Abstract
Nowadays, numerous skincare routines are used to rejuvenate aging skin. Retinoids are one of the most popular ingredients used in antiaging treatments. Among the representatives of retinoids, tretinoin is considered the most effective agent with proven antiaging effects on the skin and can be found in formulations approved as medicines for topical treatment of acne, facial wrinkles, and hyperpigmentation. Other retinoids present in topical medicines are used for various indications, but only tazarotene is also approved as adjunctive agent for treatment of facial fine wrinkling and pigmentation. The most commonly used retinoids such as retinol, retinaldehyde, and retinyl palmitate are contained in cosmeceuticals regulated as cosmetics. Since clinical efficacy studies are not required for marketing cosmetic formulations, there are concerns about the efficacy of these retinoids. From a formulation perspective, retinoids pose a challenge to researchers as a result of their proven instability, low penetration, and potential for skin irritation. Therefore, novel delivery systems based on nanotechnology are being developed to overcome the limitations of conventional formulations and improve user compliance. In this review, the clinical evidence for retinoids in conventional and nanoformulations for topical antiaging treatments was evaluated. In addition, an overview of the comparison clinical trials between tretinoin and other retinoids is presented. In general, there is a lack of evidence from properly designed clinical trials to support the claimed efficacy of the most commonly used retinoids as antiaging agents in cosmeceuticals. Of the other retinoids contained in medicines, tazarotene and adapalene have clinically evaluated antiaging effects compared to tretinoin and may be considered as potential alternatives for antiaging treatments. The promising potential of retinoid nanoformulations requires a more comprehensive evaluation with additional studies to support the preliminary findings.
Keywords: Antiaging, Clinical evidence, Cosmeceuticals, Nanoformulations, Retinoids, Retinol, Tretinoin
Key Summary Points
| Tretinoin is the retinoid present in medicines for topical application with the strongest clinical evidence of antiaging activity. | |
| Tazarotene and adapalene may be considered as tretinoin alternatives for antiaging treatments. | |
| Clinical evidence is lacking for retinoids contained in cosmeceuticals for topical antiaging treatments. | |
| Novel formulations based on nanotechnology are being developed to overcome the major drawbacks of retinoid use. | |
| Further evaluation with in vivo testing and clinical trials are needed to support the preclinical results for the developed nanoformulations containing retinoids. |
Introduction
Skin aging is manifested in clinical features such as wrinkles, dermal atrophy, and decreased elasticity as a result of various processes at the molecular level stimulated by intrinsic and extrinsic sources, leading to degenerative changes and decreased amount of functional components of the skin [1, 2]. Although skin aging is a natural process due to the chronological aging of the human body, photoaging by UV radiation is identified as the most important extrinsic factor that accelerates the process through the effects of the generated reactive oxygen species [3–5].
Nowadays, antiaging skin treatments are a hot topic that encompasses various approaches to rejuvenate and improve skin condition and appearance. Among the variety of treatments, topical retinoids are one of the most commonly used active ingredients for which there is clinical evidence. The well-known strategy against photoaging includes regular use of sunscreens and a combination of retinoids and antioxidants [6, 7].
The retinoid family consists of both naturally occurring and synthetic analogues of retinol (vitamin A). The anti-wrinkle effects of topically applied formulations containing retinoids are based on the promotion of keratinocyte proliferation and collagen synthesis, improvement of the epidermal barrier, inhibition of collagen degradation, transdermal water loss (TEWL), and metalloproteinase activity [8, 9]. Retinoids act through retinoic acid (RAR-α, -β, -γ) and retinoid-X receptors (RXR-α, -β, -γ) [1]. Considering the molecular structure and receptor selectivity, retinoids are classified into four generations as shown in Table 1.
Table 1.
Retinoid generations
| 1st generation | 2nd generation | 3rd generation | 4th generation |
|---|---|---|---|
|
Tretinoin (all-trans-retinoic acid) Isotretinoin (13-cis-retinoic acid) Alitretinoin (9-cis-retinoic acid) Retinol (all-trans-retinol, vitamin A) Retinal (retinaldehyde) Retinyl palmitate Retinyl propionate Retinyl acetate Retinyl retinoate Retinyl N-formyl aspartamate |
Etretinate Acitretin Motretinate |
Tazarotene Adapalene Bexarotene |
Trifarotene Seletinoid G |
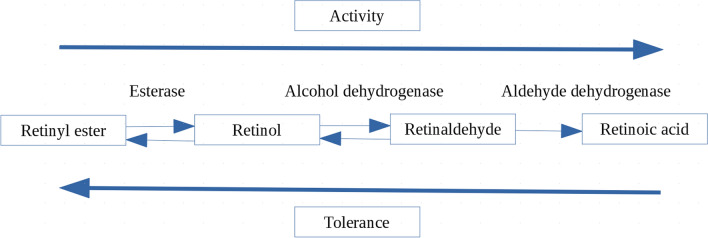
After topical application, retinyl esters are hydrolyzed to retinol; consequently, retinol is converted to biologically active retinoic acid (tretinoin) via retinaldehyde by dehydrogenases in a two-step oxidation process [10, 11]. According to their metabolic pathway, retinoid activity of the first-generation representatives increases in the following order: retinyl esters << retinol < retinaldehyde < retinoic acid, while the tolerance ranking is reversed: retinyl esters > retinol = retinaldehyde >> retinoic acid (Fig. 1) [11]. Therefore, when topically applied, retinol, retinaldehyde, and retinol esters must be converted to a biologically active form—tretinoin, while other retinoids are active compounds in their specific form [12].
Fig. 1.
Representation of retinoid activity and tolerance considering the metabolic pathway
Tretinoin, alitretinoin, adapalene, tazarotene, bexarotene, and trifarotene are found in topical formulations approved as medicines for various indications such as acne or psoriasis. In addition, tretinoin and tazarotene are the only retinoids with approved indications for treatment of photoaged skin as an adjunctive treatment.
Commercial cosmetics (cosmeceuticals) usually contain retinol, retinaldehyde, or retinyl esters. The term cosmeceuticals is used for cosmetic products with active ingredients for which “drug-like” effects on skin structure and function are claimed and are distinguished as a subgroup between cosmetic products and topical prescription medicines [13]. However, because of their classification as cosmeceuticals, no rigorous pre-market safety or efficacy testing is required [14]. Because retinoids present as active ingredients in cosmeceuticals must be converted to tretinoin after application, researchers question their efficacy compared to tretinoin [14].
Although retinoids are among the most commonly used antiaging and depigmenting ingredients in cosmetics [15], their use is associated with the issues of low penetration and common side effects such as skin irritation due to their physicochemical properties and proven instability. The side effects of topically applied retinoids are generally dose-dependent and manifest as “retinoid dermatitis” or skin irritation at the application site [16]. In addition to dermatitis, the application of retinoids around the eyes may cause ocular discomfort and dry eye syndrome because of their influence on the ocular surface and meibomian gland structure [17].
Another issue associated with the use of retinoids is potential teratogenicity. Retinoid teratogenicity has been confirmed for orally ingested retinoids, while concerns about retinoid embryopathy after topical application are based on published case reports [18]. Panchaud et al. [18] conducted a prospective, controlled, multicenter observational study and evaluated the incidence of congenital malformations associated with topical retinoid use during the first trimester. Results did not confirm an increased risk of retinoid embryopathy. Other authors also conducted meta-analyses and reviews of epidemiologic data and concluded that retinoid embryopathy or other adverse pregnancy outcomes are unlikely with topical exposure during pregnancy [19, 20]. However, on the basis of current knowledge and available data, it can be concluded that the use of topical retinoids during pregnancy cannot be advised because of lack of evidence to support this decision.
The mechanisms of retinoid side effects at the molecular level are not fully elucidated [21]. Generally, in cases where the active ingredient is used in the formulation at a low concentration and the patient’s skin shows intolerance, it is recommended to stop retinoid treatment [22]. Representatives of non-selective retinoids (first generation) are associated with a higher incidence of side effects [23].
Researchers are continuously focusing on the development of new retinoid molecules and stable formulations that are effective and well tolerated to overcome the drawbacks associated with the use of retinoids.
Nanoformulations
Novel nanotechnology-based formulations are being developed and tested to improve retinoid efficacy and overcome post-topical application problems. In general, nanotechnology approaches are used to improve the chemical and photochemical stability of the active ingredient, increase penetration, modify its release from the formulation, and achieve satisfactory efficacy with minimal skin irritation [24–26].
The use of nanotechnology in antiaging cosmetics is the fastest growing segment in the skin care industry. These formulations include lipid-based, polymer-based, metal-based, and other nanosystems (dendrimers, fullerenes, and nanocrystals) [27, 28]. Among them, lipid-based delivery systems (solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), liposomes, and nanoemulsions) are considered advantageous compared to other nanosystems owing to their low toxicity, high drug loading capacity, biodegradability, large-scale manufacturing, and diverse chemical and formulation landscape [29]. Various lipid-based delivery systems have been investigated for different retinoids [30–32]. Liposomes are the most studied nanomaterial [26], while hydrogels and nanoemulgels are the most promising semisolid cosmetic formulations for dermal application of lipid-based nanoparticles [33]. The lipid-based nanoparticles are considered safe colloidal carriers for the delivery of poorly soluble active ingredients through the skin, because the excipients used for the formulation are biodegradable and approved for topical application [34, 35]. However, there is still controversy about the toxicity and safety of using nanomaterials in cosmeceuticals [36].
Although nanomaterials may be present in formulations as active ingredients, rheology modifiers, or carriers, their use and role in marketed cosmetics generally remain undisclosed because of the current mistrust towards their application [26]. Usually, nanoparticles are mentioned in some technical information about the manufacturing process or on the product packaging if they are intentionally synthesized or added to the formulation [26]. Consequently, the identification of commercially available cosmetics based on nanotechnology is quite challenging. Moreover, there is a recognized need for research into the efficacy and safety with precise guidelines for manufacturing, advertising, nanotoxicological testing, and safe use of nanocosmetics [27, 28, 33, 36, 37].
In this review, the clinical evidence for retinoids used for topical antiaging treatments is critically discussed. Also, an overview of the comparison clinical trials between the topical formulations with tretinoin as a gold standard and other retinoids is presented. In addition, the clinical evidence for the antiaging efficacy of recently developed retinoid nanoformulations is addressed.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Clinical Evidence
1st Generation
Tretinoin
Tretinoin (all-trans-retinoic acid) is considered the gold standard for clinically effective topical retinoids used in antiaging treatments. Tretinoin is the most potent and best studied retinoid for its antiaging properties [38]. Topical tretinoin formulations (concentrations ranging from 0.01% to 0.1%; the most commonly used are 0.025%, 0.05%, or 0.1%) are approved as prescription medicines for the treatment of acne vulgaris, facial wrinkles, and hyperpigmentation. Numerous clinical studies have confirmed the efficacy of tretinoin in the treatment of photoaging skin [39–45]. The long-term safety and efficacy of 0.05% tretinoin in the treatment of moderate to severe facial photodamage were evaluated in a 2-year placebo-controlled clinical trial involving 204 subjects [45]. The study results suggest that long-term use of 0.05% tretinoin emollient cream with a once-daily application is a safe and effective treatment.
Although the most commonly used formulations for the treatment of aging skin contain 0.05% tretinoin, the optimal concentration, application regimen, and duration of treatment must be individualized by considering the balance between efficacy and irritation [12]. Generally, tretinoin at a concentration of 0.025% is considered therapeutically effective with a reasonable safety profile [46]. Because the retinoid response is dose-dependent, some studies compared the performance of formulations with different concentrations [42–44]. Generally, the formulations with lower concentrations resulted in less pronounced but significant improvement in the clinical and histologic characteristics of the treated photoaged skin with a lower incidence of adverse effects compared to the higher concentrations. To avoid a retinoid dermatitis reaction, it is recommended to start treatment with a lower concentration of the active ingredient and gradually increase the concentration to a tolerable level by using moisturizers to keep the skin hydrated [47]. There is no consensus on the duration of tretinoin therapy. It is recommended that initial treatment is followed by long-term maintenance therapy with regular application of the retinoid at lower concentrations and/or less frequently [46].
Besides formulations with low tretinoin content, nanotechnology-based formulations are also being tested to provide sustained release and minimize adverse effects. Another reason for developing nanoformulations of tretinoin is its proven instability in the presence of light and oxygen [48]. Isotretinoin is the main degradation product of tretinoin when exposed to light [49].
Different types of nanocarriers with tretinoin have been developed [50–60]. In most studies, in vitro permeation studies and drug release tests were performed. Some authors have also performed in vivo skin irritation tests in rats [50, 56] or rabbits [52] comparing the developed nanoformulations with conventional formulations containing tretinoin. Overall, prolonged release of the active ingredient, improved stability, and reduced irritation were achieved. In addition, Yamaguchi et al. [56], who developed CaCO3-coated nanomicelles containing tretinoin, evaluated the anti-wrinkle properties of the formulation by performing in vivo study in mice. The results showed better pharmacological effects of the nanoformulation (0.1%) compared to the tretinoin–Vaseline ointment (0.1%), including thicker epidermis and overexpression of heparin-binding epidermal growth factor mRNA as well as increased production of hyaluronan between epidermal layers [56].
Nasrollahi et al. [60] also studied for the first time in vivo the safety of two formulations containing tretinoin (0.05%)—nanoemulsions and NLCs—by conducting a 1-week controlled clinical trial with 20 volunteers. The tested formulations were reasonably safe for topical application on the basis of the evaluated parameters: skin pH, erythema index, skin hydration, and TEWL. Similarly, Lima et al. [55] conducted a clinical study and evaluated skin irritation of the developed gel formulation containing tretinoin NLCs (0.05%) compared to the marketed tretinoin (0.05%). Twenty-eight participants were enrolled in the study over a 4-week period, and by measuring TEWL, as a non-invasive physiological measure of skin barrier function, it was shown that skin irritation was higher with the conventional tretinoin formulation. Since the performed studies included only a small number of participants over a short time with application to the forearm, the formulations need further investigation.
The main goal of developing innovative nano-delivery systems containing tretinoin is to provide a safe formulation with satisfactory user compliance [61]. However, to date, there is no commercially available nanoformulation with tretinoin on the market.
Isotretinoin
Isotretinoin is a cis-isomer of retinoic acid approved for oral administration in capsules for the treatment of acne, with a well-documented adverse effect profile ranging from xerosis to teratogenicity [62]. The efficacy of isotretinoin (0.1% or 0.05%) for topical application in antiaging treatments of photodamaged skin has been investigated in several studies [63–66]. Maddin et al. [63] conducted a 36-week, multicenter, double-blind, placebo-controlled study with 800 patients and showed that the application of 0.1% isotretinoin cream improved the overall appearance of photodamaged skin compared to the vehicle-controlled group. Similar results were presented by Griffiths et al. [66], who conducted a 6-month, multicenter, randomized, double-blind, vehicle-controlled clinical trial involving 346 subjects with photodamaged skin. It was confirmed that the application of 0.05% isotretinoin in combination with sunscreens improved the appearance of the fine wrinkles associated with photoaged skin [66]. The results were consistent with previously performed studies by other authors [64, 65]. It can be concluded that most of the performed studies about topical application of isotretinoin for photodamaged skin showed statistically significant improvement in the overall appearance of the skin including fine lines and pigmentation.
However, the use of topical isotretinoin formulations is limited because of skin irritation potential and physicochemical instability [67]. Therefore, some authors have developed nanoformulations with isotretinoin to overcome the problems associated with the use of conventional topical formulations, but most are intended for acne treatment [67–73].
Raza et al. [69] developed a hydrogel formulation containing isotretinoin-loaded NLCs (0.05%) for the treatment of photoaged skin. The formulation was evaluated by in vivo study for its antiaging efficacy on extrinsically photoaged mouse skin by assessing the biochemical and macroscopic properties of the skin in comparison to two commercially available formulations containing tretinoin and isotretinoin [69]. The nanoformulation with isotretinoin was better tolerated and more effective compared to the evaluated commercial formulations. However, the concentration of the active ingredients in the compared formulations was not clearly stated. Further studies are needed to confirm the promising potential of the developed nanoformulation.
Alitretinoin
Alitretinoin is a retinoid that binds to all subclasses of RARs and RXRs [74]. Alitretinoin gel at doses of 0.1% and 0.05% is approved as a medicine for the topical treatment of Kaposi’s sarcoma. To our knowledge, the efficacy of 0.1% alitretinoin gel in the treatment of photoaged skin has been studied only in one small, 16-week open-label pilot study with 20 participants [75]. The formulation improved evaluated skin parameters, but with a high incidence of side effects.
Retinol
Retinol is a compound with multifunctional effects on photodamaged skin, including induced production of hyaluronic acid, collagen, and elastin, as well as epidermal proliferation and differentiation [76–78].
Compared to tretinoin, retinol is about tenfold less potent [10, 14]. For example, retinol at a concentration of 0.25% is considered to be as effective as 0.025% tretinoin, without significant side effects and irritation [79]. The maximum recommended concentration of retinol in hand and face creams and other leave-on or rinse-off cosmetics is 0.3% [80].
Compared to other retinoids in cosmeceuticals, retinol is the most evaluated by randomized, double-blind, vehicle-controlled, whole, or split-face clinical trials [81]. Formulations with different retinol concentrations (from 0.075% to 0.5%) have been evaluated for their antiaging efficacy. Some authors reported statistically significant improvements in the assessed parameters compared to vehicle [82–85], while others did not confirm positive treatment outcome [86]. However, as suggested by Spierings [81], considering the limitations of the performed studies, the wide range of concentrations used, and the declared conflicts of interest in the sponsored studies, it is questionable whether the positive study results truly reflect the efficacy of the active ingredient in the product.
To prove the efficacy of retinol, some authors have also conducted comparison clinical studies with tretinoin. Babcock et al. [87] conducted a clinical study and compared the efficacy of three sustained-release cosmetic formulations containing 0.25%, 0.5%, or 1.0% retinol with prescription creams containing 0.025%, 0.05%, or 0.1% tretinoin. No statistically significant differences were found in the assessed efficacy parameters between retinol and tretinoin formulations [87] (Table 2). Recently, a 12-week, double-blind, controlled clinical trial was performed comparing the performance of three retinol serums (0.25%, 0.5%, 1.0%) and tretinoin creams (0.025%, 0.05%, 0.1%) by following a step-up protocol to increase the dose of the applied formulation in combination with a moisturizer [88]. The retinol formulations performed equivalent to or better than the tretinoin creams.
Table 2.
Comparison clinical studies: tretinoin vs other topical retinoids in retinoid formulations used in facial antiage treatments
| Retinoid | Clinical study | Duration | Subjects | Comparison | Evaluated parameters | Results | References |
|---|---|---|---|---|---|---|---|
| Retinol | Randomized, double-blind, split-face comparison clinical study | 12 weeks | 65 | Retinol 0.25%, 0.5%, and 1.0%, vs tretinoin 0.025%, 0.05% and 0.1% | Overall photodamage, fine lines/wrinkles, coarse lines/wrinkles, skin tone brightness, mottled pigmentation, tactile roughness | Statistically significant improvements from baseline in all efficacy parameters. No statistically significant differences in efficacy between the formulations was observed | [87] |
| Retinol | Randomized, parallel, double-blind, whole-face, controlled clinical study | 3 months | 120 | Retinol 0.2%/tetrahydrojasmonic acid 2% cream vs tretinoin 0.025% cream |
Clinical efficacy evaluations of wrinkles (frontal and glabellar area, eyes area, nasolabial fold), pores, mottled pigmentation and global photodamage Three-dimensional measurements of the periorbital skin relief (wrinkles, fine lines, and skin texture) was performed |
Both products improved considerably wrinkles, mottled pigmentation, pores, and global photodamage. No statistically significant differences between both products were observed | [90] |
| Retinol | Double-blinded, single-center, parallel-arm, whole-face, comparison study | 24 weeks | 24 | Formulation with retinol (1%), retinyl acetate (0.05%), and retinyl palmitate (0.05%) vs tretinoin 0.02% | Wrinkles, pigmentation, yellowing, and overall clinical severity of the photodamage as scored on the Griffiths scale for assessment of the global photodamage | No significant differences in efficacy between the retinoid formulation and tretinoin 0.02% in Griffiths photoaging scores | [91] |
| Retinaldehyde | Randomized, double-blind, vehicle-controlled, whole-face clinical trial | 44 weeks | 125 | Retinaldehyde 0.05% vs tretinoin 0.05% vs vehicle | Optical profilometry of skin silicone replica, assessing for deepness and roughness of the wrinkles |
Significant reduction of the wrinkle and roughness features No statistically significant differences between the treatment groups at any assessment time point was observed |
[107] |
| Retinaldehyde | Randomized, double-blind, split-face comparison study | 12 weeks | 30 | Retinaldehyde proretinal hydrogel containing 0.025% retinoid vs 0.025% tretinoin hydrogel | Evaluation of skin aging (fine lines and wrinkles) and seven skin parameters, namely surface, volume, energy, entropy, homogeneity, contrast, and variance values | Retinaldehyde nanoformulation showed better antiaging efficacy in the evaluated parameters compared to tretinoin | [110] |
| Adapalene | Multicenter, randomized, investigator-blinded, whole-face, parallel-group comparison study | 24 weeks | 128 | 0.3% adapalene gel vs 0.05% tretinoin cream | Evaluation of the global cutaneous photoaging, periorbital and forehead wrinkles, ephelides/melanosis and actinic keratosis |
Significant improvement of the evaluated parameters: global cutaneous photoaging, periorbital and forehead wrinkles, ephelides/melanosis, and actinic keratosis No statistically significant difference between the treatments was observed |
[158] |
| Tazarotene | Prospective weekly multicenter, investigator-masked, whole-face, randomized, vehicle-controlled, parallel group study | 24 weeks | 349 | 0.01%, 0.025%, 0.05%, and 0.1% tazarotene cream vs 0.05% tretinoin emollient vs vehicle | Fine wrinkling, mottled hyperpigmentation, lengthiness, irregular depigmentation, tactile roughness, coarse wrinkling, telangiectasia, pore size, elastosis, actinic keratoses, and overall integrated assessment of the individual signs of photodamage | Significant improvement was observed for the mottled hyperpigmentation, fine wrinkling, and the overall integrated assessment of the individual signs of photodamage compared to vehicle. Tazarotene 0.05% and tretinoin 0.05% produced comparable results. Tazarotene 0.1% was the most effective among the tested formulations | [150] |
| Tazarotene | Multicenter, double-blind, randomized, whole-face, parallel-group study | 24 weeks | 173 | 0.1% tazarotene vs 0.05% tretinoin emollient cream | Fine wrinkling, mottled hyperpigmentation, coarse wrinkling, irregular depigmentation, lentigines, elastosis, tactile roughness, telangiectasia, and actinic keratoses |
Tazarotene 0.1% cream offers significantly faster improvement compared to tretinoin 0.05% emollient cream in the treatment of photodamaged skin Tazarotene efficacy was significantly better for fine wrinkling and the overall integrated assessment of photodamage, mottled hyperpigmentation, and coarse wrinkling |
[151] |
Kong et al. [89] evaluated the effects on the epidermis of the weekly application of 0.1% tretinoin, 0.1% retinol, and a base formulation on the forearm under occlusion for 1 day during a 4-week period in six participants. Compared to the vehicle control group, both 0.1% retinol and 0.1% tretinoin formulation significantly increased epidermal thickness and upregulated the genes for collagen type I and III [89]. However, the retinol effects were approximately two-fold lower compared to tretinoin. Additionally, the efficacy of 0.1% retinol formulation in the reduction of facial wrinkles was confirmed by facial image analysis in a 12-week study that included 41 healthy women [89]. The study did not include a control group.
Retinol is often combined with other active ingredients to achieve synergistic effects and positive clinical results. Bouloc et al. [90] compared the efficacy of a commercial cosmetic product with 0.2% retinol and 2% tetrahydrojasmonic acid to a 0.025% tretinoin formulation. Results indicated that formulations provided comparable effects but the retinol formulation was better tolerated and perceived by participants (Table 2).
Recently, Chien et al. [91] published a study in which a topical formulation containing a combination of three retinoids (1.1%: retinol (1%), retinyl acetate (0.05%), and retinyl palmitate (0.05%)) was compared in terms of antiaging efficacy and tolerability with a topical tretinoin formulation (0.02%), involving patients with moderate to severe facial photodamage. The study suggests that cosmeceuticals containing a combination of retinol and retinyl esters may have a comparable antiaging effect to tretinoin (Table 2).
Performed clinical studies for the antiaging efficacy of retinol have limitations including small sample size and lack of a vehicle control group which limits the robustness of the results and the validity of the conclusions. Studies for topical treatments need to be vehicle controlled as vehicle alone can also produce significant improvements on the skin [81]. However, in practice vehicle-controlled clinical studies are rare.
The need for stabilization of retinol formulations is well known because of its sensitivity to light, oxygen, heat, and heavy metals [80, 92]. A recent study that evaluated the stability of 12 commercially available products with declared retinoids confirmed the instability of retinoids in almost all cosmetic products by long-term and accelerated stability testing [92]. It is recommended that retinol in cosmetics is stabilized through appropriate formulation, since retinol in cosmetic formulations is generally stable for less than 6 months if manufactured under an inert atmosphere and stored, for example, in aluminum tubes at 20 °C or below [80]. Considering that such products are not manufactured and stored in this way in practice, the validity of the results from the performed studies is questionable and cannot be attributed solely to the retinol effect [81]. However, the stability of retinoids in cosmetics is formulation-dependent and must be evaluated on a case-by-case basis [92]. In addition, Temova Rakuša et al. [93] evaluated the content-related quality of 35 commercially available cosmetic products containing retinol and other retinoids (mainly retinyl palmitate) and found significant inconsistencies in labeling and deviations from the declared content, e.g., a very low content or a much higher concentration than the maximum concentration recommended by the Scientific Committee on Consumer Safety at the European Commission. Considering the study findings and the fact that most cosmetic products do not indicate the active ingredient content on the packaging, which is important for assessment of the product safety and efficacy, stricter regulations and quality controls are recommended [93].
Different methods are being investigated to improve the stability of retinol cosmetic formulations, including nanotechnology. Consequently, various nanoformulations with retinol have been developed [94–98]. Boskabadi et al. [94] developed SLNs with retinol (0.5%) by ultrasonication procedure and incorporated them into gel formulations. The results of the in vitro cytotoxicity, in vitro permeation, and in vivo skin irritation testing in rats were favorable, suggesting that SLNs could be suitable carriers for topical delivery of retinol. Jun et al. [96] developed a cream containing vacuum-assisted lipid nanoparticles with retinol (0.1% or 0.3%) and evaluated the permeation properties of the formulation in an in vitro study on porcine skin and its effects on a reconstructed 3D human skin model. The developed formulation with retinol-loaded lipid nanocarriers exhibited beneficial properties such as improved penetration, low irritation profile, and increased epidermis thickness compared to the other tested formulations in the study [96].
Clinical studies evaluating the antiaging effects of nanoformulations containing retinol are lacking.
Retinaldehyde
As a natural precursor of retinoic acid with a favorable activity/tolerance ratio after topical application, retinaldehyde is considered the most effective retinoid in cosmeceuticals [11, 99, 100]. Oxidation of retinaldehyde to tretinoin is differentiation-dependent and more efficiently performed by differentiated keratinocytes [101, 102]. The low irritation profile of retinaldehyde is due to the limited capacity of keratinocytes, since topically applied retinaldehyde does not bind to retinoid receptors, but is predominantly converted to retinyl esters and only a small fraction is metabolized to tretinoin, which is responsible for the biological activity [101, 103]. It is considered that skin irritation is partly a result of the receptors’ “overload” with non-physiological amounts of exogenous tretinoin [102] and with retinaldehyde as a precursor this process is prevented [100, 101].
Compared to retinol esters, the efficacy of retinaldehyde in antiaging treatments is supported by stronger evidence. Several studies evaluated the efficacy of retinaldehyde on aged and photoaged skin [104–106]. Only Creidi et al. [107] performed a comparison clinical study and evaluated the efficacy and tolerability of 0.05% retinaldehyde cream compared to 0.05% tretinoin cream and vehicle in patients with photodamaged skin (Table 2). Both formulations were effective on the basis of the assessed changes of the wrinkle and roughness features, but tretinoin caused more local irritation and lower compliance in patients. However, no statistically significant differences in assessed parameters were observed between retinaldehyde and tretinoin or between retinaldehyde and vehicle group (Table 2). Recently, Kwon et al. [108] conducted a 3-month randomized, double-blind, controlled clinical trial comparing the efficacy of 0.1% and 0.05% retinaldehyde creams in 40 women. The data showed improvement in photoaged skin in terms of hydration, wrinkles, skin roughness, and reduction in TEWL, although no vehicle control group was included. The only statistically significant difference between the two groups was observed in the melanin index, which was higher in 0.1% retinaldehyde group [108].
Formulations containing retinaldehyde at a concentration of 0.05% are considered effective and well tolerated [100]. Topical application of retinaldehyde, even when applied at higher concentrations or during a longer period, is also not associated with detectable change in the constitutive levels of plasma retinoids due to skin metabolism [109]. Retinaldehyde is also a highly unstable molecule prone to autooxidation and photooxidation [110]. Various formulations based on nanotechnology are being developed for retinaldehyde as a compound with high antiaging potential and physicochemical instability.
Pisetpackdeekul et al. [110] developed proretinal nanoparticles for controlled release of retinaldehyde by grafting retinaldehyde on chitosan. The formulation was stability tested and evaluated for its irritation potential and histological changes in the skin by performing tests on rats and human volunteers. In addition, the antiaging efficacy of the hydrogel formulation with proretinal nanoparticles was evaluated in comparison with a 0.025% tretinoin hydrogel (Table 2). The controlled release of the active ingredient resulted in improved stimulation of the epidermal proliferation with minimized side effects [110]. Similarly, by performing an in vitro cytotoxicity study on keratinocytes, an in vivo skin irritation, and a biological activity study on rats’ skin, Limcharoen et al. [111] suggested that proretinal nanoparticles can be used as an effective system for sustained delivery of retinaldehyde.
Nayak et al. [112] developed NLCs loaded with coenzyme Q10 and retinaldehyde (0.05%) and evaluated the efficacy and safety of different formulations with in vitro release and cytotoxicity, ex vivo skin permeation, and in vivo skin irritation and therapeutic efficacy testing on wrinkles in mice. The NLCs gel formulation with both active ingredients showed a significant reduction in wrinkles and better safety profile than other tested formulations.
Further studies are needed to evaluate the clinical efficacy of retinaldehyde in various cosmetic formulations.
Retinyl Palmitate
Retinyl palmitate (RP) is the most abundant form of retinol esters found endogenously in the skin [113]. As a thermally stable alternative to retinol, it is widely used in antiaging cosmetics. However, compared to retinol, RP is more prone to photodegradation [114]. It has been shown that RP can be a prooxidant under certain conditions, such as photo-irradiation, through its toxic photodegradation products, generation of reactive oxygen species, induction of lipid peroxidation, and DNA damage [113, 115]. In addition, there is evidence that topical application of cosmetics containing RP may enhance UVR-induced photococarcinogenesis [116]. The long-term consequences of the use of RP-containing cosmetics have not been thoroughly investigated [117]. However, on the basis of current knowledge and available data, RP is considered a safe cosmetic ingredient when used in current practice and at the recommended concentrations of 0.05% in body lotions and 0.3% (retinol equivalents) in creams and leave-on or rinse-off products [80]. RP at a concentration of 0.6% is half as active compared to 0.25% retinol [79].
No published clinical study has exclusively investigated the use of RP as an antiaging agent under normal conditions of use. There is also only one published study that compared the antiaging effects of two different commercial cosmetic products containing RP [118]. However, the RP content in the formulations was not disclosed. In general, most of the studies that evaluated the efficacy of retinol esters as antiaging agents in cosmetics have methodological issues and limitations [119, 120]. Therefore, there is a clear lack of evidence from adequately designed and controlled clinical trials to support the use of RP for antiaging purposes.
Nevertheless, RP is a very popular cosmetic ingredient and there is ongoing research to improve its stability and efficacy through various formulation approaches, including nanoformulations [54, 121–128], microencapsulation [124, 129], or combination with other ingredients such as pectin [124, 130]. Most of the developed formulations have been evaluated by various in vitro or ex vivo tests, including release and skin permeation studies [54, 121, 124, 125, 127, 128, 131], skin irritation test [123], in vitro safety profile and in vitro antioxidant activity [126]. Some authors went a step further and evaluated the developed formulations with in vivo studies. Jeon et al. [128] developed SLNs with RP and incorporated them in hydrogel (1000 IU/g and 2500 IU/g as active vitamin A, RP content). The formulations were evaluated with in vivo anti-wrinkle study in mice. On the basis of the results, it was suggested that SLNs with RP can be used for the development of effective anti-wrinkle formulations. Moreover, Pena-Rodríguez et al. [122] developed transfersomes with RP (1.1%) integrated into liposome membrane with a predicted shelf life of 36 months. The in vitro study showed increased penetration of RP into the skin compared to the formulation with free RP. In addition, an in vivo study including six volunteers evaluated the compatibility with the skin of the emulsion prepared with RP transfersomes (0.55%) [122]. Measurements of TEWL and penetration of RP into the stratum corneum showed that the emulsion with RP transfersomes did not affect the skin integrity. All studies performed showed beneficial properties of the developed topical nanoformulations such as improved formulation stability, higher penetration, and low skin irritation. Similar to other retinoids contained in nanoformulations, comprehensive in vivo and clinical studies of RP formulations are needed, as most published studies include only in vitro evaluations.
Interestingly, there is no published, well-designed clinical study that confirms the antiaging effects of RP on the skin, but compared to other retinoids, it is a very popular compound for developing new formulations. Overall, there is a lack of clinical evidence supporting the use of RP as an antiaging agent in both conventional and nanoformulations.
Retinyl Propionate
Retinyl propionate is a retinyl ester that is considered to have a stronger retinoid activity compared to retinol and RP, as shown by Bjerke et al. [132] in in vitro and ex vivo skin models. It is also the first retinyl ester that has been clinically evaluated by Green et al. [133], who performed a double-blind, randomized, placebo-controlled 48-week study with retinyl propionate cream (0.15%). Although minimal improvement trends were noted in photoaged skin, no statistically significant effects were observed compared to vehicle. Some studies showed that combining retinyl propionate with other agents may increase its efficacy in antiaging treatments [134, 135]. To our knowledge, no published studies are addressing the incorporation of retinyl propionate into nanotechnology delivery systems. In light of recent findings identifying retinyl propionate as a compound with pronounced antiaging potential, further research is needed.
Retinyl Acetate
Retinyl acetate is a popular retinoid derivative used as a commercial cosmetic ingredient in antiaging products [136]. To date, no published clinical study has evaluated the antiaging efficacy of retinyl acetate as a single active ingredient in a topical formulation. Retinyl acetate has been studied as a cocarcinogen, but the current evidence on its effects on photocarcinogenesis does not provide a consistent mechanistic explanation for the results of different studies [115]. Arayachukeat et al. [136] developed polymeric nanoparticles containing retinyl acetate to improve the photostability of the compound. The results showed that the delivery of retinyl acetate to the dermis could be achieved by a suitable polymeric carrier.
Retinyl Retinoate
Retinyl retinoate was synthesized by a condensation reaction between retinol and retinoic acid to achieve improved photostability [137]. The compound is considered more stable and less irritating compared to other first-generation retinoid derivatives with comparable ability to induce hyaluronan production [138]. The efficacy of retinyl retinoate in the treatment of fine wrinkles in photoaged skin is also supported by clinical data [139–141]. Considering that the performed studies have limitations, including a small sample size, further studies are needed to prove the potential of retinyl retinoate as an antiaging agent.
Lee et al. [142] developed different nanoformulations with retinyl retinoate and investigated in vitro the active ingredient release. The study suggests that NLCs may be a suitable delivery system for topical application of retinyl retinoate.
Retinyl N-Formyl Aspartamate
RetinylN-formyl aspartamate is a retinoid derivative with higher photostability and lower irritation potential compared to retinol [143]. Its efficacy on photoaged skin was investigated in a 24-week, prospective, single-blind, randomized, controlled split-face study with 24 participants [144]. The retinyl N-formyl aspartamate cream was well tolerated when applied twice daily and significantly improved the assessed parameters compared to vehicle. However, the study has limitations, including small sample size.
Overall, there is a lack of evidence supporting the use of the commonly used retinyl esters in antiaging cosmetics (RP, retinyl propionate, retinyl retinoate, retinyl acetate).
2nd Generation
Currently, second-generation retinoids are not used as active ingredients in formulations for topical application [145].
3rd Generation
Tazarotene
Tazarotene is a prodrug of tazarotenic acid with selectivity toward RAR-β and RAR-γ but not toward RXRs [22]. It is currently regulated as medicine and approved in topical formulations (0.045%, 0.05%, or 0.1%) for the treatment of psoriasis and acne. Also, the 0.1% formulation of tazarotene is approved as an adjunctive agent for the treatment of facial fine wrinkling, pigmentation, and benign lentigines.
The use of 0.1% tazarotene for treatment of photodamage is considered safe and effective with mild to moderate adverse effects [146]. Kang et al. [147] conducted a 24-week, multicenter, double-blind, randomized, vehicle-controlled, parallel-group study involving 568 patients with photodamaged skin. The once-daily 0.1% tazarotene cream was significantly more effective than the vehicle in reducing fine wrinkles, mottled hyperpigmentation, lentigines, irregular depigmentation, apparent pore size, elastosis, tactile roughness, and the overall assessment of photodamage [147]. The results were consistent with the previously published studies [148, 149].
Some authors also performed clinical comparison studies with tretinoin. Kang et al. [150] conducted a clinical study and compared the efficacy of different concentrations of tazarotene creams with 0.05% tretinoin cream. The 0.1% tazarotene cream was as effective as standard tretinoin cream and produced only mild to moderate side effects (Table 2). Similar results were obtained in a study conducted by Lowe et al. [151], suggesting that 0.1% tazarotene cream may provide comparable efficacy with a faster improvement compared to 0.05% tretinoin cream (Table 2). Compared to tretinoin, tazarotene has been associated with a more frequent occurrence of a burning sensation on the skin, but only during the first week of treatment [151].
To our knowledge, there are no recent clinical trials on tazarotene in antiaging treatments, and most published studies examine its efficacy in the treatment of acne.
Generally, the conducted studies suggest that topical treatments with tazarotene and tretinoin have similar tolerability and frequency of adverse effects, although tazarotene, as a representative of the selective retinoids, is expected to be better tolerated. In practice, tretinoin is well established and commonly used for the treatment of aged skin. Considering the similar performance of the two agents in antiaging treatments, perhaps the higher price is one of the reasons why tazarotene formulations are not as widely used in clinical practice as tretinoin.
Although tazarotene has confirmed potential for antiaging treatments, new nanotechnology-based formulations are being evaluated for other indications such as wound healing [152] and psoriasis [153].
Adapalene
The activity of adapalene is selectively mediated via RAR-β and RAR-γ receptors [154]. In topical formulations at a concentration of 0.1% or 0.3%, adapalene is used to treat acne with clinically confirmed efficacy and tolerability but is also used off-label for treatment of photoaging [155]. Kang et al. [156] conducted a prospective, randomized, controlled, masked, two-center, parallel-group study and evaluated the efficacy of adapalene gel at two concentrations (0.1% and 0.3%) compared with vehicle in patients with actinic keratoses and solar lentigines. The 9-month study showed that adapalene significantly improved the assessed parameters of the photoaged skin with acceptable tolerability compared to the vehicle-treated group. Herane et al. [157] also recommended 0.3% adapalene gel as an effective and safe treatment for facial photodamage, as demonstrated by the 6-month open-label study in Chilean women with cutaneous photoaging, although no vehicle control group was included in the study.
In addition, a comparison study conducted by Bagatin et al. [158] showed that the clinical efficacy of 0.3% adapalene gel was comparable to 0.05% tretinoin cream (Table 2). Therefore, 0.3% adapalene gel is recommended as an effective treatment in patients with mild or moderate photoaging [158]. Pinto et al. [54] developed NLCs formulation with 0.1% adapalene by miniemulsion method and performed an in vitro release experiment using the equilibrium dialysis method. The new formulation showed favorable properties from a technological perspective, but further studies are needed to reveal the potential of the formulation for antiaging treatments. Other nanoformulations containing adapalene are being developed and evaluated specifically for the treatment of acne [159, 160].
Bexarotene
Bexarotene is a selective agonist of all RXRs subtypes with a complex and not yet fully evaluated mechanism of action [161]. As a 1% gel, bexarotene is used for topical treatment of IA and IB persistent or refractory cutaneous T cell lymphoma. To our knowledge, there is no published study that has investigated the use of bexarotene in antiaging treatments.
4th Generation
Trifarotene
Trifarotene is currently approved in topical formulations as a 0.005% cream for the treatment of acne [162]. Its efficacy and safety profile for acne treatment is also being clinically evaluated [163, 164]. Trifarotene, as a selective RAR-γ agonist with greater than 20-fold higher selectivity over RAR-α and RAR-β receptors, has confirmed depigmenting and antipigmenting activity in animal models [165]. It is suggested that trifarotene, as a selective agonist of RAR-γ (the predominant isoform in the dermis), has potential for the treatment of photoaging, as it may activate the same pathways induced by tretinoin [166]. On the basis of the results from preclinical studies, trifarotene is expected to have a better profile compared to earlier generation retinoids. However, to date, there is no published comparison clinical study that directly evaluates the superiority of trifarotene in terms of efficacy and tolerability. Therefore, the use of trifarotene as an antiaging agent requires further research and clinical evaluation. Since it is a new generation of retinoids, new formulations are also likely to be developed in near future.
Seletinoid G
Seletinoid G is a synthetic retinoid with selectivity toward RAR-γ [22]. Kim et al. [23] conducted an in vivo study in which formulations containing 1% seletinoid G, 0.1% tretinoin, or vehicle were applied occlusively to the buttock skin of 17 participants for 4 days. The results showed that seletinoid G affected the expression of the assessed extracellular matrix proteins and connective tissue-degrading enzymes similarly to tretinoin, and it was suggested that it could repair altered connective tissue in old skin and inhibit UV-induced collagen deficiency in young skin [23]. Similarly, a skin irritation test with three different concentrations of seletinoid G (0.25%, 0.5%, and 0.1%) and tretinoin (0.025%, 0.05%, and 0.1%) was also performed on six volunteers [23]. Seletinoid G showed no irritation on the treated area compared to tretinoin. However, the results cannot be generalized because the study has some limitations. A recent in vitro study has also shown that seletinoid G is a potent compound for antiaging treatments without significant side effects, as it improves skin barrier function by promoting keratinocyte migration and restoring abnormal collagen deposition in the dermis [167]. To date, there is no published clinical study investigating the antiaging effect of seletinoid G under normal conditions of use. Although seletinoid G shows promising results as an antiaging agent, its potential has yet to be evaluated.
Discussion
Clinical Evidence for Conventional Formulations
Cosmeceuticals containing retinoids are freely available on the market compared to the prescribed formulations regulated as medicines. Customers expect cosmeceuticals to offer penetration of the active ingredient into the skin and clinical effects after topical application [13]. However, cosmeceuticals are not regulated as medicines in terms of safety, quality, and efficacy and often contain ingredients for which there is no clinical evidence to support their use [14]. The results of published studies suggest that tretinoin, included in formulations regulated as medicines, is the most well-established retinoid with solid evidence of antiaging efficacy and approved indications for treatment of wrinkles and photoaging.
Overall, there is limited evidence from clinical research evaluating the antiaging efficacy of other retinoids compared to tretinoin as the gold standard. Comparisons with tretinoin by whole or split-face clinical studies are performed for retinol, retinaldehyde, adapalene, and tazarotene. Only retinol and retinaldehyde are found in cosmeceuticals, while adapalene and tazarotene are regulated as medicines that are also used for antiaging indications. Adapalene and tazarotene are recommended as alternatives to tretinoin in patients with sensitive skin [47]. Retinol is the most clinically studied retinoid in cosmetics, while retinaldehyde is considered the most promising. On the other hand, retinyl palmitate is the most popular model compound for the development of new formulations, although its use as an antiaging agent is not supported by clinical evidence. Also, there is a lack of clinical evidence for the antiaging efficacy of the other retinyl esters since most of the performed studies have limitations, such as a small number of participants and/or a lack of a vehicle control group. In general, placebo-controlled double-blind studies as a standard in clinical research are lacking for cosmetics [168]. Considering the limitations of the clinical studies performed, the use of retinoid cosmetics as a tretinoin equivalent is not justified and evidence-based. Our findings are consistent with previous studies that also suggested that in the case of cosmeceuticals, the positive study outcome cannot be considered relevant information for clinical decision-making because most of the performed studies have significant methodological issues and deficiencies in study design that limit the relevance of the results [81, 168].
Although the cosmetic market is constantly growing and many products are marketed with unrealistic claims, there is a lack of validated methods for evaluation of the product efficacy and safety through comprehensive and standardized testing. As a result of the competitiveness of the cosmetic industry and short product development cycles, cosmeceuticals are being marketed with little or no clinical evaluation. Therefore, there is a need for trustworthy evidence from high-quality designed clinical studies that can guide clinicians in their decision-making about treatment options and not just serve as promotional material for the cosmetics industry.
Clinical Evidence for Nanoformulations
Overall there is a lack of clinical studies evaluating the safety and efficacy of nanoformulations compared to conventional formulations. From the literature review, most of the performed studies evaluated the nanoformulations containing retinoids by in vitro/in vivo permeation tests and in vivo irritation tests. To our knowledge, only one study including human volunteers clinically evaluated the antiaging effect of the developed nanoformulation with retinaldehyde and compared it with tretinoin [110].
One of the reasons for the small number of nanoformulations on the market is the lack of robust in vitro studies with confirmed in vitro/in vivo correlations that can be used instead of clinical studies [169]. Currently, the risk/benefit ratio of the topical nanoformulations is evaluated on a case-by-case basis because of the lack of harmonized quality standards and regulatory frameworks [169]. Since clinical trials are not required for cosmetics, there is also a lack of evidence to support the claimed efficacy of cosmetic nanoformulations.
The use of nanomaterials in cosmetics requires a comprehensive safety assessment, as exposure via topical application may pose a risk for adverse effects from insoluble and persistent nanoparticles that can reach unintended sites in the body and interact with biological entities at the molecular level [170]. One way to bridge the current gap between research and commercialization of innovations and realize the full potential of new delivery systems is to implement analytical and in vitro/in vivo evaluation strategies for their characterization [171].
As a result of restrictive regulations in some countries, researchers are also looking for alternatives to animal testing. For example, the Cosmetics Regulation (EC) No. 1223/2009 in Europe prohibits animal testing for cosmetics and marketing of cosmetic ingredients/products tested on animals since March 2013 [170]. Therefore, toxicological data must be obtained from scientifically validated alternatives such as in vitro and ex vivo studies, in silico models, and the use of physiologically based pharmacokinetic and toxicokinetic modeling [170]. Overall, the development of targeted delivery systems with efficiently incorporated active ingredients, low irritability when applied to the skin, and control over the potential toxicity of the used nanomaterials is complex.
In general, nanoformulations containing retinoids are advantageous compared to conventional formulations because they are more stable, penetrate the skin better, produce a modified release of the active ingredient at the site of action, and cause fewer side effects and irritation. The results of the performed studies highlight the promising potential of nanoformulations for topical delivery, but the preliminary results need to be supported by additional in vivo testing and clinical studies. However, translating the results into clinical practice and scaling up the production are challenging because most studies use small batch formulations. Since most published results are from preclinical studies, there is a need for comprehensive clinical evidence for evaluation of the benefits and risks of nanoformulations for topical use. The use of retinoids containing nanoformulations for topical antiaging treatments requires further research.
Conclusion
Most of the performed studies emphasized the importance of an appropriate combination of active ingredients, excipients, and technological processes to obtain a stable cosmetic product that is effective and well tolerated. When a suitable formulation is used, significant clinical effects on the skin are obtained as with tretinoin, but with fewer side effects. Consequently, such a formulation leads to better user compliance and satisfaction. However, considering the limitations of the performed studies, there is a need for additional studies that will provide new information and insights to support the use of different retinoids in antiaging treatments.
Acknowledgements
Funding
No funding was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and design. The literature search and data analysis were performed by Daniela Milosheska. All authors drafted and/or critically revised the work. All authors read and approved the final manuscript.
Disclosures
Daniela Milosheska and Robert Roškar have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article because no datasets were generated or analyzed during the current study.
References
- 1.Shin JW, Kwon SH, Choi JY, et al. Molecular mechanisms of dermal aging and antiaging approaches. Int J Mol Sci. 2019;20(9):E2126. doi: 10.3390/ijms20092126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27(5):729–738. doi: 10.1177/0963689717725755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganguly B, Hota M, Pradhan J. Skin aging: implications of UV radiation, reactive oxygen species and natural antioxidants. In: Ahmad R, editor. Biochemistry. IntechOpen. 2022. https://www.intechopen.com/chapters/78654. Accessed 1 Aug 2022.
- 4.Krutmann J, Schalka S, Watson REB, Wei L, Morita A. Daily photoprotection to prevent photoaging. Photodermatol Photoimmunol Photomed. 2021;37(6):482–489. doi: 10.1111/phpp.12688. [DOI] [PubMed] [Google Scholar]
- 5.Lephart ED. Skin aging and oxidative stress: equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res Rev. 2016;31:36–54. doi: 10.1016/j.arr.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Baumann L. Skin ageing and its treatment. J Pathol. 2007;211(2):241–251. doi: 10.1002/path.2098. [DOI] [PubMed] [Google Scholar]
- 7.McCullough JL, Kelly KM. Prevention and treatment of skin aging. Ann N Y Acad Sci. 2006;1067:323–331. doi: 10.1196/annals.1354.044. [DOI] [PubMed] [Google Scholar]
- 8.Kim BH. Safety evaluation and anti-wrinkle effects of retinoids on skin. Toxicol Res. 2010;26(1):61–66. doi: 10.5487/TR.2010.26.1.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zasada M, Budzisz E. Retinoids: active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postepy Dermatol Alergol. 2019;36(4):392–7. [DOI] [PMC free article] [PubMed]
- 10.Riahi RR, Bush AE, Cohen PR. Topical retinoids: therapeutic mechanisms in the treatment of photodamaged skin. Am J Clin Dermatol. 2016;17(3):265–276. doi: 10.1007/s40257-016-0185-5. [DOI] [PubMed] [Google Scholar]
- 11.Sorg O, Antille C, Kaya G, Saurat JH. Retinoids in cosmeceuticals. Dermatol Ther. 2006;19(5):289–296. doi: 10.1111/j.1529-8019.2006.00086.x. [DOI] [PubMed] [Google Scholar]
- 12.Hubbard BA, Unger JG, Rohrich RJ. Reversal of skin aging with topical retinoids. Plast Reconstr Surg. 2014;133(4):481e–e490. doi: 10.1097/PRS.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 13.Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3(2):22–41. [PMC free article] [PubMed] [Google Scholar]
- 14.Tetali B, Fahs FM, Mehregan D. Popular over-the-counter cosmeceutical ingredients and their clinical efficacy. Int J Dermatol. 2020;59(4):393–405. doi: 10.1111/ijd.14718. [DOI] [PubMed] [Google Scholar]
- 15.Resende DISP, Ferreira MS, Lobo JMS, Sousa E, Almeida IF. Skin depigmenting agents in anti-aging cosmetics: a medicinal perspective on emerging ingredients. Appl Sci. 2022;12(2):775. doi: 10.3390/app12020775. [DOI] [Google Scholar]
- 16.Szymański Ł, Skopek R, Palusińska M, et al. Retinoic acid and its derivatives in skin. Cells. 2020;9(12):E2660. doi: 10.3390/cells9122660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng A, Evans K, North RV, Jones L, Purslow C. Impact of eye cosmetics on the eye, adnexa, and ocular surface. Eye Contact Lens. 2016;42(4):211–220. doi: 10.1097/ICL.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 18.Panchaud A, Csajka C, Merlob P, et al. Pregnancy outcome following exposure to topical retinoids: a multicenter prospective study. J Clin Pharmacol. 2012;52(12):1844–1851. doi: 10.1177/0091270011429566. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan YC, Ozsarfati J, Etwel F, Nickel C, Nulman I, Koren G. Pregnancy outcomes following first-trimester exposure to topical retinoids: a systematic review and meta-analysis. Br J Dermatol. 2015;173(5):1132–1141. doi: 10.1111/bjd.14053. [DOI] [PubMed] [Google Scholar]
- 20.Williams AL, Pace ND, DeSesso JM. Teratogen update: topical use and third-generation retinoids. Birth Defects Res. 2020;112(15):1105–1114. doi: 10.1002/bdr2.1745. [DOI] [PubMed] [Google Scholar]
- 21.Yin S, Luo J, Qian A, et al. Retinoids activate the irritant receptor TRPV1 and produce sensory hypersensitivity. J Clin Invest. 2013;123(9):3941–3951. doi: 10.1172/JCI66413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1(4):327–348. doi: 10.2147/ciia.2006.1.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim MS, Lee S, Rho HS, Kim DH, Chang IS, Chung JH. The effects of a novel synthetic retinoid, seletinoid G, on the expression of extracellular matrix proteins in aged human skin in vivo. Clin Chim Acta. 2005;362(1–2):161–169. doi: 10.1016/j.cccn.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Limcharoen B, Toprangkobsin P, Banlunara W, et al. Increasing the percutaneous absorption and follicular penetration of retinal by topical application of proretinal nanoparticles. Eur J Pharm Biopharm. 2019;139:93–100. doi: 10.1016/j.ejpb.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Souto EB, Fangueiro JF, Fernandes AR, et al. Physicochemical and biopharmaceutical aspects influencing skin permeation and role of SLN and NLC for skin drug delivery. Heliyon. 2022;8(2):e08938. doi: 10.1016/j.heliyon.2022.e08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salvioni L, Morelli L, Ochoa E, et al. The emerging role of nanotechnology in skincare. Adv Coll Interface Sci. 2021;293:102437. doi: 10.1016/j.cis.2021.102437. [DOI] [PubMed] [Google Scholar]
- 27.Abu Hajleh MN, Abu-Huwaij R, Al-Samydai A, Al-Halaseh LK, Al-Dujaili EA. The revolution of cosmeceuticals delivery by using nanotechnology: a narrative review of advantages and side effects. J Cosmet Dermatol. 2021;20(12):3818–3828. doi: 10.1111/jocd.14441. [DOI] [PubMed] [Google Scholar]
- 28.Alsabeelah N, Arshad MF, Hashmi S, Khan RA, Khan S. Nanocosmeceuticals for the management of ageing: rigors and vigors. J Drug Deliv Sci Technol. 2021;63:102448. doi: 10.1016/j.jddst.2021.102448. [DOI] [Google Scholar]
- 29.Khater D, Nsairat H, Odeh F, et al. Design, preparation, and characterization of effective dermal and transdermal lipid nanoparticles: a review. Cosmetics. 2021;8(2):39. doi: 10.3390/cosmetics8020039. [DOI] [Google Scholar]
- 30.Romes NB, Abdul Wahab R, Abdul HM. The role of bioactive phytoconstituents-loaded nanoemulsions for skin improvement: a review. Biotechnol Biotechnol Equip. 2021;35(1):711–730. doi: 10.1080/13102818.2021.1915869. [DOI] [Google Scholar]
- 31.Ahmad J. Lipid nanoparticles based cosmetics with potential application in alleviating skin disorders. Cosmetics. 2021;8(3):84. doi: 10.3390/cosmetics8030084. [DOI] [Google Scholar]
- 32.Morales JO, Valdés K, Morales J, Oyarzun-Ampuero F. Lipid nanoparticles for the topical delivery of retinoids and derivatives. Nanomed (Lond) 2015;10(2):253–269. doi: 10.2217/nnm.14.159. [DOI] [PubMed] [Google Scholar]
- 33.Garcês A, Amaral MH, Sousa Lobo JM, Silva AC. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: a review. Eur J Pharm Sci. 2018;15(112):159–167. doi: 10.1016/j.ejps.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 34.Doktorovová S, Kovačević AB, Garcia ML, Souto EB. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: current evidence from in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2016;108:235–252. doi: 10.1016/j.ejpb.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Gordillo-Galeano A, Mora-Huertas CE. Solid lipid nanoparticles and nanostructured lipid carriers: a review emphasizing on particle structure and drug release. Eur J Pharm Biopharm. 2018;133:285–308. doi: 10.1016/j.ejpb.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Kaul S, Gulati N, Verma D, Mukherjee S, Nagaich U. Role of nanotechnology in cosmeceuticals: a review of recent advances. J Pharm (Cairo) 2018;2018:3420204. doi: 10.1155/2018/3420204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Souto EB, Jäger E, Jäger A, et al. Lipid nanomaterials for targeted delivery of dermocosmetic ingredients: advances in photoprotection and skin anti-aging. Nanomater (Basel) 2022;12(3):377. doi: 10.3390/nano12030377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ascenso A, Ribeiro H, Marques HC, Oliveira H, Santos C, Simões S. Is tretinoin still a key agent for photoaging management? Mini Rev Med Chem. 2014;14(8):629–641. doi: 10.2174/1389557514666140820102735. [DOI] [PubMed] [Google Scholar]
- 39.Kligman AM, Grove GL, Hirose R, Leyden JJ. Topical tretinoin for photoaged skin. J Am Acad Dermatol. 1986;15(4 Pt 2):836–859. doi: 10.1016/S0190-9622(86)70242-9. [DOI] [PubMed] [Google Scholar]
- 40.Leyden JJ, Grove GL, Grove MJ, Thorne EG, Lufrano L. Treatment of photodamaged facial skin with topical tretinoin. J Am Acad Dermatol. 1989;21(3 Pt 2):638–644. doi: 10.1016/S0190-9622(89)70231-0. [DOI] [PubMed] [Google Scholar]
- 41.Ellis CN, Weiss JS, Hamilton TA, Headington JT, Zelickson AS, Voorhees JJ. Sustained improvement with prolonged topical tretinoin (retinoic acid) for photoaged skin. J Am Acad Dermatol. 1990;23(4 Pt 1):629–637. doi: 10.1016/0190-9622(90)70265-J. [DOI] [PubMed] [Google Scholar]
- 42.Weinstein GD, Nigra TP, Pochi PE, et al. Topical tretinoin for treatment of photodamaged skin. A multicenter study. Arch Dermatol. 1991;127(5):659–665. doi: 10.1001/archderm.1991.01680040067005. [DOI] [PubMed] [Google Scholar]
- 43.Olsen EA, Katz HI, Levine N, et al. Tretinoin emollient cream for photodamaged skin: results of 48-week, multicenter, double-blind studies. J Am Acad Dermatol. 1997;37(2 Pt 1):217–226. doi: 10.1016/S0190-9622(97)80128-4. [DOI] [PubMed] [Google Scholar]
- 44.Griffiths CE, Kang S, Ellis CN, et al. Two concentrations of topical tretinoin (retinoic acid) cause similar improvement of photoaging but different degrees of irritation. A double-blind, vehicle-controlled comparison of 0.1% and 0.025% tretinoin creams. Arch Dermatol. 1995;131(9):1037–44. [PubMed]
- 45.Kang S, Bergfeld W, Gottlieb AB, et al. Long-term efficacy and safety of tretinoin emollient cream 0.05% in the treatment of photodamaged facial skin: a two-year, randomized, placebo-controlled trial. Am J Clin Dermatol. 2005;6(4):245–53. [DOI] [PubMed]
- 46.Darlenski R, Surber C, Fluhr JW. Topical retinoids in the management of photodamaged skin: from theory to evidence-based practical approach. Br J Dermatol. 2010;163(6):1157–1165. doi: 10.1111/j.1365-2133.2010.09936.x. [DOI] [PubMed] [Google Scholar]
- 47.Buchanan PJ, Gilman RH. Retinoids: literature review and suggested algorithm for use prior to facial resurfacing procedures. J Cutan Aesthet Surg. 2016;9(3):139–144. doi: 10.4103/0974-2077.191653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prow TW, Grice JE, Lin LL, et al. Nanoparticles and microparticles for skin drug delivery. Adv Drug Deliv Rev. 2011;63(6):470–491. doi: 10.1016/j.addr.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 49.Tashtoush BM, Jacobson EL, Jacobson MK. UVA is the major contributor to the photodegradation of tretinoin and isotretinoin: Implications for development of improved pharmaceutical formulations. Int J Pharm. 2008;352(1–2):123–128. doi: 10.1016/j.ijpharm.2007.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghate VM, Lewis SA, Prabhu P, Dubey A, Patel N. Nanostructured lipid carriers for the topical delivery of tretinoin. Eur J Pharm Biopharm. 2016;108:253–261. doi: 10.1016/j.ejpb.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 51.Raza K, Singh B, Lohan S, et al. Nano-lipoidal carriers of tretinoin with enhanced percutaneous absorption, photostability, biocompatibility and anti-psoriatic activity. Int J Pharm. 2013;456(1):65–72. doi: 10.1016/j.ijpharm.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 52.Ebrahimi S, Mahjub R, Haddadi R, Vafaei SY. Design and optimization of cationic nanocapsules for topical delivery of tretinoin: application of the box-behnken design, in vitro evaluation, and ex vivo skin deposition study. Biomed Res Int. 2021;2021:4603545. doi: 10.1155/2021/4603545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu J, Zheng A, Peng B, Xu Y, Zhang N. Size-dependent absorption through stratum corneum by drug-loaded liposomes. Pharm Res. 2021;38(8):1429–1437. doi: 10.1007/s11095-021-03079-9. [DOI] [PubMed] [Google Scholar]
- 54.Pinto F, de Barros DPC, Reis C, Fonseca LP. Optimization of nanostructured lipid carriers loaded with retinoids by central composite design. J Mol Liq. 2019;293:111468. doi: 10.1016/j.molliq.2019.111468. [DOI] [Google Scholar]
- 55.Lima FA, Vilela RV, Oréfice RL, et al. Nanostructured lipid carriers enhances the safety profile of tretinoin: in vitro and healthy human volunteers’ studies. Nanomed (Lond) 2021;16(16):1391–1409. doi: 10.2217/nnm-2021-0031. [DOI] [PubMed] [Google Scholar]
- 56.Yamaguchi Y, Nagasawa T, Nakamura N, et al. Successful treatment of photo-damaged skin of nano-scale atRA particles using a novel transdermal delivery. J Control Release. 2005;104(1):29–40. doi: 10.1016/j.jconrel.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 57.Mandawgade SD, Patravale VB. Development of SLNs from natural lipids: application to topical delivery of tretinoin. Int J Pharm. 2008;363(1–2):132–138. doi: 10.1016/j.ijpharm.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 58.Shah KA, Date AA, Joshi MD, Patravale VB. Solid lipid nanoparticles (SLN) of tretinoin: potential in topical delivery. Int J Pharm. 2007;345(1–2):163–171. doi: 10.1016/j.ijpharm.2007.05.061. [DOI] [PubMed] [Google Scholar]
- 59.Ourique AF, Melero A, de Bona da Silva C, et al. Improved photostability and reduced skin permeation of tretinoin: development of a semisolid nanomedicine. Eur J Pharm Biopharm. 2011;79(1):95–101. [DOI] [PubMed]
- 60.Nasrollahi SA, Hassanzade H, Moradi A, et al. Safety assessment of tretinoin loaded nano emulsion and nanostructured lipid carriers: a non-invasive trial on human volunteers. Curr Drug Deliv. 2017;14(4):575–580. doi: 10.2174/1567201813666160512145954. [DOI] [PubMed] [Google Scholar]
- 61.Raminelli ACP, Romero V, Semreen MH, Leonardi GR. Nanotechnological advances for cutaneous release of tretinoin: an approach to minimize side effects and improve therapeutic efficacy. Curr Med Chem. 2018;25(31):3703–3718. doi: 10.2174/0929867325666180313110917. [DOI] [PubMed] [Google Scholar]
- 62.Abdelmaksoud A, Lotti T, Anadolu R, et al. Low dose of isotretinoin: a comprehensive review. Dermatol Ther. 2020;33(2):e13251. doi: 10.1111/dth.13251. [DOI] [PubMed] [Google Scholar]
- 63.Maddin S, Lauharanta J, Agache P, Burrows L, Zultak M, Bulger L. Isotretinoin improves the appearance of photodamaged skin: results of a 36-week, multicenter, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2000;42(1 Pt 1):56–63. doi: 10.1016/S0190-9622(00)90009-4. [DOI] [PubMed] [Google Scholar]
- 64.Armstrong RB, Lesiewicz J, Harvey G, Lee LF, Spoehr KT, Zultak M. Clinical panel assessment of photodamaged skin treated with isotretinoin using photographs. Arch Dermatol. 1992;128(3):352–356. doi: 10.1001/archderm.1992.01680130066007. [DOI] [PubMed] [Google Scholar]
- 65.Sendagorta E, Lesiewicz J, Armstrong RB. Topical isotretinoin for photodamaged skin. J Am Acad Dermatol. 1992;27(6 Pt 2):S15–18. doi: 10.1016/S0190-9622(08)80254-X. [DOI] [PubMed] [Google Scholar]
- 66.Griffiths CEM, Maddin S, Wiedow O, Marks R, Donald AE, Kahlon G. Treatment of photoaged skin with a cream containing 0.05% isotretinoin and sunscreens. J Dermatolog Treat. 2005;16(2):79–86. [DOI] [PubMed]
- 67.Shiva G, Somaye M, Reza JM. Improved photostability, reduced skin permeation and irritation of isotretinoin by solid lipid nanoparticles. Acta Pharm. 2012;62(4):547–562. doi: 10.2478/v10007-012-0032-z. [DOI] [PubMed] [Google Scholar]
- 68.Liu J, Hu W, Chen H, Ni Q, Xu H, Yang X. Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int J Pharm. 2007;328(2):191–195. doi: 10.1016/j.ijpharm.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 69.Raza K, Singh B, Singla N, Negi P, Singal P, Katare OP. Nano-lipoidal carriers of isotretinoin with anti-aging potential: formulation, characterization and biochemical evaluation. J Drug Target. 2013;21(5):435–442. doi: 10.3109/1061186X.2012.761224. [DOI] [PubMed] [Google Scholar]
- 70.Bettoni CC, Felippi CC, de Andrade C, et al. Isotretinoin-loaded nanocapsules: stability and cutaneous penetration by tape stripping in human and pig skin. J Biomed Nanotechnol. 2012;8(2):258–271. doi: 10.1166/jbn.2012.1381. [DOI] [PubMed] [Google Scholar]
- 71.Miastkowska MG, Sikora EB, Ogonowski J, Zielina M, Łudzik A. The kinetic study of isotretinoin release from nanoemulsion. Coll Surfa A Physicochem Eng Aspects. 2016;510(1):63–8.
- 72.Patwekar SL, Pedewad SR, Gattani S. Development and evaluation of nanostructured lipid carriers-based gel of isotretinoin. Part Sci Technol. 2018;36(7):832–843. doi: 10.1080/02726351.2017.1305026. [DOI] [Google Scholar]
- 73.Gupta S, Wairkar S, Bhatt LK. Isotretinoin and α-tocopherol acetate-loaded solid lipid nanoparticle topical gel for the treatment of acne. J Microencapsul. 2020;37(8):557–565. doi: 10.1080/02652048.2020.1823499. [DOI] [PubMed] [Google Scholar]
- 74.Cheng C, Michaels J, Scheinfeld N. Alitretinoin: a comprehensive review. Expert Opin Investig Drugs. 2008;17(3):437–443. doi: 10.1517/13543784.17.3.437. [DOI] [PubMed] [Google Scholar]
- 75.Baumann L, Vujevich J, Halem M, et al. Open-label pilot study of alitretinoin gel 0.1% in the treatment of photoaging. Cutis. 2005;76(1):69–73. [PubMed]
- 76.Li WH, Wong HK, Serrano J, et al. Topical stabilized retinol treatment induces the expression of HAS genes and HA production in human skin in vitro and in vivo. Arch Dermatol Res. 2017;309(4):275–283. doi: 10.1007/s00403-017-1723-6. [DOI] [PubMed] [Google Scholar]
- 77.Romana-Souza B, Silva-Xavier W, Monte-Alto-Costa A. Topical retinol attenuates stress-induced ageing signs in human skin ex vivo, through EGFR activation via EGF, but not ERK and AP-1 activation. Exp Dermatol. 2019;28(8):906–913. doi: 10.1111/exd.13675. [DOI] [PubMed] [Google Scholar]
- 78.Shao Y, He T, Fisher GJ, Voorhees JJ, Quan T. Molecular basis of retinol anti-ageing properties in naturally aged human skin in vivo. Int J Cosmet Sci. 2017;39(1):56–65. doi: 10.1111/ics.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duell EA, Kang S, Voorhees JJ. Unoccluded retinol penetrates human skin in vivo more effectively than unoccluded retinyl palmitate or retinoic acid. J Invest Dermatol. 1997;109(3):301–305. doi: 10.1111/1523-1747.ep12335788. [DOI] [PubMed] [Google Scholar]
- 80.European Commission. Directorate general for health and food safety. Opinion on vitamin A (retinol, retinyl acetate, retinyl palmitate). 2016. https://data.europa.eu/doi/10.2875/642264. Accessed 13 Aug 2022.
- 81.Spierings NMK. Evidence for the efficacy of over-the-counter vitamin A cosmetic products in the improvement of facial skin aging: a systematic review. J Clin Aesthet Dermatol. 2021;14(9):33–40. [PMC free article] [PubMed] [Google Scholar]
- 82.Tucker-Samaras S, Zedayko T, Cole C, Miller D, Wallo W, Leyden JJ. A stabilized 0.1% retinol facial moisturizer improves the appearance of photodamaged skin in an eight-week, double-blind, vehicle-controlled study. J Drugs Dermatol. 2009;8(10):932–6. [PubMed]
- 83.Randhawa M, Rossetti D, Leyden JJ, et al. One-year topical stabilized retinol treatment improves photodamaged skin in a double-blind, vehicle-controlled trial. J Drugs Dermatol. 2015;14(3):271–280. [PubMed] [Google Scholar]
- 84.Kikuchi K, Suetake T, Kumasaka N, Tagami H. Improvement of photoaged facial skin in middle-aged Japanese females by topical retinol (vitamin A alcohol): a vehicle-controlled, double-blind study. J Dermatol Treat. 2009;20(5):276–281. doi: 10.1080/09546630902973987. [DOI] [PubMed] [Google Scholar]
- 85.Bellemère G, Stamatas GN, Bruère V, Bertin C, Issachar N, Oddos T. Antiaging action of retinol: from molecular to clinical. Skin Pharmacol Physiol. 2009;22(4):200–209. doi: 10.1159/000231525. [DOI] [PubMed] [Google Scholar]
- 86.Gold MH, Kircik LH, Bucay VW, Kiripolsky MG, Biron JA. Treatment of facial photodamage using a novel retinol formulation. J Drugs Dermatol. 2013;12(5):533–540. [PubMed] [Google Scholar]
- 87.Babcock M, Mehta RC, Makino ET. A randomized, double-blind, split-face study comparing the efficacy and tolerability of three retinol-based products vs. three tretinoin-based products in subjects with moderate to severe facial photodamage. J Drugs Dermatol. 2015;14(1):24–30. [PubMed]
- 88.Draelos ZD, Peterson RS. A double-blind, comparative clinical study of newly formulated retinol serums vs tretinoin cream in escalating doses: a method for rapid retinization with minimized irritation. J Drugs Dermatol. 2020;19(6):625–631. doi: 10.36849/JDD.2020.5085. [DOI] [PubMed] [Google Scholar]
- 89.Kong R, Cui Y, Fisher GJ, et al. A comparative study of the effects of retinol and retinoic acid on histological, molecular, and clinical properties of human skin. J Cosmet Dermatol. 2016;15(1):49–57. doi: 10.1111/jocd.12193. [DOI] [PubMed] [Google Scholar]
- 90.Bouloc A, Vergnanini AL, Issa MC. A double-blind randomized study comparing the association of retinol and LR2412 with tretinoin 0.025% in photoaged skin. J Cosmet Dermatol. 2015;14(1):40–6. [DOI] [PubMed]
- 91.Chien AL, Kim DJ, Cheng N, et al. Biomarkers of tretinoin precursors and tretinoin efficacy in patients with moderate to severe facial photodamage: a randomized clinical trial. JAMA Dermatol. 2022;158(8):879–886. doi: 10.1001/jamadermatol.2022.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Temova Rakuša Ž, Škufca P, Kristl A, Roškar R. Retinoid stability and degradation kinetics in commercial cosmetic products. J Cosmet Dermatol. 2021;20(7):2350–2358. doi: 10.1111/jocd.13852. [DOI] [PubMed] [Google Scholar]
- 93.Temova Rakuša Ž, Škufca P, Kristl A, Roškar R. Quality control of retinoids in commercial cosmetic products. J Cosmet Dermatol. 2021;20(4):1166–1175. doi: 10.1111/jocd.13686. [DOI] [PubMed] [Google Scholar]
- 94.Boskabadi M, Saeedi M, Akbari J, Morteza-Semnani K, Hashemi SMH, Babaei A. Topical gel of vitamin a solid lipid nanoparticles: a hopeful promise as a dermal delivery system. Adv Pharm Bull. 2021;11(4):663–674. doi: 10.34172/apb.2021.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goudon F, Clément Y, Ripoll L. Controlled release of retinol in cationic co-polymeric nanoparticles for topical application. Cosmetics. 2020;7(2):29. doi: 10.3390/cosmetics7020029. [DOI] [Google Scholar]
- 96.Jun SH, Kim H, Lee H, Song JE, Park SG, Kang NG. Synthesis of retinol-loaded lipid nanocarrier via vacuum emulsification to improve topical skin delivery. Polymers (Basel) 2021;13(5):826. doi: 10.3390/polym13050826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim DG, Jeong YI, Choi C, et al. Retinol-encapsulated low molecular water-soluble chitosan nanoparticles. Int J Pharm. 2006;319(1–2):130–138. doi: 10.1016/j.ijpharm.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 98.Eskandar NG, Simovic S, Prestidge CA. Chemical stability and phase distribution of all-trans-retinol in nanoparticle-coated emulsions. Int J Pharm. 2009;376(1–2):186–194. doi: 10.1016/j.ijpharm.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 99.Fluhr JW, Vienne MP, Lauze C, Dupuy P, Gehring W, Gloor M. Tolerance profile of retinol, retinaldehyde and retinoic acid under maximized and long-term clinical conditions. Dermatology. 1999;199(Suppl 1):57–60. doi: 10.1159/000051381. [DOI] [PubMed] [Google Scholar]
- 100.Saurat JH, Didierjean L, Masgrau E, et al. Topical retinaldehyde on human skin: biologic effects and tolerance. J Invest Dermatol. 1994;103(6):770–774. doi: 10.1111/1523-1747.ep12412861. [DOI] [PubMed] [Google Scholar]
- 101.Sorg O, Didierjean L, Saurat JH. Metabolism of topical retinaldehyde. Dermatology. 1999;199(Suppl 1):13–17. doi: 10.1159/000051372. [DOI] [PubMed] [Google Scholar]
- 102.Didierjean L, Carraux P, Grand D, Sass JO, Nau H, Saurat JH. Topical retinaldehyde increases skin content of retinoic acid and exerts biologic activity in mouse skin. J Invest Dermatol. 1996;107(5):714–719. doi: 10.1111/1523-1747.ep12365603. [DOI] [PubMed] [Google Scholar]
- 103.Sass JO, Didierjean L, Carraux P, Plum C, Nau H, Saurat JH. Metabolism of topical retinaldehyde and retinol by mouse skin in vivo: predominant formation of retinyl esters and identification of 14-hydroxy-4, 14-retro-retinol. Exp Dermatol. 1996;5(5):267–271. doi: 10.1111/j.1600-0625.1996.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 104.Boisnic S, Branchet-Gumila MC, Le Charpentier Y, Segard C. Repair of UVA-induced elastic fiber and collagen damage by 0.05% retinaldehyde cream in an ex vivo human skin model. Dermatology. 1999;199 Suppl 1:43–8. [DOI] [PubMed]
- 105.Diridollou S, Vienne MP, Alibert M, et al. Efficacy of topical 0.05% retinaldehyde in skin aging by ultrasound and rheological techniques. Dermatology. 1999;199 Suppl 1:37–41. [DOI] [PubMed]
- 106.Creidi P, Humbert P. Clinical use of topical retinaldehyde on photoaged skin. Dermatology. 1999;199(Suppl 1):49–52. doi: 10.1159/000051379. [DOI] [PubMed] [Google Scholar]
- 107.Creidi P, Vienne MP, Ochonisky S, et al. Profilometric evaluation of photodamage after topical retinaldehyde and retinoic acid treatment. J Am Acad Dermatol. 1998;39(6):960–965. doi: 10.1016/S0190-9622(98)70270-1. [DOI] [PubMed] [Google Scholar]
- 108.Kwon HS, Lee JH, Kim GM, Bae JM. Efficacy and safety of retinaldehyde 0.1% and 0.05% creams used to treat photoaged skin: a randomized double-blind controlled trial. J Cosmet Dermatol. 2018;17(3):471–6. [DOI] [PubMed]
- 109.Sass JO, Masgrau E, Piletta PA, Nau H, Saurat JH. Plasma retinoids after topical use of retinaldehyde on human skin. Skin Pharmacol. 1996;9(5):322–326. doi: 10.1159/000211436. [DOI] [PubMed] [Google Scholar]
- 110.Pisetpackdeekul P, Supmuang P, Pan-In P, et al. Proretinal nanoparticles: stability, release, efficacy, and irritation. Int J Nanomed. 2016;11:3277–3286. doi: 10.2147/IJN.S111748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Limcharoen B, Pisetpackdeekul P, Toprangkobsin P, Thunyakitpisal P, Wanichwecharungruang S, Banlunara W. Topical proretinal nanoparticles: biological activities, epidermal proliferation and differentiation, follicular penetration, and skin tolerability. ACS Biomater Sci Eng. 2020;6(3):1510–1521. doi: 10.1021/acsbiomaterials.9b01109. [DOI] [PubMed] [Google Scholar]
- 112.Nayak K, Katiyar SS, Kushwah V, Jain S. Coenzyme Q10 and retinaldehyde co-loaded nanostructured lipid carriers for efficacy evaluation in wrinkles. J Drug Target. 2018;26(4):333–344. doi: 10.1080/1061186X.2017.1379527. [DOI] [PubMed] [Google Scholar]
- 113.Yan J, Xia Q, Cherng SH, et al. Photo-induced DNA damage and photocytotoxicity of retinyl palmitate and its photodecomposition products. Toxicol Ind Health. 2005;21(7–8):167–175. doi: 10.1191/0748233705th225oa. [DOI] [PubMed] [Google Scholar]
- 114.Ihara H, Hashizume N, Hirase N, Suzue R. Esterification makes retinol more labile to photolysis. J Nutr Sci Vitaminol (Tokyo) 1999;45(3):353–358. doi: 10.3177/jnsv.45.353. [DOI] [PubMed] [Google Scholar]
- 115.Tolleson WH, Cherng SH, Xia Q, et al. Photodecomposition and phototoxicity of natural retinoids. Int J Environ Res Public Health. 2005;2(1):147–155. doi: 10.3390/ijerph2005010147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boudreau MD, Beland FA, Felton RP, et al. Photo-co-carcinogenesis of topically applied retinyl palmitate in SKH-1 hairless mice. Photochem Photobiol. 2017;93(4):1096–1114. doi: 10.1111/php.12730. [DOI] [PubMed] [Google Scholar]
- 117.Fu PP, Cheng SH, Coop L, et al. Photoreaction, phototoxicity, and photocarcinogenicity of retinoids. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2003;21(2):165–197. doi: 10.1081/GNC-120026235. [DOI] [PubMed] [Google Scholar]
- 118.Farooq U, Mahmood T, Shahzad Y, Yousaf AM, Akhtar N. Comparative efficacy of two anti-aging products containing retinyl palmitate in healthy human volunteers. J Cosmet Dermatol. 2018;17(3):454–460. doi: 10.1111/jocd.12500. [DOI] [PubMed] [Google Scholar]
- 119.Bradley EJ, Griffiths CEM, Sherratt MJ, Bell M, Watson REB. Over-the-counter anti-ageing topical agents and their ability to protect and repair photoaged skin. Maturitas. 2015;80(3):265–272. doi: 10.1016/j.maturitas.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 120.Babamiri K, Nassab R. Cosmeceuticals: the evidence behind the retinoids. Aesthet Surg J. 2010;30(1):74–77. doi: 10.1177/1090820X09360704. [DOI] [PubMed] [Google Scholar]
- 121.Clares B, Calpena AC, Parra A, et al. Nanoemulsions (NEs), liposomes (LPs) and solid lipid nanoparticles (SLNs) for retinyl palmitate: effect on skin permeation. Int J Pharm. 2014;473(1–2):591–598. doi: 10.1016/j.ijpharm.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 122.Pena-Rodríguez E, Moreno MC, Blanco-Fernandez B, González J, Fernández-Campos F. Epidermal delivery of retinyl palmitate loaded transfersomes: penetration and biodistribution studies. Pharmaceutics. 2020;12(2):E112. doi: 10.3390/pharmaceutics12020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pinto F, Fonseca LP, Souza S, Oliva A, de Barros DPC. Topical distribution and efficiency of nanostructured lipid carriers on a 3D reconstructed human epidermis model. J Drug Deliv Sci Technol. 2020;57:101616. doi: 10.1016/j.jddst.2020.101616. [DOI] [Google Scholar]
- 124.Ro J, Kim Y, Kim H, et al. Pectin micro- and nano-capsules of retinyl palmitate as cosmeceutical carriers for stabilized skin transport. Korean J Physiol Pharmacol. 2015;19(1):59–64. doi: 10.4196/kjpp.2015.19.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Teixeira Z, Zanchetta B, Melo BAG, et al. Retinyl palmitate flexible polymeric nanocapsules: characterization and permeation studies. Coll Surf B Biointerfaces. 2010;81(1):374–380. doi: 10.1016/j.colsurfb.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 126.Oliveira MB, do Prado AH, Bernegossi J, et al. Topical application of retinyl palmitate-loaded nanotechnology-based drug delivery systems for the treatment of skin aging. Biomed Res Int. 2014;2014:632570. [DOI] [PMC free article] [PubMed]
- 127.AlZahabi S, Sakr OS, Ramadan AA. Nanostructured lipid carriers incorporating prickly pear seed oil for the encapsulation of vitamin A. J Cosmet Dermatol. 2019;18(6):1875–1884. doi: 10.1111/jocd.12891. [DOI] [PubMed] [Google Scholar]
- 128.Jeon HS, Seo JE, Kim MS, et al. A retinyl palmitate-loaded solid lipid nanoparticle system: effect of surface modification with dicetyl phosphate on skin permeation in vitro and anti-wrinkle effect in vivo. Int J Pharm. 2013;452(1–2):311–320. doi: 10.1016/j.ijpharm.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 129.Nandy A, Lee E, Mandal A, Saremi R, Sharma S. Microencapsulation of retinyl palmitate by melt dispersion for cosmetic application. J Microencapsul. 2020;37(3):205–219. doi: 10.1080/02652048.2020.1720029. [DOI] [PubMed] [Google Scholar]
- 130.Suh DC, Kim Y, Kim H, et al. Enhanced in vitro skin deposition properties of retinyl palmitate through its stabilization by pectin. Biomol Ther (Seoul) 2014;22(1):73–77. doi: 10.4062/biomolther.2013.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Algahtani MS, Ahmad MZ, Ahmad J. Nanoemulgel for improved topical delivery of retinyl palmitate: formulation design and stability evaluation. Nanomaterials (Basel) 2020;10(5):E848. doi: 10.3390/nano10050848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bjerke DL, Li R, Price JM, et al. The vitamin A ester retinyl propionate has a unique metabolic profile and higher retinoid-related bioactivity over retinol and retinyl palmitate in human skin models. Exp Dermatol. 2021;30(2):226–236. doi: 10.1111/exd.14219. [DOI] [PubMed] [Google Scholar]
- 133.Green C, Orchard G, Cerio R, Hawk JL. A clinicopathological study of the effects of topical retinyl propionate cream in skin photoageing. Clin Exp Dermatol. 1998;23(4):162–167. doi: 10.1046/j.1365-2230.1998.00331.x. [DOI] [PubMed] [Google Scholar]
- 134.Fu JJJ, Hillebrand GG, Raleigh P, et al. A randomized, controlled comparative study of the wrinkle reduction benefits of a cosmetic niacinamide/peptide/retinyl propionate product regimen vs. a prescription 0.02% tretinoin product regimen. Br J Dermatol. 2010;162(3):647–54. [DOI] [PMC free article] [PubMed]
- 135.Lam ECS, Li R, Rodrigues MR, et al. Enhanced retinoid response by a combination of the vitamin A ester retinyl propionate with niacinamide and a flavonoid containing Ceratonia siliqua extract in retinoid responsive in vitro models. Int J Cosmet Sci. 2021;43(1):102–106. doi: 10.1111/ics.12669. [DOI] [PubMed] [Google Scholar]
- 136.Arayachukeat S, Wanichwecharungruang SP, Tree-Udom T. Retinyl acetate-loaded nanoparticles: dermal penetration and release of the retinyl acetate. Int J Pharm. 2011;404(1–2):281–288. doi: 10.1016/j.ijpharm.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 137.Kim H, Kim B, Kim H, et al. Synthesis and in vitro biological activity of retinyl retinoate, a novel hybrid retinoid derivative. Bioorg Med Chem. 2008;16(12):6387–6393. doi: 10.1016/j.bmc.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 138.Kim JE, Kim B, Kim H, et al. Retinyl retinoate induces hyaluronan production and less irritation than other retinoids. J Dermatol. 2010;37(5):448–454. doi: 10.1111/j.1346-8138.2010.00808.x. [DOI] [PubMed] [Google Scholar]
- 139.Kim H, Koh J, Baek J, et al. Retinyl retinoate, a novel hybrid vitamin derivative, improves photoaged skin: a double-blind, randomized-controlled trial. Skin Res Technol. 2011;17(3):380–385. doi: 10.1111/j.1600-0846.2011.00512.x. [DOI] [PubMed] [Google Scholar]
- 140.Kim H, Kim N, Jung S, et al. Improvement in skin wrinkles from the use of photostable retinyl retinoate: a randomized controlled trial. Br J Dermatol. 2010;162(3):497–502. doi: 10.1111/j.1365-2133.2009.09483.x. [DOI] [PubMed] [Google Scholar]
- 141.Kim M, Yang H, Kim H, Jung H, Jung H. Novel cosmetic patches for wrinkle improvement: retinyl retinoate- and ascorbic acid-loaded dissolving microneedles. Int J Cosmet Sci. 2014;36(3):207–212. doi: 10.1111/ics.12115. [DOI] [PubMed] [Google Scholar]
- 142.Lee SG, Jeong JH, Kim SR, et al. Topical formulation of retinyl retinoate employing nanostructured lipid carriers. J Pharm Investig. 2012;42(5):243–250. doi: 10.1007/s40005-012-0036-1. [DOI] [Google Scholar]
- 143.Han HS, Kwon YJ, Park MS, et al. Efficacy validation of synthesized retinol derivatives In vitro: stability, toxicity, and activity. Bioorg Med Chem. 2003;11(17):3839–3845. doi: 10.1016/S0968-0896(03)00334-1. [DOI] [PubMed] [Google Scholar]
- 144.Lee MS, Lee KH, Sin HS, Um SJ, Kim JW, Koh BK. A newly synthesized photostable retinol derivative (retinyl N-formyl aspartamate) for photodamaged skin: profilometric evaluation of 24-week study. J Am Acad Dermatol. 2006;55(2):220–224. doi: 10.1016/j.jaad.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 145.Motamedi M, Chehade A, Sanghera R, Grewal P. A clinician’s guide to topical retinoids. J Cutan Med Surg. 2022;26(1):71–78. doi: 10.1177/12034754211035091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ogden S, Samuel M, Griffiths CEM. A review of tazarotene in the treatment of photodamaged skin. Clin Interv Aging. 2008;3(1):71–76. doi: 10.2147/CIA.S1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kang S, Krueger GG, Tanghetti EA, et al. A multicenter, randomized, double-blind trial of tazarotene 0.1% cream in the treatment of photodamage. J Am Acad Dermatol. 2005;52(2):268–74. [DOI] [PubMed]
- 148.Phillips TJ, Gottlieb AB, Leyden JJ, et al. Efficacy of 0.1% tazarotene cream for the treatment of photodamage: a 12-month multicenter, randomized trial. Arch Dermatol. 2002;138(11):1486–93. [DOI] [PubMed]
- 149.Machtinger LA, Kaidbey K, Lim J, et al. Histological effects of tazarotene 0.1% cream vs. vehicle on photodamaged skin: a 6-month, multicentre, double-blind, randomized, vehicle-controlled study in patients with photodamaged facial skin. Br J Dermatol. 2004;151(6):1245–52. [DOI] [PubMed]
- 150.Kang S, Leyden JJ, Lowe NJ, et al. Tazarotene cream for the treatment of facial photodamage: a multicenter, investigator-masked, randomized, vehicle-controlled, parallel comparison of 0.01%, 0.025%, 0.05%, and 0.1% tazarotene creams with 0.05% tretinoin emollient cream applied once daily for 24 weeks. Arch Dermatol. 2001;137(12):1597–604. [DOI] [PubMed]
- 151.Lowe N, Gifford M, Tanghetti E, et al. Tazarotene 0.1% cream versus tretinoin 0.05% emollient cream in the treatment of photodamaged facial skin: a multicenter, double-blind, randomized, parallel-group study. J Cosmet Laser Ther. 2004;6(2):79–85. [DOI] [PubMed]
- 152.Liu P, Yang X, Han J, et al. Tazarotene-loaded PLGA nanoparticles potentiate deep tissue pressure injury healing via VEGF-Notch signaling. Mater Sci Eng C Mater Biol Appl. 2020;114:111027. doi: 10.1016/j.msec.2020.111027. [DOI] [PubMed] [Google Scholar]
- 153.Nasr M, Abdel-Hamid S, Moftah NH, Fadel M, Alyoussef AA. Jojoba oil soft colloidal nanocarrier of a synthetic retinoid: preparation, characterization and clinical efficacy in psoriatic patients. Curr Drug Deliv. 2017;14(3):426–432. doi: 10.2174/1567201813666160513132321. [DOI] [PubMed] [Google Scholar]
- 154.Shroot B, Michel S. Pharmacology and chemistry of adapalene. J Am Acad Dermatol. 1997;36(6 Pt 2):S96–103. doi: 10.1016/S0190-9622(97)70050-1. [DOI] [PubMed] [Google Scholar]
- 155.Rusu A, Tanase C, Pascu GA, Todoran N. Recent advances regarding the therapeutic potential of adapalene. Pharmaceuticals. 2020;13(9):217. doi: 10.3390/ph13090217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kang S, Goldfarb MT, Weiss JS, et al. Assessment of adapalene gel for the treatment of actinic keratoses and lentigines: a randomized trial. J Am Acad Dermatol. 2003;49(1):83–90. doi: 10.1067/mjd.2003.451. [DOI] [PubMed] [Google Scholar]
- 157.Herane MI, Orlandi C, Zegpi E, Valdés P, Ancić X. Clinical efficacy of adapalene (Differin®) 0.3% gel in Chilean women with cutaneous photoaging. J Dermatolog Treat. 2012;23(1):57–64. [DOI] [PubMed]
- 158.Bagatin E, de Sá Gonçalves H , Sato M, Almeida LMC, Miot HA. Comparable efficacy of adapalene 0.3% gel and tretinoin 0.05% cream as treatment for cutaneous photoaging. Eur J Dermatol. 2018;28(3):343–50. [DOI] [PubMed]
- 159.Najafi-Taher R, Jafarzadeh Kohneloo A, Eslami Farsani V, et al. A topical gel of tea tree oil nanoemulsion containing adapalene versus adapalene marketed gel in patients with acne vulgaris: a randomized clinical trial. Arch Dermatol Res. 2022;314(7):673–679. doi: 10.1007/s00403-021-02267-2. [DOI] [PubMed] [Google Scholar]
- 160.Prasad S, Mukhopadhyay A, Kubavat A, et al. Efficacy and safety of a nano-emulsion gel formulation of adapalene 0.1% and clindamycin 1% combination in acne vulgaris: a randomized, open label, active-controlled, multicentric, phase IV clinical trial. Indian J Dermatol Venereol Leprol. 2012;78(4):459–67. [DOI] [PubMed]
- 161.Schadt CR. Topical and oral bexarotene. Dermatol Ther. 2013;26(5):400–403. doi: 10.1111/dth.12087. [DOI] [PubMed] [Google Scholar]
- 162.Scott LJ. Trifarotene: first approval. Drugs. 2019;79(17):1905–1909. doi: 10.1007/s40265-019-01218-6. [DOI] [PubMed] [Google Scholar]
- 163.Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80(6):1691–1699. doi: 10.1016/j.jaad.2019.02.044. [DOI] [PubMed] [Google Scholar]
- 164.Blume-Peytavi U, Fowler J, Kemény L, et al. Long-term safety and efficacy of trifarotene 50 μg/g cream, a first-in-class RAR-γ selective topical retinoid, in patients with moderate facial and truncal acne. J Eur Acad Dermatol Venereol. 2020;34(1):166–173. doi: 10.1111/jdv.15794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Aubert J, Piwnica D, Bertino B, et al. Nonclinical and human pharmacology of the potent and selective topical retinoic acid receptor-γ agonist trifarotene. Br J Dermatol. 2018;179(2):442–456. doi: 10.1111/bjd.16719. [DOI] [PubMed] [Google Scholar]
- 166.Cosio T, Di Prete M, Gaziano R, et al. Trifarotene: a current review and perspectives in dermatology. Biomedicines. 2021;9(3):237. doi: 10.3390/biomedicines9030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lee ES, Ahn Y, Bae IH, et al. Synthetic retinoid seletinoid G improves skin barrier function through wound healing and collagen realignment in human skin equivalents. Int J Mol Sci. 2020;21(9):E3198. doi: 10.3390/ijms21093198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Imhof L, Leuthard D. Topical over-the-counter antiaging agents: an update and systematic review. Dermatology. 2021;237(2):217–229. doi: 10.1159/000509296. [DOI] [PubMed] [Google Scholar]
- 169.Schlich M, Musazzi UM, Campani V, et al. Design and development of topical liposomal formulations in a regulatory perspective. Drug Deliv Transl Res. 2022;12(8):1811–1828. doi: 10.1007/s13346-021-01089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.European Commission. Directorate General for Health and Food Safety. Guidance on the safety assessment of nanomaterials in cosmetics. 2019. https://data.europa.eu/doi/10.2875/40446. Accessed 13 Aug 2022.
- 171.Mahant S, Rao R, Souto EB, Nanda S. Analytical tools and evaluation strategies for nanostructured lipid carrier-based topical delivery systems. Expert Opin Drug Deliv. 2020;17(7):963–992. doi: 10.1080/17425247.2020.1772750. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no datasets were generated or analyzed during the current study.