Abstract
In view of frequent present use of invasive procedures on limb joints, it is astonishing that articular joint inflammation is a rare event. We questioned whether antimicrobial peptides play a role in protecting human articular cartilage and synovial membrane against inflammatory agents. Our results implicate defensins in the protection of human articular joints against pathogens.
Septic arthritis has shown no change in incidence, and despite advances in antimicrobial therapy it is often responsible for residual functional impairment and for a high mortality rate among debilitated patients (1). However, in view of the frequency of joint punctures and other invasive methods presently applied in limb joints, it is astonishing that articular joint infection and inflammation are rare events. Natural peptide antibiotics have been evolutionarily conserved and show a broad range of antimicrobial activity that contributes to the innate resistance of insects, plants, and mammals to invading pathogens (5). Antimicrobial peptides of the defensin family with broad activity against gram-negative bacteria, fungi, mycobacteria, and enveloped viruses have been isolated from neutrophil granules, macrophages, and various epithelial cells (5). We questioned whether antimicrobial peptides also play a role in articular cartilage by protecting it against inflammatory agents. With a combination of PCR and immunohistochemical procedures, we have identified and localized expression of a member of the defensin family to chondrocytes in human osteoarthritic cartilage.
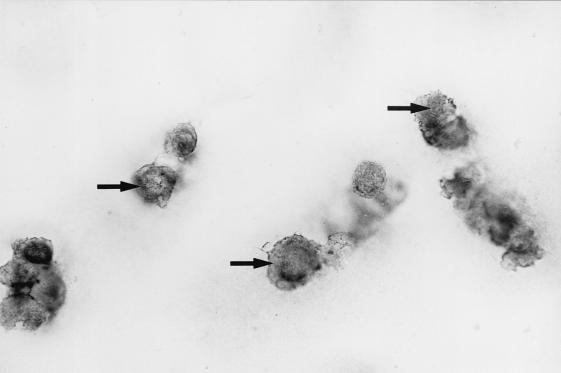
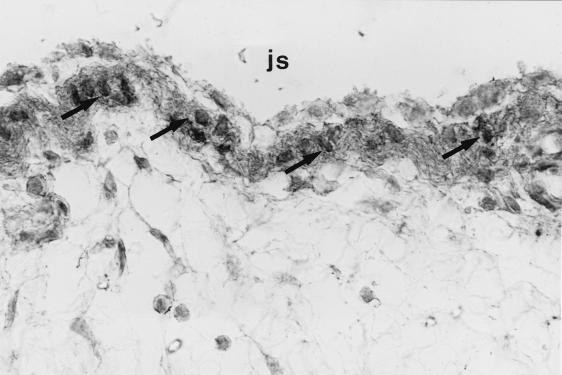
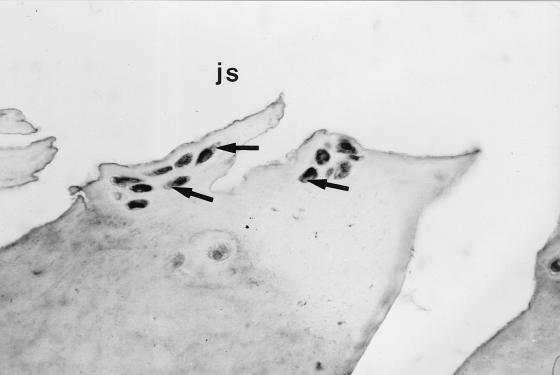
Human beta defensin 1 (HBD1) is a recently identified constitutively expressed epithelial antimicrobial peptide (9). We investigated the expression of HBD1 in human osteoarthritic cartilage as well as synovial membrane using reverse transcription-PCR and immunohistochemistry to determine whether this gene is expressed and to determine the distribution of transcripts within the articular cartilage. Reverse transcription-PCR amplified the predicted 253-bp fragment from osteoarthritic cartilage and synovia of the same patients as well as from cultured keratinocytes (as a positive control) using HBD1-specific primers (primer 1, 5′ TTGTCTGAGATGGCCTCAGGTGGTAAC; primer 2, 5′ ATACTTCAAAAGCAATTTTCCTTTAT). For immunohistochemistry we used an antibody against HBD1 (1:200 in Tris-buffered saline for 60 min; courtesy of T. Ganz, Pulmonary Research Laboratory, Los Angeles, Calif.). The specificity of the reaction was confirmed by two negative controls and one positive control with defined antigen sites (normal human skin). Immunohistochemistry of paraffin sections of paraformaldehyde-fixed osteoarthritic cartilage and synovia with a standard peroxidase-labeled streptavidin-biotin technique revealed localization of HBD1 within the chondrocytes (Fig. 1) of the superficial cartilage layers of different osteoarthritic stages as well as within type B synoviocytes (Fig. 2). Solitary chondrocytes of chondrocyte clusters in late stages of osteoarthritis showed particularly strong reactivity (Fig. 3). Not all cartilage samples revealed expression of immunoreactive defensin, and not all chondrocytes in chondrocyte clusters demonstrated reactivity with the antibody. It is not clear whether this is due to technical factors or whether it reflects site or interindividual differences in the expression level.
FIG. 1.
Immunohistochemical staining of osteoarthritic cartilage with a peroxidase-labeled antibody against HBD1. Chondrocytes in the superficial layer of the cartilage show positive reactivity (arrows).
FIG. 2.
Immunohistochemical staining of synovial membrane (the patient suffered from osteoarthritis) with a peroxidase-labeled antibody against HBD1. Type B synoviocytes reveal strong positive reactivity (arrows). js, joint space.
FIG. 3.
Immunohistochemical staining of osteoarthritic cartilage in a late stage with a peroxidase-labeled antibody against HBD1. Chondrocytes in chondrocyte clusters show strong reactivity (arrows). js, joint space.
Given the bactericidal activity of HBD1, our findings point to the existence of a previously unrecognized defense mechanism in human articular cartilage that may contribute to the remarkable innate resistance of the articular cartilage to infection. An understanding of the exact mechanism of production and regulation of defensins in the articular cartilage and synovial membrane will provide further insight into prevention of septic arthritis, which often leads to residual functional impairment. Risk factors for septic arthritis include old age, diabetes mellitus, rheumatoid arthritis, immunodeficiency, and preexisting joint disease (e.g., osteoarthritis) to which symptoms of septic arthritis are sometimes ascribed (1).
The importance of antimicrobial peptides in healing and disease processes on epithelial surfaces (8) warrants further investigation of articular cartilage and synovia. Antimicrobial peptides may therefore offer a more refined approach to the management of joint infection. Support for this hypothesis comes from the recent cloning of a second beta defensin, HBD2 (2), which is strongly upregulated by contact with the mucoid form of gram-negative Pseudomonas aeruginosa (3). Factors controlling the production of joint-associated antimicrobial peptides are unknown, and it is likely that some of the infection risk factors mentioned above are associated with downregulation of antimicrobial peptide production. It is interesting to hypothesize that purified or recombinant antimicrobial peptides may be ideal therapeutic agents in the joint in all forms of septic arthritis, as they could be applied directly to the site of infection on the joint surface. Identification of the full arsenal of antimicrobial peptides in normal human articular cartilage and elucidation of the way in which these genes are regulated may facilitate identification of new antimicrobial agents. An evaluation of antimicrobial peptide production in articular cartilage disease states that cause a predisposition to infection will provide the means to initiate a new approach in management of the conditions. Nevertheless, there is a lack of experience in the clinical use of cationic peptides, and this important aspect should be addressed in future investigations, especially in view of the fact that some reports have revealed that defensins may accelerate a key event in joint infection, i.e., by promoting cellular proliferation (6, 7) and fibrin formation (4).
REFERENCES
- 1.Dubost J J, Soubrier M, Sauvezie B. Pyogenic arthritis in adults. Joint Bone Spine. 2000;67:11–21. [PubMed] [Google Scholar]
- 2.Harder J, Bartels J, Christophers E, Schröder J M. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 3.Harder J, Meyer-Hoffert U, Teran L M, Schwichtenberg L, Bartels J, Maune S, Schröder J M. Mucoid Pseudomonas aeruginosa, TNF-alpha, and IL-1-beta, but not IL-6, induce human beta-defensin-2 in respiratory epithelia. Am J Respir Cell Mol Biol. 2000;22:714–721. doi: 10.1165/ajrcmb.22.6.4023. [DOI] [PubMed] [Google Scholar]
- 4.Higazi A A-R, Ganz T, Kariko K, Cines D B. Defensin modulates tissue-type plasminogen activator and plasminogen binding to fibrin and endothelial cells. J Biol Chem. 1996;271:17650–17655. doi: 10.1074/jbc.271.30.17650. [DOI] [PubMed] [Google Scholar]
- 5.Kagan B L, Ganz T, Lehrer R I. Defensins: a family of antimicrobial and cytotoxic peptides. Toxicology. 1994;87:131–149. doi: 10.1016/0300-483x(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 6.Kudryashov B A, Kondashevskay M V, Lyapina L A. Action of defensin on healing of aseptic skin wounds and vascular permeability. Bull Exp Biol Med. 1990;109:513–515. [Google Scholar]
- 7.Murphy C J, Foster B A, Mannis M J, Selsted M E, Reid T W. Defensins are mitogenic for epithelial cells and fibroblasts. J Cell Physiol. 1993;155:408–413. doi: 10.1002/jcp.1041550223. [DOI] [PubMed] [Google Scholar]
- 8.Schonwetter B S, Stolzenberg E D, Zasloff M A. Epithelial antibiotics induced at sites of inflammation. Science. 1995;267:1645–1648. doi: 10.1126/science.7886453. [DOI] [PubMed] [Google Scholar]
- 9.Zaho C, Wang I, Lehrer R I. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996;396:319–322. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]