Abstract
Infertility negatively impacts the overall health and social life of affected individuals and couples. Female infertility is their inability to perceive pregnancy. To date, polycystic ovary syndrome, primary ovarian insufficiency, fallopian tube obstruction, endometriosis, and intrauterine synechiae have been identified as the primary causes of infertility in women. However, despite the mutual efforts of clinicians and research scientists, the development of an effective treatment modality has met little success in combating female infertility. Intriguingly, significant research has demonstrated mesenchymal stem cells as an optimal source for treating infertility disorders. Therefore, here we attempted to capsulize to date available studies to summarize the therapeutic potential of mesenchymal stem cells in combating infertility in women by focusing on the underlying mechanism through which stem cells can reduce the effects of ovarian disorders. Furthermore, we also discussed the preclinical and clinical application of stem cell therapy, their limitation, and the future perspective to minimize these limitations.
Keywords: Pregnancy, Infertility, Female, Stem cell transplantation, Uterine diseases, Mesenchymal stem cells.
1. Introduction
Infertility affects 8-12% of couples and has become a common problem worldwide (1-3). According to the epidemiological categorization, a female is considered infertile if she remains unsuccessful even after several attempts to be pregnant (4, 5). An approximate estimation for the prevalence of overall infertility has indicated that 33-41% of infertility could be due to female cause, while male causes contribute 25-39%, and mixed causes are about 9-39% (6). The statistics reveal that women's infertility is among the most significant factors (7) due to its increasing epidemiology, affects millions of females worldwide (7, 8). Accordingly, ovulation disorders, i.e., hypothalamic dysfunction, primary ovarian insufficiency, or polycystic ovary (PCOS), are considered the major cause of female infertility. At the same time, endometriosis and tubal infertility are also among the preeminent cause of female infertility (7). The ovary is a complex and highly regulated organ where minor dysregulation can ultimately lead to infertility (9). While ovulation and hormonal testing are more commonly used methods for diagnosing female infertility.
Moreover, further advent in technology has introduced some assisted reproduction strategies i.e., intrauterine insemination, and in-vitro fertilization to minimize the damaging effects of ovarian dysfunction (1, 10-12). Other available treatment options include the intervention of supplements or antioxidants, such as zinc, vitamin E, and L-carnitine. Despite their frequent application, these treatment strategies have shown their limitations and side effects (1, 13). Hence, to date, there is a darn lack of an effective treatment strategy for curing female infertility (14). Therefore, researchers and clinician have been investigating more efficient and novel therapeutic measures for infertility. Among these strategies, stem cell therapy holds significant importance, being undifferentiated cells, their self-renewal for longer duration without developing any change (7). Nonetheless, as compared to other group of stem cell, mesenchymal stem cells (MSCs) have been indicated to possess advantages over other types of stem cells for being free of ethical concerns and teratoma formation (7, 15).
Herein we will discuss the published studies about the cause of infertility, to date available therapies, and application of MSCs to treat the infertility in women.
2. Prevalence and etiology of female infertility
Infertility is defined as primary and secondary infertility; the primary is attributed to a woman who never perceives pregnancy, while secondary infertility indicates a woman who perceives one successful pregnancy but later becomes incapable of perceiving pregnancy (14). The most common prevalence of primary infertility occurs in developed countries, while secondary infertility has been found as a major cause of infertility in developing countries (16). The other reason for infertility could be attributed to the presence of oocytes in a limited defined number (14) which naturally tend to decline with aging and ultimately enhance the risk of miscarriage (17). In addition to the abnormality of reproductive organs, the other major reason for female infertility is a disturbance of the central nervous system that controls the secretion of hormones in the reproductive system (14) further suggest the correlation between infertility and endocrine disorders that is attributed with the hormonal imbalance in the reproductive system. Though several factors attributed to female infertility remained unexplained to date (18), major research have reported the association of infertility with the complex intrinsic events which build on various factors such as the environmental or genetic factors, the age of patient, and etiology (1). Therefore, it is important to consider these factors to develop an efficient therapeutic strategy. However, considering the complexity of underlying signaling pathways of reproductive system disorders multiple molecular factors could be involved which not only cause ovarian dysfunctions but also impact individual's overall systemic health (9, 19). However, to date, the major factors and comprehensive mechanism with peculiar biomarkers that result in infertility remains unknown. Therefore, not a single efficient or effective treatment is available to inhibit the causes of female infertility disorder. Further, we will brief on the currently available treatment.
3. Currently available combat tactics for infertility fighting
As reported in the previous section, endocrine system disorder is among the major contributing factor to female infertility. Therefore, to date, hormone replacement therapy has been widely implicated to treat various types of infertility disorders. For instance, Clomid (a widely used gonadotropin) has been reported to trigger an excess generation of luteinizing hormone and follicle-stimulating hormone to be released by the pituitary gland, which induce the ovulation and promotes follicular growth (20) and ultimately results in the production of multiple eggs (1, 21). However, this therapeutic strategy carries the risk of breast cancer (22, 23). The other treatment option is fertility drugs to treat ovulation disorders (1). However, these drugs have exhibited some setbacks, such as preterm birth, ovarian hyperstimulation syndrome, multiple births, and ovarian tumor (20). Though surgical therapeutics strategies, i.e., assisted reproductive technologies, ovulation induction, hysteroscopic, laparoscopy, and fallopian tube surgeries (1), and superovulation have shown efficiency in optimizing the condition for perceiving pregnancy. However, the efficacy and safety of these strategies have not been completely evaluated (7), and the outcome of these strategies also remained unsatisfactory.
Nonetheless, despite the advancement in treatment strategies for fertility disorders, the overall percentage of infertility remained more than 80% (24). While the development of an effective therapeutic strategy not only requires considerable attention to the physical, psychological, economic, and time-related factors but also needs to apply the novel technology at the cellular level to understand the underlying molecular mechanism completely. Hence, it is essential to establish an alternative and novel therapeutic modality to cure female infertility. While despite being in their infancy, regenerative medicines offers a promising therapeutic candidate, a future set of comprehensive study is required for its clinical application. Accordingly, further, there will be a discussion about the function, applications, and limitations of stem cell therapeutic strategies.
4. Stem cell therapy in treating female infertility and significance of using MSCs
Based on the preclinical study data, stem cell therapy is the most promising candidate for treating infertility disorder. At the same time, further insight into its differentiation potential characterized stem cells as either pluripotent or multipotent. However, some studies have indicated that pluripotent stem cells or embryonic stem, i.e., germ-line stem cells or ovarian surface epithelial cells, can be used as cell sources for neo-oogenesis and oogenesis (25). Such as, an interesting recent finding has revealed the immunomodulatory properties of embryonic stem cells and demonstrated that by altering the gene expression profiles of mesenchymal stem-like cells, human embryonic stem cells (hESCs) can be generated from both normal (diploid) and abnormal (triploid) hESCs lines (26). However, due to an incomplete understanding of the major molecular events of oogenesis, i.e., follicle formation and meiosis, these results could not be reproduced in-vivo culture system (25). Hence, despite having a good experimental evidence, it is not ethically possible to use embryonic stem cells in a clinical setting, largely due to its risk of developing cancer.
However, on the other hand, MSCs, that are categorized as multipotent adult stem cells, have been extensively applied for research and clinical use due to their advantage of differentiation into multiple lineages of tissues/cells and having no ethical issue for its application (27-28). Thus, recent years have seen remarkable advancement in the clinical application of MSCs due to their safety and high efficacy against various disorders (29-32). For instance, MSCs therapy has been reported to positively affects chemotherapy-induced lesions, premature ovarian failure, and PCOS (33-37). Besides, the underlying mechanism indicates that stem cells can be used therapeutically for a variety of ailments that is attributed to the secretion of various mediators particularly cytokines that could effectively modulate the inflammatory and immune activation processes that significantly reduce inflammation-associated tissue damage (38-40). Hence, MSCs aid in the reduction of ovarian damage by targeting inflammation.
Nonetheless, it is also important to note that most of the above-mentioned research has been carried out using the rodent's model, which significantly differs from humans, particularly regarding the female reproductive system. Collectively, the above-mentioned studies suggest that MSCs therapy can provide longer reproductive life in larger animals, peculiarly, cattle that are more identical to humans, and present an economically admissible model (30). Accordingly, some latest preclinical trial studies and their outcome have been summarized (Table I). While preclinical trials and their application in large farm animals remained under consideration (41, 42). In the next section, we will discuss the various sources of stem cells and their role in the treatment of female infertility in light of previously reported studies.
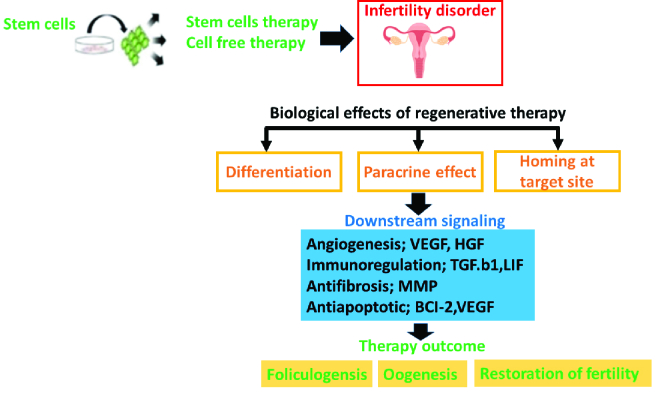
Although various types of MSCs, such as bone marrow-derived MSCs (BM-MSCs), umbilical cord-derived MSCs (UC-MSCs), and endometrial derived MSCs (EnMSCs) have been reported to demonstrate significant therapeutic efficiency against infertility, however, regarding female infertility, some unusual and less researched stem cell lines such as peritoneum MSCs (PeMSC, which is derived from peritoneum mesothelium consisting of intraperitoneal or intestinal space) have been found to differentiate into ovarian cell-like cells (43). While another study has demonstrated the positive outcome of PeMSCs in clinical trials (44). Accordingly, we have presented a schematic presentation of a general overview of the effects of MSCs based cell therapy for infertility disorders in figure 1.
Hence, collectively stating that despite having the unique ability to restore the quality and quantity of oocytes MSC possesses, there is a lack of clinical evidence that could prove and validate the treatment of MSCs as a safe clinical therapeutic option for infertility disorders (30). Hence, the further sections, based on previously reported studies, will briefly highlight the role of a few important MSCs, i.e., UC-MSCs, BM-MSCs, and EnMSCs cell lines that have been widely reported in the recent literature for the treatment of infertility and have shown promising outcome to suggest a future set of study. This presented the cell-free therapeutic approach to treat PCOS, or maybe other poor-quality oocytes. Accordingly, we have summarized this few recent preclinical research in table I.
Table 1.
Summary of recently reported preclinical trials using stem cell therapy to treat female infertility
|
| |||||||
| Author, yr (Ref) | Types of models | Source of stem cells | Time of administration | Dose | Delivery route | Efficacy | Important findings |
| Xie et al. , 2019 (35) | PCOS in C57BL/6 mice was induced with DHEA | hUC-MScs | 21 days after the modeling | (2 106 passage 4 hUC-MSCs suspended in 0.2 ml normal saline | Tail vein injection | By inhibiting local (ovaries and uterus) and systemic inflammatory responses, hUC-MSC treatment effectively improved the pathological changes and function of PCOS in mice | A mouse model of DHEA-induced PCOS was alleviated by MScs by inhibiting inflammation |
| Kalhori et al. , 2018 (45) | NMRI mice PCOS was induced through daily subcutaneous injections of testosterone enanthate dissolved in sesame oil | BM-MSCs | At 1 and 14 day after experimental induction of PCOS | (106 MSCs/animal) | Injected into the mice through the tail vein | The number of oocytes increased significantly as folliculogenesis increased whereas the number of primary and preantral follicles decreased significantly | Polycystic ovaries in mice injected with BM-MSCs showed improved folliculogenesis, oocyte quality and endocrine function due to their antioxidant, anti-inflammatory and anti-apoptotic properties |
| Jafarzadeh et al. , 2018 (46) | PCOS, 21-day-old female NMRI mice received daily subcutaneous injections of 6 mg/100 g bodyweight dehydroepiandrosterone | hBM-MSC derived CM | Condition medium was exposed to oocytes at the GV stage | The cells in the third passage were seeded at a density of 1 104 cells/cm2. Then, the CM was concentrated 10 times (10 ) by centrifugation | Condition medium was exposed to oocytes at the GV stage | hBM-MSC-CM improved the oocyte -IVM, cytoplasmic maturation, fertilization and early embryo development rates | This study suggest that the cell-free therapeutic strategy can be efctive in the treatment of PCOS |
| Chugh et al. , 2021 (47) | For in vitro experiment, conditioned media from BM-hMSC was exposed to androgen-producing H293R cells and analyzed androgen-producing gene expression. For in vivo experiment, BM-hMSC were implanted into a letrozole-induced PCOS mouse model | hBM-MSCs | 5 wk after modeling | 5.0 105 cells per ovary resuspended in 10 μl PBS | Intra-ovarian injection of BM-hMSC via laparotomy | This research indicated the efficacy of intra-ovarian injection of secretome of BM-hMSC that effectively improved the PCOS-related symptoms | BM-hMSC or its secretome potentially reversed the inflammation caused by PCOS-via IL-10 secretion |
| PCOS: Polycystic ovary syndrome, DHEA: Dehydroepiandrosterone, hUC-MScs: Human umbilical cord-mesenchymal stem cells, BM-MSCs: Bone marrow mesenchymal stem cells, hBM-MSC: Human bone marrow mesenchymal stromal cells, CM: Condition medium, GV: Germinal vesicle, IVM: In vitro maturation, PBS: Phosphate buffered saline, IL-10: Interleukin-10 | |||||||
Figure 1.
Schematic presentation of general overview of the effects of mesenchymal stem cell (MSC) based cell therapy for infertility disorders by demonstrating the downstream signaling factors. Shortly, graphic presentation theoretically indicates that after exposure to the stem cell-based therapy or cell-free to ovarian or endometrial disorder, its biological effects (i.e., paracrine factors, differentiation, and migration to the target site) effectively aid in reducing the deleterious effects by aiding in folliculogenesis, oogenesis and ultimate recovery due to the production of vital downstream signaling factors.
UC-MSCs and their underlying mechanism for fertility disorders
The most prominent properties of UC-MSCs that make these cells a reliable option are easy to collect, self-renewal, and lower risk of tumor formation (48). According to previously reported study UC-MSCs can repair the premature ovarian failure by reducing the apoptosis in ovarian cell which exhibited the improvement in the function of ovary in animal model as well as in human trials (49-51). Besides, accumulated studies attempted to unravel the underlying mechanism which aid UC-MSCs in diminishing the damaging effects of ovarian disorders. Though number of signaling mechanism have been reported to date, however, the mitogen-activated protein kinase signaling pathway and insulin signaling pathway have been found to possess central role in treating the impact of ovarian damage (52). Moreover, UC-MSCs play their role in angiogenesis by stimulating the secretion of vital growth factors such as placental growth factor, transforming growth factor-beta 1, vascular endothelial growth factor, and hepatocyte growth factor (53). Moreover, UC-MSCs have also been reported to control fibrosis and enhance cell proliferation by upregulating the expression of vascular marker's which aid in alleviating the inflammation (54).
For instance, it has been found that UC-MSCs can overhaul the injured endometrium in the rat model. Intriguingly, UC-MSCs have been also been reported to overturn apoptosis in ovarian cells either through customary adjustment of the tunica albuginea and surface epithelium or by downregulating the caspase-3 and elevation in the expression of proliferating cell nuclear antigen, transforming growth factor-beta, and cytokeratin 8/18 (55). Furthermore, studies have illustrated that UC-MSCs could aid in the recovery of perimenopausal rats by accelerating the release of cytokines (53). At the same time, another study found that intramuscular injection of UC-MSCs can alleviate the marring effects on human endometrial cells (56). Importantly, a phase-I clinical trial has stated that collagen scaffolds containing UC-MSCs can effectively restore endometrial differentiation, vascularization, and proliferation by elevating the estrogen receptor α and angiogenic factors (57). These studies indicates UC-MSCs as the most reliable and widely used stem cells for not only research purpose to treat female infertility but also for preclinical and clinical trials; however, further clinical evidence is a prerequisite for their clinical application (9).
BM-MSCs
Stem cells derived from bone-marrow have been extensively used for multiple therapeutic purposes, yet its clinical application for the treatment of infertility in women remained elusive to date. Although, a previously reported study has used mice model of PCOS and found the improvement in endocrine function, oocyte quality, and folliculogenesis due to the anti-inflammatory, anti-apoptotic, and anti-oxidative properties of PCOS (45). Another interesting recent evidence from the larger animal has indicated that the autologous BM-MSCs isolated from juvenile macaques significantly restore the ovarian structure by reduction of cell apoptosis and aid in the regeneration of blood vessels and follicles which ultimately inhibited the fibrosis and prevent the ovarian aging (58). Besides, chemotherapy is considered one of the significant causes of infertility in female cancer survivals, which results in a drastic reduction of primordial follicles and ultimately causes premature ovarian failure. Moreover, another study has indicated that aged women (with history of being exposed to chemotherapy) after intra-ovarian administration of BM-MSCs exhibited the significant restoration of ovarian function (42). However, a comparative study suggested that ovarian stromal cells could significantly promote the maturation of ovarian follicle in the damaged ovary as compared to the BM-MSCs (59). However, there is a lack of data to support these findings. Thus, speculating that regarding female infertility disorder, it is necessary to seek out alternative novel sources of stem cells (more supportive to female reproductive system) rather than traditional applied common sources for stem cells therapy.
EnMSCs
Since EnMSCs are a promising candidate for treating infertility disorders due to their ability of self-renewal, high proliferation, and differentiation (60). It has been found that basal layer of the endometrium is an ample source EnMSCs; however, menstrual blood has also been indicated to contain EnMSCs, known as menstrual derived endometrial stem cells (MenSCs). Hence, representing a novel and promising source of MSCs to treat infertility in women and drawed the attention of clinicians as well as researchers (9).
Due to their convenient harvesting procedure with a high proliferation rate using noninvasive techniques, EnMSCs present no risk of rejection by the autoimmune system (61, 62). While EnMSCs have also been indicated to improve the proliferation and growth of injured endometrium by minimizing inflammation and fibrosis by elevating various growth differentiation factors 5 and signaling pathways (63, 64). EnMSCs, and hormonal stimulation, could preeminently restore the fertility potential in women affected by intrauterine synechiae (65), thus suggesting the significance of a combined treatment strategy.
Accordingly, a recent study has found that Yazd endometrial MSCs can not only increase, but also possess the potential to differentiate into various types of cells which make these cells an attractive source for clinical application particularly for the cure of patoents suffering from uterine-factor based infertility (66). Moreover, further studies have indicated that angiogenic and anti-inflammatory factors are the major contributor to the therapeutic effects of endometrial or MenSCs in eliminating the detrimental effects of intrauterine synechiae in an experimental rodent model (67). It could be due to the ability of MenSCs to regulate the protein kinase B signaling pathways that not only aid in the survival of MenSCs (68) but also provide a suitable environment for implanting embryos (69). Moreover, it has also been demonstrated that MenSCs enhance the endometrial thickness by elevating the expression of cell cycle inducers and reducing the expression of DNA damaging factors, which ultimately aids in perceiving the pregnancy for patients suffering from a uterus disorder such as intrauterine synechiae (67, 70-71). Notably, another piece of evidence has suggested that MenSCs can cure premature ovarian failure (POF) in young female patients (with a history of receiving chemotherapy). Hence, this suggesting the significant reparation potential of endometrial or MenSCs. Nonetheless, the underlying molecular mechanism which aid EnMSCs in curing infertility in women remain elusive to date, therefore, a precise set of comprehensive study design is prerequisite in future (72).
5. Role of cell-free therapy and female infertility disorders
SCs possess a powerful paracrine effect (contributing to their therapeutic effects), which not only pave the way for developing novel techniques also enhance the regenerative potential of SCs (73, 74). Importantly, due to the presence of micro vesicles, exosomes, and a variety of signaling factors, SCs-derived secretomes is considered as an effective therapeutic options (75), with the ability to repair of damaged tissue even in the absence of parent cells (76). Intriguingly, recent studies have also reported the effectiveness of condition medium from different cell sources for improving female infertility treatments. For instance, for assisted reproductive technology, in vitro maturation has reported being improved by using conditioned media from different sources (in vitro maturation). A higher level of female germ cell markers and granulosa cells was observed in female stem cells exposed to cumulus cells condition medium (CCCM) (44). Moreover, granulosa CCM has also been shown to improve follicle development in primordial mice. As a result, this provides evidence that human cumulus cells condition medium can support the IVM of mouse GV oocytes when derived from cultures of adherent cumulus cells (77). The effects testicular CCM has also been evaluated in vitro from embryonic stem cells on the development of female germ cells (78-80). For instance, testicular CCM was found to increase oocyte maturation in both mice and buffalo by secreting a variety of growth factors which enhance in vitro oogenesis (80). Intriguingly, humans also exhibit these factors (81).
Further research into the paracrine properties of stem cells has revealed that exosomes derived from stem cells have therapeutic potential to cure infertility in women. Exosomes are considered an optimal source for research due to their convenient isolation method and no risk of tumor formation (82, 83). For instance, it would be interesting to add that not only human UC-MSCs themselves can aid in the recovery of perimenopausal rats by accelerating the release of cytokines (51), but a study examining rat model of preeclampsia has reported that the UC-MSC-derived exosomes remarkably ameliorate the placenta by improving its morphology and angiogenesis in a dose-dependent manner (84). Adipocyte-derived exosomes injections have been shown to enhance ovarian function by reducing apoptosis and increasing follicle counts (70). On the other hand, a study on mice model of POF (that have been exposed to chemotherapy) demonstrated that exosomes derived from human amniotic epithelial cells contain transferring miRNAs that potentially aid in the repair of damaged ovary (85, 86).
Moreover, the use of exosomes (secreted by MSC) is also considered an alternative strategy to overcome the limitations of stem cell exposure, thus determining the advantage of cell-free therapy (43) and cell therapy (87). Hence, despite these promising experimental data, exosomes derived from stem cells have no reported clinical trial for the treatment of women's infertility. Therefore, to determine the precise clinical outcome of exosomes-based therapy for the cure of infertility in women, further preclinical data is required to establish the base for clinical trials.
6. Constraints in the clinical application of MSCs therapy for infertility disorders
Although there are few registered clinical trials studies in the NIH clinical trial database (www.clinicaltrials.gov) that exhibit the complete therapeutic outcome of stem cell therapy, although very few clinical studies have used stem cells to treat female infertility. However, to date available clinical trial results have shown the effectiveness of stem cell-based therapy to reviving the function of the damaged ovary to improve the regulation of hormonal and menstruation function (88). Accordingly, we have attempted to summarize some important clinical trial studies evaluating the potential of stem cells to cure infertility in females (Table II). This suggests that all studies remained under investigation and could not reach phase III for their practical application.
Several factors could determine the slow progress of stem cell therapy towards practical application. However, appropriate selection of participants, inclusion, and exclusion criteria is an important factors for the positive outcome of clinical trials. Advancing the clinical translation of preclinical studies is mainly constrained by the exclusion of pregnant or lactating patients, patients suffering from ovaries or breast cancer, with autoimmune diseases, and those with ovarian diseases such as endometriosis (89). However, it could be due to ethical and clinical concerns in each country regarding the healthcare policy (90).
Besides, the efficient clinical translation of preclinical trials remained the major challenge. This suggests that due to the difference in the microenvironment of human and animal models, the majority of animal trial studies could not be efficiently translated into human patients. Inadequacy for clinical translation of preclinical trials (which mainly carried on animals models) can be attributed to the difference in intrinsic microenvironments between animals and humans (91). While another study has presented the risk of developing autoimmune responses after stem cell transplantation, this aspect needs further research (92). Hence, at the moment, it is pretty hard to assume whether stem cell therapy could be effective in curing infertility in women or not. Therefore, a more comprehensive research design and study plans are required, which could preferably benefit patients with minimum risk of an immune response. Besides, it is also important to overcome various risk factors associated with the clinical application of MSCs. This could be attributed by establishing the safety parameters for the clinical application of MSCs (43) and by designing a real-time professional set up in preclinical trials to determine its outcome on clinical system, yet it remained challenging to date (93).
Table 2.
Summary of clinical trials using stem cell therapy to treat female infertility disorders
|
| ||||||||
| Author, yr | ClinicalTrials.gov Identifier | Study type | Type of cell | Type of infertility disorder | Patient's demography | Delivery route | Dose | Study outcome |
| Valeria Muller, 2017 | NCT03166189 | Interventional phase-II | BM-MSC | Female with Ashermen syndrome and infertility of uterine origin | Female at the age of 20-44 yr | endometrial injection of cells | 1 ml of suspension containing 5 million BM-MSCs | Open randomized trial of clinical efficiency and safety of cell product BM-MSC for reparative treatment of destructively changed endometrium in patients with repeated IVF failures |
| Hesham Elshaer, 2014 | NCT02043743 | Interventional phases 1 and 2 | huCART-meso cells | Female with POF | Female at the age of 18-40 yr | Intraovarian injection | 3-5 million MSCs injected into ovarian tissue | Autologous stem cells transplantation in patients with idiopathic and drug-induced premature ovarian failure |
| Guangzhi Liu, 2022 | NCT03816852 | Interventional phase 2 | Human UC-MSCs | Female up to 19-40 yr (Adult) | POI or POF | Intravenous infusion | From a high dose of 9*10 7 cells, 30 ml, to a low dose of 3*10 7 | The safety and efficiency study of MSCs in POI |
| Stem Cells Arabia, 2018 | NCT03069209 | Interventional phase 1 and phase 2 | Human BM-MSC | Females between the age of 20 to 39 yr | POF | Intraovarian transplantation | 6*10 7 cells, 30 ml | Autologous BM-MSC transplantation in patients with POF) aids folliculogenesis, normalizes the FSH level, and pregnancy occurred within 12 months of follow up |
| Hongmei Wang, 2021 | NCT03877471 | Interventional | Embryonic stem cell-derived MSC-like cell transplantation directly into bilateral ovaries | Females up to the age of 40 | POI | Ovary injection with MSC-like cells through transvaginal ultrasound | Patients received from low dose of 0.2 107 to a high dose of (1.0 107) cells | MSCs - like cell transplantation in women with primary ovarian insufficiency |
| BM-MSC: Bone marrow-derived mesenchymal stem cells, IVF: In vitro fertilization, huCART-meso cells: Human chimeric antigen receptor-modified T-meso cells, POF: Premature ovarian failure, MSCs: Mesenchymal stem cells, UC-MSCs: Umbilical cord mesenchymal stem cells, POI: Premature ovarian insufficiency, FSH: Follicle stimulating hormone | ||||||||
7. Challenges and future perspective for application of stem cell therapy infertility disorder
The fact that engineered MSCs combined with the scaffold technique are considered a promising candidate to cure infertility-related disorders in females (43). However, therapeutic application of stem cell therapy remained restricted to few countries due to several technical and ethical limitations (89). Thus, this section will determine the challenges and some potential novel strategies to overcome these challenges. Besides, optimizing extraction and transplantation method of stem cells also remained the major concern for its therapeutic application (92). Besides, the route for administration of stem cell injection is also an important factors effecting the outcome of stem cell therapy (93). For instance, direct administration of stem cells into the ovaries is applied when the target is to restore ovarian function, while intravenous (i.v.) or intraperitoneal (i.p.) that allows fair distribution of stem cells through blood strea, can be used to evaluate the effects of stem cell transplantation for multiple organ system (89).
To overcome the limitations of stem cell exposure, recently, cell-free therapy has been developed (43) and reported to hold several advantages in cell therapy (88). For this purpose, the most practical approach is using exosomes (secreted by MSC), as discussed in the previous section. In addition to cellular therapy, the therapeutic sequel of stem cell treatment can also be improved by combined therapeutic strategies. For instance, biomaterials are increasingly being integrated for fertility disorders, not only to reduce the shear stress caused by stem cell injection but also enhance the probability of cell survival after administration (93). It has been demonstrated that the combined use of collagen scaffolds and stem cells aids in the rapid spread of stem cells to the targeted tissues or organs and enhances the probability of transplanted cells survival at the initial phase of transplantation in-vivo (43). Consistently, its has been shown in an experimental study that combined application of collagen scaffold with UC-MSCs in patients suffering from POF exhibited the successful activation of follicles in dormant ovaries (43). Although integrated therapeutic strategies are considered a potential candidate for developing a safe and efficient therapeutic strategy to overcome the clinical challenges associated with infertility disorders, precise understanding of the signaling mechanism remained elusive. Nonetheless, stem cells-derived exosomes and their vital content, such as miRNAs have also been suggested as the promising therapeutic candidate in the treatment of various ovarian dysfunction, particularly via folliculogenesis and genetic stability and vascular formation (91). However, further studies are required to unveil the exact mechanism. Taken together, despite of these novel molecular therapeutic modalities, further advent in the research and comprehensive knowledge of the underlying molecular mechanism is prerequisite to treat female fertility disorders.
8. Conclusions
Developing an efficient therapeutic strategy based on the regenerative properties of stem cells holds the confidence and hope of scientists, clinicians, and patients suffering from fertility disorders. However, to obtain significant breakthroughs for the cure of infertility in women and for successful clinical translation of precise and well-designed study plan (from the isolation of stem cells or stem cell-derived molecules to informed, voluntary consent and model of cell delivery including) is essentially required for the success of initial clinical trials in the light of previously reported studies to overcome the limitations. Taken together, the present study attempted to present an overview of previous continued attempts and research studies to sort out the complex web of stem cell-derived therapeutic strategies and their role in treating female fertility disorders.
Conflicts of Interest
The authors declare that they have no competing interest.
References
- Wang J, Liu Ch, Fujino M, Tong G, Zhang Q, Li XK, et al. Stem cells as a resource for treatment of infertility-related diseases. Curr Mol Med. 2019;19:519–546. doi: 10.2174/1566524019666190709172636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra BL, Skandhan KP, Prasad BS, Pawankumar G, Singh G, Jaya V. Male infertility rate: A retrospective study. Urologia. 2018;85:22–24. doi: 10.5301/uj.5000254. [DOI] [PubMed] [Google Scholar]
- Hansen KR, He AL, Styer AK, Wild RA, Butts S, Engmann L, et al. Predictors of pregnancy and live-birth in couples with unexplained infertility after ovarian stimulation-intrauterine insemination. Fertil Steril. 2016;105:1575–1583. doi: 10.1016/j.fertnstert.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurunath S, Pandian Z, Anderson RA, Bhattacharya S. Defining infertility: A systematic review of prevalence studies. Hum Reprod Update. 2011;17:575–588. doi: 10.1093/humupd/dmr015. [DOI] [PubMed] [Google Scholar]
- Bushnik T, Cook JL, Yuzpe AA, Tough S, Collins J. Estimating the prevalence of infertility in Canada. Hum Reprod. 2012;27:738–746. doi: 10.1093/humrep/der465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroux A, Dumestre-Perard Ch, Dunand-Faure C, Bouillet L, Hoffmann P. Female infertility and serum auto-antibodies: A systematic review. Clin Rev Allergy Immunol. 2017;53:78–86. doi: 10.1007/s12016-016-8586-z. [DOI] [PubMed] [Google Scholar]
- Zhao Y-X, Chen Sh-R, Su P-P, Huang F-H, Shi Y-Ch, Shi Q-Y, et al. Using mesenchymal stem cells to treat female infertility: An update on female reproductive diseases. Stem Cells Int. 2019;2019:9071720. doi: 10.1155/2019/9071720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull MG, Glazener CM, Kelly NJ, Conway DI, Foster PA, Hinton RA, et al. Population study of causes, treatment, and outcome of infertility. Br Med J. 1985;291:1693–1697. doi: 10.1136/bmj.291.6510.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfandyari S, Chugh RM, Park H-S, Hobeika E, Ulin M, Al-Hendy A. Mesenchymal stem cells as a bio organ for treatment of female infertility. Cells. 2020;9:2253. doi: 10.3390/cells9102253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn DD, Bates GW. Evidence-based approach to unexplained infertility: A systematic review. Fertil Steril. 2016;105:1566–1574. doi: 10.1016/j.fertnstert.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Ohannessian A, Loundou A, Gnisci A, Paulmyer-Lacroix O, Perrin J, Courbiere B. Unexplained infertility: Live-birth's prognostic factors to determine the ART management. Minerva Ginecol. 2017;69:526–532. doi: 10.23736/S0026-4784.17.04085-0. [DOI] [PubMed] [Google Scholar]
- Cissen M, Bensdorp A, Cohlen BJ, Repping S, de Bruin JP, van Wely M. Assisted reproductive technologies for male subfertility. Cochrane Database Syst Rev. 2016;2:CD000360. doi: 10.1002/14651858.CD000360.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inhorn MC, Patrizio P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21:411–426. doi: 10.1093/humupd/dmv016. [DOI] [PubMed] [Google Scholar]
- Rungsiwiwut R, Virutamasen P, Pruksananonda K. Mesenchymal stem cells for restoring endometrial function: An infertility perspective. Reprod Med Biol. 2021;20:13–19. doi: 10.1002/rmb2.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv Cancer Res. 2008;100:133–158. doi: 10.1016/S0065-230X(08)00005-5. [DOI] [PubMed] [Google Scholar]
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: A systematic analysis of 277 health surveys. PLoS Med. 2012;9:e1001356. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May-Panloup P, Boucret L, Chao de la Barca JM, Desquiret-Dumas V, Ferré-L'Hotellier V, Morinière C, et al. Ovarian ageing: The role of mitochondria in oocytes and follicles. Hum Reprod Update. 2016;22:725–743. doi: 10.1093/humupd/dmw028. [DOI] [PubMed] [Google Scholar]
- Al-Inany H. Female infertility. Clin Evid. 2006;15:2465–2487. [PubMed] [Google Scholar]
- Jobling Ph, O'Hara K, Hua S. Female reproductive tract pain: Targets, challenges, and outcomes. Front Pharmacol. 2014;5:17. doi: 10.3389/fphar.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourad S, Brown J, Farquhar C. Interventions for the prevention of OHSS in ART cycles: An overview of Cochrane reviews. Cochrane Database Syst Rev. 2017;1:CD012103. doi: 10.1002/14651858.CD012103.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaparrotta A, Chiarelli F, Verrotti A. Potential teratogenic effects of clomiphene citrate. Drug Saf. 2017;40:761–769. doi: 10.1007/s40264-017-0546-x. [DOI] [PubMed] [Google Scholar]
- Holmberg L, Iversen OE, Rudenstam CM, Hammar M, Kumpulainen E, Jaskiewicz J, et al. Increased risk of recurrence after hormone replacement therapy in breast cancer survivors. J Natl Cancer Inst. 2008;100:475–482. doi: 10.1093/jnci/djn058. [DOI] [PubMed] [Google Scholar]
- Vermeulen RFM, Korse CM, Kenter GG, Brood-van Zanten MMA, Beurden MV. Safety of hormone replacement therapy following risk-reducing salpingo-oophorectomy: Systematic review of literature and guidelines. Climacteric. 2019;22:352–360. doi: 10.1080/13697137.2019.1582622. [DOI] [PubMed] [Google Scholar]
- Balen AH, Morley LC, Misso M, Franks S, Legro RS, Wijeyaratne CN, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: An analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. 2016;22:687–708. doi: 10.1093/humupd/dmw025. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Hikabe O, Obata Y, Hirao Y. Reconstitution of mouse oogenesis in a dish from pluripotent stem cells. Nat Protoc. 2017;12:1733–1744. doi: 10.1038/nprot.2017.070. [DOI] [PubMed] [Google Scholar]
- Javidpou M, Seifati SM, Farashahi-Yazd E, Hajizadeh-Tafti F, Golzadeh J, Akyash F, et al. Mesenchymal stem/stromal-like cells from diploid and triploid human embryonic stem cells display different gene expression profiles. Iran Biomed J. 2021;25:99–105. doi: 10.29252/ibj.25.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM. Concise review: Multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl Med. 2017;6:2173–2185. doi: 10.1002/sctm.17-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah JS, Sabouni R, Cayton Vaught KC, Owen CM, Albertini DF, Segars JH. Biomechanics and mechanical signaling in the ovary: A systematic review. J Assist Reprod Genet. 2018;35:1135–1148. doi: 10.1007/s10815-018-1180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PK, Krishna M, Chullikana A, Desai S, Murugesan R, Dutta S, et al. Administration of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells in critical limb ischemia due to Buerger's disease: Phase II study report suggests clinical efficacy. Stem Cells Transl Med. 2017;6:689–699. doi: 10.5966/sctm.2016-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malard PF, Peixer MAS, Grazia JG, Brunel HDSS, Feres LF, Villarroel CL, et al. Intraovarian injection of mesenchymal stem cells improves oocyte yield and in vitro embryo production in a bovine model of fertility loss. Sci Rep. 2020;10:8018. doi: 10.1038/s41598-020-64810-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball LM, Bernardo ME, Roelofs H, van Tol MJ, Contoli B, Zwaginga JJ, et al. Multiple infusions of mesenchymal stromal cells induce sustained remission in children with steroid-refractory, grade III-IV acute graft-versus-host disease. Br J Haematol. 2013;163:501–509. doi: 10.1111/bjh.12545. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Gallia A, Sgarella A, Kruzliak P, Gobbi PG, Corazza GR. Long-term follow-up of Crohn disease fistulas after local injections of bone marrow-derived mesenchymal stem cells. Mayo Clin Proc. 2015;90:747–755. doi: 10.1016/j.mayocp.2015.03.023. [DOI] [PubMed] [Google Scholar]
- Kim TH, Choi JH, Jun Y, Lim SM, Park S, Paek JY, et al. 3D-cultured human placenta-derived mesenchymal stem cell spheroids enhance ovary function by inducing folliculogenesis. Sci Rep. 2018;8:15313. doi: 10.1038/s41598-018-33575-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Lu J, Ding C, Zou Q, Wang W, Li H. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. Stem Cell Res Ther. 2018;9:216. doi: 10.1186/s13287-018-0953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Xiong X, Xiao N, He K, Chen M, Peng J, et al. Mesenchymal stem cells alleviate DHEA-induced polycystic ovary syndrome (PCOS) by inhibiting inflammation in mice. Stem Cells Int. 2019;2019:9782373. doi: 10.1155/2019/9782373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Yu Q, Huang H, Deng W, Cao X, Adu-Frimpong M, et al. Human chorionic plate-derived mesenchymal stem cells transplantation restores ovarian function in a chemotherapy-induced mouse model of premature ovarian failure. Stem Cell Res Ther. 2018;9:81. doi: 10.1186/s13287-018-0819-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SY, Yoon JA, Park M, Shin EY, Jung S, Lee JE, et al. Recovery of ovarian function by human embryonic stem cell-derived mesenchymal stem cells in cisplatin-induced premature ovarian failure in mice. Stem Cell Res Ther. 2020;11:255. doi: 10.1186/s13287-020-01769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. Why are MSCs therapeutic? New data: New insight. J Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Bravo M, Carvalho JL, Saldanha-Araujo F. Adenosine production: A common path for mesenchymal stem-cell and regulatory T-cell-mediated immunosuppression. Purinergic Signal. 2016;12:595–609. doi: 10.1007/s11302-016-9529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad R, Saldanha-Araujo F. Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: What do we know so far? Biomed Res Int 2014; 2014: 216806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L-B, Peng S-Y, Chou C-J, Chen Y-J, Shiu J-S, Tu P-A, et al. Therapeutic potential of amniotic fluid stem cells to treat bilateral ovarian dystrophy in dairy cows in a subtropical region. Reprod Domest Anim. 2018;53:433–441. doi: 10.1111/rda.13123. [DOI] [PubMed] [Google Scholar]
- Grady ST, Watts AE, Thompson JA, Penedo MCT, Konganti K, Hinrichs K. Effect of intra-ovarian injection of mesenchymal stem cells in aged mares. J Assist Reprod Genet. 2019;36:543–556. doi: 10.1007/s10815-018-1371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YX, Ji J, Shan F, Li J, Hu R. Human mesenchymal stem cell treatment of premature ovarian failure: New challenges and opportunities. Stem Cell Res Ther. 2021;12:161. doi: 10.1186/s13287-021-02212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaeian L, Eftekhari-Yazdi P, Esfandiari F, Eivazkhani F, Rezazadeh Valojerdi M, Moini A, et al. Induction of mouse peritoneum mesenchymal stem cells into germ cell-like cells using follicular fluid and cumulus cells-conditioned media. Stem Cells Dev. 2019;28:554–564. doi: 10.1089/scd.2018.0149. [DOI] [PubMed] [Google Scholar]
- Kalhori Z, Azadbakht M, Soleimani Mehranjani M, Shariatzadeh MA. Improvement of the folliculogenesis by transplantation of bone marrow mesenchymal stromal cells in mice with induced polycystic ovary syndrome. Cytotherapy. 2018;20:1445–1458. doi: 10.1016/j.jcyt.2018.09.005. [DOI] [PubMed] [Google Scholar]
- Jafarzadeh H, Nazarian H, Ghaffari Novin M, Shams Mofarahe Z, Eini F, Piryaei A. Improvement of oocyte in vitro maturation from mice with polycystic ovary syndrome by human mesenchymal stromal cell-conditioned media. J Cell Biochem. 2018;119:10365–10375. doi: 10.1002/jcb.27380. [DOI] [PubMed] [Google Scholar]
- Chugh RM, Park HS, El Andaloussi A, Elsharoud A, Esfandyari S, Ulin M, et al. Mesenchymal stem cell therapy ameliorates metabolic dysfunction and restores fertility in a PCOS mouse model through interleukin-10. Stem Cell Res Ther. 2021;12:388. doi: 10.1186/s13287-021-02472-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J Stem Cells. 2014;6:195–202. doi: 10.4252/wjsc.v6.i2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed SA, Shalaby S, Brakta S, Elam L, Elsharoud A, Al-Hendy A. Umbilical cord blood mesenchymal stem cells as an infertility treatment for chemotherapy induced premature ovarian insufficiency. Biomedicines. 2019;7:7. doi: 10.3390/biomedicines7010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Zhong Y, Qian C, Zou Q, Ou J, Shi Y, et al. Human umbilical cord mesenchymal stem cells therapy in cyclophosphamide-induced premature ovarian failure rat model. Biomed Res Int. 2016;2016:2517514. doi: 10.1155/2016/2517514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu SF, Hu HB, Xu HY, Fu XF, Peng DX, Su WY, et al. Human umbilical cord mesenchymal stem cell transplantation restores damaged ovaries. J Cell Mol Med. 2015;19:2108–2117. doi: 10.1111/jcmm.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Sh, Yu L, Sun M, Mu S, Wang C, Wang D, et al. The therapeutic potential of umbilical cord mesenchymal stem cells in mice premature ovarian failure. Biomed Res Int. 2013;2013:690491. doi: 10.1155/2013/690491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Ch The roles of different stem cells in premature ovarian failure. Curr Stem Cell Res Ther. 2020;15:473–481. doi: 10.2174/1574888X14666190314123006. [DOI] [PubMed] [Google Scholar]
- Li J, Mao QX, He JJ, She HQ, Zhang Zh, Yin ChY. Human umbilical cord mesenchymal stem cells improve the reserve function of perimenopausal ovary via a paracrine mechanism. Stem Cell Res Ther. 2017;8:55. doi: 10.1186/s13287-017-0514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li Y, Guan CY, Tian S, Lv XD, Li JH, et al. Therapeutic effect of human umbilical cord-derived mesenchymal stem cells on injured rat endometrium during its chronic phase. Stem Cell Res Ther. 2018;9:36. doi: 10.1186/s13287-018-0777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfayomy AK, Almasry SM, El-Tarhouny SA, Eldomiaty MA. Human umbilical cord blood-mesenchymal stem cells transplantation renovates the ovarian surface epithelium in a rat model of premature ovarian failure: Possible direct and indirect effects. Tissue Cell. 2016;48:370–382. doi: 10.1016/j.tice.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Yang X, Zhang M, Zhang Y, Li W, Yang B. Mesenchymal stem cells derived from wharton jelly of the human umbilical cord ameliorate damage to human endometrial stromal cells. Fertil Steril. 2011;96:1029–1036. doi: 10.1016/j.fertnstert.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Cao Y, Sun H, Zhu H, Zhu X, Tang X, Yan G, et al. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: A phase I clinical trial. Stem Cell Res Ther. 2018;9:192. doi: 10.1186/s13287-018-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Ch, He J, An Y, Yang Z, Yan D, Pan H, et al. Bone marrow mesenchymal stem cells derived from juvenile macaques reversed ovarian ageing in elderly macaques. Stem Cell Res Ther. 2021;12:460. doi: 10.1186/s13287-021-02486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besikcioglu HE, Saribas GS, Ozogul C, Tiryaki M, Kilic S, Pinarli FA, et al. Determination of the effects of bone marrow derived mesenchymal stem cells and ovarian stromal stem cells on follicular maturation in cyclophosphamide induced ovarian failure in rats. Taiwan J Obstet Gynecol. 2019;58:53–59. doi: 10.1016/j.tjog.2018.11.010. [DOI] [PubMed] [Google Scholar]
- Xu Sh, Chan RWS, Ng EHY, Yeung WSB. Spatial and temporal characterization of endometrial mesenchymal stem-like cells activity during the menstrual cycle. Exp Cell Res. 2017;350:184–189. doi: 10.1016/j.yexcr.2016.11.020. [DOI] [PubMed] [Google Scholar]
- Meng X, Ichim TE, Zhong J, Rogers A, Yin Z, Jackson J, et al. Endometrial regenerative cells: A novel stem cell population. J Transl Med. 2007;5:57. doi: 10.1186/1479-5876-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Zh, Patel AN, Ichim TE, Riordan NH, Wang H, Min WP, et al. Feasibility investigation of allogeneic endometrial regenerative cells. J Transl Med. 2009;7:15. doi: 10.1186/1479-5876-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li P, Yuan Z, Tan J. Platelet-rich plasma improves therapeutic effects of menstrual blood-derived stromal cells in rat model of intrauterine adhesion. Stem Cell Res Ther. 2019;10:61. doi: 10.1186/s13287-019-1155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Sh-X, Wang J, Wang X-L, Ali A, Wu L-M, Liu Y-Sh. Feasibility analysis of treating severe intrauterine adhesions by transplanting menstrual blood-derived stem cells. Int J Mol Med. 2018;41:2201–2212. doi: 10.3892/ijmm.2018.3415. [DOI] [PubMed] [Google Scholar]
- Tan J, Li P, Wang Q, Li Y, Li X, Zhao D, et al. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman's syndrome. Hum Reprod. 2016;31:2723–2729. doi: 10.1093/humrep/dew235. [DOI] [PubMed] [Google Scholar]
- Akyash F, Javidpou M, Farashahi Yazd E, Golzadeh J, Hajizadeh-Tafti F, Aflatoonian R, et al. Characteristics of the human endometrial regeneration cells as a potential source for future stem cell-based therapies: A lab resources study. Int J Reprod BioMed. 2020;18:943–950. doi: 10.18502/ijrm.v13i11.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domnina A, Novikova P, Obidina J, Fridlyanskaya I, Alekseenko L, Kozhukharova I, et al. Human mesenchymal stem cells in spheroids improve fertility in model animals with damaged endometrium. Stem Cell Res Ther. 2018;9:50. doi: 10.1186/s13287-018-0801-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Song K, Zhang J, Zhang Y, Tan BZh. Effects of menstrual bloodderived stem cells on endometrial injury repair. Mol Med Rep. 2019;19:813–820. doi: 10.3892/mmr.2018.9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Jiang Y, Pan Y, Shi L, Zhang S. Human menstrual blood-derived stem cells promote the repair of impaired endometrial stromal cells by activating the p38 MAPK and AKT signaling pathways. Reprod Biol. 2018;18:274–281. doi: 10.1016/j.repbio.2018.06.003. [DOI] [PubMed] [Google Scholar]
- Yan Zh, Guo F, Yuan Q, Shao Y, Zhang Y, Wang H, et al. Endometrial mesenchymal stem cells isolated from menstrual blood repaired epirubicin-induced damage to human ovarian granulosa cells by inhibiting the expression of Gadd45b in cell cycle pathway. Stem Cell Res Ther. 2019;10:4. doi: 10.1186/s13287-018-1101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Zh, Wang Y, Yang T, Li J, Yang X. Study of the reparative effects of menstrual-derived stem cells on premature ovarian failure in mice. Stem Cell Res Ther. 2017;8:49. doi: 10.1186/s13287-016-0458-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherqui S, Kingdon KM, Thorpe C, Kurian SM, Salomon DR. Lentiviral gene delivery of vMIP-II to transplanted endothelial cells and endothelial progenitors is proangiogenic in vivo. Mol Ther. 2007;15:1264–1272. doi: 10.1038/sj.mt.6300183. [DOI] [PubMed] [Google Scholar]
- Huang J, Zhang Z, Guo J, Ni A, Deb A, Zhang L, et al. Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ Res. 2010;106:1753–1762. doi: 10.1161/CIRCRESAHA.109.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhang SH, Lee S, Shin JY, Lee TJ, Jang HK, Kim BS. Efficacious and clinically relevant conditioned medium of human adipose-derived stem cells for therapeutic angiogenesis. Mol Ther. 2014;22:862–872. doi: 10.1038/mt.2013.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med. 2014;92:387–397. doi: 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- Adib M, Seifati SM, Ashkezari MD, Khoradmehr A, Rezaee-Ranjbar-Sardari R, Tahajjodi SS, Aflatoonian B. The effect of the human cumulus cells-conditioned medium on in vitro maturation of mouse oocyte: An experimental study. Int J Reprod BioMed. 2020;18:1019–1028. doi: 10.18502/ijrm.v18i12.8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adib M, Seifati SM, Dehghani Ashkezari M, Akyash F, Khoradmehr A, Aflatoonian B. Effect of human testicular cells conditioned medium on in vitro maturation and morphology of mouse oocytes. Int J Fertil Steril. 2020;14:175–184. doi: 10.22074/ijfs.2020.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacham-Kaplan O, Chy H, Trounson A. Testicular cell conditioned medium supports differentiation of embryonic stem cells into ovarian structures containing oocytes. Stem Cells. 2006;24:266–273. doi: 10.1634/stemcells.2005-0204. [DOI] [PubMed] [Google Scholar]
- Shah SM, Saini N, Singh MK, Manik R, Singla SK, Palta P, et al. Testicular cell-conditioned medium supports embryonic stem cell differentiation toward germ lineage and to spermatocyte-and oocyte-like cells. Theriogenology. 2016;86:715–729. doi: 10.1016/j.theriogenology.2016.02.025. [DOI] [PubMed] [Google Scholar]
- Sutton-McDowall ML, Mottershead DG, Gardner DK, Gilchrist RB, Thompson JG. Metabolic differences in bovine cumulus-oocyte complexes matured in vitro in the presence or absence of folliclestimulating hormone and bone morphogenetic protein 15. Biol Reprod. 2012;87:87. doi: 10.1095/biolreprod.112.102061. [DOI] [PubMed] [Google Scholar]
- Lou G, Chen Zh, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 2017;49:e346. doi: 10.1038/emm.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Lyden D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat Protoc. 2019;14:1027–1053. doi: 10.1038/s41596-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong ZH, Wei J, Lu MQ, Jin MY, Geng HL. Protective effect of human umbilical cord mesenchymal stem cell exosomes on preserving the morphology and angiogenesis of placenta in rats with preeclampsia. Biomed Pharmacother. 2018;105:1240–1247. doi: 10.1016/j.biopha.2018.06.032. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Sun J, Huang Y, Bu Sh, Guo Y, Gu T, et al. Human amniotic epithelial cell-derived exosomes restore ovarian function by transferring MicroRNAs against apoptosis. Mol Ther Nucleic Acids. 2019;16:407–418. doi: 10.1016/j.omtn.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao GY, Cheng Ch-Ch, Chiang Y-Sh, Cheng WT-K, Liu I-H, Wu Sh-Ch. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci Rep. 2016;6:23120. doi: 10.1038/srep23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33:2158–2168. doi: 10.1002/stem.1771. [DOI] [PubMed] [Google Scholar]
- Na J, Kim GJ. Recent trends in stem cell therapy for premature ovarian insufficiency and its therapeutic potential: A review. J Ovarian Res. 2020;13:74. doi: 10.1186/s13048-020-00671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pean ChA, Kingery MT, Strauss E, Bosco JA, Halbrecht J. Direct-to-consumer advertising of stem cell clinics: Ethical considerations and recommendations for the health-care community. J Bone Joint Surg Am. 2019;101:e103. doi: 10.2106/JBJS.19.00266. [DOI] [PubMed] [Google Scholar]
- Chen L, Guo S, Wei C, Li H, Wang H, Xu Y. Effect of stem cell transplantation of premature ovarian failure in animal models and patients: A meta-analysis and case report. Exp Ther Med. 2018;15:4105–4118. doi: 10.3892/etm.2018.5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Cantore A, Chaimov D, Orlando G. Regenerative medicine: The red planet for clinicians. Intern Emerg Med. 2019;14:911–921. doi: 10.1007/s11739-019-02126-z. [DOI] [PubMed] [Google Scholar]
- Herraiz S, Buigues A, Díaz-García C, Romeu M, Martínez S, Gómez-Seguí I, et al. Fertility rescue and ovarian follicle growth promotion by bone marrow stem cell infusion. Fertil Steril. 2018;109:908–918. doi: 10.1016/j.fertnstert.2018.01.004. [DOI] [PubMed] [Google Scholar]
- Aguado BA, Mulyasasmita W, Su J, Lampe KJ, Heilshorn SC. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A. 2012;18:806–815. doi: 10.1089/ten.tea.2011.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]