Abstract
Purpose
To analyze clinical safety and efficacy of flow re-direction endoluminal device (FRED) Jr flow diverter for treatment of unruptured, ruptured, or recanalyzed aneurysms.
Materials and methods
Between October 2019 and February 2022, 25 patients with 31 aneurysms treated with FRED Jr were included in the study. Clinical and radiological records, procedural details, and follow-up outcomes were retrospectively evaluated. Eighteen (72%) patients were female. Median age was 48.8 (age range 9–85). Mean follow-up was 21 months (6–28 months). Location of the aneurysms were as follows; 13 in middle cerebral artery (MCA), 7 in anterior cerebral artery (ACA), 4 in posterior cerebral artery (PCA), 3 in true posterior communicating artery (PCom), 2 in anterior communicating artery (ACom), 1 in superior cerebellar artery (SCA), 1 in true ophthalmic artery. Five patients (20%) presented with acute subarachnoid hemorrhage (aSAH).
Results
In all procedures, FRED Jr was successfully deployed. Three true Pcom aneurysms and a true ophthalmic aneurysm were treated with FRED Jr. Three patients with two adjacent aneurysms were treated with a single FRED Jr. In two (8%) patients in-stent thrombosis occurred intraoperatively, they were treated with iv tirofiban and thrombectomy without any sequelae. Post-discharge 2 weeks later, intraparenchymal hemorrhage occurred in a patient. He was treated with surgical drainage, the clinical course was modified Rankin score (mRS) 2. Digital subtraction angiography (DSA) was performed on 16 (64%) patients with 21 (67%) aneurysms. Near complete–complete occlusion (O’Kelly–Morata grading scale (OKM C-D) was documented in 15/16 (93.7%) patients, 20/21 (95.2%) aneurysms. In nine (36%) patients, no residual filling was observed in the magnetic resonance angiography (MRA). Good clinical outcome (mRS 0–1) was achieved in 24/25 (96%) of patients.
Conclusion
Endovascular treatment of small cerebral aneurysms with FRED Jr is safe and effective even in complex and challenging morphologies allowing high rates of aneurysm occlusion with low periprocedural complications. Our cohort, consisting of a rate 20% acute ruptured aneurysms, is the major additive data to the published literature.
Keywords: FRED Jr, Low-profile flow diverter, Small cerebral artery aneurysm, Subarachnoid hemorrhage
Introduction
In this study, we reported on cerebral and cerebellar aneurysms arising from small-sized arteries beyond A2 anterior cerebral artery (ACA), M2 middle cerebral artery (MCA), P2 posterior cerebral artery (PCA), s2 superior cerebellar artery (SCA), a2 anterior inferior cerebellar artery (AICA), p2 posterior inferior cerebellar artery (PICA) segments. [1]. In the past, microsurgical clipping was preferred in the treatment of distal aneurysms because of relatively superficial anatomy. Some studies revealed good outcome in both endovascular and surgical management of distal anterior cerebral artery aneurysms [2]. Endovascular treatment of distal complex aneurysms is a challenging issue. Due to technical difficulties in accessing distal small and convoluted vessels and the necessity of using dual antiplatelets after treatment, the risk of complications such as bleeding, dissection, and thrombogenic events is higher than primary coiling [1–3].
Recently marketed low-profile flow diverters (FDs) are recommended in selected distal vascular pedicle and bifurcation aneurysms [4, 5]. The Flow Re-Direction Endoluminal Device Junior-FRED Jr (MicroVention, Tustin, California) is one of the low-profile FDs dedicated for distally located aneurysms whose parent vessel diameter is less than 3 mm [6]. Reports on the results of treatment with FRED Jr in complex and challenging aneurysms of small diameter arteries, especially ruptured aneurysms and rare localized aneurysms, are scarce. [7–9]. “In this single-center retrospective study, we evaluated long-term safety and efficacy of FRED Jr in treating challenging intracranial aneurysms of various morphologic types. We compared our results with the published FRED Jr and other low profile FD stent series in similar settings”.
Material and methods
Study design and data collection
Twenty-five consecutive patients with thirty-one aneurysms who were treated with FRED Jr by a single research team between October 2019 and February 2022 were retrospectively evaluated.
A written informed consent was taken that implies their medical records and images can be used for research in future. Ethical committee approval was assigned with a reference number of E1-20–1445 at our hospital.
The decision of treatment was taken jointly by at least two neurosurgeons and two interventional neuroradiologists. After examination of 2D and 3D angiograms of the aneurysm morphologic and morphometric criteria, the procedure was planned.
Data about patients’ demographics (age, sex), aneurysm morphology (location, sizes, shape, vessel branch incorporating aneurysm, ruptured or unruptured), periprocedural details and clinical status, clinical and angiographic follow-up outcomes were collected from the hospital database.
Device description
The FRED Jr is a self-expandable, double layer, can be resheathed, nitinol braided flow diverter used for 2.5–3.0 mm vessels. Unlike other flow diverters, FRED Jr has a unique combination consisting of two nitinol layers including flow diverter (90% of its length) and stent formation. Inner flow diverter part has tightly braided 36 wires, outer layer has wider braided 16 wires and no flow diverter effect. This unique design protects adjacent critical branches, provides good navigability and enhanced wall apposition. There are four platinum markers on both ends and tantalum spiral wires surrounding the stent which enhance visibility. Available stent sizes are 2.5–3 mm in diameter and up to 41 mm in length with a dual layer coverage of 8–37 mm. Headway 21 (MicroVention, Tustin, California) is its compatible microcatheter.
Antiplatelet regimen
Elective patients with unruptured aneurysms were premedicated with dual antiplatelet therapy (DAPT) with 10 mg prasugrel or 75 mg clopidogrel and 100 mg acetylsalicylic acid (ASA) for 5 days before the procedure. Some patients had taken a loading dose of clopidogrel (300–450 mg) or prasugrel (30–60 mg) depending on weight. In patients with high clopidogrel resistance, which is empirically defined as < 10% reduction in aggregation in response to 5 μmol/L ADP compared with pretreatment values, was seen in 63% of patients at 2 h, 31% at 24 h, 31% at 5 days, and 15% at 30 days according to VerifyNow testing, clopidogrel cut-off and prasugrel was administered [3]. Because of the acute settings for ruptured aneurysms, prasugrel was initiated just before the procedure as a loading dose. Perioperatively 25mcg/kg intravenous (iv) tirofiban infusion was administered in patients with a high risk of thromboembolic events and it was discontinued within 24 h. DAPT was maintained for the first 6 months, 75 mg clopidogrel or10 mg prasugrel with ASA 100 mg and then antiplatelet regimen was continued with ASA.
Endovascular procedure
All procedures were performed under general anesthesia with systemic heparinization (50–70 units per kilogram bolus followed by infusion). First, we placed a long introducer sheath via femoral artery. Then through the long sheath, we reached internal carotid artery or distal V2 segment of the vertebral artery using an intermediate guiding catheter. After detailed evaluation of the arterial anatomy and the aneurysm with 2D and 3D rotational angiograms, we planned the treatment. Since FRED Jr had a unique design to protect adjacent critical branches, we preferred to use FRED Jr FD. FRED Jr was deployed via Headway 21 over a micro guidewire (Synchro 0.014, Stryker Neurovascular, US). If cerebral vasospasm (a focal or diffuse temporary narrowing of cerebral arteries and delaying intra-arterial blood flow) occurred due to catheterization, we used nimodipine (Nimotop, Bayer, Germany) via catheter. Haemostasias was maintained at the access site with an arterial closure device.
Follow-up
Post-operative computer tomography (CT) was performed at 24 h to exclude hemorrhagic complications. Clinical follow-up was evaluated with modified Rankin scale (mRS). Major procedural complications were accepted as resulting in death or morbidity with mRS ≥ 2. Good clinical outcome was defined as mRS 0–1. Radiological follow-up was performed at 1, 6, 12 months postoperatively. It is a part of our routine practice to have a MR imaging at the post-operative first month to check for asymptomatic ischemic findings. It is our clinical policy to proceed with a digital subtraction angiography (DSA) postoperatively at 6–12 months to evaluate the efficacy of the procedure as a gold standard. Follow-up DSA images were classified according to O’Kelly–Marotta grading scale (OKM) [10]. The grades of occlusion were as follows; A = total filling (> 95%), B = subtotal filling (5–95%), C = entry remnant (< 5%) and D = no filling (0%). Adequate aneurysm occlusion was defined as OKM C and OKM D. In patients with sufficient occlusion in the first year DSA, radiological follow-up was performed yearly by magnetic resonance angiography (MRA). Some of the patients had been followed with TOF-MRA because of patient’s reluctance to DSA. TOF-MRA examinations were performed at 1.5 T (Siemens Healthineers, Germany). T1-weighted spoiled gradient echo sequences were used. The examination parameters were as follows: TR/TE = 25/7.0 ms, FA (flip angle) = 25, FOV = 230 × 195.5 × 112.5 mm3, acquisition matrix = 480 × 234, reconstruction matrix = 512 × 512, acquisition voxel = 0.48 × 0.84 × 1.5 mm3, reconstruction voxel = 0.45 × 0.45 × 0.75 mm3, and pixel bandwidth = 108.5 Hz.
Results
Baseline population and aneurysm features
Twenty-five patients with thirty-one aneurysms were included in this study. Eighteen (72%) patients were female. Median age was 48.8 years old (age range 9–85 years old). Location of the 31 aneurysms were as follows; 13 (41%) in middle cerebral artery (MCA), 7 (22%) in anterior cerebral artery (ACA), 4 (14.2%) in posterior cerebral artery (PCA), 3 (6%) in true posterior communicating artery (PCom), 2 (6%) in anterior communicating artery (ACom), 1 (3%) in superior cerebellar artery (SCA), 1 (3%) in true ophthalmic artery. One patient had four (Patient 10), three patients had two (Patient 4, 14, 21) and twenty-one patients had a single aneurysm. Five (20%) patients presented with acute subarachnoid hemorrhage (aSAH) due to a ruptured aneurysm (2 true PCom, 2 ACA, PCA). Twenty patients had unruptured aneurysms. Four patients (16%) had been treated previously for a ruptured aneurysm, they had residual filling. There were 21 saccular, 6 fusiform, and 4 dissecting aneurysms. Five patients had six fusiform aneurysms. Treatment was decided because two patients had a previous history of SAH, one patient presented with aSAH, and two patients had severe headache and relatively large aneurysms. Median aneurysm dome height was 5.98 mm, median neck size was 6.18 mm. Mean proximal and distal parent artery diameter was 2.43 mm (range 1.7–3.5) and 2.13 mm (range 1.5–3.5), respectively.
Demographics of patients and aneurysm features are summarized in Table 1.
Table 1.
Demographics of patients and aneurysm features
| No | Age | Sex | Acute SAH | Location | Number | Morphology |
|---|---|---|---|---|---|---|
| 1 | 58 | M | – | Left MCA | 1 | Saccular |
| 2 | 69 | F | – | Left MCA | 1 | Saccular |
| 3 | 27 | F | + | Left ACA | 1 | Saccular |
| 4 | 13 | F | – | Left PCA | 2 | Dissecting |
| 5 | 44 | F | – | Right MCA | 1 | Fusiform |
| 6 | 63 | F | – | Right MCA | 1 | Saccular |
| 7 | 9 | M | + | Left PCA | 1 | Dissecting |
| 8 | 85 | F | – | Left ACA | 1 | Saccular |
| 9 | 69 | M | + | Left true PCom | 1 | Fusiform |
| 10 | 53 | F | – | Right ACA | 2 | Saccular |
| 10 | 53 | F | – | Left ACA | 1 | Fusiform |
| 10 | 53 | F | – | Left SCA | 1 | Fusiform |
| 11 | 67 | M | – | Right MCA | 1 | Saccular |
| 12 | 66 | F | – | Right MCA | 1 | Saccular |
| 13 | 57 | F | – | Left MCA | 1 | Saccular |
| 14 | 49 | M | – | Left ACA A2, ACom | 2 | Saccular |
| 15 | 72 | F | + | Left ACA | 1 | Saccular |
| 16 | 46 | F | – | Left MCA | 1 | Fusiform |
| 17 | 67 | F | – | ACom | 1 | Saccular |
| 18 | 41 | F | – | Left true PCom | 1 | Saccular |
| 19 | 40 | F | – | Left MCA | 1 | Saccular |
| 20 | 15 | F | – | Left PCA | 1 | Fusiform |
| 21 | 43 | F | – | Right MCA | 2 | Saccular |
| 22 | 52 | M | – | Right MCA | 1 | Saccular |
| 23 | 32 | F | + | Left true PCom | 1 | Saccular |
| 24 | 73 | F | – | Left MCA | 1 | Saccular |
| 25 | 38 | M | – | Right ophthalmic | 1 | Dissecting |
Procedural technical details
In all procedures, FRED Jr was successfully deployed. In three patients, two adjacent aneurysms were treated with a single FRED Jr deployment. Patient 10 had four aneurysms totally. Two adjacent aneurysms in right ACA A2-3 segment were not suitable for coiling, they were treated using a single FRED Jr (Fig. 1). This patient also had a left A2-A3 and superior cerebellar artery segment aneurysms that were treated with a second and third FRED Jr deployments.
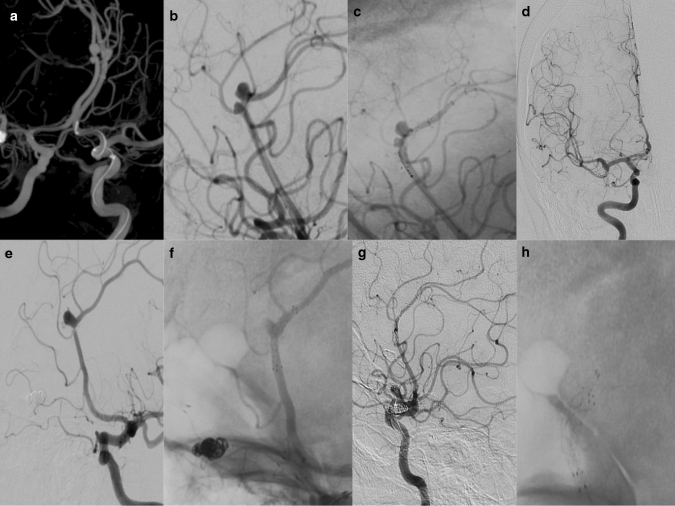
Fig. 1.
a Preprocedural 3D DSA demonstrated two aneurysms in the right ACA A2-3 segment and an aneurysm in the left ACA A2-3 segment in a 53-year-old-female. b, c First, two adjacent aneurysms in the right A2-3 segment were treated by placing a single Fred Jr on their neck. While FRED Jr covered both aneurysms, stasis was observed in the aneurysms after FD. d No residual filling was observed in both aneurysms located in the right ACA in the 9th-month follow-up AP angiogram. e, f Then in the left A2-3 segment aneurysm was treated with a second FRED Jr deployment in the same session. g In a follow-up lateral angiogram, no residual filling (OKM D) was observed for three aneurysms. h Lateral angiograms showed FRED Jr located bilaterally in A2-3
We had five patients with ruptured aneurysms (2 true PCom, 2 ACA, PCA). Two true PCom and PCA aneurysms were fusiform–dissecting with severe vessel wall damage. Since the parent artery wall was not healthy and the neck width was not suitable for primary coiling, we preferred to cover both the dissecting segment and the aneurysm neck using FD. All patients with ruptured aneurysms were discharged from the hospital without any complication.
Two patients (4 and 7) had larger than > 1 cm dissecting aneurysms located in the proximal part of the PCA and collateral perfusion from the MCA region was not prominent. We did not prefer occluding parenteral artery so as not to cause a neurological deficit. To avoid mass effect at special locations, we preferred FD only instead of FD with coiling.
In patients with dissecting aneurysms, since the parent artery wall was not healthy, we preferred to cover both the dissecting segment and the aneurysm neck using FD. The patient 4 had two dissecting aneurysms located in PCA and were treated by a single long-sized FRED Jr (Fig. 2).
Fig. 2.
a, b, c In the AP, lateral, and 3D angiograms of a 13-year-old female patient, two dissecting giant aneurysms were observed proximal to the PCA. d The procedural lateral angiogram shows both aneurysms treated with a single long dimensional FRED Jr. e Significant stasis was observed in the post-procedural immediate angiogram. f No residual filling(OKM D) was observed in the 6th-month follow-up angiograms
In patient seven, we had to deploy a second FRED Jr to achieve stasis for the ruptured dissecting giant aneurysm in PCA (Fig. 3). Rest of the aneurysms were either too small or neck width was not suitable for coiling. Patient 14 had two aneurysms located in left A2 duplication segment and ACom artery and was treated with a single FRED Jr.
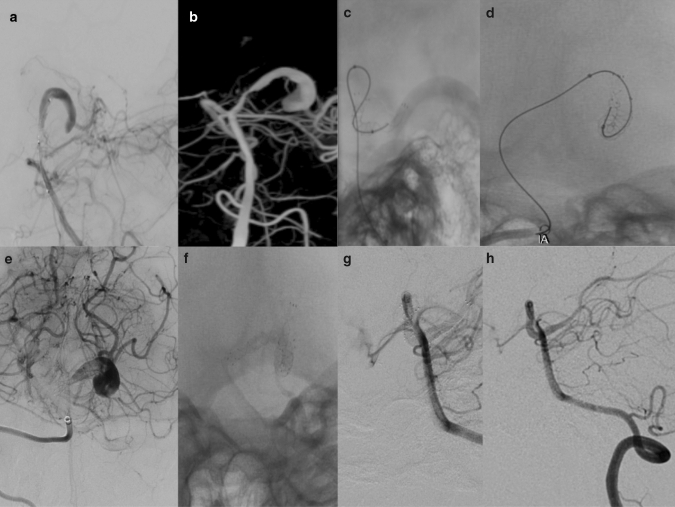
Fig. 3.
a A ruptured dissecting giant aneurysm in PCA was detected in a 9-year-old boy in the AP angiogram. b 3D angiogram shows dissecting PCA aneurysm. c, d After crossing the dissecting aneurysm with a microguidewire, a FRED Jr was placed in the aneurysmal segment. d Since there was not enough stasis in the immediate angiogram, sufficient stasis was obtained by placing a second FRED Jr. e Post-operative significant stasis was observed in immediate angiograms. f AP angiogram shows two overlapping FRED Jr in PCA. g, h No residual filling was observed (OKM D) in the 12th-month follow-up angiograms
In patient 20, the distal part of the FRED Jr did not open well (fish mouth appearance). Apposition of the distal part of the stent was established by passing a microcatheter through the stent. None of the patients required percutaneous balloon angioplasty (PTA). Patient 21 had two aneurysms in MCA bifurcation and superior trunk close to each other. This patient had to be treated with two FRED Jr because of unpredicted shortening of the first FRED Jr which was planned to cover both aneurysms. In three patients (No 9, 18, 23), we treated true PCom aneurysms (Fig. 4). Patient 25 had true ophthalmic aneurysm and was treated with FRED Jr (Fig. 5). In patient 17, coiling was applied in addition to FRED Jr into the large sized and irregular contoured ACom aneurysm. In patient 8, moderate vasospasm occurred at distal ACA after FRED Jr was deployed. Nimodipine infusion was performed, and vasospasm resolved.
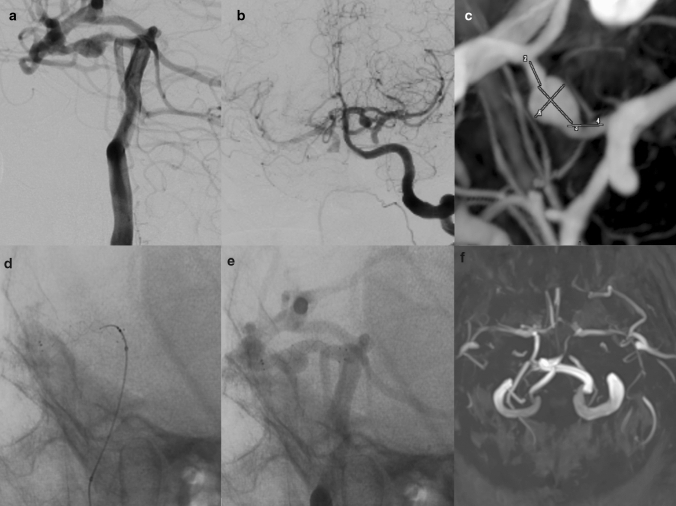
Fig.4.
a, b A true PCom aneurysm was detected on the left PCom artery in the lateral and AP angiograms in a 32-year-old patient presenting with acute SAH. c 3D DSA demonstrated true PCom aneurysm in more detail. d After the PCom artery was selectively catheterized, FRED JR was deployed into PCom artery. e Immediate DSA showed stasis in aneurysm. f No residual filling was observed in the 6th-month MRA (OKM D)
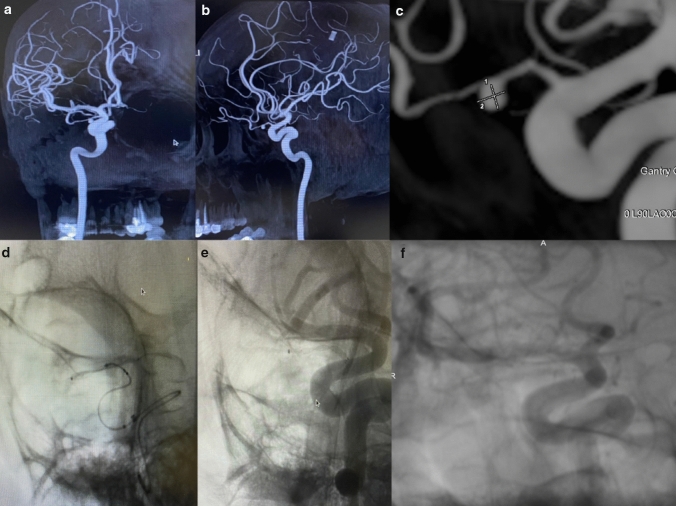
Fig. 5.
a, b Ophthalmic artery intracanalicular segment aneurysm was detected in AP and lateral DSA images. c True ophthalmic artery aneurysm was evaluated in detail by taking a procedural 3D angiogram. d Procedural lateral angiogram showed FRED Jr insertion into the neck of the intracanal true ophthalmic artery aneurysm. e Aneurysm occluded immediately after insertion of FRED Jr into the ophthalmic artery. f No residual filling was observed in 6th-month follow up angiogram (OKM D)
Periprocedural complications
In this study, complication rate was 3/25 (12%). In 2 (8%) patients with unruptured aneurysms, intra-operative intra-stent thrombosis occurred after deployment of FRED Jr. We observed intra-stent thrombosis after stent deployment in patient 4 who had two giant aneurysms in the left PCA. Intravenous (iv) tirofiban infusion was started immediately and then thrombus resolved. In patient 19 with MCA bifurcation aneurysm, parent artery occluded just after stent deployment. We performed thromboaspiration immediately. After passing distally through the occluded stent with Headway 21 microcatheter over the Synchro 0.014 (Stryker Neurovascular, US) micro guidewire, Sofia 5f (Microvention, Terumo, Japan) aspiration catheter was advanced, and thrombus was aspirated. Arterial lumen was partially opened; therefore, we deployed Neuroform Atlas stent (Stryker, USA) into the FRED Jr. Finally normal blood flow was established in the parent artery. Both patients were discharged from the hospital without any significant morbidity (mRS 0). Patient 24, 73 years old was successfully discharged from the hospital. Two weeks later, the patient presented with an intraparenchymal hemorrhage. Since it was a delayed hemorrhage, we thought that it might have happened because of dual antiplatelet treatment or increased pressure in the aneurysmal sac after the treatment with FD. Parenchymal hemorrhage was drained surgically, and the patient was followed in the intensive care unit for 1 week with endotracheal intubation. Finally, the patient was discharged with mRS 2 from the hospital at the first month of follow-up.
Procedural details and complications are summarized in Table 2.
Table 2.
Procedural details and complications
| No | Neck width (mm) | Dome width (mm) | Dome height (mm) | Distal PA* mm | Proximal PA* mm | Stent size (FRED Jr) | Additional device | Complications/treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | 3.1 | 3.3 | 4.7 | 2.5 | 3.2 | 3 × 14×19 | None | None |
| 2 | 1.9 | 3 | 3 | 2.5 | 3 | 2.5 × 8×13 | None | None |
| 3 | 4.2 | 4 | 3 | 2.6 | 2.8 | 2.5×13 × 8 | None | None |
| 4 | 21 | 23 | 21 | 3.2 | 3.5 | 3.0 × 27×32 | None | Intra-stent thrombosis/tirofiban |
| 4 | 16 | 11 | 12 | 3.2 | 3.5 | 3.0 × 27×32 | None | Intra-stent thrombosis/tirofiban |
| 5 | N/A | 7 | 8 | 2.8 | 3 | 2.5 × 13×18 | None | None |
| 6 | 2.2 | 3 | 3.5 | 2.5 | 3.2 | 2.5 × 8×13 | None | None |
| 7 | 16.4 | 38 | 13 | 1.8 | 2.1 | 3.0 × 32×27 | FRED Jr 3 × 27×30 | None |
| 8 | 6.1 | 6 | 5 | 1.8 | 2.2 | 2.5×18 × 13 | None | Vasospasm/nimodipine |
| 9 | N/A | 6 | 7 | 2.0 | 2.2 | 3.0 × 14×19 | None | None |
| 10 | 1.9 | 4 | 4 | 1.8 | 2.1 | 2.5 × 13×18 | None | None |
| 10 | N/A | 2 | 5 | 1.3 | 1.5 | 2.5 × 8×13 | None | None |
| 10 | N/A | 3 | 2 | 2.1 | 3.1 | 2.5 × 8×13 | None | None |
| 11 | 4.5 | 4 | 5 | 2.1 | 2.5 | 3.0 × 27×21 | None | None |
| 12 | 8.7 | 13 | 11 | 2.3 | 2.5 | 2.5 × 20×25 | None | None |
| 13 | 2.1 | 3 | 2 | 1.7 | 2.4 | 3.0 × 9×13 | None | None |
| 14 | 3.5 | 2 | 1.5 | 1.7 | 2 | 2.5 × 30×26 | None | None |
| 15 | 1.5 | 3 | 2 | 2.4 | 2.9 | 2.5 × 8×13 | None | None |
| 16 | 5.9 | 11 | 5 | 2.1 | 2.7 | 3.0 × 27×21 | None | None |
| 17 | 8.3 | 11.5 | 8.5 | 2.1 | 2.2 | 3.0 × 14×19 | Coil | None |
| 18 | 1.8 | 1.3 | 1.5 | 1.3 | 1.7 | 2.5 × 13×8 | None | None |
| 19 | 2.8 | 3.1 | 3.1 | 2.1 | 2.2 | 2.5 × 8×13 | Atlas 3 × 24, Sofia 5f | Intra-stent thrombosis/thrombectomy |
| 20 | N/A | 4.2 | 2.5 | 2.7 | 3.2 | 3.0 × 18×14 | None | Distal fish mouth appearance/opened |
| 21 | 3.8 | 3.3 | 2.3 | 2.1 | 2.5 | 2.5 × 18×13 | FRED Jr 2.5 × 18×13 | Stent shortened/second stent deployed |
| 22 | 3.5 | 3.7 | 6.2 | 2.2 | 2.4 | 2.5 × 18×13 | None | None |
| 23 | 3.8 | 5 | 4 | 2.1 | 2.5 | 2.5 × 13×8 | None | None |
| 24 | 2.8 | 3 | 5 | 2.8 | 3.2 | 3.0 × 18×14 | None | Delayed rupture/drained |
| 25 | 2.7 | 2.9 | 2.5 | 0.7 | 1.8 | 2.5 × 13×8 | None | None |
*PA parent artery
Periprocedural outcomes
Immediate periprocedural angiography of 31 aneurysms revealed significant stasis in 21, moderate stasis in 6, and no filling in 4 of the total aneurysms according to OKM classification.
Follow-up outcomes
In this study, mean follow-up was 21 months (6–28 months). Control DSA imaging was performed on 16/25 (64%) patients with 21/31 (67%) aneurysms. Near complete–complete occlusion (OKM C-D) was observed in 15/16 (93.7%) patients, 20/21 (95.2%) aneurysms on the follow-up angiograms. One (6.25%) patient had subtotal filling in the aneurysm (OKM B). In patients who had both follow-up with MRA and DSA angiograms, an analysis with respect to aneurysm occlusion rate revealed no discrepancy between the imaging tools. Nine patients were followed with only MRA, no residual filling was observed.
The clinical outcome scores were evaluated according to the mRS, and at the end of an average of 21 months, 24 (96%) patients were mRS 0 and one (4%) (Patient 24) was mRS 2. During the follow-up period, one patient died of a heart attack in the seventh month and another patient died of Covid-19 pneumonia in the ninth month. No residual filling was observed in the control MRA examination of these patients.
Angiographic and clinical follow-up outcomes are summarized in Table 3.
Table 3.
Angiographic and clinical follow-up outcomes
| No | Pre-op/post-op OKM* | Follow up OKM* | MRA RROC** | Pre-op GCS*** | Post-op GCS*** | 1/6/12 month mRS**** |
|---|---|---|---|---|---|---|
| 1 | A1/A3 | – | 1 | 15 | 15 | 0/0/0 |
| 2 | A1/A3 | – | 1 | 15 | 15 | 0/0/0 |
| 3 | A1/A3 | D | 1 | 15 | 15 | 0/0/0 |
| 4 | A2/B3 | D | 1 | 15 | 15 | 0/0/0 |
| 4 | A1/B3 | D | 1 | 15 | 15 | 0/0/0 |
| 5 | B1/B3 | D | 1 | 15 | 15 | 0/0/0 |
| 6 | A1/A3 | – | 1 | 15 | 15 | 0/0/0 |
| 7 | A1/B3 | D | 1 | 15 | 15 | 0/0/0 |
| 8 | A1/B3 | – | 1 | 15 | 15 | 0/0/0 |
| 9 | A1/C3 | – | 2 | 15 | 15 | 0/0/0 |
| 10 | A1/C3 | D | 1 | 15 | 15 | 0/0/0 |
| 10 | A1/B2 | D | 1 | 15 | 15 | 0/0/0 |
| 10 | A1/A3 | D | 1 | 15 | 15 | 0/0/0 |
| 11 | A1/A3 | B2 | 2 | 15 | 15 | 0/0/0 |
| 12 | A1/B3 | B2 | 2 | 15 | 15 | 0/0/0 |
| 13 | B2/B2 | D | 1 | 15 | 15 | 0/0/0 |
| 14 | A1/A3 | D | 1 | 15 | 15 | 0/0/0 |
| 15 | A1/B3 | – | 1 | 14 | 14 | 0/0/0 |
| 16 | A1/A3 | D | 1 | 15 | 15 | 0/0/0 |
| 17 | A1/A3 | D | 1 | 15 | 15 | 0/0/0 |
| 18 | A1/A3 | D | 1 | 15 | 15 | 0/0/0 |
| 19 | A1/A3 | – | 1 | 15 | 15 | 0/0 |
| 20 | A1/A3 | D | 1 | 15 | 15 | 0/0 |
| 21 | B2/C3 | C2 | 1 | 15 | 15 | 0/0 |
| 22 | A1/A3 | D | 1 | 15 | 15 | 0/0 |
| 23 | A1/A2 | – | 1 | 15 | 15 | 0/0 |
| 24 | A1/A3 | – | 1 | 15 | 15 | 2/2 |
| 25 | A1/A3 | D | 1 | 15 | 15 | 0/0 |
*OKM O’Kelly–Morata grading scale
**RROC Raymond–Roy classification
***GCS Glasgow Coma Scale
****mRS Modified Rankin scale
Discussion
Wide-necked, complex, irregular aneurysms and giant fusiform, dissecting, blister-like, or recanalyzed aneurysms are not suitable for primary coiling or clipping. These types of aneurysms are suitable for endovascular treatment with FD [7]. FD placed at the neck of the aneurysm reduces blood flow to the aneurysm by diverting the flow to the main artery and induces thrombosis [5, 11–13]. FDs have been used increasingly due to its efficacy and safety profile [14, 15]. A recent meta-analysis by Briganti et al. [16] indicated that FD stenting of intracranial aneurysms achieved a good percentage of occlusion 81.5% with a low incidence of major complications (mean 8.3% range 0–23).
Use of low-profile FDs in small arteries with a diameter of < 3 mm have increased recently [2, 17–19]. FRED Jr’s distinctive design provides the advantage of side branch protection in complex aneurysms. Less amount of outer stent wires provides advantage in navigability in tortuous vessels [8]. Studies with the FRED Jr stents are few in the literature. The multicenter studies published by Jesser J et al. [7] with 150 patients, Möhlenbruch MA et al. [8] with 42 patients, and Rautio R et al. [9] with 15 patients demonstrate the efficacy and safety of the FRED Jr stent in un-ruptured aneurysms. Using FRED Jr for ruptured aneurysms is rarely reported in the relevant literature. In the series of Sivasankar R et al. [20], only one patient was treated with FRED Jr during acute SAH.
In our series, five (20%) patients had acute ruptured aneurysms. Two of them were true PCom aneurysms with no option of treatment other than a FD. One patient with a PCA aneurysm had severe vessel wall damage. So we decided to perform a FRED Jr to protect side branches and treat the severely diseased vessel wall. Two distal ACA aneurysms were wide-necked and not accessible for surgical approach, they were treated with FD. During their follow-up, no residual filling was observed in angiograms or MRA. To the best of our knowledge, there is a paucity of data about treatment of ruptured aneurysms with FDs. Although numbers are small to draw a conclusion, outcome of ruptured and unruptured aneurysms was similar. In this respect, treatment of ruptured aneurysms with FD in small vessels may be an optional treatment if other methods are not suitable.
In case of insufficient aneurysmal stasis, overlapped flow diverters could achieve more flow reduction than a single flow diverter [21]. This technique is most effective for large orifice fusiform aneurysms. Damiano RJ et al. [22] reported that using overlapping pipeline embolization devices (PED) (Medtronic, California, USA) helped reducing an additional aneurysmal flow velocity of 30% with respect to deploying one device. Yu J et al. [23] reported that two overlapping PEDs were used to cover the aneurysm neck in 3/22 cases. Awad et al. [24] reported eight patients, treated with two overlapping PEDs. Using overlapping Silk Vista Baby (Balt, France) were reported in six patients [3]. In one patient with giant, dissecting PCA aneurysm, we had to use two overlapping FRED Jr to achieve stasis.
Parent vessel occlusion has been reported with coil embolization if an aneurysm located in distal parent artery; eg PICA, AICA, PCA [25]. In our series, we had three dissecting and one fusiform aneurysm located in proximal PCA. Because of poor collateral perfusion from the MCA territory, we did not prefer to occlude parenteral artery; it could be risky.
To provide occlusion of tandem aneurysms, it is possible to use a single FD. Awad et al. [24] reported a series of 9/17 patients with tandem aneurysms treated using a single PED. To the best of our knowledge, using a single FRED Jr to cover adjacent aneurysms is not reported in the relevant literature. We deployed a single FRED Jr to cover two aneurysms in three patients. True PCom aneurysms are defined as aneurysms arising from the PCom itself rather than the junction of the internal carotid artery (ICA) and the PCom [26] These aneurysms represent about 1.3% of all intracranial aneurysms and 6.8% of all PCom artery aneurysms [27]. True PCom aneurysms are rare, and this subtype of aneurysms is more prone to rupture [20]. Some authors prefer microsurgical management to endovascular treatment for the PCom aneurysms because of difficulties in navigation of microcatheter and potential risk of intra-operative rupture [27, 28]. Endovascular treatment for true PCom aneurysm is limited in the literature [28–30]. Our series included three patients with true PCom artery aneurysms, two of the three were ruptured aneurysms. All three patients were discharged from the hospital without any complication. Intracanalicular true ophthalmic artery aneurysm is rare [31]. Only one true ophthalmic aneurysm was previously reported to be treated with FD [32]. We treated true ophthalmic aneurysm via deployment of FRED Jr into the ophthalmic artery in one patient. The patient was discharged from the hospital without any complication and no residual filling (OKM D) in the first month follow-up angiograms.
Complications of endovascular FD treatments are ischemic stroke, intra-operative rupture, stent thrombosis, stent restenosis, delayed aneurysm rupture, and intraparenchymal hemorrhage. In the literature, Jesser J et al. [2], reported a periprocedural complication, technical complication and in-stent thrombosis rate of 16, 3 and 7%, respectively. Jesser J et al. [7] have reported periprocedural ischemic events with FRED Jr within the first 30 days as 6% (two major strokes and seven minor strokes). Möhlenbruch et al. [8] have reported 47 aneurysms treated with FRED Jr with a complication rate of 7% and mortality rate of 2%. Rautio et al. [9] and Sivasankar R et al. [20] have reported no periprocedural complication and mortality in 15 unruptured aneurysms treated with FRED Jr. In the literature, some studies were including both FRED and FRED Jr. In the study published by Guimaraens L et al. [33], 19.5% of aneurysms were treated with FRED Jr, major complications were reported in 6.5% of cases (three strokes, six in-stent thrombosis, three hemorrhage) and asymptomatic minor complications in 5.4% of cases (three stent shortening, two arterial dissection, two arterial occlusion, and three intra-stent stenosis). The intracerebral hemorrhage rate has been reported as 1.42% within the series of PED and Silk [34]. In multi-center SAFE study by Pierot et al. [14], 103 patients were treated with FRED (85%) and FRED Jr (15%) stents, they reported 1.9% mortality, 2.9% morbidity, and 6.8% thromboembolic complications rates at 1-year follow-up. In a meta-analysis published by Yao X et al. [35], procedure-related neurologic mortality, neurologic morbidity, and ischemic complication rates were reported as 0.87, 5.22, and 2.35% for distal aneurysms treated with Silk and PED, respectively.
In our series of periprocedural mortality, major or minor stroke did not occur. Our complication rate is 12%, similar with the biggest series in the given literature [7]. In-stent thrombosis occurred in two patients (7.6%) with unruptured aneurysm, shortly after the FRED Jr placement. These patients were discharged from the hospital without any neurologic deficit. In a 73 year-old patient, delayed intraparenchymal hemorrhage occurred 2 weeks later post-procedure who was discharged from the hospital with mRS 0. Delayed hemorrhage post-discharge might be related with increased pressure in the aneurysmal sac or due to dual antiplatelet treatment.
Large prospective studies and meta-analysis reported a complete occlusion rate of 75% for different FDs [34]. Pierot et al. [14] reported an occlusion/near occlusion rate of 81% in their FRED and FRED Jr series during 6–12 months follow-up. Yao X et al. [35] reported a complete aneurysm occlusion rate of 84.23% in patients treated with Silk and PED. Searching about literature for FRED Jr; Rautio et al. [9] reported a complete occlusion rate of 87% between 6 and 24 months of follow-up in 15 patients. Möhlenbruch et al. [8] have reported 100% occlusion in the 12 months. Sivasankar R et al. [20] have reported that OKM C-D was seen in 80% of the aneurysms on follow-up angiograms. Jesser J et al. [7] have reported a complete occlusion rate of 68% in the first year. In our series, near complete–complete occlusion (OKM C-D) rate was 93.7% of patients with 95.2% of aneurysms at 12 months angiograms. Aneurysm occlusion results were similar when MRA and DSA angiograms of patients who underwent DSA. In nine patients who were followed with TOF-MRA, no residual filling was observed. However, some studies indicate that MRA is a reliable modality for the follow-up of aneurysms treated using endovascular techniques [36]. Van Amerongen et al. reported the sensitivity and specificity of CE-MRA as 85 and 88%, and for TOF-MRA as 86 and 86% for assessing aneurysm occlusion [37]. Actually ferromagnetic metals include iron, nickel, and cobalt, all of which distort magnetic fields, thereby causing susceptibility artifacts on MR images [36–38]. MRA outcomes were classified according to Raymond–Roy occlusion classification (RROC) [11, 12]. A recently published article comparing TOF-MRA and DSA imaging as a follow up tool to evaluate aneurysm occlusion of FDs stated that nitinol FDs appear to be advantageous for TOF-MRA follow-up so as not to miss small aneurysm remnants or clinically relevant parent artery stenosis. However, there was a clear concordance of the results where we disclosed a high rate of artifacts particularly in chromium–cobalt FDs [38, 39]. Therefore, it is our clinical policy to proceed with a DSA imaging postoperatively at 6 months to evaluate the efficacy of the procedure as a gold standard.
Our cohort, consisting of 20% acute ruptured aneurysms, is the major additive data to the published literature. Including a higher percentage of ruptured aneurysms represents even a riskier patient profile than the average series in the literature. Even with such a cohort, our complication rate was similar to the relevant series [7, 20].
Limitations of the study
Our study has limitations intrinsic to single-center series and it is not a population-based study. The number of patients was relatively small, the group of aneurysms was heterogeneous; therefore, we did not perform inferential statistics. Further analysis is needed in a larger sample. The data were analyzed retrospectively. In addition, the imaging outcome was assessed by the operators and not independently. A neurosurgeon provided clinical follow-up but was not blinded to the procedure used, and there was no external evaluation of the angiographic results. There was a lack of standardization of radiological follow-up.
Conclusion
We believe that, our study group including aneurysms with different locations, various morphology, and rare types reflect actual daily practice of a busy neurointerventional laboratuary. Endovascular treatment in both acute and elective situations with FRED Jr is a safe and effective technique allowing high rates of aneurysm occlusion. Larger studies with long-term follow-up are needed to draw conclusions about the security and efficacy of these stents especially in acute ruptured aneurysm treated with FRED Jr.
Abbreviations
- ACA
Anterior cerebral artery
- ACom
Anterior communicating artery
- AICA
Anterior inferior cerebellar artery
- MCA
Middle cerebral artery
- PCom
Posterior communicating artery
- PICA
Posterior inferior cerebellar artery
- CT
Computer tomography
- CTA
Computer tomography angiography
- DAPT
Dual antiplatelet treatment
- DSA
Digital subtraction angiography
- FD
Flow diverter
- FRED
Flow re-direction endoluminal device
- MRA
Magnetic resonance angiography
- mRS
Modified Rankin score
- OKM
O’Kelly–Morata grading scale
- PCA
Posterior cerebral artery
- PED
Pipeline embolization device
- PTA
Percutaneous balloon angioplasty
- RROC
Raymond–Roy occlusion classification
- SCA
Superior cerebellar artery
- CE
Contrast enhanced
- TOF-MRA
Time-of-flight magnetic resonance angiography
Author contributions
BS: conceptualization, methodology, formal analysis, writing—original draft. YCŞ: investigation, formal analysis, data curation, visualization. Ergün Daglioglu:conceptualization, methodology, supervision, project administration. MOÖ: validation, investigation. GO: supervision, validation, formal analysis. İA: methodology, visualization, data curation, writing—original draft.
Declarations
Conflict of interest
There is no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This manuscript is an original article and has no studies with human participants or animals performed by any of the authors. A written informed consent was taken that implies their medical records and images can be used for research in future. Ethical committee approval was assigned with a reference number of E1-20–1445 at our hospital.
Informed consent
The requirement for informed consent was waived for this type of review article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sturiale CL, Brinjikji W, Murad MH, Cloft HJ, Kallmes DF, Lanzino G. Endovascular treatment of distal anterior cerebral artery aneurysms: single-center experience and a systematic review. Am J Neuroradiol. 2013;34:2317–2320. doi: 10.3174/ajnr.A3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schob S, Hoffmann KT, Richter C, et al. Flow diversion beyond the circle of Willis: endovascular aneurysm treatment in peripheral cerebral arteries employing a novel low-profile flow diverting stent. J Neurointerv Surg. 2019;11(12):1227–1234. doi: 10.1136/NEURINTSURG-2019-014840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiviott SD, Antman EM. Clopidogrel resistance: a new chapter in a fast-moving story. Circulation. 2004;109(25):3064–3067. doi: 10.1161/01.CIR.0000134701.40946.30. [DOI] [PubMed] [Google Scholar]
- 4.Furtado SV, Jayakumar D, Perikal PJ, Mohan D. Mohan contemporary management of distal anterior cerebral artery aneurysms: a dual-trained neurosurgeon’s perspective. J Neurosci Rural Pract. 2021;12:711–717. doi: 10.1055/s-0041-1735823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schob S, Kläver M, Richter C, Scherlach C, Maybaum J, Mucha S, et al. Single-center experience with the bare p48mw low-profile flow diverter and its hydrophilically covered version for treatment of bifurcation aneurysms in distal segments of the anterior and posterior circulation. Front Neurol. 2020;11:1050. doi: 10.3389/fneur.2020.01050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierot L, Spelle L, Berge J, Januel AC, Herbreteau D, Aggour M, et al. Feasibility, complications, morbidity, and mortality results at 6 months for aneurysm treatment with the flow re-direction endoluminal device: report of SAFE study. J Neurointerv Surg. 2018;10:765–770. doi: 10.1136/neurintsurg-2017-013559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jesser J, Alberalar ND, Kizilkilic O, Saatci I, Baltacioglu F, Ozlük E, et al. Safety and efficacy of the FRED Jr Flow Re-Direction endoluminal device for intracranial aneurysms: retrospective multicenter experience with emphasis on midterm results. Front Neurol. 2021;12:722183. doi: 10.3389/fneur.2021.722183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Möhlenbruch MA, Kizilkilic O, Killer-Oberpfalzer M, Baltacioglu F, Islak C, Bendszus M, et al. Multicenter experience with FRED Jr flow re-direction endoluminal device for intracranial aneurysms in small arteries. Am J Neuroradiol. 2017;38:1959–1965. doi: 10.3174/ajnr.A5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rautio R, Rahi M, Katila A, Rinne J. Single-center experience with six-month follow-up of FRED Jr® flow diverters for intracranial aneurysms in small arteries. Acta radiol. 2019;60:917–924. doi: 10.1177/0284185118805266. [DOI] [PubMed] [Google Scholar]
- 10.O’Kelly CJ, Krings T, Fiorella D, Marotta TR. A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol. 2010;16:133–137. doi: 10.1177/159101991001600204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raymond J, Guilbert F, Weill A, Georganos SA, Juravsk L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398–1403. doi: 10.1161/01.STR.0000073841.88563.E9. [DOI] [PubMed] [Google Scholar]
- 12.Mascitelli JR, Moyle H, Oermann EK, Polykarpou MF, Patel AA, Doshi AH, et al. An update to the Raymond-Roy occlusion classification of intracranial aneurysms treated with coil embolization. J Neurointerv Surg. 2015;7:496–502. doi: 10.1136/neurintsurg-2014-011258. [DOI] [PubMed] [Google Scholar]
- 13.Henkes H, Weber W. The past, present and future of endovascular aneurysm treatment. Clin Neuroradiol. 2015;25:317–324. doi: 10.1007/s00062-015-0403-1. [DOI] [PubMed] [Google Scholar]
- 14.Pierot L, Spelle L, Berge J, Januel AC, Herbreteau D, Aggour M, et al. SAFE study (safety and efficacy analysis of FRED embolic device in aneurysm treatment): 1-year clinical and anatomical results. J Neurointerv Surg. 2019;11:184–189. doi: 10.1136/neurintsurg-2018-014261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schüngel MS, Quäschling U, Weber E, Struck MF, Maybaum J, Bailis N, et al. Endovascular treatment of intracranial aneurysms in small peripheral vessel segments—efficacy and intermediate follow-up results of flow diversion with the silk vista baby low-profile flow diverter. Front Neurol. 2021;12:671915. doi: 10.3389/fneur.2021.671915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briganti F, Leone G, Marseglia M, Mariniello G, Caranci F, Brunetti A, et al. Endovascular treatment of cerebral aneurysms using flow-diverter devices: a systematic review. Neuroradiol J. 2015;28:365–375. doi: 10.1177/1971400915602803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhogal P, Makalanda HLD, Wong K, Keston P, Downer J, Du Plessis JC, et al. The silk vista baby-the UK experience. Interv Neuroradiol. 2022;28:201–212. doi: 10.1177/15910199211024061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van den Bergh F, De Beule T, van Rooij WJ, Voormolen MH, Van der Zijden T, Stockx L, et al. The p48 flow diverter: first clinical results in 25 aneurysms in three centers. Interv Neuroradiol. 2021;27:339–345. doi: 10.1177/1591019920972213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mou K, Zhou Z, Yin J, Yang H, Liu J. Clinical features and treatment of distal intracranial aneurysms. J Craniofac Surg. 2016;27:244–247. doi: 10.1097/SCS.0000000000002439. [DOI] [PubMed] [Google Scholar]
- 20.Sivasankar R, Shrivastava M, Limaye US. Experience with FRED junior flow diverter in treatment of cerebral aneurysms at or distal to the circle of Willis. J Clin Neurosci. 2019;69:166–169. doi: 10.1016/j.jocn.2019.07.079. [DOI] [PubMed] [Google Scholar]
- 21.Damiano RJ, Tutino VM, Paliwal N, Ma D, Davies JM, Siddiqui AH, et al. Compacting a single flow diverter versus overlapping flow diverters for intracranial aneurysms: a computational study. AJNR Am J Neuroradiol. 2017;38:603–610. doi: 10.3174/ajnr.A5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Damiano RJ, Ma D, Xiang J, Siddiqui AH, Snyder KV, Meng H. Finite element modeling of endovascular coiling and flow diversion enables hemodynamic prediction of complex treatment strategies for intracranial aneurysm. J Biomech. 2015;48:3332–3340. doi: 10.1016/j.jbiomech.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu J, Lv X. Flow Diversion for intracranial aneurysms beyond the circle of Willis. Front Neurol. 2021;12:674966. doi: 10.3389/fneur.2021.674966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Awad AW, Moon K, Yoon N, Mazur MD, Kalani MYS, Taussky P, et al. Flow diversion of tandem cerebral aneurysms: a multi-institutional retrospective study. Neurosurg Focus. 2017;42:E10. doi: 10.3171/2017.2.FOCUS1731. [DOI] [PubMed] [Google Scholar]
- 25.Cui L, Peng Q, Ha W, Zhou D, Xu Y. Parent artery occlusion for intracranial aneurysms. Interv Neuroradiol. 2009;15:309–315. doi: 10.1177/159101990901500308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Zhang Y, Li W, Wang K, Zhang Y, Yang X. Treatment of true posterior communicating artery aneurysms: Endovascular experience in a single center. Interv Neuroradiol. 2020;26:55–60. doi: 10.1177/1591019919874603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golshani K, Ferrell A, Zomorodi A, Smith TP, Britz GW. A review of the management of posterior communicating artery aneurysms in the modern era. Surg Neurol Int. 2010;1:88. doi: 10.4103/2152-7806.74147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He W, Gandhi CD, Quinn J, Karimi R, Prestigiacomo CJ. True aneurysms of the posterior communicating artery: a systematic review and meta-analysis of individual patient data. World Neurosurg. 2011;75:64–72. doi: 10.1016/j.wneu.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Yang ZG, Liu J, Ge J, Li ZF, Tian CO, Han J, et al. A novel proximal end stenting technique for assisting embolization of a complex true posterior communicating aneurysm. J Clin Neurosci. 2016;28:148–151. doi: 10.1016/j.jocn.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Su W, Meng Q. Endovascular treatment of ruptured true posterior communicating artery aneurysms. Turk Neurosurg. 2015;25:73–77. doi: 10.5137/1019-5149.JTN.10137-13.1. [DOI] [PubMed] [Google Scholar]
- 31.Piché SL, Haw CS, Redekop GJ, Heran MKS. Rare intracanalicular ophthalmic aneurysm: endovascular treatment and review of the literature. Am J Neuroradiol. 2005;26:1928–1931. [PMC free article] [PubMed] [Google Scholar]
- 32.Sirakov S, Sirakov A, Tsonev H, Hristov H. Ruptured intracanalicular ophthalmic artery aneurysm treated with low profile flow diverter device: case report. Clin Neuroradiol. 2020;30:177–180. doi: 10.1007/s00062-019-00792-2. [DOI] [PubMed] [Google Scholar]
- 33.Guimaraens L, Vivas E, Saldaña J, Llibre JC, Gil A, Balaguer E, et al. Efficacy and safety of the dual-layer flow-diverting stent (FRED) for the treatment of intracranial aneurysms. J Neurointerv Surg. 2020;12:521–525. doi: 10.1136/neurintsurg-2019-015371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cagnazzo F, Perrini P, Dargazanli C, Lefevre PH, Gascou G, Morganti R, et al. Treatment of unruptured distal anterior circulation aneurysms with flow-diverter stents: a meta-analysis. AJNR Am J Neuroradiol. 2019;40:687–693. doi: 10.3174/ajnr.A6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao X, Ma J, Li H, Shen H, Lu X, Chen G. Safety and efficiency of flow diverters for treating small intracranial aneurysms: a systematic review and meta-analysis. J Int Med Res. 2017;45:11–21. doi: 10.1177/0300060516671600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmed SU, Mocco J, Zhang X, Kelly M, Doshi A, Nael K, et al. MRA versus DSA for the follow-up imaging of intracranial aneurysms treated using endovascular techniques: a meta-analysis. J Neurointerv Surg. 2019;11:1009–1014. doi: 10.1136/neurintsurg-2019-014936. [DOI] [PubMed] [Google Scholar]
- 37.van Amerongen MJ, Boogaarts HD, de Vries J, Verbeek ALM, Meijer FJA, Prokop M, et al. MRA versus DSA for followup of coiled intracranial aneurysms: a meta-analysis. AJNR Am J Neuroradiol. 2014;35:1655–1661. doi: 10.3174/ajnr.A3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batur H, Sayin B, Balci S, Akmangit I, Daglioglu E, Alagoz F, et al. The implications of magnetic resonance angiography artifacts caused by different types of intracranial flow diverters. J Cardiovasc Magn Reson. 2021;23:69. doi: 10.1186/s12968-021-00753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oishi H, Fujii T, Suzuki M, Takano N, Teranishi K, Yatomi K, et al. Arai. Usefulness of silent MR Angiography for intracranial aneurysms treated with a flow-diverter device. AJNR Am J Neuroradiol. 2019;40(5):808–814. doi: 10.3174/ajnr.A6047. [DOI] [PMC free article] [PubMed] [Google Scholar]