Abstract
Background:
Approximately 30% of childhood cancer survivors (CCSs) will develop chronic kidney disease (CKD) or hypertension 15 to 20 years after treatment ends. The incidence of CKD and hypertension in the 5-year window after cancer therapy is unknown. Moreover, extent of monitoring of CCS with CKD and associated complications in current practice is underexplored. To inform the development of new and existing care guidelines for CCS, the epidemiology and monitoring of CKD and hypertension in the early period following cancer therapy warrants further investigation.
Objective:
To describe the design and methods of the KIdney aNd blooD prESsure ouTcomes in Childhood Cancer Survivors study, which aims to evaluate the burden of late kidney and blood pressure outcomes in the first ~10 years after cancer therapy, the extent of appropriate screening and complications monitoring for CKD and hypertension, and whether patient, disease/treatment, or system factors are associated with these outcomes.
Design:
Two distinct, but related studies; a prospective cohort study and a retrospective cohort study.
Setting:
Five Ontario pediatric oncology centers.
Patients:
The prospective study will involve 500 CCS at high risk for these late effects due to cancer therapy, and the retrospective study involves 5,000 CCS ≤ 18 years old treated for cancer between January 2008 and December 2020.
Measurements:
Chronic kidney disease is defined as Estimated glomerular filtration rate <90 mL/min/1.73 m2 or albumin-to-creatinine ratio ≥ 3mg/mmol. Hypertension is defined by 2017 American Academy of Pediatrics guidelines.
Methods:
Prospective study: we aim to investigate CKD and hypertension prevalence and the extent to which they persist at 3- and 5-year follow-up in CCS after cancer therapy. We will collect detailed biologic and clinical data, calculate CKD and hypertension prevalence, and progression at 3- and 5-years post-therapy. Retrospective study: we aim to investigate CKD and hypertension monitoring using administrative and health record data. We will also investigate the validity of CKD and hypertension administrative definitions in this population and the incidence of CKD and hypertension in the first ~10 years post-cancer therapy. We will investigate whether patient-, disease/treatment-, or system-specific factors modify these associations in both studies.
Limitations:
Results from the prospective study may not be generalizable to non-high-risk CCS. The retrospective study is susceptible to surveillance bias.
Conclusions:
Our team and knowledge translation plan is engaging patient partners, researchers, knowledge users, and policy group representatives. Our work will address international priorities to improve CCS health, provide the evidence of new disease burden and practice gaps to improve CCS guidelines, implement and test revised guidelines, plan trials to reduce CKD and hypertension, and improve long-term CCS health.
Keywords: cancer survivors, children, chronic kidney disease, hypertension, protocol
Introduction
Chronic Kidney Disease and Hypertension Among Childhood Cancer Survivors
Long-term complications are common in childhood cancer survivors (CCSs).1-3 Over 30% of adult CCS have chronic kidney conditions more than 15 years post-diagnosis and are observed to have an increased risk of chronic kidney disease (CKD) and hypertension compared with non-CCS.4-6 Nephrotoxic cancer therapies in children can trigger acute or chronic kidney outcomes such as acute kidney injury (AKI), CKD, and hypertension.7-9 Chronic kidney disease, proteinuria, and hypertension prevalence rates range from 2% to 32%, 4% to 84%, and 50%, respectively, in CCS at varying follow-up time-points,2,10 and there is evidence that these cardiovascular risk factors may increase morbidity, mortality, and reduce quality of life (QoL).11-14
Research on long-term effects of cancer therapy on kidney function in CCS has been historically limited by small sample size and suboptimal definitions for CKD and hypertension with resulting uncertainty of the onset, severity, and characteristics of these conditions in CCS.2,15-17 Furthermore, most studies on kidney or blood pressure (BP) outcomes in CCS are performed more than 5 years after cancer therapy. The first 5 years post-therapy may be a critical window to mitigate long-term progression and adverse effects of CKD and hypertension in CCS.
Current kidney and BP follow-up guidelines (eg, Children’s Oncology Group [COG]),18 lack specific or actionable recommendations for detecting, preventing, or treating CKD and hypertension soon after cancer therapy completion. The “Kidney Disease: Improving Global Outcomes (KDIGO)”19,20 and Pediatric Hypertension21 guidelines do provide actionable recommendations on kidney disease and BP monitoring, although not specific to CCS. To our knowledge, there have been no attempts to harmonize these guidelines to optimize practice. Moreover, the extent of CKD and hypertension monitoring being performed in CCS during the first 5 years after cancer therapy completion is unknown.
Study Aims
To investigate CKD and hypertension epidemiology during the first 5 years after cancer therapy and identify evidence-practice gaps in contemporary screening and prevention of CKD and hypertension, and of complications from CKD and hypertension in CCS.
Methods
KINDEST-CCS Study Overview
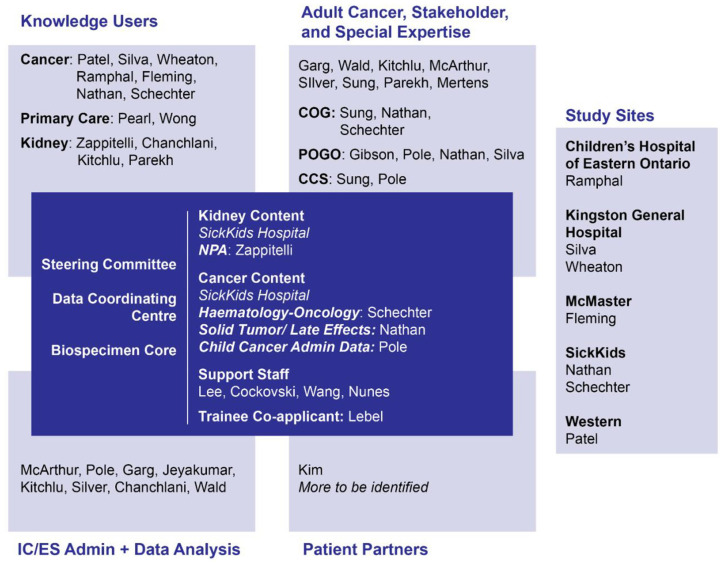
We will perform 2 related multi-center studies in Ontario, Canada. This pair of studies involves a network of clinicians, researchers, biostatisticians, stakeholders, and patient partners (Figure 1 and Supplementary Table 1). Both studies will be conducted at 5 pediatric oncology sites in Ontario, Canada: Hamilton, Kingston, London, Ottawa, and Toronto.
Figure 1.
Study organizational structure of KINDEST-CCS.
A prospective cohort study will investigate CKD and hypertension epidemiology in CCS during the first 5 years post-cancer therapy. A retrospective cohort study will use provincial administrative healthcare data and institutional health records to highlight kidney monitoring and practice gaps in CCS and to validate administrative CKD and hypertension definitions. We will also investigate the extent to which patient, disease, treatment, or system factors are associated with or modify kidney and BP outcomes and/or monitoring practices. These studies are necessary first steps toward developing and reshaping evidence-based guidelines, for better kidney outcomes in CCS.
Study Organization
The Hospital for Sick Children (SickKids) and the SickKids Research Institute will act as a participating site, study and data coordinating center, and the biorepository. ICES (a not-for-profit provincial research institute holding Ontario administrative health data) scientists and analysts will be integral members of the study team, receiving secure study data for linkage and analysis with administrative healthcare data.22-24
SickKids will provide comprehensive training on the study protocol via video-teleconference covering data collection, biospecimen collection and processing, standardized physical exams and placement of 24-hour ambulatory blood pressure monitoring (ABPM) devices. The study will be submitted to the Ontario Cancer Research Ethics Board (OCREB) for approval, prior to study start (at this time, the Team is finalizing case report forms and online database). Upon approval, we anticipate recruitment for the prospective study will begin in 2022, with a recruitment target of 500 patients.
Prospective Study: Epidemiology of Kidney and BP Outcomes in the 5 Years After Therapy
Prospective Study Aims
Evaluate CKD and hypertension prevalence at 3 and 5 years after cancer therapy and prevalence of 24-hour ABPM abnormalities at 5 years after cancer therapy, in CCS at high risk for late kidney and BP outcomes.
Investigate changes in estimated glomerular filtration rate (GFR; eGFR), albuminuria, BP, and of CKD and hypertension prevalence from 3 to 5 years post-cancer therapy in CCS at high risk for late kidney and BP outcomes.
Investigate whether AKI occurrence during cancer therapy, cardiometabolic risk factors, and/or patient-, condition-, system-, and treatment-specific factors are associated with and/or modify kidney outcomes in CCS at high risk for late kidney and BP outcomes.
A secondary but important aim will be to expand our patient representative committee. We will prioritize ongoing engagement with patient partners to ensure their voices are heard and promoted in research planning. An example of this will be to explore ways of communicating information on long-term kidney and BP outcomes to patients and their families, during cancer therapy.
Study Design
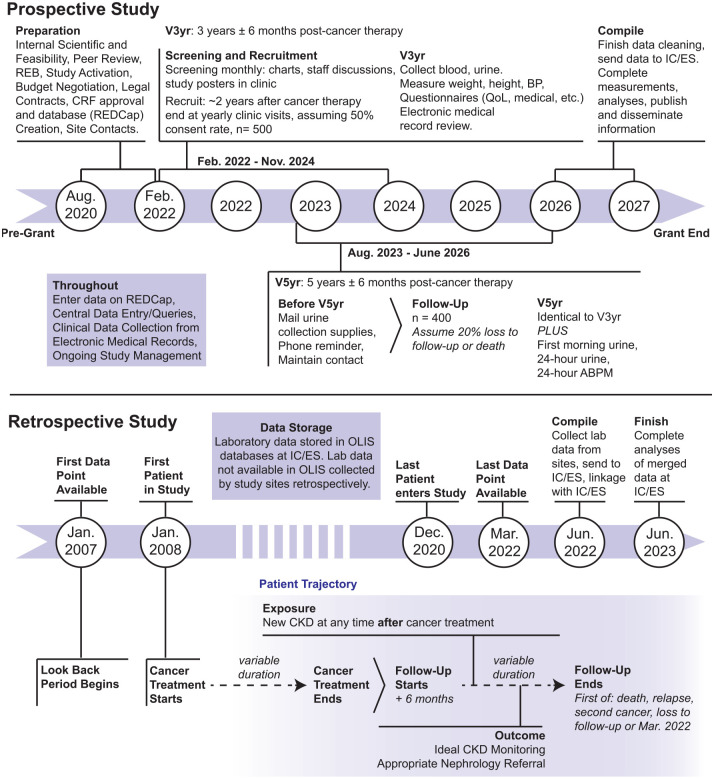
This is a prospective cohort study of ~500 CCS followed in pediatric oncology clinics, including “AfterCare” clinics devoted to follow-up of CCS, across 5 Ontario sites over a period of approximately 3.5 years (Figure 2). Study visits will be conducted 3 and 5 years after cancer therapy end, defined as the date of last chemotherapy treatment, radiation, stem cell transplant (SCT) administration (end of therapy for SCT will be 2 years post SCT), or surgery before remission of the patient’s first cancer. The last 5-year follow-up visit will occur in approximately 2026.
Figure 2.
Summary of KINDEST-CCS study timeline and procedures.
Cohort Participants and Recruitment
The study will enroll CCS who will be ≤18.9 years old at 3 years ± 6 months after cancer therapy completion and received potentially nephrotoxic therapies (eg, platinums, ifosfamide, high dose methotrexate, abdominal or total body radiation, SCT, nephrectomy or other therapies which may be known to cause late kidney and/or BP effects). Patients with pre-cancer CKD diagnoses will be excluded. Given the challenges differentiating underlying hypertension and hypertension secondary to cancer diagnosis, patients with pre-cancer hypertension diagnoses will be included. The definition for cancer therapy end may vary based on cancer treatment group. For example, cancer treatment end will be at 2 years after transplant for SCT patients. Detailed eligibility criteria are outlined in Table 1.
Table 1.
Prospective Study Inclusion and Exclusion Criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| • 3 years ± 6 months after therapy for first cancer
• Received high-risk therapy for first cancer, as defined by the COG (platinums, ifosfamide, high dose methotrexate, abdominal radiation, stem cell transplant, nephrectomy or other therapies which may be known to cause late kidney and/or BP effects)a, b |
• Pre-cancer illness CKD (by chart review or by interview with patient/parent/guardian) • Previous kidney transplant • Will be >18.9 years old at date of 3-year follow-up, as determined at screening ~2 years after cancer therapy end |
Abbreviations: CKD = chronic kidney disease; COG = Children’s Oncology Group.
Literature on nephrotoxicity of chemotherapeutic agents (particularly those either known to cause AKI via various mechanisms and/or to cause CKD or hypertension in the long-term) will be reviewed annually and the list of “high-risk chemotherapies” will be reviewed and potentially modified by the principal investigator accordingly.
Modified lists will be sent to site investigators for agreement and development of a final list. This list may grow as new data/clinical experience become available.
Site coordinators will screen CCS at approximately 2 years post-cancer therapy (using electronic health records and hematology-oncology department databases). Eligible participants will be approached for informed consent either virtually or in person. To support recruitment, study posters with contact information will be advertised in clinics.
Follow-Up Visits, Data Collection, and Participant Retention
Study visits are scheduled 3- and 5-years post-cancer therapy (Figure 2). For Toronto and Ottawa participants whose 3-year follow-up visit occurs at age >16 years old, conduct of 5-year visit may prove challenging as patients are often transferred to separate adult centers at 18 years of age. At other sites, where pediatric and adult care is provided in the same or nearby institution, the issue of “aging out” as a reason for loss to follow-up (expect < 5% of the total study population) may be mitigated.
Variables of interest collected at 3, 4, and 5 years after cancer treatment are summarized in Table 2. Triplicate measures of weight, height, and BP will be taken at the 3-year visit to calculate adjusted BP percentiles and height-adjusted z-scores.21 Sociodemographics, family history of kidney/BP diagnoses, medications, questionnaires,25,26 QoL measures,27,28 a 5 mL blood sample, and a 30 mL urine sample will also be collected at this time.
Table 2.
Summary of Visit Schedules and Study Components Occurring After Recruitment at Approximately 2 Years After Cancer Therapy End.
| 3-Year Visit | 4-Year Contact | 5-Year Visit | |
|---|---|---|---|
| Blood sample (5 mL) | X | X | |
| Urine sample (30 mL) | X | X | |
| Phone call to maintain contact | X | ||
| Mailing of 2 first-morning urine cups and 24-hour urine collection materials a | X | ||
| Optional 24-hour urine collection b | X | ||
| First-morning urine sample (30 mL) b | X | ||
| Blood pressure (3 measurements) | X | X | |
| Optional 24-hour ABPM | X | ||
| Weight, Height (3 measurements) | X | X | |
| Questionnaires | X c | X d | X c |
| Phone call to maintain contact | X | ||
| Chart review | X | X | X |
Abbreviations: ABPM = ambulatory blood pressure monitoring.
Approximately 2 months prior to 5-year visit.
Within approximately 1 week before the 5-year visit.
Questionnaires at 3- and 5- year visits include case report form, outcomes of interest, lifestyle questions and PedsQL (including cancer-specific form).
Questionnaire at 4-year contact include questions regarding new medication, kidney issues, BP issues, or receiving dialysis.
At the 4-year visit, participants will be contacted (phone, email, video conference, in-person during clinic visits per family preference) to maintain correspondence and collect minimal data. Then 2 months before the 5-year visit, participants will be mailed 2 urine cups for first-morning samples and a 24-hour urine collection container with instructions; samples will be collected within a week before 5-year follow-up.
The 5-year visit will mirror the 3-year visit with the addition of: urine samples (2 first-morning and one 24-hour sample) and 24-hour ABPM data for patients 5 years + as per guidelines.29 Families will choose whether to have the ABPM device (Ultralight 90217, Spacelabs Med. Inc., Issaquash) placed during the study visit or given instructions on how to place the device and perform ABPM within a week of follow-up. Ambulatory blood pressure monitoring will record wake and sleep BP every 20 and 30 minutes, respectively, and participant diaries will be kept throughout the 24-hour period.30
Biospecimen Handling, Analysis, and Storage
Blood will be centrifuged at 4°C (2000 g × 10 minutes) and serum aliquoted into 1 mL cryovials. Urine will be poured into 2 x 15 mL conical tubes for collection and a dipstick urinalysis. Coordinators will scan specimens into REDCap with barcode readers as they collect/store/ship them for real-time monitoring and quality assurance evaluations. Biospecimens will be stored on-site at −80°C until quarterly shipments to SickKids on dry ice. The central biochemistry lab at SickKids will measure several kidney and cardiovascular markers: serum creatinine (SCr) using isotope-dilution mass spectrometry-traceable assay electrolytes, glucose, cholesterol, triglycerides, serum C-reactive protein, urine albumin to creatinine ratio (ACR), and urine protein to creatinine ratio. Yearly, the Ottawa site biochemistry lab will receive serum shipments for Cystatin C measurement (particle-enhanced turbidimetric immunoassay). Leftover biospecimens will be stored at SickKids at −80°C for repeat measurements as needed and future studies.31
Data Sources and Management
On top of data collected at recruitment and follow-up, clinical data (eg, key medical history, comorbidities, medication lists, recent laboratory values as part of routine care) will be abstracted from patients’ electronic medical records and entered onto paper case report forms (CRFs). Each site will upload de-identified data forms to a secure REDCap database located on the SickKids server. A SickKids data coordinator will enter these data into REDCap and send monthly queries to sites. In previous work, we found central data entry yields fewer errors and reduces study site burden. At the time of participant recruitment, permission will be sought for future data abstraction from patient charts (up to 10 years) to update existing study records.
Only at SickKids, 2 sets of laboratory values will be collected retrospectively, as SickKids only recently (2021) started contributing data to Ontario Laboratories Information System (OLIS): SCr values throughout cancer therapy to define AKI, monthly SCr, urine albumin, and protein values between cancer therapy end and 3-year follow-up and between 3-year and 5-year timepoints.
Following data collection, a database containing all measurements will be sent to ICES for linkage with administrative healthcare data, as done previously.32-35 The main patient, system, and treatment factors we will utilize from ICES databases include pre-cancer diagnosis and previously diagnosed cardiac/diabetes/liver disease, dialysis procedures/CKD and/or hypertension diagnoses per administrative healthcare data, birthweight, income quintile, rural vs. urban community, and cancer-variables (diagnoses, therapies, SCT, radiation).
ICES Databases
Administrative health data sources used in the study include The Pediatric Oncology Group of Ontario Networked Information System (POGONIS),36-38 a database of clinical information for pediatric oncology patients in the 5 Ontario tertiary care centers, providing rich cancer data39-43; Discharge Abstract Database (DAD), Same Day Surgery (SDS), and MOMBABY, documenting administrative, clinical, and demographic characteristics of patients39,44; The Ontario Health Insurance Plan (OHIP), ICES-derived Physicians Database (IPDB), and Canadian Organ Replacement Register (CORR), databases used to track physician billing, procedures, and specialist referrals45,46; The Registered Persons Database (RPDB), providing demographic information on all Ontarians with health cards47 and when they leave the Ontario healthcare system,48 providing a method to account for some loss to follow-up; and the OLIS, used to collect laboratory data. A detailed list of data collected from each administrative health data source are provided (Table 3). These databases will also be used for the retrospective study.
Table 3.
Non-exhaustive Summary of Variables to be Used From ICES Databases and Extracted by Health Records Review for Both the Prospective and Retrospective Studies.
| ICES Database | Brief description of how we will use the database | Variables of interest |
|---|---|---|
| Pediatric Oncology Group of Ontario Networked Information System (POGONIS) Available from 1985 Data collected by all cancer sites |
• Cancer patient identification, sex, age at cancer diagnosis date • Cohort entry date (diagnosis date/date of definitive diagnostic procedure) • Cancer treatment start/end date (first/last treatment given) • Cancer diagnosis type • Treatment type (eg, chemotherapy, radiation, SCTx with details) • Treatment site (hospital) • Death date (linked to other databases, eg, RPDB) • Previous cancer (will be supplemented with Ontario Cancer Registry linkage, not described in proposal) |
• Date of Birth • Sex • Pathologically Confirmed Diagnosis • Date of Diagnosis • Radiation Type + Technique • Amount of Radiation Used • Date of Death • Treatment Start/End Date • Date of Receiving Stem Cell • Transplant • Treatment (including “watch and wait” versus palliative versus treatment) • Drug Name and doses for alkylating agents and platinums |
| Ontario Cancer Registry (OCR) Available from 1964 + National Ambulatory Care Reporting System (NACRS) + Cancer Care Ontario Activity Level Reporting (ALR) + Records within the New Drug Funding Plan + OHIP, DAD, SDS below |
• Collectively, to supplement POGONIS with treatments (including outpatient and systemic therapies), diagnosis, death, pathologies, and consultation records for patients ≥15 years old who are not found in POGONIS (as per Kitchlu et al36,37). | • Age at Diagnosis • Date of Diagnosis • Sex • Diagnosis (and body part), treatments, locations • Relapse, second malignancy |
| Discharge Abstract Database (DAD) + Same Day Surgery Database (SDS) Available from 1991 |
• Identify hospitalizations and dates, used for comorbidities assessments, diagnoses and procedures (eg, dialysis—for AKI during cancer therapy), demographic information | • Admission/Discharge date • Birth date/Weight/Age • Dialysis, mechanical ventilation, • Radiotherapy, chemotherapy, y/n • Diagnosis/Procedure codes • Region/Education/Residence |
| MOMBABY database Available from 1988 |
• Contribute to identify admissions prior to cohort entry in infants to evaluate comorbidities • Birth weight |
• Birth Date, sex • Birth weight (grams) • Diagnoses |
| Ontario Health Insurance Plan Claims Database (OHIP) Available from 1991 |
• Identify nephrology consultations through Ontario physician billings • Maintenance dialysis codes—Contribute to end stage renal disease/kidney transplant (pre-cancer CKD- defining) • Define most baseline comorbidities listed in proposal |
• Diagnostics Code (includes codes for consultation, dialysis, cardiac, etc.) • OHIP fee code (as above) • Location of OHIP claim • Physician number (for consult type through link with IPDB) • Physician specialty |
| Registered Persons Database (RPDB) Available from 1991 |
• Date of last contact (and OHIP coverage end date) • Birth date • Death date (with POGONIS) • Socioeconomic status and rural variables |
• Birth date • Death date • Neighborhood Income Quintile • Rurality Index • Date of last contact |
| Canadian Organ Replacement Registry (CORR) Available from 1981 |
• Past kidney transplant, dialysis • Kidney transplant, maintenance dialysis (for CKD definition, pre-cancer) |
• Kidney Transplant • Death date • Hemodialysis/Peritoneal dialysis |
| ICES Physician’s Database (IPDB) Available from 1991 |
• Physician specialty for visits (eg, oncology care, AfterCare, primary care; nephrology) | • Physician specialty (first, second, etc.) • Primary care (yes/no) |
| Ontario Laboratory Information System (OLIS) Database Available from 2007 |
• Serum creatinine, cystatin C, urinary protein/albumin, vitamin D, hemoglobin, parathyroid hormone measures • Ordering center, provider, and lab destination • For prospective study: Goal is mainly to identify AKI during cancer therapy, as we have previously defined, using serum creatinine criteria of the KDIGO definition. OLIS will provide serum creatinine data to define AKI during cancer therapy for all sites except SickKids, which has not yet contributed to OLIS. For the retrospective study (need labs as far back as 2007), OLIS data available for sites in Kingston, Hamilton, London, and Ottawa from as far back as 2015, 2014, 2013 and 2016, respectively. |
• Birth Date • Destination Lab • Measure type (eg, albumin, protein) • Measure value (+ ref range, units) • Ordering Facility / Provider |
| Variables to be collected by site staff via chart abstraction | Labs above and dates; whether inpatient or outpatient. Prospective study: Baseline and study visit medical, medication and family history characteristics; inclusion and exclusion criteria (including relevant chemotherapies received); routinely collected labs above performed closest to the study visits. At SickKids only: serum creatinine values throughout cancer therapy to define AKI and monthly serum creatinine and urine albumin/protein performed during routine care from cancer therapy end to date of first study visit. Retrospective study: Lab values described above will need to be collected by site research staff during the following time periods (years not available in OLIS provincial database): Kingston: 2007-2014 Hamilton: 2007-2013 London: 2007-2012 Ottawa: 2007-2015 SickKids: 2007-2020 |
• Destination Lab • Measure type (eg, albumin, protein) • Measure value (+ ref range, units) • Ordering Facility / Provider • CKD y/n, adherence y/n (from measures) • Together with other databases, determine if inpatient or outpatient lab test. |
Abbreviations: POGONIS = Pediatric Oncology Group of Ontario Networked Information System; SCTx = stem cell treatment; OCR = Ontario Cancer Registry; NACRS = National Ambulatory Care Reporting System; OHIP = Ontario Health Insurance Plan; DAD = Discharge Abstract Database; SDS = Same Day Surgery Database; AKI = acute kidney injury; CKD = chronic kidney disease; IPDB = ICES Physician Database; RPBD = Registered Persons Database; OLIS = Ontario Laboratory Information System; CORR = Canadian Organ Replacement Registry; KDIGO = Kidney Disease Improving Global Outcomes; ALR = activity level reporting.
Outcome and Exposure Definitions
The primary outcomes in this study are post-cancer therapy CKD and hypertension. Chronic kidney disease will be defined as low eGFR [eGFR<90 mL/min/1.73 m2; Grade 2 CKD or worse] or albuminuria [ACR >3 mg/mmol] per KDIGO guidelines19 (Table 4). Grade 3 CKD will also be described. In this prospective study, GFR will be estimated using a validated equation including SCr and Cystatin C.49,50 In secondary analyses, 5-year follow-up visit GFR will be defined using the creatinine clearance measured from 24-hour urine collection. Hypertension will be defined according to American Academy of Pediatrics (AAP) using height-, sex-, and age-adjusted BP percentile tables21,29 (Table 5). At the 5-year study visit the presence of either ambulatory hypertension or masked hypertension will be the main ABPM outcome; however, presence/absence of any ABPM abnormality and the prevalence of individual ABPM component abnormalities will also be treated as outcomes (Supplementary Table 2).
Table 4.
Simplified Version of the KDIGO Definition for Chronic Kidney Disease (CKD) Used in This Study, Based on Low Estimated Glomerular Filtration Rate (eGFR) or Albuminuria (ACR).
| CKD Grading | Estimated glomerular filtration rate (eGFR) | Albumin-to-creatinine ratio (ACR) | Time Frame (only retrospective study) |
|
|---|---|---|---|---|
| Grade 1 | ≥90 mL/min/1.73 m2 | OR | <3 mg/mmol | For ≥3 months |
| Grade 2 or worse | <90 mL/min/1.73 m2 | ≥3 mg/mmol | ||
| Grade 3 or worse | <60 mL/min/1.73 m2 | ≥30 mg/mmol |
Notes: Albuminuria= ≥3 mg/mmol; Grade 1 CKD = known kidney problems, but eGFR is still normal and there is no significant albuminuria.
Abbreviations: KDIGO = Kidney Disease: Improving Global Outcomes Guidelines; CKD = chronic kidney disease; eGFR = Estimated glomerular filtration rate; ACR = Albumin-to-creatinine ratio.
Table 5.
American Academy of Pediatrics Classification of Blood Pressure According to Casual Blood Pressure Measures Guidelines.
| Children 1 to <13 years old | Children ≥ 13 years old | |
|---|---|---|
| Normal | <90th percentile | <120/< 80 mmHg |
| Elevated blood pressure | ≥90th to <95th percentile or 120/80 mmHg to <95th percentile (whichever is lower) |
120/< 80 to 129/< 80 mmHg |
| Stage 1 hypertension | ≥95th to <95th percentile + 12 mmHg or 130/80 to 139/89 mmHg (whichever is lower) |
130/80 to 139/89 mmHg |
| Stage 2 hypertension | ≥ 95th percentile + 12mmHg or ≥140/90 mmHg (whichever is lower) |
≥140/90 mmHg |
Abbreviations: mmHg = millimeters of mercury.
Changes in eGFR, albuminuria, and BP percentile from the 3-year to the 5-year visits, persistent CKD, and persistent hypertension at 3- and 5-year visits, and new CKD or hypertension at the 5-year visit will be recorded. Aim 3 outcomes will be the same as those described above.
Study exposures of interest pertain mainly to aim 3: AKI during cancer therapy and presence of cardiometabolic risk factors. Acute kidney injury will be defined based on KDIGO guidelines for AKI definition, as a ≥50% SCr rise throughout cancer therapy, from baseline, as previously described.19,51,52 Cardiometabolic risk factors will be ascertained at the study visits and will include glucose, body mass index (BMI) z-score (with secondary analyses using overweight/obesity classifications), non-fasting lipid profile (triglycerides, high- and low-density lipoprotein cholesterol), and C-reactive protein.
Statistical Analysis
Multivariable analyses described below will include variables of cancer diagnosis classified by a previously described classification system,53 age at diagnosis, birthweight, sex (gender), income quintile, rural vs. urban, and baseline cardiac, diabetes or liver disease, unless stated otherwise. Sex-stratified analyses will also be conducted. Effect estimates will be described with 95% confidence intervals.
Three- and 5-year prevalence of CKD, hypertension, all categorical 5-year ABPM outcomes, and continuous outcome measures (eg, eGFR; BP percentile) will be calculated. We will compare 3- vs. 5- year CKD and hypertension prevalence using the McNemar test, calculate 3- to 5-year eGFR, ACR, and BP percentile change and proportions of participants with persistent CKD, hypertension and status change. We will use mixed effect models (for repeat measures) to evaluate adjusted associations of AKI and cardiometabolic measures with 3- and 5-year outcomes to evaluate if they modify trajectories by including interaction terms for these measures with time in models. We will also evaluate univariable and multivariable associations of AKI and cardiometabolic measures (lipids, glucose, BMI) with individual 3- and 5-year binary outcomes (eg, CKD) using log-binomial regression and continuous outcomes using linear regression (eg, eGFR). In exploratory analyses, we will determine if analyses above differ by treatment groups (eg, platinums, radiation, etc.), treatment combinations (eg, receiving cisplatin and radiation), and diagnosis classification53 in univariable analyses, and if event rates allow, in multivariable analyses. Sample size justification is provided in Supplementary Information.
Retrospective Study: CKD and Hypertension Screening, Complications Monitoring, and Administrative Data Validation in Childhood Cancer Survivors
Aims and Objectives
In a retrospective cohort of Ontario CCS treated for cancer between 2008 and 2020, we will evaluate:
1a. Chronic kidney disease screening, complications monitoring (Vitamin D, parathyroid hormone, hemoglobin, SCr, proteinuria) and nephrology referral;
1b. Hypertension screening, complications monitoring (BP follow-up measures, 24-hour BP monitoring, echocardiogram) and nephrology referral.
2. The validity of several pediatric specific CKD and hypertension administrative algorithms
3. Chronic kidney disease and hypertension incidence in the ~10 years after cancer therapy.
4. Whether patient, disease/treatment or system factors are associated with each of the above outcomes.
Study Design and Cohort
The retrospective multi-center cohort study consists of approximately 5,000 CCS treated for cancer across 5 Ontario sites between January 2008 and December 2020. Hypertension outcome analyses will include SickKids patients only (approximately 2500 CCS) due to data collection feasibility. The inclusion and exclusion criteria are shown in Table 6. Briefly, all children treated for cancer during the study period, who survive at least 6 months post-cancer treatment end will be included. Figure 2 shows that cohort entry date is at cancer diagnosis (first patient enrolled January 2008, allowing for a 1-year look-back period to January 2007 for labs and baseline characteristics from administrative healthcare data); only patients surviving ≥6 months from cancer therapy end will be evaluated for outcomes (eg, adequate CKD monitoring and nephrology referral); those dying or censored before then will be described to ascertain potential biases. Childhood cancer survivor with CKD at <6 months post-therapy end will be classified as CKD at follow-up start. The last patients to enter the cohort will be those with diagnosis occurring on or before December 2020 (Figure 2; Table 6) to allow at least 1.5 years of data available from administrative healthcare data after diagnosis (date of last data point to be used: March 2022). Patients will be censored on March 2022, date of death, second cancer, relapse, or loss to follow-up—whichever comes first.54 In sensitivity analyses, we will consider including second cancer and relapse as binary variables in the multivariable analyses described below, rather than as censoring events (not described further).
Table 6.
Retrospective Study Inclusion and Exclusion Criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| All patients treated for cancera | Invalid/missing health number, birth date or sex |
| ≤18 years old at cancer diagnosis, Jan 1, 2008-Dec 31,2020.b | Children surviving <6 months after treatment end |
The Pediatric Oncology Group of Ontario Networked Information System (POGONIS) will be used to identify these patients, as previously described.
Dates selected to ensure necessary administrative health data is available as far back as 2007 and at least 1-2 years post-cancer diagnosis.
Data Sources
Much of the data used will be obtained from ICES databases (described in Table 3). One important exception will be laboratory data; all sites will collect labs onto a paper CRF as far back as 2007 (for entry into REDCap at the study coordinating center) or receive spreadsheets of desired laboratory variables from Health Records departments (depending on site approved processes). If unavailable, these data may then be supplemented by OLIS. Dates of laboratory tests availability will vary by site (Table 3). Other variables of interest (eg, cancer diagnosis, treatment variables) available at sites will be abstracted from patient’s electronic medical records at all sites and entered onto paper CRFs, to be later entered into REDCap. Outpatient BP values and echocardiogram data will be collected at SickKids only. Following data collection, study data will be sent to ICES for linkage with administrative healthcare databases.
Exposures and Outcomes
Aim 1 primary outcomes include ideal CKD and hypertension monitoring and appropriate nephrology referral. As shown in Table 4, ideal CKD monitoring can change yearly depending on the CKD severity, as per KDIGO guidelines.19 Ideal CKD monitoring in CCS will be categorized as yes/no according to CKD stage as described in Table 7. Appropriate nephrology referral will be defined as a referral within 1 year of CKD appearance or within 1 year of CKD progression (≥3 months of CKD Grade worsening or 25% eGFR drop or eGFR <60 mL/min/1.73m2 or proteinuria ≥50 mg/mmol or albuminuria ≥30 mg/mmol). Ideal hypertension monitoring will be defined as performance of a 24-hour ABPM and echocardiogram within 6 months of hypertension appearance, as defined in Table 5 based on age-, height- and sex- percentiles. Appropriate hypertension nephrology referral will be defined as nephrology referral within 6 months of hypertension appearance.21
Table 7.
Simplified Summary of Ideal Chronic Kidney Disease (CKD) Monitoring (in Children, Combined Recommendations From KDIGO and the KDOQI Quidelines Are Used, as Recommended).
| CKD Categories | Monitoring Targetsa | Laboratory Measures | ||||
|---|---|---|---|---|---|---|
| Vitamin D | Parathyroid Hormone | Hemoglobin | Serum Creatinine (eGFR) |
Urine Protein (ACR) |
||
| Grade 1 | None indicated | |||||
| Grade 2 | ≥1 measures at least once after Grade 2 CKD appearance | X | X | X | ||
| ≥1 measure(s) within 2 years of an abnormal value | X | X | X | |||
| ≥yearly measures after Grade 2 CKD | X | X | ||||
| Grade 3 | ≥1 measure(s) within 1 year of Grade 3 CKD appearance | X | X | X | ||
| ≥1 measure(s) within 1 year of an abnormal value | X | X | X | |||
| ≥yearly measures after Grade 3 CKD appearance | X | X | X | |||
Abbreviations: KDIGO = Kidney Disease: Improving Global Outcomes Guidelines; KDOQI = Kidney Disease Outcomes Quality Initiative; eGFR = estimated glomerular filtration rate; ACR = albumin-to-creatinine ratio.
Patients with no CKD will not be labeled as requiring monitoring. Patients with CKD will only be classified as “no” for ideal monitoring if enough time has passed. This allows CKD Grade (and ideal monitoring) to change.
The primary exposures for aim 1 are CKD and hypertension. CKD will be defined in 2 ways, detailed in Table 4: Grade 2 CKD or worse (binary; and also staged by CKD severity); and a stricter definition requiring ≥2 outpatient, abnormal eGFRs or albuminuria (or proteinuria) results, greater than 3 months apart, with no normal results in between.55-57 eGFR will be calculated with only SCr if cystatin C is unavailable; a sensitivity analysis for patients with both analytes available will be conducted. Should height be unavailable (for pediatric GFR estimation), validated height-independent eGFR equations will be used.50,58-60 Hypertension will be defined as 2 or more consecutive hypertensive values, defined in Table 5 based on age-, height- and sex- percentiles, on 2 separate days. Similar to other outcomes, patient-, disease/treatment-, and system-related factors will be evaluated. CKD and hypertension will also be defined in this way to attain aim 3, describing the incidence of CKD and hypertension in the ~10 years after cancer therapy.
Aim 2 (validation of administrative healthcare data for identifying CKD and hypertension) outcomes will be CKD and hypertension, defined using laboratory data and BP measures, respectively. These outcomes will be defined using the strict criteria described above (ie, reference standard method) and considered the “gold standard.” The diagnostic test will be algorithms based on diagnosis and procedure codes available from ICES databases (described in previous work). These CKD and hypertension algorithms in the ICES databases will be evaluated for their ability to identify or detect CKD and hypertension defined by reference standard-methods.61,62
Statistical Analysis
We will calculate yearly proportions of patients with CKD and hypertension who undergo ideal monitoring, and appropriate nephrology referral, separately. We will also evaluate individual CKD and hypertension monitoring components (eg, Vitamin D measurement). Yearly measurement rates of eGFR and urine protein measures from the end of therapy will also be calculated. We will compare characteristics and monitoring in CCS with vs. without CKD and hypertension to investigate factors related to variation in clinical practice. Using log-binomial regression (with generalized estimating equations, accounting for within subject correlation), we will estimate the yearly change in ideal monitoring. We will use multivariable log-binomial regression with generalized estimating equations to yield adjusted rate ratios of patient, disease, treatment, and system covariates for ideal monitoring. Analyses will be repeated, adjusting for time as well as provider-level and center-level clustering in monitoring practices.
Various diagnosis and procedure code-based algorithms for CKD and hypertension will be evaluated for detecting reference-standard-based CKD and hypertension (Aim 2). Algorithms will be evaluated for validity and level of agreement with the following measures: sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), kappa (κ) statistic, and area under the receiver operating characteristic (ROC) curve.
We will use a sub-distribution hazards model accounting for competing risk of death (Fine and Gray method)63 to estimate cumulative incidence (including at pre-defined time intervals: 1, 3, 5, 7, 10 years) and generate cumulative incidence function curves to understand burden and time of CKD and hypertension onset (aim 3). Censoring will occur at system leave date (ie, emigration), relapse, second malignancy, or follow-up end.54,64 Sample size calculation is provided in supplementary information.
Timeline and Knowledge Translation (KT) Plans
Table 8 shows the overall timeline. The benefit gained from these 2 studies will be magnified by a thoughtful and iteratively planned KT strategy. Knowledge dissemination at meetings and in publications will target kidney, oncology, and general pediatrics audience. Early on, we will engage kidney, oncology and pediatric stakeholder and patient groups (eg, Canadian Society of Nephrology; Canadian Cancer Society; Pediatric Oncology Group of Ontario; Canadian Pediatric Society; Cancer Care Ontario), on their research missions. We hope to foster partnerships between these groups (eg, nephrology groups with pediatric oncology groups) by identifying common goals for CCS cardiovascular and kidney health. In year 1, we will begin creating a larger patient advisory board and approach international investigators performing similar work, to collaborate on common CCS health goals. Furthermore, patient and provider surveys will explore perspectives, practice gaps, and research priorities to inform KT for the foreseeable future.
Table 8.
Study Timeline Over 6 Years, From Pre-study Start to April 2027.
| Item | 2020-2021 Pre |
2021-2022 Year 1 |
2022-2023 Year 2 |
2023-2024 Year 3 |
2024-2025 Year 4 |
2025-2026 Year 5 |
2026-2027 Year 6 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SickKids Research Ethics Board (REB), then other sites + contracts | |||||||||||||
| Develop forms, REDCap, validity/reliability | |||||||||||||
| Aim 1: Train sites staff, recruit, 3- and 5-year visits | |||||||||||||
| Aim 1: Finish lab measures, ICES analysis plan, list to ICES | |||||||||||||
| Aim 1: SickKids data cleaning, ICES linkage, analyses | |||||||||||||
| Aim 1: Initial manuscripts | |||||||||||||
| Aim 2: REB, ICES analysis plan | |||||||||||||
| Aim 2: Get supplemental institution lab data, clean data | |||||||||||||
| Aim 2: Institution lab data linkage to ICES data and analyses | |||||||||||||
| Aim 2: Initial manuscripts | |||||||||||||
| Monthly KDT, bi-monthly investigator team meetings | |||||||||||||
| Core Patient partner/Knowledge user quarterly meetings | |||||||||||||
| Knowledge Translation specific timeline (excluding manuscripts) | |||||||||||||
| Identify stakeholder/policy maker groups to contact | |||||||||||||
| Stakeholder/policy maker meetings (year 1 and throughout) | |||||||||||||
| Identify patient partners for larger group | |||||||||||||
| Create larger patient partner group, regular meetings | |||||||||||||
| Contact other country common-goal groups/societies/cardiology | |||||||||||||
| Create links between kidney-cancer groups | |||||||||||||
| Patient, knowledge user, stakeholder surveys | |||||||||||||
| In-person/telecon planning meeting (trials, guideline, knowledge translation) | |||||||||||||
| Present at cancer, kidney, pediatrics scientific meetings | |||||||||||||
| Present to patient, stakeholder, Knowledge user groups | |||||||||||||
| Plan next grants | |||||||||||||
Abbreviations: REB = research ethics board; KDT = Kidney, Dialysis and Transplantation Research Program.
At years 3 and 5, we will host videoconference meetings with investigators, patients, stakeholders, and policy representatives to discuss progress, surveys, and update/unify priorities. We also plan to hold working groups to discuss action plans in response to actual and potential findings (eg, Prospective study: If hypertension is common, how to proceed? Clinical trial? Guideline changes/ implementation trial? What are success measures?), discuss steps to impact CCS guidelines based on burden findings from the prospective study and practice gaps from the retrospective study. We will know normative practice patterns, gaps, and associated factors of CKD and CKD-related complications. Our cohort, data and biobank will open countless avenues of novel cross-disciplinary research endeavors and training. Our team will be ideally placed to inform and apply CCS kidney and BP guidelines within the greater context of CCS health and maximize patient and system beneficial impact from the knowledge we generate.
Discussion
While it is known that CCS are at increased risk of adverse kidney effects, limitations in our understanding of long-term effects of cancer therapy may track as late complications. CKD and hypertension burden in the first 5 years after cancer therapy is unclear, as are current recommendations on follow-up for CCS.
The involvement of all major cancer centers in Ontario, facilitating diverse patient recruitment including a spectrum of therapies is a certain strength of this study. Our use of robust definitions for CKD, hypertension, and AKI than currently available will increase the validity of results and applicability to current clinical practice. To our knowledge, this will be the first study to implement 24-hour ABPM, gold-standard for detection of hypertension in a large cohort of CCS, permitting comprehensive diagnosis of types of hypertension.65
A major goal is to describe current management of CKD and hypertension complications in CCS and establish practice gaps in this patient population. When consulting best practices in managing CCS at higher risk for CKD, clinicians may turn to KDIGO19 or COG.9,18 KDIGO provides management objectives for patients with or at risk of CKD, but is not adapted to the CCS health context, whereas COG kidney guidelines are used internationally to identify and manage cancer therapy effects in CCS, but does not provide actionable recommendations on managing kidney complications.18,19 We believe that data generated from this study may enable improvements in both pediatric oncology and of overall CKD and hypertension guidelines, especially with a targeted KT strategy.
Another strength is our assessment of whether cancer-, patient-, therapy-, and system-specific factors influence kidney and BP outcomes or screening and follow-up practices when treating CCS.66-68 Mothers experiencing high stress and/or with residences far from neonatal clinics were less likely to attend follow-up appointments.68 If specific barriers are identified, an informed, targeted approach may lead to improved quality of care and health outcomes. Our aim to validate administrative CKD and hypertension definitions in this population would dramatically enhance understanding of disease burdens in this population, using administrative data.
Both studies have limitations. In the prospective study, a proportion of CCS will have a cancer relapse, which may prove difficult to discern between worsening disease- and treatment-associated kidney dysfunction. Survivor bias will be an issue when analyzing risk factors for treatment-associated CKD and hypertension. We can compare survivors’ vs. non-survivors’ characteristics, conduct sensitivity analyses to assess the impact of differential loss to follow-up, and if needed, propensity scores (eg, for AKI) can be used in multivariable analyses to mitigate these biases. There may be small sample sizes for subgroup analyses of specific treatments; however, our patient partners stressed these subgroup analyses are priorities.
We acknowledge including study visits before 3 years would provide insight on early post-therapy development of CKD and hypertension. We chose 3-year follow-up for cohort entry to improve feasibility/cost and allow time for resolution of immediate therapy-related effects, while capturing patients early enough to identify early detection and intervention opportunities.15 By design, conclusions in the prospective study only apply to high-risk CCS; however, this is a priority population for evidence-based guideline development and will benefit the most from early CKD and hypertension diagnosis and intervention. Moreover, our high-risk groups definitions are aligned with risk groups delineated by the COG long-term follow-up guidelines, which will enhance future KT. One potential issue is 3 and 5-year prospective study event rates may be lower than expected; however, previous work69 demonstrates high event rates and including only high-risk CCS makes this problem unlikely. If low event rates are found, this will direct future research on the timing of CKD and hypertension onset in CCS. We may not see much change between the 3- and 5-year outcomes, but this first-of-its-kind evaluation of sustained kidney or BP abnormalities will establish rate-of-change data for future research. ABPM will only be done at the 5-year visit to reduce attrition bias. Similar to most albuminuria studies, ACR is susceptible to postural proteinuria and overestimation. This is a challenge in all pediatric studies evaluating proteinuria. We attempt to mitigate this by providing participants with instructions to effectively perform first-morning urine prior to the 5-year visit. We acknowledge that the study aim is descriptive in measuring changes in the outcomes.
In the retrospective study, practice drift may be evident. However, we do not expect this as COG guidelines have not changed significantly in recent editions in 2013.18,70 We will evaluate pre vs. post 2013 eras and will include this variable in analyses if differences are found. The retrospective study is susceptible to surveillance bias; sicker patients are tested more often for CKD, thus included in analyses. We will use this large database to calculate CKD incidence using routinely collected data, but because of ascertainment bias concerns, this was not the primary goal of this study. A possible challenge for the retrospective study relates to using tests (eg, hemoglobin) to determine if complication monitoring occurs; it will not be possible to know if a test is specifically done to monitor for CKD. For atypical tests (eg, parathyroid hormone), this is likely not an issue. We also may be unable to infer the specific intentions of clinicians when ordering the test, nor if treatments were given in response to abnormal test results; we can only know whether or not tests were done. In future work, we may determine if treatments were given in response to test abnormalities by incorporating healthcare provider surveys with prospective data collection.
Conclusions
Guidelines to monitor CKD19 and hypertension21 exist but are not adapted to the natural history of CKD and hypertension specifically in CCS. We aim to prospectively study 3-year and 5-year CKD and hypertension outcomes and retrospectively determine whether appropriate screening practices are used for kidney outcomes in CCS. With this information we will be able to identify practice gaps within the current CCS healthcare context in Ontario and inform modifications to current guidelines for monitoring late kidney complications in CCS.18,19,21,71,72
Supplemental Material
Supplemental material, sj-docx-1-cjk-10.1177_20543581221130156 for KIdney aNd blooD prESsure ouTcomes in Childhood Cancer Survivors: Description of Clinical Research Protocol of the KINDEST-CCS Study by Adree Khondker, Michael Groff, Sophia Nunes, Carolyn Sun, Natasha Jawa, Jasmine Lee, Vedran Cockovski, Yasmine Hejri-Rad, Rahul Chanchlani, Adam Fleming, Amit Garg, Nivethika Jeyakumar, Abhijat Kitchlu, Asaf Lebel, Eric McArthur, Luc Mertens, Paul Nathan, Rulan Parekh, Serina Patel, Jason Pole, Raveena Ramphal, Tal Schechter, Mariana Silva, Samuel Silver, Lillian Sung, Ron Wald, Paul Gibson, Rachel Pearl, Laura Wheaton, Peter Wong, Kirby Kim and Michael Zappitelli in Canadian Journal of Kidney Health and Disease
Footnotes
List of Abbreviations: AKI, Acute kidney injury; ACR, Albumin to creatinine ratio; ABPM, Ambulatory blood pressure monitoring; AAP, American Academy of Pediatrics; BMI, Body mass index; BP, Blood pressure; CORR, Canadian Organ Replacement Register; CKD, Chronic kidney disease; CCS, Childhood cancer survivors; COG, Children’s Oncology Group; CRF, Case Report Form; DAD, Discharge Abstract Database; eGFR, Estimated glomerular filtration rate; GFR, Glomerular filtration rate; SickKids, Hospital for Sick Children; IPDB, ICES Physician Database; KDIGO, International Kidney Disease: Improving Global Outcomes; KT, Knowledge translation; OHIP, Ontario Health Insurance Plan; OLIS, Ontario Laboratory Information System; QOL, Quality of life; POGO, Pediatric Oncology Group of Ontario; POGONIS, Pediatric Oncology Group of Ontario Networked Information System; RPDB, Registered Persons Database; REB, Research Ethics Board; SDS, Same Day Surgery; SCr, Serum creatinine.
Author Contributions: AK and MG drafted the manuscript. SN, CS, NJawa, JL, VC, and YH provided feedback, assembled the study protocol and related documents, and acted as points of contact for participating sites. RC contributed ICES child kidney research and ABPM-specific expertise to protocol development. AG provided input on design and methods and revised the ICES components. AK defined non-kidney outcomes and covariates in the protocol, and contributed knowledge to recruit older teens. AL provided expertise for Aim 2 and offered input on the ABPM portion of the manuscript. EM helped develop the analysis plan, and will supervise data analysis at ICES. LM revised sections on cardiovascular and cardiometabolic risk factors, and provided knowledge translation expertise. PN and JP contributed expertise to the section on ICES data sources, in particular POGONIS. RP contributed to the conception and design of the study, providing expertise in ICES and cohort research. TS is a liaison for COG SCT groups, a site investigator, and provided a critical eye on the stem cell-related portions of the study. SS is a site investigator, who reviewed the protocol and provided advice on quality of care. LS revised the manuscript and provided feedback on feasibility and utilization of results for knowledge translation. RW revised the manuscript and provided feedback on clinical and epidemiological aspects. PG revised the manuscript and acted as a liaison for POGO and other cancer societies. RP and PW revised the manuscript and provided insight on CCS follow-up practices. MZ designed the study and reviewed all portions of the study protocol. NJeyakumar advised on study design, dataset creation plan, and acted as a data management and logistics liaison to ICES. SP, RR, MS, AF, and LW are site investigators who reviewed and revised the protocol, providing site-specific feedback. KK provided a patient partner perspective during study design and feedback on the manuscript. All authors read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study has been awarded support by a Canadian Institutes of Health Research (CIHR) project operating grant [App#427154]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethics Approval and Consent to Participate: Written consent will be obtained from all participants recruited to the prospective study. Ethics approval for the prospective study will be obtained via the Ontario Cancer Research Ethics Board (OCREB). The SickKids Research Ethics Board approved the retrospective study (1000079220).
ORCID iDs: Amit Garg  https://orcid.org/0000-0003-3398-3114
https://orcid.org/0000-0003-3398-3114
Samuel Silver  https://orcid.org/0000-0002-1843-6131
https://orcid.org/0000-0002-1843-6131
Michael Zappitelli  https://orcid.org/0000-0002-9977-0536
https://orcid.org/0000-0002-9977-0536
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Knijnenburg SL, Mulder RL, Schouten-Van Meeteren AY, et al. Early and late renal adverse effects after potentially nephrotoxic treatment for childhood cancer. Cochrane Database Syst Rev. 2013;10:Cd008944. doi: 10.1002/14651858.CD008944.pub2. [DOI] [PubMed] [Google Scholar]
- 2. Kooijmans EC, Bökenkamp A, Tjahjadi NS, et al. Early and late adverse renal effects after potentially nephrotoxic treatment for childhood cancer. Cochrane Database Syst Rev. 2019;3:Cd008944. doi: 10.1002/14651858.CD008944.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skinner R. Late renal toxicity of treatment for childhood malignancy: risk factors, long-term outcomes, and surveillance. Pediatr Nephrol. 2018;33(2):215-225. doi: 10.1007/s00467-017-3662-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hingorani S, Guthrie KA, Schoch G, Weiss NS, McDonald GB. Chronic kidney disease in long-term survivors of hematopoietic cell transplant. Bone Marrow Transplant. 2007;39(4):223-229. doi: 10.1038/sj.bmt.1705573. [DOI] [PubMed] [Google Scholar]
- 5. Hoffmeister PA, Hingorani SR, Storer BE, Baker KS, Sanders JE. Hypertension in long-term survivors of pediatric hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16(4):515-524. doi: 10.1016/j.bbmt.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Latcha S, Jaimes EA, Patil S, et al. Long-term renal outcomes after cisplatin treatment. Clin J Am Soc Nephrol. 2016;11:1173-1179. doi: 10.2215/cjn.08070715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Menon S, Kirkendall ES, Nguyen H, Goldstein SL. Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months. J Pediatr. 2014;165(3):522-527. doi: 10.1016/j.jpeds.2014.04.058. [DOI] [PubMed] [Google Scholar]
- 8. Sigurjonsdottir VK, Chaturvedi S, Mammen C, Sutherland SM. Pediatric acute kidney injury and the subsequent risk for chronic kidney disease: is there cause for alarm. Pediatr Nephrol. 2018; 33(11):2047-2055. doi: 10.1007/s00467-017-3870-6. [DOI] [PubMed] [Google Scholar]
- 9. Jones DP, Spunt SL, Green D, Springate JE, Children's Oncology Group. Renal late effects in patients treated for cancer in childhood: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2008;51(6):724-731. doi: 10.1002/pbc.21695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davidoff AM, Interiano RB, Wynn L, et al. Overall survival and renal function of patients with synchronous bilateral wilms tumor undergoing surgery at a single institution. Ann Surg. 2015;262:570-576. doi: 10.1097/sla.0000000000001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nottage KA, Ness KK, Li C, Srivastava D, Robison LL, Hudson MM. Metabolic syndrome and cardiovascular risk among long-term survivors of acute lymphoblastic leukaemia—from the St. Jude Lifetime Cohort. Br J Haematol. 2014;165(3):364-374. doi: 10.1111/bjh.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kavey RE, Allada V, Daniels SR, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2006;114:2710-2738. doi: 10.1161/circulationaha.106.179568. [DOI] [PubMed] [Google Scholar]
- 13. Janjua HS, Mahan JD. Growth in chronic kidney disease. Adv Chronic Kidney Dis. 2011;18:324-331. doi: 10.1053/j.ackd.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 14. James MT, Hemmelgarn BR, Tonelli M. Early recognition and prevention of chronic kidney disease. Lancet. 2010;375:1296-1309. doi: 10.1016/s0140-6736(09)62004-3. [DOI] [PubMed] [Google Scholar]
- 15. Skinner R, Parry A, Price L, Cole M, Craft AW, Pearson AD. Persistent nephrotoxicity during 10-year follow-up after cisplatin or carboplatin treatment in childhood: relevance of age and dose as risk factors. Eur J Cancer. 2009;45(18):3213-3219. doi: 10.1016/j.ejca.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 16. Knijnenburg SL, Jaspers MW, van der Pal HJ, Schouten-van Meeteren AY, Bouts AH, Lieverst JA, et al. Renal dysfunction and elevated blood pressure in long-term childhood cancer survivors. Clin J Am Soc Nephrol. 2012;7(9):1416-1427. doi: 10.2215/cjn.09620911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gibson TM, Li Z, Green DM, Armstrong GT, Mulrooney DA, Srivastava D, et al. Blood pressure status in adult survivors of childhood cancer: a report from the St.Jude Lifetime Cohort Study. Cancer Epidemiol Biomarkers Prev. 2017;26(12):1705-1713. doi: 10.1158/1055-9965.EPI-17-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers [Version 5.0]. Monrovia, CA: Children’s Oncology Group; 2018. [Google Scholar]
- 19. KDIGO Group. KDIGO 2012 clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1-138. [Google Scholar]
- 20. Eknoyan G, Lameire N, Eckardt K, et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Supp. 2013;3:5-14. [DOI] [PubMed] [Google Scholar]
- 21. Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904. [DOI] [PubMed] [Google Scholar]
- 22. Dormuth CR, Hemmelgarn BR, Paterson JM, et al. Use of high potency statins and rates of admission for acute kidney injury: multicenter, retrospective observational analysis of administrative databases. Bmj. 2013;346:f880. doi: 10.1136/bmj.f880. [DOI] [PubMed] [Google Scholar]
- 23. Garg AX, Nevis IF, McArthur E, et al. Gestational hypertension and preeclampsia in living kidney donors. N Engl J Med. 2015;372:124-133. doi: 10.1056/NEJMoa1408932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Institute for Clinical Evaluative Sciences. ICES Data Dictionary, datadictionary.ices.on.ca (accessed 10 July 2020).
- 25. Bruni O, Ottaviano S, Guidetti V, et al. The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res. 1996;5:251-261. doi: 10.1111/j.1365-2869.1996.00251.x. [DOI] [PubMed] [Google Scholar]
- 26. Romeo DM, Bruni O, Brogna C, Ferri R, Galluccio C, De Clemente V, et al. Application of the sleep disturbance scale for children (SDSC) in preschool age. Eur J Paediatr Neurol. 2013;17(4):374-382. doi: 10.1016/j.ejpn.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 27. Varni JW, Burwinkle TM, Katz ER, et al. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer. 2002;94:2090-2106. doi: 10.1002/cncr.10428. [DOI] [PubMed] [Google Scholar]
- 28. Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800-812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 29. Flynn JT, Daniels SR, Hayman LL, et al. Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension. 2014;63:1116-1135. doi: 10.1161/hyp.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dasgupta K, Quinn RR, Zarnke KB, Rabi DM, Ravani P, Daskalopoulou SS, et al. The 2014 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2014;30(5):485-501. doi: 10.1016/j.cjca.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 31. Djudjaj S, Boor P. Cellular and molecular mechanisms of kidney fibrosis. Mol Aspects Med. 2019;65:16-36. doi: 10.1016/j.mam.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 32. Hessey E, Morissette G, Lacroix J, Perreault S, Samuel S, Dorais M, et al. Long-term mortality after acute kidney injury in the pediatric ICU. Hosp Pediatr. 2018;8(5):260-268. doi: 10.1542/hpeds.2017-0215. [DOI] [PubMed] [Google Scholar]
- 33. James MT, Pannu N, Hemmelgarn BR, et al. Derivation and external validation of prediction models for advanced chronic kidney disease following acute kidney injury. Jama. 2017;318:1787-1797. doi: 10.1001/jama.2017.16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nash DM, Brimble S, Markle-Reid M, McArthur E, Tu K, Nesrallah GE, et al. Quality of care for patients with chronic kidney disease in the primary care setting: a retrospective cohort study from Ontario, Canada. Can J Kidney Health Dis. 2017;4:2054358117703059. doi: 10.1177/2054358117703059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hessey E, Perreault S, Dorais M, Roy L, Zappitelli M. Acute kidney injury in critically ill children and subsequent chronic kidney disease. Can J Kidney Health Dis. 2019;6: 2054358119880188. doi: 10.1177/2054358119880188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kitchlu A, Dixon S, Dirk JS, Chanchlani R, Vasilevska-Ristovska J, Borges K, et al. Elevated risk of cancer after solid organ transplant in childhood: a population-based cohort study. Transplantation. 2019;103(3):588-596. doi: 10.1097/TP.0000000000002378. [DOI] [PubMed] [Google Scholar]
- 37. Kitchlu A, McArthur E, Amir E, et al. Acute kidney injury in patients receiving systemic treatment for cancer: a population-based cohort study. J Natl Cancer Inst. 2019;111:727-736. doi: 10.1093/jnci/djy167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kitchlu A, Shapiro J, Amir E, et al. Representation of patients with chronic kidney disease in trials of cancer therapy. Jama. 2018;319:2437-2439. doi: 10.1001/jama.2018.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chellapandian D, Pole JD, Nathan PC, Sung L. Congestive heart failure among children with acute leukemia: a population-based matched cohort study. Leuk Lymphoma. 2019;60(2):385-394. doi: 10.1080/10428194.2018.1474522. [DOI] [PubMed] [Google Scholar]
- 40. Greenberg ML, Barr RD, DiMonte B, et al. Childhood cancer registries in Ontario, Canada: lessons learned from a comparison of two registries. Int J Cancer. 2003;105:88-91. doi: 10.1002/ijc.11004. [DOI] [PubMed] [Google Scholar]
- 41. Nathan PC, Bremner KE, Liu N, et al. Resource utilization and costs in adolescents treated for cancer in pediatric vs adult institutions. J Natl Cancer Inst. 2019;111:322-330. doi: 10.1093/jnci/djy119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nathan PC, Nachman A, Sutradhar R, et al. Adverse mental health outcomes in a population-based cohort of survivors of childhood cancer. Cancer. 2018;124:2045-2057. doi: 10.1002/cncr.31279. [DOI] [PubMed] [Google Scholar]
- 43. Pole JD, Gibson P, Ethier MC, et al. Evaluation of treatment-related mortality among paediatric cancer deaths: a population based analysis. Br J Cancer. 2017;116:540-545. doi: 10.1038/bjc.2016.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lucyk K, Lu M, Sajobi T, et al. Administrative health data in Canada: lessons from history. BMC Med Inform Decis Mak. 2015;15:69. doi: 10.1186/s12911-015-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chan B. Supply of physicians’ services in Ontario. Hosp Q. 1999;3:17. doi: 10.12927/hcq.16734. [DOI] [PubMed] [Google Scholar]
- 46. Moist LM, Richards HA, Miskulin D, Lok CE, Yeates K, Garg AX, et al. A validation study of the Canadian Organ Replacement Register. Clin J Am Soc Nephrol. 2011;6(4):813-818. doi: 10.2215/CJN.06680810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chiu M, Lebenbaum M, Lam K, et al. Describing the linkages of the immigration, refugees and citizenship Canada permanent resident data and vital statistics death registry to Ontario’s administrative health database. BMC Med Inform Decis Mak. 2016;16:135. doi: 10.1186/s12911-016-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McIsaac KE, Farrell MacDonald S, Chong N, Moser A, Moineddin R, Colantonio A, et al. Creating a powerful platform to explore health in a correctional population: a record linkage study. Plos One. 2016;11(8):e0161173. doi: 10.1371/journal.pone.0161173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zappitelli M, Parvex P, Joseph L, Paradis G, Grey V, Lau S, et al. Derivation and validation of cystatin C-based prediction equations for GFR in children. Am J Kidney Dis. 2006;48(2):221-230. doi: 10.1053/j.ajkd.2006.04.085. [DOI] [PubMed] [Google Scholar]
- 50. Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629-637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McMahon KR, Rassekh SR, Schultz KR, et al. Epidemiologic characteristics of acute kidney injury during cisplatin infusions in children treated for cancer. JAMA Netw Open. 2020;3:e203639. doi: 10.1001/jamanetworkopen.2020.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hessey E, Ali R, Dorais M, Morissette G, Pizzi M, Rink N, et al. Evaluation of height-dependent and height-independent methods of estimating baseline serum creatinine in critically ill children. Pediatr Nephrol. 2017;32(10):1953-1962. doi: 10.1007/s00467-017-3670-z. [DOI] [PubMed] [Google Scholar]
- 53. Steliarova-Foucher E, Stiller C, Lacour B, et al. International Classification of Childhood Cancer, third edition. Cancer. 2005;103:1457-1467. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 54. Pole JD, Darmawikarta D, Gassas A, Ali M, Egler M, Greenberg ML, et al. Subsequent malignant neoplasms in pediatric cancer patients treated with and without hematopoietic SCT. Bone Marrow Transplant. 2015;50(5):721-726. doi: 10.1038/bmt.2015.4. [DOI] [PubMed] [Google Scholar]
- 55. Marcoux S, Drouin S, Laverdière C, Alos N, Andelfinger GU, Bertout L, et al. The PETALE study: late adverse effects and biomarkers in childhood acute lymphoblastic leukemia survivors. Pediatr Blood Cancer. 2017;64(6):e26361. doi: 10.1002/pbc.26361. [DOI] [PubMed] [Google Scholar]
- 56. Skinner R, Cotterill SJ, Stevens MC. Risk factors for nephrotoxicity after ifosfamide treatment in children: a UKCCSG Late Effects Group study. Br J Cancer. 2000;82(10):1636-1645. doi: 10.1054/bjoc.2000.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol. 2015;26(8):1765-1776. doi: 10.1681/ASN.2015010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zappitelli M, Zhang X, Foster BJ. Estimating glomerular filtration rate in children at serial follow-up when height is unknown. Clin J Am Soc Nephrol. 2010;5(10):1763-1769. doi: 10.2215/CJN.08741209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rink N, Zappitelli M. Estimation of glomerular filtration rate with and without height: effect of age and renal function level. Pediatr Nephrol. 2015;30(8):1327-1336. doi: 10.1007/s00467-015-3063-0. [DOI] [PubMed] [Google Scholar]
- 60. Mulder RL, Knijnenburg SL, Geskus RB, et al. Glomerular function time trends in long-term survivors of childhood cancer: a longitudinal study. Cancer Epidemiol Biomarkers Prev. 2013;22:1736-1746. doi: 10.1158/1055-9965.Epi-13-0036. [DOI] [PubMed] [Google Scholar]
- 61. Dart A, Chartier M, Komenda P, Walld R, Koseva I, Burchill C, et al. Evaluation of administrative case definitions for chronic kidney disease in children. Pediatr Res. 2020;87(3):569-575. doi: 10.1038/s41390-019-0595-1. [DOI] [PubMed] [Google Scholar]
- 62. D’Arienzo D, Hessey E, Ali R, Perreault S, Samuel S, Roy L, et al. A validation study of administrative health care data to detect acute kidney injury in the pediatric intensive care unit. Can J Kidney Health Dis. 2019;6:2054358119827525. doi: 10.1177/2054358119827525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496-509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 64. Pole JD, Gu LY, Kirsh V, Greenberg ML, Nathan PC. Subsequent malignant neoplasms in a population-based cohort of pediatric cancer patients: a focus on the first 5 years. Cancer Epidemiol Biomarkers Prev. 2015;24(10):1585-1592. doi: 10.1158/1055-9965.EPI-15-0360. [DOI] [PubMed] [Google Scholar]
- 65. Shatat IF, Flynn JT. Hypertension in children with chronic kidney disease. Adv Chronic Kidney Dis. 2005;12:378-384. doi: 10.1053/j.ackd.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 66. Nathan PC, Agha M, Pole JD, Hodgson D, Guttmann A, Sutradhar R, et al. Predictors of attendance at specialized survivor clinics in a population-based cohort of adult survivors of childhood cancer. J Cancer Surviv. 2016;10(4):611-618. doi: 10.1007/s11764-016-0522-y. [DOI] [PubMed] [Google Scholar]
- 67. Alexander S, Pole JD, Gibson P, Lee M, Hesser T, Chi SN, et al. Classification of treatment-related mortality in children with cancer: a systematic assessment. Lancet Oncol. 2015;16(16):e604-10. doi: 10.1016/S1470-2045(15)00197-7. [DOI] [PubMed] [Google Scholar]
- 68. Ballantyne M, Benzies K, Rosenbaum P, Lodha A. Mothers’ and health care providers’ perspectives of the barriers and facilitators to attendance at Canadian neonatal follow-up programs. Child Care Health Dev. 2015;41(5):722-733. doi: 10.1111/cch.12202. [DOI] [PubMed] [Google Scholar]
- 69. McMahon KR, Harel-Sterling M, Pizzi M, et al. Long-term renal follow-up of children treated with cisplatin, carboplatin, or ifosfamide: a pilot study. Pediatr Nephrol. 2018;33:2311-2320. doi: 10.1007/s00467-018-3976-5. [DOI] [PubMed] [Google Scholar]
- 70. Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancer [Version 4.0]. Monrovia, CA: Children’s Oncology Group; 2013. [Google Scholar]
- 71. Loeffen EAH, Kremer LCM, Mulder RL, Font-Gonzalez A, Dupuis LL, Sung L, et al. The importance of evidence-based supportive care practice guidelines in childhood cancer-a plea for their development and implementation. Support Care Cancer. 2017;25(4):1121-1125. doi: 10.1007/s00520-016-3501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pediatric Oncology Group of Ontario (POGO). Practice Guidelines. Toronto, ON, Canada: POGO; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cjk-10.1177_20543581221130156 for KIdney aNd blooD prESsure ouTcomes in Childhood Cancer Survivors: Description of Clinical Research Protocol of the KINDEST-CCS Study by Adree Khondker, Michael Groff, Sophia Nunes, Carolyn Sun, Natasha Jawa, Jasmine Lee, Vedran Cockovski, Yasmine Hejri-Rad, Rahul Chanchlani, Adam Fleming, Amit Garg, Nivethika Jeyakumar, Abhijat Kitchlu, Asaf Lebel, Eric McArthur, Luc Mertens, Paul Nathan, Rulan Parekh, Serina Patel, Jason Pole, Raveena Ramphal, Tal Schechter, Mariana Silva, Samuel Silver, Lillian Sung, Ron Wald, Paul Gibson, Rachel Pearl, Laura Wheaton, Peter Wong, Kirby Kim and Michael Zappitelli in Canadian Journal of Kidney Health and Disease