Abstract
In response to consumer demands, plant protein ingredients are increasingly being used in the formulation of plant-based alternatives to cheese. The aim of this study was to determine the influence of protein concentration on key quality attributes of chickpea-based alternatives to cheese. Moreover, the age-induced changes in such attributes were assessed, with samples analysed after 1 month of storage. After characterisation of the ingredients, the chickpea-based formulations were prepared by blending chickpea flour and protein concentrate in different proportions to obtain four samples of increasing protein content (i.e., 8.68–21.5%). Formulations were developed at pH ∼4.5, and a moisture content of 50%, with shea butter used to obtain 15% fat content. The differential scanning calorimetry thermograms of the samples showed a main peak around 30 °C, corresponding to transition of the shea butter, and a smaller peak around 70 °C related to starch gelatinisation. Analysis of microstructure showed formation of a protein matrix with more extensive protein structure at high protein concentration. Furthermore, none of the chickpea-based samples melted under the testing conditions and all samples showed increasing values for adhesiveness, springiness and cohesiveness with increasing protein content. However, hardness was the highest for the sample with the lowest protein content, likely due to starch retrogradation. After storage, hardness increased further for all samples. This work improves our understanding of the role of chickpea protein in developing plant-based alternatives to cheese and the challenges therein.
Keywords: Plant-based cheese, Chickpea protein ingredients, Microstructure, Texture, Meltability
Graphical abstract
Highlights
-
•
Protein concentration influenced the texture of chickpea-based cheese alternatives.
-
•
Samples showed increased protein network formation with increasing protein content.
-
•
All samples displayed poor melting properties under the testing conditions.
-
•
Samples stored for 1 month at 4 °C had altered colour and texture.
1. Introduction
Food systems (i.e., production, processing, distribution, preparation and consumption of food) are responsible for between 21 and 37% of all net anthropogenic greenhouse gas emissions (IPCC et al., 2019). In particular, due to their impact on global emissions, animal-based systems are currently major contributors to climate change and, in turn, biodiversity loss (Notarnicola et al., 2017; Benton et al., 2021). The growing global population and the corresponding need to increase food supply, combined with the high environmental impact of animal food production, are driving growth in the development of plant-based alternatives to animal-based products, such as cheese.
Due to increasing consumer awareness of the impact of food production on the environment, animal welfare and human health, consumption of plant-based food is increasing globally, with a growth in sales of 27% in the US in 2020 (SPINS and Good Food Institute, 2020). In particular, the US dollar sales for plant-based alternatives to cheese grew by 42% in 2020, and the sector is projected to reach almost $4 billion by 2024 (Bharat Book Bureau, 2017; SPINS and Good Food Institute, 2020). However, most commercially available products currently rely on starch and solid fats (e.g., coconut oil) as their principal components, and have low protein and high saturated fat contents, making them nutritionally inferior to traditional cheese. Furthermore, from a consumers perspective, the taste and price of such commercial plant-based alternatives to cheese do not meet consumer expectations, and in fact represent the plant-based product category with the highest potential demand (i.e., product type that consumers would like to see more of in supermarkets) (ProVeg International, 2020). To formulate plant-based alternatives to cheese with improved nutritional profiles and low environmental impact, a number of research groups are currently investigating the suitability of plant protein ingredients (Mattice and Marangoni, 2020; Ferawati et al., 2021; Grossmann and McClements, 2021; Mefleh et al., 2021). The aim of several of these recent studies is mainly to develop alternatives to non-protein ingredients (e.g., polysaccharides) that are often used to build structure and mimic dairy proteins and fat in such applications. These are frequently used in cheese analogues formulations as inexpensive alternatives to protein, to partially replace casein (Bachmann, 2001). However, in addition to nutritional quality, dairy proteins provide cheese products with unique sensory and textural properties and the replication of such properties using plant proteins is challenging (Grossmann and McClements, 2021; Short et al., 2021).
Among the plant protein sources available, soy has been extensively used in plant-based cheese alternative applications for its ability to form a curd under specific processing conditions; more recently new ingredients such as zein have also been studied, showing promising results for such applications (Mattice and Marangoni, 2020).
Pulses are part of traditional diets in many countries and represent important sources of dietary proteins; thus, pulse flours, protein concentrates and isolates offer opportunities for novel food product development and can contribute to achieving recommended daily protein requirements (Boye et al., 2010). In particular, chickpeas are considered highly nutritious, with a protein content of 20–25% and high levels of fat, starch and fibre, as well as significant concentrations of minerals, vitamins and bioactive compounds (e.g., phenolic acid and isoflavones) (Hall et al., 2017). Due to their nutritional value and functional properties, chickpea protein ingredients (i.e., flour, protein concentrate and isolate) show great potential in the development of new and reformulated food products. Previous studies investigated functional properties of chickpea proteins of relevance in plant-based alternatives to cheese applications, such as oil absorption capacity, emulsifying and gelling properties (Kaur and Singh, 2007; Papalamprou et al., 2009; Withana-Gamage et al., 2011). Chickpea protein ingredients showed good performance in such functional properties, probably due to the high levels of globulins (53–60% of total chickpea proteins), which, because of their highly structured nature due to disulphide bonds and hydrophobic interactions, strongly influence functionality (Ghumman et al., 2016). In addition, as for other pulses, chickpea protein ingredients have been employed in the development of plant-based milk alternatives (Wang et al., 2018; Lopes et al., 2020).
To the authors’ knowledge, there are no published scientific studies available that investigated the use of chickpea protein ingredients in the development of plant-based alternatives to cheese. In this work, chickpea-based alternatives to cheese were formulated using chickpea flour and chickpea protein concentrate in different ratios. The aim of this study was to determine the influence of protein concentration on chickpea-based alternatives to cheese, in terms of key quality attributes, such as structure and texture. Moreover, the age-induced changes in such attributes were assessed after 1 mo of storage. The results of this work will enhance our understanding of the role, and potential, of chickpea protein ingredients in formulating and developing high protein content chickpea-based alternatives to cheese, and the challenges therein.
2. Materials and methods
2.1. Ingredients
Commercially available chickpea flour (CF) (Müller's Mühle GmbH, Gelsenkirchen, Germany), with 20% protein, 10.4% moisture, 60.8% carbohydrate, 37.8% starch, 6.15% fat and 2.67% ash, and chickpea protein concentrate (CPC) (Artesa, PLT Health Solutions, Morristown, NJ, US), with 53.1% protein, 7.73% moisture, 33.3% carbohydrate, 2.86% starch, 1.37% fat and 4.47% ash, were used in this study to formulate the chickpea-based alternatives to cheese. The composition of the CF and CPC were typical of pulse flours and concentrates. A shea butter ingredient (Zenitex M 50 G) kindly provided by Fuji Oil Europe (Gent, Belgium), was used as fat source, and was composed of 99.9% fat, with 49% of the fatty acids being saturated and 45% mono-unsaturated. The shea butter ingredient was chosen in this study due to its solid nature at room temperature and high ratio of unsaturated to saturated fatty acids and the lower content of saturated fats compared to coconut oil, which represents the most used source of fat in commercially available plant-based cheeses. All chemicals were purchased from Sigma-Aldrich (St Louis, MO, US), unless stated otherwise.
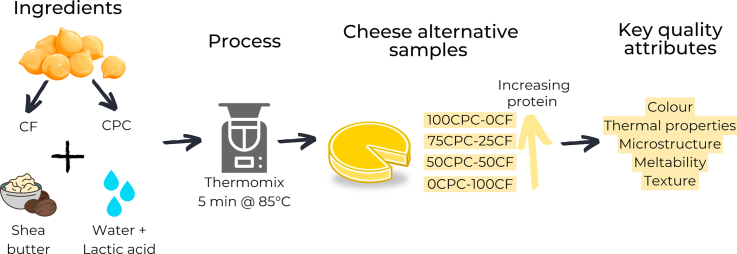
2.2. Formulation of cheese alternatives
The protein components of chickpea-based alternatives to cheese, hereafter referred to as chickpea-based samples, were formulated by blending CF and CPC in different proportions. Selected additions of CPC were used to obtain four chickpea-based samples of increasing protein, and consequently decreasing carbohydrate, contents (Table 1). An ingredient ratio based on protein contribution of 0:100, 50:50, 75:25 and 100:0 from CPC and CF was used to obtain the 4 samples, 0CPC-100CF, 50CPC-50CF, 75CPC-25CF and 100CPC-0CF, respectively. Lactic acid was added to water to achieve a pH of ∼4.5 and 50% moisture content in the chickpea-based samples. Shea butter was added to obtain 15% fat content for all the samples, which was set as a target to align with the typical fat content of commercially available “reduced-fat” cheese products. The final formulation and processing conditions described here were confirmed after numerous preliminary and optimisation trials. Samples were prepared by mixing the CF and CPC with water in a Thermomix (TM 5, Vorwerk, Wuppertal, Germany) at speed 1 (100 rpm). The temperature was set to 85 °C and when 45 °C was reached, shea butter was added to the mixture at speed 2 (200 rpm) for 5 min. After 2.5 min, the speed was increased to 3.5 (800 rpm) for 10 s to ensure that all ingredients were uniformly dispersed and incorporated in the mixture. Following this, samples were poured into moulds and stored for 24 h at 4 °C before analysis and for 1 mo at 4 °C to assess the influence of storage on selected properties of the chickpea-based samples.
Table 1.
Formulation (%) of the chickpea-based samples made using chickpea flour (CF) and chickpea protein concentrate (CPC).
| CF | CPC | Shea butter | Lactic acid | Water | |
|---|---|---|---|---|---|
| 0CPC-100CF | 43.3 | 0 | 12.3 | 5.10 | 40.4 |
| 50CPC-50CF | 30.9 | 11.6 | 12.9 | 7.25 | 38.6 |
| 75CPC-25CF | 19.6 | 22.2 | 13.5 | 9.20 | 37.0 |
| 100CPC-0CF | 0 | 40.5 | 14.4 | 12.5 | 34.3 |
2.3. Compositional analysis of chickpea flour and protein concentrate ingredients and cheese alternatives
The composition of the CF and CPC was analysed prior to formulating the chickpea-based samples. Protein content of chickpea ingredients and chickpea-based cheese alternatives was measured using the Kjeldahl method and a nitrogen-to-protein conversion factor of 6.25, according to method 930.29 (AOAC, 1930) and 2001.14 (AOAC, 2002), respectively. Moisture of protein ingredients and samples was determined using oven drying at 103 °C for 5 h, according to method 925.10 (AOAC, 1925) and 926.08 (AOAC, 1990), respectively. Ash content of CF and CPC was measured by incineration in a muffle furnace to 700 °C for 5 h, according to method 923.03 (AOAC, 1923); for chickpea-based samples, ash content was analysed by incineration at 800 °C for 5 h after pre-ashing in crucibles for 10 min, according to method 935.42 (AOAC, 1990a). Fat content of protein ingredients and samples was assessed using the Soxhlet method with SoxCap and Soxtec (Foss UK Ltd, UK) according to the AACC method 30–25.01 (AACC, 2009); activated silica was used to absorb moisture in the chickpea-based samples. Moreover, total starch content of CF and CPC was analysed using the enzyme kit K-TSTA (Megazyme, Ireland) according to method 996.11 (AOAC, 2005). Total carbohydrate of protein ingredients and chickpea-based samples was calculated by difference (i.e., 100 – sum of protein, fat, ash and moisture). Moreover, the pH of the chickpea-based samples was measured using a pH meter equipped with a FC200B Foodcare pH electrode for semi-solid foods (Hanna Instruments, Woonsocket, RI, US) and the water activity (aw) was measured at 20 °C using a water activity meter after calibration (Aqua Lab, Decagon Devices, Inc., Pullman, WA, US).
2.4. Colour assessment
The colour of the chickpea-based samples was assessed by measuring the CIE LAB coordinates (L*, a* and b*) with a Chroma Meter CR-400 (Konica Minolta Sensing, Inc., Osaka, Japan), calibrated using a white tile. The colour assessment was performed after 24 h of storage at 4 °C and after meltability measurement (Section 2.8) and repeated on 1 mo old samples before and after meltability measurement.
2.5. Electrophoretic protein profile analysis of chickpea flour and protein concentrate ingredients
The protein profile of CF and CPC was measured using a Capillary Electrophoresis (CE) instrument (PA 800 plus Pharmaceutical Analysis System, Sciex, Kildare, Ireland) equipped with a photo diode array (PDA) detector. The powder samples were mixed directly with the sodium dodecyl sulphate (SDS) molecular weight (MW) sample buffer (Sciex, Kildare, Ireland) containing 100 mM Tris-HCl, pH 9.0, 1% SDS at a protein concentration of 2 mg/mL and mixed over 14 h at 4 °C and over 6 h at 20 °C. After rehydration, 95 μL of sample was mixed with 2 μL of 10 kDa internal marker, and 5 μL 2-iodoacetamide (IAM) and heated at 70 °C for 3 min for non-reducing conditions. While under reducing conditions, samples (95 μL) were mixed with 2 μL of 10 kDa internal marker, and 5 μL 2-mercaptoethanol (2 ME) and heated at 100 °C for 3 min. After heating, samples were cooled at room temperature and transferred into micro sample tubes.
Separation was obtained using a 50-μm bare fused-silica capillary of 30 cm with a 20.2 cm effective length from the inlet to the detection window. All CE-grade reagents were obtained as part of the ProteomeLab™ SDS-MW Analysis Kit (Beckman Coulter, CA, US), designed for the separation of protein-SDS complexes using a replaceable gel matrix. The separating gel was formulated to provide an effective sieving range of approximately 10–225 kDa. The SDS-MW size standard (from 10 to 225 kDa, Beckman Coulter, CA, US) was used to estimate the protein MW distribution of the sample, with a 10 kDa protein (Beckman Coulter, CA, US) used as a mobility marker. A capillary conditioning method was run before analysing each sample, which consisted of a basic rinse (0.1 N NaOH, 10 min, 20 psi), followed by an acidic rinse (0.1 N HCl, 5 min, 20 psi), a water rinse (CE-grade H2O, 2 min, 20 psi) and finally an SDS gel separation buffer rinse (10 min, 70 psi). The voltage equilibration (15 kV for 10 min, with 5 min ramping time) was then applied to the filled SDS gel. The total protein concentration of each sample was 2 mg/mL after the addition of the SDS-MW sample buffer (Beckman Coulter, CA, US). Each sample was injected into the gel-filled capillary by pressure injection in reverse polarity at −5 kV for 20 s. The separation was performed at 15 kV for 30 min with reverse polarity in filled SDS gel. All CE steps were carried out at room temperature. UV detection of migrating proteins was monitored at 220 nm. Data were analysed using 32 Karat™ software (version 8.0, Beckman Coulter, CA, US).
2.6. Differential scanning calorimetry analysis of the ingredients and cheese alternatives
Thermograms of the CF, CPC, shea butter and chickpea-based samples were obtained using a Mettler DSC821 (Mettler-Toledo, Schwerzenbach, Switzerland) differential scanning calorimeter (DSC) equipped with liquid nitrogen cooling. The shea butter ingredient was weighed (12.5–18.1 mg) into standard aluminium pans (Mettler, 40 μL) which were hermetically sealed. The powder ingredients (i.e., CF and CPC) were weighed (5.2–8.6 mg) into aluminium pans and ∼10 mg of water was added to hydrate the powders. Chickpea-based samples were also weighted (17.2–21.1 mg) into aluminium pans. The calorimeter was calibrated for temperature and heat flow using indium. The thermal behaviour of the ingredients and chickpea-based samples was recorded from 0 to 100 °C at a heating rate of 5 °C/min. The DSC curves were analysed using Mettler-Toledo STARe system version 8.10 for thermal analysis. Samples were analysed after 24 h at 4 °C and after 1 mo of storage at 4 °C.
2.7. Confocal laser scanning microscopy
The microstructural observations of the chickpea-based samples were performed using an OLYMPUS FV1000 confocal laser scanning biological microscope (Olympus Corporation, Japan) with a 40× objective lens. The chickpea-based samples were placed onto a glass slide and fat and protein were stained as previously described by Le Tohic et al. (2018) with ∼50 μL of a mixture of Nile Red in 1,2-propanediol (600 μL of 0.1 g/L) and Fast Green FCF aqueous solution (200 μL of 0.1 g/L), respectively. Images were obtained after exciting the Nile Red and Fast Green FCF at 488 and 633 nm, using Ar and He-Ne lasers, respectively (Auty et al., 2001). Representative images of the chickpea-based samples after ∼5 d at 4 °C and after 1 mo at 4 °C were reported.
2.8. Schreiber meltability test
Meltability of the chickpea-based samples was measured after 24 h at 4 °C and after 1 mo, using the Schreiber test (Altan et al., 2005). Cylinders, of height 5 mm and diameter 41 mm, were prepared by pouring the chickpea-based mixture into stainless steel moulds after preparation in the Thermomix and stored. After storage, the samples were placed in a covered glass Petri dish, pictures were taken, and the samples were heated at 232 °C for 5 min in an oven (Memmert, Schwabach, Germany). After cooling the samples at room temperature for 30 min, pictures were taken again, and specimen expansion was measured with a ruler along six lines marked on a set of concentric circles.
2.9. Texture profile analysis
Texture profile analysis (TPA) of the chickpea-based samples, defined as the compression of a bite-size piece of food, two times in a reciprocating motion, imitating the action of the human jaw (Bourne, 2002a), was performed using a Texture Analyser TA-XT2i (Stable Micro Systems, Godalming, Surrey, UK), as previously described by Grasso et al. (2021), with minor modifications. Cylinders of 12 mm height and 20 mm diameter were prepared by pouring the chickpea-based mixture, after the Thermomix step, in glass moulds precoated with siliconizing reagent for glass (Sigmacote®, Sigma-Aldrich, MO, US). The samples were kept at room temperature in the moulds for at least 4 h, after which they were removed from the moulds and stored at 4 °C for 24 h and for 1 mo. After removal from storage, samples were compressed to 30% of their original height in a double compression at a rate of 1.0 mm/s. Hardness, adhesiveness, springiness and cohesiveness, as previously defined by Fox et al. (2017) and Kasapis and Bannikova (2017), were measured for each sample.
2.10. Statistical data analysis
Compositional analysis of the CF and CPC ingredients, and of chickpea-based samples, was performed in triplicate, as well as DSC analysis of the ingredients (i.e., CF, CPC and shea butter). Electrophoretic protein profile analysis of the powder ingredients was performed in duplicate. Two independent trials were conducted to develop the chickpea-based samples and three independent replicates from each trial were used for all the analyses, except for the DSC analysis of the chickpea-based samples which was performed with two independent replicates from each of the two trials. Results are expressed as mean ± standard deviation, unless otherwise stated. Levene's test was used to check the homogeneity of variance and one-way analysis of variance (ANOVA) was carried out using SPSS version 25 (SPSS Inc., Chicago, IL, USA). A Tukey's paired comparison post-hoc test was used to determine statistically significant differences (p < 0.05) between mean values for samples with different formulations, at a 95% confidence level. The paired t-test was used to identify statistically significant differences (p < 0.05) between fresh and aged (1 mo) samples, at a 95% confidence level.
3. Results and discussion
3.1. Composition of chickpea flour and protein concentrate ingredients and cheese alternatives, and physical appearance of cheese alternatives
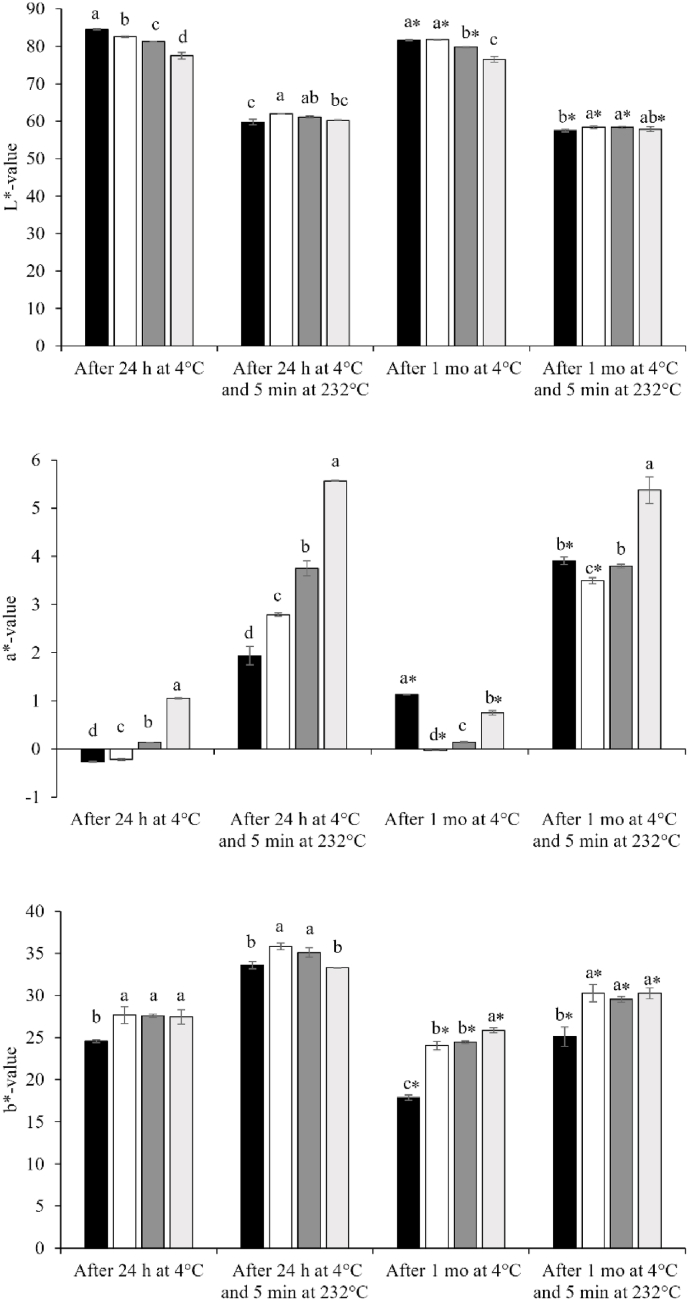
The chickpea-based samples were formulated as described in Section 2.2, using the compositional information available for the CF and CPC ingredients (Section 2.1), with the target compositional parameters provided in Table 1. In good agreement with the predicted formulation, measured protein content of the chickpea-based samples ranged from 8.68 to 21.5% (Table 2). Measured moisture contents were slightly lower than those values from formulation prediction (50%), probably due to water evaporation during the thermo-mechanical processing. Consequently, the carbohydrate content of the samples, calculated by difference, was higher than the predicted values. Fat contents, as expected from the formulations, were not significantly different among the chickpea-based samples. Ash values were in agreement with the ash content found in the powder ingredients, with total ash content increasing with increasing addition level of CPC. The addition of lactic acid led to pH values ranging from 4.39 to 4.50, similar to commercial plant-based cheeses (Grasso et al., 2021). To achieve these pH values, higher amounts of acid were added with increasing protein contents, probably due to the buffering capacity of the globulin fractions of chickpea protein (Martínez-Villaluenga et al., 2008). After 1 mo of storage at 4 °C, pH values ranged from 4.42 to 4.50. The pH value for the 0CPC-100CF sample (i.e., 4.50) did not differ from the value measured after 24 h at 4 °C, again, likely due to the higher buffering capacity of the chickpea-based samples at higher protein contents; indeed, for the 100CPC-0CF sample, the pH increased very slightly from 4.39 to 4.42. The aw values for chickpea-based samples ranged from 0.974 to 0.981, with the values being similar to those for plant-based cheeses available commercially (Grasso et al., 2021). The colour space values of the chickpea-based samples after 24 h and 1 mo of storage at 4 °C are reported in Fig. 1. The L* value, representing brightness with values ranging from 0 to 100, was significantly higher for the 0CPC-100CF sample compared to the other samples, with values for L* decreasing with increasing protein content. The a* value measures the degree of redness (associated with positive values) or greenness (associated with negative values), and increased with increasing protein content in samples stored for 24 h at 4 °C. The b* value, representing the degree of yellowness (associated with positive values) or blueness (associated with negative values), was significantly lower for the 0CPC-100CF sample than the other chickpea-based samples. After 1 mo of storage, all samples showed lower L* and b* values compared to fresh samples stored for 24 h.
Table 2.
Composition of the chickpea-based samples made using chickpea flour (CF) and chickpea protein concentrate (CPC).
| Protein (%) | Fat (%) | Carbohydrates (%) | Ash (%) | Moisture (%) | pH (−) | aw (−) | |
|---|---|---|---|---|---|---|---|
| 0CPC-100CF | 8.68 ± 0.10d | 15.8 ± 0.30a | 27.8 | 1.07 ± 0.07d | 46.7 ± 0.40ab | 4.50 ± 0.00a | 0.981 ± 0.001a |
| 50CPC-50CF | 12.2 ± 0.20c | 15.8 ± 0.25a | 23.2 | 1.33 ± 0.11c | 47.4 ± 0.94a | 4.42 ± 0.00c | 0.979 ± 0.001a |
| 75CPC-25CF | 15.7 ± 0.01b | 15.6 ± 0.84a | 21.4 | 1.64 ± 0.02b | 45.6 ± 0.24b | 4.45 ± 0.01b | 0.976 ± 0.00b |
| 100CPC-0CF | 21.5 ± 0.65a | 15.0 ± 0.91a | 16.0 | 2.03 ± 0.07a | 45.4 ± 0.39b | 4.39 ± 0.00d | 0.974 ± 0.001b |
Values followed by different superscript letters in a column (a-d) are significantly different (p < 0.05).
Fig. 1.
Colour space values before and after 5 min at 232 °C of chickpea-based samples after 24 h at 4 °C and after 1 mo of storage at 4 °C are shown. Bars represent 0CPC-100CF ( ), 50CPC-50CF (
), 50CPC-50CF ( ), 75CPC-25CF (
), 75CPC-25CF ( ) and 100CPC-0CF (
) and 100CPC-0CF ( ) samples. Different letters on bars of the group (a–d) indicate significant differences between samples (p < 0.05), with significance of differences between samples after 24 h at 4 °C and after 1 mo of storage at 4 °C identified with independent t-test and ∗ indicates significant differences (p < 0.05).
) samples. Different letters on bars of the group (a–d) indicate significant differences between samples (p < 0.05), with significance of differences between samples after 24 h at 4 °C and after 1 mo of storage at 4 °C identified with independent t-test and ∗ indicates significant differences (p < 0.05).
3.2. Protein profile of chickpea flour and protein concentrate ingredients
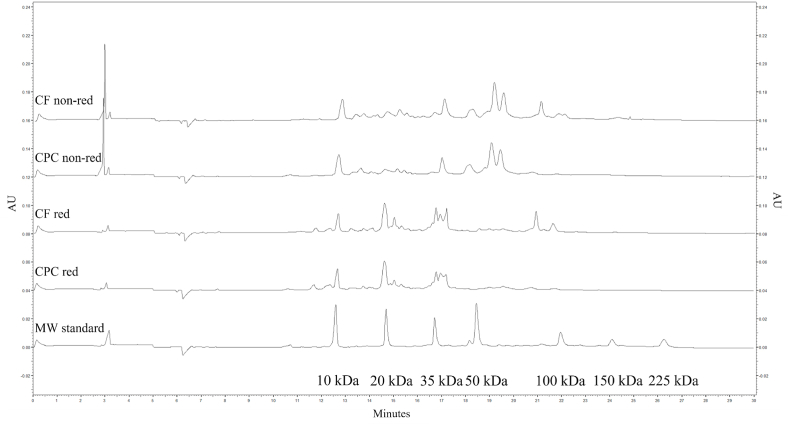
The protein profiles of the CF and CPC under reducing and non-reducing conditions are shown in Fig. 2. Chickpea protein fractions are classified as globulins, 53–60% of total protein, glutelins, 19–25%, albumins, 8–12%, and prolamins, 3–7% (Osborne, 1924; Day, 2013). The peaks around 35–40 and 20 kDa of the CF and CPC electropherograms under reducing conditions (Fig. 2), corresponded to the 11S legumin (the main globulin in chickpeas) acidic (α-legumin) and basic (β-legumin) chains, respectively, probably due to the dissociation of legumin into its acidic and basic subunits under reducing conditions, in agreement with previous studies (Sánchez-Vioque et al., 1999; Papalamprou et al., 2009). Indeed, under non-reducing conditions, such peaks were smaller and a peak at higher MW (i.e., around 60 kDa) was visible (Papalamprou et al., 2009; Vogelsang-O’Dwyer et al., 2020). Other than legumin, another globulin found in chickpeas is 7S vicilin, a trimeric protein, and its subunits corresponded to the peaks around 50 kDa (i.e., major fraction) and around 15, 32 and 70 kDa (i.e., several minor subunits) of the CF and CPC electropherograms, particularly visible under non-reducing conditions, as also reported by Chang et al. (2012). Peaks around 20 and 55 kDa might be associated with glutelin fractions, as observed by Chang et al. (2011); indeed, the same authors reported similarities between these MWs of chickpea protein fractions and those for rice glutelins. While generally similar protein profiles were evident for both the CF and CPC ingredients, two peaks situated between 60 and 100 kDa, were more intense for the CF than the CPC ingredient, under both reducing and non-reducing conditions. The first of the two peaks, with lower MW, may be attributed to convicilin, a globular protein with MW ∼70 kDa. The proportion of convicilin, and more generally the protein profile of chickpea, may vary according to the agronomic practices used for chickpea seed production (e.g., conventional vs organic) and to the exact chickpea genotype (De Santis et al., 2021). The higher MW protein is possibly lipoxygenase, which normally has MW of 92–94 kDa. The lipoxygenase enzyme, an albumin protein, might be partially lost during protein enrichment, which is why its peak is less intense on the CPC electropherograms, in agreement with results from previous research (Sánchez-Vioque et al., 1999).
Fig. 2.
Protein profile of chickpea flour (CF) and chickpea protein concentrate (CPC) under reducing (red) and non reducing (non-red) conditions. From top to bottom, the first two electropherograms represent CF and CPC under non-reducing conditions, respectively, third and fourth electropherograms represent CF and CPC under reducing conditions, respectively. The bottom electropherogram represents the MW standard.
3.3. Thermal behaviour of the ingredients and cheese alternatives
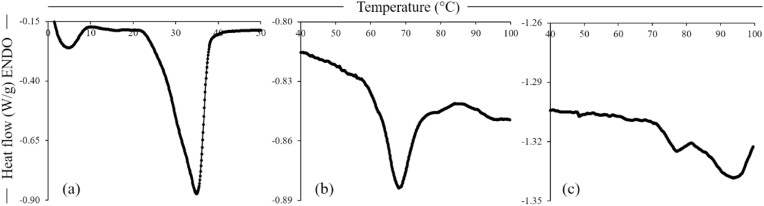
Differential scanning calorimetry (DSC) analysis was performed on the ingredients to develop an understanding, and ultimately support prediction, of the behaviour of these ingredients during the thermal processing involved in the manufacture of the chickpea-based samples, with the results presented in Fig. 3. The shea butter ingredient (Fig. 3a) showed a main peak at 35 °C and smaller peaks at lower temperatures (around 5, 15 and 25 °C), due to the polymorphic nature of shea butter, in agreement with the thermograms previously reported by Lawer-Yolar et al. (2019). The main peak of CF was at 68.1 °C (Fig. 3b), corresponding to starch gelatinisation, with starch representing 37.8% of the CF ingredient. This temperature was comparable to the peak temperatures for desi and kabuli chickpea starches reported by Miao et al. (2009). According to the same authors, some of the main factors influencing gelatinisation temperature of chickpea starch are amylose content, size, form and distribution of starch granules, as well as distribution of amylopectin short chains. The CPC showed 2 peaks, the first at 77.1 °C, which was smaller compared to the second peak at 93.7 °C (Fig. 3c). The presence of a shoulder peak at 77.1 °C was probably associated with denaturation of the (7S) vicilin, while the major peak (93.7 °C) corresponded to denaturation of the (11S) legumin fraction, as also previously reported by Withana-Gamage et al. (2011). Denaturation temperatures reported in the literature for chickpea protein ingredients range between 78.7 and 99.8 °C, with protein structure and composition, chickpea variety (i.e., desi or kabuli) and the processing conditions used to concentrate the proteins, influencing the thermal properties of the ingredient (Paredes-Lopez et al., 1991; Kaur and Singh, 2007; Mousazadeh et al., 2018).
Fig. 3.
Differential scanning calorimetry thermograms of shea butter (a), chickpea flour (CF) (b) and chickpea protein concentrate (CPC) (c).
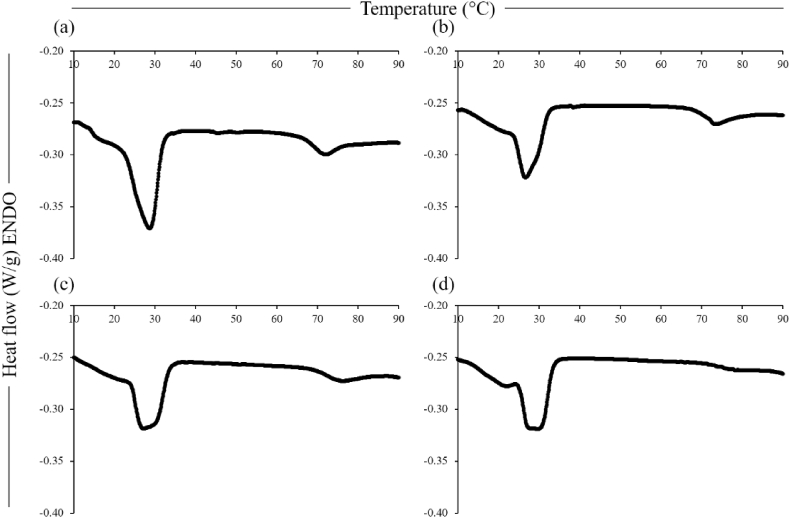
The thermograms of the chickpea-based samples are shown in Fig. 4. All four samples displayed a main peak around 30 °C, corresponding to transition of the shea butter ingredient, with a smaller peak around 70 °C related to starch gelatinisation. This second peak decreased with decreasing carbohydrate content (i.e., mainly starch) in the chickpea-based samples, according to the formulations (Table 1). The starch component was gelatinised during the thermal process, due to the processing temperature of 85 °C. However, the samples were stored for 24 h at 4 °C before analysis (Section 2.6), leading to starch retrogradation and consequent re-gelatinisation during the heating ramp of the DSC analysis, as previously observed for native potato starch analysed before and after 5 d of storage (Morikawa and Nishinari, 2000). The profile of the shea butter transition peak in the chickpea-based samples was narrower for the 0CPC-100CF sample (Fig. 4a), comparable to the thermogram of the shea butter ingredient (Fig. 3a), with the respective component showing wider profiles for the samples with higher protein contents. This was probably due to the different distribution of protein and fat among the samples; indeed, for the 100CPC-0CF sample, the protein formed a matrix surrounding the fat globules, as evident from the microstructural analysis (Fig. 5g). No differences were observed between the thermograms before and after 1 mo of storage at 4 °C (data not shown).
Fig. 4.
Differential scanning calorimetry thermograms of the chickpea-based samples, 0CPC-100CF (a), 50CPC-50CF (b), 75CPC-25CF (c) and 100CPC-0CF (d).
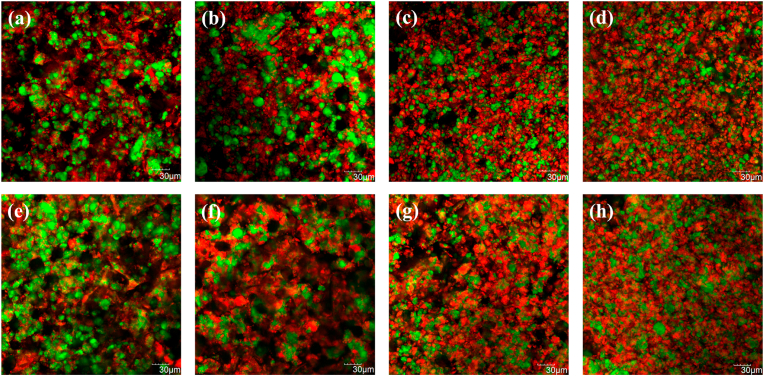
Fig. 5.
Confocal laser scanning microscopy images of chickpea-based samples, after ∼5 d at 4 °C 0CPC-100CF (a), 50CPC-50CF (b), 75CPC-25CF (c) and 100CPC-0CF (d) and samples after 1 mo of storage at 4 °C 0CPC-100CF (e), 50CPC-50CF (f), 75CPC-25CF (g) and 100CPC-0CF (h) are shown. Fat and protein are represented in green and red, respectively.
3.4. Microstructure
Microstructural images of the chickpea-based samples are reported in Fig. 5. Samples after ∼5 d of storage (Fig. 5a, b, c, d) showed formation of a protein matrix and a low occurrence of the carbohydrate components, associated with increasing protein contents. Similar observations were recorded for samples stored for 1 mo (Fig. 5e, f, g, h). Fat globules showed both spherical and non-spherical coalesced pools in all samples. However, the size of fat globules, as well as coalescence of same, decreased with increasing protein contents and a homogeneous distribution throughout the protein matrix was observed in the 75CPC-25CF and 100CPC-0CF samples. The 0CPC-100CF sample showed many black areas, indicating that the carbohydrate constituents gave structure to the sample and, in turn, influenced its physicochemical properties. No major differences in the microstructure were observed between samples before and after storage.
3.5. Meltability
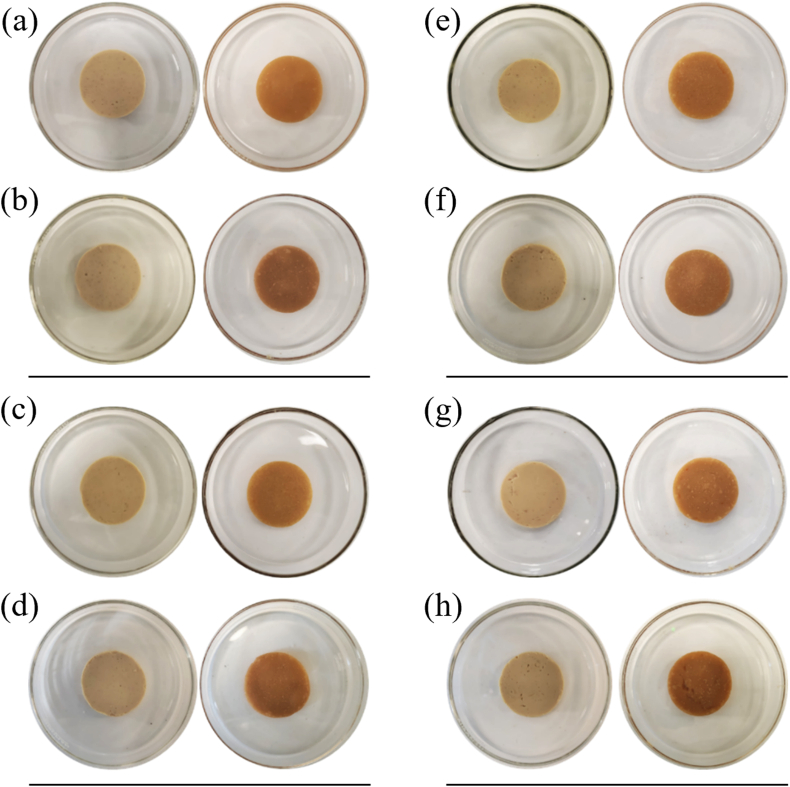
As evident in Fig. 6 (a, c, e, g), none of the chickpea-based samples melted under the testing conditions, since no differences in diameter were observed between the samples before and after the test. The same behaviour was noted for the samples after 1 mo of storage (Fig. 6b, d, f, h). A dry surface of the samples after oven heating was visually observed, and this increased with increasing protein content. On heating, samples stored for 1 mo were dryer compared to samples stored for 24 h at 4 °C, with sample 100CPC-0CF (Fig. 6h) showing fractures on the surface. During oven heating, in the lower protein content samples water was probably absorbed by the starch granules to gelatinise, while the high protein samples showed more dehydration. Furthermore, with temperature increasing over the gelatinisation temperature, water continues to be absorbed by starch, leading to disorganisation of the crystalline structure and more solid-like texture, affecting meltability, and this is probably due to the high levels of amylose in chickpea starch (Lertphanich et al., 2013; Zhang et al., 2016). Poor melting characteristics were previously observed for commercial plant-based cheese products (Grasso et al., 2021). Improvements of the melting behaviour of chickpea-based systems will be necessary for application of same in the formulation of alternatives to cheese products. Moreover, as evident from the data for colour analysis reported in Fig. 1, thermal processing greatly affected the colour of the samples, which had lower L* and higher a* values (i.e., more intense red colour), and higher b* values (i.e., more intense yellow colour), with L* values of heated samples decreasing after 1 mo of storage.
Fig. 6.
Photographs of chickpea-based samples before (left) and after 5 min at 232 °C (right), samples after 24 h at 4 °C 0CPC-100CF (a), 50CPC-50CF (c), 75CPC-25CF (e) and 100CPC-0CF (g) and samples after 1 mo of storage at 4 °C 0CPC-100CF (b), 50CPC-50CF (d), 75CPC-25CF (f) and 100CPC-0CF (h) are shown.
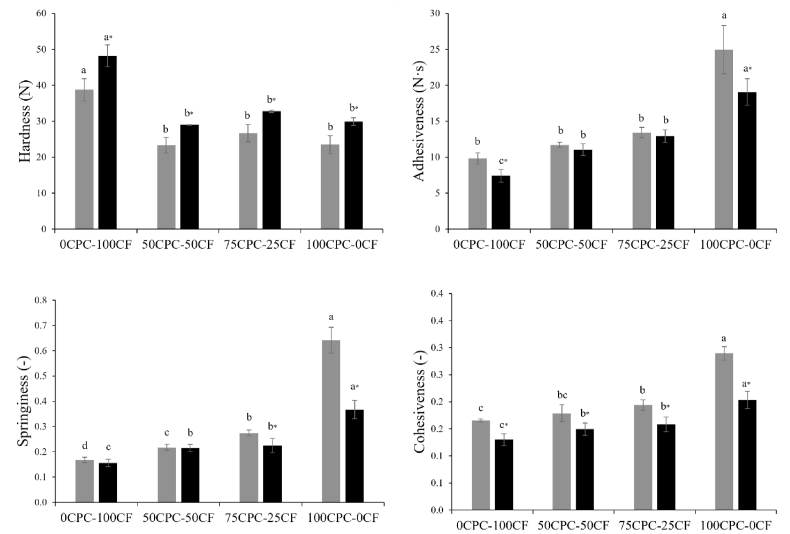
3.6. Textural properties
Texture is one of the principal quality features of food and is defined as the response of the tactile sense to physical stimuli, resulting from contact between the food and some part of the body (Bourne, 2002b). The texture parameters hardness, adhesiveness, springiness and cohesiveness, derived from TPA analysis, of the chickpea-based samples after 24 h at 4 °C and after 1 mo of storage, are shown in Fig. 7. All the samples showed increasing values of adhesiveness, springiness and cohesiveness with increasing protein contents, with the same general trend evident after storage. Adhesiveness is related to the structure of the protein matrix and to the interactions between fat and protein, which influence the adherence between the product and the contact surface (Cunha et al., 2010); increasing adhesiveness with increasing protein content was previously observed in processed cheese (Sołowiej et al., 2015). The values for adhesiveness of chickpea-based samples were higher than those observed previously for commercial plant-based cheeses and Cheddar, being more similar to commercial processed cheese, with the same observed for the cohesiveness results (Grasso et al., 2021). The high cohesiveness for the 100CPC-0CF sample, which is a measure of the strength of the internal bonds within the product, was attributed to the strong protein matrix, as observed from microstructural analysis (Section 3.4). Hardness was highest for the 0CPC-100CF sample, being significantly different from the other samples; this is possibly due to retrogradation of the starch component of the 0CPC-100CF sample. After 1 mo at 4 °C, a slight increase in hardness was observed for all samples, in particular for the 0CPC-100CF sample, likely due to rearrangement of starch (e.g., retrogradation) and protein fractions during storage. This is in agreement with the results reported by Zhang et al. (2016), where chickpea starch gels showed increasing firmness over time. Indeed, in combination with the high proportion of amylose in chickpea starch, the authors related this firm texture to the crystallisation of amylopectin within the starch paste. Indeed, amylose can form junction zones quickly, re-associate, and then re-create intermolecular hydrogen bonds (Zhang et al., 2016). In general, the hardness values reported in the current study for chickpea-based samples were lower compared to commercial plant-based and dairy cheese products previously studied, with only the 0CPC-100CF sample being similar to processed cheese (Grasso et al., 2021), with adhesiveness, springiness and cohesiveness decreasing during storage for all samples.
Fig. 7.
Texture profile analysis parameters hardness, adhesiveness, springiness and cohesiveness of chickpea-based samples, after 24 h at 4 °C ( ) and after 1 mo of storage at 4 °C (
) and after 1 mo of storage at 4 °C ( ) are shown. Different letters on bars of the same colour (a–d) indicate significantly different samples (p < 0.05), with significance of differences between samples after 24 h at 4 °C and after 1 mo of storage at 4 °C identified with independent t-test and ∗ indicates significant differences (p < 0.05).
) are shown. Different letters on bars of the same colour (a–d) indicate significantly different samples (p < 0.05), with significance of differences between samples after 24 h at 4 °C and after 1 mo of storage at 4 °C identified with independent t-test and ∗ indicates significant differences (p < 0.05).
4. Conclusion
The influence of protein concentration on key quality attributes of chickpea-based alternatives to cheese was studied. The samples showed differences based on protein content, particularly in terms of microstructure and texture. Microstructural analysis of the samples demonstrated that formation of a stronger protein matrix, which surrounded fat globules and reduced coalescence, was intensified with increasing protein content. The samples showed higher values for adhesiveness, springiness and cohesiveness with increasing protein content, while hardness was highest for the sample with lowest protein content, associated with the high starch content of that sample. None of the samples melted under the testing conditions; further research should focus on improving the melting behaviour of such formulations for application as alternatives to cheese. The effect of storage for 1 mo was mainly only evident in terms of colour and texture analyses, with lower brightness and higher hardness observed after storage. The results of this work showed the effect of chickpea protein concentration on quality attributes in the development of chickpea-based alternatives to cheese and improved the understanding of the challenges related to such applications. Furthermore, these new insights will help inform future research questions in this area.
CRediT authorship contribution statement
N. Grasso: Conceptualization, Methodology, Investigation, Visualization, Writing – original draft, Software. F. Bot: Methodology, Investigation, Writing – original draft, Software. Y.H. Roos: Methodology, Writing – review & editing. S.V. Crowley: Supervision, Writing – review & editing. E.K. Arendt: Investigation, Writing – review & editing. J.A. O'Mahony: Funding acquisition, Conceptualization, Supervision, Writing – review & editing, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Author Nadia Grasso was supported by the Lauritzson Foundation with the Lauritzson Research Scholarship, through the College of Science, Engineering and Food Science at University College Cork.
Handling Editor: Professor A.G. Marangoni
References
- AACC . 2009. Approved Methods of Analysis-Crude Fat in Wheat, Corn, and Soy Flour, Feeds, and Mixed Feeds 30-25.01. [Google Scholar]
- Altan A., Turhan M., Gunasekaran S. Short communication: comparison of covered and uncovered Schreiber test for cheese meltability evaluation. J. Dairy Sci. 2005;88:857–861. doi: 10.3168/jds.S0022-0302(05)72751-X. [DOI] [PubMed] [Google Scholar]
- AOAC . 1923. Official Methods of Analysis- Ash of Flour 923.03. [Google Scholar]
- AOAC . 1925. Official Methods of Analysis- Solids (Total) and Loss on Drying (Moisture) in Flour. Air Oven Method 925.10. [Google Scholar]
- AOAC . 1930. Official Methods of Analysis-Protein in Dried Milk 930; p. 29. [Google Scholar]
- AOAC . 1990. Official Methods of Analysis- Moisture in Cheese 926.08. [Google Scholar]
- AOAC . 2002. Official Methods of Analysis- Determination of Nitrogen (Total) in Cheese 2001; p. 14. [Google Scholar]
- AOAC . 2005. Official Methods of Analysis- Starch (Total) in Cereal Products. Amyloglucosidase-α-amylase method 996.11. [Google Scholar]
- Auty M.A.E., Twomey M., Guinee T.P., Mulvihill D.M. Development and application of confocal scanning laser microscopy methods for studying the distribution of fat and protein in selected dairy products. J. Dairy Res. 2001;68(3):417–427. doi: 10.1017/S0022029901004873. [DOI] [PubMed] [Google Scholar]
- Bachmann H.P. Cheese analogues: a review. Int. Dairy J. 2001;11(4–7):505–515. doi: 10.1016/S0958-6946(01)00073-5. [DOI] [Google Scholar]
- Benton T.G., Bieg C., Harwatt H., Pudasaini R., Wellesley L. Chatham House: Energy, Environment and Resources Programme. 2021. Food system impacts on biodiversity loss- Three levers for food. [Google Scholar]
- Bharat Book Bureau . Trend and Forecast; 2017. Vegan Cheese Market - Global Scenario, Market Size, Outlook; pp. 2015–2024. [Google Scholar]
- Bourne M.C. In: Food Texture and Viscosity. second ed. Bourne M.C., editor. Academic Press; New York and San Diego: 2002. Principles of objective texture measurements; pp. 107–188. [DOI] [Google Scholar]
- Bourne M.C. In: Food Texture and Viscosity. second ed. Bourne M.C., editor. Academic Press; New York and San Diego: 2002. Texture, viscosity, and food; pp. 1–32. [DOI] [Google Scholar]
- Boye J., Zare F., Pletch A. Pulse proteins: processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010;43(2):414–431. doi: 10.1016/j.foodres.2009.09.003. [DOI] [Google Scholar]
- Chang Y.W., Alli I., Konishi Y., Ziomek E. Characterization of protein fractions from chickpea (Cicer arietinum L.) and oat (Avena sativa L.) seeds using proteomic techniques. Food Res. Int. 2011;44(9):3094–3194. doi: 10.1016/j.foodres.2011.08.001. [DOI] [Google Scholar]
- Chang Y.W., Alli I., Molina A.T., Konishi Y., Boye J.I. Isolation and characterization of chickpea (Cicer arietinum L.) seed protein fractions. Food Bioprocess Technol. 2012;5(2):618–625. doi: 10.1007/s11947-009-0303-y. [DOI] [Google Scholar]
- Cunha C.R., Dias A.I., Viotto W.H. Microstructure, texture, colour and sensory evaluation of a spreadable processed cheese analogue made with vegetable fat. Food Res. Int. 2010;43(3):723–729. doi: 10.1016/j.foodres.2009.11.009. [DOI] [Google Scholar]
- Day L. Proteins from land plants - potential resources for human nutrition and food security. Trends Food Sci. Technol. 2013;32:25–42. doi: 10.1016/j.tifs.2013.05.005. [DOI] [Google Scholar]
- De Santis M.A., Rinaldi M., Menga V., Codianni P., Giuzio L., Fares C., Flagella Z. Influence of organic and conventional farming on grain yield and protein composition of chickpea genotypes. Agronomy. 2021;11(2):191. doi: 10.3390/agronomy11020191. [DOI] [Google Scholar]
- Ferawati F., Hefni M., Östbring K., Witthöft C. The application of pulse flours in the development of plant-based cheese analogues: proximate composition, color, and texture properties. Foods. 2021;10:2208. doi: 10.3390/foods10092208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P.F., Guinee T.P., Cogan T.M., McSweeney P.L.H. In: Fundamentals of Cheese Science. second ed. Fox P.F., Guinee T.P., Cogan T.M., McSweeney P.L.H., editors. Springer; Boston: 2017. Cheese structure, rheology and texture; pp. 475–532. [DOI] [Google Scholar]
- Ghumman A., Kaur A., Singh N. Functionality and digestibility of albumins and globulins from lentil and horse gram and their effect on starch rheology. Food Hydrocolloids. 2016;61:843–850. doi: 10.1016/j.foodhyd.2016.07.013. [DOI] [Google Scholar]
- Grasso N., Roos Y.H., Crowley S.V., Arendt E.K., O'Mahony J.A. Composition and physicochemical properties of commercial plant-based block-style products as alternatives to cheese. Futur. Foods. 2021;4(December 2020) doi: 10.1016/j.fufo.2021.100048. [DOI] [Google Scholar]
- Grossmann L., McClements D.J. The science of plant-based foods: Approaches to create nutritious and sustainable plant-based cheese analogs. Trends Food Sci. Technol. 2021;118(PA):207–229. doi: 10.1016/j.tifs.2021.10.004. [DOI] [Google Scholar]
- Hall C., Hillen C., Robinson J.G. Composition, nutritional value, and health benefits of pulses. Cereal Chem. 2017;94(1):11–31. doi: 10.1094/CCHEM-03-16-0069-FI. [DOI] [Google Scholar]
- IPCC . In: Climate Change and Land: an IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems. Shukla P.R., Skea J., Buendia E.C., Masson-Delmotte V., Pörtner H.-O., Roberts D.C., Zhai P., Slade R., Connors S., van Diemen R., Ferrat M., Haughey E., Luz S., Neogi S., Pathak M., Petzold J., Pereira J.P., Vyas P., Huntley E., et al., editors. 2019. Summary for policymakers. (in press) [Google Scholar]
- Kasapis S., Bannikova A. In: Advances in Food Rheology and its Applications. Ahmed J., Ptaszek P., Basu S., editors. Woodhead Publishing; Sawston: 2017. Rheology and food microstructure; pp. 7–46. [DOI] [Google Scholar]
- Kaur M., Singh N. Characterization of protein isolates from different Indian chickpea (Cicer arietinum L.) cultivars. Food Chem. 2007;102(1):366–374. doi: 10.1016/j.foodchem.2006.05.029. [DOI] [Google Scholar]
- Lawer-Yolar G., Dawson-Andoh B., Atta-Obeng E. Novel phase change materials for thermal energy storage: evaluation of tropical tree fruit oils. Biotechnol. Rep. 2019;24 doi: 10.1016/j.btre.2019.e00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Tohic C., O'Sullivan J.J., Drapala K.P., Chartrin V., Chan T., Morrison A.P., Kerry J.P., Kelly A.L. Effect of 3D printing on the structure and textural properties of processed cheese. J. Food Eng. 2018;220:56–64. doi: 10.1016/j.jfoodeng.2017.02.003. [DOI] [Google Scholar]
- Lertphanich S., Wansuksri R., Tran T., Da G., Nga L.H., Dufour D., Piyachomkwan K., Sriroth K. Comparative study on physicochemical properties of ensete and water caltrop with other root, tuber, and legume starches. Starch/Staerke. 2013;65(11–12):1038–1050. doi: 10.1002/star.201300026. [DOI] [Google Scholar]
- Lopes M., Pierrepont C., Duarte C.M., Filipe A., Medronho B., Sousa I. Legume beverages from chickpea and lupin, as new milk alternatives. Foods. 2020;9:1458. doi: 10.3390/foods9101458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Villaluenga C., Gulewicz P., Frias J., Gulewicz K., Vidal-Valverde C. Assessment of protein fractions of three cultivars of Pisum sativum L.: effect of germination. Eur. Food Res. Technol. 2008;226:1465–1478. doi: 10.1007/s00217-007-0678-9. [DOI] [Google Scholar]
- Mattice K.D., Marangoni A.G. Physical properties of plant-based cheese products produced with zein. Food Hydrocolloids. 2020;105(February) doi: 10.1016/j.foodhyd.2020.105746. [DOI] [Google Scholar]
- Mefleh M., Pasqualone A., Caponio F., Faccia M. Legumes as basic ingredients in theproduction of dairy-free cheesealternatives: a review. J. Sci. Food Agric. 2021;102(1):8–18. doi: 10.1002/jsfa.11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao M., Zhang T., Jiang B. Characterisations of kabuli and desi chickpea starches cultivated in China. Food Chem. 2009;113(4):1025–1032. doi: 10.1016/j.foodchem.2008.08.056. [DOI] [Google Scholar]
- Morikawa K., Nishinari K. Effects of concentration dependence of retrogradation behaviour of dispersions for native and chemically modified potato starch. Food Hydrocolloids. 2000;14:395–401. doi: 10.1016/S0268-005X(00)00021-7. [DOI] [Google Scholar]
- Mousazadeh M., Mousavi M., Askari G., Kiani H., Adt I., Gharsallaoui A. Thermodynamic and physiochemical insights into chickpea protein-Persian gum interactions and environmental effects. Int. J. Biol. Macromol. 2018;119:1052–1058. doi: 10.1016/j.ijbiomac.2018.07.168. [DOI] [PubMed] [Google Scholar]
- Notarnicola B., Tassielli G., Alexander P., Castellani V., Sala S. Environmental impacts of food consumption in Europe. J. Clean. Prod. 2017;140:753–765. doi: 10.1016/j.jclepro.2016.06.080. [DOI] [Google Scholar]
- Osborne T.B. The Vegetable Proteins. 2nd ed. Longmans Green and Co; 1924. [Google Scholar]
- Papalamprou E.M., Doxastakis G.I., Biliaderis C.G., Kiosseoglou V. Influence of preparation methods on physicochemical and gelation properties of chickpea protein isolates. Food Hydrocolloids. 2009;23(2):337–343. doi: 10.1016/j.foodhyd.2008.03.006. [DOI] [Google Scholar]
- Paredes-Lopez O., Ordorica-Falomir C., Olivarez-Vazquez M.R. Chickpea protein isolates: physicochemical, functional and nutritional characterization. J. Food Sci. 1991;56(3):3–6. doi: 10.1111/j.1365-2621.1991.tb05367.x. [DOI] [Google Scholar]
- ProVeg International . 2020. European Consumer Survey on Plant-Based Foods- Describing the Product Landscape and Uncovering Priorities for Product Development and Improvement. [Google Scholar]
- Sánchez-Vioque R., Clemente A., Vioque J., Bautista J., Millán F. Protein isolates from chickpea (Cicer arietinum L.): chemical composition, functional properties and protein characterization. Food Chem. 1999;64(2):237–243. doi: 10.1016/S0308-8146(98)00133-2. [DOI] [Google Scholar]
- Short E.C., Kinchla A.J., Nolden A.A. Plant-based cheeses: a systematic review of sensory evaluation studies and strategies to increase consumer acceptance. Foods. 2021;10(4):725. doi: 10.3390/foods10040725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sołowiej B., Glibowski P., Muszyński S., Wydrych J., Gawron A., Jeliński T. The effect of fat replacement by inulin on the physicochemical properties and microstructure of acid casein processed cheese analogues with added whey protein polymers. Food Hydrocolloids. 2015;44:1–11. doi: 10.1016/j.foodhyd.2014.08.022. [DOI] [Google Scholar]
- SPINS. Good Food Institute . 2020. State of the Industry Report: Plant-Based Meat, Eggs, and Dairy. [Google Scholar]
- Vogelsang-O’Dwyer M., Petersen I.L., Joehnke M.S., Sørensen J.C., Bez J., Detzel A., Busch M., Krueger M., O’Mahony J.A., Arendt E.K., Zannini E. Comparison of faba bean protein ingredients produced using dry fractionation and isoelectric precipitation: techno-functional, nutritional and environmental performance. Foods. 2020;9(3):322. doi: 10.3390/foods9030322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Chelikani V., Serventi L. Short Communication: evaluation of chickpea as alternative to soy in plant-based beverages, fresh and fermented. LWT--Food Sci. Technol. 2018;97(July):570–572. doi: 10.1016/j.lwt.2018.07.067. [DOI] [Google Scholar]
- Withana-Gamage T.S., Wanasundara J.P., Pietrasik Z., Shand P.J. Physicochemical, thermal and functional characterisation of protein isolates from Kabuli and Desi chickpea (Cicer arietinum L.): a comparative study with soy (Glycine max) and pea (Pisum sativum L.) J. Sci. Food Agric. 2011;91(6):1022–1031. doi: 10.1002/jsfa.4277. [DOI] [PubMed] [Google Scholar]
- Zhang H., Yin L., Zheng Y., Shen J. Rheological, textural, and enzymatic hydrolysis properties of chickpea starch from a Chinese cultivar. Food Hydrocolloids. 2016;54:23–29. doi: 10.1016/j.foodhyd.2015.09.018. [DOI] [Google Scholar]