Abstract
The reactivities of sera from chronic chagasic patients against the trypomastigote excreted-secreted antigens (TESA) of Trypanosoma cruzi strains with different biodemes were analyzed by TESA-blot and TESA–enzyme-linked immunosorbent assay (ELISA). Although both tests presented high sensitivity and specificity, TESA-ELISA is more appropriate for screening a larger number of samples.
Chagas' disease, caused by Trypanosoma cruzi, is still a major health problem in Latin America, where some 16 to 18 million people are infected (23). There is high morbidity among these infected individuals, as there is no vaccine and chemotherapy is not very effective.
In the acute phase of Chagas' disease, when parasitemia is high, diagnosis can be easily made using conventional parasitological methods. During the chronic phase (low parasite levels), diagnosis is performed mainly by immunological methods (9). At present, the serological diagnosis of Chagas' disease relies upon the widely used indirect immunofluorescence, indirect hemagglutination, and enzyme-linked immunosorbent assay (ELISA). None of these methods can be regarded as sensitive enough for blood bank screening. Furthermore, with the low prevalence of infected donors (<2%), one has to expect low positive predictive values leading to a high false-positive rate of results that must be confirmed by other methods (22). In addition, contradictory results have been obtained by different methods and laboratories, probably due to the use of different strains of T. cruzi, different antigenic fractions, and nonstandardized procedures, causing variations in sensitivity and specificity (9).
Whole epimastigotes or epimastigote fractions are commonly used in the serological diagnosis. These antigens may give rise to false-positive reactions mainly due to cross-reactivity with antibodies developed against other parasitic diseases (5). These problems may be overcome by using defined antigens containing specific T. cruzi epitopes that can be recognized by the majority of chagasic patients. The World Health Organization has long emphasized the need for defined antigens to improve serodiagnosis of Chagas' disease. In an attempt to solve this problem, several research groups have used recombinant and/or synthetic and biochemically purified antigens (1, 7, 12, 14–16, 19). In order to be useful, these antigens must meet the following criteria: (i) they should be present in T. cruzi isolates from different areas of endemicity and absent in other infectious disease agents; (ii) they should be highly immunogenic in populations with different immunogenetic backgrounds, regardless of the clinical phase of Chagas' disease; and (iii) they should be stable and easily amenable to quality control tests, to guarantee reproducibility (18, 24).
Recently, an immunoblot assay using trypomastigote excreted-secreted antigens (TESA) of T. cruzi Y strain was proposed as a sensitive and specific diagnostic assay (TESA-blot) in cases of suspected acute or congenital T. cruzi infection and as a general confirmatory test for conventional Chagas' disease serology (20). However, this assay was not performed with T. cruzi strains of different biodemes and may not meet the criteria described above.
In the present paper we report on a 150- to 170-kDa T. cruzi excreted-secreted polypeptide obtained from different strains that is recognized by 100% of chronic chagasic patients tested and was not recognized by sera of patients with other parasitic diseases. Furthermore, we used TESA in ELISA, resulting in high sensitivity and specificity.
Serum samples were collected from patients referred to Hospital Universitário Oswaldo Cruz (Recife, Brazil) living in areas of Chagas' disease endemicity of the State of Pernambuco, Brazil. The diagnosis of Chagas' disease was supported by clinical, epidemiological, and serological (ELISA, indirect immunofluorescence, or indirect hemagglutination) evidence. Serum samples were classified as negative when two serological tests gave nonreactive results against T. cruzi antigens and identified as positive when two tests were reactive.
We analyzed by TESA-blot assay the sera of 42 chagasic patients (14 with the cardiac form, 18 with the asymptomatic form, 7 with the mixed form, and 3 with the digestive form)and of 14 individuals with negative serology, as well as serum samples of patients with the following other parasitic diseases: cutaneous (n = 5) and visceral (n = 5) leishmaniasis, toxoplasmosis (n = 5), amebiasis (n = 5), and filariasis (n = 5). Serum samples of 124 chronic chagasic patients, 205 normal individuals, 14 patients with cutaneous leishmaniasis, and 17 patients with visceral leishmaniasis were analyzed by TESA-ELISA. Blood samples were taken by venipuncture, and the sera were stored at −20°C.
TESA from T. cruzi strains with different biodemes, designated Y for type I, WSL and 12SF for type II, and Colombiana for type III (Table 1), were obtained from the supernatant of infected Vero cells according to the method of Umezawa et al. (20). Protein concentrations were determined by the method of Bradford (4). TESA corresponding to each T. cruzi strain were solubilized in sample buffer and run in 7.5% polyacrylamide gel (13) using a minigel system (Hoefer Scientific Instruments, San Francisco, Calif.). The TESA-blot assay was carried out as previously described (20).
TABLE 1.
Trypanosoma cruzi strains with different biodemes
| Strain | Source | Region | Type | Reference(s) |
|---|---|---|---|---|
| Y | Human | São Paulo State, Brazil | I | 17 |
| WSL | Guinea pig | Pernambuco State, Brazil | II | 8 |
| 12S | Human | Bahia State, Brazil | II | 2, 3 |
| Colombiana | Human | Bogota, Colombia | III | 6 |
TESA of each strain were also used in ELISA. Microtiter plates (Nunc-Immuno Plates, MaxiSorp, 96 wells; Nalge Nunc International Corporation) were coated with 5 μg of TESA (100 μl/well) per ml diluted in 0.05 M Na2CO3 buffer, pH 9.6, and incubated overnight at 4°C. The plates were blocked for 2 h with phosphate-buffered saline–Tween 20 (0.05%) (PBS-Tw) containing 5% defatted milk (Nestlé), prior to incubation of 100 μl of sera diluted (1:100) in PBS-Tw (2 h, room temperature). The bound antibodies were detected with peroxidase-conjugated goat anti-human immunoglobulin G (Fc specific) (Sigma Chemical Co., St. Louis, Mo.). The immune complexes were revealed by addition of orthophenyldiamine and H2O2. Optical density (OD) was measured at 490 nm. The cutoff was established as the OD mean of the negative controls + 3 standard deviations. The sensitivity and specificity were estimated according to the method of Camargo (5). The gray zone was established as ±10%. The confidence interval (CI) was calculated at the level of 95%.
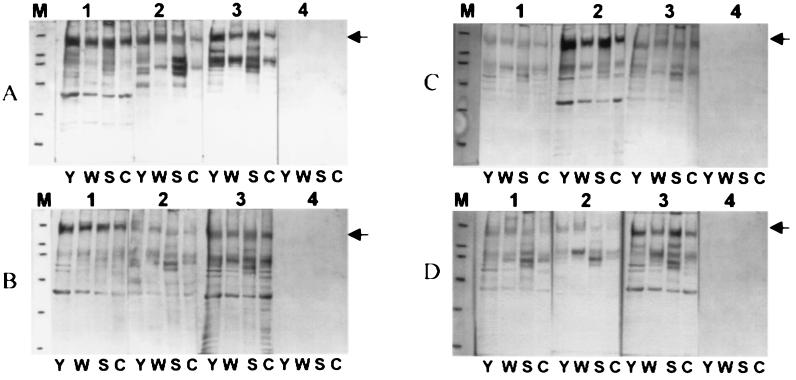
The intensity of the reaction to different antigens was variable, but similar patterns of reactivity were observed by Western blot analysis for almost all analyzed sera (Fig. 1). Chronic chagasic sera (100%) recognized a 150- to 170-kDa band on TESA-blot from Y, WSL, 12SF, and Colombiana strains. Cross-reactions with visceral and cutaneous leishmaniasis sera were observed with some polypeptides below 150 kDa (data not shown). No reaction was observed when the nitrocellulose membrane was incubated with normal human control sera. The discrimination of different clinical forms of the disease was not possible based upon the analysis of the antigenic profiles. The data reported herein confirm previous studies (10, 11, 20, 21) and show that the high sensitivity of TESA-blot in chronic cases in combination with the absence of cross-reactions with leishmaniasis makes this test attractive as an alternative to conventional tests. On the other hand, it may be used as a specific confirmatory assay to exclude cross-reactions after serum screening by conventional serology (20). An additional, favorable feature of TESA is its stability when stored either at −40 or 4°C, resulting in similar immunoblot antigenic patterns (11). The 150- to 170-kDa band identified herein probably corresponds to the 150- to 160-kDa band described previously (11, 20). In agreement with other studies (10, 11, 20, 21), the recognition of the 150- to 170-kDa band in different strains provides indirect evidence that this molecule is not very polymorphic among T. cruzi isolates and contains conserved epitopes that are highly immunogenic in the chronic phase of Chagas' disease. Thus, this antigenic preparation (TESA) meets the criteria proposed by Zingales et al. (24) and Stolf (18).
FIG. 1.
Representative TESA-blots using 15 μg of excreted-secreted antigens from T. cruzi with different biodemes and sera from patients with different forms of chronic Chagas' disease: cardiac form (A), asymptomatic form (B), digestive form (C), and mixed form (D). Numbers 1, 2, and 3 designate sera from different patients; number 4 designates normal serum. Lanes: Y, Y strain; W, WSL strain; S, 12SF strain; C, Colombiana strain; M, molecular mass markers (205, 116, 97.4, 66, 45, and 29 kDa).
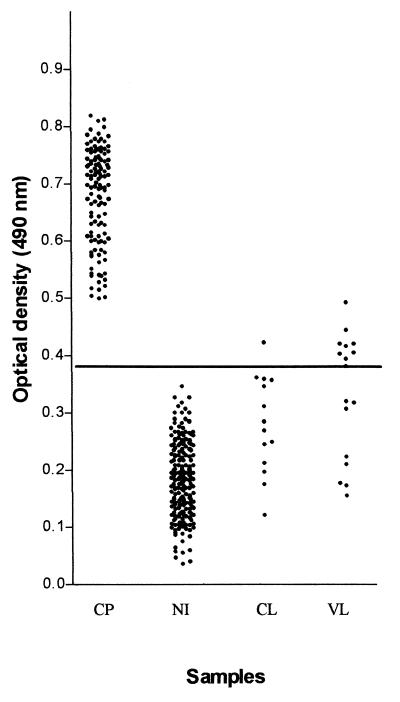
Western blot format is not very appropriate for the assay of a larger number of samples. For this purpose, the ELISA format is more convenient, as it is simple, easily performed, and amenable to automation. Thus, we developed and evaluated an ELISA using trypomastigote excreted-secreted antigens (TESA-ELISA). The TESA-ELISA results for individual sera are shown in Fig. 2. All 124 cases of confirmed Chagas' disease, presenting ODs above the cutoff value (0.378), were considered positive, and all 204 sera from nonchagasic individuals, which presented ODs below the cutoff value, were considered negative. Although TESA-ELISA showed high sensitivity (100%; 95% CI, 96.3 to 100%) and specificity (96%; 95% CI, 92.5 to 97.9%), cross-reactions were observed with cutaneous and visceral leishmaniasis (caused by organisms closely related to T. cruzi). Only 1 of 14 patients with cutaneous leishmaniasis showed an OD above the cutoff, while 9 of 17 patients with visceral leishmaniasis showed ODs above the cutoff value. However, it must be observed that most of those OD values are in the gray zone (0.340 to 0.416) above the cutoff value (0.378) and are considered borderline results. On the other hand, OD values in the gray zone below the cutoff range (0.340 to 0.378) were observed only with cutaneous leishmaniasis sera. In Fig. 2, results on TESA-ELISA using antigens of T. cruzi strain Y are shown. Nevertheless, similar results were obtained with antigens from other strains (WSL, 12SF, and Colombiana). The cross-reactivity observed may be due to the reactivity with polypeptides below 150 kDa. To verify this hypothesis, we isolated by preparative electrophoresis the 150- to 170-kDa band using TESA from T. cruzi Y strain. The fraction obtained, of 150 to 170 kDa, was evaluated by ELISA as described above using sera of chagasic patients, of normal individuals, and of patients with cutaneous and visceral leishmaniasis. Sensitivity and specificity were 100%. These results are currently being further evaluated by testing a larger sample.
FIG. 2.
TESA-ELISA using excreted-secreted antigens of T. cruzi Y strain. The horizontal line indicates the cutoff (0.378). CP, chagasic patients; NI, normal individuals; CL, patients with cutaneous leishmaniasis; VL, patients with visceral leishmaniasis.
In conclusion, both TESA-blot and TESA-ELISA presented high sensitivity and specificity. However, the latter is more appropriate for assaying a large number of samples, such as screening in blood banks. In addition, we provided preliminary evidence that the 150- to 170-kDa fraction of TESA is potentially relevant for the diagnosis of Chagas' disease and deserves further investigation.
Acknowledgments
We thank Sonia G. Andrade for critical reading of the manuscript and valuable suggestions. We are grateful to Maria Edileuza F. Brito and Otamires A. Silva for providing some sera of cutaneous and visceral leishmaniasis used in this study.
This work was supported by Fundação de Amparo à Pesquisa do Estado de Pernambuco (FACEPE), Brazilian National Research Council (CNPq), and FIOCRUZ.
REFERENCES
- 1.Affranchino J L, Ibanez C F, Luquetti A O, Rassi A, Reyes M B, Macina R A, Aslund L, Petterson U, Frasch A C C. Identification of a Trypanosoma cruzi antigen that is shed during the acute phase of Chagas' disease. Mol Biochem Parasitol. 1989;34:221–228. doi: 10.1016/0166-6851(89)90050-9. [DOI] [PubMed] [Google Scholar]
- 2.Andrade S G, Andrade V, Rocha Filho F D, Barral Neto M. Análise antigênica de diferentes cepas do Trypanosoma cruzi. Rev Inst Med Trop São Paulo. 1981;23:245–250. [PubMed] [Google Scholar]
- 3.Andrade S G. Trypanosoma cruzi: clonal structure of parasite strains and the importance of principal clones. Mem Inst Oswaldo Cruz. 1999;94(Suppl. I):185–187. doi: 10.1590/s0074-02761999000700026. [DOI] [PubMed] [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Camargo M E. An appraisal of Chagas' disease serodiagnosis. In: Wendel S, Brener Z, Camargo M E, Rassi A, editors. Chagas' disease (American trypanosomiasis): its impact on transfusion and clinical medicine. São Paulo, Brazil: ISBT Brazil'92; 1992. pp. 165–168. [Google Scholar]
- 6.Federici E E, Abelmann W H, Neva E A. Chronic and progressive myocarditis and myositis in C3H infected with Trypanosoma cruzi. Am J Trop Med Hyg. 1964;13:272–280. doi: 10.4269/ajtmh.1964.13.272. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg S, Krieger M A, Lafaille J J, Almeida E, Oelemann W. Use of Trypanosoma cruzi antigens in the immunological diagnosis of Chagas' disease. Mem Inst Butantan. 1991;53:71–76. [Google Scholar]
- 8.Gomes Y M, Leal T C, Silva M R, Coutinho E M. Characterization of Trypanosoma cruzi strain isolated from a non-endemic area in northeast Brazil. Rev Inst Med Trop São Paulo. 1995;37:87–89. doi: 10.1590/s0036-46651995000100014. [DOI] [PubMed] [Google Scholar]
- 9.Gomes Y M. PCR and sero-diagnosis in chronic Chagas' disease: biotechnological advances. Appl Biochem Biotechnol. 1997;66:107–119. doi: 10.1007/BF02788756. [DOI] [PubMed] [Google Scholar]
- 10.Jazín E E, Luquetti A O, Rassi A, Frasch A C C. Shift of excretory-secretory immunogens of Trypanosoma cruzi during human Chagas' disease. Infect Immun. 1991;59:2189–2191. doi: 10.1128/iai.59.6.2189-2191.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kesper N, Jr, Almeida K A, Stolf M A S, Umezawa E S. Immunoblotting analysis of trypomastigote excreted-secreted antigens as a tool for the characterization of Trypanosoma cruzi strains and isolates. J Parasitol. 2000;86:862–867. doi: 10.1645/0022-3395(2000)086[0862:IAOTES]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Krieger M A, Almeida E, Oelemann W, Lafaille J J, Pereira J B, Carvalho M R, Goldenberg S. Use of recombinant antigens for the accurate immunodiagnosis of Chagas' disease. Am J Trop Med Hyg. 1992;46:427–434. doi: 10.4269/ajtmh.1992.46.427. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Lafaille J J, Linss J, Krieger M A, Souto-Padron T, de Souza W, Goldenberg S. Structure and expression of two Trypanosoma cruzi genes encoding antigenic proteins bearing repetitive epitopes. Mol Biochem Parasitol. 1989;35:127–136. doi: 10.1016/0166-6851(89)90115-1. [DOI] [PubMed] [Google Scholar]
- 15.Levin M, Mesri E, Benarous R, Levitos G, Schijman A, Leyati P, Chiale P, Ruiz A M, Khan A, Rosenbaum M, Torres H N, Segura E L. Identification of major Trypanosoma cruzi antigenic determinants in chronic Chagas' disease. Am J Trop Med Hyg. 1989;41:530–538. doi: 10.4269/ajtmh.1989.41.530. [DOI] [PubMed] [Google Scholar]
- 16.Luquetti A O. Use of Trypanosoma cruzi defined proteins for diagnosis-multicentre trial. Serological and technical aspects. Mem Inst Oswaldo Cruz. 1990;85:497–505. doi: 10.1590/s0074-02761990000400021. [DOI] [PubMed] [Google Scholar]
- 17.Silva H P S, Nussenzweig V. Sobre uma cepa de Trypanosoma cruzi altamente virulenta para o camundongo branco. Folia Clin Biol. 1953;20:191–208. [Google Scholar]
- 18.Stolf M A S. Trypanosoma cruzi antigen in serodiagnosis. In: Wendel S, Brener Z, Camargo M E, Rassi A, editors. Chagas' disease (American trypanosomiasis): its impact on transfusion and clinical medicine. São Paulo, Brazil: ISBT Brazil'92; 1992. pp. 195–205. [Google Scholar]
- 19.Umezawa E S, Bastos S, Camargo M E, Yamauchi L M, Santos M R, Gonzalez A, Zingales B, Levin M J, Souza O, Rangel-Aldao R, da Silveira J F. Evaluation of recombinant antigens for serodiagnosis of Chagas' disease in South and Central America. J Clin Microbiol. 1999;37:1554–1560. doi: 10.1128/jcm.37.5.1554-1560.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umezawa E S, Nascimento M S, Kesper N, Jr, Coura J R, Borges-Pereira J, Junqueira A C V, Camargo M E. Immunoblot assay using excreted-secreted antigens of Trypanosoma cruzi in serodiagnosis of congenital, acute, and chronic Chagas' disease. J Clin Microbiol. 1996;34:2143–2147. doi: 10.1128/jcm.34.9.2143-2147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Umezawa E S, Shikanai-Yasuda M A, Stolf M A S. Changes in isotype composition and antigen recognition of anti-Trypanosoma cruzi antibodies from acute to chronic Chagas disease. J Clin Lab Anal. 1996;10:407–413. doi: 10.1002/(SICI)1098-2825(1996)10:6<407::AID-JCLA16>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Wendel S, Gonzaga A L. Chagas' disease and blood transfusion: a new universe problem? Vox Sang. 1993;64:1–12. doi: 10.1111/j.1423-0410.1993.tb02507.x. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Control of tropical disease. Chagas' disease. A disease whose days are numbered. Geneva, Switzerland: World Health Organization; 1996. [Google Scholar]
- 24.Zingales B, Gruber A, Ramalho C B, Umezawa E S, Colli W. Use of recombinant proteins of Trypanosoma cruzi in the serological diagnosis of Chagas' disease. Mem Inst Oswaldo Cruz. 1990;85:519–522. doi: 10.1590/s0074-02761990000400024. [DOI] [PubMed] [Google Scholar]