Abstract
Background
Cognitive impairment is a major challenge for elderlies, as it can progress in a rapid manner and effective treatments are limited. Sarcopenic elderlies have a higher risk of dementia. This scoping review aims to reveal whether muscle is a mediator of cognitive function from pre-clinical evidence.
Methods
PubMed, Embase, and Web of Science were searched to Feb 2nd, 2022, using the keywords (muscle) AND (cognition OR dementia OR Alzheimer) AND (mouse OR rat OR animal). The PRISMA guideline was used in this study.
Results
A total of 17 pre-clinical studies were selected from 7638 studies. 4 studies reported that muscle atrophy and injury harmed memory, functional factors, and neurons in the brain for rodents with or without Alzheimer's disease (AD). 3 studies observed exercise induced muscle to secrete factors, including lactate, fibronectin type III domain-containing protein 5 (FNDC5), and cathepsin B, which plays essential roles in the elevation of cognitive functions and brain-derived neurotrophic factor (BDNF) levels. Muscle-targeted treatments including electrical stimulation and intramuscular injections had effective remote effects on the hippocampus. 6 studies showed that muscle-specific overexpression of scFv59 and Neprilysin, or myostatin knockdown alleviated AD symptoms. 1 study showed that muscle insulin resistance also led to deficient hippocampal neurogenesis in MKR mice.
Conclusions
The skeletal muscle is involved in the mediation of cognitive function. The evidence was established by the response in the brain (altered number of neurons, functional factors, and other AD pathological characteristics) with muscle atrophy or injury, muscle secretory factors, and muscle-targeted treatments.
The translational potential of this paper
This study summarizes the current evidence in how muscle affects cognition in molecular levels, which supports muscle-specific treatments as potential clinical strategies to prevent cognitive dysfunction.
Keywords: Muscle, Cognitive function, Brain, Animal model, Review
1. Introduction
The brain is a vital part of the central nervous system (CNS), dominating our cognitive function and movements. On the other hand, the skeletal muscle is the largest organ of peripheral tissues, and responds to instructions from the brain via signal transductions by motor neurons. With the ageing population, patients suffering from dementia and sarcopenia has risen significantly [1,2]. The estimated global prevalence of dementia has been projected to 152.8 million by 2050, which is almost 3-fold higher than in 2019 [2]. The healthcare cost for Alzheimer's disease (AD) was also estimated to be $305 billion in 2020 [3]. Sarcopenia is as an age-related muscle disorder, characterized by progressive loss of muscle mass and function [4]. The prevalence of sarcopenia is approximately 27% in the elderly [1]. Similar to dementia and AD, sarcopenia is also strongly associated with higher mortality, disability and poor quality of life [[5], [6], [7], [8]]. Interestingly, cross-sectional studies have shown that muscle strength and functional performance were inversely related to cognitive function in the older population [9,10]. Recent literature has reported that sarcopenia was independently associated with cognitive impairment [11], with odds ratio 2.85 times higher compared to control subjects [12]. Brain disorders contribute to malnutrition and muscle disuse, and may ultimately lead to sarcopenia [13,14]. A cohort study also reported that sarcopenic individuals had a faster rate of cognitive decline and higher risks of incident AD (hazard ratio 1.50, 95% CI 1.20 to1.86) after following an average of 5.6 years [15]. Recently, a larger cohort involving over 8000 elderlies demonstrated that the 3-year cognitive decline was faster in old people with lower lean mass [16]. This had led to postulations of the mediating role of muscle in cognitive function.
Irreversible memory loss and brain dysfunctions brought by AD has led to substantial research. Unfortunately, there are still limited efficient strategies to suspend the progression of AD. Similar to the brain, the skeletal muscle has an abundant amount of nerves, and degenerate together with the CNS with age [17]. Recent evidence from pre-clinical studies also showed that muscle loss promoted cognitive dysfunction in mice with AD [18]. As of now, numerous treatments have dual effects on the muscle and brain, especially the function of the hippocampus, including exercise and dietary supplements. For instance, aerobic and resistance training, alpha-ketoglutarate supplements, and ketogenic diets improve both muscle and brain status [[19], [20], [21], [22]]. However, it is still unclear whether muscle is a mediator of cognition remodeling [23]. The alteration of gut microbiota and its metabolites can also influence remote organs under disease states or during interventions [24,25]. In patients with congestive heart failure, those with gut microbiota dysbiosis and less beneficial microbial metabolites (short-chain fatty acids) were associated with lower muscle quality and cognitive function [26]. The gut microbiota may also be a medium between muscle and cognition [27]. Previous reviews lacked a comprehensive summary in how muscle affects cognitive function [11,[28], [29], [30], [31], [32]]. Whether muscle can be a potential target to enhance cognitive function should be proven in pre-clinical studies for further clinical translation. To explore how muscle affects cognitive function, we performed a scoping review to summarize the underlying pathways, and the muscle-targeted interventions that can alter cognitive function.
2. Methods
2.1. Search strategy
3 databases PubMed, Embase, Web of Science were utilized for study extraction. The latest search access was Feb 22nd, 2022. The keyword search was “(muscle) AND (cognition OR dementia OR Alzheimer) AND (mouse OR rat OR animal)”. Additional studies from other reviews were also manually searched. This review complied with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [33].
2.2. Search criteria
The inclusion criteria of this review were: 1) pre-clinical studies, 2) studies in how skeletal muscle affects the brain or cognition, 3) muscle-targeted treatments that alter brain function or structure. The exclusion criteria were: 1) review articles 2) conference and abstract publication, 3) no mechanism on how muscle affects the brain, 4) rare genetic diseases. The screening of studies was conducted by two reviewers (C. Liu, and P.Y. Wong) independently. Briefly, the two reviewers screened the titles and abstracts to include as many potential eligible studies. The full text of these studies were screened, and final eligible studies were included. Any disagreements were decided with a third researcher (R.M.Y. Wong) according to the inclusion and exclusion criteria.
2.3. Data extraction and analysis
The data extraction including author, year, animal model, intervention, end-points, assessments, and outcomes (cognitive function, brain, or muscle), as well as key findings were executed. As the heterogeneity was high in the pre-clinical studies, a qualitative review was performed. SYRCLE's risk of bias was performed to evaluate the risk of bias in the included animal studies [34]. The indicators with p < 0.05 were shown by arrows in Supplemental Table 1, while those without significant differences were described.
3. Results
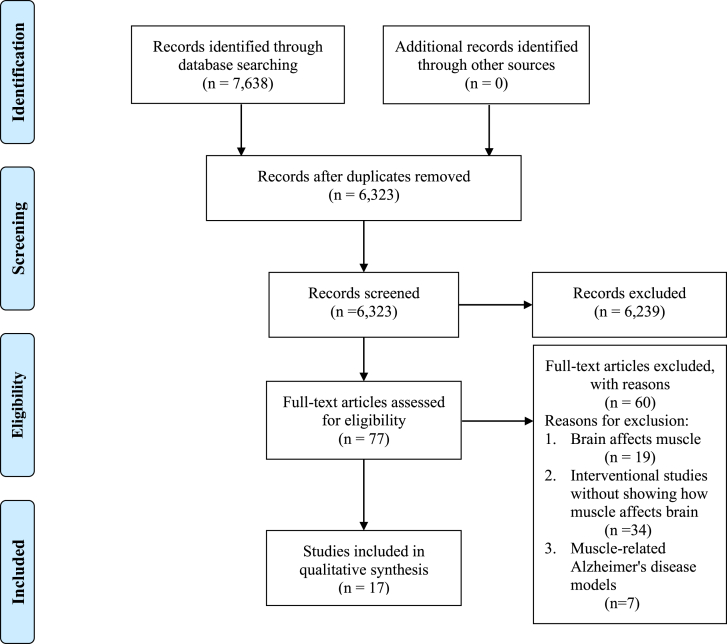
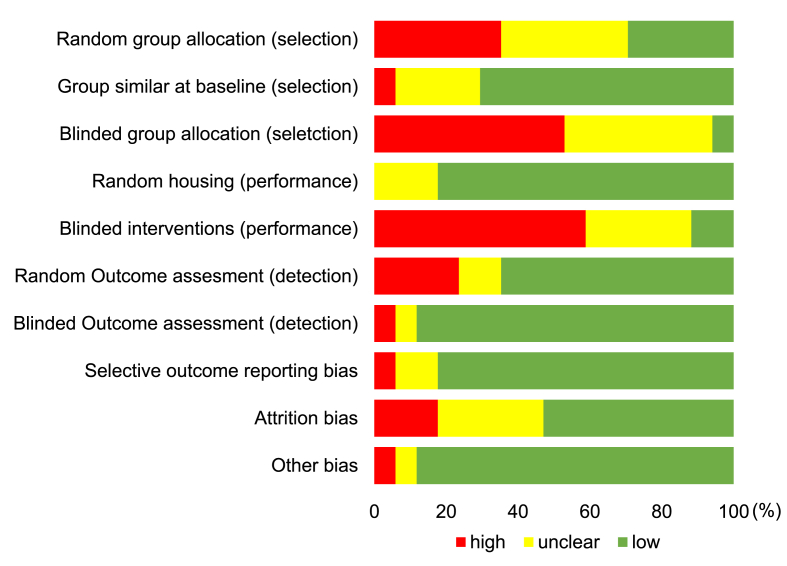
A total of 7638 records were identified through the database search (Fig. 1). After removal of duplicates, 6323 studies were selected for further screening. 77 studies with full texts were reviewed. 17 pre-clinical studies meeting the inclusion and exclusion criteria were finally included. Details of selected studies were shown in Supplemental Table 1. The overview of bias assessment was displayed as percentages (Fig. 2) and details of each study were shown in Supplemental Table 2. Most types of bias had relatively low risks except for blinded group allocation and intervention with more than 50% studies judged as high risks.
Fig. 1.
PRISMA flow diagram.
Fig. 2.
Results of SYRCLE's tool for assessing the risk of bias.
3.1. Animal models
Amongst the 17 studies, 8 studies used non-transgenic mice and rats, including C57BL/6 mice in 5 studies [22,[35], [36], [37], [38]], BALB/C mice in 2 studies [38,39], Wistar rats in 1 study [40], and Sprague Dawley rats in 1 study [41]. AD animal models were shown in 5 studies. 3 studies used amyloid precursor protein/presenilin-1 (APP/PS1) mice [[42], [43], [44]], 1 study used 5 × FAD mice [18], and 1 study used 3xTg-AD mice [45]. Muscle-specific gene knockout mice were also utilized in 3 studies. 2 studies used MCK-peroxisome proliferator-activated receptor co-activator-1α (PGC1α) mice with the overexpression of PGC1α in skeletal muscle [46,47], whilst 1 study used MKR transgenic mice with impaired insulin-like growth factor 1 (IGF-1) and insulin signaling in skeletal muscle [48]. The total body gene knockout CX3CR1GFP/+ (green fluorescent microglial cells) and cathepsin B (CTSB) −/− mice were used in 2 studies [22,49]. 1 study utilized Rhesus monkeys to verify the findings from mouse studies [22].
3.2. Study designs to explore how muscle affects cognitive function
There were 3 main strategies to investigate how muscle affects the cognitive function. Firstly, brain function changes after muscle atrophy and injury interventions can be considered as affected by skeletal muscle, which were performed in 4 animal studies. The outcomes of brain included the composition of neurons, brain functional factors, behavioral performances, signaling pathways, and other AD symptoms, such as amyloid beta (Aβ) deposits. 3 studies conducted tail suspension or hindlimb cast-immobilization to induce muscle atrophy [18,40,41], and 1 study used the muscle injury animal model by freezing the tibialis anterior [49]. Nagase et al. detected that muscle secreted hemopexin demonstrated the potential molecular pathways of factors delivery from muscle to brain [18].
Secondly, 3 studies discovered muscle-secreted factors after exercises, which play significant roles in brain function [22,36,38]. Amongst the secreted factors, two were found from the preconditioned muscle cell culture conditional mediums. Through confirmation by exogenous or endogenous periphery supplements, inhibitors, and gene knockout, the effects of the muscle secretions after exercise or immobilization on the brain could be established.
Thirdly, muscle-targeted treatments also aid in the exploration of mechanisms, which were shown in 11 studies. 2 studies used electrical stimulation to induce muscle contraction [35,40]. 1 study treated the muscle of older mice with mechanical strained muscle-resident mesenchymal stem/stromal cells (mMSCs) from young mice [37]. 1 study injected the muscle with BOTOX® [39]. 4 studies utilized adeno-associated viruses (AAV), plasmid, and short hairpin RNA (shRNA) to interfere gene expressions of muscle [[42], [43], [44], [45]], whilst 3 studies directly used muscle-specific transgenic mice [[46], [47], [48]]. After the alterations of skeletal muscle, the corresponding changes of brain were the primary outcomes to show if there was a related pathway from the muscle to cognitive change.
3.3. Outcome assessments of cognition
All 17 studies assessed cognitive indicators, such as cognitive functional tests, brain histology, as well as the expression of mRNA and protein levels. Cognitive functional tests were performed in 10 studies. Morris water maze test, fear conditioning test, open field test, and object recognition test were most widely used. Brain tissue staining were shown in 13 studies to recognize different cells in brain regions by staining with Nissl, or antibodies against Ki67, doublecortin (DCX), neuronal nuclear protein (NeuN), bromodeoxyuridine (BrdU), glial fibrillary acidic protein (GFAP), cFos, S100, Nestin, ionized calcium binding adaptor molecule 1 (Iba1) and CD11b. Table 1 shows the cell types corresponding to staining results. To detect the small molecules, 10 studies evaluated the brain levels of brain derived neurotrophic factor (BDNF), nerve growth factor (β-NGF), hemopexin, Aβ, lactate, CTSB, or fibronectin type III domain-containing protein 5 (FNDC5). 8 studies executed polymerase chain reaction (PCR) or western blot analysis of brain tissue. As the amount of the extracted protein from brain tissue, especially in the hippocampus was lower, enzyme-linked immunosorbent assay (ELISA) was used in 6 studies to accurately measure protein contents. Please refer to Supplemental Table 1.
Table 1.
Brain tissue staining: reagent, results, and corresponding cells.
| Staining reagent | Results and corresponding cells |
|---|---|
| Immunohistochemistry (IHC) staining with anti-Ki67, and anti-DCX antibodies | DAPI+/Ki67+ cells = cells in mitotic phase DCX + cells = immature neurons DAPI+/Ki67+/DCX + cells = mitotic immature neurons |
| IHC staining with anti-Nestin, anti-DCX, and anti-GFAP antibodies | Nestin+/DCX- cells = neural progenitor cells Nestin+/DCX + cells = proliferating neuroblasts Nestin-/DCX + cells = immature neurons Nestin+/GFAP + cells = neural stem cell Nestin-/GFAP + cells = mature astrocytes GFAP + cells = astrocytes |
| IHC staining with anti-BrdU, and anti-NeuN antibodies | BrdU + cells = newly generated cells NeuN + cells = neuronal cell bodies NeuN+/BrdU + cells = new mature neurons |
| IHC staining with anti-BrdU, and anti-S100ß/S100 antibodies | BrdU+/S100ß+ cells = new astrocytes S100+ cells = astrocytes |
| IHC staining with anti-Iba1 or anti-CD11b antibodies | Iba1+ or CD11b + cells = activated microglia |
| IHC staining with anti-cFos antibody | cFos + cells = cells with neuronal activation |
| Cresyl Violet | Nissl substance in neurons |
3.4. Key findings of underlying pathways and muscle-targeted interventions affecting cognitive function
4 studies revealed that muscle atrophy or injury induced memory decline, learning capacity reduction, decreased immature neurons and normal microglial, increased Aβ deposit, and lower levels of β-NGF in brain [18,40,41,49]. In contrast to muscle atrophy, muscle injury enhanced BDNF concentrations in brain [49]. Muscle secreted hemopexin after immobilization, and the cerebrospinal fluid injection of hemopexin reproduced the accelerated cognitive dysfunction [18]. The regulations of brain lipocalin-2 (Lcn2), ERK1/2, BDNF, β-NGF, hemopexin, and Aβ levels were potential pathways when muscle was under an unfavorable status.
The effects of exercise on the brain with muscle as a mediator was shown in 3 studies. The well-known lactate was released by muscle, and raised in the plasma and hippocampus after exercise [36]. Mice with exercise or lactate injection had activation of Sirt1, enhanced levels of PGC1α and FNDC5 expression, as well as hippocampal BDNF levels and cognitive functions. The injection of lactate transporter inhibitor weakened the benefits of exercise. The lactate-induced BDNF improvement were Sirt1-dependent proven by in vitro studies. Running also related to the increased CTSB in the muscle of mice, and CTSB could cross the blood–brain barrier [22]. CTSB−/− mice showed depression-like behavior, decreased hippocampal P11, and attenuated exercise-induced DCX + cells increment. The recombinant CTSB administration of neural cells facilitated the Dcx and Bdnf expression. In monkey and human, the plasma concentration of CTSB also elevated after exercise. Exercised mice had higher expressions of Fndc5 and Pgc1α in muscle, as well as Fndc5 and Bdnf in hippocampus compared to sedentary mice [38]. FNDC5 is known to secrete irisin after proteolysis, and plays positive roles in hippocampal functions. The expression of peripheral FNDC5 increased the plasma irisin levels, and the hippocampal gene expressions of Bdnf, Npas4, cFos, Arc, and Zif268. Therefore, the increments of brain BDNF and other functions by exercise were associated with muscle secretory factors.
Additionally, 2 studies showed that common methods of promoting muscle contraction by dynamic foot stimulation (DFS) and sciatic nerve electrical stimulation could benefit immature migrating neurons and astrogliogenesis in the brain [35,40]. The intramuscular injection of BOTOX® and preconditioned mMSCs benefited memory and hippocampal neurons of mice [37,39]. Genetic interventions were performed in 7 studies. Skeletal muscle Neprilysin (an Aβ peptide-degrading enzyme) overexpression ameliorated the Aβ load and memory decline of AD mouse models [42,45]. The inhibition of myokine myostatin enhanced the contextual freezing time of APP/PS1 mice [43]. The muscle delivery of scFv59 (an anti-Aβ antibody) to the AD mice increased muscle scFv59 expression, and alleviated Aβ deposits in hippocampus and neocortex [44]. 2 studies reported that overexpressed PGC1α in skeletal muscle could not promote the neurogenesis and brain Bdnf expression in older mice as exercise did [46,47]. MKR mice as the obesity-independent type-2 diabetes mellitus model, displayed insulin resistance of skeletal muscle and a lower number of new neurons in the hippocampus [48]. Numerous muscle-related treatments improved the brain function and neurogenesis, although some failed.
4. Discussion
Although skeletal muscle and brain are physically distant, there is increasing evidence showing that there is a bi-directional interaction [50]. The CNS controls muscle movement via neural and chemical signal transduction. Specifically, motor neuron endings and motor endplates of muscle fibers form the neuromuscular junction (NMJ). Electrical signals from lower motor neurons stimulate the release of neurotransmitters which bind with the receptor of endplates to induce muscle contraction [51]. In addition to the well-known muscle regulation by motor neurons, cognitive dysfunction induces physical inactivity and appetite loss, leading to a higher risk of sarcopenia [52,53]. The sensory nerve is also a bridge between muscle and brain. For instance, muscle spindle and Golgi tendon organ as proprioceptors can inform muscle activity to the CNS via sensory feedback [54]. Clinical studies have recently presented that muscle also affects cognitive function, but the underlying mechanisms are unclear [15]. In this review, we comprehensively reviewed how muscle affects cognition from the evidence of pre-clinical studies. Mice and rats were mainly used to study the relationship between muscle and brain. AD animal models were specifically used in 5 studies. With the development of gene editing technology, muscle-specific gene knockdown, knockout, or overexpression is favorable for investigations in this field. Therefore, AD animal models and muscle-specific gene alteration models can be considered in future relevant studies.
There are various studies that focused on both muscle and brain, however, only few can explain the effects of muscle on cognition due to the study design. Despite the obvious association of muscle loss and cognitive dysfunction in clinical findings, the causality and underlying mechanisms should be conducted by interventional studies [11]. 3 main interventional methods were conducted. The simplest approach is to alter muscle status, such as atrophy and injury inductions. Considering ethical issues, utilizing muscle atrophy or injury animal models appears to be more appropriate. Diverse whole-body treatments have been raised to promote both muscle and brain functions, and exercise is one of the hotspots [21,50]. As muscle is an endocrine organ, the key points in observing the role of muscle in these kinds of treatments is to discover the muscle-derived molecules, which can be assisted by modern sequencing analysis [28]. In addition to muscle, exercise can also benefit other organs, such as liver and adipose tissue [50]. Fibroblast growth factor 21 (FGF21) and insulin-like growth factor 1 (IGF1) as exercise factors instead of muscle factors contributed to cognitive enhancements, since they are not mainly secreted by muscle [50]. Therefore, it is essential to ensure the interested factors are secreted by skeletal muscle in order to confirm the mediator role of muscle. Another approach is to use muscle-specific interventions, including gene editing, injections of AAV, small interfering RNA (siRNA), shRNA, MSCs, plasmid DNA vector, or special biomaterials. It should be noticed that muscle is also a medium of drug injection, called intramuscular injection [55]. If treatments do not change muscle characteristics, the skeletal muscle would not be able to mediate the brain or cognition. Although neuroscience advances rapidly, and novel techniques including neural tracing are proposed, there is little evidence indicating that muscle can regulate cognitive function in turn via the neural connection [56]. In addition to humoral and circulatory systems, the underlying neural pathways of muscle and brain are necessary in future investigations. The gut microbiota is also an important regulator of various systems including musculoskeletal system and nervous system. Germ-free AD mice had reduced AD-like pathologies and microbiota transplantation of AD patients aggravated the symptoms of AD mice [57], whilst germ-free mice had lower muscle mass, and could be improved after the microbial transplantation from pathogen-free mice [58]. Therefore, the gut microbiota, muscle, and brain might interact with each other [27]. In summary, current evidence shows that muscle atrophy models, muscle-derived factors, and muscle-specific treatments are the important components to build the relationship between muscle and cognitive function.
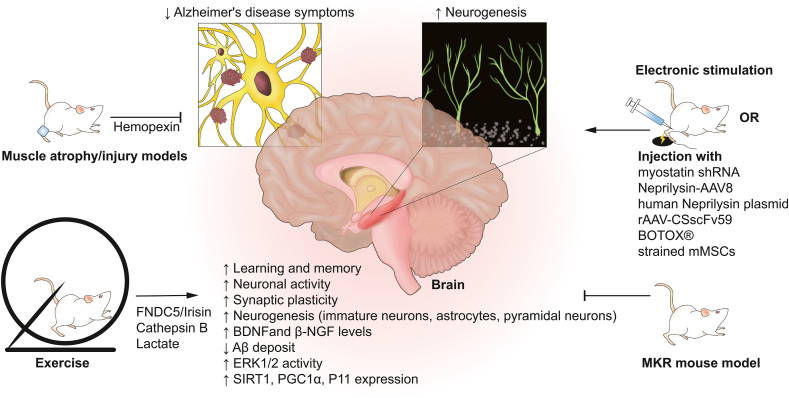
The skeletal muscle plays an important role in different subregions of the brain (cerebrum, hippocampus, neocortex, dentata gyrus, entorhinal cortex, subgranular zone) by regulating learning capacity and memory, numbers of immature neurons, astrocytes and pyramidal neurons, neuronal activity, synaptic plasticity (ARC, ZIF268), BDNF and β-NGF levels, Aβ deposit, as well as the brain expression of ERK1/2, SIRT1, PGC1α and P11 (Fig. 3). Postoperative muscle disuse and cognitive dysfunction are prevalent in old patients [59,60]. Rodents with preoperative or perioperative muscle atrophy or injury suffered poor memory, decreased brain functional factor levels and cRaf/ERK1/2 pathway activation indicating that muscle may be a regulator of neuronal differentiation and cognitive function [41,49]. However, tail suspension method to induce muscle atrophy in animals also increases the cerebral vascular resistance, which may be a confounding factor of relationship between muscle and brain [61]. Hence, the immobilization of muscle may be a superior approach to induce atrophy [18]. Up to now, only muscle-secreted hemopexin was observed to be involved in this crosstalk [18]. This secretion was detected as the most differential protein between non-cast and cast-immobilized muscle-derived conditioned media [18]. After the intracerebroventricular infusion of hemopexin, Lcn2 mRNA expression was upregulated in the hippocampus and induced memory impairment of young 5XFAD mice. However, hemopexin as a plasma protein, is primarily synthesized in the liver. Whether the changes of hemopexin expression in liver during muscle immobilization is unclear, warranting further studies. Although muscle atrophy and injury mainly led to regional inflammatory response, the exudation of inflammatory cells to the circulatory system and peripheral blood flow changes may also influence brain function [62]. One recent research reported that proteasome stress in skeletal muscle induced muscle-secreted amylase Amyrel, which could delay brain aging via disaccharide transport pathways [63]. This confirms the relationship between muscle status and brain neurodegeneration. Exercise-linked muscle-derived FDNC5/irisin and CTSB were noted as the potential treatments of AD in pre-clinical studies [22,38,[64], [65], [66]]. Fndc5 knock-out had impaired neurotrophin signaling pathway and dendrite development, as well as cognitive function, which could be rescued via the delivery of irisin into the dentate gyrus [66]. Although exercise-induced higher CTSB and irisin are associated with cognition improvement, the muscle expression of Ctsb and Fndc5 upregulated in muscle-specific PGC1α overexpression mice did not affect hippocampal neurogenesis in aging [47]. The elevation of beneficial muscle secretions might be dose-dependent and with discrepant effects in different population. The discovery of lactate receptor G-protein-coupled receptor 81 (GPR81) in brain also provided evidence of how muscle-derived lactate activated brain cells [67]. The inhibition of lactate transporter reduced BDNF, Sirt1, PGC1α and FNDC5 levels in the hippocampus of exercised mice [36]. Unfortunately, a randomized controlled trial revealed that a 12-month moderate to high intensity aerobic and strength exercise program failed to improve the cognitive function in patients with mild to moderate dementia [68]. Since low levels of physical activity are risk factors of dementia, exercise may mainly prevent against the occurrence of cognitive impairment [69,70]. The effects of myokines on brain were shown by performing exercise, activity restriction, extrinsic supplement, or conditional knockout in animal studies. However, to increase the value of clinical translation, the dose-dependent effects of muscle secretory supplements on cognitive function should be further investigated, especially in older adults. In addition to AD, patients with Parkinson's disease (PD) can also suffer from cognitive impairment and sarcopenia [71,72]. Although the cognitive abilities of PD mice could be elevated by exercise, the role of muscle has not been reported or well-validated in this disease [73,74]. This field should be explored in the future.
Fig. 3.
Pre-clinical evidences of the highway from muscle to brain. Muscle atrophy/injury animal models and diabetic MKR mouse (muscle insulin resistance) showed cognitive dysfunction and abnormal neurogenesis. Exercise, electronic stimulation, intramuscular injection of muscle-targeted agents attenuated symptoms of Alzheimer's disease and enhanced the neurogenesis, especially in hippocampus. The transport of muscle-secreted hemopexin after atrophy and FNDC5/Irisin, Cathepsin B, as well as lactate after exercise played roles in the brain.
Other muscle treatments also enhanced brain function, such as electronic stimulation and BOTOX®. Pre-clinical studies demonstrated that electronic stimulation increased the immature neurons and astrocytes of hippocampus, but not changed the behavior performance of rodents [35,40]. Recent clinical evidence showed neuromuscular electrical stimulation improved clinical outcomes except for cognitive functions in dementia patients [75,76]. People with vascular dementia may suffer paratonia [77]. The intramuscular injection of botulinum toxin relieved muscle tone abnormalities shown in patients [78], and enhanced hippocampal plasticity shown in mice via the proper inhibition of cholinergic neurotransmission [39]. The peripheral nerves that exist in muscle might be the essential bridge between muscle treatments and brain changes [79,80]. The enhanced physical activity in patients with paratonia by botulinum toxin injection may increase BDNF levels and lead to stronger brain neuroplasticity [39]. Novel cell and gene therapies could also benefit brain at the pre-clinical exploratory stage. The brain is not easily intervened as the muscle, especially in invasive ways. Intramuscular injection of mechanical strained mMSCs produced various regenerative growth factors, such as hepatocyte growth factor (HGF) and epidermal growth factor (EGF) to promote neurogenesis and astrogliogenesis in the hippocampus of aged mice [37]. The muscle-specific overexpressed Neprilysin and scFv59, myostatin knockdown, as well as normalizing insulin sensitivity were all beneficial to the brain in mouse studies. Skeletal muscle increased the expression of hNEP which could cross the blood–brain barrier to reduce Aβ deposits in brain [42]. rAAV1-mediated scFv59 increased muscle scFv59 expression and reduced Aβ levels in cerebrospinal fluid [44]. Muscle dysfunction may induce abnormal insulin and glucose metabolism, which negatively regulates cognitive function [81]. The reduction of muscle myostatin and insulin resistance might enhance cognitive functions [43,48]. These muscle-targeted strategies have clinical translation values to treat cognitive impairment. Nevertheless, muscle-specific PGC1α overexpression fails to increase brain functions as exercise and peroxisome proliferator-activated receptor δ agonist did [46,47,82]. This phenomenon reminds us that some effects of whole-body treatment did not come from the altered expression of a single muscle gene. Although various pre-clinical evidence revealed direct and indirect effects of muscle on cognitive function and related pathways, there is no favorable result shown in clinical interventional trials based on old population [83]. It is important for elderly people to maintain muscle mass and strength, which can not only prevent against sarcopenia, but also have the potential to slow down cognitive impairment via the above pathways. The combination of cognition and muscle therapeutic approaches may play a greater role in cognition improvement. Pilot studies of other novel muscle treatments should be conducted to observe the effects on brain in the future [84].
To our knowledge, this is the first scoping review to summarize the current pre-clinical evidence that shows how skeletal muscle affects cognitive function (mainly AD-related) with our search criteria. We classified the study designs into three main types to provide a reference for future research. The muscle-targeted strategies as potential brain function enhancers were proposed. There are several limitations of this study. The pre-clinical studies had high heterogeneity so that only qualitative analysis has been done. Although the risk bias of included animal studies was not high, some studies only showed the phenotype changes of the brain or behaviors without in-depth mechanism exploration. Furthermore, publication bias may exist, with only publishment of the positive roles played by muscle. The evidence were all from animal models, and the feasibility in human remains unclear. As in-vitro studies hardly illustrate the relationship across organs, we did not include simple cell studies. Furthermore, we did not include studies that reported muscle-brain connection of other conditions, such as anxiety, fear, and depression, which should be summarized in the future.
In conclusion, current pre-clinical evidence shows that skeletal muscle is an important mediator of cognitive function. Interventions that induce muscle atrophy, hypertrophy, or regulate muscle gene expression have aided in the discovery of the remote effects of muscle on brain (cognitive behaviors, brain functional factors, hippocampal neurogenesis, and Aβ load) through the transportation of muscle-secreted lactate, FNDC5/irisin, hemopexin, and CTSB. In addition to humoral and circulatory systems, whether muscle can affect cognition via neural connection should be investigated in the future.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2022.10.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Petermann-Rocha F., Balntzi V., Gray S.R., Lara J., Ho F.K., Pell J.P., et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13(1):86–99. doi: 10.1002/jcsm.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichols E., Steinmetz J.D., Vollset S.E., Fukutaki K., Chalek J., Abd-Allah F., et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105–e125. doi: 10.1016/S2468-2667(21)00249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong W. Economic burden of Alzheimer disease and managed care considerations. Am J Manag Care. 2020;26(8 Suppl):S177. doi: 10.37765/ajmc.2020.88482. s83. [eng] [DOI] [PubMed] [Google Scholar]
- 4.Anker S.D., Morley J.E., von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016;7(5):512–514. doi: 10.1002/jcsm.12147. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J., Wan C.S., Ktoris K., Reijnierse E.M., Maier A.B. Sarcopenia is associated with mortality in adults: a systematic review and meta-analysis. Gerontology. 2021:1–16. doi: 10.1159/000517099. [DOI] [PubMed] [Google Scholar]
- 6.Liang C.-S., Li D.-J., Yang F.-C., Tseng P.-T., Carvalho A.F., Stubbs B., et al. Mortality rates in Alzheimer's disease and non-Alzheimer's dementias: a systematic review and meta-analysis. Lancet Health Longev. 2021;2(8):e479–e488. doi: 10.1016/S2666-7568(21)00140-9. [DOI] [PubMed] [Google Scholar]
- 7.Nichols E., Szoeke C.E.I., Vollset S.E., Abbasi N., Abd-Allah F., Abdela J., et al. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):88–106. doi: 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanimoto Y., Watanabe M., Sun W., Tanimoto K., Shishikura K., Sugiura Y., et al. Association of sarcopenia with functional decline in community-dwelling elderly subjects in Japan. Geriatr Gerontol Int. 2013;13(4):958–963. doi: 10.1111/ggi.12037. [DOI] [PubMed] [Google Scholar]
- 9.Sui S.X., Holloway-Kew K.L., Hyde N.K., Williams L.J., Leach S., Pasco J.A. Muscle strength and gait speed rather than lean mass are better indicators for poor cognitive function in older men. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-67251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tou N.X., Wee S.L., Pang B.W.J., Lau L.K., Jabbar K.A., Seah W.T., et al. Associations of fat mass and muscle function but not lean mass with cognitive impairment: the Yishun Study. PLoS One. 2021;16(8) doi: 10.1371/journal.pone.0256702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang K.V., Hsu T.H., Wu W.T., Huang K.C., Han D.S. Association between sarcopenia and cognitive impairment: a systematic review and meta-analysis. J Am Med Dir Assoc. 2016;17(12):1164 e7–e64 e15. doi: 10.1016/j.jamda.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Peng T.C., Chen W.L., Wu L.W., Chang Y.W., Kao T.W. Sarcopenia and cognitive impairment: a systematic review and meta-analysis. Clin Nutr. 2020;39(9):2695–2701. doi: 10.1016/j.clnu.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Waite S.J., Maitland S., Thomas A., Yarnall A.J. Sarcopenia and frailty in individuals with dementia: a systematic review. Arch Gerontol Geriatr. 2021;92 doi: 10.1016/j.archger.2020.104268. [DOI] [PubMed] [Google Scholar]
- 14.Fostinelli S., De Amicis R., Leone A., Giustizieri V., Binetti G., Bertoli S., et al. Eating behavior in aging and dementia: the need for a comprehensive assessment. Front Nutr. 2020;7 doi: 10.3389/fnut.2020.604488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beeri M.S., Leugrans S.E., Delbono O., Bennett D.A., Buchman A.S. Sarcopenia is associated with incident Alzheimer's dementia, mild cognitive impairment, and cognitive decline. J Am Geriatr Soc. 2021;69(7):1826–1835. doi: 10.1111/jgs.17206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tessier A.J., Wing S.S., Rahme E., Morais J.A., Chevalier S. Association of low muscle mass with cognitive function during a 3-year follow-up among adults aged 65 to 86 Years in the Canadian longitudinal study on aging. JAMA Netw Open. 2022;5(7) doi: 10.1001/jamanetworkopen.2022.19926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bao Z., Cui C., Chow S.K., Qin L., Wong R.M.Y., Cheung W.H. AChRs degeneration at NMJ in aging-associated sarcopenia-A systematic review. Front Aging Neurosci. 2020;12 doi: 10.3389/fnagi.2020.597811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagase T., Tohda C. Skeletal muscle atrophy-induced hemopexin accelerates onset of cognitive impairment in Alzheimer's disease. J Cachexia Sarcopenia Muscle. 2021;12(6):2199–2210. doi: 10.1002/jcsm.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suijo K., Inoue S., Ohya Y., Odagiri Y., Takamiya T., Ishibashi H., et al. Resistance exercise enhances cognitive function in mouse. Int J Sports Med. 2013;34(4):368–375. doi: 10.1055/s-0032-1323747. [DOI] [PubMed] [Google Scholar]
- 20.Gyanwali B., Lim Z.X., Soh J., Lim C., Guan S.P., Goh J., et al. Alpha-Ketoglutarate dietary supplementation to improve health in humans. Trends Endocrinol Metabol. 2022;33(2):136–146. doi: 10.1016/j.tem.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Zhu H., Bi D., Zhang Y., Kong C., Du J., Wu X., et al. Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations. Signal Transduct Targeted Ther. 2022;7(1) doi: 10.1038/s41392-021-00831-w. (no pagination) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon H.Y., Becke A., Berron D., Becker B., Sah N., Benoni G., et al. Running-induced systemic cathepsin B secretion is associated with memory function. Cell Metabol. 2016;24(2):332–340. doi: 10.1016/j.cmet.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burtscher J., Millet G.P., Place N., Kayser B., Zanou N. The muscle-brain Axis and neurodegenerative diseases: the key role of mitochondria in exercise-induced neuroprotection. Int J Mol Sci. 2021;22(12) doi: 10.3390/ijms22126479. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C., Cheung W.H., Li J., Chow S.K., Yu J., Wong S.H., et al. Understanding the gut microbiota and sarcopenia: a systematic review. J Cachexia Sarcopenia Muscle. 2021;12(6):1393–1407. doi: 10.1002/jcsm.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morais L.H., Schreiber HLt, Mazmanian S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19(4):241–255. doi: 10.1038/s41579-020-00460-0. [DOI] [PubMed] [Google Scholar]
- 26.Kirschner S.K., Deutz N.E.P., Rijnaarts I., Smit T.J., Larsen D.J., Engelen M. Impaired intestinal function is associated with lower muscle and cognitive health and well-being in patients with congestive heart failure. JPEN - J Parenter Enter Nutr. 2022;46(3):660–670. doi: 10.1002/jpen.2193. [DOI] [PubMed] [Google Scholar]
- 27.Schlegel P., Novotny M., Klimova B., Valis M. Muscle-gut-brain Axis": can physical activity help patients with alzheimer's disease due to microbiome modulation? J Alzheimers Dis. 2019;71(3):861–878. doi: 10.3233/JAD-190460. [DOI] [PubMed] [Google Scholar]
- 28.Isaac A.R., Lima-Filho R.A.S., Lourenco M.V. How does the skeletal muscle communicate with the brain in health and disease? Neuropharmacology. 2021;197 doi: 10.1016/j.neuropharm.2021.108744. [eng] [DOI] [PubMed] [Google Scholar]
- 29.Kilgour A.H., Todd O.M., Starr J.M. A systematic review of the evidence that brain structure is related to muscle structure and their relationship to brain and muscle function in humans over the lifecourse. BMC Geriatr. 2014;14:85. doi: 10.1186/1471-2318-14-85. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oudbier S.J., Goh J., Looijaard S., Reijnierse E.M., Meskers C.G.M., Maier A.B. Pathophysiological mechanisms explaining the association between low skeletal muscle mass and cognitive function. J Gerontol A Biol Sci Med Sci. 2022;77(10):1959–1968. doi: 10.1093/gerona/glac121. PMID: 35661882; PMCID: PMC9536455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sui S.X., Balanta-Melo J., Pasco J.A., Plotkin L.I. Musculoskeletal deficits and cognitive impairment: epidemiological evidence and biological mechanisms. Curr Osteoporos Rep. 2022;20(5):260–272. doi: 10.1007/s11914-022-00736-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sui S.X., Williams L.J., Holloway-Kew K.L., Hyde N.K., Pasco J.A. Skeletal muscle health and cognitive function: a narrative review. Int J Mol Sci. 2020;22(1) doi: 10.3390/ijms22010255. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hooijmans C.R., Rovers M.M., de Vries R.B., Leenaars M., Ritskes-Hoitinga M., Langendam M.W. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardner J.C., Dvoretskiy S.V., Yang Y., Venkataraman S., Lange D.A., Li S., et al. Electrically stimulated hind limb muscle contractions increase adult hippocampal astrogliogenesis but not neurogenesis or behavioral performance in male C57BL/6J mice. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-76356-z. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El Hayek L., Khalifeh M., Zibara V., Abi Assaad R., Emmanuel N., Karnib N., et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF) J Neurosci. 2019;39(13):2369–2382. doi: 10.1523/JNEUROSCI.1661-18.2019. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huntsman H.D., Rendeiro C., Merritt J.R., Pincu Y., Cobert A., De Lisio M., et al. The impact of mechanically stimulated muscle-derived stromal cells on aged skeletal muscle. Exp Gerontol. 2018;103:35–46. doi: 10.1016/j.exger.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Wrann C.D., White J.P., Salogiannnis J., Laznik-Bogoslavski D., Wu J., Ma D., et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metabol. 2013;18(5):649–659. doi: 10.1016/j.cmet.2013.09.008. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yesudhas A., Roshan S.A., Radhakrishnan R.K., Abirami G.P.P., Manickam N., Selvaraj K., et al. Intramuscular injection of BOTOX (R) boosts learning and memory in adult mice in association with enriched circulation of platelets and enhanced density of pyramidal neurons in the Hippocampus. Neurochem Res. 2020;45(12):2856–2867. doi: 10.1007/s11064-020-03133-9. [DOI] [PubMed] [Google Scholar]
- 40.Berezovskaya A.S., Tyganov S.A., Nikolaeva S.D., Naumova A.A., Shenkman B.S., Glazova M.V. Plantar stimulations during 3-day hindlimb unloading prevent loss of neural progenitors and maintain ERK1/2 activity in the rat Hippocampus. Life-Basel. 2021;11(5) doi: 10.3390/life11050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nemoto A., Goyagi T., Nemoto W., Nakagawasai O., Tan-No K., Niiyama Y. Low skeletal muscle mass is associated with perioperative neurocognitive disorder due to decreased neurogenesis in rats. Anesth Analg. 2022;134(1):194–203. doi: 10.1213/ANE.0000000000005681. [eng] [DOI] [PubMed] [Google Scholar]
- 42.Li Y.L., Wang Y.D., Wang J., Chong K.Y., Xu J.J., Liu Z.H., et al. Expression of Neprilysin in skeletal muscle by ultrasound-mediated gene transfer (sonoporation) reduces amyloid burden for AD. Mol Ther-Meth Clin Dev. 2020;17:300–308. doi: 10.1016/j.omtm.2019.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin Y.S., Lin F.Y., Hsiao Y.H. Myostatin is associated with cognitive decline in an animal model of alzheimer's disease. Mol Neurobiol. 2019;56(3):1984–1991. doi: 10.1007/s12035-018-1201-y. [DOI] [PubMed] [Google Scholar]
- 44.Yang J.L., Pattanayak A., Song M., Kou J.H., Taguchi H., Paul S., et al. Muscle-directed anti-A beta single-chain antibody delivery via AAV1 reduces cerebral A beta load in an alzheimer's disease mouse model. J Mol Neurosci. 2013;49(2):277–288. doi: 10.1007/s12031-012-9877-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y.X., Studzinski C., Beckett T., Guan H.J., Hersh M.A., Murphy M.P., et al. Expression of Neprilysin in skeletal muscle reduces amyloid burden in a transgenic mouse model of alzheimer disease. Mol Ther. 2009;17(8):1381–1386. doi: 10.1038/mt.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karlsson L., González-Alvarado M.N., Motalleb R., Wang Y., Wang Y., Börjesson M., et al. Constitutive PGC-1α overexpression in skeletal muscle does not contribute to exercise-induced neurogenesis. Mol Neurobiol. 2021;58(4):1465–1481. doi: 10.1007/s12035-020-02189-6. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karlsson L., González-Alvarado M.N., Motalleb R., Blomgren K., Börjesson M., Kuhn H.G. Constitutive PGC-1α overexpression in skeletal muscle does not protect from age-dependent decline in neurogenesis. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-48795-w. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonds J.A., Shetti A., Stephen T.K.L., Bonini M.G., Minshall R.D., Lazarov O. Deficits in hippocampal neurogenesis in obesity-dependent and -independent type-2 diabetes mellitus mouse models. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-73401-9. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guéniot L., Lepere V., De Medeiros G.F., Danckaert A., Flamant P., Le Dudal M., et al. Muscle injury induces postoperative cognitive dysfunction. Sci Rep. 2020;10(1):2768. doi: 10.1038/s41598-020-59639-3. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pedersen B.K. Physical activity and muscle-brain crosstalk. Nat Rev Endocrinol. 2019;15(7):383–392. doi: 10.1038/s41574-019-0174-x. [eng] [DOI] [PubMed] [Google Scholar]
- 51.Yin X., Yu T., Chen B., Xu J., Chen W., Qi Y., et al. Spatial distribution of motor endplates and its adaptive change in skeletal muscle. Theranostics. 2019;9(3):734–746. doi: 10.7150/thno.28729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kimura A., Sugimoto T., Niida S., Toba K., Sakurai T. Association between appetite and sarcopenia in patients with mild cognitive impairment and early-stage alzheimer's disease: a case-control study. Front Nutr. 2018;5:128. doi: 10.3389/fnut.2018.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogawa Y., Kaneko Y., Sato T., Shimizu S., Kanetaka H., Hanyu H. Sarcopenia and muscle functions at various stages of alzheimer disease. Front Neurol. 2018;9:710. doi: 10.3389/fneur.2018.00710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oliver K.M., Florez-Paz D.M., Badea T.C., Mentis G.Z., Menon V., de Nooij J.C. Molecular correlates of muscle spindle and Golgi tendon organ afferents. Nat Commun. 2021;12(1):1451. doi: 10.1038/s41467-021-21880-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greenblatt D.J. Intramuscular injection of drugs. N Engl J Med. 1976;295(10):542–546. doi: 10.1056/NEJM197609022951006. [DOI] [PubMed] [Google Scholar]
- 56.Coulon P., Bras H., Vinay L. Characterization of last-order premotor interneurons by transneuronal tracing with rabies virus in the neonatal mouse spinal cord. J Comp Neurol. 2011;519(17):3470–3487. doi: 10.1002/cne.22717. [DOI] [PubMed] [Google Scholar]
- 57.Chen C., Liao J., Xia Y., Liu X., Jones R., Haran J., et al. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut. 2022;71(11):2233–2252. doi: 10.1136/gutjnl-2021-326269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lahiri S., Kim H., Garcia-Perez I., Reza M.M., Martin K.A., Kundu P., et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci Transl Med. 2019;11(502) doi: 10.1126/scitranslmed.aan5662. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Safavynia S.A., Goldstein P.A. The role of neuroinflammation in postoperative cognitive dysfunction: moving from hypothesis to treatment. Front Psychiatr. 2018;9:752. doi: 10.3389/fpsyt.2018.00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rasch A., Dalen N., Berg H.E. Muscle strength, gait, and balance in 20 patients with hip osteoarthritis followed for 2 years after THA. Acta Orthop. 2010;81(2):183–188. doi: 10.3109/17453671003793204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilkerson M.K., Colleran P.N., Delp M.D. Acute and chronic head-down tail suspension diminishes cerebral perfusion in rats. Am J Physiol Heart Circ Physiol. 2002;282(1):H328–H334. doi: 10.1152/ajpheart.00727.2001. [eng] [DOI] [PubMed] [Google Scholar]
- 62.Smith C., Kruger M.J., Smith R.M., Myburgh K.H. The inflammatory response to skeletal muscle injury. Sports Med. 2008;38(11):947–969. doi: 10.2165/00007256-200838110-00005. [DOI] [PubMed] [Google Scholar]
- 63.Rai M., Coleman Z., Curley M., Nityanandam A., Platt A., Robles-Murguia M., et al. Proteasome stress in skeletal muscle mounts a long-range protective response that delays retinal and brain aging. Cell Metabol. 2021;33(6):1137–11354 e9. doi: 10.1016/j.cmet.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lourenco M.V., Frozza R.L., de Freitas G.B., Zhang H., Kincheski G.C., Ribeiro F.C., et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer's models. Nat Med. 2019;25(1):165–175. doi: 10.1038/s41591-018-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pena G.S., Paez H.G., Johnson T.K., Halle J.L., Carzoli J.P., Visavadiya N.P., et al. Hippocampal growth factor and myokine cathepsin B expression following aerobic and resistance training in 3xTg-AD mice. Int J Chronic Dis. 2020;2020 doi: 10.1155/2020/5919501. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Islam M.R., Valaris S., Young M.F., Haley E.B., Luo R., Bond S.F., et al. Exercise hormone irisin is a critical regulator of cognitive function. Nat Metab. 2021;3(8):1058–1070. doi: 10.1038/s42255-021-00438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morland C., Lauritzen K.H., Puchades M., Holm-Hansen S., Andersson K., Gjedde A., et al. The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: expression and action in brain. J Neurosci Res. 2015;93(7):1045–1055. doi: 10.1002/jnr.23593. [eng] [DOI] [PubMed] [Google Scholar]
- 68.Lamb S.E., Sheehan B., Atherton N., Nichols V., Collins H., Mistry D., et al. Dementia and Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial. BMJ. 2018;361:k1675. doi: 10.1136/bmj.k1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De la Rosa A., Olaso-Gonzalez G., Arc-Chagnaud C., Millan F., Salvador-Pascual A., García-Lucerga C., et al. Physical exercise in the prevention and treatment of Alzheimer's disease. J Sport Health Sci. 2020;9(5):394–404. doi: 10.1016/j.jshs.2020.01.004. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu W., Ding D., Zhao Q., Xiao Z., Luo J., Ganguli M., et al. Dose-response relationship between late-life physical activity and incident dementia: a pooled analysis of 10 cohort studies of memory in an international consortium. Alzheimers Dement. 2022 doi: 10.1002/alz.12628. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fang C., Lv L., Mao S., Dong H., Liu B. Cognition deficits in Parkinson's disease: mechanisms and treatment. Parkinsons Dis. 2020;2020 doi: 10.1155/2020/2076942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cai Y., Feng F., Wei Q., Jiang Z., Ou R., Shang H. Sarcopenia in patients with Parkinson's disease: a systematic review and meta-analysis. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.598035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goes A.T., Souza L.C., Filho C.B., Del Fabbro L., De Gomes M.G., Boeira S.P., et al. Neuroprotective effects of swimming training in a mouse model of Parkinson's disease induced by 6-hydroxydopamine. Neuroscience. 2014;256:61–71. doi: 10.1016/j.neuroscience.2013.09.042. [eng] [DOI] [PubMed] [Google Scholar]
- 74.Zhou W., Barkow J.C., Freed C.R. Running wheel exercise reduces alpha-synuclein aggregation and improves motor and cognitive function in a transgenic mouse model of Parkinson's disease. PLoS One. 2017;12(12) doi: 10.1371/journal.pone.0190160. (no pagination) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nishikawa Y., Takahashi T., Kawade S., Maeda N., Maruyama H., Hyngstrom A. The effect of electrical muscle stimulation on muscle mass and balance in older adults with dementia. Brain Sci. 2021;11(3) doi: 10.3390/brainsci11030339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hahm S.-C., Suh H.R., Cho H-y. The effect of transcutaneous electrical nerve stimulation on pain, muscle strength, balance, and gait in individuals with dementia: a double blind, pilot randomized controlled trial. Eur J Integrat Med. 2019;29 [Google Scholar]
- 77.Drenth H., Zuidema S., Bautmans I., Marinelli L., Kleiner G., Hobbelen H. Paratonia in dementia: a systematic review. J Alzheimers Dis. 2020;78(4):1615–1637. doi: 10.3233/JAD-200691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Quinn T.J., Kleiner-Fisman G., Khoo E., Moncrieffe N., Forbell T., Gryfe P., et al. A randomized, placebo controlled pilot trial of botulinum toxin for paratonic rigidity in people with advanced cognitive impairment. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0114733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Javeed S., Faraji A.H., Dy C., Ray W.Z., MacEwan M.R. Application of electrical stimulation for peripheral nerve regeneration: stimulation parameters and future horizons. Interdisciplin Neurosurg. 2021:24. [Google Scholar]
- 80.Luvisetto S. Botulinum toxin and neuronal regeneration after traumatic injury of central and peripheral nervous system. Toxins. 2020;12(7) doi: 10.3390/toxins12070434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cholerton B., Baker L.D., Craft S. Insulin, cognition, and dementia. Eur J Pharmacol. 2013;719(1–3):170–179. doi: 10.1016/j.ejphar.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kobilo T., Yuan C.Y., van Praag H. Endurance factors improve hippocampal neurogenesis and spatial memory in mice. Learn Mem. 2011;18(2):103–107. doi: 10.1101/lm.2001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Balbim G.M., Falck R.S., Barha C.K., Starkey S.Y., Bullock A., Davis J.C., et al. Effects of exercise training on the cognitive function of older adults with different types of dementia: a systematic review and meta-analysis. Br J Sports Med. 2022 doi: 10.1136/bjsports-2021-104955. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 84.Lo J.H., U K.P., Yiu T., Ong M.T., Lee W.Y. Sarcopenia: current treatments and new regenerative therapeutic approaches. J Orthop Translat. 2020;23:38–52. doi: 10.1016/j.jot.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.