Abstract
Calorie restriction (CR) is known to prolong the life span and maintain an active immune function in aged mice, but it is still not known if rodents under CR can respond optimally to bacterial infection. We report here on the influence of CR on the response of peritoneal macrophages to lipopolysaccharide, splenic NF-κB and NF–interleukin-6 (IL-6) activities, and mortality in polymicrobial sepsis induced by cecal ligation and puncture (CLP). Macrophages from 6-month-old C57BL/6 mice on a calorie-restricted diet were less responsive to lipopolysaccharide, as evidenced by lower levels of IL-12 and IL-6 protein and mRNA expression. Furthermore, in vitro lipopolysaccharide-stimulated macrophages from mice under CR also expressed decreased lipopolysaccharide receptor CD14 levels as well as Toll-like receptor 2 (TLR2) and TLR4 mRNA levels. In addition, the phagocytic capacity and class II (I-Ab) expression of macrophages were also found to be significantly lower in mice under CR. Mice under CR died earlier (P < 0.005) after sepsis induced by CLP, which appeared to be a result of increased levels in serum of the proinflammatory cytokines tumor necrosis factor alpha and IL-6 and splenic NF-κB and NF–IL-6 activation 4 h after CLP. However, mice under CR survived significantly (P < 0.005) longer than mice fed ad libitum when injected with paraquat, a free radical-inducing agent. These data suggest that young mice under CR may be protected against oxidative stress but may have delayed maturation of macrophage function and increased susceptibility to bacterial infection.
Calorie restriction (CR; level of restriction, 25 to 50%) is known to increase the life span in rodents by 30 to 40% (23, 63). One of the well-known protective effects of CR is enhanced T-cell-mediated immune function including the prolonged maintenance of naive T cells and the delay of the early rise of memory cells (16, 26, 58). Numerous studies have reported that CR significantly delays the onset of malignancy and autoimmune renal disease in autoimmune-susceptible and -resistant mice and rats (27, 37, 58, 64, 65). Furthermore, CR is known to increase the numbers of cytotoxic T cells, increase the level of resistance to influenza viral infection, reduce the incidence of ulcerative dermatitis, and preserve spleen interleukin-2 (IL-2) production (16, 26, 28, 43, 45, 58). There is evidence that calorie-restricted rodents are more resistant to a variety of stress-inducing agents or insults (37) including surgical trauma (38), heat shock (32), and the toxicities of a variety of drugs (20). Despite the wealth of most supportive evidence describing the cellular and molecular changes that occur with CR, it is still not known whether rodents on CR can initiate a protective response against bacterial infection. There are also no studies that have assessed the effects of CR in an experimental model that mimics the clinical disease symptoms of polymicrobial sepsis and septic shock. The studies of CR with bacterial pathogens carried out so far are also inconclusive due to variability in experimental conditions such as diet and strain variations (19, 44).
Macrophages constitute the first line of defense against infection and are primarily responsible for the clearance of gram-positive or -negative bacteria from the blood via phagocytosis and intracellular killing. In addition, macrophages are central to both immune effects and autoregulatory function and are critical to defense mechanisms. Also, macrophages play an important role in the pathogenesis of sepsis and septic shock (11). However, the effects of CR on sepsis and/or macrophage function have not yet been fully addressed. We first reported on the impaired antigen-presenting function of macrophages and decreased antigen-specific T-cell proliferation in C57BL/6 mice fed calorie-restricted diets (17); Shi et al. (54) later confirmed these effects in energy-restricted BALB/c mice. We therefore formulated a hypothesis that although CR increases T-cell-mediated cellular immune function against viral infection (25), it may impair antigen presentation and/or phagocytosis by macrophages. A recent review by Weindruch et al. (62) on CR has pointed out the paucity of studies in the area of CR and infection. Since studies of CR are already being carried out with primates (14, 30, 34, 60, 62) and plans are under way to initiate studies of CR with humans (49), it is urgent that the effects of CR on the immune response to commonly occurring pathogens be addressed as soon as possible to prevent any infection-related fatalities in humans during CR or weight-reduction studies.
In light of this, it was the aim of this particular study to examine first the effects of CR on the responsiveness of peritoneal macrophages to lipopolysaccharide (LPS), a major cell wall constituent of gram-negative bacterial organisms in vitro. Additional studies were conducted to examine the effects of CR on splenic NF-κB and NF–IL-6 activities and mortality in a murine model of polymicrobial sepsis induced by cecal ligation and puncture (CLP). Since oxidative damage has been implicated in the pathogenesis of septic shock (9, 73) and animals fed calorie-restricted diets have been reported to have enhanced antioxidant enzyme capacity (13, 69), we have also determined the susceptibility to free radical-induced death by paraquat poisoning of mice fed ad libitum (AL) and mice fed calorie-restricted diets.
MATERIALS AND METHODS
Materials.
Fetal bovine serum, RPMI 1640 medium, and penicillin-streptomycin were purchased from GIBCO (Great Island, N.Y.). LPS (Escherichia coli O111:B4) was supplied by Sigma Chemical Company (St. Louis, Mo.). All fluorescence-labeled monoclonal antibodies for CD14, I-Ab, tumor necrosis factor alpha (TNF-α), IL-6, and IL-12 were obtained from Pharmingen, San Diego, Calif. Perm-wash buffer was purchased from Becton Dickinson, San Jose, Calif. All other chemicals were reagent grade and were purchased from Sigma Chemical Co. Double-stranded consensus binding site oligonucleotides for NF-κB and NF–IL-6 (CCAT enhancer-binding protein) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.).
Animals.
Inbred, weight-matched, female C57BL/6 weanling mice were obtained from Taconic Farms (Germantown, N.Y.) at 4 weeks of age. The mice were grouped and housed at five mice per cage in a temperature-controlled room at 24°C and were maintained on a 12-h dark and 12-h light cycle in the laboratory animal care facility at the University of Texas Health Science Center at San Antonio. The mice were fed a nutritionally adequate semipurified diet (AIN76 formula) containing 5% (wt/wt) corn oil either AL or in a gradually restricted manner (CR) until the number of calories in the diet was 40% of the calories fed to the mice fed AL from 6 weeks of age, as described earlier (57). Briefly, the mice were initially fed 5 to 10% less than mice fed AL for the first 2 weeks and were then fed 10 to 20% less for the 3rd and 4th weeks and 40% less thereafter. The composition of the diet was 20% casein, 50% dextrose, 15% starch, 5% cellulose, 5% corn oil, 3.5% AIN (American Institute of Nutrition) salt mixture, 1% AIN vitamin mixture, 0.3% dl-methionine, and 0.2% choline chloride. Mice fed a calorie-restricted diet were not given additional vitamin or mineral mixture supplements (48), primarily to prevent excess vitamin and mineral intake per gram of body weight, which may alter gene expression between mice fed AL and mice fed a calorie-restricted diet. After 20 weeks of feeding, the mice were used for the experiments. All animal care and surgical procedures were approved by the Institutional Animal Care and Use Committee.
Peritoneal macrophage isolation and culture.
Ninety-six hours before the mice were killed, the mice received an intraperitoneal injection of 2.0 ml of thioglycolate broth (Sigma Chemical Co.). The mice were killed by carbon dioxide inhalation. The macrophages in the peritoneal exudate were harvested by lavage with 10 ml of ice-cold phosphate-buffered saline (PBS) under aseptic conditions. After extensive washing in PBS, the cells were resuspended in RPMI 1640 (Gibco, Grand Island, N.Y.) supplemented with 1% penicillin-streptomycin, 2 mM l-glutamine, 10 μM β-mercaptoethanol, and 10% heat-inactivated fetal bovine serum and counted. After incubation for 2 h in 5% CO2 at 37°C in a plastic tissue culture dish, nonadherent cells were removed by vigorous washing. The resultant adherent cell population consisted of 95% macrophages, with viability being >97%, as demonstrated by trypan blue exclusion. Viability remained >97% at all times in culture.
Phagocytosis assay with latex beads.
Macrophage monolayers were preincubated for 30 min in a buffered salt solution (140 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES ]pH 7.5[). Macrophages were incubated with the latex beads (Sigma Chemical Co.) at a density of 107/dish for 30 min at 21 to 30°C. The monolayers were then washed five times with cold PBS to remove uningested, freely suspended particles. The contents of each dish were then incubated in PBS containing 0.25% trypsin for 0.5 h at 37°C on a shaker to detach the macrophages from the bottom of the culture dishes and to remove uningested particles attached to the macrophage's membrane surface. The macrophages were then fixed with 1% paraformaldehyde (PFA).
Phagocytosis assay with zymosan.
Macrophages were incubated with zymosan (Molecular Probes) at a ratio of 1:10 at 37°C for 15 min and were then washed with 0.9% NaCl–0.02 M EDTA (pH 5.9) and fixed with 1% PFA at 4°C for 1 h. The macrophage phagocytic function was analyzed with a FACScan (Becton Dickinson) flow cytometer.
Flow cytometry analysis.
Macrophages obtained from thioglycolate-treated mice were stimulated with LPS at 1 μg/ml for 12 h. Both LPS-stimulated and nonstimulated macrophages were harvested and washed with PBS. The Fc receptor was blocked with anti-CD16/CD32 by incubation for 10 min at room temperature with shaking. After two washes in wash buffer, 105 cells were incubated in 200 μl of wash buffer for 30 min at room temperature with either phycoerythrin (PE)–anti-CD14 or PE–anti-I-Ab antibody. The samples were analyzed with a FACScan (Becton Dickinson) flow cytometer with CellQuest software, as described earlier (1).
Intracellular cytokine staining.
Intracellular cytokine analysis was carried out as described previously (22). Briefly, peritoneal macrophages were preincubated with LPS (1 μg/ml) for 18 h, and then brefeldin A (10 μg/ml) was added and the incubation was continued for 6 h. The cells were harvested and fixed with 1% PFA at 4°C for 1 h and then permeabilized by incubation with PBS containing 0.5% saponin for 30 min at room temperature to allow the antibodies to penetrate the cell membrane. The cells were then incubated with PE-conjugated anticytokine antibodies for 30 min at room temperature. Stained cells were analyzed on the FACScan flow cytometer with CellQuest software.
Cytokine and other protein mRNA expression.
Total cellular RNA was isolated with the Trizol reagent (Gibco BRL) by the protocol recommended by the manufacturer. Aliquots of 2 μg of RNA were transcribed in a total volume of 20 μl with random primers and superSCRIPT II reverse transcriptase. Aliquots of 2 μl of the product obtained by reverse transcription were taken as substrates for PCR, as described previously (35, 42). The other PCR components were added as a master mixture containing 10 mM Tris · HCl (pH 8.0); 1.5 mM MgCl2; 50 mM KCl; dATP, dCTP, dTTP, and dGTP, each at a concentration of 200 mM; 100 ng of primer; sterile water; and 2.5 U of Taq polymerase in a final volume of 50 μl. The sequences for the primers for Toll-like receptor 2 (TLR2), TLR4, TNF-α, IL-6, IL-12, and β-actin were obtained from published literature (39, 46, 52). β-Actin was used as a housekeeping gene to control for the differences in mRNA quantity and/or level of degradation.
Aliquots of 10 μl of PCR product were analyzed by electrophoresis in a 1.5% agarose gel in TBE (Tris-borate-EDTA) buffer (pH 8.0) containing ethidium bromide and were visualized by UV illumination. Densitometric analysis for semiquantitation of the PCR products was performed with an Alpha Imager 2000 analyzer.
Preparation of nuclear extracts.
Spleens were homogenized in solution A (0.6% Nonidet P-40, 10 mM KCl, 10 mM HEPES [pH 7.9], 0.1 mM EDTA, 0.1 mM EGTA, 0.1 mM dithiothreitol [DTT], 1.0 μg of leupeptin per ml, 5.0 μg of pepstatin ml, 0.5 mM phenylmethylsulfonyl fluoride, 5.0 μg of trypsin per ml, 5.0 μg of aprotinin per ml, 0.1 mM benzamidine). After transfer to a 15-ml tube, the debris was pelleted by centrifugation at 360 × g for 30 s. The supernatant containing intact nuclei was incubated on ice for 10 min and then centrifuged for 10 min at 2,240 × g. The nuclear pellets were then resuspended in solution C (10 mM HEPES [pH 7.9], 450 mM NaCl, 0.1 mM EDTA, 0.1 mM EGTA, 0.1 mM DTT, 1.0 μg of leupeptin per ml, 5.0 μg of pepstatin l per ml, 0.5 mM phenylmethylsulfonyl fluoride, 5.0 μg of trypsin per ml, 5.0 μg of aprotinin per ml, 0.1 mM benzamidine) and incubated on ice for 30 min. The nuclei were then centrifuged for 5 min at 17,560 × g. Supernatants containing nuclear proteins were aliquoted and stored at −70°C. The protein concentration was determined by the Bradford assay.
Electrophoretic mobility shift assay.
Oligonucleotides for NF-κB and NF–IL-6 were end labeled by treatment with T4 kinase in the presence of [γ-32P]ATP. Labeled oligonucleotides were purified on a Sephadex G-25 M column. Aliquots of 2.5 μg of nuclear protein were added to a binding reaction mixture containing 10 mM Tris-HCl (pH 7.5), 100 mM NaCl, 1 mM EDTA, 0.5 mM DTT, 1 μg of bovine serum albumin per μl, and 0.1 μg of poly(dI-dC)(dI-dC) per μl; and the mixture was incubated on ice for 10 min. After incubation, 30,000 to 50,000 cpm of labeled double-stranded oligonucleotide was added to each sample. The mixtures were incubated for 10 min at room temperature and separated on a 5% polyacrylamide gel in 0.5× TBE buffer. The gels were vacuum dried and subjected to autoradiography. To eliminate nonspecific binding, cold competition was done by adding 100 ng of unlabeled double-stranded NF-κB and NF–IL-6 oligonucleotides to the reaction mixture before adding the labeled probes. Nonspecific competition was done by adding 100 ng of unlabeled double-stranded Sp-1 oligonucleotide before the addition of the labeled probes.
Cytokine immunoassays.
Plasma TNF-α and IL-6 levels were measured by standard enzyme-linked immunosorbent assay (ELISA) techniques. In brief, each well of flat-bottom 96-well microtiter plates were coated with 50 μl of purified anti-TNF-α and anti-IL-6 antibodies (4 μg/ml in binding solution) overnight at 4°C. The plates were rinsed four times with washing buffer, and diluted serum was added, followed by incubation for 2 h at room temperature. The plates were washed four times with washing buffer, followed by the addition of biotinylated anticytokine antibodies. The plates were incubated in room temperature for 1 h and then washed four times with washing buffer. Streptavidin-alkaline phosphatase conjugate was added, and the plates were incubated for 30 min at room temperature. The plates were again washed four times with washing buffer, and the chromogen substrate was added. The plates were then incubated at room temperature to achieve the desired maximum absorbance and were read at 410 nM in an ELISA reader.
Model of polymicrobial sepsis.
Polymicrobial sepsis was induced by using a CLP model described for mice by Baker et al. (7) and Ayala et al. (6). In brief, after anesthesia with methoxyflurane, a midline incision was made. The cecum was isolated and ligated and was then punctured twice with a 25-gauge needle. A small amount of the bowel contents was extruded through the puncture holes to maintain their patency. Upon returning the bowel to the abdomen, the midline incision was closed in layers with 5-0 ethicon sutures. The animals were resuscitated with saline (40 ml/kg of body weight) administered subcutaneously. Mice that underwent a sham operation underwent the same procedure but without ligation and puncture of the cecum. The animals that were not subjected to anesthesia or surgery served as negative controls. Spleens and blood were harvested 4 h after the operation for assessment of NF-κB and NF–IL-6 activation and for analysis of the levels of cytokine TNF-α and IL-6 mRNA expression. To examine the mice for mortality after CLP, the mice were observed four times daily over a period of 72 h.
Paraquat injection.
Oxidative stress was induced as described by Migliaccio et al. (40). Briefly, mice were injected intraperitoneally with paraquat (methylviologen; 1,1′-dimethyl-4,4′-bipyridinium dichloride) prepared in PBS at a dose of 70 mg/kg of body weight, and their health status was observed every 2 h.
Statistics.
All data were analyzed by a factorial analysis of variance (ANOVA) with NCSS software (version 5.01; NCSS, Kaysville, Utah) or one-way ANOVA with Bonferroni's posttest with GraphPad Prism software (version 3.00; GraphPad Software, San Diego, Calif.). A P value of <0.05 was considered significant. Survival analysis was done with NCSS statistical software (version 5.5). The data were fit to two different survival distributions, and the parameters for the two groups were compared by the Peto-Wilcoxon test and Gehan's Wilcoxon test for the Weibull distribution and by the log rank test and the Cox-Mantel test for the exponential distribution. Results for the distribution of best fit were used to determine significance. Data for mice that had not died at the end of the observation period were treated as censored datum points.
RESULTS
Animal weights.
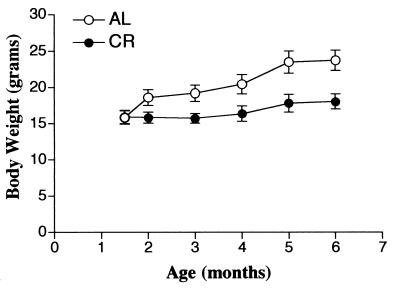
Body weight was significantly affected by calorie restriction. Mice in the group fed AL tended to gain weight with age, whereas animals fed the calorie-restricted diet gained very little body weight. The data are shown in Fig. 1.
FIG. 1.
Effect of CR on body weight. Six-month-old C57BL/6 mice were fed a semipurified diet containing 5% (wt/wt) corn oil AL or the same diet whose calories were restricted 40% (CR).
Peritoneal macrophage responsiveness to LPS ex vivo.
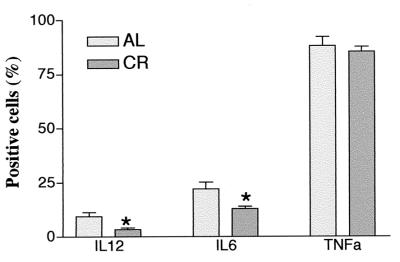
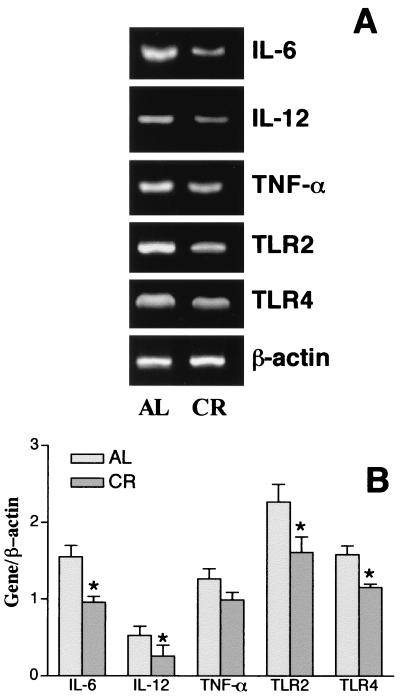
Since macrophages are the first line of defense during the innate immune response and play an important role in the pathogenesis of sepsis and septic shock, we assessed the responsiveness of macrophages from animals fed a calorie-restricted diet and AL to LPS ex vivo. Figure 2 shows the result of flow cytometric analysis of peritoneal macrophages stained for intracellular cytokines. After 24 h of LPS stimulation ex vivo, peritoneal macrophages from mice fed a calorie-restricted diet produced significantly less IL-6 and IL-12 than macrophages from mice fed AL. Although the percentage of TNF-α-positive cells in mice fed a calorie-restricted diet was less, there was no statistically significant difference between these two groups. To determine the effect of CR on inflammatory cytokine gene expression, reverse transcription-PCR was carried out with the isolated RNA. Cytokine TNF-α, IL-6, and IL-12 mRNAs were present at low basal levels in unstimulated macrophages; and there were no differences between mice fed AL and mice fed a calorie-restricted diet. After LPS stimulation, TNF-α, IL-6, and IL-12 mRNA levels were lower in peritoneal macrophages from animals on a calorie-restricted diet than those isolated from mice fed AL (Fig. 3). These results show that the responsiveness to LPS of macrophages from mice fed a calorie-restricted diet was lower than that of macrophages from mice fed AL.
FIG. 2.
Effect of CR on macrophage cytokine production after LPS stimulation. Peritoneal macrophages from 6-month-old C57BL/6 mice fed a calorie-restricted diet were stimulated with LPS ex vivo. Twenty-four hours after stimulation, macrophages were incubated with fluorescent antibodies to TNF-α, IL-6, and IL-12. The cells were analyzed by flow cytometry. Each bar represents the mean ± standard error of the mean of six independent measurements. ∗, significantly different from controls fed AL (P < 0.05).
FIG. 3.
Effect of CR on cytokine, TLR2, and TLR4 gene expression in peritoneal macrophages of C57BL/6 mice after LPS stimulation. Peritoneal macrophages were isolated as described in Material and Methods and were stimulated with LPS (1 μg) for 12 h in vitro. Total RNA was isolated and transcribed to cDNA and was then amplified by PCR with primers specific for TLR2, TLR4, IL-12, TNF-α, IL-6, and β-actin, as described in Materials and Methods.
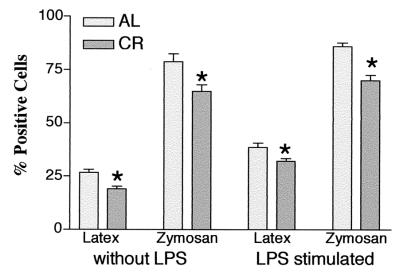
Peritoneal macrophage phagocytic function.
In order to define the effects of CR on the phagocytic function of peritoneal macrophages, the levels of ingestion of fluorescence-labeled latex beads and zymosan were measured by flow cytometry. Figure 4 presents these measurements. Both before and after LPS stimulation, macrophage phagocytic function in mice fed a calorie-restricted diet remained lower than that in the group fed AL.
FIG. 4.
Peritoneal macrophage phagocytosis in mice fed a calorie-restricted diet. Six-month-old C57BL/6 mice were fed a semipurified diet containing 5% (wt/wt) corn oil AL or the same diet whose calories were restricted 40% (CR). Peritoneal macrophages were harvested 96 h after injection of thioglycolate intraperitoneally. Phagocytosis of latex beads and zymosan was assessed before and 4 h after LPS stimulation (1 μg/ml) (n = 6). ∗, significant differences between the group fed AL and the group fed a calorie-restricted diet (P < 0.05).
Peritoneal macrophage CD14 and I-Ab antigen expression.
The constitutive expression of LPS receptor CD14 (mice fed AL, 26.9% ± 2.8%; mice fed a calorie-restricted diet, 19.9% ± 3.2% [P, not significant]) and major histocompatibility complex class II (MHC II) antigen I-Ab (mice fed AL, 53.2% ± 0.9%; mice fed a calorie-restricted diet, 37.6% ± 6.7% [P < 0.05]) on macrophages from mice fed a calorie-restricted diet was lower than that on macrophages from mice fed AL. After stimulation by LPS in vitro, CD14 expression (mice fed AL, 35.3% ± 3.1%; mice fed a calorie-restricted diet, 24.3% ± 3.5% [P < 0.05]) and I-Ab expression (mice fed AL, 85.2% ± 0.9%; mice fed a calorie-restricted diet, 69.1% ± 3.0% [P < 0.05]) on macrophages increased in the group of mice fed a calorie-restricted diet, but to a lesser extent than that on macrophages from the group fed AL.
Expression of TLR2 and TLR4 mRNAs.
Our primary data showed that TLR2 and TLR4 mRNA expression peaked at 12 h after LPS stimulation (data not shown). As shown in Fig. 3, after LPS stimulation, TLR2 and TLR4 mRNA expression in mice fed a calorie-restricted diet was significantly lower than that in mice fed AL.
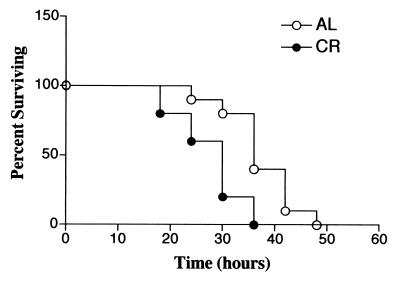
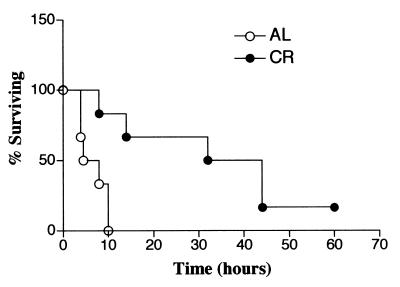
Survival trends of mice fed AL and mice fed a calorie-restricted diet with CLP-induced polymicrobial sepsis.
Figure 5 shows the 48-h survival pattern of C57BL/6 mice with CLP-induced sepsis. The first death was observed at 18 h in the group of mice fed a calorie-restricted diet, and all of the animals in the group of mice fed a calorie-restricted diet died within 36 h. Mice fed AL began to die later and survived significantly longer than mice fed a calorie-restricted diet (P = 0.004).
FIG. 5.
Effects of CR on the survival of C57BL/6 mice after CLP. Six-month-old C57BL/6 mice fed AL and a calorie-restricted diet were subjected to polymicrobial sepsis induced by CLP. The survival rate was monitored 72 h after CLP. There is a significant difference between these survival curves (P = 0.004 by the log rank test).
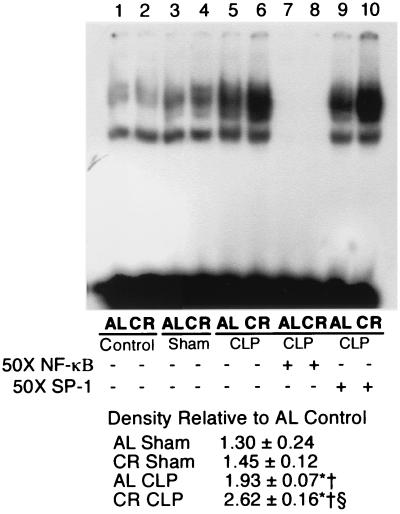
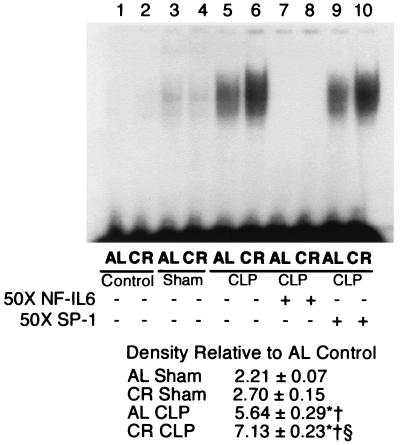
Splenic NF-κB and NF–IL-6 activation after polymicrobial sepsis induced by CLP. The effect of CLP on splenic NF-κB activation is presented in Fig. 6. Since splenic NF-κB nuclear binding activity peaked at 3 to 6 h after CLP (data not shown), we took the spleens at 4 h after CLP. The magnitude of the increase in NF-κB-binding activity was much greater in the group of mice fed a calorie-restricted diet than in the mice fed AL. Figure 7 shows the effect of CLP on splenic NF–IL-6 activation in the mice fed AL and the mice fed a calorie-restricted diet. Polymicrobial sepsis induces splenic NF–IL-6 activation in both the group fed AL and the group fed a calorie-restricted diet, but the NF–IL-6-binding activity was much higher in the group fed a calorie-restricted diet than in the group fed AL.
FIG. 6.
Electophoretic mobility shift assay of NF-κB-binding activity. Nuclear extracts were isolated as described in Materials and Methods. Nuclear extracts (2.5 μg) were incubated with radiolabeled probes specific for NF-κB, separated on a 5% polyacrylamide gel, and then exposed to film. The gel is representative of gels from five independent experiments. Band intensity was measured by densitometry and expressed as the mean ± standard error of the mean. ∗, significantly different from the corresponding control group (P < 0.05); †, significantly different from the corresponding group that underwent a sham operation (P < 0.05); §, significantly different from the group that underwent CLP and that was fed AL (P < 0.05).
FIG. 7.
NF–IL-6 activation and binding activity in murine spleen 4 h after CLP. Nuclear extracts were isolated as described in Materials and Methods. Nuclear extract (2.5 μg) was incubated with radiolabeled probes specific for NF–IL-6 and separated on a 5% polyacrylamide gel and then exposed to film. The gel is representative of gels from five independent experiments. Band intensity was measured by densitometry and is expressed as the mean ± standard error of the mean. ∗, Significantly different from corresponding control group (P < 0.05); †, significantly different from the corresponding group that underwent a sham operation (P < 0.05); §, significantly different from the group that underwent CLP and that was fed AL (P < 0.05).
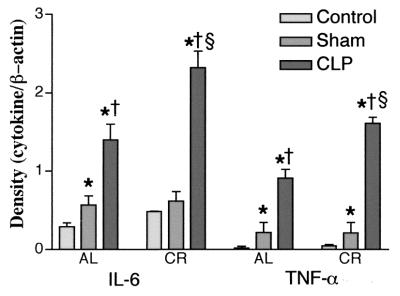
Effects of CR on TNF-α and IL-6 mRNA expression in splenic tissue of septic mice.
The levels of TNF-α and IL-6 mRNA expression in splenic tissue were examined 4 h after CLP. We selected only these cytokines because of their potential role in sepsis, and they are known to be transcriptionally regulated by NF-κB and/or NF–IL-6. TNF-α and IL-6 mRNA levels were found to be significantly higher in all mice that underwent CLP but were increased more in mice fed a calorie-restricted diet than in mice fed AL (Fig. 8).
FIG. 8.
Effects of CR on cytokine gene expression after polymicrobial peritonitis induced by CLP puncture. Polymicrobial peritonitis was induced by CLP in C57BL/6 mice. Four hours after the operation total RNA was isolated from the spleens, as described in Material and Methods. The RNA was transcribed to cDNA and was then amplified by PCR with primers specific for TNF-α, IL-6, or β-actin, as described in Materials and Methods. The resulting samples were subjected to electrophoresis on agarose gels and stained with ethidium bromide. Band intensity was quantitated by densitometry. The values are the means ± standard errors of the means for five gels. ∗, significantly different from corresponding control group (P < 0.05); †, significantly different from corresponding group that underwent a sham operation (P < 0.05); §, significantly different from group that underwent CLP and that was fed AL (P < 0.05).
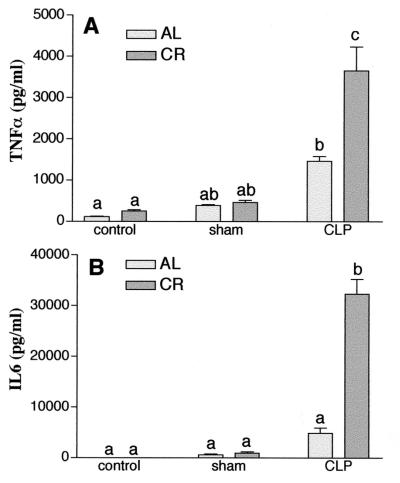
Generation of TNF-α and IL-6 in sera after CLP.
Previous investigations demonstrated that the levels of certain cytokines, such TNF-α, IL-1β, and IL-6, are elevated in serum during the evolution of CLP-induced peritonitis and peak at 3 to 6 h after sepsis. In the present studies, serum TNF-α and IL-6 levels were higher in mice that underwent CLP than in control mice and mice that underwent a sham operation (Fig. 9). Interestingly, 4 h after induction of polymicrobial sepsis by CLP, the levels of TNF-α and IL-6 in mice fed a calorie-restricted diet, and especially the level of IL-6, were extremely high. These data indicate that TNF-α and IL-6 levels in systemic tissues in general were augmented much more during the evolution of CLP-induced peritonitis in mice fed a calorie-restricted diet than in mice fed AL, which may have caused earlier deaths due to sepsis in mice fed a calorie-restricted diet.
FIG. 9.
Effect of calorie restriction on serum cytokine TNF-α and IL-6 levels of 6-month-old C57BL/6 mice fed a calorie-restricted diet. Mice were treated as described in the legend to Fig. 8. Serum TNF-α (A) and IL-6 (B) levels were quantitated by ELISA. Each bar represents the mean ± standard error of the mean of five independent measurements. The means for bars labeled with different letters are significantly different (P < 0.05).
Effect of CR on oxidative stress.
It is known that the numbers of oxidative stress-induced deaths are higher in mice fed AL than in mice fed a calorie-restricted diet. Since death from sepsis was found earlier in mice fed a calorie-restricted diet than in mice fed AL, we chose to compare oxidative stress-induced death in mice fed AL and mice fed a calorie-restricted diet. To examine the ability of mice fed a calorie-restricted diet to resist oxidative stress in vivo, animals were treated with paraquat, which generates superoxide anions upon cellular metabolism. Groups of six 6-month-old mice were injected intraperitoneally with 70 mg of paraquat per kg. All of the mice fed AL died within 12 h after injection, whereas of the six animals fed a calorie-restricted diet and injected with paraquat, only one died within 12 h, four died within 48 h, and one survived for 3 days after injection (Fig. 10). These differences between the two survival curves were found to be significantly different (P = 0.0048). Furthermore, these data also suggest that mice fed a calorie-restricted diet were not malnourished and that their antioxidant defense mechanisms were elevated compared to those of mice fed AL.
FIG. 10.
Survival of mice fed a calorie-restricted diet and mice fed AL after challenge with the oxidizing agent paraquat. Six-month-old C57BL/6 mice fed a calorie-restricted diet and mice fed AL were injected intraperitoneally with paraquat at 70 mg/kg. Mice fed AL died significantly earlier than mice fed a calorie-restricted diet (P = 0.0048).
DISCUSSION
In the present experiments, we first examined the effects of CR on the responsiveness of peritoneal macrophages to LPS ex vivo. The data indicated that the peritoneal macrophages of young mice fed a calorie-restricted diet show a significantly suppressed response to LPS. Intracellular cytokine analysis revealed that the percentage of Il-12-, IL-6-, and TNF-α-positive cells from mice in the group fed a calorie-restricted diet is lower after stimulation with LPS ex vivo. Similarly, macrophages from mice fed a calorie-restricted diet had significant reductions in IL-6, IL-12, and TNF-α mRNA levels.
Surprisingly, very few studies have attempted to investigate the effects of CR on the responsiveness of macrophages to LPS. Dong et al. (19) addressed the effects of 25% CR on rat alveolar macrophages. In vitro, LPS-stimulated alveolar macrophages from mice fed a calorie-restricted diet generated less NO and TNF-α as well as less TNF-α and IL-6 mRNA than animals maintained on a diet fed AL. From these results, it seems that the response to LPS of macrophages from mice on calorie-restricted regimens depends on various factors including the duration and extent of CR, the strain of animal used, and the anatomical site from which the macrophages are isolated.
It is well known that LPS mediates many of the pathophysiologic events in sepsis by stimulating the release of host-derived proinflammatory cytokines (18). In response to endotoxin exposure, TNF-α, IL-1β, and IL-6 are produced by tissue macrophages and peripheral monocytes. These cytokines have been shown to produce many of the hemodynamic and inflammatory changes of sepsis either directly or indirectly. It has only recently been reported that CD14 participates in the mediation of LPS responsiveness by high-affinity binding of LPS during the innate immune response (67). We therefore analyzed the CD14 expression in peritoneal macrophages by fluorescence-activated cell sorter analysis and found no difference between the groups fed a calorie-restricted diet and AL. However, after LPS stimulation, the level of CD14 expression in the macrophages of both groups was increased, but the percentage of CD14+ cells was less in mice fed a calorie-restricted diet than in mice fed AL, which may be one of the mechanisms involved in the impairment of macrophage function in mice fed a calorie-restricted diet.
Along with the CD14 receptor, several mammalian Toll homologues have recently been identified. Toll was first identified as a protein that controls dorsoventral pattern formation in the early development of Drosophila (10). Since that time it has been shown to participate in the antimicrobial immune response. Two of these human Toll homologues, TLR2 and TLR4, have been found to be involved in LPS signaling (36, 70). Because of a lack of availability of TLR2 and TLR4 antibodies for mice, we chose to analyze the expression of mRNA for these two receptors by reverse transcription-PCR and found it to be significantly lower in macrophages from mice fed a calorie-restricted diet than from mice fed AL. These results suggest that the animals fed a calorie-restricted diet are likely to respond less to bacterial pathogens, whereas the response to an external insult is known to be intact in animals fed a calorie-restricted diet (53). Furthermore, the macrophages from mice fed a calorie-restricted diet showed hyporesponsiveness to the acute stress, as evidenced by down regulation of the expression of mRNAs for the LPS receptors TLR2 and TLR4 and the cytokines TNF-α, IL-6, and IL-12 as well as the release of decreased levels of TNF-α, IL-6, and IL-12 peptides.
It was established that IL-12 plays a crucial role in both the innate and the acquired immune responses. Although first recognized as a cytokine critical for activation of cytotoxic lymphocytes, the most important biological significance of IL-12 secretion relates to its effects on T-helper cells. IL-12 is known to drive Th1 cytokine production during physiological immune responses, mediated principally by its ability to stimulate proliferation and gamma interferon production in T cells and natural killer (NK) cells (29, 33). Furthermore, clinical evidence reveals a critical role for IL-12 in early resistance to postoperative infection (31). We also noted that macrophages from mice fed a calorie-restricted diet express less MHC class II antigen than those from mice fed AL. LPS-induced augmentation of MHC class II antigen I-Ab was also found to be less in macrophages from mice fed a calorie-restricted diet. In addition to decreased levels of CD14, TLR2, and TLR4 expression, MHC class II also modifies LPS responsiveness. A lack of or a low level of expression of MHC class II molecules results in diminished secretion of proinflammatory cytokines by macrophages following stimulation with LPS (47). These results are consistent with those described in our first report that CR leads to impaired antigen processing and presentation by macrophages and/or defects in recognition of antigen in vivo by T cells in young C57BL/6 mice (17).
Thus, in support of our previous observation, our present results also show that CR either suppresses the macrophage phagocytic function or delays macrophage maturation. We used two different approaches to assess the macrophage phagocytic function: phagocytosis of latex beads and phagocytosis of zymosan. Both approaches showed similar trends. These results, however, do not agree with the findings of Dong et al. (19), who examined alveolar macrophages in rats fed a calorie-restricted diet. They found that CR enhanced phagocytic function in both control and ozone-exposed rats. The discrepancy may be due to two factors. First, the duration of CR and the species of animals used were different. They restricted calories only by 25% for 21 days, whereas we restricted calories by 40% for 5 months. Second, macrophages in different anatomical sites behave differently (5).
In addition to studying the functional activity of macrophages, we also used the CLP model to assess the effect of CR on septic shock. The CLP model in rodents is analogous to perforated appendicitis in humans (7). It produces peritonitis that leads to 95% mortality within 48 to 72 h (15). This is a widely used model that has been found to mimic the clinical disease symptoms of polymicrobial sepsis and septic shock (15). Our present results show that CR renders young C57BL/6 mice more susceptible to sepsis than mice fed AL. Animals in the group fed a calorie-restricted diet died earlier than animals in the group fed AL. Peck et al. (44) investigated the effects of CR and protein restriction on mortality in A/J mice subjected to salmonella infection. They showed that after 3 weeks of feeding of a calorie-restricted diet, CR improved the survival rate. This discrepancy may again be due to strain differences and differences in the duration of CR. For instance, the mice fed a calorie-restricted diet were supplemented with additional levels of minerals and a vitamin mixture to maintain their intake equal to that of the mice fed AL, whereas in the present study the mice fed a calorie-restricted diet were not supplemented with additional mineral and vitamin mixtures, which may cause increased numbers of deaths among the mice fed a calorie-restricted diet. However, this needs to be confirmed in studies with and without vitamin supplements to determine the protective effects of vitamins and minerals in preventing the immune deficiency in sepsis and/or other bacterial infections.
In our present studies, the earlier deaths due to sepsis in mice fed a calorie-restricted diet appear to be due to increased levels of TNF-α and IL-6 mRNA expression in the spleen and activation of the transcriptional factors NF-κB and NF–IL-6, whose levels we measured 4 h after induction of CLP in both groups. These results are in agreement with previous observations made in CLP studies by other investigators (12, 66). The levels of both TNF-α and IL-6 mRNA expression and NF-κB and NF–IL-6 activation in the spleen were found to be higher in the group fed a calorie-restricted diet than in the group fed AL. Several lines of evidence show that activation, nuclear translocation, and binding of NF-κB or NF–IL-6 are pivotal steps in controlling the transcription of genes coding for proinflammatory and immunoregulatory cytokines (41, 61, 68, 72). Activation of NF-κB and NF–IL-6 in the early stages of sepsis accompanies bacteremia, cytokine expression, and mortality (12, 66). Furthermore, we observed that systemic TNF-α and IL-6 levels were significantly increased after CLP. Of greatest interest is that systemic TNF-α and IL-6 levels were much higher in mice fed a calorie-restricted diet than in mice fed AL. This finding is somewhat in contrast to the findings of Spaulding et al. (56), who reported that CR prevents the rise in serum TNF-α and IL-6 levels during aging. Those investigators, however, did not measure TNF-α and IL-6 levels after stimulation either in vivo or in vitro to establish the differences between young and old mice fed a calorie-restricted diet and young and old mice fed AL. TNF-α and IL-6 are the typical proinflammatory mediators, and it is well known that they are released during polymicrobial sepsis (2, 4). High levels of IL-6 in the circulation have been reported to be one of the more consistent markers associated with increased rates of morbidity and mortality in traumatic injury and/or sepsis (8). Salkowski et al. investigated the production of cytokines and chemokines during the early phase of sepsis induced by CLP (51). The data indicate that polymicrobial sepsis stimulates a broad range of cytokines during the early periods of sepsis (51). In the present study, we did not examine cytokine expression in other organs, which needs to be carried out. Other studies have shown that, besides macrophages from the spleen, macrophages from the liver are another predominant source of proinflammatory cytokines during polymicrobial sepsis (3).
The results of our present CLP study suggest that CR, initiated at 6 weeks, increases the susceptibility of mice to polymicrobial sepsis and suppresses macrophage function, as evidenced by a decrease in the levels of expression of the LPS receptors CD14, TLR2, and TLR4 after stimulation by LPS and suppression of macrophage phagocytic function and MHC class II antigen expression. After infection is established, it is quite possible that macrophages in animals fed a calorie-restricted diet are unable to function adequately to prevent the rise in the number of invading pathogens, and the pathogens may spill over into the system due to the impairment of the activation of immune cells (T cells, NK cells, neutrophils). Furthermore, it is also possible that the regulation of Th-1 and Th-2 cytokines may be altered by CR, thereby releasing more proinflammatory cytokines, which leads to failure to confine the infection and, consequently, induction of more severe sepsis, septic shock, and/or multiorgan dysfunction.
The earlier deaths from sepsis in mice fed a calorie-restricted diet are quite surprising since CR is known to prolong the life span in virus-infected mice (25). We and others have reported higher cytotoxic T-cell function in mice fed a calorie-restricted diet (21, 24). We speculate that CR initiated at 6 to 8 weeks of age may have a selective immunosuppresive effect on macrophages due to increased levels of cortisol, which are known to increase in animals fed a calorie-restricted diet (50). It is possible that if CR is started in adult mice aged 4 to 6 months, the macrophage function may be less impaired compared to that in 6- to 8-week-old mice, although the survival rate is known to be much less in adult animals fed a calorie-restricted diet than in 6- to 8-week-old animals fed a calorie-restricted diet (63). However, this needs to be studied in detail soon.
Oxidant damage is known to be increased in septic shock and endotoxemia and has been implicated in the pathogenic mechanism (9, 73). Several previous studies showed that CR can increase the levels of antioxidant enzymes superoxide dismutase and catalase and decrease the levels of free radicals (13, 55, 71). In order to determine the effect of CR on oxidative stress in vivo, both animals fed AL and animals fed a calorie-restricted diet were also treated with paraquat. In contrast to the deleterious effects of CR on mice with sepsis, the results from paraquat administration show that CR enhances or maintains the increased level of resistance to oxidative stress in vivo. Thus, the results of the present studies are similar to those reported earlier by others and show that mice fed a calorie-restricted diet have increased resistance to oxidative stress (53); the greater susceptibility to infection is not due to dietary deficiency and/or the effect of young age.
Although our present findings suggest that CR increases susceptibility to CLP in young mice, experiments are urgently needed to establish the role of CR during aging. It is quite possible that with age mice fed a calorie-restricted diet may develop increased resistance to bacterial infection, whereas older mice (24 months or older) fed AL may become more susceptible than mice fed a calorie-restricted diet due to the age-related decline in immune function (23, 59). We and several other investigators have shown a decline in IL-2 production with age in mice fed AL (23, 26, 43, 58), whereas CR maintains better cell-mediated immune function during aging. Thus, additional studies with aged mice fed a calorie-restricted diet and mice fed AL are needed to measure the response to CLP as well as resistance to well-known gram-negative and gram-positive bacterial pathogens.
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grants AG14541 and AG13693 from the National Institute on Aging.
REFERENCES
- 1.Avula C P, Fernandes G. Modulation of antioxidant enzymes and apoptosis in mice by dietary lipids and treadmill exercise. J Clin Immunol. 1999;19:35–44. doi: 10.1023/a:1020562518071. [DOI] [PubMed] [Google Scholar]
- 2.Ayala A, Deol Z K, Lehman D L, Herdon C D, Chaudry I H. Does endotoxin play a major role in inducing the depression of macrophage function during polymicrobial sepsis? Arch Surg. 1995;130:1178–1184. doi: 10.1001/archsurg.1995.01430110036007. (discussion, 1184–1185). [DOI] [PubMed] [Google Scholar]
- 3.Ayala A, O'Neill P J, Uebele S A, Herdon C D, Chaudry I H. Mechanism of splenic immunosuppression during sepsis: key role of Kupffer cell mediators. J Trauma. 1997;42:882–888. doi: 10.1097/00005373-199705000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Ayala A, Perrin M M, Kisala J M, Ertel W, Chaudry I H. Polymicrobial sepsis selectively activates peritoneal but not alveolar macrophages to release inflammatory mediators (interleukins-1 and -6 and tumor necrosis factor) Circ Shock. 1992;36:191–199. [PubMed] [Google Scholar]
- 5.Ayala A, Perrin M M, Wang P, Ertel W, Chaudry I H. Hemorrhage induces enhanced Kupffer cell cytotoxicity while decreasing peritoneal or splenic macrophage capacity. Involvement of cell-associated tumor necrosis factor and reactive nitrogen. J Immunol. 1991;147:4147–4154. [PubMed] [Google Scholar]
- 6.Ayala A, Urbanich M A, Herdon C D, Chaudry I H. Is sepsis-induced apoptosis associated with macrophage dysfunction? J Trauma. 1996;40:568–573. doi: 10.1097/00005373-199604000-00008. (discussion, 573–574). [DOI] [PubMed] [Google Scholar]
- 7.Baker C C, Chaudry I H, Gaines H O, Baue A E. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983;94:331–335. [PubMed] [Google Scholar]
- 8.Barriere S L, Lowry S F. An overview of mortality risk prediction in sepsis. Crit Care Med. 1995;23:376–393. doi: 10.1097/00003246-199502000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Basu S, Eriksson M. Oxidative injury and survival during endotoxemia. FEBS Lett. 1998;438:159–160. doi: 10.1016/s0014-5793(98)01290-3. [DOI] [PubMed] [Google Scholar]
- 10.Belvin M P, Anderson K V. A conserved signaling pathway: the Drosophila toll-dorsal pathway. Annu Rev Cell Dev Biol. 1996;12:393–416. doi: 10.1146/annurev.cellbio.12.1.393. [DOI] [PubMed] [Google Scholar]
- 11.Bone R C, Balk R A, Cerra F B, Dellinger R P, Fein A M, Knaus W A, Schein R M, Sibbald W J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 12.Browder W, Ha T, Chuanfu L, Kalbfleisch J H, Ferguson D A, Jr, Williams D L. Early activation of pulmonary nuclear factor kappaB and nuclear factor interleukin-6 in polymicrobial sepsis. J Trauma. 1999;46:590–596. doi: 10.1097/00005373-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Byun D S, Venkatraman J T, Yu B P, Fernandes G. Modulation of antioxidant activities and immune response by food restriction in aging Fischer-344 rats. Aging (Milan) 1995;7:40–48. doi: 10.1007/BF03324291. [DOI] [PubMed] [Google Scholar]
- 14.Cefalu W T, Wagner J D, Wang Z Q, Bell-Farrow A D, Collins J, Haskell D, Bechtold R, Morgan T. A study of caloric restriction and cardiovascular aging in cynomolgus monkeys (Macaca fascicularis): a potential model for aging research. J Gerontol A Biol Sci Med Sci. 1997;52:B10–B19. doi: 10.1093/gerona/52a.1.b10. [DOI] [PubMed] [Google Scholar]
- 15.Chaudry I H. Sepsis: lessons learned in the last century and future directions. Arch Surg. 1999;134:922–929. doi: 10.1001/archsurg.134.9.922. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Astle C M, Harrison D E. Delayed immune aging in diet-restricted B6CBAT6 F1 mice is associated with preservation of naive T cells. J Gerontol A Biol Sci Med Sci. 1998;53:B330–B337. doi: 10.1093/gerona/53a.5.b330. (discussion, B338–B339). [DOI] [PubMed] [Google Scholar]
- 17.Christadoss P, Talal N, Lindstrom J, Fernandes G. Suppression of cellular and humoral immunity to T-dependent antigens by calorie restriction. Cell Immunol. 1984;88:1–8. doi: 10.1016/0008-8749(84)90046-7. [DOI] [PubMed] [Google Scholar]
- 18.Davies M G, Hagen P O. Systemic inflammatory response syndrome. Br J Surg. 1997;84:920–935. doi: 10.1002/bjs.1800840707. [DOI] [PubMed] [Google Scholar]
- 19.Dong W, Selgrade M K, Gilmour I M, Lange R W, Park P, Luster M I, Kari F W. Altered alveolar macrophage function in calorie-restricted rats. Am J Respir Cell Mol Biol. 1998;19:462–469. doi: 10.1165/ajrcmb.19.3.3114. [DOI] [PubMed] [Google Scholar]
- 20.Duffy P H, Feuers R J, Pipkin J L, Berg T F, Leal L M C C, Turturro A, Hart R W. The effect of dietary restriction and aging on the physiological response of rodents to drugs. In: Hart R W, Neuman D A, Robertson R T, editors. Dietary restriction: implications for the design and interpretation of toxicity and carcinogenicity studies. Washington, D.C.: ILSI Press; 1995. pp. 127–140. [Google Scholar]
- 21.Effros R B, Casillas A, Walford R L. Influenza immunity in aged mice: effects of dietary restriction. Aging Immunol Infect Dis. 1990;2:111–116. [Google Scholar]
- 22.Fernandes G. Methods for the study of immune cell in aging. In: Yu B P, editor. Methods in aging research. Boca Raton, Fla: CRC Press, Inc.; 1999. pp. 549–567. [Google Scholar]
- 23.Fernandes G. Nutritional factors: modulating effects on immune function and aging. Pharmacol Rev. 1984;36:123S–129S. [PubMed] [Google Scholar]
- 24.Fernandes G, Friend P, Yunis E J, Good R A. Influence of dietary restriction on immunologic function and renal disease in NZBxNZWF1 mice. Proc Natl Acad Sci USA. 1978;75:1500–1504. doi: 10.1073/pnas.75.3.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandes G, Tomar V, Mohan N, Venkatraman J T. Potentials of diet therapy on murine AIDS. J Nutr. 1992;122:716–722. doi: 10.1093/jn/122.suppl_3.716. [DOI] [PubMed] [Google Scholar]
- 26.Fernandes G, Venkatraman J, Turturro A, Attwood V G, Hart R W. Effect of food restriction on life span and immune functions in long-lived Fischer-344 × Brown Norway F1 rats. J Clin Immunol. 1997;17:85–95. doi: 10.1023/a:1027344730553. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes G, Yunis E J, Good R A. Influence of diet on survival of mice. Proc Natl Acad Sci USA. 1976;73:1279–1283. doi: 10.1073/pnas.73.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandes G, Yunis E J, Good R A. Suppression of adenocarcinoma by the immunological consequences of calorie restriction. Nature. 1976;263:504–507. doi: 10.1038/263504b0. [DOI] [PubMed] [Google Scholar]
- 29.Gately M K, Desai B B, Wolitzky A G, Quinn P M, Dwyer C M, Podlaski F J, Familletti P C, Sinigaglia F, Chizonnite. Gubler R U, et al. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor) J Immunol. 1991;147:874–882. [PubMed] [Google Scholar]
- 30.Hansen B C, Ortmeyer H K, Bodkin N L. Prevention of obesity in middle-aged monkeys: food intake during body weight clamp. Obes Res. 1995;3(Suppl. 2):199s–204s. doi: 10.1002/j.1550-8528.1995.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 31.Hensler T, Heidecke C D, Hecker H, Heeg K, Bartels H, Zantl N, Wagner H, Siewert J R, Holzmann B. Increased susceptibility to postoperative sepsis in patients with impaired monocyte IL-12 production. J Immunol. 1998;161:2655–2659. [PubMed] [Google Scholar]
- 32.Heydari A R, Wu B, Takahashi R, Strong R, Richardson A. Expression of heat shock protein 70 is altered by age and diet at the level of transcription. Mol Cell Biol. 1993;13:2909–2918. doi: 10.1128/mcb.13.5.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O'Garra A, Murphy K M. Listeria-induced Th1 development in a,b-TCR transgenic CD4+ T cells occurs through macrophage production of IL-12. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 34.Ingram D K, Cutler R G, Weindruch R, Renquist D M, Knapka J J, April M, Belcher C T, Clark M A, Hatcherson C D, Marriott B M, Roth G S. Dietary restriction and aging: the initiation of a primate study. J Gerontol Biol Sci. 1990;45:B148–B163. doi: 10.1093/geronj/45.5.b148. [DOI] [PubMed] [Google Scholar]
- 35.Jolly C A, Fernandez R, Muthukumar A R, Fernandes G. Calorie restriction modulates Th-1 and Th-2 cytokine-induced immunoglobulin secretion in young and old C57BL/6 cultured submandibular glands. Aging (Milan) 1999;11:383–389. doi: 10.1007/BF03339817. [DOI] [PubMed] [Google Scholar]
- 36.Kirschning C J, Wesche H, Merrill Ayres T, Rothe M. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masoro E J. Caloric restriction and aging: an update. Exp Gerontol. 2000;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 38.Masoro E J. Influence of caloric intake on aging and on the response to stressors. J Toxicol Environ Health B Crit Rev. 1998;1:243–257. doi: 10.1080/10937409809524554. [DOI] [PubMed] [Google Scholar]
- 39.Matsuguchi T, Takagi K, Musikacharoen T, Yoshikai Y. Gene expressions of lipopolysaccharide receptors, toll-like receptors 2 and 4, are differently regulated in mouse T lymphocytes. Blood. 2000;95:1378–1385. [PubMed] [Google Scholar]
- 40.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi P P, Lanfrancone L, Pelicci P G. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 41.Murphy K, Haudek S B, Thompson M, Giroir B P. Molecular biology of septic shock. New Horiz. 1998;6:181–193. [PubMed] [Google Scholar]
- 42.Muthukumar A R, Jolly C A, Zaman K, Fernandes G. Calorie restriction decreases proinflammatory cytokines and polymeric Ig receptor expression in the submandibular glands of autoimmune prone (NZB × NZW)F1 mice. J Clin Immunol. 2000;20:354–361. doi: 10.1023/a:1006620130114. [DOI] [PubMed] [Google Scholar]
- 43.Pahlavani M A, Harris M D, Richardson A. The increase in the induction of IL-2 expression with caloric restriction is correlated to changes in the transcription factor NFAT. Cell Immunol. 1997;180:10–19. doi: 10.1006/cimm.1997.1155. [DOI] [PubMed] [Google Scholar]
- 44.Peck M D, Babcock G F, Alexander J W. The role of protein and calorie restriction in outcome from Salmonella infection in mice. J Parenter Enteral Nutr. 1992;16:561–565. doi: 10.1177/0148607192016006561. [DOI] [PubMed] [Google Scholar]
- 45.Perkins S N, Hursting S D, Phang J M, Haines D C. Calorie restriction reduces ulcerative dermatitis and infection-related mortality in p53-deficient and wild-type mice. J Investig Dermatol. 1998;111:292–296. doi: 10.1046/j.1523-1747.1998.00270.x. [DOI] [PubMed] [Google Scholar]
- 46.Peterson V M, Adamovicz J J, Elliott T B, Moore M M, Madonna G S, Jackson III W E, Ledney G D, Gause W C. Gene expression of hematoregulatory cytokines is elevated endogenously after sublethal gamma irradiation and is differentially enhanced by therapeutic administration of biologic response modifiers. J Immunol. 1994;153:2321–2330. [PubMed] [Google Scholar]
- 47.Piani A, Hossle J P, Birchler T, Siegrist C A, Heumann D, Davies G, Loeliger S, Seger R, Lauener R P. Expression of MHC class II molecules contributes to lipopolysaccharide responsiveness. Eur J Immunol. 2000;30:3140–3146. doi: 10.1002/1521-4141(200011)30:11<3140::AID-IMMU3140>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 48.Pugh T D, Klopp R G, Weindruch R. Controlling caloric consumption: protocols for rodents and rhesus monkeys. Neurobiol Aging. 1999;20:157–165. doi: 10.1016/s0197-4580(99)00043-3. [DOI] [PubMed] [Google Scholar]
- 49.Roberts S, Pi-Sunyer X, Kuller L, Ellison P, Prior J, Shapses S. Physiologic effects of lowering caloric intake in nonhuman primates and nonobese persons. J Gerontol Biol Sci. 2001;56A:66–75. doi: 10.1093/gerona/56.suppl_1.66. [DOI] [PubMed] [Google Scholar]
- 50.Sabatino F, Masoro E J, McMahan C A, Kuhn R W. Assessment of the role of the glucocorticoid system in aging processes and in the action of food restriction. J Gerontol. 1991;46:B171–B179. doi: 10.1093/geronj/46.5.b171. [DOI] [PubMed] [Google Scholar]
- 51.Salkowski C A, Detore G, Franks A, Falk M C, Vogel S N. Pulmonary and hepatic gene expression following cecal ligation and puncture: monophosphoryl lipid A prophylaxis attenuates sepsis-induced cytokine and chemokine expression and neutrophil infiltration. Infect Immun. 1998;66:3569–3578. doi: 10.1128/iai.66.8.3569-3578.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schoenhaut D S, Chua A O, Wolitzky A G, Quinn P M, Dwyer C M, McComas W, Familletti P C, Gately M K, Gubler U. Cloning and expression of murine IL-12. J Immunol. 1992;148:3433–3440. [PubMed] [Google Scholar]
- 53.Scrofano M M, Shang F, Nowell T R, Jr, Gong X, Smith D E, Kelliher M, Dunning J, Mura C V, Taylor A. Calorie restriction, stress and the ubiquitin-dependent pathway in mouse livers. Mech Age Dev. 1998;105:273–290. doi: 10.1016/s0047-6374(98)00097-9. [DOI] [PubMed] [Google Scholar]
- 54.Shi H N, Scott M E, Stevenson M M, Koski K G. Energy restriction and zinc deficiency impair the functions of murine T cells and antigen-presenting cells during gastrointestinal nematode infection. J Nutr. 1998;128:20–27. doi: 10.1093/jn/128.1.20. [DOI] [PubMed] [Google Scholar]
- 55.Sohal R S, Weindruch R. Oxidative stress, calorie restriction and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spaulding C C, Walford R L, Effros R B. Calorie restriction inhibits the age-related dysregulation of the cytokines TNF-alpha and IL-6 in C3B10RF1 mice. Mech Age Dev. 1997;93:87–94. doi: 10.1016/s0047-6374(96)01824-6. [DOI] [PubMed] [Google Scholar]
- 57.Troyer D A, Chandrasekar B, Thinnes T, Stone A, Loskutoff D A, Fernandes G. Effects of energy intake on type I plasminogen activator inhibitor levels in glomeruli of lupus-prone B/W mice. Am J Pathol. 1995;146:111–120. [PMC free article] [PubMed] [Google Scholar]
- 58.Venkatraman J T, Attwood V G, Turturro A, Hart R W, Fernandes G. Maintenance of virgin T cells and immune functions by food restriction during aging in long-lived B6D2F1 female mice. Aging, Immunol. Infect Dis. 1994;5:13–25. [Google Scholar]
- 59.Walford R L, Liu R K, Gerbase-DeLima M, Mathies M, Smith G S. Long-term dietary restriction and immune function in mice: response to sheep red blood cells and to mitogenic agents. Mech Age Dev. 1973;2:447. doi: 10.1016/0047-6374(73)90035-3. [DOI] [PubMed] [Google Scholar]
- 60.Wanagat J, Allison D B, Weindruch R. Caloric intake and aging: mechanisms in rodents and a study in nonhuman primates. Toxicol Sci. 1999;52:35–40. doi: 10.1093/toxsci/52.2.35. [DOI] [PubMed] [Google Scholar]
- 61.Wedel A, Ziegler-Heitbrock H W. The C/EBP family of transcription factors. Immunobiology. 1995;193:171–185. doi: 10.1016/s0171-2985(11)80541-3. [DOI] [PubMed] [Google Scholar]
- 62.Weindruch R, Keenan K P, Carney J M, Fernandes G, Feuers R J, Floyd R A, Halter J B, Ramsey J J, Richardson A, Roth G S, Spindler S R. Caloric restriction mimetics: metabolic interventions. J Gerontol Biol Sci. 2001;56A:20–33. doi: 10.1093/gerona/56.suppl_1.20. [DOI] [PubMed] [Google Scholar]
- 63.Weindruch R, Walford R. The retardation of aging and disease by dietary restriction. Springfield, Ill: Charles C Thomas, Publisher; 1988. [Google Scholar]
- 64.Weindruch R, Walford R L. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215:1415–1418. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- 65.Weindruch R, Walford R L, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 66.Williams D L, Ha T, Li C, Kalbfleisch J H, Ferguson D A., Jr Early activation of hepatic NF-kappaB and NF-IL6 in polymicrobial sepsis correlates with bacteremia, cytokine expression, and mortality. Ann Surg. 1999;230:95–104. doi: 10.1097/00000658-199907000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 68.Xia C, Cheshire J K, Patel H, Woo P. Cross-talk between transcription factors NF-kappa B and C/EBP in the transcriptional regulation of genes. Int J Biochem Cell Biol. 1997;29:1525–1539. doi: 10.1016/s1357-2725(97)00083-6. [DOI] [PubMed] [Google Scholar]
- 69.Xia E, Rao G, Van Remmen H, Heydari A R, Richardson A. Activities of antioxidant enzymes in various tissues of male Fischer 344 rats are altered by food restriction. J Nutr. 1995;125:195–201. doi: 10.1093/jn/125.2.195. [DOI] [PubMed] [Google Scholar]
- 70.Yang R B, Mark M R, Gray A, Huang A, Xie M H, Zhang M, Goddard A, Wood W I, Gurney A L, Godowski P J. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 71.Yu B P, Lee D W, Choi J W. Prevention of free radical damage by food restricition. In: Fishbein L, editor. Biological effects of dietary restriction. New York, N.Y: Springer-Verlag; 1991. pp. 185–190. [Google Scholar]
- 72.Zagariya A, Mungre S, Lovis R, Birrer M, Ness S, Thimmapaya B, Pope R. Tumor necrosis factor alpha gene regulation: enhancement of C/EBPbeta-induced activation by c-Jun. Mol Cell Biol. 1998;18:2815–2824. doi: 10.1128/mcb.18.5.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang H, Slutsky A S, Vincent J L. Oxygen free radicals in ARDS, septic shock and organ dysfunction. Intensive Care Med. 2000;26:474–476. doi: 10.1007/s001340051185. [DOI] [PubMed] [Google Scholar]