Hypertrophic cardiomyopathy (HCM) is genetic cardiomyopathy with the risk of sudden death characterized by abnormal sarcomere protein.[1] Although some pathogenic genes related to HCM have been reported,[2] the disease phenotype and pathogenesis cannot be fully elucidated, which brings great difficulties to the diagnosis and treatment of HCM, especially the exploration of HCM treatment strategies.[3] Studies have shown that sarcomere protein mutation may affect the normal development of sarcomere structure and lead to abnormal cardiac function, indicating that HCM is insufficient in research in many cases.[4,5] The pathogenesis of HCM disease mainly includes haploinsufficiency (“loss of function”) or shortened nonfunctional proteins (“toxic peptides”).[6] MYH7 and other sarcomere proteins mainly cause haploinsufficiency (“loss of function”) by missense mutations, while 90% of MYBPC mutations cause shortened nonfunctional proteins (“toxic peptides”).[7]
Protein quality control (PQC) system mainly includes molecular chaperones, proteases and regulatory factors to promote protein folding, prevent protein aggregation and remove improperly folded polypeptides.[8] Protein misfolding is a process in which proteins obtain various non-native contacts to affect their structure and properties.[9] Protein misfolding is characterized by the formation of nonfunctional protein structures, which leads to cardiomyopathy.[8] The misfolded proteins usually occur in the following two situations: one is the degradation of misfolded proteins, resulting in the loss of protein function; the other is the aggregation of misfolded proteins, resulting in increased functional toxicity (Figure 1). Aggregation occurs when the folded intermediate or partially folded state exposes hydrophobic amino acid residues or areas mostly embedded in the natural state.[9] However, molecular chaperones may help break down tight aggregates to form naturally folded monomer proteins. Misfolded monomers are polymerized into dimers, which are the initial building blocks for the formation of amyloid fibers. The dimers can further polymerize to form oligomers, and then the oligomers further combine to form fibrils.[9] “Protein homeostasis” is an interrelated phenomenon in PQC, which plays a key role in the process of protein synthesis, transportation and transfer.[10] The function of the classification task of the PQC system is to remove proteins to increase the possibility of unfolded and misfolded conformations, resulting in a large part of newly synthesized proteins being sorted out before the synthesis process is completed through cotranslation degradation.[11] However, replacing old and misfolded proteins is a complex process, which requires an increasing number of effective sarcomere PQC systems, as well as the regulation of various protein degradation pathways and numerous molecular chaperones.[12]
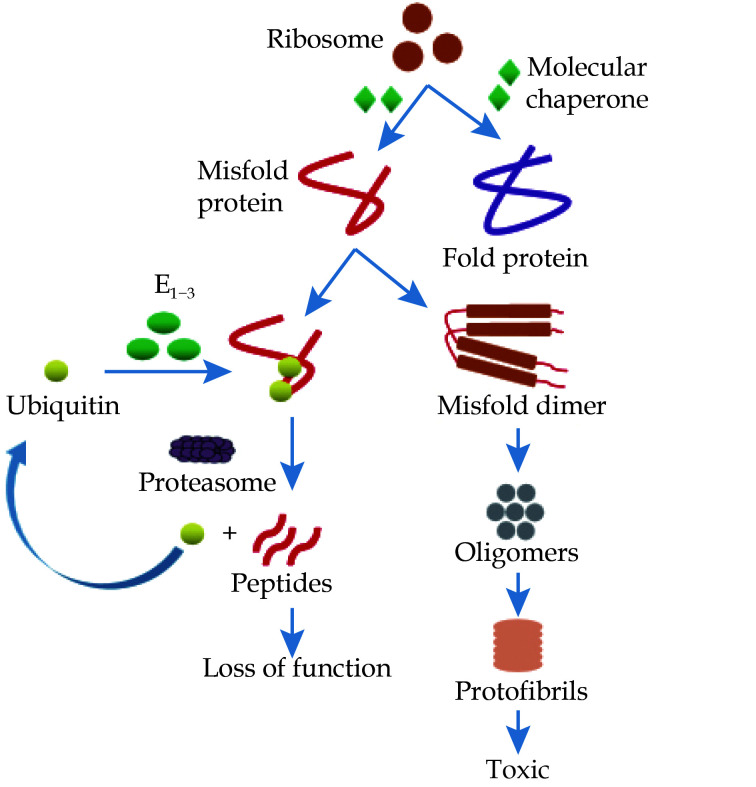
Figure 1.
Protein misfolding and degradation pathways.
Proteins are folded by molecular chaperones, and polypeptide chains synthesized by mutant genes form misfolded proteins. The misfolded mutant protein is ubiquitinated, and the ubiquitinated protein complex is then degraded into small peptides by the proteasome system and releases ubiquitin. Another way is that misfolded monomers are polymerized into dimers, further polymerized to form oligomers, and finally combined to form protofibrils.
Sarcomere contraction is mediated by molecular motion myosin, which converts energy from adenosine triphosphate into mechanical work and moves along actin filaments.[10] The folding of myosin polypeptide into its functional conformation requires the assistance of chaperone protein in the PQC system to assemble the hexameric myosin complex (Figure 2).[13] UNC-45 is a highly conserved key myosin partner, which associates myosin through its domain to prevent protein aggregation.[14] UNC-45 mainly acts as a chaperone by linking the head region of myosin, and even plays a key role with HSP90 chaperone.[14] HSP70 or HSP90 molecular chaperones ensure the stability of proteins in the early stage by affecting myosin folding and assembly.[13] UNC-45 enhances the myosin folding activity of HSP70 and HSP90, and UNC-45 interacts with HSP70/HSP90 through its co-chaperone helix trans helix motif.[15] In the case of missense MYBPC mutants, the role of HSP70 in regulating MYBPC degradation may be a mechanism to prevent the “poison peptide” effect. Meanwhile, HSP70 molecular chaperone may prevent MYBPC-HCM disease by rapidly degrading “toxic peptide”.[16,17] Based on the destruction of UNC-45/HSP90/myosin complex associated with Smyd1, knockout of the Smyd1 gene will inhibit myosin accumulation in early sarcomeres, resulting in myosin instability and protein degradation.[17] However, the abnormality of Smyd1 leads to the decrease of protein stability, and indirectly leads to the increase of UNC-45 and HSP90 expression, suggesting that Smyd1 was the third member of the typical myosin chaperone complex.[12]
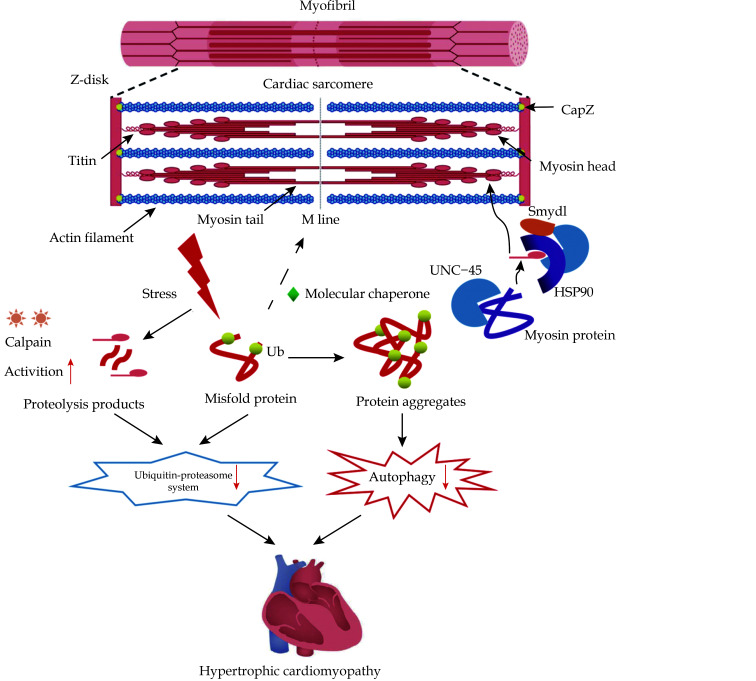
Figure 2.
Schematic diagram of action mechanism of sarcomere protein quality control in hypertrophic cardiomyopathy.
BAG3/HSC70 chaperone complex maintains CapZ stability in sarcomeres. UNC-45 coordinates the folding of myosin ATPase domain and is a key chaperone for thick filament assembly and maintaining its stability. UNC-45 interacts with HSP90 to participate in myosin folding and early myofibril assembly. Lysine methyltransferase Smyd1 acts on HSP90/UNC-45/myosin complex to maintain sarcomere stability. Abnormal protein misfolding caused by stressors is accompanied by increased calpain activity, resulting in excessive protein hydrolysates. In hypertrophic cardiomyopathy, ubiquitin-proteasome system and autophagy-lysosomal system activities are down-regulated. These factors ultimately lead to the toxic level of protein aggregation, which may lead to sarcomere structural disorder and mechanical dysfunction common in failed myocardium.
Sarcomere protein turnover is very important for maintaining function and promoting cardiomyocyte life. Obsolete proteins are mainly removed by the following ways (calpain, ubiquitin-proteasome and autophagy-lysosomal) to maintain the stability of sarcomere proteins.[12] Calpain is a conserved calcium-dependent cysteine protease.[18] Chaperone mediates the cleavage site of sarcomere protein to regulate the sensitivity of protein to calpain hydrolysis. The activation of calpain is related to the increase of protein ubiquitination and is considered to be a reaction before the ubiquitin-proteasome system (UPS) degrades myofilament protein.[12] UPS mediated protein degradation is the result of the synergistic action of multiple enzymes, which is finally catalyzed by E3 ubiquitin ligase and transports ubiquitin to the place where it is needed.[19] Many studies have shown that UPS is the basis of sarcomeric PQC.[12] Macroautophagy mainly degrades proteins that fail to fold and ubiquitinate, which forms membrane encapsulated vesicles (autophagosomes) around some cytoplasm containing protein aggregates and/or other toxic cellular components.[20] Ubiquitin can also regulate the selectivity of autophagy, and its function is similar to UPS.[21] Meanwhile, autophagosomes recover goods by fusing with lysosomes, so that the contents of autophagosomes are exposed to lysosomal hydrolases. Autophagy is crucial in maintaining the normal structure of sarcomere. The damage of autophagy is related to the abnormality of the sarcomere structure, but little is known about autophagy is associated with sarcomere PQC system. Chaperone assisted selective autophagy, is the only autophagy pathway clearly shown to play a role in sarcomere so far, which can form complex with BAG3 in sarcomere Z disk, degrade misfolded filamin C and prevent their aggregation, and provide scaffold function for HSPB8 and HSC70/HSP70.[22]
In HCM end-stage heart failure, there is an imbalance in the PQC mechanism (Figure 2), resulting in the aggregation of old/misfolded proteins, which may lead to the damage of sarcomere structure and function. The activity of calpain in patients with cardiomyopathy is increased, resulting in the increase of calpain activity in patients with UPS. The results showed that in the late stage of HCM, the degradation of misfolded protein in vivo was slow, and its ups activity decreased significantly. In normal myocardium, protein aggregates that cannot be cleared by two basic approaches (UPS and calpain) can be cleared by autophagy. Autophagy is up-regulated under insufficient proteasome function, elevated mechanical strain and other stress conditions (including stress conditions related to sarcomere protein gene mutation).[23] However, the PQC system in patients with HCM is abnormal, resulting in reduced autophagy activity and ultimately unable to clear protein aggregates.[23]
Due to protein misfolding, a defective PQC system may lead to various diseases, including HCM. Conceptually, the following three methods can be used to treat HCM with protein misfolding (Table 1):[8] (1) directly targeting misfolded proteins; (2) improve post-translational modification of misfolded proteins; and (3) combine with other chaperone proteins or molecules to prevent protein aggregation. Among them, the molecular chaperone is considered a potential therapeutic molecule for targeted treatment of protein misfolding diseases and is widely used in many diseases.
Table 1. Application of protein quality control in the treatment of hypertrophic cardiomyopathy.
| Target for therapy | Mechanisms | References |
| HSP70 | Prevent protein aggregation | 24 |
| HSP40 | Achieve these diverse functions such as protein folding and protein transport activities | 8,25 |
| Cytosolic chaperonin T-complex protein 1-ring complex | Maintain the normal kinetic level and sarcomere protein structure | 26 |
| Heat shock transcription factors | Ensure effective assembly and protein stability to maintain adequate protein degradation | 27 |
| Autophagy-based | Prevent protein aggregation and indirectly regulate protein quality control system | 19,28 |
| Adeno-associated virus serotype 9-BAG3 | Save myofilament contraction function and restore sarcomere protein stability | 29 |
HSP70 is the most conserved chaperone, which acts on the newborn polypeptide chain during folding and prevents protein aggregation,[24] may be a potential specific target against myocardial hypertrophy and fibrosis. The HSP40 (DnaJ like) family helps proteins fold, assemble and transport within cells.[25] Regulating the molecular chaperones HSP40 protein and HSP70 protein in the PQC system can improve the HCM-related phenotype by mediating the normal folding of sarcomere protein, maintaining the normal kinetic level and sarcomere protein structure.[8] The cytosolic chaperonin T-complex protein 1-ring complex or chaperonin containing T-complex protein 1 is a cytosolic chaperone whose main function is to maintain the stability of polypeptides in the early stage of folding and binding.[26] Heat shock transcription factors are actively involved in the process of transcription and expression under normal, stress or abnormal mutation conditions, while the combined action of different transcription factors leads to the differences of many kinds of stress induction or development. Increasing HSP expression in sarcomeres may ensure efficient assembly and stable synthesis of proteins, and maintain the balance of protein degradation.[27] Abnormal PQC may be a common pathological event in HCM, and mTOR inhibition and increased autophagy may play their therapeutic role by regulating PQC in cardiomyocytes.[28] Drugs that induce autophagy are also expected to restore cardiac function in HCM animal models. They mainly affect sarcomere by indirectly regulating the PQC system, but the specific mechanism is not clear.[19] By analyzing the clinical and pathological characteristics of patients with abnormal molecular chaperone at the later stage of HCM, we suspect that gene therapy based on adeno-associated virus may restore the stability of its protein and become a new treatment strategy.[29]
PQC system is the basis of maintaining normal sarcomere structure. The exact mechanism of sarcomere formation (sarcomere genesis) is controversial. The normal sarcomere structure requires the participation of multiple chaperones and co-chaperones to maintain protein stability and prevent protein aggregation. Future work may aim to determine how the assembly and degradation of sarcomere proteins in HCM are regulated on sarcomere and how to ensure that new proteins are accurately transported to sarcomere in time and space, which will further clarify the mechanism of dynamic PQC system in sarcomere, and provide a good theoretical basis and research direction for HCM disease research.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (No.82070352), the Natural Science Foundation of Hunan Province (2022JJ40765), and the Natural Science Foundation of Changsha City, China (kq2202366). All authors had no conflicts of interest to disclose.
References
- 1.Makavos G, Kairis C, Tselegkidi ME, et al Hypertrophic cardiomyopathy: an updated review on diagnosis, prognosis, and treatment. Heart Fail Rev. 2019;24:439–459. doi: 10.1007/s10741-019-09775-4. [DOI] [PubMed] [Google Scholar]
- 2.Wang B, Wang J, Wang LF, et al Genetic analysis of monoallelic double MYH7 mutations responsible for familial hypertrophic cardiomyopathy. Mol Med Rep. 2019;20:5229–5238. doi: 10.3892/mmr.2019.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ommen SR, Mital S, Burke MA, et al 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020;142:e533–e557. doi: 10.1161/CIR.0000000000000938. [DOI] [PubMed] [Google Scholar]
- 4.Tyska MJ, Hayes E, Giewat M, et al Single-molecule mechanics of R403Q cardiac myosin isolated from the mouse model of familial hypertrophic cardiomyopathy. Circ Res. 2000;86:737–744. doi: 10.1161/01.RES.86.7.737. [DOI] [PubMed] [Google Scholar]
- 5.Maron BJ Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med. 2018;379:1977. doi: 10.1056/NEJMc1812159. [DOI] [PubMed] [Google Scholar]
- 6.Parbhudayal RY, Garra AR, Götte MJW, et al Variable cardiac myosin binding protein-C expression in the myofilaments due to MYBPC3 mutations in hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2018;123:59–63. doi: 10.1016/j.yjmcc.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Burke MA, Cook SA, Seidman JG, et al Clinical and mechanistic insights into the genetics of cardiomyopathy. J Am Coll Cardiol. 2016;68:2871–2886. doi: 10.1016/j.jacc.2016.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yadav K, Yadav A, Vashistha P, et al Protein misfolding diseases and therapeutic approaches. Curr Protein Pept Sci. 2019;20:1226–1245. doi: 10.2174/1389203720666190610092840. [DOI] [PubMed] [Google Scholar]
- 9.Dill KA, MacCallum JL The protein-folding problem, 50 years on. Science. 2012;338:1042–1046. doi: 10.1126/science.1219021. [DOI] [PubMed] [Google Scholar]
- 10.Ellis RJ Macromolecular crowding: an important but neglected aspect of the intracellular environment. Curr Opin Struct Biol. 2001;11:114–119. doi: 10.1016/S0959-440X(00)00172-X. [DOI] [PubMed] [Google Scholar]
- 11.Turner GC, Varshavsky A Detecting and measuring cotranslational protein degradation in vivo. Science. 2000;289:2117–2220. doi: 10.1126/science.289.5487.2117. [DOI] [PubMed] [Google Scholar]
- 12.Martin TG, Kirk JA Under construction: the dynamic assembly, maintenance, and degradation of the cardiac sarcomere. J Mol Cell Cardiol. 2020;148:89–102. doi: 10.1016/j.yjmcc.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saibil H Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol. 2013;14:630–642. doi: 10.1038/nrm3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barral JM, Hutagalung AH, Brinker A, et al Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science. 2002;295:669–671. doi: 10.1126/science.1066648. [DOI] [PubMed] [Google Scholar]
- 15.Scheufler C, Brinker A, Bourenkov G, et al Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2. [DOI] [PubMed] [Google Scholar]
- 16.Marston S, Copeland O, Jacques A, et al Evidence from human myectomy samples that MYBPC3 mutations cause hypertrophic cardiomyopathy through haploinsufficiency. Circ Res. 2009;105:219–222. doi: 10.1161/CIRCRESAHA.109.202440. [DOI] [PubMed] [Google Scholar]
- 17.Just S, Meder B, Berger IM, et al The myosin-interacting protein SMYD1 is essential for sarcomere organization. J Cell Sci. 2011;124:3127–3136. doi: 10.1242/jcs.084772. [DOI] [PubMed] [Google Scholar]
- 18.Ono Y, Sorimachi H Calpains: an elaborate proteolytic system. Biochim Biophys Acta. 2012;1824:224–236. doi: 10.1016/j.bbapap.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Lecker SH, Goldberg AL, Mitch WE Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol. 2006;17:1807–1819. doi: 10.1681/ASN.2006010083. [DOI] [PubMed] [Google Scholar]
- 20.Henning RH, Brundel BJJM Proteostasis in cardiac health and disease. Nat Rev Cardiol. 2017;14:637–653. doi: 10.1038/nrcardio.2017.89. [DOI] [PubMed] [Google Scholar]
- 21.Willis MS, Townley-Tilson WH, Kang EY, et al Sent to destroy: the ubiquitin proteasome system regulates cell signaling and protein quality control in cardiovascular development and disease. Circ Res. 2010;106:463–478. doi: 10.1161/CIRCRESAHA.109.208801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ulbricht A, Gehlert S, Leciejewski B, et al Induction and adaptation of chaperone-assisted selective autophagy CASA in response to resistance exercise in human skeletal muscle. Autophagy. 2015;11:538–546. doi: 10.1080/15548627.2015.1017186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taneike M, Yamaguchi O, Nakai A, et al Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy. 2010;6:600–606. doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- 24.Pilon M, Schekman R Protein translocation: how Hsp70 pulls it off. Cell. 1999;97:679–682. doi: 10.1016/S0092-8674(00)80780-1. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Qian X, Sha B Heat shock protein 40: structural studies and their functional implications. Protein Pept Lett. 2009;16:606–612. doi: 10.2174/092986609788490159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vang S, Corydon TJ, Børglum AD, et al Actin mutations in hypertrophic and dilated cardiomyopathy cause inefficient protein folding and perturbed filament formation. FEBS J. 2005;272:2037–2049. doi: 10.1111/j.1742-4658.2005.04630.x. [DOI] [PubMed] [Google Scholar]
- 27.Treiber A, Morand O, Clozel M The pharmacokinetics and tissue distribution of the glucosylceramide synthase inhibitor miglustat in the rat. Xenobiotica. 2007;37:298–314. doi: 10.1080/00498250601094543. [DOI] [PubMed] [Google Scholar]
- 28.Bu H, Ding Y, Li J, et al Inhibition of mTOR or MAPK ameliorates vmhcl/myh7 cardiomyopathy in zebrafish. JCI Insight. 2021;6:e154215. doi: 10.1172/jci.insight.154215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin TG, Myers VD, Dubey P, et al Cardiomyocyte contractile impairment in heart failure results from reduced BAG3-mediated sarcomeric protein turnover. Nat Commun. 2021;12:2942. doi: 10.1038/s41467-021-23272-z. [DOI] [PMC free article] [PubMed] [Google Scholar]