Abstract
Background
Currently, different types of mazes are used to assess spatial learning and memory of rodents. The typical disadvantage is the inability to separate and exclude coincidences of the result of random choice with the correct one. The other problem is the impossibility of knowing whether the animal is guided by particular cues of the environment, or a map.
New method
Our novel transformer maze can be used to test learning and memory of rodents and their navigation. It is a multiple T-maze with passages in the interior walls. Its modular design allows to quickly change routes. The task can include external signals; for example, the colors of the interior walls, or it can be used without any cues.
Results
We compared Wistar and dopamine transporter heterozygous (DAT-HET) rats’ behavior in this novel paradigm using the black color of the wall as a cue. Entering a cul-de-sac compartment was considered an error. While Wistar rats learned the rule abruptly with the total number of errors rapidly decreasing, DAT-HET rats’ errors decreased gradually. We suppose that this reflects different strategies: insightful learning behavior is typical for Wistar rats, and trial-and-error learning is typical for DAT-HET rats.
Comparison with existing methods
The diversity of the chains of choices gives us confidence that trained animals do not make a choice randomly and are guided precisely by the cues. Moreover, we propose to use the same arena for a task with route-based navigation without any cues, and for a task with a visible and invisible feeder to study the path integration navigation within one box.
Conclusions
We suggest that the transformer maze could be a valuable tool for behavioral and pharmacological research to study learning, memory and navigation mechanisms.
Keywords: Maze, Rat, Behavior
Maze; Rat; Behavior.
1. Introduction
Mazes are commonly used to study animal behavior, such as spatial navigation and memory, and the brain mechanisms underlying such behavior. Some methods are more focused on allocentric navigation (Morris, 1984) and some on egocentric navigation (Vorhees and Williams, 2016). However, it is obvious that any navigation relies on the integration of both mechanisms. One of the most widely used methods for spatial memory testing is the Morris water maze, where the animal is trained to find a platform hidden underwater (Morris, 1981). Rats simultaneously use several strategies to solve spatial navigation tasks, and find a visible or hidden platform in a swimming pool (Whishaw and Mittleman, 1986). Another model is the Cincinnati water maze which focuses on remembering the path. The animal makes a chain of alternative choices leading to the platform. However, the maze layout is constant, which limits its use (Vorhees and Williams, 2016). One limitation of water mazes is that not all rodents solve cognitive tasks well when under stressful conditions, and being in the water, they are stressed. The Barnes maze is similar to the Morris water maze, but less stressful for animals, as it’s an overland maze. In this maze, rats tend to try to get out of open space and bright light. This maze consists of an elevated circular platform with 18 holes along its edge. Rats search for an escape hole using distal visual cues to determine the spatial location of the escape hole (Barnes, 1979). Another advantage of this labyrinth is that there is no need of food deprivation. Food reinforcement is used in other non-swimming mazes. The T-maze, the Y-maze and radial arm mazes allow measurement of decision making abilities and short-term memory (Swonger and Rech, 1972; Olton and Samuelson, 1976; Zhang et al., 2018). One of the disadvantages of the Barnes maze and radial mazes isthe possibility for animals to use non-spatial strategies, like a serial strategy. The paired-associate learning was explicitly designed to investigate the recall of foodcache locations and sequential memory of two paired associations: flavors of food and their spatial locations (Day et al., 2003; Tse et al., 2007).
We use a two-ring maze, which is a looped T-maze, to study neural mechanisms of decision-making (Filatova et al., 2015). The disadvantage of a bilateral choice is the inability to distinguish random choices from a correct one, which affects the results of the experiment. It always remains a guess what strategy the animal uses to solve the problem. The Hebb-Williams maze provides different routes between fixed start and finish points, but the set of maze layouts is not equivalent in terms of route difficulty levels (Hebb and Williams, 1946; Pritchett and Mulder, 2004).
Different navigational systems are mediated by different neural networks (O'Keefe and Nadel, 1978; Buzsáki and Moser, 2013; Sherrill et al., 2013). One of the methodical goals in investigating navigation is to model tasks which require different navigation strategies (Vorhees and Williams, 2016). In nature, all navigation systems overlap extensively. To navigate, animals simultaneously use external cues, like distal landmarks located outside, and internal determinants such feedback from limb movements, direction and turns, and also signposts.
The aim of the current study was to develop a maze which can be used to test spatial learning and memory of rodents and different types of navigation. Its modular design allows for creation of different routes, comparable in difficulty level. Moreover, the task can either include external cues or not. Thus, the maze can be used to assess both allocentric and egocentric navigation.
We decided to compare cognitive and motor performance in the new transformer maze using the dopamine dysfunction model rats. It has been shown that striatal dopamine (DA) dysfunction induces spatial information processing deficits (De Leonibus et al., 2007), and neostriatal DA modulates, in both egocentric (route-based) and allocentric (spatial, map-based) learning (Braun et al., 2012). Manipulation of the dopamine transporter (DAT) gene in animal models results in delayed clearance of DA and down-regulation of its receptors, leading to behavioral abnormalities. Genetically-modified rats and mice that lack DAT are hyperdopaminergic, and often are used as models for attention deficit hyperactivity disorder, obsessive-compulsive disorder, addiction and mania (Cinque et al., 2018).
Also, we used DAT-heterozygous (DAT-HET) and Wistar rats to compare their learning patterns and navigation in the novel task, using the color of the interior walls as a cue in the new transformer maze.
2. Material and methods
2.1. Apparatus
2.1.1. Design №1
The maze is made of opaque white plexiglass. The first design of the rat maze is a square 42 cm × 42 cm arena with 30 cm high walls. The maze consists of 9 square compartments (3 × 3) divided by interior partitions. Each compartment is a 14 × 14 cm square, formed by removable barrier walls. With the barriers’ position changed, the maze is reconfigured for each trial. Removable barriers are fixed using grooves on the inner surface of the side walls of the maze, and on the 4 columns located in the corners of the central square compartment. Two different types of interior barriers are used to build the routes of the maze. There are solid white barriers and barriers with arch-shaped openings for animal passage that are white on one side and black on the other side, so that the black color is used as a cue, and there is only one black wall with a hole in each compartment. The route is arranged in such a way, that each maze compartment is either a cul-de-sac or a correct choice. The first compartment, where the rat is placed at the beginning, has two passages, one through the black barrier and one through the white barrier. Choosing the black passage leads to the next compartment with the next choice. Choosing the white passage leads to the cul-de-sac compartment which has only one black passage for return. Figure 1 shows one possible route.
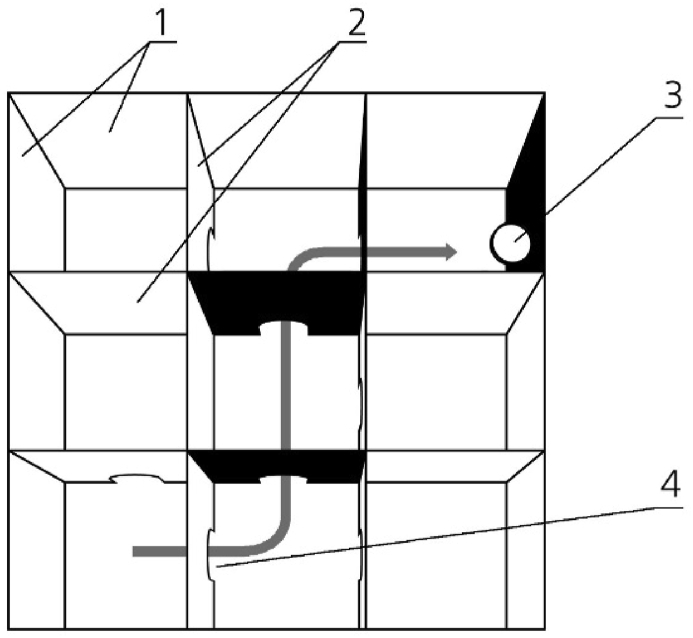
Figure 1.
Maze schematic. Example of one possible route. 1 – external walls, 2 – interior walls, 3 – feeder, 4 – arch passage. The gray arrow shows the correct path to the finish compartment with positive reinforcement. The feeder is mounted on the black side of the barrier wall 10 cm above the floor.
The modular design allows us to build 4 different routes in this maze. All these routes (Figure 2a–d) are of the same length, and consist of the same number of choice compartments (four), cul-de-sac compartments (four), and two turns per route.
Figure 2.
Four different routes. Ch1 – first choice compartment, F – finish compartment with a feeder, C – cul-de-sac compartment, Ch – choice compartment. The white circle - passage through the white barriers, the black circle - passage through the black barriers. The grey line shows the correct route to the feeder.
The position of the first choice compartment and finish compartment relative to the external environment can be changed by rearranging the partitions or rotating the maze. Figure 3 shows the options for turning one of the routes (Figure 3a1) by 90 (Figure 3a2), 180 (Figure 3a3) and 270 (Figure 3a4) degrees.
Figure 3.
Four options for one route (a1) after a 90 (a2), 180 (a3) or 270 (a4) degrees rotation. Ch1 – first choice compartment, F – finish compartment with a feeder, C – cul-de-sac compartment, Ch – choice compartment.
2.1.2. Design №2
The second sample of the rat maze is a square 56 cm × 56 cm arena separated into 16 square compartments (4 × 4) by interior partitions. Each compartment is also a 14 × 14 cm square.
White and black interior barriers are used in this maze, with the black color used as a cue. This design allows us to build many different routes of the same or different length, consisting of the same or different number of choice and cul-de-sac compartments. Moreover, some compartments may not have an entrance and may remain unused, or may have a through-passage without any choice. This design gives an opportunity to add a separate start chamber to our 3 × 3 maze (Figure 4).
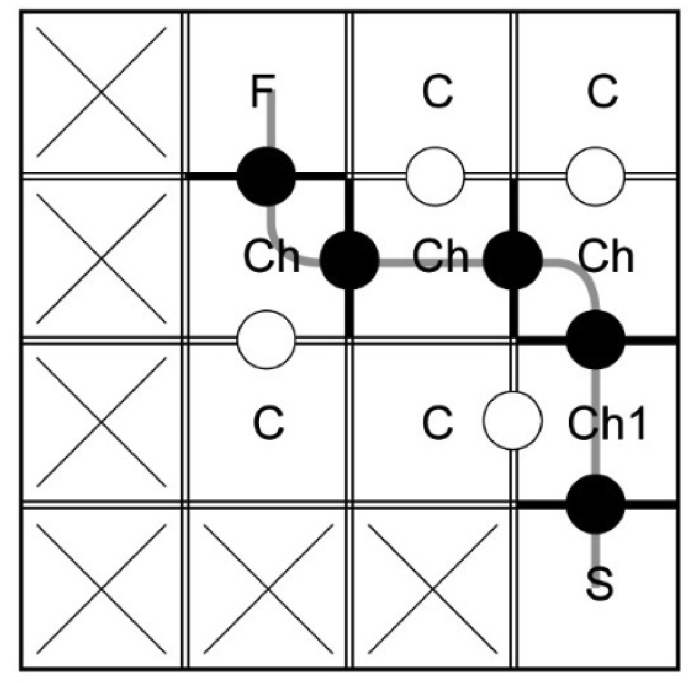
Figure 4.
An example of a 3 × 3 maze with an additional start compartment built in a 4 × 4 maze. S-start compartment, Ch1 – first choice compartment, F – finish compartment with a feeder, C – cul-de-sac compartment, Ch – choice compartment. Crosses indicate inaccessible compartments.
In this case, the start chamber has only one black barrier with an opening (passage) to the first choice compartment. Using this kind of a start chamber helps to avoid the impulsive choice, which is possible in the first choice compartment in the 3 × 3 maze.
Any new longer routes can be used for testing after learning in a 3 × 3 maze. Figure 5(a, b) shows two examples of routes with six choice compartments and three turns.
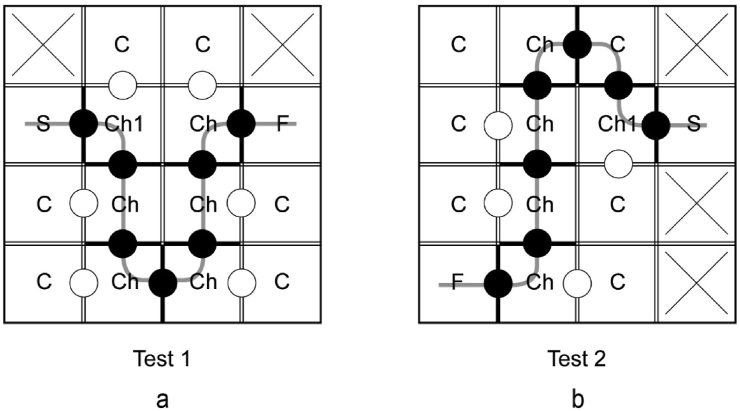
Figure 5.
Examples of the test routes in a 4 × 4 maze, a-test1, b-test2. S – start compartment, Ch1 – first choice compartment, F – finish compartment with a feeder, C – cul-de-sac compartment, Ch – choice compartment. Crosses indicate inaccessible compartments.
For illustration, the video of the route assembling and test execution in the 4 × 4 maze is represented (Supplemental file video 1).
2.2. Optional equipment
The maze is equipped with a video camera located above. Recording and tracking analysis is carried out by a blinded observer. Tracking animal coordinates at each point of time allows analysis of the trajectory, timing and errors. During subsequent analysis, the video recording was viewed by the experimenter to eliminate tracking errors; episodes of rear and head entrances were also manually noted. Returns and visits to the cul-de-sac compartments were counted as errors.
2.3. Subjects
The subjects used in this study were 10 outbred Wistar rats and 10 DAT-HET 4-month-old male rats. Wistar rats were bred in house at the Sechenov Institute of Evolutionary Physiology and Biochemistry. DAT-HET rats were obtained from the Institute of Translational Biomedicine, St. Petersburg State University, St. Petersburg, Russia. The rats, initially weighing between 200-270 g (Wistar) and 250–320 g (DAT-HET) were group housed (5 rats per cage). Animals were maintained on a 12/12 h light/dark cycle at a constant temperature of 21 ± 1 °C. Behavioral testing was carried out during the light phase.
All experimental procedures were conducted in accordance with European, national, and institutional guidelines for animal care (EU Directive, 2010/63/EU), and were performed in conformity with the Ethics Committee for Animal Research of the Sechenov Institute of Evolutionary Physiology and Biochemistry, Saint-Petersburg, Russia.
2.4. Training procedure
Before the training, all animals were food-restricted and kept at 90% of their free-feeding body weight. During the whole duration of training, the rats’ weight was measured in order to monitor and control the level of food restriction.
One peeled sunflower seed per trial was used as a food reward.
At the beginning of training, an animal is placed in one compartment with solid walls without any exits, with a feeder attached to the black wall by double sided tape. The rat is taken out after emptying the feeder. When food reinforcement is replaced, the procedure is repeated. Usually less than 10 trials are needed to habituate rats to the feeder and type of reinforcement.
During the next experimental session, an animal is placed in the start compartment of the maze and the reinforcement is placed in the feeder in the finish compartment. After consumption of reinforcement, the animal is removed and placed into an individual cell for 2–5 min to wait for the next trial. If the rat does not move and does not leave one compartment within 10 min, it is removed and the trial is considered a failure. The test is also considered not complete if the rat moves in the maze for 10 min but does not consume food reinforcement. Then the route or position of the route is changed, and a rat is placed again in the start compartment. During subsequent experimental sessions, the rat is offered other maze configurations, trials are carried out after maze rotations. Thus, different tests are carried out every day, using different routes in different positions. Rotating the maze allows exclusion of distal environmental cues. The final goal is to get each rat acquainted with all 4 routes in 4 different positions. A total of 16 maze variants were used during training (all routes a, b, c, d (Figure 2) with a start compartment (Figure 4) and with 4 rotation options). Figure 6 shows the protocol of successive trials during the 5 learning sessions (Figure 6(a–e)) and one testing session (Figure 6f). Trials are presented so that during one experimental session there are at least two different routes from one starting position. After the learning sessions in all modifications of the 3 × 3 maze, rats undergo two final tests with unfamiliar routes in the 4 × 4 maze (test 1 and test 2). These routes are longer than the ones used for training, and include more turns (Figure 5a, b). Each rat is given one attempt at 2 test routes.
Figure 6.
The protocol of successive trials, (a–e)-training sessions, f-final tests session. S-start compartment, Ch1-first choice compartment, F-finish compartment with a feeder, C-cul-de-sac compartment, Ch-choice compartment. Crosses indicate inaccessible compartments.
Between trials, the floor and walls are cleaned by 70% ethanol to minimize the possibility of any marks being used as reference points. It should be noted, that it is also possible to obtain the same route placement options in space without turning the maze by changing the start and finish compartments' placement and replacing barrier walls. It takes more time, but in this case, you can be sure that the rat did not use any external cues except the walls’ color.
2.5. Analysis
For the analysis, a custom-written “seeker” program (Shevelev_pro) was used. This software analyzes movement tracks and calculates the number of visits to the cul-de sac and choice compartments, the number of returns, time spent in each compartment, total distance traveled and time to reach the feeder. It is important to note, that visits and peeps into the compartments are distinguished. The rat is considered to have entered the next compartment, if two of the rat's limbs cross the border. Just the rat's head crossing the border is considered as peeking. Error-free execution does not involve returns and visits to the cul-de-sac compartments.
The track can be drawn manually while watching the video, or automatically if animal recognition is used. Behavioral acts such as standing upright, peeking, grooming and defecation are also manually marked. If the rat has not reached the feeder in 10 min, the test is considered a failure and excluded from analysis.
2.6. Statistical analysis
Analyses were performed using Statistica software, version 8.0, StatSoft, ink, Tulsa, USA. A Shapiro-Wilk test was used to assess the normality of data distribution. Because of the non-normal distribution of some parameters, the significant differences in parameters between groups of the Wistar and DAT-HET rats were identified using the nonparametric Mann-Whitney U test. To compare repeated measures of learning parameters in the same animals, nonparametric Wilcoxon Matched Pairs Test was used. The chi-square statistical procedure for a 2 × 2 research design was used to test for differences in the number of errors and correct executions in different groups. Differences were considered statistically significant at p ≤ 0.05. Data is presented as mean ± standard error of the mean (SEM).
3. Results
5 training sessions and one test session were performed with Wistar and DAT-HET rats. The first training session consisted of 4 trials, the rest of the training sessions had three trials. The test session included two trials. Each time, the mazes were new or in a new position. Outcomes of each trial were combined for each animal. Examples of one rat's tracks in different mazes during the training are shown in Figure 7.
Figure 7.
The superposition of the rat tracks and maze schemes. Samples of the same rat in different routes at the beginning (a), middle (b) and the end of training (c). S – start compartment, F – finish compartment. Crosses indicate inaccessible compartments.
Out of a total of 180 trials of 10 Wistar rats, only two rats did not complete the task within 13 trials; in the DAT-HET group, only 5 trials were not completed by three rats. So, out of the total number of 360 trials, only 5% were not finished, which did not affect final learning outcomes, since there were no such cases in the final tests, during which all rats reached the finish and got food reinforcement. Analysis of the number of the error-free executions, when the animal did not visit cul-de-sac compartments and there were no returns, was carried out for each test session. Figure 8 shows the percentage of error-free executions. Significant differences were observed for the groups in the last training session (t5) (p = 0.0003, Chi-square test) and the final test session (t6) (p = 0.0003, Chi-square test).
Figure 8.
Error-free task execution. Ordinate - percentage of the error-free executions to a total number of the trials. Abscissa – experimental sessions (training sessions – t1–t5, final tests – t6). Statistical significance ∗∗∗ – p < 0,0001 (Chi-square test).
Wistar rats have abruptly improved their accuracy levels, in contrast to DAT-HET rats, only about 10% of which trials performed the task accurately both during the training and during the final testing. More than 60% trials of Wistar rats had no errors during the final test. If we reduce the criteria so that one error (one visit to a cul-de-sac or one return) per trial is allowed, differences between the groups become significant only in the last training session (Figure 9) (p = 0.019, Chi-square test). The percentage of error-free and error-one tests for Wistar rats reached 85% and 70% for DAT-HET rats.
Figure 9.
The task executed without mistakes or with one mistake. Ordinate – trials with ≤1 error, % to the total number of the trials. Abscissa – experimental sessions (training sessions – t1–t5, final tests – t6). Statistical significance ∗ – p < 0.05 (Chi-square test).
The main characteristic of the learning curve is the change in the total number of errors. Figure 10 shows the average number of visits to cul-de-sac compartments for each experimental session for rats of both groups.
Figure 10.
Number of visits to the cul-de-sac compartments. Ordinate – number of errors (mean ± SEM). Abscissa – experimental sessions (training sessions – t1–t5, final tests – t6). Statistical significance ∗∗ – p < 0.001 (differences between groups – Mann-Whitney U test and between different sessions – Wilcoxon test).
Wistar rats demonstrated a significant decrease in the number of errors during the 5th learning session, in contrast to DAT-HET rats (t4–t5, p = 0.008, Wilcoxon test). At the same time, there were significant differences between strains in their performance during the last training session and the final test (p = 0.002 and p = 0.002, Mann-Whitney U test).
The average velocity of rats’ movement was analyzed (Figure 11). A significant increase in velocity was detected during the learning phase, and it happened earlier in the Wistar rats (t3–t4, p = 0.0002, t4–t5, p = 0.013, Wilcoxon test) and later in the DAT-HET rats (t4–t5, p = 0,004, Wilcoxon test). There was a trend towards higher velocity in Wistar rats in comparison with DAT-HET rats in the t5 (p = 0.057, Mann-Whitney U test).
Figure 11.
Velocity analysis results. Ordinate – average velocity (mm/s) (mean ± SEM). Abscissa - experimental sessions (training sessions – t1–t5, final tests – t6). Statistical significance ∗ – p < 0.05; ∗∗ – p < 0.001; ∗∗∗ – p < 0,0001 (differences between different sessions -Wilcoxon test).
There are 4 types of compartments in the maze: start, finish, cul-de sac and choice compartments. An animal has to decide which passage to choose in the choice compartments. Analysis of the time spent in the choice compartments showed a sharp decrease of the decision-making time starting with the t4 learning session in Wistar rats (t3–t4, p = 0.001, Wilcoxon test) in contrast to the DAT-HET rats (t4, p = 0.014 and t5, p = 0.0005, Mann-Whitney U test) (Figure 12). Only successful trials were included in this analysis. In the final test session, the decision-making time did not differ between the groups.
Figure 12.
Decision-making time (s). Ordinate – average time, seconds (mean ± SEM). Abscissa – experimental sessions (training sessions – t1–t5, final tests – t6). Statistical significance ∗– p < 0.05; ∗∗ – p < 0.001; ∗∗∗ – p < 0,0001 (differences between groups -Mann-Whitney U test and between different sessions -Wilcoxon test).
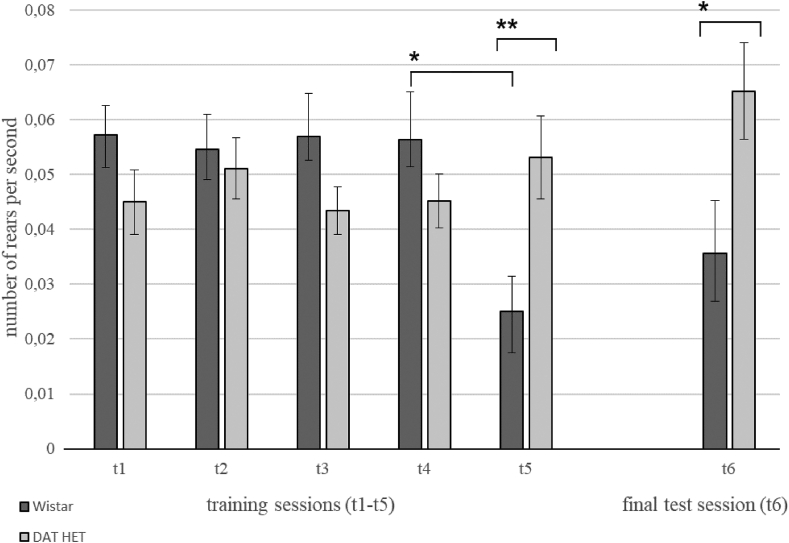
Analysis of the number of rears (Figure 13) showed its decrease by the end of the training in Wistar rats (t4–t5, p = 0.024, Wilcoxon test), in contrast to the DAT-HET rats, where significant differences between the groups were observed on day 5 of training and during final test session (t5, p = 0.005 and t6, p = 0.036, Mann-Whitney U test).
Figure 13.
Rears. Ordinate – average number of rears per second (mean ± SEM). Abscissa – experimental session (training sessions – t1–t5, final test day – t6). Statistical significance ∗ – p < 0.05; ∗∗ – p < 0.001 (differences between groups -Mann-Whitney U test and between different sessions -Wilcoxon test).
The number of head entrances did not differ between groups during training sessions, however, Wistar rats peeked significantly more than DAT-HET rats during the final tests (t6, p = 0.0044, Mann-Whitney U test) (Figure 14).
Figure 14.
Head entrances. Ordinate – average number of head entrances per second (mean ± SEM). Abscissa – experimental sessions: training sessions – t1–t5, test day – t6. Statistical significance ∗∗ – p < 0.001 (differences between groups – Mann-Whitney U).
4. Discussion
4.1. Training procedure and its advantages
In this paper, we present a new technique as an alternative to the currently available mazes. The transformer maze is a compact, land-based and multifunctional device. It allows us to quickly change the route and orientation of the maze, to use different types of cues, or avoid them. When we use our cue (black side of the barrier with the arch) and present non-recurring routes, we make sure that the cue serves as the only reference point. Protocol presented in this paper provides fast and reliable learning. Animals can receive different tasks under identical conditions. In the current experiment, training consisted of 5 learning sessions and one test session. On average, total time spent by one animal in the maze during the whole experiment was about 40 min. Taking into account the time it takes to rebuild routes and cleaning, the needed experimental total time spent can be calculated.
Analysis of the time spent in the maze and total distance traveled allows us to assess memory and the level of general locomotor activity. Analysis of rears, head entrances, grooming, and defecation can indicate the level of anxiety and exploratory behavior (Seibenhener and Wooten, 2015). Time spent in cul-de-sac compartments and choice compartments may indirectly indicate different search strategies.
4.2. Comparison Wistar and DAT-HET rats learning patterns
Comparison between Wistar and DAT-HET rats did not reveal differences at the initial stages of learning in the 3 × 3 maze, but showed significant differences at the late training stage and during final tests. DAT-HET rats’ inferior performance in new conditions may be associated with worse memory or lower cognitive flexibility. The final test does not just involve solving the same problem while taking a new route within the same space, but forces the animal to make more steps to make the choices. If there were 4 steps in the training mazes, in the test trials there were 6 steps. The essential characteristic of short routes in the 3 × 3 maze is that they always pass through the central compartment of the maze, and this passage is always straight and never includes a turn. Test routes in the 4 × 4 maze include longer sections with no turns. Reactions to the met with these new route features also may indicate flexibility.
Spatial navigation requires egocentric and allocentric strategies, meaning that the animal is guided simultaneously by the cues and features of the routes. These mechanisms are mediated by various neurochemical pathways (Rubio et al., 2012; Gutierrez et al., 2019). Disturbances of DA metabolism in DAT-HET rats may lead to impaired functioning and integration of these different systems. Previously it has been shown, that methamphetamine impairs both egocentric and allocentric learning and memory (Gutierrez et al., 2019).
A fundamental difference was found between Wistar and DAT-HET rats in the learning patterns and transformer maze test performances. While Wistar rats learned the rule quickly with the total number of errors rapidly decreasing, DAT-HET rats’ errors decreased gradually.
In the final tests, 65% of Wistar rats trials were error-free, with rats perfectly following visual cues. At the same time, only in Wistar rats was there an increase in the number of peeks in the final test. Apparently, they checked their options without fully going into the cul-de-sac compartments.
Increased velocity during the 4th training session and decreased time of decision making in the choice compartments precedes a sharp decrease in the number of errors observed in the 5th training session in Wistar rats. Also, during the 5th training session, Wistar rats showed a decrease in the number of rears, which may indirectly indicate a change in the strategy of their choice. Perhaps it is connected with the switch to the cue-based orientation. The absence of this shift in DAT-HET rats may indicate different learning mechanisms in these animals.
There are studies that were aimed at separating allocentric and egocentric navigation strategies. For example, a four-arm plus-shaped maze was used for this (Ramos, 2017). To determine a predominant strategy, there 10 consecutive trials were carried out, in the probe test, and there was used a mean percentage of correct responses obtained by an allocentric or egocentric strategy. In this maze, it is difficult to distinguish between a random and a conscious choice. In the present transformer maze, the animal has got to make a sequence of choices, so the possibility of a random choice is minimized. When we observe animals making error-free successive choices without any mistakes, we can be sure that they have used the cue.
Two forms of learning have been described: trial and error strategy and “insightful learning”, which manifests as a sudden change in behavior (Wolfensteller and Ruge, 2012; Neves Filho et al., 2015). In Wistar rats, the observed learning behavior is closer to insightful learning, leading to successful cue-based performance. They showed sudden changes of behavior parameters while learning, and error-free trials in the final tests can be taken as a criterion of the animal using cues. In contrast to that, DAT-HET rats continue to use trial and error strategy, and demonstrate gradually changing behavior parameters.
There are various models describing the role of DA in learning and memory. The prediction error model suggests that dopamine is important for matching expectation and reward, while the stimulus model suggests that dopamine promotes motivated behavior (Saddoris et al., 2015). DA also plays a role in novelty and the significance of stimuli (Kutlu et al., 2021).
Any of these roles of DA may be considered as a cause of DAT-HAT rats’ inferior performance and an absence of insightful learning.
Overall, the new transformer maze makes it possible to discover the differences in learning and performance patterns of rats.
4.3. Comparison of the transformer maze and its additional variations with other mazes
The transformer maze has several advantages in comparison with other mazes. Being a multiple T-maze, like the Cincinnati water maze, it can be quickly modified. Using visual cues like The Morris water maze, it does not require a large volume of warm water, which makes it possible to precisely evaluate the use of cues by animals. In addition, during the training, there is no need to stress the animals by making them swim, although food deprivation and weight monitoring are required. Rats explore the space and find a feeder without human intervention. Thus, the behavior execution is under less stressful conditions as compared to with water mazes.
The layout of the maze can be changed to contain any number of compartments, and it can be used to assess egocentric route-based navigation by placing same-colored barriers and setting up one route. In order to change the location of the route relative to the external environment, it can be turned at any angle. The principle of choosing between cul-de-sac and next choice compartments on the way to the feeder is preserved. Figure 15 shows an example of one route built this way.
Figure 15.
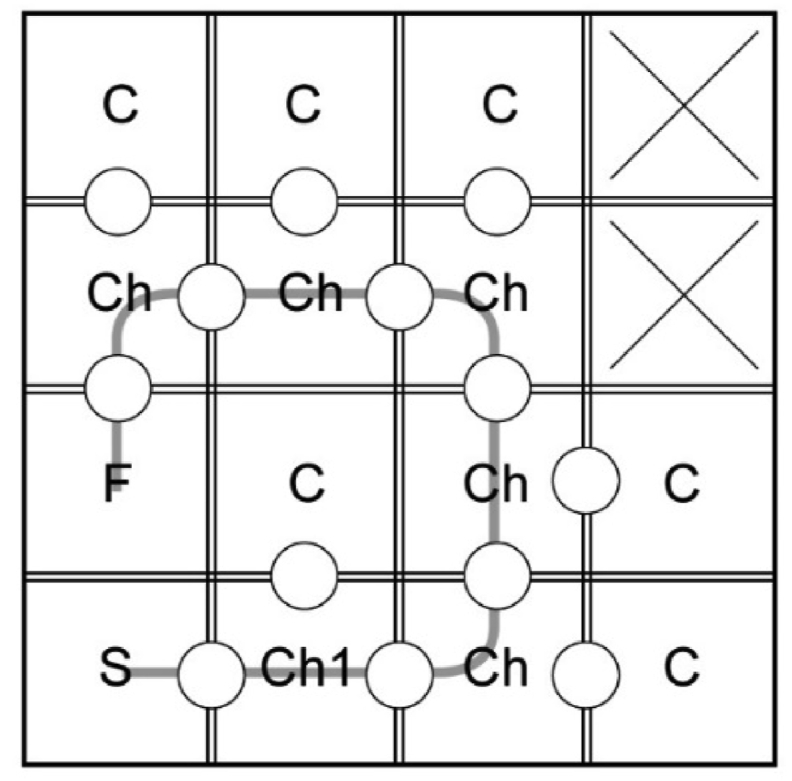
An example of the route without any cues built in a 4 × 4 maze that allows us to assess egocentric navigation. F – finish compartment with a feeder, C – cul-de-sac compartments, Ch – choice compartment; crosses indicate inaccessible compartments.
In this maze, there are no proximal or distal landmarks that could help the animal to navigate. The rat has got to remember that it needs to go straight-straight-left-straight-left-straight-left. We only started to use this configuration in our experiments, but now we already have native recording of the tracks at the start of learning and at the finish. Figure 16 shows the samples of these tracks for one rat. It is obvious, that after a series of training trials when the animal wanders through the maze, the trained animal follows the route and uses_ route-based navigation without any landmarks.
Figure 16.
The superposition of the rat tracks in the route-based navigation task in the maze configuration without any cues. Samples of the same rat in different turns of the route at the beginning (var1), middle (var2, var3, var4) and the end of training (var1). Var.2, 3, 4 are route turns of 90, 180 and 270°. S – start compartment, F – finish compartment. Crosses indicate inaccessible compartments.
We also suggest using the same arena to study path integration navigation (Figure 17). To do this, the environment above the maze changes. Landmarks are added above the maze and learning find-the–feeder sessions occur in the empty arena without any barriers (similar to searching for the visible platform in the Morris water maze) (Figure 17a). Then the animal's ability to navigate is tested by placing white barriers with holes, so that the animal does not see the feeder, but still sees distal landmarks (similar to searching for the invisible platform in the Morris water maze) (Figure 17b). You can also change the starting point (Figure 17c). Figure 17 shows the samples of these tracks for one rat. It can be seen that the trained animal uses the vector, finding the shortest path.
Figure 17.
The superposition of the rat tracks in the path integration navigation task in the maze configuration with cues above the maze, “a” – arena without any barriers, except start and finish compartment barriers, “b” and “c” – arena with white barriers with holes, without any cul-de-sac compartments with different place of the start point. S – start compartment, F – finish compartment. Crosses indicate inaccessible compartments.
We supposed, that when animals in the transformer maze choose the black passages, it can be estimated that they are moving by signposts. In this case, the animal must refuse to remember the routes or form a map, because it only interfere with the work. This allows us to study mechanisms of navigation without using spatial memory. The Barnes maze or radial arm mazes (Barnes, 1979; Olton and Samuelson, 1976) allow us to study different aspects of spatial navigation; however, they can't provide a task where the animal, on its way to the goal point, should use only external cues, without any location-related skills.
Researchers use different mazes in one experiment to evaluate different cognitive aspects. But different motivations, different ways of moving, and different environments influence the result. (Lewejohann et al., 2004). Comparing the behavior in three different tasks — moving by signposts, remembering the route, and the task using the arena with a visible and invisible feeder within one box—could be a precise instrument to study the brain processes.
5. Limitations
However, the design of this maze leads to certain limitations in research. The animal has to go through holes, so it's impossible to use any equipment connecting its skull to an external device, such as dialysis tubing or electrophysiological wires. To record brain processes, you can use only wireless methods. In addition, it is necessary to consider the animals' physiological ability to recognize the cues, so only relevant signals should be used.
6. Conclusion
We show that the new transformer maze can serve as a tool to evaluate the learning and decision-making patterns of rats. The comparison of Wistar and DAT-HET rats performance in this novel task revealed insightful learning typical for Wistar rats and trial and error-based learning in DAT-HET rats.
Declarations
Author contribution statement
Elena Filatova: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Sechenov Institute of Evolutionary Physiology and Biochemistry of the Russian Academy of Sciences, the State assignment No. 075-0152-22-00.
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Acknowledgements
I express my gratitude to the Institute of Translational Biomedicine, St. Petersburg State University, St. Petersburg, Russia that provided DAT-HET rats. I would like to express my appreciation to Shevelev Artem for excellent software development, patience and invaluable assistance. I am grateful to Galina Gromova for her help and support in the work. I am also immensely grateful to Dr. Maria Dorofeikova for the comments and editorial work on this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2022.e11211.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Sample of the 4 × 4 maze assembling and test execution.
References
- Barnes C.A. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979;93(1):74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Braun A., Graham D.L., Schaefer T.L., Vorhees C.V., Williams M.T. Dorsal striatal dopamine depletion impairs both allocentric and egocentric navigation in rats. Neurobiol. Learn. Mem. 2012;97(4):402–408. doi: 10.1016/j.nlm.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G., Moser E.I. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 2013;16(2):130–138. doi: 10.1038/nn.3304. Epub 2013 Jan 28. PMID: 23354386; PMCID: PMC4079500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinque S., Zoratto F., Poleggi A., Leo D., Cerniglia L., Cimino S., Tambelli R., Alleva E., Gainetdinov R.R., Laviola G., Adriani W. Behavioral phenotyping of dopamine transporter knockout rats: compulsive traits, motor stereotypies, and Anhedonia. Front. Psychiatr. 2018:9–43. doi: 10.3389/fpsyt.2018.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M., Langston R., Morris R.G. Glutamate-receptor-mediated encoding and retrieval of paired-associate learning. Nature. 2003;424(6945):205–209. doi: 10.1038/nature01769. PMID: 12853960. [DOI] [PubMed] [Google Scholar]
- Filatova E.V., Orlov A.A., Afanas'ev S.V. A two-ring maze for studies of the behavior of laboratory animals. Neurosci. Behav. Physiol. 2015;45(7):765–770. [Google Scholar]
- Gutierrez A., Regan S.L., Hoover C.S., Williams M.T., Vorhees C.V. Effects of intrastriatal dopamine D1 or D2 antagonists on methamphetamine-induced egocentric and allocentric learning and memory deficits in Sprague-Dawley rats. Psychopharmacology. 2019;236(7):2243–2258. doi: 10.1007/s00213-019-05221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb D.O., Williams K.A. A method of rating animal intelligence. J. Gen. Psychol. 1946;34:59–65. doi: 10.1080/00221309.1946.10544520. [DOI] [PubMed] [Google Scholar]
- Kutlu M.G., Zachry J.E., Melugin P.R., Cajigas S.A., Chevee M.F., Kelly S.J., Kutlu B., Tian L., Siciliano C.A., Calipari E.S. Dopamine release in the nucleus accumbens core signals perceived saliency. Curr. Biol. 2021;31(21):4748–4761. doi: 10.1016/j.cub.2021.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leonibus E., Pascucci T., Lopez S., Oliverio A., Amalric M., Mele A. Spatial deficits in a mouse model of Parkinson disease. Psychopharmacology. 2007;194(4):517–525. doi: 10.1007/s00213-007-0862-4. [DOI] [PubMed] [Google Scholar]
- Lewejohann L., Skryabin B.V., Sachser N., Prehn C., Heiduschka P., Thanos S., Jordan U., Dell'Omo G., Vyssotski A.L., Pleskacheva M.G., Lipp H.P., Tiedge H., Brosius J., Prior H. Role of a neuronal small non-messenger RNA: behavioural alterations in BC1 RNA-deleted mice. Behav. Brain Res. 2004;154(1):273–289. doi: 10.1016/j.bbr.2004.02.015. PMID: 15302134. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris R.G.M. Spatial localization does not require the presence of local cues. Learn. Motiv. 1981;12(2):239–260. [Google Scholar]
- Neves Filho H.B., Dos Reis Stella L., Harder Ferro Dicezare R., Garcia-Mijares M. Insight in the white rat: spontaneous interconnection of two repertoires in Rattus norvegicus. Eur. J. Behav. Anal. 2015;16(2):8. [Google Scholar]
- O’Keefe J., Nadel L. Clarendon Press; Oxford, UK: 1978. The Hippocampus as a Cognitive Map. [Google Scholar]
- Olton D.S., Samuelson R.J. Remembrance of places passed: spatial memory in rats. J. Exp. Psychol. Anim. Behav. Process. 1976;2(2):97–116. [Google Scholar]
- Pritchett K., Mulder G. Hebb-Williams mazes. Contemp. Top. Lab. Anim. Sci. 2004;43(5):44–45. [PubMed] [Google Scholar]
- Ramos J.M.J. Perirhinal cortex involvement in allocentric spatial learning in the rat: evidence from doubly marked tasks. Hippocampus. 2017;27(5):507–517. doi: 10.1002/hipo.22707. [DOI] [PubMed] [Google Scholar]
- Rubio S., Begega A., Méndez M., Méndez-López M., Arias J.L. Similarities and differences between the brain networks underlying allocentric and egocentric spatial learning in rat revealed by cytochrome oxidase histochemistry. Neuroscience. 2012;223:174–182. doi: 10.1016/j.neuroscience.2012.07.066. [DOI] [PubMed] [Google Scholar]
- Saddoris M.P., Cacciapaglia F., Wightman R.M., Carelli R.M. Differential dopamine release dynamics in the nucleus accumbens core and shell reveal complementary signals for error prediction and incentive motivation. J. Neurosci. 2015;35(33):11572–11582. doi: 10.1523/JNEUROSCI.2344-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibenhener M.L., Wooten M.C. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. JoVE. 2015;96 doi: 10.3791/52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill K.R., Erdem U.M., Ross R.S., Brown T.I., Hasselmo M.E., Stern C.E. Hippocampus and retrosplenial cortex combine path integration signals for successful navigation. J. Neurosci. 2013;33(49):19304–19313. doi: 10.1523/JNEUROSCI.1825-13.2013. PMID: 24305826; PMCID: PMC3850045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swonger A.K., Rech R.H. Serotonergic and cholinergic involvement in habituation of activity and spontaneous alternation of rats in a Y maze. J. Comp. Physiol. Psychol. 1972;81(3):509–522. doi: 10.1037/h0033690. PMID: 4265289. [DOI] [PubMed] [Google Scholar]
- Tse D., Langston R.F., Kakeyama M., Bethus I., Spooner P.A., Wood E.R., Witter M.P., Morris R.G. Schemas and memory consolidation. Science. 2007;316(5821):76–82. doi: 10.1126/science.1135935. PMID: 17412951. [DOI] [PubMed] [Google Scholar]
- Vorhees C.V., Williams M.T. Cincinnati water maze: a review of the development, methods, and evidence as a test of egocentric learning and memory. Neurotoxicol. Teratol. 2016;57:1–19. doi: 10.1016/j.ntt.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw I.Q., Mittleman G. Visits to starts, routes, and places by rats (Rattus norvegicus) in swimming pool navigation tasks. J. Comp. Psychol. 1986;100(4):422–431. [PubMed] [Google Scholar]
- Wolfensteller U., Ruge H. Frontostriatal mechanisms in instruction-based learning as a hallmark of flexible goal-directed behavior. Front. Psychol. 2012;3:192. doi: 10.3389/fpsyg.2012.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Kobayashi Y., Goto H., Itohara S. An automated T-maze based Apparatus and protocol for analyzing delay- and effort-based decision making in free. Moving rodents. JoVE. 2018;138 doi: 10.3791/57895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sample of the 4 × 4 maze assembling and test execution.
Data Availability Statement
Data will be made available on request.