS; Sigma Aldrich, G6767-19B409) in 0.5% PBST with 1% dimethyl sulfoxide (DMSO) for 1 h, and then with the primary antibodies solution (Nrgn: Rabbit Anti-Neurogranin Polyclonal Antibody; Chemicon, AB5620, Lot #3,091,673, 1:500 dilution; SV2: Mouse Anti-synaptic vesicle protein 2; DSHB AB2315387, 1:250 dilution) overnight at 4 °C. Then, fish were washed in PBST (4 times, 30 min each) and incubated with appropriate secondary antibodies (Goat Anti Rabbit IgG-Alexa Fluor 488, Invitrogen, A11008 for Nrgn immunohistochemistry and Goat Anti Rabbit IgG-Alexa Fluor 568, Invitrogen, A1104 for SV2; 1:500 dilutions) at room temperature for 1 h. After two washes in PBST (30 min each), fish immunoreacted against Nrgn antibody were counterstained with Sytox Orange Nucleic Acid Stain (Invitrogen, S11368, 1:104 dilution) for 7 min at room temperature. After two washes in PBST (30 min each), fish were maintained in 50% glycerol in PB and stored at 4 °C. For imaging, embryos and larvae were transferred to 80% glycerol (30 min) and mounted in 1% low melting point agarose in 80% glycerol.
Immunocytochemistry in cryosections
Whole larvae (6 dpf) were kept on 30% sucrose in PB overnight at 4 °C. The next day, larvae were embedded in Tissue-Tek mounted media (Cell Path, KMA-0100-00A), frozen in methylbutane cooled in liquid nitrogen. Next, transverse sections (12–14 µm thick) were obtained using a cryostat (MICROM; HM 500 M) and collected in gelatin-coated slides. To remove autofluorescence, sections were incubated in 0.2% sodium borohydride in PBS (30 min). Sections were preincubated with normal goat serum (1 h) and then incubated with the primary antibody solution as indicated above for whole-mount immunocytochemistry (4 °C; overnight). Then, sections were washed four times in PBST (15 min each) and incubated with Goat Anti-Rabbit IgG coupled to Alexa Fluor 488 (Sigma Aldrich, A11008, 1:500 dilution) at room temperature for 1 h. After two washes with PBST (10 min each), slides were mounted using 50% glycerol in PB and maintained at 4 °C in darkness until observation.
Imaging
Embryos and larvae were imaged using a laser scanning confocal microscope Nikon A1R equipped with Nikon Plan Fluor 10x (0.30 NA) and 20x (0.50 NA) objectives. An argon ion laser (488 nm) and a diode laser (561 nm) provided the excitation light for the fluorophores. Emission light was sequentially acquired for each channel. Confocal z-stacks were processed and analyzed using Fiji software (Schindelin et al. 2012). Red channel is shown as magenta in the figures. Sections of zebrafish larvae (6 dpf) were imaged using an Epifluorescence microscope (Nikon Eclipse 90i) coupled to an Olympus DP71 digital camera.
Results
Neurogranin distribution in the embryo and larva
We investigated Nrgn-like immunoreactive (Nrgn-like-ir) structures at various stages of embryonic (1–3 dpf) and postembryonic/ larval (5, 6, 16 and 21 dpf) development of zebrafish.
Neurogranin expression in the embryo
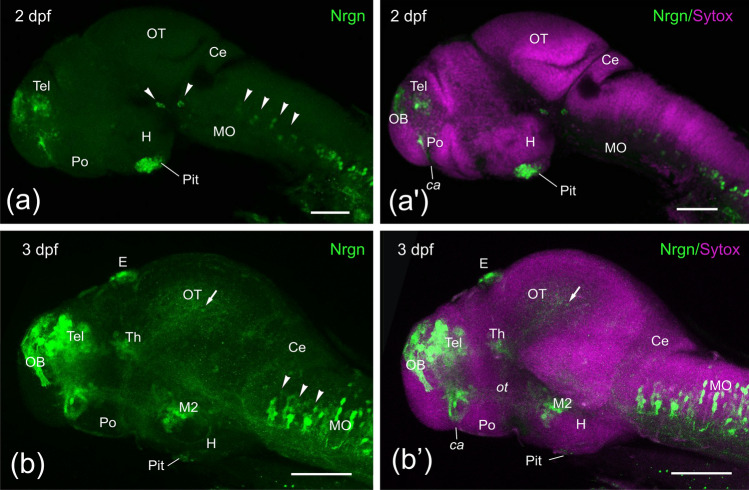
No Nrgn immunoreactivity was observed in the central nervous system at 1 dpf embryos. The first Nrgn-like-ir structures appeared at 2 dpf, showing the first immunoreactive cell bodies in the pallium, the epiphyseal cluster (not shown) and the hypophysis (Fig. 1a, a’). A few sparsely distributed Nrgn-like-ir cell bodies were also observed in the preoptic region close to the anterior commissure. In addition, in the prosencephalon, immunoreactive fibers were observed in the olfactory bulb (glomeruli) and the anterior commissure. Two small compact groups of Nrgn-like-ir cell bodies were observed in the ventral region (basal plate) of the mesencephalon and the isthmus, which could represent the IIIrd (oculomotor) and IVth (trochlear) motor nuclei, respectively (see discussion). In addition, many Nrgn-like-ir cell bodies and fibers were located along the medulla oblongata and spinal cord, with some hindbrain neurons forming discrete groups in a segmental pattern (Fig. 1a, a’).
Fig. 1.
Side view of confocal projections from 2 (a, a’) and 3 (b, b’) dpf zebrafish embryos showing Neurogranin-like (Nrgn-like) immunoreaction (in green) and counterstained with Sytox Orange Nucleic Acid (in magenta). Arrowheads point to mesencephalic and rhombencephalic positive cell groups. A faintly labeled cell was also pointed in the optic tectum (arrow). Rostral is to the left and dorsal to the top. For abbreviations, see the list. Scale bars, 100 µm
By 3 dpf, in addition to Nrgn-like-ir fibers in the olfactory glomeruli, we observed a few large Nrgn-like-ir cell bodies in the olfactory bulbs. We also observed strong immunoreactivity in cell bodies in the pallium, subpallium and preoptic region close to the anterior commissure (Fig. 1b, b’). More caudally, in addition to the expression in epiphyseal and hypophyseal cell bodies, Nrgn-like expression was also seen in the tubercular area (M2 of Mueller and Wullimann 2003) and faint Nrgn-like expression in cell bodies of the thalamus and optic tectum. Although no new immunoreactive cell groups were seen in the mesencephalic tegmentum and isthmus, an increasing number of Nrgn-like-ir cell bodies were observed in the rhombencephalic tegmentum (Fig. 1b, b’). Strongly immunostained fibers were also seen coursing the olfactory tract and the anterior commissure, while faintly labeled fibers could be observed in the supraoptic tract/ forebrain bundle (see Wilson et al. 1990; Chitnis and Kuwada 1990), the optic tract and the ventral longitudinal tract through the medulla and rostral spinal cord (Fig. 1b, b’). Only scarce tectal cell bodies showed faint Nrgn-like immunoreactivity.
Neurogranin expression in larvae
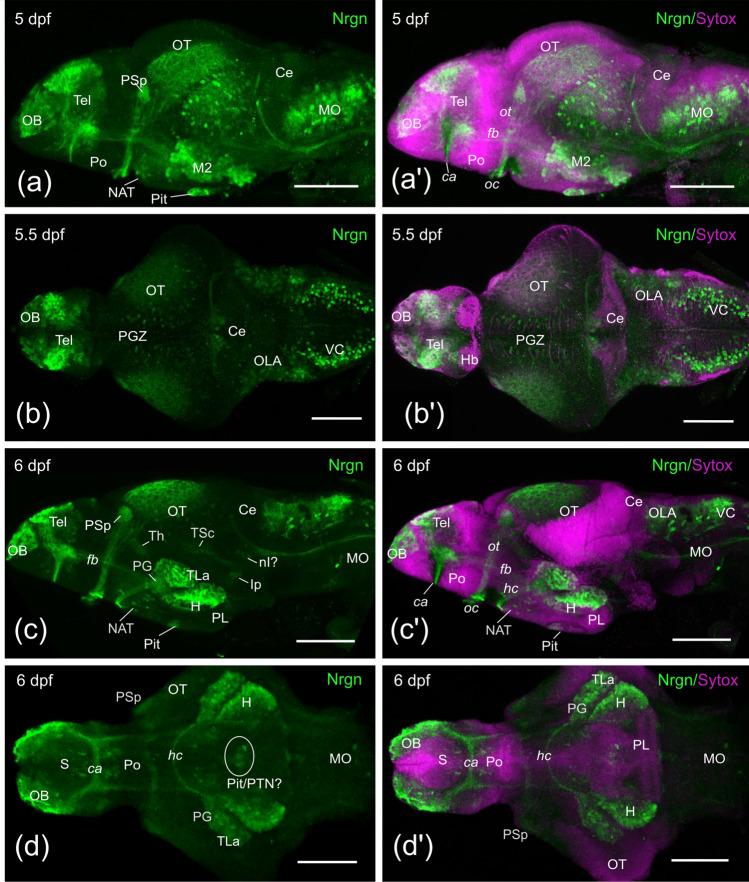
By 5 dpf, in addition to the Nrgn-like-ir structures described previously in embryos, Nrgn-like-ir cell bodies appeared in the tuberal area, hypothalamus and in the cerebellum, the later likely representing Purkinje cells of the cerebellar valvula. A significant increase in the number of Nrgn-like-ir cell bodies was noticed in the optic tectum, in the pallium and in M2 (Fig. 2a, a’, b, b’).
Fig. 2.
Side (a, a’, c, c’) dorsal (b, b’) and ventral (d, d’) confocal projections of zebrafish brains from 5 (a), 5.5 (b) and 6 (c, d) dpf larvae showing Neurogranin-like (Nrgn) immunoreaction of zebrafish brain (in green) and counterstained with Sytox Orange Nucleic Acid (in magenta). Rostral is to the left. For abbreviations, see the list. Scale bars, 100 µm
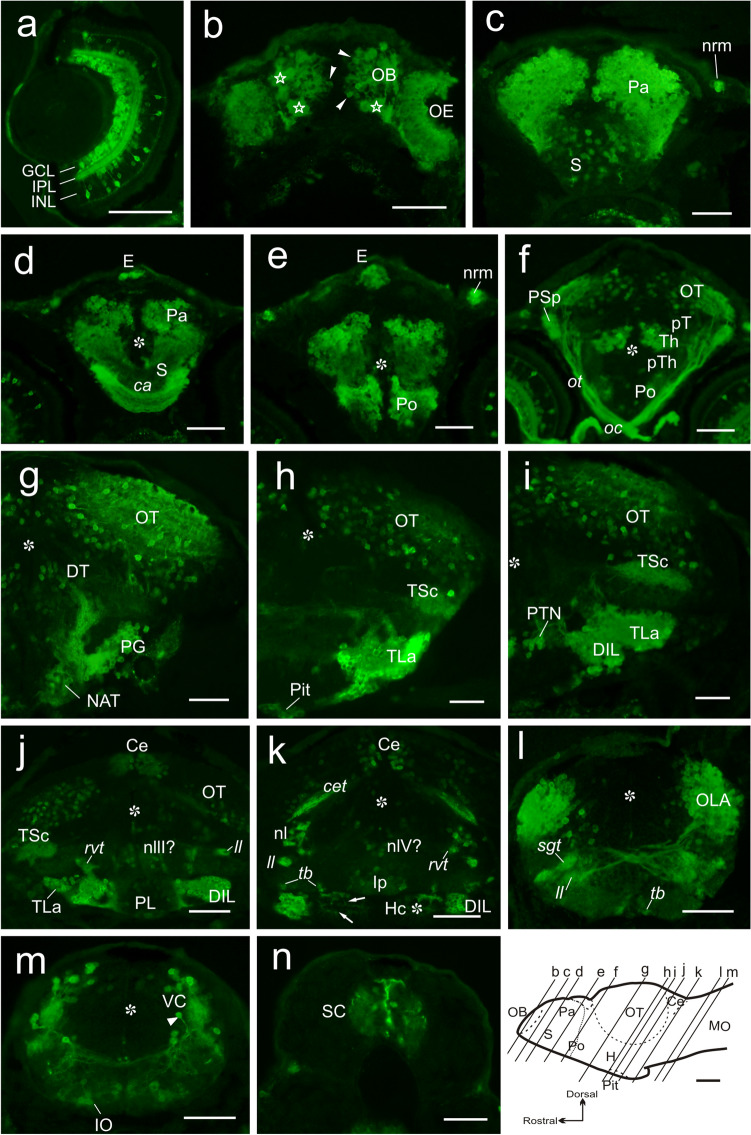
At 6 dpf, Nrgn-like immunoreactivity was studied both in whole-mount brain and in transverse sections from non-dissected larvae for better neuroanatomical characterization. We observed Nrgn-like immunoreactivity in the central nervous system and sensory organs. Well-developed Nrgn-like-ir cell bodies were observed in developing sensory organs as the retina, the olfactory epithelium, cranial neuromasts (Fig. 3a, c, e) and inner ear (hair cells; not shown). In the retina, Nrgn-like immunoreactivity was noticed in several populations, namely, some bipolar and amacrine cells and in most, if not all, ganglion cells. Nrgn-like-ir processes of these cells were also observed forming organized strata in the inner nuclear layer (INL) and coursing in the optic nerve and tract (Figs. 2c, c’, 3a). In the brain, we could observe a number of Nrgn-like-ir fibers in the olfactory glomeruli coming from Nrgn-like-ir receptor cells in the olfactory rosette. Double immunostaining against Nrgn and the synaptic marker SV2 confirmed that all olfactory glomeruli received Nrgn-like-ir fibers (not shown). Fibers both in the olfactory nerve and bulb were strongly labeled (Figs. 2c, c’, 3b). In the telencephalic lobes, we observed increased numbers of intensely labeled Nrgn-like-ir cell bodies in the pallium, the precommissural subpallium and the preoptic area (Fig. 3c–f). Periventricular cells of the preoptic area send projections to the ventrolateral margin and course caudally (Fig. 3e). Caudal to the anterior commissure, a large group of intensely immunolabeled cell bodies was also observed. Nrgn-like-ir cell bodies were also observed in the epiphysis, dorsal thalamus, posterior tubercle (anterior and posterior tuberal nuclei), torus lateralis, the inferior hypothalamic lobes, pituitary (probably adenohypophysis) and, more faintly stained, in the preglomerular complex and the caudal hypothalamic lobes (Figs. 2c, c’, 3d–k). Faintly immunolabeled cell bodies were also seen in the posterior lobe (Hc), showing CSF-contacting morphology around the posterior recess (Fig. 3k). Compared with previous stages, the number of Nrgn-like-ir fibers increased both in the posterior tubercle and hypothalamus. As in previous stages, Nrgn-like-ir fibers were observed in the optic tract, coursing to a conspicuous neuropil area in the pretectum (likely to be the parvocellular superficial pretectal nucleus) and also entering the optic tectum (Figs. 2c, c’, 3f). In the alar mesencephalon, the number of Nrgn-like-ir cell bodies and fibers increased in the optic tectum. Some Nrgn-like-ir cell bodies, together with fibers likely originated from the lateral lemniscus, were also observed in the torus semicircularis (Fig. 3h–j). In the mesencephalic tegmentum, Nrgn-like-ir cell bodies were observed medially close to the ventricle (Fig. 3j). In the rostral rhombencephalon, immunoreactive cell bodies were observed in the cerebellar valvula (Fig. 3h–k), and in the medial and lateral regions of the isthmic tegmentum, including the interpeduncular nucleus (Fig. 3k). Caudally, a number of Nrgn-like-ir cell bodies were observed in the octavolateralis area, the primary viscerosensory column, the reticular formation and the inferior olive (Fig. 3l–m). Some Nrgn-like-ir cell bodies were also observed in the spinal cord (Fig. 3n). In addition to the labeled fibers and cell bodies described above, we observed Nrgn-like-ir fibers in several tracts and commissures: in the anterior and horizontal commissures and in the olfactory, telencephalic, optic, tectobulbar, cerebellar and secondary gustatory/visceral tracts (Fig. 2c, c’), most of them already present in previous stages (Figs. 2, 3).
Fig. 3.
a–n Photomicrographs of transverse sections showing Nrgn-like immunoreaction (in green) in the retina (a), brain (b–m) and spinal cord (n) of a 6 dpf zebrafish larvae. Section levels are indicated in the longitudinal schema of the brain at the bottom. In b, outlined stars show the olfactory glomeruli. Note in j the slightly mismatch between both sides of the brain at mesencephalic level. Arrows in k point to the CSF-contacting cell processes directed towards the posterior recess of the hypothalamus. Asterisk: ventricle. For abbreviations, see the list. Scale bars, 200 µm
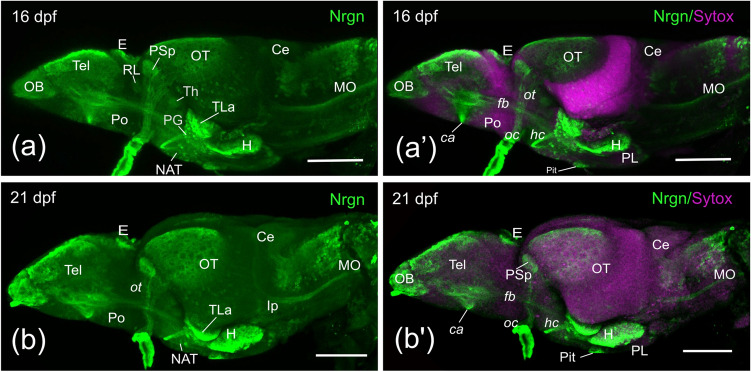
Finally, we analyzed expression by 16 and 21 dpf in whole-mount (Fig. 4a-a’, b-b’). We observed little qualitative differences in Nrgn-like immunoreactivity compared to 6 dpf larvae. Noteworthy, the region of the pallium with cells with intense Nrgn-like expression was broader than in previous stages.
Fig. 4.
Side view of confocal projections from 16 (a, a’) and 21 (b, b’) dpf zebrafish brains showing Nrgn-like immunoreaction (in green) and counterstained with Sytox Orange Nucleic Acid (in magenta). Rostral is to the left and dorsal to the top. For abbreviations, see the list. Scale bars, 100 µm
Discussion
This study reports the appearance and changes in distribution of Nrgn-like immunoreactivity in the brain and sensory organs of zebrafish during development. Nrgn-like immunoreactivity is late appearing, since its expression starts by 2 dpf in very restricted areas of the brain but increases noticeably from 3 to 6 dpf. By 5 dpf-6 dpf, the regional expression of Nrgn-like peptides resembles that observed in the adult (Alba-González et al. 2022). We observed expression in cell bodies and fibers of specific regions of the forebrain, midbrain and hindbrain. These results expand considerably the neuronal distribution reported previously by nrgna mRNA in situ hybridization (Zada et al. 2014). Based on the location of the Nrgn-like-ir cells, away from the ventricular zone, it seems probable that they correspond to differentiated neurons, which would agree with observations in the cerebral cortex of rat (Represa et al. 1990; Houben et al. 2000).
Nrgn shares important biochemical similarities with other members of the calpacitin family, such as neuromodulin (Coggins et al. 1993; Gerendasy and Sutcliffe 1997) and pcp-4 (Mione et al. 2006). They share an IQ domain and, at least in mammals, they are substrates for PKC phosphorylation (Baudier et al. 1991; Deloulme et al. 1991; Watson et al. 1992; Huang et al. 2000; Kumar et al. 2013, Alba-González et al. 2022). While Nrgn seems to be mainly postsynaptic in mammals (Represa et al. 1990; Coggins et al. 1993; Watson et al. 1994; Neuner-Jehle et al. 1996), neuromodulin seems to be presynaptic and located in axons (Snipes et al. 1987; McGuire et al. 1988; Gerendasy and Sutcliffe 1997). It is noteworthy that Nrgn appears to be a marker for specific cell populations, allowing to track these populations during development (present results), as it is also the case for pcp-4, another member of the calpacitin family (Mione et al. 2006). We have observed Nrgn-like expression throughout the brain and sensory organs, which suggests that in zebrafish Nrgn-like peptides could be both pre- and post-synaptic, as shown in the rat spinal cord (Houben et al. 2000). It would be necessary a detailed study of the different Nrgn-like peptides to confirm how they relate to the synapse and their relation to neuromodulin. Below, we will discuss the main findings in specific brain and sensory systems populations.
Olfactory system. Present results reveal Nrgn-like expression in cells of the olfactory epithelium, as well as in the olfactory nerve and terminal fields (glomeruli) in the olfactory bulb, i.e., in the primary olfactory neurons. However, the neurons of the olfactory bulb (mitral cells, granule cells), as well as the olfactory tracts, appear to be negative in embryos/larvae, suggesting that Nrgn is presynaptic in zebrafish primary olfactory fibers. Some Nrgn-like-ir olfactory bulb neurons (likely to represent granule cells) were also observed in adults (Alba-González et al. 2022). Results in zebrafish larvae differ from those reported by Gribaudo et al. (2012) in the olfactory bulbs of developing mouse, which lack Nrgn-like immunoreactivity in olfactory fibers, but prominently express it in tufted cells and in granule cells. This suggests that Nrgn is involved in different tasks in olfactory circuits of zebrafish and mouse.
Telencephalon. An interesting result is the strong Nrgn-like expression in cell bodies of the primordial pallium of zebrafish larvae, which agrees with results reported with nrgna in situ hybridization (Zada et al. 2014). Our results of Nrgn-like expression in larval pallium correspond with that observed in some pallium regions of the adult (Alba-González et al. 2022). The larval pallium appears to originate a conspicuous Nrgn-like-ir forebrain tract that is recognizable in whole mount stained brains extending toward the hypothalamus–posterior tubercle. In addition, the pallium originates Nrgn-like-ir fibers coursing in the anterior commissure. These projections correspond with those reported in detail with DiI tracing from some pallial regions of the adult zebrafish (Yáñez et al. 2022). However, it was not possible to identify the different adult pallial regions in larvae, which precludes more detailed comparisons. The strong expression of Nrgn found in projection neurons of the zebrafish pallium reminds the distribution of Nrgn in principle neurons of various pallial areas of rodents (Álvarez-Bolado et al. 1996), although projection neurons appear morphologically much more specialized in rodents. The pallium of two oscine birds also expresses high Nrgn mRNA levels (Clayton et al. 2009).
The development of the Nrgn-like expression in zebrafish telencephalon shows some differences with that reported in the developing rat (Álvarez-Bolado et al. 1996). In the rat telencephalon, Nrgn expression starts in the primordium of the amygdala and the piriform cortex at embryonic stage 18, increasing the areas of expression on postnatal week 1, when Nrgn immunoreactivity appears in olfactory cortex, isocortex, subiculum, hippocampus, striatum (caudoputamen) and parts of the globus pallidus and septum (Álvarez-Bolado et al. 1996). In the zebrafish nervous system, Nrgn-like expression is observed from 2 dpf, before the animal hatched from the chorion and is capable of independent feeding (Kimmel et al. 1995; Strähle et al. 2012; Filosa et al. 2016). At this stage, we observe Nrgn-like expression in cell bodies of the pallium, which is similar the situation in the rat.
Visual system. In the retina of developing zebrafish, Nrgn-like is expressed largely in ganglion cells but also in numerous amacrine cells and some bipolar cells. Both inner and outer plexiform layers show Nrgn-like immunoreactivity, which is prominent in sublayers of the inner plexiform layer, unlike its poor expression in adults (Alba-González et al. 2022). As far as we are aware, there are no reports of distribution or development of Nrgn expression in the retina of other vertebrates. The strong Nrgn-like expression in retinal ganglion cells during development is also observed in their axons. In toto staining reveals Nrgn-like positivity in the optic nerve and optic tract since 3 dpf, as well as conspicuous immunoreactivity in two visual afferent fields, that is, AF7 (corresponding to the adult parvocellular superficial pretectal nucleus, PSp, located in p1) and the optic tectum. Other afferent fields of the optic pathway were less easily recognizable (for a description of the different AFs in larval zebrafish see Robles et al. 2014, and Baier and Wullimann, 2021). As reported in adults, the AF7 neuropil has no associated Nrgn-like-ir cells, nor the conspicuous ventral commissure linking the PSp of both sides (Castro et al. 2006a; Yáñez et al. 2018) was labeled. The optic tectum, as in the adult zebrafish (Alba-González et al. 2022), showed abundant Nrgn-like-ir cells, most showing their somas in the thick periventricular cell layer. Whereas the zebrafish visual system shows abundant expression of Nrgn-like early on in development, suggesting it is an important peptide for this system, the lack of data on this system in other vertebrates precludes further comparison.
Diencephalon and segmental distribution of Nrgn. The diencephalon of vertebrates, including zebrafish, consists of three prosomeres (p1–p3), from caudal to rostral, with several alar and basal plate derivatives (Puelles and Rubenstein 1993; Wullimann and Puelles 1999; Hauptmann et al. 2002; Mueller, 2012). Convenient sections as that presented in Fig. 3f, show that the three prosomeres are different with respect to Nrgn-like expression in neuronal populations. The most conspicuous Nrgn-like expressing population corresponds to that of the thalamus (alar region of p2), whereas alar p3 (prethalamus) and p1 (pretectum) lack similar populations. The neurons of the zebrafish thalamus are mostly glutamatergic and at least some nuclei project to the pallium (Mueller 2012; Yáñez et al. 2022). Unlike the thalamus, the most dorsal region of p2 (the habenula) neither shows Nrgn-like-ir neurons, nor the habenular commissure shows Nrgn-like-ir fibers. The alar region of p1 (pretectum) mostly consists of GABAergic populations (Mueller et al. 2006; Mueller 2012). Pretectal neurons do not express Nrgn, nor Nrgn-like-ir fibers are observed in the posterior commissure, the most conspicuous of the brain dorsal commissures. The Nrgn-like-ir cells of the epiphysis probably are not projection neurons, because fibers of the epiphysis tract were not labeled. This thin but conspicuous tract appears very early in zebrafish development (Wilson et al. 1990).
Hypothalamus. The hypothalamus is considered the ventral region of the secondary prosencephalon in neuromeric models of the brain (Puelles and Rubenstein 1993; Affaticati et al. 2015). In zebrafish and other teleosts, its caudal (ventral) region evaginates to form lateral and posterior recesses of the infundibulum around which become organized the hypothalamic lobes (inferior and posterior), an impar saccus vasculosus (in some teleosts but not in zebrafish), as well as outstanding groups of neurons, some protruding laterally or caudally (preglomerular complex, torus lateralis, diffuse nucleus, mammillary nucleus). The origin of these neuronal populations of teleosts is complex, because they originate from the primordial hypothalamic walls and from cells migrating tangentially from posterior tubercular/midbrain regions (Bergqvist 1932; Corujo and Anadón 1990; Bloch et al. 2019, 2020). The origin of some migrating populations was recently traced in transgenic zebrafish to the midbrain (Bloch et al. 2019, 2020), and these migrating cells travel during several days before reaching its hypothalamic location. Present results reveal that the conspicuous hypothalamic populations of Nrgn-like-ir cells at 5–6 dpf appeared by 3 dpf, i.e., before the arrival to the torus lateralis and hypothalamic lobes of the midbrain migrating population. Moreover, these Nrgn-like-ir populations of the torus lateralis and diffuse nucleus give rise to a conspicuous Nrgn-like-ir tract (tract of the horizontal commissure) that decussates ventrally and caudally to the optic chiasm in the horizontal commissure, which is characteristic of teleost fishes. Instead, the preglomerular population originated in the midbrain projects ipsilaterally to the pallium without forming any commissure (see Fig. 2c, d in Bloch et al. 2020). This suggests that these Nrgn-like-ir embryonic populations originate from the hypothalamic primordia, mixing with those tangentially migrating from the midbrain demonstrated by Bloch et al. (2019, 2020).
Hindbrain. The expression of Nrgn-like immunoreactivity during hindbrain development shows a segmental pattern of the first positive neurons. This is clearly appreciable in 2 dpf and 3 dpf embryos, where small groups of Nrgn-like-ir cell bodies can be ascribed to rhombomeres 2 to 6. Segmental patterns of early hindbrain populations have been reported for reticulospinal and motoneurons (Metcalfe et al. 1986; Hanneman et al. 1988), which develop much earlier than the Nrgn-like-ir cells. In 5 dpf and 6 dpf larvae the number of Nrgn-like-ir cells increased considerably, and the segmental groups have coalesced longitudinally forming two partially overlapped columns in the dorsolateral hindbrain. Careful observation of these columns and comparison with topographical expression of key markers in 6 dpf brain reveals that Phoxb2 and VGlut expressions in the zebrafish brain browser application (http://vis.arc.vt.edu/projects/zbb/) (Marquart et al. 2015) allows to easily distinguish between the octavolateralis column (OLA) (VGlut + , Phoxb2-) and the viscerosensory column (VGlut-, Phoxb2 +) at dorsal hindbrain regions. The dorsolateral Nrgn-like-ir population corresponding to the viscerosensory column extends between r4 (rhombomere 4) and the obex (caudal hindbrain, where both sides fuse) and that of the octavolateralis column (OLA) extends between r2 and r4–r5. In its rostral level the viscerosensory column becomes thinner and shifts to locate medial to the OLA. The Nrgn-like-ir viscerosensory column coincides with a cellular band that expresses Phoxb2 (Coppola et al. 2012), whereas this marker is not expressed in the OLA. In adult zebrafish, the viscerosensory column shows three lobes (facial, glossopharyngeal and vagal sensory lobes), two of them conspicuous (Wullimann et al. 1996; Castro et al. 2006b; Yáñez et al. 2017). In larvae, a conspicuous Nrgn-like-ir ipsilateral ascending tract ending in the cerebellar region can be identified as the secondary gustatory tract projecting to the secondary gustatory nucleus, as reported with tract tracing in adults (Yáñez et al. 2017), although the cells of this nucleus were Nrgn negative.
As indicated above, combination of Phoxb2 and VGlut expressions in the Z Brain Browser application (http://vis.arc.vt.edu/projects/zbb/) (Marquart et al. 2015) can be used for easily distinguishing between the Nrgn-like-ir OLA (VGlut + , Phoxb2-) and the Nrgn-like-ir viscerosensory column (VGlut- and Phoxb2 +) at dorsal hindbrain regions. The location of the hindbrain area responsible to auditory stimuli in 6 dpf larvae (Constantin et al. 2020) appears to match with that the OLA reported here. In the case of the OLA, in transverse hindbrain sections it can be appreciated how the Nrgn-like-ir OLA gives rise to abundant arcuate fibers crossing the midline and coursing in the lateral lemniscus toward the midbrain (maybe torus semicircularis), i.e., in the reported main efferent pathway of the OLA (Vanwalleghem et al. 2017; Constantin et al. 2020). With regards the developing cerebellum, Nrgn-like expression is low in most cerebellar regions in contrast with that observed in the OLA. The zebrafish cerebellum has been molecularly characterized in adults and development by Bae et al. (2009). As in the case of the viscerosensory column, we have not found reports of Nrgn distribution in these hindbrain regions of other vertebrates, which precludes comparative comparisons.
Possible roles of Nrgn during brain development. The fact that Nrgn is expressed in the brain from early stages in zebrafish (present results) and rat (Gerendasy et al. 1994; Álvarez-Bolado et al. 1996) suggests it plays some roles during development, maybe in axonal growth, plasticity and synaptogenesis. In zebrafish, pathfinding and synaptogenesis has already started by 2 dpf, and thus Nrgn could have a role in these processes under regulation by thyroid hormones and other signals (Muñoz et al. 1991; Iñiguez et al. 1992, 1993, 1994, 1996; Enderlin et al. 1997; Husson et al. 2003, 2004; Féart et al. 2005; Buaud et al. 2010). In fact, zebrafish larva shows a 46% increase in nrgna transcript after T3 thyroid hormone administration (Zada et al. 2014), which points to thyroid regulation of Nrgn expression during development, as shown in mammals (Iñiguez et al. 1992, 1996; Piosik et al. 1995; Martínez de Arrieta et al. 1999; Dowling and Zoeller 2000; Zoeller et al. 2005; Stepien and Huttner 2019). In the rat brain, a peak of Nrgn expression has been described between postnatal days 10 and 20 (Represa et al. 1990; Watson et al. 1990; Álvarez-Bolado et al, 1996), which may be coupled with a peak in synaptogenesis. Our data does not allow analyzing differences in expression levels between different stages, as levels of confocal signal (gain) was adjusted individually for every imaged specimen, so levels of expression between stages are not comparable. We did observe a sustained expression of Nrgn-like throughout development, without transient expression in any area or cell type, i.e., once Nrgn-like expression is observed in one area it is maintained in development and in the adult (Alba-González et al. 2022). This is similar to results in the mammalian brain (Represa et al. 1990; Watson et al. 1990; Álvarez-Bolado et al. 1996; Guadaño-Ferraz et al. 2005), because loss of expression at a given area or cell type has only been reported in the mouse olfactory bulb (Gribaudo et al. 2012).
Comparison with the expression of pcp4a in zebrafish. It is worth noting that our results show that Nrgn is a marker for specific neuronal populations, allowing tracing these populations during development. This is also the case for pcp4a, another member of the calpacitin family studied by in situ hybridization in developing and adult zebrafish (Mione et al. 2006). Some parallelisms can be noted in the distribution of pcp4a mRNA (Mione et al. 2006) and Nrgn-like peptides (present results). In both cases, first expression is observed in differentiating neurons, and not in proliferating zones. Some neuronal populations show expression of both pcp4a and Nrgn in development, but there are others expressing one or the other, suggesting only partial codistribution. Among populations expressing both substances, the most outstanding are the retinal ganglion cells. Other areas showing possible colocalization or codistribution of both pcp4a and Nrgn are the pallium, the dorsal thalamus, the optic tectum, the torus semicircularis, cerebellum and the viscerosensory area (Mione et al. 2006; present results). The dorsal habenula is pcp4a positive but Nrgn negative, and the same appears to occur with the preglomerular complex, pseudoglomerular nucleus, mammillary bodies and reticulospinal neurons (Mione et al. 2006; present results). Among the Nrgn-like-ir populations that are largely pcp4a negative during development, it is worth mentioning the amacrine and bipolar cells of the retina, and the inferior lobes. Thus, although both Nrgn and pcp4a interact with calmodulin in neurons, facilitating adaptation, they appear to be selectively used by some centers. Further comparison of pcp4a and Nrgn-like expression in developing zebrafish is precluded because of the different nature of the methods used by Mione et al. (2006) and in the present study. For instance, whereas Nrgn immunohistochemistry allowed studying tracts and neuropil regions, the techniques applied in Mione et al. (2006) did not allow showing these important anatomical features. Further studies should address in detail possible relations between pcp4a and Nrgn, as well as other calpacitins, in developing zebrafish neurons.
Final consideration. There are many aspects of Nrgn function in the adult brain and during development yet to be clarified, research in which zebrafish will most certainly contribute, given the number of tools available to work in these species. Study of Nrgn function has attracted little attention so far, but this may change soon, as a Nrgn mutant has been generated as part of a project investigating the phenotype of zebrafish carrying mutations in human schizophrenia-associated genes (Thyme et al. 2019). This project highlights the potential implication of Nrgn and zebrafish in understanding human disease.
Conclusions
Our study of Nrgn-like immunoreactivity in neural tissues during the development of the zebrafish reveals positive cells in both sensory organs of the head (retina, olfactory rosette, neuromasts) and in the brain. Nrgn-like expression appears late in the positive populations, suggesting that it is expressed in cells that are differentiated functionally. Main Nrgn-like-ir populations in the brain were observed in the pallium, hypothalamic lobes, thalamus, optic tectum, octavolateralis area and viscerosensory column, suggesting close relationship of Nrgn with processing sensory information, probably contributing to adaptative responses in larval stages. The restriction of its expression to specific neuronal populations, combined with observations in toto, allowed to use Nrgn-like immunoreactivity to reveal the origin of some tracts and commissures.
Abbreviations
- ca
Anterior commissure
- Ce
Cerebellum
- cet
Cerebellar tract
- DiL
Diffuse nucleus of the inferior hypothalamic lobe
- DT
Dorsal thalamus
- E
Epiphysis
- fb
Forebrain bundle
- GCL
Retinal ganglion cell layer
- H
Hypothalamus
- Hb
Habenulae
- Hc
Caudal zone of periventricular hypothalamus
- hc
Horizontal commissure
- INL
Retinal inner nuclear layer
- IO
Inferior olive
- Ip
Interpeduncular nucleus
- IPL
Retinal interplexiform layer
- ll
Lateral lemniscus
- M2
Posterior tubercular area
- MO
Medulla oblongata
- NAT
Anterior tuberal nucleus
- nI
Nucleus isthmi
- nIII
Oculomotor nucleus
- nIV
Trochlear nucleus
- nrm
Neuromasts
- OB
Olfactory bulb
- oc
Optic chiasm
- OE
Olfactory epithelium
- OLA
Octavolateralis area
- OT
Optic tectum
- ot
Optic tract
- Pa
Pallium
- PG
Preglomerular complex
- PGZ
Periventricular grey zone
- Pit
Pituitary
- PL
Posterior lobe
- Po
Preoptic area
- PSp
Parvocellular superficial pretectal nucleus
- pT
Pretectum
- pTh
Prethalamus
- PTN
Posterior tuberal nucleus
- RL
Rostrolateral nucleus
- rvt
Rostral visceral tract (Yáñez et al. 2017)
- S
Subpallium
- SC
Spinal cord
- sgt
Secondary gustatory tract
- tb
Tectobulbar tract
- Tel
Telencephalic lobes
- Th
Thalamus
- TLa
Torus lateralis
- TSc
Torus semicircularis
- VC
Viscerosensory column
Author contributions
Study concept and design: AA-G, MF and JY. Immunohistochemistry data: AA-G and MF. Analysis and interpretation of data: AA-G, JY, RA, and MF. Drafting of the manuscript: AA-G, JY, RA and MF. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Alba-González A. is recipient of a Predoctoral Fellowship from Xunta de Galicia (Grant number ED481A-2019/003).
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Declarations
Conflict of interest
The authors have no conflict of interest to declare. All authors had access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Ethical approval
All experimental procedures, handling, use and care of the animals used in this study were conducted following the Spanish (Royal Decree 53/2013) and the European Union (Directive 2010/63/EU) legislations regarding the protection of animals used for scientific research purposes.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/12/2022
Missing Open Access funding information has been added in the Funding Note.
Contributor Information
Julián Yáñez, Email: julian.yanez@udc.es.
Mónica Folgueira, Email: m.folgueira@udc.es.
References
- Adams MM, Kafaligonul H. Zebrafish-A model organism for studying the neurobiological mechanisms underlying cognitive brain aging and use of potential interventions. Front Cell Dev Biol. 2018 doi: 10.3389/fcell.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affaticati P, Yamamoto K, Rizzi B, Bureau C, Peyriéras N, Pasqualini C, Demarque M, Vernier P. Identification of the optic recess region as a morphogenetic entity in the zebrafish forebrain. Sci Rep. 2015 doi: 10.1038/srep08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba-González A, Folgueira M, Castro A, Anadón R, Yáñez J. Distribution of Neurogranin-like immunoreactivity in the brain and sensory organs of the adult zebrafish. J Comp Neurol. 2022;530:1569–1587. doi: 10.1002/cne.25297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleström P, D’Angelo L, Midtlyng PJ, Schorderet DF, Schulte-Merker S, Sohm F, Warner S. Zebrafish: Housing and husbandry recommendations. Lab Anim. 2019 doi: 10.1177/0023677219869037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez-Bolado G, Rodríguez-Sánchez P, Tejero-Diez P, Fairén A, Diez-Guerra FJ. Neurogranin in the development of the rat telencephalon. Neurosci. 1996;73:565–580. doi: 10.1016/0306-4522(96)00061-9. [DOI] [PubMed] [Google Scholar]
- Bae YK, Kani S, Shimizu T, Tanabe K, Nojima H, Kimura Y, Higashijima S, Hibi M. Anatomy of zebrafish cerebellum and screen for mutations affecting its development. Develop Biol. 2009;330:406–426. doi: 10.1016/j.ydbio.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Baier H, Wullimann MF. Anatomy and function of retinorecipient arborization fields in zebrafish. J Comp Neurol. 2021;529:3454–3476. doi: 10.1002/cne.25204. [DOI] [PubMed] [Google Scholar]
- Bao W, Volgin AD, Alpyshov ET, Friend AJ, Strekalova TV, de Abreu MS, Collins C, Amstislavskaya TG, Demin KA, Kalueff AV. Opioid neurobiology, neurogenetics and neuropharmacology in zebrafish. Neurosci. 2019;404:218–232. doi: 10.1016/j.neuroscience.2019.01.045. [DOI] [PubMed] [Google Scholar]
- Baudier J, Bronnerll C, Kligman D, David Cole R. Protein kinase C substrates from bovine brain. J Biol Chem. 1989;264:1824–1828. doi: 10.1016/S0021-9258(18)94262-6. [DOI] [PubMed] [Google Scholar]
- Baudier J, Deloulme JC, Van DA, Black D, Matthes HWD. Purification and characterization of a brain-specific protein kinase C substrate, Neurogranin (p 17): identification of a consensus amino acid sequence between neurogranin and neuromodulin (GAP-43) that corresponds to the protein kinase C phosphorylation site and the calmodulin-binding domain. J Biol Med. 1991;266:229–237. doi: 10.1016/S0021-9258(18)52425-X. [DOI] [PubMed] [Google Scholar]
- Bereczki E, Francis PT, Howlett D, Pereira JB, Höglund K, Bogstedt A, Cedazo-Minguez A, Baek JH, Hortobágyi T, Attems J, Ballard C, Aarsland D. Synaptic proteins predict cognitive decline in Alzheimer`s disease and Lewy body dementia. Alzheimers Dement. 2016;12:1149–1158. doi: 10.1016/j.jalz.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Bereczki E, Bogstedt A, Höglund K, Tsitsi P, Brodin L, Ballard C, Svenningsson P, Aarsland D. Synaptic proteins in CSF relate to Parkinson’s disease stage markers. Npj Parkinson’s Dis. 2017;3:1–5. doi: 10.1038/s41531-017-0008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergqvist H. Zur Morphologie des Zwischenhirns bei niederen Wir-beltieren. Acta Zool. 1932;13:57–303. doi: 10.1111/j.1463-6395.1932.tb00485.x. [DOI] [Google Scholar]
- Blennow K, Zetterberg H. Biomarkers for Alzheimer’s disease: current status and prospects for the future. J Internal Med. 2018;284:643–663. doi: 10.1111/joim.12816. [DOI] [PubMed] [Google Scholar]
- Blennow K, Zetterberg H. The past and the future of Alzheimer’s disease fluid biomarkers. J Alzheimers Dis. 2018;62:1125–1140. doi: 10.3233/JAD-170773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch S, Thomas M, Colin I, Galant S, Machado E, Affaticati P, Jenett A, Yamamoto K. Mesencephalic origin of the inferior lobe in zebrafish. BMC Biol. 2019;17:22. doi: 10.1186/s12915-019-0631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch S, Hagio H, Thomas M, Heuzé A, Hermel JM, Lasserre E, Colin I, Saka K, Affaticati P, Jenett A, Kawakami K, Yamamoto N, Yamamoto K. Non-thalamic origin of zebrafish sensory nuclei implies convergent evolution of visual pathways in amniotes and teleosts. Elife. 2020;9:e54945. doi: 10.7554/eLife.54945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buaud B, Esterle L, Vaysse C, Alfos S, Combe N, Higueret P, Pallet V. A high-fat diet induces lower expression of retinoid receptors and their target genes GAP-43/neuromodulin and RC3/neurogranin in the rat brain. Brit J Nutr. 2010;103:1720–1729. doi: 10.1017/S0007114509993886. [DOI] [PubMed] [Google Scholar]
- Casaletto KB, Elahi FM, Bettcher BM, Neuhaus J, Bendlin BB, Asthana S, Johnson SC, Yaffe K, Carlsson C, Blennow K, Zetterberg H, Kramer JH. Neurogranin, a synaptic protein, is associated with memory independent of Alzheimer biomarkers. Am Acad Neurol. 2017;89:1782–1788. doi: 10.1212/WNL.0000000000004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro A, Becerra M, Manso MJ, Anadón R. Calretinin immunoreactivity in the brain of the zebrafish, Danio rerio: distribution and comparison with some neuropeptides and neurotransmitter-synthesizing enzymes. I. Olfactory organ and forebrain. J Comp Neurol. 2006;494:435–459. doi: 10.1002/cne.20782. [DOI] [PubMed] [Google Scholar]
- Castro A, Becerra M, Manso MJ, Anadón R. Calretinin immunoreactivity in the brain of the zebrafish, Danio rerio: distribution and comparison with some neuropeptides and neurotransmitter-synthesizing enzymes. II. Midbrain, hindbrain, and rostral spinal cord. J Comp Neurol. 2006;494:792–814. doi: 10.1002/cne.20843. [DOI] [PubMed] [Google Scholar]
- Chang JW, Schumacher E, Coulter PM, Vinters HV, Watson JB. Dendritic translocation of RC3/Neurogranin mRNA in Normal Aging, Alzheimer Disease and Fronto-Temporal Dementia. J Neuropath Exp Neurol. 1997;56:1105–1118. doi: 10.1097/00005072-199710000-00004. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Sweatt JD, Klann E. Enhanced phosphorylation of the postsynaptic protein kinase C substrate RC3 neurogranin during long-term potentiation. Brain Res. 1997;749:181–187. doi: 10.1016/s0006-8993(96)01159-6. [DOI] [PubMed] [Google Scholar]
- Chitnis AB, Kuwada JY. Axonogenesis in the Brain of Zebrafish Embryos. J Neurosci. 1990;10:1892–1905. doi: 10.1523/JNEUROSCI.10-06-01892.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton DF, George JM, Mello CV, Siepka SM. Conservation and expression of iq-domain-containing calpacitin gene products (neuromodulin/GAP-43, neurogranin/RC3) in the adult and developing oscine song control system. Dev Neurobiol. 2009;69:124–140. doi: 10.1002/dneu.20686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggins PJ, Mclean K, Zwiers H. Neurogranin, a B-50/GAP-43-immunoreactive C-kinase substrate (BICKS), is ADP-ribosylated. FEBS. 1993;335:109–113. doi: 10.1016/0014-5793(93)80450-9. [DOI] [PubMed] [Google Scholar]
- Cohen RW, Margulies JE, Coulter PM, Watson JB. Functional consequences of expression of the neuron-specific, protein kinase C substrate RC3 (neurogranin) in Xenopus oocytes. Brain Res. 1993;627:147–152. doi: 10.1016/0006-8993(93)90758-f. [DOI] [PubMed] [Google Scholar]
- Constantin L, Poulsen RE, Scholz LA, Favre-Bulle IA, Taylor MA, Sun B, Goodhill GJ, Vanwalleghem GC, Scott EK. Altered brain-wide auditory networks in a zebrafish model of fragile X syndrome. BMC Biol. 2020;16:125. doi: 10.1186/s12915-020-00857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola E, D’Autréaux F, Nomaksteinsky M, Brunet JF. Phox2b expression in the taste centers of fish. J Comp Neurol. 2012;520:3633–3649. doi: 10.1002/cne.23117. [DOI] [PubMed] [Google Scholar]
- Corujo A, Anadón R. The development of the diencephalon of the rainbow trout (Salmo gairdneri Richardson) Thalamus and hypothalamus. J Hirnforsch. 1990;31:669–680. [PubMed] [Google Scholar]
- Deloulme J, Sensenbrenner M, Baudier J. A rapid purification method for neurogranin, a brain specific calmodulin-binding protein kinase C substrate. FEBS. 1991;282:183–188. doi: 10.1016/0014-5793(91)80473-g. [DOI] [PubMed] [Google Scholar]
- DiFiglia M. Excitotoxic injury of the neostriatum: a model for Huntington’s disease. Trends Neurosci. 1990;13:286–290. doi: 10.1016/0166-2236(90)90111-m. [DOI] [PubMed] [Google Scholar]
- Domínguez-González I, Vázquez-Cuesta SN, Algaba A, Díez-Guerra FJ. Neurogranin binds to phosphatidic acid and associates to cellular membranes. Biochem J. 2007;404:31–43. doi: 10.1042/BJ20061483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling ALS, Zoeller RT. Thyroid hormone of maternal origin regulates the expression of RC3/neurogranin mRNA in the fetal rat brain. Mol Brain Res. 2000;82:126–132. doi: 10.1016/s0169-328x(00)00190-x. [DOI] [PubMed] [Google Scholar]
- Enderlin V, Pallet V, Alfos S, Dargelos E, Jaffard R, Garcin H, Higueret P. Age-related decreases in mRNA for brain nuclear receptors and target genes are reversed by retinoic acid treatment. Neurosci Lett. 1997;229:125–129. doi: 10.1016/s0304-3940(97)00424-2ers. [DOI] [PubMed] [Google Scholar]
- Féart C, Mingaud F, Enderlin V, Husson M, Alfos S, Higueret P, Pallet V. Differential effect of retinoic acid and triiodothyronine on the age-related hypo-expression of neurogranin in rat. Neurobiol Aging. 2005;26:729–738. doi: 10.1016/j.neurobiolaging.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Fedorov NB, Pasinelli P, Oestreiches AB, Degraan PNE, Reymann’ KG. Antibodies to postsynaptic PKC substrate Neurogranin prevent long-term potentiation in hippocampal CAI neurons. EurJ Neurosci. 1995;7:819–822. doi: 10.1111/j.1460-9568.1995.tb00685.x. [DOI] [PubMed] [Google Scholar]
- Filosa A, Barker AJ, Dal Maschio M, Baier H. Feeding state modulates behavioral choice and processing of prey stimuli in the zebrafish tectum. Neuron. 2016;90:596–608. doi: 10.1016/j.neuron.2016.03.014. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Jacobson GA, Zhu P. Circuit neuroscience in zebrafish. Curr Biol. 2010;20:371–381. doi: 10.1016/j.cub.2010.02.039. [DOI] [PubMed] [Google Scholar]
- Gerendasy D. Homeostatic tuning of Ca2+ signal transduction by members of the calpacitin protein family. J Neurosci Res. 1999;58:107–119. doi: 10.1002/(SICI)1097-4547(19991001)58:1<107::AID-JNR11>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Gerendasy DD, Sutcliffe JG. RC3/Neurogranin, a postsynaptic Calpacitin for setting the response threshold to calcium influxes. Mol Neurobiol. 1997;15:131–163. doi: 10.1007/BF02740632. [DOI] [PubMed] [Google Scholar]
- Gerendasy DD, Herron SR, Watson JB, Sutcliffe JG. Mutational and biophysical studies regulates Calmodulin availability. J Biol Chem. 1994;269:22420–22426. doi: 10.1016/S0021-9258(17)31806-9. [DOI] [PubMed] [Google Scholar]
- Giegling I, Genius J, Benninghoff J, Rujescu D. Genetic findings in schizophrenia patients related to alterations in the intracellular Ca-homeostasis. Prog Neuro-Psychopharmacol Biol Psychiatry. 2010;34:1375–1380. doi: 10.1016/j.pnpbp.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Gribaudo S, Bovetti S, Friard O, Denorme M, Oboti L, Fasolo A, De Marchis S. Transitory and activity-dependent expression of neurogranin in olfactory bulb tufted cells during mouse postnatal development. J Comp Neurol. 2012;520:3055–3069. doi: 10.1002/cne.23150. [DOI] [PubMed] [Google Scholar]
- Guadaño-Ferraz A, Viñuela A, Oeding G, Bernal J, Rausell E. RC3/neurogranin is expressed in pyramidal neurons of motor and somatosensory cortex in normal and denervated monkeys. J Comp Neurol. 2005;493:554–570. doi: 10.1002/cne.20774. [DOI] [PubMed] [Google Scholar]
- Gurung R, Prata DP. What is the impact of genome-wide supported risk variants for schizophrenia and bipolar disorder on brain structure and function? A systematic review. Psychol Med. 2015;45:2461–2480. doi: 10.1017/S0033291715000537. [DOI] [PubMed] [Google Scholar]
- Hanneman E, Trevarrow B, Metcalfe WK, Kimmel CB, Westerfield M. Segmental pattern of development of the hindbrain and spinal cord of the zebrafish embryo. Development. 1988;103:49–58. doi: 10.1242/dev.103.1.49. [DOI] [PubMed] [Google Scholar]
- Hauptmann G, Söll I, Gerster T. The early embryonic zebrafish forebrain is subdivided into molecularly distinct transverse and longitudinal domains. Brain Res Bull. 2002;57:371–375. doi: 10.1016/s0361-9230(01)00691-8. [DOI] [PubMed] [Google Scholar]
- Hellwig K, Kvartsberg H, Portelius E, Andreasson U, Oberstein TJ, Lewczuk P, Blennow K, Kornhuber J, Maler JM, Zetterberg H, Spitzer P. Neurogranin and YKL-40: Independent markers of synaptic degeneration and neuroinflammation in Alzheimer’s disease. Alzheimer Res Therapy. 2015;7:74. doi: 10.1186/s13195-015-0161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben MPWA, Lankhorst AJ, Van Dalen JJW, Veldman H, Joosten EAJ, Hamers FPT, Gispen WH, Schrama LH. Pre-and Postsynaptic Localization of RC3/ Neurogranin in the Adult Rat Spinal Cord: An Immunohistochemical Study. J Neurosci Res. 2000;59:750–759. doi: 10.1002/(SICI)1097-4547(20000315)59:6<750::AID-JNR7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Huang KP, Huang FL, Chen HC. Characterization of a 7.5-kDa protein kinase C substrate (RC3 Protein, Neurogranin) from rat brain. Arch Biochem Biophys. 1993;305:570–580. doi: 10.1006/abbi.1993.1463. [DOI] [PubMed] [Google Scholar]
- Huang KP, Huang FL, Li J, Schuck P, McPhie P. Calcium-sensitive interaction between calmodulin and modified forms of rat brain neurogranin/RC3. Biochemistry. 2000;39:7291–7299. doi: 10.1021/bi000336l. [DOI] [PubMed] [Google Scholar]
- Huang KP, Huang FL, Jäger T, Li J, Reymann KG, Balschun D. Neurogranin/RC3 enhances long-term potentiation and learning by promoting calcium-mediated signaling. J Neurosci. 2004;24:10660–10669. doi: 10.1523/JNEUROSCI.2213-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson M, Enderlin V, Alfos S, Féart C, Higueret P, Pallet V. Triiodothyronine administration reverses vitamin A deficiency-related hypo-expression of retinoic acid and triiodothyronine nuclear receptors and of neurogranin in rat brain. Brit J Nutr. 2003;90:191–198. doi: 10.1079/bjn2003877. [DOI] [PubMed] [Google Scholar]
- Husson M, Enderlin V, Alfos S, Boucheron C, Pallet V, Higueret P. Expression of neurogranin and neuromodulin is affected in the striatum of vitamin A-deprived rats. Mol Brain Res. 2004;123:7–17. doi: 10.1016/j.molbrainres.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Iñiguez MA, Rodríguez-Pelia A, Ibarrola N, Morreale De Escobar G, Bernal J. Adult rat brain is sensitive to Thyroid Hormone. regulation of RC3/ Neurogranin mRNA. J Clin Invest. 1992;90:554–558. doi: 10.1172/JCI115894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez MA, Rodríguez-Pelia A, Ibarrola N, Aguilera M, Bernal J. Thyroid hormone regulation of RC3, a brain-specific gene encoding a protein kinase-C substrate. Endocrinology. 1993;133:467–473. doi: 10.1210/endo.133.2.8344193. [DOI] [PubMed] [Google Scholar]
- Iñiguez MA, Morte B, Rodriguez-Pefia A, Mufioz A, Gerendasy D, Sutcliffe G, Bernal J. Characterization of the promoter region and flanking sequences of the neuron-specific gene RC3 (neurogranin) Mol Brain Res. 1994;27:205–214. doi: 10.1016/0169-328X(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Iñiguez MA, De Lecea L, Guadtio-Ferraz A, Morte B, Gerendasy D, Sutcliffe JG, Bernal J. Cell-specific effects of thyroid hormone on RC3/Neurogranin expression in rat brain. Endocrinology. 1996;137:1032–1041. doi: 10.1210/endo.137.3.8603571. [DOI] [PubMed] [Google Scholar]
- Jin M, Cao L, Dai YP. Role of Neurofilament Light chain as a potential biomarker for Alzheimer’s disease: a correlative meta-analysis. Front Aging Neurosci. 2019;11:1–10. doi: 10.3389/fnagi.2019.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key B, Devine CA. Zebrafish as an experimental model: strategies for developmental and molecular neurobiology studies. Methods Cell Sci. 2003;25:1–6. doi: 10.1023/B:MICS.0000006849.98007.03. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Develop Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Koob AO, Shaked GM, Bender A, Bisquertt A, Rockenstein E, Masliah E. Neurogranin binds α-synuclein in the human superior temporal cortex and interaction is decreased in Parkinson’s disease. Brain Res. 2014;1591:102–110. doi: 10.1016/j.brainres.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Chichili VP, Zhong L, Tang X, Velazquez-Campoy A, Sheu FS, Seetharaman J, Gerges NZ, Sivaraman J. Structural basis for the interaction of unstructured neuron specific substrates neuromodulin and neurogranin with Calmodulin. Sci Rep. 2013;3:1392. doi: 10.1038/srep01392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvartsberg H, Lashley T, Murray CE, Brinkmalm G, Cullen NC, Höglund K, Zetterberg H, Blennow K, Portelius E. The intact postsynaptic protein neurogranin is reduced in brain tissue from patients with familial and sporadic Alzheimer’s disease. Acta Neuropathol. 2019;137:89–102. doi: 10.1007/s00401-018-1910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashley T, Schott JM, Weston P, Murray CE, Wellington H, KeshavanA FSC, Foiani M, Toombs J, Rohrer JD, Heslegrave A, Zetterberg H. Molecular biomarkers of Alzheimer’s disease: progress and prospects. Dis Model Mech. 2018;11:1–9. doi: 10.1242/dmm.031781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK. Synaptic plasticity and phosphorylation. Pharmacol Therap. 2006;112:810–832. doi: 10.1016/j.pharmthera.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Lai M, Cole S, Le Novère N, Edelstein SJ. Neurogranin stimulates Ca2+/calmodulin-dependent kinase II by suppressing calcineurin activity at specific calcium spike frequencies. PLoS Comput Biol. 2020;16:1–29. doi: 10.1371/journal.pcbi.1006991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lista S, Hampel H. Synaptic degeneration and neurogranin in the pathophysiology of Alzheimer’s disease. Expert Rev Neurother. 2017;17:47–57. doi: 10.1080/14737175.2016.1204234. [DOI] [PubMed] [Google Scholar]
- Marquart GD, Tabor KM, Brown M, Strykowski JL, Varshney GK, LaFave MC, Mueller T, Burgess SM, Higashijima S, Burgess HA. A 3D searchable database of transgenic zebrafish Gal4 and cre lines for functional neuroanatomy studies. Front Neural Circuits. 2015;9:78. doi: 10.3389/fncir.2015.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez de Arrieta C, Pé L, Jurado R, Bernal J, Coloma A. Structure, organization, and chromosomal mapping of the human Neurogranin gene (NRGN) Genomics. 1997;41:243–249. doi: 10.1006/geno.1997.4622. [DOI] [PubMed] [Google Scholar]
- Martínez de Arrieta C, Morte B, Coloma A, Bernal J. The Human RC3 Gene Homolog, NRGN Contains a Thyroid Hormone-Responsive Element Located in the First Intron. Endocrinology. 1999;140:335–343. doi: 10.1210/endo.140.1.6461. [DOI] [PubMed] [Google Scholar]
- Martzen MR, Slemmon TLR. The Dendritic Peptide Neurogranin can regulate a Calmodulin-dependent target. J Neurochem. 1995;64:92–100. doi: 10.1046/j.1471-4159.1995.64010092.x. [DOI] [PubMed] [Google Scholar]
- McGuire CB, Snipes GJ, Norden JJ. Light-microscopic immunolocalization of the growth-and plasticity-associated protein GAP-43 in the developing rat brain. Dev Brain Res. 1988;41:277–291. doi: 10.1016/0165-3806(88)90189-7. [DOI] [PubMed] [Google Scholar]
- Metcalfe WK, Mendelson B, Kimmel CB. Segmental homologies among reticulospinal neurons in the hindbrain of the zebrafish larva. J Comp Neurol. 1986;251:147–159. doi: 10.1002/cne.902510202. [DOI] [PubMed] [Google Scholar]
- Mione M, Lele Z, Kwong CT, Concha ML, Clarke JD. Expression of pcp4a in subpopulations of CNS neurons in zebrafish. J Comp Neurol. 2006;495:769–787. doi: 10.1002/cne.20907. [DOI] [PubMed] [Google Scholar]
- Morte B, Martínez De Arrieta C, Manzano J, Coloma A, Bernal J. Identification of a cis-acting element that interferes with thyroid hormone induction of the neurogranin (NRGN) gene. FEBS. 1999;464:179–183. doi: 10.1016/s0014-5793(99)01706-8. [DOI] [PubMed] [Google Scholar]
- Mueller T. What is the Thalamus in Zebrafish? Front Neurosci. 2012;6:64. doi: 10.3389/fnins.2012.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller T, Wullimann MF. Anatomy of neurogenesis in the early zebrafish brain. Brain Res Dev Brain Res. 2003;140:137–155. doi: 10.1016/s0165-3806(02)00583-7. [DOI] [PubMed] [Google Scholar]
- Mueller T, Vernier P, Wullimann MF. A phylotypic stage in vertebrate brain development: GABA cell patterns in zebrafish compared with mouse. J Comp Neurol. 2006;494:620–634. doi: 10.1002/cne.20824. [DOI] [PubMed] [Google Scholar]
- Muñoz A, Rodriguez-Pena A, Perez-Castillo A, Ferreiro B, Sutcliffe JG, Bernal J. Effects of neonatal hypothyroidism on rat brain gene expression. Mol Endocrinol. 1991;5:273–280. doi: 10.1210/mend-5-2-273. [DOI] [PubMed] [Google Scholar]
- Neel VA, Young MW. Igloo, a GAP-43 related gene expressed in the developing nervous system of Drosophila. Development. 1994;120:2235–2243. doi: 10.1242/dev.120.8.2235. [DOI] [PubMed] [Google Scholar]
- Neuner-Jehle M, Rhyner TA, Borbey AA. Sleep deprivation differentially alters the mRNA and protein levels of neurogranin in rat brain. Brain Res. 1995;685:143–153. doi: 10.1016/0006-8993(95)00416-n. [DOI] [PubMed] [Google Scholar]
- Neuner-Jehle M, Denizot JP, Mallet J. Neurogranin is locally concentrated in rat cortical and hippocampal neurons. Brain Res. 1996;733:149–154. doi: 10.1016/S0006-8993(96)00786-X. [DOI] [PubMed] [Google Scholar]
- Pak JH, Huang FL, Li J, Balschun D, Reymann KG, Chiang C, Westphal H, Huang KP. Involvement of neurogranin in the modulation of calcium/calmodulin-dependent protein kinase II, synaptic plasticity, and spatial learning: A study with knockout mice. PNAS. 2000;97:11232–11237. doi: 10.1073/pnas.210184697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel HK, Zwiers H, Wang JH. Kinase phosphorylates the calmodulin-binding regulatory regions of neuronal tissue-specific proteins B-50 (GAP-43) and Neurogranin. J Biol Chem. 1993;268:6207–6213. doi: 10.1016/S0021-9258(18)53240-3. [DOI] [PubMed] [Google Scholar]
- Piosik PA, Van Groenigen M, Ponne NJ, Bolhuis PA, Baas F. RC3/neurogranin structure and expression in the caprine brain in relation to congenital hypothyroidism. Mol Brain Res. 1995;29:119–130. doi: 10.1016/0169-328x(94)00237-9. [DOI] [PubMed] [Google Scholar]
- Prichard L, Deloulme JC, Storm DR. Interactions between Neurogranin and Calmodulin in vivo. J Biol Chem. 1999;274:7689–7694. doi: 10.1074/jbc.274.12.7689. [DOI] [PubMed] [Google Scholar]
- Puelles L, Rubenstein JL. Expression patterns of homeobox and other putative regulatory genes in the embryonic mouse forebrain suggest a neuromeric organization. Trends Neurosci. 1993;16:472–479. doi: 10.1016/0166-2236(93)90080-6. [DOI] [PubMed] [Google Scholar]
- Ramakers GMJ, De Graan PNE, Urban IJA, Kraay D, Tang T, Pasinelli P, Oestreicher B, Gispen WH. Temporal differences in the phosphorylation state of pre- and postsynaptic protein kinase C substrates B-50/GAP-43 and neurogranin during long term potentiation. J Biol Chem. 1995;270:13892–13898. doi: 10.1074/jbc.270.23.13892. [DOI] [PubMed] [Google Scholar]
- Ramakers GM, Pasinelli P, Hens JJ, Hendrik Gispen W, Graan NDE. Protein kinase C in synaptic plasticity; changes in the in situ phosphorylation state of identified pre- and postsynaptic substrates. Prog Neuro-Psychopharmacology Biol Psychiatry. 1997;21:455–486. doi: 10.1016/s0278-5846(97)00013-4. [DOI] [PubMed] [Google Scholar]
- Represa A, Deloulme JC, Sensenbrenner M, Ben-Ari Y, Baudier J. Neurogranin: immunocytochemical localization of a brain-specific protein kinase C substrate. J Neurosci. 1990;10:3782–3792. doi: 10.1523/JNEUROSCI.10-12-03782.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhyner TA, Borbely AA, Mallet J. Molecular cloning of forebrain mRNAs which are modulated by sleep deprivation. Eur J Neurosci. 1990;2:1063–1073. doi: 10.1111/j.1460-9568.1990.tb00018.x. [DOI] [PubMed] [Google Scholar]
- Robles E, Laurell E, Baier H. The retinal projectome reveals brain-area-specific visual representations generated by ganglion cell diversity. Curr Biol. 2014;24:2085–2096. doi: 10.1016/j.cub.2014.07.080. [DOI] [PubMed] [Google Scholar]
- Sato T, Xiao DM, Li H, Huang FL, Huan KP. Structure and regulation of the gene encoding the neuron-specific Protein kinase C substrate Neurogranin (RC3 Protein) J Biol Chem. 1995;270:10314–10322. doi: 10.1074/jbc.270.17.10314. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: An open-source platform for biological-image analysis. Nat Meth. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selnes P, Stav AL, Johansen KK, Bjørnerud A, Coello C, Auning E, Kalheim L, Almdahl IS, Hessen E, Zetterberg H, Blennow K, Aarsland D, Fladby T. Impaired synaptic function is linked to cognition in Parkinson’s disease. Ann Clin Transl Neurol. 2017;4:700–713. doi: 10.1002/acn3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singec I, Knoth R, Ditter M, Frotscher M, Volk B. Neurogranin expression by cerebellar neurons in rodents and non-human primates. J Comp Neurol. 2003;459:278–289. doi: 10.1002/cne.10600. [DOI] [PubMed] [Google Scholar]
- Singleman C, Holtzman NG. Growth and maturation in the zebrafish, Danio rerio: A staging tool for teaching and research. Zebrafish. 2014;11:396–406. doi: 10.1089/zeb.2014.0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes GJ, Chan SY, Mcguire CB, Costello BR, Norden JJ, Freeman JA, Routtenberg A. Evidence for the coidentification of GAP-43, a growth-associated protein, and Fl, a plasticity-associated protein. J Neurosci. 1987;7:4066–4075. doi: 10.1523/JNEUROSCI.07-12-04066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepien BK, Huttner WB. Transport, metabolism, and function of thyroid hormones in the developing mammalian brain. Front Endocrinol. 2019;10:1–16. doi: 10.3389/fendo.2019.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strähle U, Scholz S, Geisler R, Greiner P, Hollert H, Rastegar S, Schumacher A, Selderslaghs I, Weiss C, Witters H, Braunbeck T. Zebrafish embryos as an alternative to animal experiments-A commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod Toxicol. 2012;33:128–132. doi: 10.1016/j.reprotox.2011.06.121. [DOI] [PubMed] [Google Scholar]
- Thyme SB, Pieper LM, Li EH, Pandey S, Wang Y, Morris NS, Sha C, Choi JW, Herrera KJ, Soucy ER, Zimmerman S, Randlett O, Greenwood J, McCarroll SA, Schier AF. Phenotypic landscape of schizophrenia-associated genes defines candidates and their shared functions. Cell. 2019;177:478–491. doi: 10.1016/j.cell.2019.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner KJ, Bracewell TG, Hawkins TA. Anatomical dissection of zebrafish brain development. Meth Mol Biol. 2014;1082:197–214. doi: 10.1007/978-1-62703-655-9_14. [DOI] [PubMed] [Google Scholar]
- Vanwalleghem G, Heap LA, Scott EK. A profile of auditory-responsive neurons in the larval zebrafish brain. J Comp Neurol. 2017;525:3031–3043. doi: 10.1002/cne.24258. [DOI] [PubMed] [Google Scholar]
- Vanwalleghem GC, Ahrens MB, Scott EK. Integrative whole-brain neuroscience in larval zebrafish. Curr Opin Neurobiol. 2018;50:136–145. doi: 10.1016/j.conb.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Watson JB, Battenberg EF, Wong KK, Bloom FE, Sutcliffe JG. Subtractive cDNA cloning of RC3, a rodent cortex-enriched mRNA encoding a novel 78 residue protein. J Neurosci Res. 1990;26:397–408. doi: 10.1002/jnr.490260402. [DOI] [PubMed] [Google Scholar]
- Watson JB, Sutcliffe JG, Fishert RS. Localization of the protein kinase C phosphorylation/calmodulin-binding substrate RC3 in dendritic spines of neostriatal neurons. Neurobiology. 1992;89:8581–8585. doi: 10.1073/pnas.89.18.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JB, Szijan I, Coulter PM. Localization of RC3 (neurogranin) in rat brain subcellular fractions. Mol Brain Res. 1994;27:323–328. doi: 10.1016/0169-328X(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Watson JB, Margulies JE, Coulter PM, Gerendasy DD, Sutcliffe JG, Cohen RW. Functional studies of single-site variants in the calmodulin-binding domain of RC3/neurogranin in Xenopus oocytes. Neurosci Lett. 1996;219:183–186. doi: 10.1016/s0304-3940(96)13203-1. [DOI] [PubMed] [Google Scholar]
- Wen Z, Chen J, Khan RA, Wang M, Son Z, Li Z, Shen J, Li W, Shi Y. Polymorphisms in NRGN are associated with schizophrenia, major depressive disorder and bipolar disorder in the Han Chinese population. J Affect Disord. 2016;194:180–187. doi: 10.1016/j.jad.2016.01.034. [DOI] [PubMed] [Google Scholar]
- Wilson SW, Ross LS, Parrett T, Easter SS., Jr The development of a simple scaffold of axon tracts in the brain of the embryonic zebrafish, Brachydanio rerio. Development. 1990;108:121–145. doi: 10.1242/dev.108.1.121. [DOI] [PubMed] [Google Scholar]
- Van Winkel R, Rutten BP, Peerbooms O, Peuskens J, van Os J, De Hert M. MTHFR and risk of metabolic syndrome in patients with schizophrenia. Schizophr Res. 2010;131:193–198. doi: 10.1016/j.schres.2010.05.030. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Rupp B, Reichert H (1996) Neuroanatomy of the zebrafish brain: A topological atlas. Birkhäuser. Basel.
- Wullimann MF, Puelles L. Postembryonic neural proliferation in the zebrafish forebrain and its relationship to prosomeric domains. Anat Embryol. 1999;199:329–348. doi: 10.1007/s004290050232. [DOI] [PubMed] [Google Scholar]
- Wyatt C, Bartoszek EM, Yaksi E. Methods for studying the zebrafish brain: Past, present and future. Eur J Neurosci. 2015;42:1746–1763. doi: 10.1111/ejn.12932. [DOI] [PubMed] [Google Scholar]
- Yáñez J, Souto Y, Piñeiro L, Folgueira M, Anadón R. Gustatory and general visceral centers and their connections in the brain of adult zebrafish: a carbocyanine dye tract-tracing study. J Comp Neurol. 2017;525:333–362. doi: 10.1002/cne.24068. [DOI] [PubMed] [Google Scholar]
- Yáñez J, Suárez T, Quelle A, Folgueira M, Anadón R. Neural connections of the pretectum in zebrafish (Danio rerio) J Comp Neurol. 2018;526:1017–1040. doi: 10.1002/cne.24388. [DOI] [PubMed] [Google Scholar]
- Yáñez J, Folgueira M, Lamas I, Anadón R. The organization of the zebrafish pallium from a hodological perspective. J Comp Neurol. 2022;530:1164–1194. doi: 10.1002/cne.25268. [DOI] [PubMed] [Google Scholar]
- Yang J, Korley FK, Dai M, Everett AD. Serum neurogranin measurement as a biomarker of acute traumatic brain injury. Clin Biochem. 2015;48:843–848. doi: 10.1016/j.clinbiochem.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zada D, Tovin A, Lerer-Goldshtein T, Vatine GD, Appelbaum L. Altered behavioral performance and live imaging of circuit-specific neural deficiencies in a zebrafish model for psychomotor retardation. PLoS Genet. 2014;10:e10046151121. doi: 10.1371/journal.pgen.1004615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakowski W. Animal Use in Neurobiological Research. Neuroscience. 2020;433:1–10. doi: 10.1016/j.neuroscience.2020.02.049. [DOI] [PubMed] [Google Scholar]
- Zhabotinsky AM, Camp RN, Epstein IR, Lisman JE. Role of the neurogranin concentrated in spines in the induction of long-term potentiation. J Neurosci. 2006;26:7337–7347. doi: 10.1523/JNEUROSCI.0729-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gong X, Yin Z, Cui L, Yang J, Wang P, Zhou Y, Jiang X, Wei S, Wang F, Tang Y. Association between NRGN gene polymorphism and resting-state hippocampal functional connectivity in schizophrenia. BMC Psychiatry. 2019;19:1–9. doi: 10.1186/s12888-019-2088-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Gerges NZ. Neurogranin Regulates Metaplasticity. Front Mol Neurosci. 2020;12:1–9. doi: 10.3389/fnmol.2019.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Cherry T, Bies CE, Florence MA, Gerges NZ. Neurogranin enhances synaptic strength through its interaction with calmodulin. EMBO J. 2009;28:3027–3039. doi: 10.1038/emboj.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Kaleka KS, Gerges NZ. Neurogranin phosphorylation fine-tunes long-term potentiation. Eur J Neurosci. 2011;33:244–250. doi: 10.1111/j.1460-9568.2010.07506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT, Bansal R, Parris C. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology. 2005;146:607–612. doi: 10.1210/en.2004-1018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.