Abstract
Background:
No study has yet systematically evaluated the effect of antidiabetic therapy on clinical outcomes of COVID-19 patients with type 2 diabetes (T2D).
Objective:
We aimed to evaluate the effect of different antidiabetic therapy on clinical outcomes of COVID-19 patients with T2D.
Methods:
We comprehensively retrieved the published research which examined the effect of antidiabetic therapy on clinical outcomes of COVID-19 patients with T2D. The odds ratio (OR) and its 95% confidence interval (95% CI) for clinical outcomes were calculated using the random-effects model, and meta-regression was adopted to evaluate the potential sources of heterogeneity between studies.
Results:
A total of 54 studies were included in this study. We found that the use of metformin (OR = 0.66, 95% CI: 0.58-0.75), SGLT-2i (OR = 0.80, 95% CI: 0.73-0.88), and GLP-1ra (OR = 0.83, 95% CI: 0.70-0.98) were significantly associated with lower mortality risk in COVID-19 patients with T2D, while insulin use might unexpectedly increase the ICU admission rate (OR = 2.32, 95% CI: 1.34-4.01) and risk of death (OR = 1.52, 95% CI: 1.32-1.75). No statistically significant associations were identified for DPP-4i, SUs, AGIs, and TZDs.
Conclusion and Relevance:
We demonstrated that the usage of metformin, SGLT-2i, and GLP-1ra could significantly decrease mortality in COVID-19 patients with T2D. The heterogeneity across the studies, baseline characteristics of the included patients, shortage of dosage and the duration of antidiabetic drugs and autonomy of drug selection might limit the objectivity and accuracy of results. Further adequately powered and high-quality randomized controlled trials are warranted for conclusive findings.
Keywords: COVID-19, SARS-CoV-2, meta-analysis, diabetes, antidiabetic therapy
Introduction
To date, the coronavirus disease-2019 (COVID-19) pandemic has resulted in more than 614 million confirmed infections, including 6.5 million deaths, up to September 30, 2022, as reported by the World Health Organization (WHO). 1 Amongst these rapidly increasing cases of confirmed COVID-19 infection, type 2 diabetes (T2D) has been found to be the second comorbidity.2-6 COVID-19 patients with T2D are more prone to requiring hospital care, developing severe respiratory symptoms, and COVID-19-related death.7-9 A large population-based cohort study from England showed that patients with T2D have twice the risk of COVID-19 mortality than those without. 10 Some studies have also noted that hyperglycemia is significantly associated with death and poor clinical prognosis in patients with T2D and COVID-19.11,12 Interest in the potential effect of antidiabetic therapy on clinical outcomes of COVID-19 patients with T2D has increased.11,13,14
There are a wide variety of antidiabetic drugs with different mechanisms, however, no study has yet systematically evaluated the effect of antidiabetic therapy on clinical outcomes of COVID-19 patients with T2D. At present, these antidiabetic drugs, including metformin, insulin, thiazolidinediones (TZDs), dipeptidyl-peptidase 4 inhibitors (DPP-4i), sulfonylureas (SUs), sodium-glucose cotransporter-2 inhibitors (SGLT-2i), glucagon-like peptide-1 receptor agonists (GLP-1ra), and α-glycosidase inhibitors (AGIs), are being used to control blood glucose level and improve clinical outcomes of COVID-19 patients with T2D.15-18 However, so far the results of these trials were inconsistent due to the limited sample size and heterogeneous methodological quality.15,19-23 Some studies found that metformin could improve the clinical outcomes of COVID-19 patients with T2D,24-27 while some reported no difference.18,28-30 For other antidiabetic drugs, the results are variable and contradictory. For example, a multinational retrospective cohort study reported the use of GLP-1RA, DPP-4i, or pioglitazone could improve outcomes for COVID-19 patients with T2D, 15 while another study found that DPP-4i was not associated with improved clinical outcomes in Dutch patients. 23 Thus, up-to-date and comprehensive summaries of available evidence are crucial.
A systematic review and meta-analysis could summarize and evaluate a comprehensive and up-to-date view of the evidence, as well as reveal more robust estimates, or even form clinical practice guidelines. In this study, we comprehensively retrieved the published research which examined the effect of antidiabetic therapy on clinical outcomes of COVID-19 patients with T2D to update the pre-existing evidence which may contribute to the selection of appropriate antidiabetic drugs for these COVID-19 patients.
Methods
Selection Criteria
This study was conducted and reported according to the guidelines proposed by the Meta-analysis of Observational Studies in Epidemiology (MOOSE) and Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).31,32 Selection criteria for studies were based on the Patient-Intervention-Comparison-Outcome-Study (PICOS) framework is as follows: (1) P: COVID-19 patients with T2D; (2) I: taking one of the anti-diabetes drugs; (3) C: the control group that didn’t take the candidate anti-diabetes drugs; (4) O: COVID-19 related deaths and other poor clinical outcomes including ICU admission and invasive mechanical ventilation; (5) S: cohort, case-control, cross-sectional design in human beings. Excluded studies, on the other hand, fall into one of the following categories: (1) case report; (2) unrelated article; (3) no complete data available; (4) no full text available; (5) no related outcomes; (6) published in non-English; (7) duplicated cohort.
Search Strategy
We searched PubMed, EMBASE, and the Cochrane Library for eligible studies from December 1, 2019 until July 10, 2022. The search strategy used the following terms: “COVID-19,” “diabetes,” “metformin,” “DDP-4i,” “Insulin,” “SGLT-2i,” “SUs,” “GLP-1RAs,” “TZDs,” and “AGIs,” as well as their synonyms, full name, and related keywords. Each search item was a combination of one antidiabetic drug, COVID-19, and diabetes. Details of the search strategies were listed in the Supplementary Materials.
Data Extraction and Quality Assessment
All the relevant studies included in this analysis were imported into Endnote X9 software (developed by Clarivate Analytics, Philadelphia, PA, USA), and a team of paired reviewers independently performed further filtering of the retrieved studies. Duplicate records were removed by Endnote X9. The remaining studies were then further screened, first for title, abstract, and then for full text, leaving only those studies that met our criteria.
At least two researchers independently performed the data extraction from the eligible studies, and any disagreements were solved by discussion or, when necessary, by third-party adjudication. We developed a standardized extraction form to collect the following data: first author, publication year, country, study design, sample size, characteristic of the population (age, gender, co-hypertension, and antidiabetic agent use), the definition of control groups, information about the adjustment for confounds, and clinical endpoints. When extracting outcome data, we extracted the reported OR or adjusted OR that had been calculated by the study authors if it was available, and we also extracted the number of events in each group whenever possible. All of the medications documented in the patients were taken before admission. The studies’ quality was evaluated with the New-castle–Ottawa Scale (NOS) by assessing the selection, comparability, and outcome of each study, assigning each study a total score from zero to nine.
Outcomes
The primary study outcome was COVID-19-related death (defined by the total number of COVID-19 patients with T2D who died during the follow-up period). The secondary outcomes for our study were other clinical outcomes including (1) intensive care unit (ICU) admission; (2) invasive mechanical ventilation.
Statistical Analysis
Meta-analysis was performed with statistical packages “meta” and “metafor” in R (version 4.0.5). All tests were two-sided, and a P value of less than .05 for any test or model was considered statistically significant unless otherwise stated. The odds ratio (OR) and its 95% confidence interval (95% CI) for clinical outcomes were calculated using the random-effects model to promote the universality of the results. I2 statistics were used to assess the degree of heterogeneity, and we deemed I2 values at around 25% as low heterogeneity, I2 values at around 50% as moderate heterogeneity, and I2 values at around 75% high heterogeneity. Furthermore, we performed meta-regression analysis to evaluate the potential sources of heterogeneity between studies. The funnel plot was used to evaluate the qualitative risk of publication bias, while Egger’s regression method was used to assess the quantitative risk of publication bias.
Results
Description of Included Studies
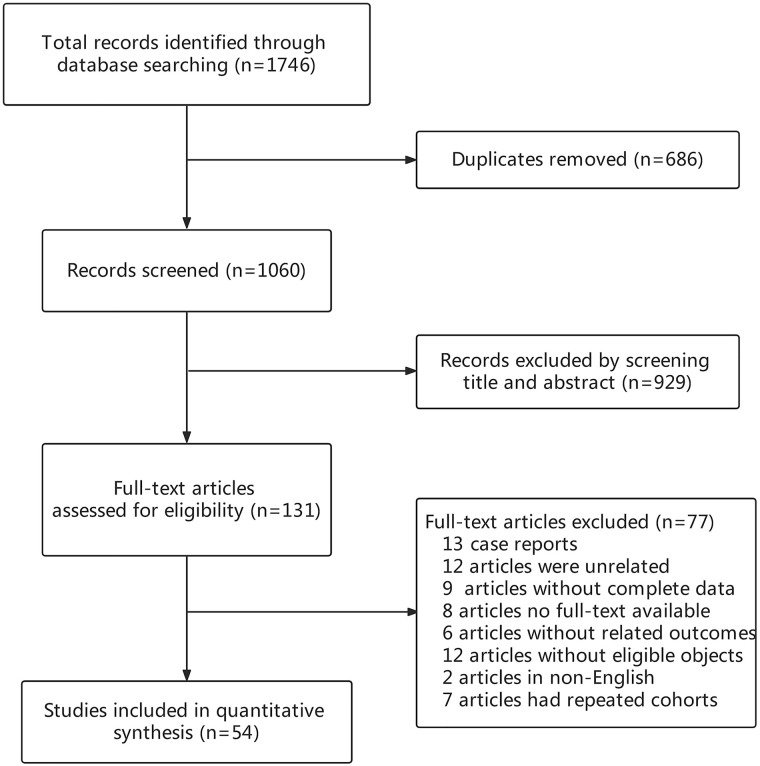
The flow chart of the selection process of this study is shown in Figure 1. By electronic searching up to July 10, 2022, a total of 1746 records were identified. After eliminating 686 duplicates, 1060 records remained. A total of 929 articles were excluded for the titles and abstracts, 131 articles were further evaluated by full text, and 77 records were excluded for following reasons: (1) case report (n = 13); (2) unrelated article (n = 12); (3) no complete data available (n = 9); (4) no full text available (n = 8); (5) no related outcomes (n = 6); (6) the objects did not meet the inclusion criteria (n = 12); (7) published in non-English (n = 2); (8) duplicate study cohorts (n = 7). Finally, the remaining 54 studies were included in this meta-analysis (Details were presented in Supplementary Table 2). Of the 54 studies included in this analysis, 34 studies were related to metformin, 28 to insulin, 24 to DPP-4i, 13 to SUs, 11 to GLP-1RA, 10 to SGLT-2i, 5 to AGIs, and 3 to TZDs. The basic characteristics of the studies are presented in Supplementary Table 1. The NOS scores of all studies were no less than 6, indicating high quality.
Figure 1.
Flow chart of the study.
Metformin
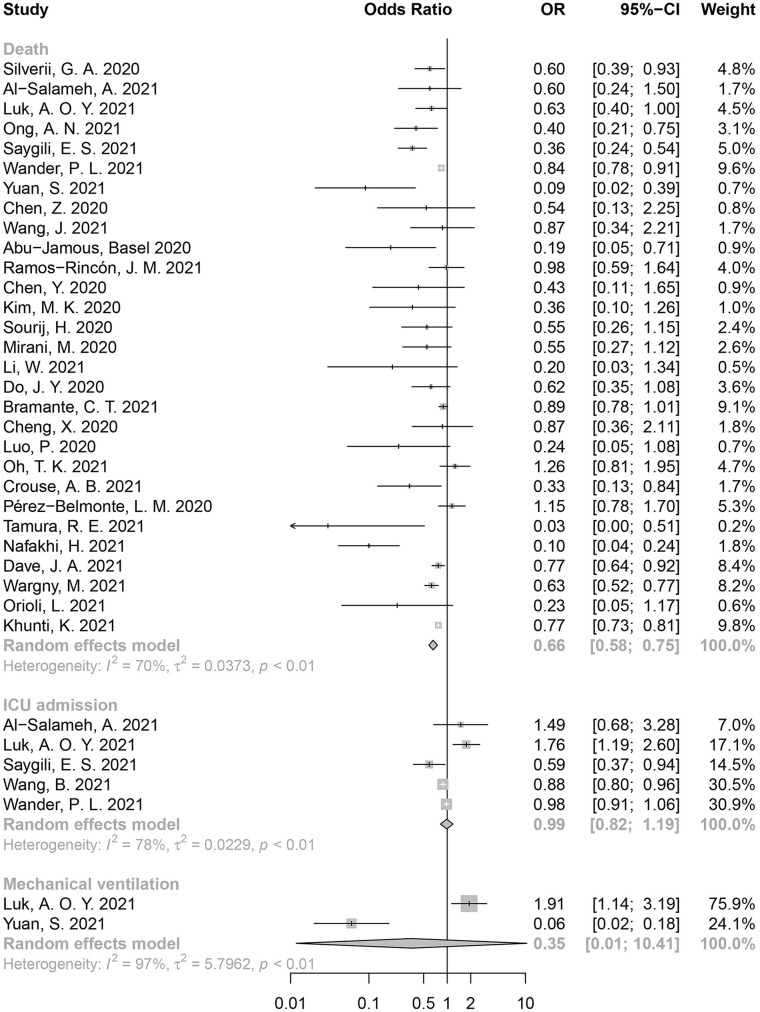
Totally 34 studies related to metformin were included in the quantitative analysis. Among them, 29 reported mortality, 5 reported ICU admission rate, and 2 reported incidence of mechanical ventilation. Metformin use could reduce mortality significantly in COVID-19 patients with T2D (OR = 0.66, 95% CI: 0.58-0.75, I2 = 70%; Figure 2). However, as opposed to our expectation, no statistically significant reduction in ICU admission or mechanical ventilation was observed (ICU admission: OR = 0.99, 95% CI: 0.82-1.19, I2 = 78%; mechanical ventilation: OR = 0.35, 95% CI: 0.01-10.41, I2 = 97%).
Figure 2.
Forest plot for the association between metformin use and poor clinical outcomes in COVID-19 patients with T2D.
Insulin
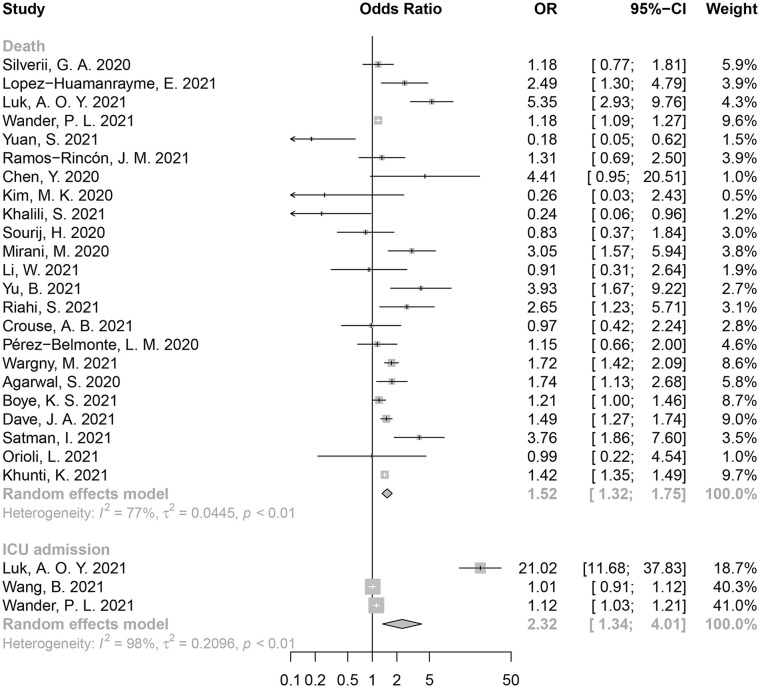
Of the 28 insulin-related studies, 23 reported mortality from COVID-19, 3 reported ICU admissions, and 2 reported the incidence of mechanical ventilation. We noted that insulin use could increase the risk of death in COVID-19 patients with T2D (OR = 1.52, 95% CI: 1.32-1.75, I2 = 77%) (Figure 3). Insulin was also associated with a higher ICU admission rate (OR = 2.32, 95% CI: 1.34-4.01, I2 = 98%).
Figure 3.
Forest plot for the association between insulin use and poor clinical outcomes in COVID-19 patients with T2D.
DPP-4i
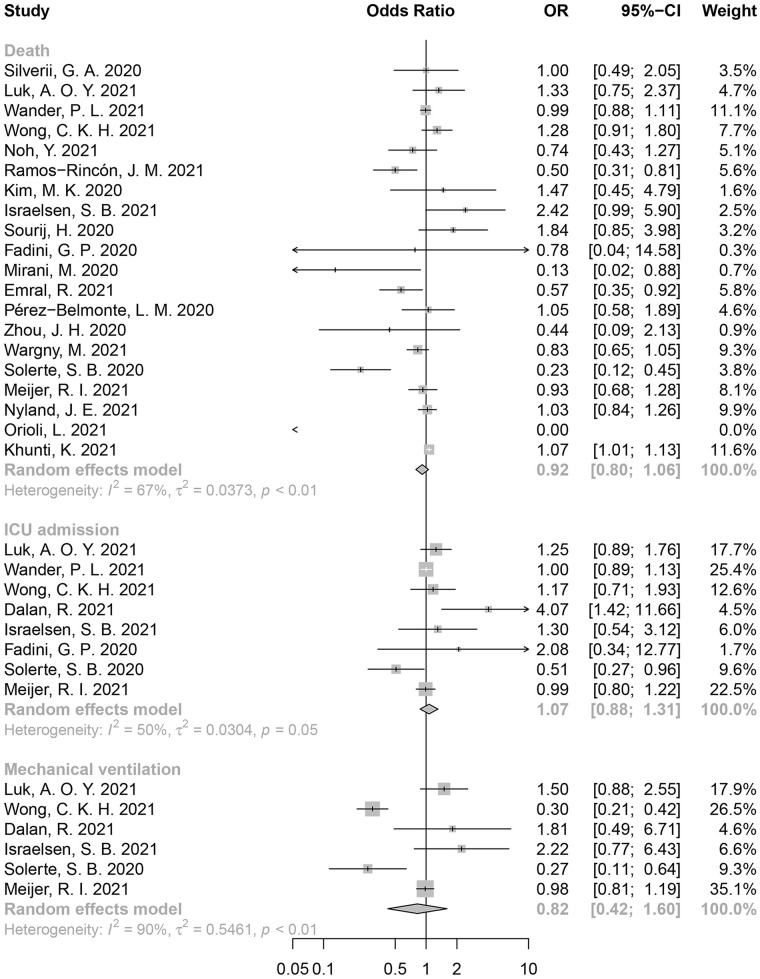
Totally 24 studies related to DPP-4i were included in the quantitative analysis. Among them, 20 reported mortality, 8 reported ICU admission rate, and 6 reported incidence of mechanical ventilation. No statistically significant association was identified between DPP-4i use and mortality (OR = 0.92, 95% CI: 0.80-1.06, I2 = 67%) (Figure 4), ICU admission (OR = 1.07, 95% CI: 0.88-1.31, I2 = 50%), or mechanical ventilation (OR = 0.82, 95% CI: 0.42-1.60, I2 = 90%).
Figure 4.
Forest plot for the association between DPP-4i use and poor clinical outcomes in COVID-19 patients with T2D.
Other Antidiabetic Drugs
There were 13 studies related to SUs, 11 studies related to GLP-1RA, 10 studies related to SGLT-2i, 5 studies related to AGIs, and 3 studies related to TZDs included in our meta-analysis. Both SGLT-2i use (OR = 0.80, 95% CI: 0.73-0.88, I2 = 12%) and GLP-1ra use (OR = 0.83, 95% CI: 0.70-0.98, I2 = 47%) were associated with lower mortality risk in COVID-19 patients with T2D. However, studies related to SUs (OR = 0.97, 95% CI: 0.85-1.09, I2 = 50%), AGIs (OR = 0.30, 95% CI: 0.05-1.85, I2 = 88%) and TZDs (OR = 0.88, 95% CI: 0.61-1.27, I2 = 55%) did not reveal a significant effect. The relevant results are shown in Supplementary Figures 1 to 5.
Sensitivity Analysis
Furthermore, we considered that insulin use might be associated with a patient’s initial poorer physiological status and might be more likely to be admitted to the hospital. Thus, we re-examined both metformin and insulin in patients who received in-hospital treatment and obtained similar results to the main analysis, and it is reasonable to assume that hospitalization did not play a confounding role in our study (Supplementary Figures 6–7).
Meta-Regression
The full results of the meta-regression are shown in Supplementary Figures 8 to 13 with bubble plots. Among metformin users, age (P = .04) had a significant effect on the association between metformin use and mortality. Among GLP-1ra users, hypertension (P = .01) was associated with a higher risk of death.
Publication Bias
Funnel plot and Egger’s test were used to evaluate the publication bias of the included studies on the candidate antidiabetic drugs (Supplementary Figures 14–21). Asymmetry was noted in funnel plot examining the studies related to metformin use, and validated by Egger’s test (P < .01), indicating a potential threat of publication bias.
Discussion
Based on this systematic review and meta-analysis of the available evidence, we found that usage of metformin, SGLT-2i, and GLP-1ra use were associated with a lower risk of mortality in COVID-19 patients with T2D, while insulin use could unexpectedly increase the ICU admission rate and risk of death. Meta-regression analysis demonstrated that age had a direct impact on the association between metformin use and reduced mortality. Older age was associated with a higher risk of death in metformin users. Among GLP-1ra users, hypertension was associated with a higher risk of death. However, no statistically significant associations were identified for DPP-4i, SUs, AGIs, and TZDs. To our knowledge, this study provides the most comprehensive and up-to-date overview of the evidence for the effect of antidiabetic therapy on clinical outcomes of COVID-19 patients with T2D to date.
Metformin is one of the most widely used antidiabetic drugs. In this study, metformin was identified to be associated with a lower risk of mortality in COVID-19 patients with T2D. Interestingly, a large retrospective cohort study 33 (n = 6256) demonstrated that metformin use was significantly associated with decreased mortality in female COVID-19 patients with T2D, but not in males. Another retrospective study (n = 1213) by Cheng et al 28 showed a 41% reduction of heart failure risk in metformin users compared to the nonusers in COVID-19 patients with T2D. Research by Crouse et al 34 revealed that metformin use before the diagnosis of COVID-19 has more potential benefits compared to in-hospital use. Except for glycemic control, metformin also has roles of attenuating endothelial dysfunction, inhibiting viral entry and infection, and modifying inflammatory and immune responses. 35 Considering the multiple protective effects of metformin, further exploration should also expand the usage of metformin in COVID-19 patients without T2D.
Unexpectedly, we found that insulin use may increase the risk of mortality and ICU admission among COVID-19 patients with T2D in our analysis. However, these results should be interpreted with caution because insulin is usually given to patients in a late stage of diabetes. 36 It is difficult to rule out the negative effect of the more advanced diabetes on poor outcomes, especially in severe cases. Riahi et al 37 indicated that the association between insulin use and poor outcomes did not necessarily mean causation, as the severity of diabetes might play a bigger role in determining clinical outcomes among patients with COVID-19.16,38 At present, the basal-bolus insulin regimen is still encouraged to be adopted in COVID-19 patients with T2D, especially in patients with acute hyperglycemia.39,40 It is worth noting that diabetic patients on insulin have a higher risk of developing hypoglycemia during treatment,41,42 and the real-time blood glucose of patients should be monitored in clinical practice to adjust their insulin dose in a timely manner.
We considered that the use of glucocorticoids during the treatment of COVID-19 patients might have a potential effect on our results. Glucocorticoids are potent anti-inflammatory and immunosuppressive drugs, and are now widely used in COVID-19 patients with respiratory failure.43,44 However, glucocorticoids could exacerbate hyperglycemia in patients with diabetes, even cause hyperglycemia in nondiabetics, 45 and the adverse effects of hyperglycemia on patients’ clinical prognosis are obvious.46,47 There is no clear evidence that the choice of antidiabetic drugs is significantly beneficial for glucocorticoid-induced hyperglycemia. Insulin is often recommended in clinical practice as the drug of choice for inpatients with COVID-19 on glucocorticoids with hyperglycemia,43,48 and it is clearly unreasonable to convert all patients to insulin without clear evidence.
In our pooled analysis, we did not find a significant association between DPP-4i use and risk of mortality or other clinical outcomes in COVID-19 patients with T2D. Initially, DPP-4i was considered to affect the progression of COVID-19 via their anti-inflammatory actions, which may be beneficial for patients exposed to cytokine storms due to COVID-19.49,50 However, several large cohort studies have reported no association between DPP-4i use and mortality of COVID-19 among COVID-19 patients with T2D.15,51,52 Interestingly, Nyland et al 15 reported that use of DPP-4i was associated with a reduction in respiratory complications (24.0% vs 29.2%; RR 0.82; 95% CI 0.74-0.90), and continued use of DPP-4i after hospitalization was associated with a decrease in mortality compared with those who discontinued use (9% vs 19%, OR 0.45, 95% CI 0.28-0.72) in a multicenter multinational retrospective cohort study.
There was also a significant association between GLP-1RA use and reduced risk of mortality in COVID-19 patients with T2D. GLP-1 exerts significant anti-inflammatory and antiatherogenic properties, supporting that GLP-1RA might attenuate acute lung disease.53-55 Due to the cardiovascular protective action of GLP-1RA, it was used as a first-line drug to glycemic control in diabetic patients at high risk for cardiovascular diseases. 56 We also found that SGLT-2i use was associated with lower odds of mortality in COVID-19 patients with T2D. In addition, two meta-analyses reported that SGLT-2i was associated with reduced risk of cardiovascular events and chronic kidney disease progression in diabetic patients.57,58 SGLT-2i was mainly adopted in nonsevere patients with COVID-19. 59
This study has several limitations. First, the heterogeneity across the included studies cannot be ignored, although we have used meta-regression to explore the potential causes of heterogeneity. Second, clinical characteristics, particularly complications during hospitalization, are critical factors contributing to adverse clinical endpoints in COVID-19 patients, which may confound our results. We assessed as many patients as possible for co-existing hypertension, but not for other comorbidities or factors such as the length of diabetes. Third, most of the included studies do not include information regarding the dosage and the duration of antidiabetic drug use which might be an independent source of potential bias. Fourth, the choice of antidiabetic drugs was affected by the different status of the patients, and this could further affect the conclusion on the association between antidiabetic drugs and the prognosis of COVID-19 patients with T2D.
Conclusion and Relevance
In summary, this systematic review and meta-analysis provides the most up-to-date and comprehensive evidence for a range of antidiabetic drugs in the management of COVID-19 patients with T2D. We established that usage of metformin, SGLT-2i, and GLP-1ra could significantly decrease the mortality in COVID-19 patients with T2D. The heterogeneity across the studies, baseline characteristics of the included patients, shortage of dosage and the duration of antidiabetic drugs and autonomy of drug selection might limit the objectivity and accuracy of results. Further adequately powered and high-quality randomized controlled trials are warranted to provide a more solid evidence-based basis for developing treatment guidelines.
Supplemental Material
Supplemental material, sj-docx-1-aop-10.1177_10600280221133577 for Effect of Antidiabetic Therapy on Clinical Outcomes of COVID-19 Patients With Type 2 Diabetes: A Systematic Review and Meta-Analysis by Kegang Zhan, Liuqi Weng, Li Qi, Luhan Wang, Hao Lin, Xiaoyu Fang, Hong Jia and Xiangyu Ma in Annals of Pharmacotherapy
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Outstanding Youth Science Foundation of Chongqing (grant no. cstc2020jcyj-jqX0014), Chongqing Talents: Exceptional Young Talents Project (grant no. CQYC202005003), and the Science Foundation for Outstanding Young People of the Army Medical University (grant to Pro Xiangyu Ma).
ORCID iDs: Luhan Wang  https://orcid.org/0000-0001-7917-9071
https://orcid.org/0000-0001-7917-9071
Xiangyu Ma  https://orcid.org/0000-0001-7967-3950
https://orcid.org/0000-0001-7967-3950
Supplemental Material: Supplemental material for this article is available online.
References
- 1.WHO. WHO COVID-19 dashboard 2022. https://covid19.who.int/.
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, Wang F, Shen Y, et al. Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw Open. 2021;4(9):e2127403. doi: 10.1001/jamanetworkopen.2021.27403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang X, Ming C, Cen Y, et al. Post-sequelae one year after hospital discharge among older COVID-19 patients: a multi-center prospective cohort study. J Infect. 2022;84(2):179-186. doi: 10.1016/j.jinf.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Fang X, Cheng L, et al. Development and validation of a prognostic nomogram for predicting in-hospital mortality of COVID-19: a multicenter retrospective cohort study of 4086 cases in China. Aging. 2021;13(3):3176-3189. doi: 10.18632/aging.202605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang X, Li S, Yu H, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging. 2020;12(13):12493-12503. doi: 10.18632/aging.103579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813-822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhan K, Zhang X, Wang B, et al. Short and long-term prognosis of glycemic control in COVID-19 patients with type 2 diabetes [published online ahead of print January 31, 2022]. QJM. doi: 10.1093/qjmed/hcac020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6):1068-1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhan K, Zhang X, Wang B, et al. Response to letter to editor by Dr. Pranav Ish entitled “COVID-19 and diabetes-double whammy” [published online ahead of print February 19, 2022]. QJM. doi: 10.1093/qjmed/hcac048. [DOI] [Google Scholar]
- 14.Zhan K, Zhang X, Wang B, et al. Response to letter to editor by Dr. Rohan Magoon entitled “Glycemic control and COVID-19 outcomes: the missing metabolic players” [published online ahead of print February 16, 2022]. QJM. doi: 10.1093/qjmed/hcac044. [DOI] [Google Scholar]
- 15.Nyland JE, Raja-Khan NT, Bettermann K, et al. Diabetes, drug treatment and mortality in COVID-19: a multinational retrospective cohort study. Diabetes. 2021;70(12):2903-2916. doi: 10.2337/db21-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z, Bai X, Han X, et al. The association of diabetes and the prognosis of COVID-19 patients: a retrospective study. Diabetes Res Clin Pract. 2020;169:108386. doi: 10.1016/j.diabres.2020.108386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverii GA, Monami M, Cernigliaro A, et al. Are diabetes and its medications risk factors for the development of COVID-19? Data from a population-based study in Sicily. Nutr Metab Cardiovasc Dis. 2021;31(2):396-398. doi: 10.1016/j.numecd.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pérez-Belmonte LM, Torres-Peña JD, López-Carmona MD, et al. Mortality and other adverse outcomes in patients with type 2 diabetes mellitus admitted for COVID-19 in association with glucose-lowering drugs: a nationwide cohort study. BMC Med. 2020;18(1):359. doi: 10.1186/s12916-020-01832-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bossi AC, Forloni F, Colombelli PL. Lack of efficacy of SGLT2-i in severe pneumonia related to novel coronavirus (nCoV) infection: no little help from our friends. Diabetes Ther. 2020;11(7):1605-1606. doi: 10.1007/s13300-020-00844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han T, Ma S, Sun C, et al. The association between anti-diabetic agents and clinical outcomes of COVID-19 in patients with diabetes: a systematic review and meta-analysis. Arch Med Res. 2021;53(2):186-195. doi: 10.1016/j.arcmed.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta R, Hussain A, Misra A. Diabetes and COVID-19: evidence, current status and unanswered research questions. Eur J Clin Nutr. 2020;74(6):864-870. doi: 10.1038/s41430-020-0652-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pal R, Banerjee M, Mukherjee S, Bhogal RS, Kaur A, Bhadada SK. Dipeptidyl peptidase-4 inhibitor use and mortality in COVID-19 patients with diabetes mellitus: an updated systematic review and meta-analysis. Ther Adv Endocrinol Metab. 2021;12:2042018821996482. doi: 10.1177/2042018821996482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meijer RI, Hoekstra T, van den Oever NCG, et al. Treatment with a DPP-4 inhibitor at time of hospital admission for COVID-19 is not associated with improved clinical outcomes: data from the COVID-PREDICT cohort study in The Netherlands. J Diabetes Metab Disord. 2021;20(2):1155-1160. doi: 10.1007/s40200-021-00833-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poly TN, Islam MM, Li YJ, Lin M-C, Hsu M-H, Wang Y-C. Metformin use is associated with decreased mortality in COVID-19 patients with diabetes: evidence from retrospective studies and biological mechanism. J Clin Med. 2021;10(16):3507. doi: 10.3390/jcm10163507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varghese E, Samuel SM, Liskova A, Kubatka P, Büsselberg D. Diabetes and coronavirus (SARS-CoV-2): molecular mechanism of Metformin intervention and the scientific basis of drug repurposing. PLoS Pathog. 2021;17(6):e1009634. doi: 10.1371/journal.ppat.1009634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang W, Sun X, Zhang J, Zhang K. The effect of metformin on mortality and severity in COVID-19 patients with diabetes mellitus. Diabetes Res Clin Pract. 2021;178:108977. doi: 10.1016/j.diabres.2021.108977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganesh A, Randall MD. Does metformin affect outcomes in COVID-19 patients with new or pre-existing diabetes mellitus? A systematic review and meta-analysis. Br J Clin Pharmacol. 2022;88(6):2642-2656. doi: 10.1111/bcp.15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng X, Liu YM, Li H, et al. Metformin is associated with higher incidence of acidosis, but not mortality, in individuals with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;32(4):537-547.e3. doi: 10.1016/j.cmet.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Salameh A, Bennis Y, Cariou B, Lalau J-D. The association between metformin treatment and COVID-19 outcomes according to metformin continuation during hospitalisation. Diabetes Metab. 2021;47(6):101297. doi: 10.1016/j.diabet.2021.101297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Cooper JM, Gokhale K, et al. Association of metformin with susceptibility to COVID-19 in people with type 2 diabetes. J Clin Endocrinol Metab. 2021;106(5):1255-1268. doi: 10.1210/clinem/dgab067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting: meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 32.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bramante CT, Ingraham NE, Murray TA, et al. Metformin and risk of mortality in patients hospitalised with COVID-19: a retrospective cohort analysis. Lancet Healthy Longev. 2021;2(1):e34-e41. doi: 10.1016/S2666-7568(20)30033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crouse AB, Grimes T, Li P, Might M, Ovalle F, Shalev A. Metformin use is associated with reduced mortality in a diverse population with COVID-19 and diabetes. Front Endocrinol. 2020;11:600439. doi: 10.3389/fendo.2020.600439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samuel SM, Varghese E, Busselberg D. Therapeutic potential of metformin in COVID-19: reasoning for its protective role. Trends Microbiol. 2021;29(10):894-907. doi: 10.1016/j.tim.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gianchandani R, Esfandiari NH, Ang L, et al. Managing hyperglycemia in the COVID-19 inflammatory storm. Diabetes 2020;69(10):2048-2053. doi: 10.2337/dbi20-0022. [DOI] [PubMed] [Google Scholar]
- 37.Riahi S, Lo KB, Anastasopoulou C, et al. Insulin use and poor COVID-19 outcomes among diabetes patients: association not necessarily causation. Endocr Res. 2021;46(2):53-54. doi: 10.1080/07435800.2021.1894821. [DOI] [PubMed] [Google Scholar]
- 38.Shang J, Wang Q, Zhang H, et al. The relationship between diabetes mellitus and COVID-19 prognosis: a retrospective cohort study in Wuhan, China. Am J Med. 2021;134(1):e6-e14. doi: 10.1016/j.amjmed.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dandona P, Ghanim H. Diabetes, obesity, COVID-19, insulin, and other antidiabetes drugs. Diabetes Care 2021;44(9):1929-1933. doi: 10.2337/dci21-0003. [DOI] [PubMed] [Google Scholar]
- 40.Attri B, Goyal A, Gupta Y, Tandon N. Basal-bolus insulin regimen for hospitalised patients with COVID-19 and diabetes mellitus: a practical approach. Diabetes Ther. 2020;11(9):2177-2194. doi: 10.1007/s13300-020-00873-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalra S, Mukherjee JJ, Venkataraman S, et al. Hypoglycemia: the neglected complication. Indian J Endocrinol Metab. 2013;17(5):819-834. doi: 10.4103/2230-8210.117219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan NC, Goh SY, Khoo EY, et al. Self-reported hypoglycaemia in insulin-treated patients with diabetes mellitus: results from the Singapore cohort of the International Operations Hypoglycaemia Assessment Tool study. Singapore Med J. 2020;61(3):129-136. doi: 10.11622/smedj.2019081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mendes TB, Câmara-de-Souza AB, Halpern B. Hospital management of hyperglycemia in the context of COVID-19: evidence-based clinical considerations. Diabetol Metab Syndr. 2022;14(1):37. doi: 10.1186/s13098-022-00808-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alessi J, de Oliveira GB, Schaan BD, Telo GH. Dexamethasone in the era of COVID-19: friend or foe? An essay on the effects of dexamethasone and the potential risks of its inadvertent use in patients with diabetes. Diabetol Metab Syndr. 2020;12:80. doi: 10.1186/s13098-020-00583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suh S, Park MK. Glucocorticoid-induced diabetes mellitus: an important but overlooked problem. Endocrinol Metab 2017;32(2):180-189. doi: 10.3803/EnM.2017.32.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Z, Wang Z, Wang S, et al. The impact of type 2 diabetes and its management on the prognosis of patients with severe COVID-19. J Diabetes. 2020;12(12):909-918. doi: 10.1111/1753-0407.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110-118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Das S, Rastogi A, Harikumar KVS, et al. Diagnosis and management considerations in steroid-related hyperglycemia in COVID-19: a position statement from the endocrine society of India. Indian J Endocrinol Metab. 2021;25(1):4-11. doi: 10.4103/ijem.ijem_227_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen CF, Chien CH, Yang YP, et al. Role of dipeptidyl peptidase-4 inhibitors in patients with diabetes infected with coronavirus-19. J Chin Med Assoc. 2020;83(8):710-711. doi: 10.1097/JCMA.0000000000000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dalan R. Is DPP4 inhibition a comrade or adversary in COVID-19 infection. Diabetes Res Clin Pract. 2020;164:108216. doi: 10.1016/j.diabres.2020.108216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wargny M, Potier L, Gourdy P, et al. Predictors of hospital discharge and mortality in patients with diabetes and COVID-19: updated results from the nationwide CORONADO study. Diabetologia. 2021;64(4):778-794. doi: 10.1007/s00125-020-05351-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wander PL, Lowy E, Beste LA, et al. Risk factors for adverse outcomes among 35 879 veterans with and without diabetes after diagnosis with COVID-19. BMJ Open Diabetes Res Care. 2021;9(1):e002252. doi: 10.1136/bmjdrc-2021-002252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee YS, Jun HS. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediators Inflamm. 2016;2016:3094642. doi: 10.1155/2016/3094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mirabelli M, Chiefari E, Puccio L, et al. Potential benefits and harms of novel antidiabetic drugs during COVID-19 crisis. Int J Environ Res Public Health. 2020;17(10):3664. doi: 10.3390/ijerph17103664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sachinidis A, Nikolic D, Stoian AP, et al. Cardiovascular outcomes trials with incretin-based medications: a critical review of data available on GLP-1 receptor agonists and DPP-4 inhibitors. Metabolism. 2020;111:154343. doi: 10.1016/j.metabol.2020.154343. [DOI] [PubMed] [Google Scholar]
- 56.Maranta F, Cianfanelli L, Rizzo M, Cianflone D. Filling the gap between Guidelines and Real World in the cardiovascular approach to the diabetic patients: the need for a call to action. Int J Cardiol. 2021;329:205-207. doi: 10.1016/j.ijcard.2020.12.074. [DOI] [PubMed] [Google Scholar]
- 57.Rados DV, Viecceli C, Pinto LC, et al. Metabolic effects of antihyperglycemic agents and mortality: meta-analysis of randomized controlled trials. Sci Rep. 2020;10(1):12837. doi: 10.1038/s41598-020-69738-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papadopoulos VP, Koutroulos MV, Zikoudi DG, et al. Diabetes-related acute metabolic emergencies in COVID-19 patients: a systematic review and meta-analysis. Diabetol Int. 2021;12(4):445-459. doi: 10.1007/s13340-021-00502-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koufakis T, Metallidis S, Zebekakis P, Ajjan RA, Kotsa K. Sodium-glucose cotransporter 2 inhibitors in the era of COVID-19 pandemic: is the benefit to risk ratio still favorable. J Diabetes Sci Technol. 2020;14(4):745-747. doi: 10.1177/1932296820932155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-aop-10.1177_10600280221133577 for Effect of Antidiabetic Therapy on Clinical Outcomes of COVID-19 Patients With Type 2 Diabetes: A Systematic Review and Meta-Analysis by Kegang Zhan, Liuqi Weng, Li Qi, Luhan Wang, Hao Lin, Xiaoyu Fang, Hong Jia and Xiangyu Ma in Annals of Pharmacotherapy