Abstract
After introduction of the first human 7 tesla (7T) system in 1999, 7T MR systems have been employed as one of the most advanced platforms for human MR research for more than 20 years. Currently, two 7T MR models are approved for clinical use in the U.S.A. The approval facilitated introduction of the 7T system, summing up to around 100 worldwide. The approval in Japan is much awaited. As a clinical MR scanner, the 7T MR system is drawing attention in terms of safety.
Several large-sized studies on bioeffects have been reported for vertigo, dizziness, motion disturbances, nausea, and others. Such effects might also be found in MR workers and researchers. Frequency and severity of reported bioeffects will be presented and discussed, including their variances. The high resonance frequency and shorter RF wavelength of 7T increase the concern about the safety. Homogeneous RF pulse excitation is difficult even for the brain, and a multi-channel parallel transmit (pTx) system is considered mandatory. However, pTx may create a hot spot, which makes the estimation of specific absorption rate (SAR) to be difficult. The stronger magnetic field of 7T causes a large force of displacement and heating on metallic implants or devices, and the scan of patients with them should not be conducted at 7T. However, there are some opinions that such patients might be scanned even at 7T, if certain criteria are met. This article provides a brief review on the effect of the static magnetic field on humans (MR subjects, workers, and researchers) and neurons, in addition to scan sound, SAR, and metal implants and devices. Understanding and avoiding adverse effects will contribute to the reduction in safety risks and the prevention of incidents.
Keywords: safety, 7 tesla, magnetic resonance imaging
Introduction
Currently, two 7 tesla (7T) MR scanner models have received 510k approval for clinical imaging by the Food and Drug Administration (FDA) in the U.S.A. However, they are not yet approved in Japan, and the first-level controlled operating mode is required by the International Electrotechnical Commission (IEC) standard 60601-2-33.1 At this level, an MR scanner of 8T or less is recognized as a medical device with non-significant risk, where certain imaging parameters may cause physiological stress, such as peripheral nerve stimulation or tissue heating. The static magnetic field may also exert its effects on MR workers and researchers.
The safety issues can be sorted into several categories. In addition to the increased static magnetic field, additional safety issues stem from the transmit RF power and its inhomogeneity in distribution. RF power deposition increases quadratically to magnetic field strength or resonant frequency.2 The RF power deposition monitored as the specific absorption rate (SAR) imposes a significant restriction on pulse sequences that use large flip angles, especially on spin echo and inversion recovery sequences. Nonuniform RF transmit fields cause variations in the excitation flip angle distribution, and its correction using a multi-channel parallel transmit (pTx) system may create a hot spot. Large scan sound (noise) may also matter. Basically, subjects with metal devices or implants are not scanned at 7T, but there are some opinions that such patients can be scanned at 7T, when some criteria are met. This review article is focused on the recent reports on the safety of the MR scan at 7T. For a comprehensive safety information (in Japanese), please refer to the website of JSMRM issued in 2017 (http://www.jsmrm.jp/modules/other/index.php).
Static Magnetic Field
Effects on subjects
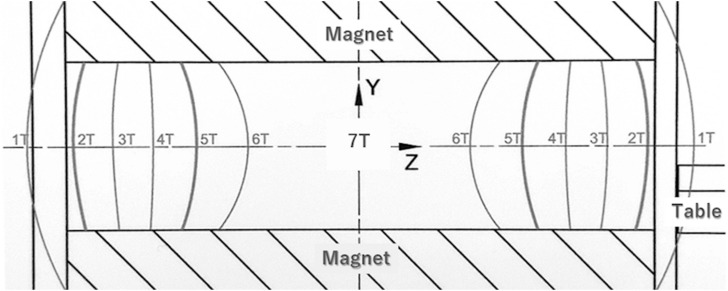
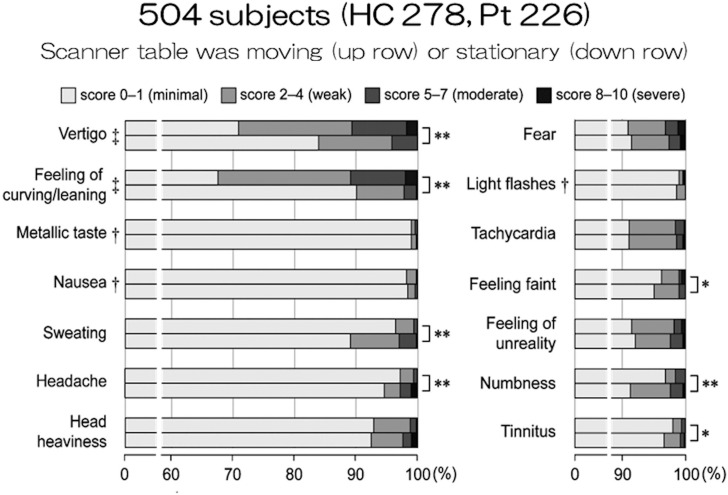
Many short-term effects have been reported in relation to exposure to time-varying or static magnetic fields, but the effects have been considered as temporary and non-serious. However, at 7T, the static magnetic field and its spatial gradient have become too large to neglect. Most of the current MR magnets are actively shielded, and the strength of the magnetic field changes rapidly at the entry of the magnet (Fig. 1). The static magnetic field changes from 1T to 6T within a distance of around 1m.3 Abrupt head motion even at the magnet entry may cause vertigo. Therefore, when the patient table is moved as fast as in 3T, patients frequently complain of vertigo. In extreme cases, nausea and vomiting might be caused. The table speed should be reduced, and it takes 1 min from the entry to the isocenter in our institution. Uwano et al. summarized sensations of 504 subjects in 7T MRI.4 Moderate-to-severe vertigo and feeling of curving/leaning were observed in more than 10% of subjects when the table was moving (Fig. 2).
Fig. 1.
Magnetic field distribution at the entry to the center of a 7T magnet. T, tesla.
Fig. 2.
Sensations in MR imaging at 7T. The results are adopted and modified from reference #4. HC, Healthy Control; Pt, Patients; T, tesla.
Transient vertigo and dizziness have been noted as effects promoted by the disturbance of the vestibular organ that becomes stronger at 3T and above.5 Possible causes for vertigo are magnetic susceptibility differences between vestibular organs and surrounding fluid6 or direct stimulation of hair cells.7 Vestibulopathy is a significant concern at 7T. Theysohn et al. assessed vestibular performance before and after exposure to different magnetic fields of 7T, 1.5T, and 0T in 46 volunteers.8 They found that exposure to the 7T static magnetic field causes only a temporary dysfunction or over-compensation of the vestibular system, which was not detected at 1.5T or 0T. RF fields, gradient switching, and orthostatic dysregulation do not seem to affect the results.
Uwano et al. also reported many biological effects other than vertigo, including headache, fear, tachycardia, and so on, but almost all of them occurred in less than 10% of the subjects.4 These symptoms were significantly mitigated when the table was stationary. When questionnaire was collected after scan in nearly 1000 subjects, dizziness of moderate to very high degree was observed in roughly 1/3, especially when going into the magnet.9 Other symptoms of the same degree included headache (up to 10%) and nausea (less than 5%).
However, some of the symptoms may not be attributable entirely to the effect of the magnetic field. Friebe et al. conducted an interesting study using a mock scanner of 0T with an actual scanner table and recorded background sound.10 They compared subjective sensation at 0T and 7T in 44 young subjects. Vertigo was apparently more frequent and severe at 7T, but unpleasantness by moving in and out of the scanners was noted even at 0T, although the severity was higher at 7T. Moreover, five subjects noticed mild magnetic field at 0T. Some factors other than magnetic field may exert certain effects on the subjective evaluations. Despite of such observations, it should be noted that MR operators should always pay attention to the patients and subjects and take every necessary action to avoid/alleviate any adverse effect.
Effects on neurons and cognitive function
No serious adverse effect has been reported on magnetic field itself, and exposure of the brain to high magnetic fields of up to 8 T does not appear to alter human cognitive performance.11 There was a significantly better performance of recognition memory in 0.05T compared with 8T, but the difference was extremely small, not clinically meaningful, and attributed to statistical artifact.
However, a recent study showed suppressed excitability of the human motor cortex using transcranial static magnetic field stimulation (tSMS).12 The intensity of the static magnetic field was 0.1–0.2T using a strong cylindrical neodymium, iron, and boron (NdFeB) permanent magnet. Application of tSMS over the supplementary motor area increased resting-state functional MRI (fMRI) activity and bilateral functional connectivity between the supplementary motor area and related brain areas.13 A higher human cognition has also been reported to be affected by tSMS, where misjudgment of pre-bisected lines was observed immediately after tSMS stimulation of 20 mins over the right superior temporal gyrus.14 At the cellular level, tSMS seems to alter the function of membrane ion channels, utilizing the diamagnetic anisotropic properties of phospholipids.15 The effect of tSMS for 10 mins at the primary motor area was found to be temporary and recovered within 20 mins.12 However, a recent study showed a long-lasting decrease in corticospinal excitability with an increased intracortical excitability caused by tSMS for 30 mins.16,17 Even a weak static magnetic field may affect the brain function, and caution should be provided. These findings on tSMS suggest some temporary changes in the brain function. Such changes can be found in detailed experiments but are not elucidated on subjects’ behavior after MR scans. We should keep such possibility in mind and pay attention to it.
Effects on operators
The International Commission on Non-Ionizing Radiation Protection (ICNIRP) defines guidelines for exposure in the controlled and uncontrolled working environments. In the latter environment, the limitations are 2000 mT for the peak static magnetic flux density and 2000 mT change over any 3s motion in the head for protection against vertigo.18,19 When exposure to static magnetic fields during activities around human MRI scanners was investigated on five researchers at a research center, the maximum exposure values were 2057 mT and 2890 mT around the 3T and 7T MR system, respectively. The dB/dt values were 4347 mT/s and 3900 mT/s.20 Occasional exceeding over the limitation was observed, but it was only for a short time and highly variable among the individuals. No correlation was found between reporting the MRI-related sensory effects and exceeding the reference values.
Despite such results, more than 10% of 7T MR workers felt vertigo (27%), feeling of instability (11%), and metallic taste (21%) in equal to or more than half of their working time, when 66 workers were examined at multiple 7T MR centers.21 It is somewhat odd that no metal taste was reported in the study conducted by Friebe.10 When the effects of stray magnetic field outside of a whole-body 7T MRI scanner combined with head motion were investigated, the performance of a visual tracking task was decreased by 1.3% per 100 mT exposure.22 In addition, there was a trend for performance reduction in eye–hand coordination tasks to be decreased. Even working within the ICNIRP guideline, some effects have been observed. Operators should pay attention so that they go near the magnet only when it is necessary and move slowly.
Effects on pregnancy
Ray et al. evaluated the risk of stillbirth or neonatal death within 28 days of birth and any congenital anomaly, neoplasm, and hearing or vision loss from birth to age 4 years.23 In their comparison between first trimester MRI (n = 1737) and no MRI (n = 1418451), no increased risk of harm to the fetus or in early childhood was found associated with MR examinations in the first trimester of pregnancy. High SAR levels may also matter, but studies failed to demonstrate substantial heating in fetal tissues or amniotic fluid when imaging was conducted at 3T within normal-operating-mode SAR levels and a maximum scan time of 30 mins.24,25 Therefore, American College of Radiology wrote in their manual on MR safety that 3T MR examinations performed within normal operating mode for durations less than 30 mins should be considered safe in pregnant patients.26 Ultimately, the decision to image a pregnant patient at 3T should be based on local institutional policies, medical needs, and accessibility to 1.5T versus 3T MR scanners. At this point, the safety of imaging pregnant patients at field strengths greater than 3T (i.e., 7T) remains unclear.
Scan Sound (noise)
One of the advantages of 7T is higher spatial resolution, but it requires larger gradient integral areas. For a fixed gradient amplitude, the gradient pulse length and the TE become longer, resulting in a lower SNR owing to the shorter T2 relaxation times at 7T. A larger gradient strength that is switched quickly makes a larger noise. Applying a large current that changes at a high frequency to the gradient coil creates a Lorentz force, and the gradient coil cylinder starts to vibrate and mechanically deform at frequencies above 100 Hz.27 Using a rampable magnet, Moelker et al. acquired acoustic data during various pulse sequences at the field strengths of 0.5, 1.0, 1.5, and 2.0T.28 They found that most of the acoustic energy was in the 1 kHz frequency band, irrespective of magnetic field strength. The relation between field strength and sound pressure level (SPL) was slightly less than linear to the field strengths.29
This relationship can be explained by a newly observed phenomenon called Lorentz damping.30 The intrinsically stronger vibrations of gradient coil conductors exposed to higher field strengths lead to greater mechanical damping compared with lower fields. In addition, there exists an acoustic barrier material behind the rigid bore wall and mechanical interfaces to block the transmission of the acoustic noise.
In spite of these mechanisms, a significantly higher frequency of general discomfort has been reported during 7T than 3T scans (P = 0.031), with the most common discomfort at 7T being noisiness (43%).31 Hansson et al. reported that approximately 10% of subjects considered scan noise to be unacceptable and scan condition to be uncomfortable.9 However, when they were asked if they are willing to have a future 7T MR scan, 5.7% and 1.5% disagreed as a study subject and a patient, respectively. These results suggest that most of the subjects will take 7T MRI scans if it is required in clinical practice.
SAR
SAR measures the rate of RF power deposition to the human body. Independent of the static magnetic field strength, IEC limits head SAR to 3.2 W/kg in a 6-min average, and the 10-s average must not exceed the double of the limit value.32 In addition, the local SAR levels averaged over 10 g of tissue also need to be restricted.33 Subject age may also matter. The 7T scan might be limited for children < 30 kg, primarily because of difference in SAR modeling from adults.34,35
The effect of SAR limitation is larger at 7T than at 3T. Many of the turbo spin echo (TSE) scans cannot be safely operated below the limit of SAR without increasing TR or decreasing the number of slices. The variable flip angle sequence can be a solution, but the current sequence is for 3T and requires optimization for 7T. Some gradient-echo sequences can be adopted to attain similar contrast, such as fluid and white matter suppression (FLAWS)36 contrast with the magnetization-prepared 2 rapid acquisition gradient echoes (MP2RAGE)37–39 readout compared with double inversion recovery (DIR)40 acquired using the TSE. DIR is frequently used to detect the lesions of multiple sclerosis41,42 and epilepsy.43,44 Also, in MRS, scans are frequently limited by SAR due to multiple RF pulses for water and outer-volume suppression. The use of stimulated echo acquisition mode (STEAM)45 sequence can reduce RF deposition and enables efficient scan with short TR values at 7T.46,47
Multi-coil transmit and SAR
The RF transmit (B1+) field inhomogeneity also raises additional SAR-related issues.48 Due to constructive interference of a single-transmit RF pulse, B1+ is highest at the central area of the brain, whereas it is lower at the periphery.49 The relative standard deviation of the B1+ field over the whole cerebrum is 11% and 22% at 3T and 7T, respectively. The relative B1+ becomes as low as 20%–30% of the intended RF power at the vicinity of the skull base,50 and some method to increase B1+ homogeneity is required. One of the solutions is to use multi-channel pTx mode.51–53 The pTx has been shown to overcome B1+ inhomogeneities that are present at high field and to reduce SAR by a factor of 2 or more.54 It enables spatial field manipulation, but at the same time, it may create hotspots by focusing the RF fields.55 This means that accurate subject-specific SAR measurement is mandatory to operate pTx appropriately.
Subject motion and SAR
It also should be noted from the viewpoint of safety that local SAR can be increased by patient head motion. Kopanoglu et al. applied RF pulses to a movable head phantom in an 8-channel transmit array coil and analyzed the effect of relative position shift/rotation.56 They compared conventional quadrature excitation and multi-spoke parallel-transmit excitation after RF shimming and found that the local SAR increased by more than 100% in 1 and 4 cm3 for quadrature and parallel-transmit excitation, respectively, in the worst case where the head model was shifted by 20 and 10 mm in right and posterior directions, respectively. Considering that patients may not keep still during an MR scan, comprehensive evaluation for safety would be required. As a user of the parallel transmit system, the counter measures would be limited, but the subject should be fixed using soft foam pads, etc., and instructed not to move for securing safety, as well as maintaining high image quality.
Use of an original pTx coil
Development of RF coils is frequently required, and this is an active field of research at 7T. Safety of RF coil is an important issue, especially when a custom-built transmit RF coil is used. Hoffmann et al. presented a comprehensive overview of the testing procedures for electrical and mechanical safety tests, SAR simulation and verification, risk analysis, and operational procedures.57 However, it should be noted that simulations need to match actual measurements as close as possible because coupling between transmit channels significantly influences peak SAR.58
Implant/device Safety
Almost all of the MR safe/conditional devices and implants are limited to their use in up to 3T. Conservative operation may exclude all subjects with metallic implants and tattoos from the 7T scan. Some clinical research sites conduct their own safety tests for cranial fixation plates59 and aneurysm clips,60 but such an evaluation requires extensive numerical RF and temperature simulations in addition to their direct measurements. The results may also be affected by the RF coil design, and formulation of a generalized approach would be required.
Attraction force can be compared to the gravity by measuring the deflection angle. Some vascular clips and implants show deflection angles larger than 45 degrees at 7T, i.e. attracted more than the gravity.61 Non-approved vascular clips and implants need to be avoided, but dental metals are usually fixed well and may not be problematic, except for orthodontics that frequently cause large signal defects. Oriso et al. examined metallic dental materials at 7T.62 Alloys of platinum, gold, and silver were not attracted, but some cobalt/nickel–chromium alloys were attracted by the MR magnet, although their deflection angles were less than 45 degrees.
Barisano reported a research subject with bilateral knee replacement implants made of cobalt–chromium–molybdenum and titanium–aluminum–vanadium alloys scanned at 7T.63 No significant effect was observed on their brain images, and no discomfort, pain, or other sensations were complained by the subject. Safety comes first, but all metallic implants may not be required to be excluded from the 7T scan.
A practical consensus
There is a practical report by Noureddine et al. on their experience in 7T imaging.64 Their decision-making process on imaging subjects with metal implants that were not approved for 7T was dependent on the distance from the RF coil. This is because there is no whole-body coil for 7T. Instead, transmit–receive coils are used for head and knee for 7T. When an implant is more than 30 cm away from the RF coil and approved for MR safe/conditional labeling at 3T, the subject can be scanned at 7T. This is because interaction between the RF field and the implant may be negligible, as long as attractive force and gradient-induced heating do not cause a safety concern. When the implant is within 30 cm but outside of the RF coil, there is potential interaction of a stray field and the implant needs to be investigated. These recommendations have been published as a consensus within the German Ultrahigh Field Imaging (GUFI) network.65 It suggests that subjects with implants may be imaged at 7T, when the implants are outside a local RF transmit coil and labeled with 3T MR safe/conditional without magnetizable components.
Conclusion
The 7T MRI system will be gradually accepted in clinical practice owing to numerous methodological and hardware developments. It can be safely used for human subjects including patients, but its operators and users should be aware of its limitations and potential adverse effects that are more frequently encountered at 7T. This review article is focused on the brain scan, but 7T application is also investigated for other body regions. It should be advised to keep the safety knowledge updated by forthcoming research for MR safety at the ultra-high field.
Acknowledgments
This review article is based on the talk on the safety of 7T MRI at Symposium 6 of JSMRM2020. Authors are grateful to Drs. Kagayaki Kuroda and Takayuki Obata for the opportunity to review the safety issues at 7T. Part of this article is supported by a grant from Siemens Healthcare K.K. and JSPS KAKENHI Grant Number 21H03806.
Footnotes
Conflicts of interest
Tomohisa Okada and Tadashi Isa received a research grant from Siemens Healthcare K.K., Japan. The other authors have no conflicts of interest related to this work.
References
- 1.International Electrotechnical Commission (IEC). Medical electrical equipment - Part 2–33: Particular requirements for the basic safety and essential performance of magnetic resonance equipment for medical diagnosis. 2015. https://webstore.iec.ch/publication/2647. (Accessed: April 20, 2021)
- 2.Hoult DI, Phil D. Sensitivity and power deposition in a high-field imaging experiment. J Magn Reson Imaging 2000; 12:46–67. [DOI] [PubMed] [Google Scholar]
- 3.Siemens Japan K.K. MR compatibility data sheet. System owner’s manual 7T. 2015: Supplement. (in Japanese)
- 4.Uwano I, Metoki T, Sendai F, et al. Assessment of sensations experienced by subjects during MR imaging examination at 7T. Magn Reson Med Sci. 2015; 14:35–41. [DOI] [PubMed] [Google Scholar]
- 5.Schenck JF, Dumoulin CL, Redington RW, et al. Human exposure to 4.0-Tesla magnetic fields in a whole-body scanner. Med Phys 1992; 19:1089–1098. [DOI] [PubMed] [Google Scholar]
- 6.Glover PM, Cavin I, Qian W, et al. Magnetic-field-induced vertigo: a theoretical and experimental investigation. Bioelectromagnetics 2007; 28:349–361. [DOI] [PubMed] [Google Scholar]
- 7.Roberts DC, Marcelli V, Gillen JS, et al. MRI magnetic field stimulates rotational sensors of the brain. Curr Biol 2011; 21:1635–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theysohn JM, Kraff O, Eilers K, et al. Vestibular effects of a 7 Tesla MRI examination compared to 1.5 T and 0 T in healthy volunteers. PLoS One 2014; 9:e92104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansson B, Markenroth Bloch K, Owman T, et al. Subjectively reported effects experienced in an actively shielded 7T MRI: a large-scale study. J Magn Reson Imaging 2020; 52:1265–1276. [DOI] [PubMed] [Google Scholar]
- 10.Friebe B, Wollrab A, Thormann M, et al. Sensory perceptions of individuals exposed to the static field of a 7T MRI: a controlled blinded study. J Magn Reson Imaging 2015; 41:1675–1681. [DOI] [PubMed] [Google Scholar]
- 11.Chakeres DW, Bornstein R, Kangarlu A. Randomized comparison of cognitive function in humans at 0 and 8 Tesla. J Magn Reson Imaging 2003; 18:342–345. [DOI] [PubMed] [Google Scholar]
- 12.Oliviero A, Mordillo-Mateos L, Arias P, et al. Transcranial static magnetic field stimulation of the human motor cortex. J Physiol 2011; 589(Pt 20):4949–4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pineda-Pardo JA, Obeso I, Guida P, et al. Static magnetic field stimulation of the supplementary motor area modulates resting-state activity and motor behavior. Commun Biol 2019; 2:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirimoto H, Watanabe T, Kubo N, et al. Influence of static magnetic field stimulation on the accuracy of tachystoscopically presented line bisection. Brain Sci 2020; 10:1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen AD. Mechanism of action of moderate-intensity static magnetic fields on biological systems. Cell Biochem Biophys 2003; 39:163–173. [DOI] [PubMed] [Google Scholar]
- 16.Dileone M, Mordillo-Mateos L, Oliviero A, et al. Long-lasting effects of transcranial static magnetic field stimulation on motor cortex excitability. Brain Stimul 2018; 11:676–688. [DOI] [PubMed] [Google Scholar]
- 17.Takamatsu Y, Koganemaru S, Watanabe T, et al. Transcranial static magnetic stimulation over the motor cortex can facilitate the contralateral cortical excitability in human. Sci Rep 2021; 11:5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. International Commission on Non-Ionizing Radiation Protection. Guidelines on limits of exposure to static magnetic fields. Health Phys 2009; 96:504–514. [DOI] [PubMed] [Google Scholar]
- 19. International Commission on Non-Ionizing Radiation Protection. Guidelines for limiting exposure to electric fields induced by movement of the human body in a static magnetic field and by time-varying magnetic fields below 1 Hz. Health Phys 2014; 106:418–425. [DOI] [PubMed] [Google Scholar]
- 20.Fatahi M, Karpowicz J, Gryz K, et al. Evaluation of exposure to (ultra) high static magnetic fields during activities around human MRI scanners. Magn Reson Mater Phy 2017; 30:255–264. [DOI] [PubMed] [Google Scholar]
- 21.Fatahi M, Demenescu LR, Speck O. Subjective perception of safety in healthy individuals working with 7 T MRI scanners: a retrospective multicenter survey. Magn Reson Mater Phy 2016; 29:379–387. [DOI] [PubMed] [Google Scholar]
- 22.de Vocht F, Stevens T, Glover P, et al. Cognitive effects of head-movements in stray fields generated by a 7 Tesla whole-body MRI magnet. Bioelectromagnetics 2007; 28:247–255. [DOI] [PubMed] [Google Scholar]
- 23.Ray JG, Vermeulen MJ, Bharatha A, et al. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA 2016; 316:952–961. [DOI] [PubMed] [Google Scholar]
- 24.Cannie MM, De Keyzer F, Van Laere S, et al. Potential heating effect in the gravid uterus by using 3-T MR imaging protocols: experimental study in miniature pigs. Radiology 2016; 279:754–761. [DOI] [PubMed] [Google Scholar]
- 25.Levine D, Zuo C, Faro CB, et al. Potential heating effect in the gravid uterus during MR HASTE imaging. J Magn Reson Imaging 2001; 13:856–861. [DOI] [PubMed] [Google Scholar]
- 26. ACR Committee on MR Safety. ACR Manual on MR Safety. American college of radiology 2020. https://www.acr.org/-/media/ACR/Files/Radiology-Safety/MR-Safety/Manual-on-MR-Safety.pdf. (Accessed: April 20, 2021)
- 27.Winkler SA, Schmitt F, Landes H, et al. Gradient and shim technologies for ultra high field MRI. Neuroimage 2018; 168:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moelker A, Wielopolski PA, Pattynama PM. Relationship between magnetic field strength and magnetic-resonance-related acoustic noise levels. MAGMA 2003; 16:52–55. [DOI] [PubMed] [Google Scholar]
- 29.Winkler SA, Wade TP, McKenzie CA, et al. Lorentz damping and the field dependence of gradient coil vibroacoustics. Proceedings of the 23th Annual Meeting of ISMRM, Toronto, 2015; 1020. [Google Scholar]
- 30.Winkler SA, Alejski A, Wade T, et al. On the accurate analysis of vibroacoustics in head insert gradient coils. Magn Reson Med 2017; 78:1635–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou IJ, Tench CR, Gowland P, et al. Subjective discomfort in children receiving 3 T MRI and experienced adults’ perspective on children’s tolerability of 7 T: a cross-sectional questionnaire survey. BMJ Open 2014; 4:e006094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allison J, Yanasak N. What MRI sequences produce the highest specific absorption rate (SAR), and is there something we should be doing to reduce the SAR during standard examinations? AJR Am J Roentgenol 2015; 205:W140. [DOI] [PubMed] [Google Scholar]
- 33.Seo Y, Wang ZJ. Measurement and evaluation of specific absorption rate and temperature elevation caused by an artificial hip joint during MRI scanning. Sci Rep 2021; 11:1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burkett BJ, Fagan AJ, Felmlee JP, et al. Clinical 7-T MRI for neuroradiology: strengths, weaknesses, and ongoing challenges. Neuroradiology 2021; 63:167–177. [DOI] [PubMed] [Google Scholar]
- 35.Malik SJ, Hand JW, Satnarine R, et al. Specific absorption rate and temperature in neonate models resulting from exposure to a 7T head coil. Magn Reson Med 2021; 86:1299–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urushibata Y, Kuribayashi H, Fujimoto K, et al. Advantages of fluid and white matter suppression (FLAWS) with MP2RAGE compared with double inversion recovery turbo spin echo (DIR-TSE) at 7T. Eur J Radiol 2019; 116:160–164. [DOI] [PubMed] [Google Scholar]
- 37.Marques JP, Kober T, Krueger G, et al. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 2010; 49:1271–1281. [DOI] [PubMed] [Google Scholar]
- 38.Okubo G, Okada T, Yamamoto A, et al. MP2RAGE for deep gray matter measurement of the brain: a comparative study with MPRAGE. J Magn Reson Imaging 2016; 43:55–62. [DOI] [PubMed] [Google Scholar]
- 39.Okubo G, Okada T, Yamamoto A, et al. Relationship between aging and T1 relaxation time in deep gray matter: a voxel-based analysis. J Magn Reson Imaging 2017; 46:724–731. [DOI] [PubMed] [Google Scholar]
- 40.Redpath TW, Smith FW. Use of a double inversion recovery pulse sequence to image selectively grey or white brain matter. Br J Radiol 1994; 67:1258–1263. [DOI] [PubMed] [Google Scholar]
- 41.de Graaf WL, Zwanenburg JJ, Visser F, et al. Lesion detection at seven Tesla in multiple sclerosis using magnetisation prepared 3D-FLAIR and 3D-DIR. Eur Radiol 2012; 22:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tallantyre EC, Morgan PS, Dixon JE, et al. 3 Tesla and 7 Tesla MRI of multiple sclerosis cortical lesions. J Magn Reson Imaging 2010; 32:971–977. [DOI] [PubMed] [Google Scholar]
- 43.Morimoto E, Kanagaki M, Okada T, et al. Anterior temporal lobe white matter abnormal signal (ATLAS) as an indicator of seizure focus laterality in temporal lobe epilepsy: comparison of double inversion recovery, FLAIR and T2W MR imaging. Eur Radiol 2013; 23:3–11. [DOI] [PubMed] [Google Scholar]
- 44.Morimoto E, Okada T, Kanagaki M, et al. Evaluation of focus laterality in temporal lobe epilepsy: a quantitative study comparing double inversion-recovery MR imaging at 3T with FDG-PET. Epilepsia 2013; 54:2174–2183. [DOI] [PubMed] [Google Scholar]
- 45.Haase A, Frahm J, Matthaei D, et al. MR imaging using stimulated echoes (STEAM). Radiology 1986; 160:787–790. [DOI] [PubMed] [Google Scholar]
- 46.Wang AM, Pradhan S, Coughlin JM, et al. Assessing brain metabolism with 7-T proton magnetic resonance spectroscopy in patients with first-episode psychosis. JAMA Psychiatry 2019; 76:314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okada T, Kuribayashi H, Kaiser LG, et al. Repeatability of proton magnetic resonance spectroscopy of the brain at 7 T: effect of scan time on semi-localized by adiabatic selective refocusing and short-echo time stimulated echo acquisition mode scans and their comparison. Quant Imaging Med Surg 2021; 11:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Buck MHS, Jezzard P, Jeong H, et al. An investigation into the minimum number of tissue groups required for 7T in-silico parallel transmit electromagnetic safety simulations in the human head. Magn Reson Med 2021; 85:1114–1122. [DOI] [PubMed] [Google Scholar]
- 49.Collins CM, Liu W, Schreiber W, et al. Central brightening due to constructive interference with, without, and despite dielectric resonance. J Magn Reson Imaging 2005; 21:192–196. [DOI] [PubMed] [Google Scholar]
- 50.Pohmann R, Speck O, Scheffler K. Signal-to-noise ratio and MR tissue parameters in human brain imaging at 3, 7, and 9.4 tesla using current receive coil arrays. Magn Reson Med 2016; 75:801–809. [DOI] [PubMed] [Google Scholar]
- 51.de Greef M, Ipek O, Raaijmakers AJ, et al. Specific absorption rate intersubject variability in 7T parallel transmit MRI of the head. Magn Reson Med 2013; 69:1476–1485. [DOI] [PubMed] [Google Scholar]
- 52.Seifert F, Wübbeler G, Junge S, et al. Patient safety concept for multichannel transmit coils. J Magn Reson Imaging 2007; 26:1315–1321. [DOI] [PubMed] [Google Scholar]
- 53.Graesslin I, Homann H, Biederer S, et al. A specific absorption rate prediction concept for parallel transmission MR. Magn Reson Med 2012; 68:1664–1674. [DOI] [PubMed] [Google Scholar]
- 54.Guérin B, Gebhardt M, Cauley S, et al. Local specific absorption rate (SAR), global SAR, transmitter power, and excitation accuracy trade-offs in low flip-angle parallel transmit pulse design. Magn Reson Med 2014; 71:1446–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Padormo F, Beqiri A, Hajnal JV, et al. Parallel transmission for ultrahigh-field imaging. NMR Biomed 2016; 29:1145–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kopanoglu E, Deniz CM, Erturk MA, et al. Specific absorption rate implications of within-scan patient head motion for ultra-high field MRI. Magn Reson Med 2020; 84:2724–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoffmann J, Henning A, Giapitzakis IA, et al. Safety testing and operational procedures for self-developed radiofrequency coils. NMR Biomed 2016; 29:1131–1144. [DOI] [PubMed] [Google Scholar]
- 58.Restivo M, Raaijmakers A, van den Berg C, et al. Improving peak local SAR prediction in parallel transmit using in situ S-matrix measurements. Magn Reson Med 2017; 77:2040–2047. [DOI] [PubMed] [Google Scholar]
- 59.Kraff O, Wrede KH, Schoemberg T, et al. MR safety assessment of potential RF heating from cranial fixation plates at 7 T. Med Phys 2013; 40:042302. [DOI] [PubMed] [Google Scholar]
- 60.Noureddine Y, Kraff O, Ladd ME, et al. In vitro and in silico assessment of RF-induced heating around intracranial aneurysm clips at 7 Tesla. Magn Reson Med 2018; 79:568–581. [DOI] [PubMed] [Google Scholar]
- 61.Dula AN, Virostko J, Shellock FG. Assessment of MRI issues at 7 T for 28 implants and other objects. AJR Am J Roentgenol 2014; 202:401–405. [DOI] [PubMed] [Google Scholar]
- 62.Oriso K, Kobayashi T, Sasaki M, et al. Impact of the static and radiofrequency magnetic fields produced by a 7T MR imager on metallic dental materials. Magn Reson Med Sci 2016; 15:26–33. [DOI] [PubMed] [Google Scholar]
- 63.Barisano G, Culo B, Shellock FG, et al. 7-Tesla MRI of the brain in a research subject with bilateral, total knee replacement implants: Case report and proposed safety guidelines. Magn Reson Imaging 2019; 57:313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noureddine Y, Bitz AK, Ladd ME, et al. Experience with magnetic resonance imaging of human subjects with passive implants and tattoos at 7 T: a retrospective study. Magn Reson Mater Phy 2015; 28:577–590. [DOI] [PubMed] [Google Scholar]
- 65. German Ultra High Field Imaging (GUFI). Approval of subjects for measurements at ultra-high-field MRI. https://mr-gufi.de/images/documents/Approval_of_subjects_for_measurements_at_UHF.pdf. (Accessed: April 20, 2021)