Abstract
Clarias gariepinus collected from Lake Heritage, Crocodile River, were found to harbour camallanid nematodes. Previously, Boomker (1982) surveyed the Hartbeespoort Dam, downstream of the current study site, and identified a high prevalence of Procamallanus (Procamallanus) laeviconchus and Paracamallanus cyathopharynx. Since then, Procamallanus (Procamallanus) pseudolaeviconchus was described from C. gariepinus suggesting reconsideration of the identifications of Procamallanus species in historical studies from clariids. The aim of the current study was to definitively identify the nematodes collected from C. gariepinus in Lake Heritage, using morphological and molecular analyses. Morphological study consisted of light and scanning electron microscopy which confirmed the identity P. (P.) pseudolaeviconchus and P. cyathopharynx. This included descriptions of the detailed morphology of isolated buccal capsules for both species using soft tissue digestion, notably for the first time for P. (P.) pseudolaeviconchus. The morphology of isolated spiculae of both species was described for the first time using SEM. Molecular analyses included genetic characterisation of the small ribosomal subunit (18S) rDNA and cytochrome oxidase 1 (CO1) mtDNA. Genetic data supported the morphological identification of both species, however, divergence was detected in CO1 mtDNA data for P. cyathopharynx indicating two distinct lineages. Due to this variation, the morphometry of P. cyathopharynx specimens were revisited including statistical re-evaluation. No robust morphological traits were identified to support CO1 mtDNA lineages and all specimens were considered conspecific. In terms of camallanid biodiversity in the Crocodile River system, it is similar to that in Boomker (1982), despite the altered water quality from past acid mine pollution in the river.
Keywords: Aquaculture, Clean water and sanitation, DNA barcoding, Life below water, Principal component analysis, Sclerotised structure isolation
Graphical abstract
Highlights
-
•
Two camallanid species (Procamallanus (Procamallanus) pseudolaeviconchus and Paracamallanus cyathopharynx) collected from Clarias gariepinus in Lake Heritage, Crocodile River, South Africa.
-
•
Isolated buccal capsules studied for the first time for P. (P.) pseudolaeviconchus.
-
•
Isolated male spiculae studied for the first time for both species.
-
•
Genetic characterisation (18 S rDNA and CO1 mtDNA) with CO1 mtDNA genetic divergence for P. cyathopharynx.
-
•
Principal component analysis (PCA) is provided for the first time to study Paracamallanus sp. Morphometry to identify distinct morphometric traits.
1. Introduction
Clarias gariepinus (Burchell, 1822), the African sharptooth catfish, and it's endoparasitic fauna have been a growing topic of interest around the world, especially due to the global significance of this freshwater fish species in aquaculture (Akinsanya and Otubanjo, 2006; Satia, 2016; Papuc et al., 2019; Adeleke et al., 2020). These fish are also effective in water quality assessments, serving as environmental sentinels (Crafford and Avenant-Oldewage, 2009; Papuc et al., 2019). Previously, Boomker (1982) surveyed C. gariepinus from Hartbeespoort Dam in the Crocodile River, South Africa, and reported a high prevalence of camallanid nematodes and recorded Procamallanus (Procamallanus) laeviconchus (Wedl, 1862) and Paracamallanus cyathopharynx (Baylis, 1923). Since then, another Procamallanus species was described from C. gariepinus in Africa, Procamallanus (Procamallanus) pseudolaeviconchus Moravec and van As, 2015a, Moravec and van As, 2015b, highlighting the need to review previous Procamallanus spp. records from this host. Moravec and van As (2004) discuss the “taxonomic problems” resulting from an “inadequate description” of P. (P.) laeviconchus, reporting distinct differences between specimens from Synodontis spp. and those from Clarias spp. Notably, Mašová et al. (2011) noticed an unusually smooth or unlobed peribuccal flange of suspected P. (P.) laeviconchus specimens from C. gariepinus in their study using scanning electron microscopy (SEM), and suggested that the specimens may represent a distinct species. Thereafter, Moravec and van As, 2015a, Moravec and van As, 2015b separated the P. (P.) pseudolaeviconchus from P. (P.) laeviconchus mainly on the absence or presence, respectively, of a lobed peribuccal flange surrounding the oral cavity. Notably, prior to the study of Moravec and van As (2004), P. (P.) laeviconchus had not been studied using SEM, while P. (P.) pseudolaeviconchus was studied using both light microscopy (LM) and SEM during its description.
Moravec and van As (2015a) and Moravec (2019) explain that previous studies in Africa probably identified all Procamallanus specimens from clariid hosts as P. (P.) laeviconchus, prior to the description of P. (P.) pseudolaeviconchus and, therefore, likely represent P. (P.) pseudolaeviconchus. According to Moravec (2019), P. (P.) laeviconchus has been reported in Africa from 13 Synodontis spp. (Siluriformes: Mochokidae), as well as Schilbe intermedius Rüppell, 1832 (Schilbeidae), Chrysichthys sp. (Claroteidae) and Distichodus lusosso Schilthuis, 1891 (Distichodontidae: Characiformes). Importantly, Scholz et al. (2018) list P. (P.) laeviconchus from family Clariidae including C. gariepinus and Clarias anguillaris (Linnaeus, 1758), although these host species are only listed as hosts for P. (P.) pseudolaeviconchus in Moravec (2019). In contrast to P. (P.) laeviconchus, P. (P.) pseudolaeviconchus has been reported only from 11 Clarias spp. (Moravec, 2019). This highlights that, according to Moravec (2019), P. (P.) laeviconchus does not infect clariid fishes. In terms of distribution, P. (P.) laeviconchus has been reported from many African countries including Egypt, Sudan, Botswana and Central African Republic (Moravec and van As, 2004; Moravec and van As, 2015a; Moravec and Jirků, 2017). Following the opinion of Moravec (2019), P. (P.) pseudolaeviconchus has unknowingly also been reported from multiple African countries such as the Democratic Republic of the Congo, Egypt, Kenya, Tanzania and South Africa (eg. Campana-Rouget, 1961; Moravec, 1974a; Mašová et al., 2011; Mwita, 2014; Svitin et al., 2019), as well as Israel (Paperna, 1964).
In Southern Africa, Barson and Avenant-Oldewage (2006) reported P. (P.) laeviconchus from C. gariepinus in the Rietvlei Dam using light microscopy. However, this was before the description of P. (P.) pseudolaeviconchus and the specimens in their study were then later presumed to be P. (P.) pseudolaeviconchus by Svitin et al. (2019), who collected this species from C. gariepinus in South Africa (Ndumo Game Reserve). This was due to the presence of an unlobed peribuccal flange corresponding to the description of P. (P.) pseudolaeviconchus. In the afore mentioned study by Boomker (1982), no mention is made of the peribuccal flange of the Procamallanus specimens from C. gariepinus in the Crocodile River. Therefore, it is uncertain which Procamallanus species is present in this system.
Regarding the other camallanid infecting clariid fishes in Africa, Moravec (2019) states that there is only one Paracamallanus species, P. cyathopharynx, present. Originally described as Camallanus cyathopharynx Baylis, 1923, P. cyathopharynx was reassigned to Paracamallanus by Moravec (1974b). Thereafter, P. cyathopharynx and Paracamallanus senegalensis Vassiliadès, 1970 were considered synonyms (Moravec, 1974b, 2019; Boomker, 1982). To date, morphometric analyses of P. cyathopharynx include light and scanning microscopy of whole specimens (eg. Baylis, 1923; Campana-Rouget, 1961; Moravec, 1974b, 2019; Boomker, 1982; Barson et al., 2008; Moravec and van As, 2015b; Sorour and Hamouda, 2019; Svitin et al., 2019; Rindoria et al., 2020), as well as isolated buccal capsules (Rindoria et al., 2020). Even though P. cyathopharynx has been reported from various fishes, Moravec and van As (2015b) states that it appears to only infect clariid fishes as definitive hosts, C. gariepinus as the type host, and other hosts are likely postcyclic. In his review, Moravec (2019) included various siluriform catfish species (Bagridae, Mochokidae and Schilbeidae), as well as Characiformes (Alestidae). Paracamallanus cyathopharynx has a wide distribution, recorded in many localities including the Democratic Republic of Congo, Sudan, Israel, Egypt, South Africa, Nigeria, Zimbabwe, Tanzania, the Central African Republic, Ethiopia and Kenya (eg. Campana-Rouget, 1961; Paperna, 1964; Khalil, 1969; Moravec, 1974b; Boomker, 1982, 1994; Akinsanya and Otubanjo, 2006; Madanire-Moyo and Barson, 2009; Mwita and Nkwengulila, 2010; Moravec and van As, 2015b; Moravec and Jirků, 2017; Moravec and Scholz, 2017; Sorour and Hamouda, 2019; Svitin et al., 2019; Rindoria et al., 2020), with a list provided in Moravec (2019).
In the present study, camallanids were collected from C. gariepinus in Lake Heritage, upstream of Boomker's (1982) sampling site. Since his study, this river system has been exposed to severe levels of pollution, mainly from gold mine effluent releases in the form of acid mine drainage (AMD) which has exacerbated over time (Department of Water Affairs and Forestry, S.A, 2008; Abiye et al., 2015; Leketa et al., 2019; Atta et al., 2020; Shapi et al., 2021; Windisch et al., 2022). Measures to counteract this have been implemented such as water treatment plants, cedar-needle compost bags and upstream lime filtration (Durand, 2012; Atta et al., 2020). However, it is unknown whether this pollution has affected the biodiversity in this system. The present study focuses on biodiversity of enteric nematodes in C. gariepinus using LM, SEM as well as genetic characterisation.
2. Materials and methods
2.1. Fish and parasite collection
A total of 51 C. gariepinus were collected using gill nets, rod and reel, and electronarcosis during January 2019 (n = 9), February 2020 (n = 12), November 2020 (n = 10), September 2021 (n = 10), and February 2022 (n = 10) from Lake Heritage (25°57′31.7″ S, 27°51′20.2″ E) in the Crocodile River in Gauteng, South Africa (Fig. 1). The catfish were euthanized following South African National Standard: Care and Use of Animals for Scientific Purposes (2008) and obtaining the required ethical clearance from the University of Johannesburg (Protocols number: 2018-02-15/Gilbert and 2021-04-01/Nofal _Oldewage). Collection permit CPE2- 000129 from the Gauteng Department of Agriculture and Rural Development was used. Visceral organs were removed, placed in 0.8% NaCl (saline), and the gastrointestinal tract was opened and examined for the presence of endoparasitic nematodes using a dissection microscope (Zeiss Stemi 305, Jena, Germany). Nematodes were fixed in either steaming hot 70% ethanol, room temperature 70% ethanol, steaming hot triethanolamine formalin (TAF) or steaming hot 10% neutrally buffered formalin (NBF) for morphometry and electron microscopy. Some samples were fixed and stored in 96% ethanol for DNA analysis.
Fig. 1.
Maps indicating the sampling locality within South Africa. A- Map of Africa. B- Map of South Africa; red square highlighting area of interest. C- Map of Crocodile River flowing from Lake Heritage (sampling site) to Hartbeespoort Dam further downstream.
2.2. Morphological study
For morphometric analyses, 40 adult nematode specimens (ten male and ten female of each collected taxon) were cleared and mounted in glycerol. Morphometric data for each specimen was captured following standard measurements (see Moravec and van As, 2015a; 2015b; Rindoria et al., 2020). Additionally, 20 ethanol fixed specimens (five males and five females per species fixed in 96% ethanol) were temporarily mounted on glass microscope slides and studied using LM, prior to molecular analysis. Measurements given in micrometres (μm) unless stated otherwise, as a range with mean in parentheses.
For SEM, 40 whole nematodes (ten males and ten females per species) were prepared following the methods of Nation (1983) and Dos Santos and Avenant-Oldewage (2015) and studied using a TESCAN Vega 3 LMH (Tescan, Brno, Czech Republic) scanning electron microscope at 8 kV. To isolate buccal capsules, the anterior of the 20 ethanol fixed specimens (five males and five females per species; to be used for molecular analysis as discussed) were removed, digested, prepared and studied using LM (brightfield and fluorescence) as well as SEM following Dos Santos and Avenant-Oldewage (2015) and Rindoria et al. (2020). After initial study, isolated buccal capsules were micro-dissected using sharpened tungsten needles (Rindoria et al., 2020) to expose the associated internal structures.
To study isolated spiculae using SEM, spiculae of 50 male nematodes (25 per species) were removed using two approaches. Firstly, by adapting the method of Rammah and Hirschmann (1987) using lactophenol and a concavity slide. Secondly, spiculae were isolated by cutting the tail from live males and transferring it to a drop of milli-Q water on a glass slide, a coverslip placed on top and moved in small rotations with added pressure. Once completely freed, the coverslip was removed, and persistent tissue removed by enzymatic digestion if absolutely necessary. Isolated spiculae were studied using SEM.
2.3. Molecular analyses
Genomic DNA was extracted from the midbody sections of the 20 ethanol fixed specimens discussed previously using an E.Z.N.A. Tissue DNA kit (Omega Bio-Tek Inc., Norcross, U.S.A.). Polymerase chain reactions (PCR's) were used to amplify regions of 18S rDNA and CO1 (cytochrome c oxidase I) mtDNA. For 18S rDNA, NEM18SF (5′-CGC GAA TRG CTC ATT ACA ACA GC-3′) by Floyd et al. (2005) and 9r (5′-GAT CCT TCC GCA GGT TCA CCT AC-3′) by Møller et al. (2008) were used with a thermocycling profile as follows: 5 min for denaturation at 94 °C, followed by 3 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 2 min for amplification, and finally 72 °C for 10 min. Additional PCR primers JLR24 (5′-CGG AAT TCG CTA GAG GTG AAA TTC TTG G-3′) and JLR25 (5′-CCG AAT TCC GCA GGT TCA CCT ACG G-3′) as described by Campos et al. (1998) were used. For CO1 mtDNA, amplification was done using newly designed primers; CAMAF1 (5′-TAT TTD WTD TTT GCT TTT TGR TGT GG-3′) and CAMAR1 (5′-TGN CCA AAA AAT CAA AAY AAA TG-3′) with the thermocycling profile as follows: 5 min for denaturation at 95 °C, then 35 cycles of 95 °C for 30 s, 45 °C for 60 s, 72 °C for 60 s for amplification, and finally 72 °C for 10 min. Amplification verified using 1% agarose with SafeView™ Classic dye (Applied Biological Materials Inc, Richmond, Canada) and viewed using a SmartDoc™ 2.0 (Benchmark Scientific, NJ, USA) transilluminator.
2.4. Sequencing and sequence analysis
Amplicons were sequenced using PCR primers in both directions following Avenant-Oldewage et al. (2014). The produced electropherograms were inspected, manually edited if needed, and the reads merged using Geneious Prime 2022.1.1 (https://www.geneious.com). BLAST (Johnson et al., 2008) analyses were performed to check for similarity of obtained haplotypes to published sequence data. Sequence data for family Camallanidae was downloaded from GenBank, including Spirocerca lupi (Rudolphi, 1809) as outgroup following Ailán-Choke and Pereira (2021), and aligned to obtained haplotypes using MUSCLE (Edgar, 2004) as implemented in MEGA 7.0 (Kumar et al., 2015). Identical conspecific sequences and unidentified species were removed from alignments for simplicity. For 18S rDNA, the produced alignment was trimmed to primer JLR24 due to limited coverage of some available data. Genetic distances were calculated based on uncorrected p-distances and number of base pair (bp) differences using MEGA 7.0.
Phylogenetic analyses were performed using maximum-likelihood (ML) and Bayesian inference (BI) approaches as implemented in MEGA 7.0 and BEAST 2.5 (Bouckaert et al., 2019), respectively. For ML analyses of 18S rDNA, the Kimura 2-parameter (Kimura, 1980) model was used as selected using the nucleotide substitution model tool in MEGA 7.0, with discrete Gamma distribution (5 categories (+G, parameter = 0.4848)), invariant sites ([+I], 44.51% sites) and 1000 bootstrap replicate support. For CO1 mtDNA ML analyses, the General Time Reversible (GTR) (Nei and Kumar, 2000) model was used as selected using the nucleotide substitution model tool in MEGA 7.0, with discrete Gamma distribution (5 categories (+G, parameter = 0.7573)), invariant sites ([+I], 21.29% sites) and 1000 bootstrap replicate support. For BI analyses, 10 million Markov chain Monte Carlo (MCMC) generations were utilised, using the Hasegawa-Kishino-Yano (Hasegawa et al., 1985) model for 18S rDNA and the GTR model for CO1 mtDNA. Topologies were combined into a single phylogram based on BI analysis, with nodes lower than 0.75/75% support not indicated and well-supported monophyletic conspecific groupings collapsed (except where lineages were present). Nodal supports are given as BI/ML and support above 0.9/90% indicated by an asterisk (*).
2.5. Statistical analyses
Prevalence, mean intensity and mean abundance of the nematode infections were calculated following Bush et al. (1997). Confidence intervals (CI) at 95% were calculated for prevalence and are presented in parentheses after prevalence, while standard error for intensity is given alongside mean intensity values. Nematodes for which the sex could not be differentiated, either due to damaged or broken bodies, were designated as “unknown sex”. Larvae were not identified to their exact larval stage and were pooled together. Every fish was counted only once when calculating totals for prevalence. However, when a particular fish was infected with males and/or females and/or larvae, this was calculated three times and the total prevalence/intensity/abundance is therefore smaller than the sum of the parts.
To test for statistically significant morphometric variables or distinct specimens, morphometric ratios of Paracamallanus specimens (14 males and 16 females; 30 specimens total) were evaluated with multivariate statistical analyses using a principal component analysis (PCA) in Canoco (ter Braak and Smilauer, 2002) version 5.15. A second PCA was performed using only buccal capsule ratios, and the morphotype and lineage information of the specimens overlayed.
3. Results
Two species of camallanid nematodes were recovered from the African sharptooth catfish, C. gariepinus, in Lake Heritage: P. (P.) pseudolaeviconchus and P. cyathopharynx.
3.1. Morphological and morphometric results
- Family Camallanidae Railliet and Henry, 1915
- Genus Procamallanus Baylis, 1923
- Procamallanus (Procamallanus) pseudolaeviconchus Moravec and van As, 2015
Type host: African sharptooth catfish, Clarias gariepinus (Burchell, 1822); Siluriformes; Clariidae.
Other hosts: According to Moravec (2019), the following are considered definitive hosts: Clarias spp. including Clarias agboyiensis Sydenham, 1980, Clarias alluaudi Boulenger, 1906, Clarias anguillaris (Linnaeus, 1758), Clarias buthupogon Sauvage, 1879, Clarias ebriensis Pellegrin, 1920, Clarias liocephalus Boulenger, 1898, Clarias macromystax Günther, 1864, Clarias stappersi Boulenger, 1915, Clarias theodorae Weber, 1897 and Clarias werneri Boulenger, 1906.
Locality: Lake Heritage (25°57′31.7″ S, 27°51′20.2″ E), Crocodile River, Muldersdrift, South Africa.
Site of infection: Oesophagus and stomach.
Prevalence: 78.4% (CI = 67.1–89.7%); 40 out of 51 infected.
Intensity: 1–26 (8.8 ± 1).
Abundance: 0–26 (6.9).
Specimens deposited: A total of 15 voucher specimens were deposited: 5 (2 females and 3 males) in each of the 3 museums; Institute of Parasitology, Czech Academy of Sciences, České Budějovice, Czech Republic (IPCAS N-1068), the Natural History Museum, London (NHMUK 2022.10.13.1–13.5), and Iziko Museum of South Africa (SAMC-A094714–18).
Genetic data: All genetic material produced deposited to GenBank, eight 18S rDNA sequences (OP602987 to OP602994) and ten CO1 mtDNA sequences (OP602960 to OP602969).
General description. Medium-sized nematodes, sexually dimorphic, males distinctively smaller. Body long and narrow, maximum width equatorially. Cuticle thick and continuous, transverse striations along entire length of body. Anterior end with terminal, circular oral opening, characterized by unlobed or smooth peribuccal flange (Fig. 2A and B). Eight submedian cephalic papillae encompass oral opening, arranged in inner and outer rings of four papillae each (Fig. 2B). Two amphids, each lateral to oral opening (Fig. 2B). Buccal capsule elongated, sclerotized, yellow-orange, barrel-shaped, six marginal, crescent-shaped elevations anteriorly (Fig. 2D). Interior of buccal capsule smooth, narrow posterior ring followed by basal ring located at point of oesophageal attachment (Fig. 2E and F). Oesophageal cup posterior to buccal capsule, relatively small and poorly sclerotized. Muscular oesophagus triradial and claviform, preequatorially encircled by nerve ring. Glandular oesophagus cylindrical and claviform, longer than muscular oesophagus. Excretory pore posterior to nerve ring, anterior to widened part of muscular oesophagus base (Fig. 2G, G(i)). Small, simple, short, single, blunt deirids lateral on opposing sides, at level of nerve ring (Fig. 2H and I). Narrow intestine; light-brown in adults. Tail tapered, ending in rounded tip.
Fig. 2.
Scanning electron micrographs of Procamallanus (Procamallanus) pseudolaeviconchus Moravec and van As, 2015 from Clarias gariepinus (Burchell, 1822). A-anterior end, lateral view; B- anterior end, apical view, solid arrow shows smooth peribuccal flange, dashed arrow shows marginal elevation; C- isolated buccal capsule, lateral view, arrow shows oesophagus; D-isolated buccal capsule, apical view, solid arrows show marginal elevations; E−buccal capsule interior, apical view, dashed arrow shows narrow ring, solid arrow shows basal ring; F- microdissected buccal capsule, lateral view, dashed arrow shows narrow ring, solid arrow shows basal ring; G-anterior region, lateral view, arrow shows excretory pore; G(i)- excretory pore, lateral view; H- lateral deirid, lateral view; I- lateral deirid, apical view. a = amphid; s = submedian papilla.
Female (Fig. 3A–C; supplementary file A). Morphometrics based on 14 adult specimens for LM; ten whole specimens studied using SEM. Body length 3.25–8.26 (6.77) mm; maximum width 111–212 (162). Entire buccal capsule (including basal ring) 49–77 (73) long, 39–64 (55) wide. Basal ring 6–37 (15) long, 7–37 (28) wide. Oesophageal cup 5–12 (8) long, 9–20 (13) wide. Muscular oesophagus 378–544 (430) long, 5–12 (7) % of body length; 19–46 (36), 31–62 (49) and 44–99 (67) wide at anterior, mid-length and posterior region, respectively. Glandular oesophagus 586–1044 (811) long, 10–18 (12) % of body length; 38–64 (48), 40–99 (60), 42–103 (68) wide at anterior, mid-length and posterior region, respectively. Length of entire oesophagus and buccal capsule 17–32 (20) % of body length. Nerve ring 168–276 (212) from anterior end. Excretory pore 202–493 (280) from anterior end. Deirids 180–244 (213) from anterior end. Vagina directed posteriorly, causing lateral body wall protrusion near vulva. Vulva situated postequatorially on body, directed laterally with distinct lips (Fig. 3A and B), 1.77–4.78 (3.68) mm from anterior end 49–60 (54) % of body length. First-stage larva observed exiting vulva (Fig. 3C). Tail 85–220 (122) long with three digit-like processes.
Fig. 3.
Scanning electron micrographs of Procamallanus (Procamallanus) pseudolaeviconchus Moravec and van As, 2015 from Clarias gariepinus (Burchell, 1822). A-postequatorial region of female, vulva, ventral view; B- vulva, lateral view, arrows show lips; C- first-stage larva exiting vulva; D-posterior end of male, ventral view, solid arrows show pre-cloacal papillae, dashed arrows show post-cloacal papillae, circles show adcloacal papillae; D(i)- pedunculate papilla; E−isolated right spicule, lateral view, solid arrow shows shaft, dashed arrow shows spicule tip; E(i)- right spicule tip, arrow shows velum; F- posterior end of male, ventrolateral view, arrow shows right spicule; G-isolated left spicule, lateral view; G(i)- left spicule tip, arrow shows velum.
Male (Fig. 3D–G; supplementary file B). Morphometrics based on 16 adult specimens for LM; ten whole specimens and 25 specimens for spicule isolation studied using SEM. Body length 2.98–6.14 (4.10) mm; maximum width 90–162 (114). Entire buccal capsule (including basal ring) 52–67 (58) long, 37–53 (42) wide. Basal ring 5–33 (10) long, 6–51 (24) wide. Oesophageal cup 4–12 (7) long, 6–18 (11) wide. Muscular oesophagus 219–495 (368) long, 5–15 (9) % of body length; 21–46 (31), 28–65 (43) and 38–97 (59) wide at anterior, mid-length and posterior region, respectively. Glandular oesophagus 589–1051 (736) long, 15–29 (18) % of body length; 27–78 (47), 36–104 (60) and 34–97 (56) wide at anterior, mid-length and posterior region, respectively. Length of entire oesophagus and buccal capsule 24–45 (29) % of body length. Nerve ring 144–223 (178) from anterior end. Excretory pore 155–357 (250) from anterior end. Deirids 124–251 (185) from anterior end. Caudal end, ventrally curved with narrow alae and papillae: eight to nine pairs precloacal pedunculate papillae (Fig. 3D and D(i)), one pair adcloacal sessile papillae (slightly anterior to cloacal opening) (Fig. 3D), four pairs postcloacal papillae (three pairs postcloacal and ventral (Fig. 3D), one pair further dorsolateral). Two simple unequal spicules (Fig. 3E–F), both with long and thin cylindrical shaft, with blunt, rounded distal ends and thin, poorly sclerotised, wing-like velum. Right spicule with widened base. Right (large) spicule 41–148 (93) long, left (small) spicule 19–57 (41) long (Fig. 3G and G(i)). Gubernaculum indistinct, recorded in only five specimens, 36–141 (77) long. Tail 38–85 (56) long.
Remarks. The morphometry of specimens described here strongly agree with the species description of P. (P.) pseudolaeviconchus by Moravec and van As (2015a). It also agrees with other records of this taxon (Moravec, 2019; Svitin et al., 2019), as well as those reported as P. (P.) laeviconchus from the same host (Moravec, 1975; Boomker, 1982; Barson and Avenant-Oldewage, 2006). Morphology of isolated buccal capsules, right (large) and left (small) spiculae are presented for the first time. The region above the basal ring lacks a uniform term. Moravec and van As (2015a) described a thickening of the capsule wall situated anteriorly to the basal ring, whereas Svitin et al. (2019) refers to it as two step-like folds, and Moravec (2019) as a narrow ring. Following Moravec (2019), the term narrow ring was used. Vulva, with lips, shown for the first time using SEM. The number of digit-like processes varied, with adult females bearing three and fourth-stage larvae four processes. The number of cloacal papillae in males varied in previous studies, those observed in the present study overlapping with most.
- Genus Paracamallanus Yorke and Maplestone, 1926
- Paracamallanus cyathopharynx (Baylis, 1923)
- Syns. Camallanus cyathopharynx Baylis (1923); Paracamallanus senegalensis Vassiliadès, 1970
Host: African sharptooth catfish, Clarias gariepinus (Burchell, 1822); Siluriformes; Clariidae.
Other hosts: According to Moravec (2019): clariids Channallabes apus (Günther, 1873), Clariallabes teugelsi Ferraris, 2007, Clarias alluaudi Boulenger, 1906, C. anguillaris, Clarias buthupogon Sauvage, 1879, Clarias ebriensis Pellegrin, 1920, Clarias liocephalus Boulenger, 1898, Clarias stappersi Boulenger, 1915, Clarias theodorae Weber, 1897, Clarias werneri Boulenger, 1906, Clarias sp. and Heterobranchus longifilis Valenciennes, 1840. Additionally, it has been recorded from silurids Clarotes laticeps (Rüppell, 1829), Schilbe intermedius Rüppell, 1832, Synodontis schall (Bloch and Schneider, 1801), Synodontis zambezensis Peters, 1852; and a characid Hydrocynus vittatus Castelnau, 1861.
Locality: Lake Heritage (25°57′31.7″ S, 27°51′20.2″ E), Crocodile River, Muldersdrift, South Africa.
Site of infection: Distal intestine and rectum.
Prevalence: 94.1% (CI = 87.7–100%); 48 out of 51 infected.
Intensity: 1–187 (28.3 ± 4.7).
Abundance: 0–187 (26.6).
Specimens deposited: A total of 15 voucher specimens were deposited: 5 (2 females and 3 males) in each of the 3 museums; Institute of Parasitology, Czech Academy of Sciences, České Budějovice, Czech Republic (IPCAS N-12), the Natural History Museum, London (NHMUK 2022.10.13.6–13.10), and Iziko Museum of South Africa (SAMC-A094709–13).
Genetic data: All genetic material produced deposited to GenBank, five 18S rDNA sequences (OP602982 to OP602986) and twelve CO1 mtDNA sequences (OP602970 to OP602981).
General description. Medium-sized nematodes, sexually dimorphic; males smaller than females. Elongated and thin body, widest at mid-body. Cuticle continuous with prominent horizontal striations along body length. Anterior end with terminal, slit-like oral opening surrounded by eight cephalic submedian papillae (two circular arrangements of four papillae each) (Fig. 4A and B). Pair of amphids, located dorsal and ventral to mouth (Fig. 4A). Sclerotised buccal capsule, yellow-orange, consisting of anterior and posterior parts separated by isthmus (Fig. 4C(i)). Anterior buccal capsule rounded, characterized by; dorsal and ventral valves, each valve with two sclerotized plates superficially surrounding mouth (Fig. 4C), superficial pair of large posteriorly extending lateral tridents distal to mouth with middle prong longest (Fig. 4C). Interior surface of each valve with nine distinct longitudinal ridges (Fig. 4D). Posterior buccal capsule (pharynx) comprised of two parts (Fig. 4C(i)); anterior long, widened towards top and lined with thick walls; posterior substantially smaller with thin walls leading to small, sclerotized and round oesophageal cup (wider than it is tall) (Fig. 4B). Muscular oesophagus claviform with basal region wider. Nerve ring situated slightly posterior to oesophageal cup, encircling muscular oesophagus. Pair of inconspicuous, simple, blunt lateral deirids at level of nerve ring (Fig. 4E). Excretory pore posterior to nerve ring. Glandular oesophagus cylindrical and longer than muscular oesophagus. Long, straight intestine, narrow rectum. Tail tapered, rounded tip.
Fig. 4.
Scanning electron micrographs of Paracamallanus cyathopharynx (Baylis, 1923) from Clarias gariepinus (Burchell, 1822). A-anterior end, apical view; B- anterior end, apical view, arrow shows oesophagus; C- isolated buccal capsule, lateral view, arrow shows oesophagus; C(i)- isolated buccal capsule, lateral view, double arrow shows isthmus; D-microdissected anterior buccal capsule, ventral view of longitudinal ridges. E- lateral view of deirid; F, G, G(i)- posterior end of female, apical view, digit-like processes. a = amphid; s = submedian papilla; sc = sclerotised plate; t = trident; 1 = anterior buccal capsule; 2 = anterior part of posterior buccal capsule; 3 = posterior part of buccal capsule.
Female (Fig. 4D–E; supplementary file C). Morphometrics based on 16 specimens for LM; ten whole specimens studied using SEM. Body length 5–10 (8) mm; maximum width 103–187 (147). Entire buccal capsule (excluding oesophageal cup) 107–146 (122) long. Anterior buccal capsule 66–85 (74) long, 52–100 (78) wide. Posterior buccal capsule (pharynx) 34–56 (42) long, 61–98 (77) wide. Posterior part of posterior buccal capsule 12–31 (19) long, 28–53 (40) wide. Tridents 58–93 (73) long. Muscular oesophagus 456–680 (573) long, 6–12 (8) % of body length, 32–80 (50), 42–96 (66) and 12–125 (72) wide at anterior, mid-length and posterior region, respectively. Glandular oesophagus 436–989 (736) long, 5–17 (10) % of body length, 35–86 (59), 30–106 (67) and 25–104 (64) wide at anterior, mid-length and posterior region, respectively. Entire oesophagus and buccal capsule 1.2–1.8 (1.43) mm long; 14–32 (19) % of body length. Nerve ring 153–220 (181) from anterior end. Excretory pore 172–347 (245) from anterior end. Deirids 145–251 (192) from anterior end. Vagina directed posteriorly, causing slight elevation anterior to vulva. Vulva situated postequatorially on body, directed posteriorly, indistinct lips, 2–6 (4) mm from anterior end, 30–63 (55) % of body length. Tail 235–401 (320) long, three or four digit-like processes (Fig. 4F and G).
Male (Fig. 5A–F; supplementary file D). Morphometrics based on 14 specimens for LM; ten whole specimens and 25 specimens for spicule isolation, studied using SEM. Body length 3.29–6.20 (4.87) mm; maximum width 74–146 (112). Entire buccal capsule (excluding oesophageal cup) 77–101 (91) long. Anterior buccal capsule 50–67 (57) long, 46–71 (58) wide. Posterior buccal capsule (pharynx) 22–62 (32) long, 28–64 (50) wide. Posterior part of posterior buccal capsule 10–17 (12) long, 21–33 (27) wide. Tridents 49–68 (59) long. Muscular oesophagus 353–497 (423) long, 7–12 (9) % of body length, 28–64 (44), 41–74 (53) and 45–97 (67) wide at anterior, mid-length and posterior region, respectively. Glandular oesophagus 416–863 (572) long, 10–14 (12) % of body length, 40–83 (52), 36–108 (59) and 32–104 (58) wide at anterior, mid-length and posterior region, respectively. Entire oesophagus and buccal capsule 845–1397 (1086) long; 18–28 (23) % of body length. Nerve ring 113–164 (143) from anterior end. Excretory pore 140–218 (178) from anterior end. Deirids 124–186 (154) from anterior end. Ventrally curved caudal end, narrow alae and papillae: five pairs precloacal pedunculated papillae (Fig. 5A), two pairs adcloacal papillae (Fig. 5B), six pairs postcloacal papillae (three pairs grouped together short distance posterior to cloaca, two pairs at mid region of tail and one pair close to tail tip) (Fig. 5B). Two spicules, unequal in length. Right (large) spicule 157–323 (264) long, sclerotized with sharply curved, lanceolate distal part 28–56 (38) long (Fig. 5C–E), miniscule barb at ventral base of distal part 2–9 (6) long (Fig. 5E). Left (small) spicule 52–197 (99) long, acerose, less sclerotised, sharply pointed distal end (Fig. 5F). No openings or channels visible in either of the spicules. Tail 54–103 (70) long, two digit-like processes (Fig. 5A(i)).
Fig. 5.
Scanning electron micrographs of male Paracamallanus cyathopharynx (Baylis, 1923) from Clarias gariepinus (Burchell, 1822). A-posterior end, ventrolateral view, arrows show precloacal papillae; B- posterior end, ventral view, solid arrow shows right spicule, dashed arrow shows pair of adcloacal papillae, double arrows show postcloacal papillae; C- isolated right spicule, ventrolateral view, arrow shows shaft; D-right spicule tip, dorsal view; E−right spicule tip, ventral view, arrow shows ventral barb; F- isolated left spicule.
Remarks. The morphometry of specimens described here conform to the species description of P. cyathopharynx by Baylis (1923), the redescription by Moravec (1974b) and the studies by Moravec and van As (2015b), Moravec (2019), Svitin et al. (2019) and Rindoria et al. (2020). Slight differences were recorded compared to Baylis (1923) regarding the length of the tridents and number of longitudinal ridges in the anterior buccal capsule. Isolated buccal capsules in the current study are similar to those in Rindoria et al. (2020), however, the anterior small trident was not observed in any of the current specimens. Morphology of the isolated right (large) and left (small) spiculae studied using SEM are presented for the first time. Variation was observed in the caudal end of females regarding the number of digit-like processes of either three or four. In terms of male digit-like processes, some discrepancy was noted between the studies of Moravec and van As (2015b) and Rindoria et al. (2020) in which the current study corresponds with that described by Moravec and van As (2015b). Lastly, variation was also observed in the number of cloacal papillae described by Rindoria et al. (2020) to the current and other studies.
3.2. Molecular results
3.2.1. 18S rDNA
A total of 20 specimens (five males and five females of each species) were used to characterise the 18S rDNA gene region, for which eight Procamallanus samples (1557 bp to 1622 bp) and five Paracamallanus samples (1599 bp) produced useable sequence data. The entire 18S alignment of all Camallanidae data, including this study's sequences and after trimming to primer JLR24, was 874 bp long with 771 conserved sites, 96 variable sites and 42 parsimony informative sites. The calculated intraspecific range for the family is up to 1.34%, and the interspecific range 0.12%–4.87% based on 18S rDNA (supplementary file E), forming a clear overlap. However, 18S rDNA data still support the morphological identification of both P. (P.) pseudolaeviconchus and P. cyathopharynx. All P. (P.) pseudolaeviconchus sequences were identical and the obtained haplotype identical to the only sequence for this taxon (MN514770) by Svitin et al. (2019). However, sequences ON321842 and ON359914 for P. (P.) laeviconchus (“Procamallanus laevionchus”) were also identical to those from the present study. These latter two sequences are from unpublished works and were both for Procamallanus collected from C. gariepinus thus, they likely represent P. (P.) pseudolaeviconchus as well. All Paracamallanus sequences were nearly identical, only varying by one ambiguous site, and the obtained haplotype identical to sequences by Mwita and Nkwengulila (2010) [DQ813445], Rindoria et al. (2020) [MN396556] and Svitin et al. (2019) [MN514775] for P. cyathopharynx.
The phylogram in Fig. 6 shows that P. (P.) pseudolaeviconchus data from the present study groups in a well-supported monophyletic clade with other data for this taxon (in purple). Similar to sequences ON321842 and ON359914, sequence DQ813446 for P. (P.) laeviconchus (“Procamallanus laevionchus”) by Mwita and Nkwengulila (2010) groups with P. (P.) pseudolaeviconchus, and not P. laeviconchus (JF803934) by Černotíková et al. (2011), but is more distant. The P. cyathopharynx haplotype in the current study forms a monophyletic clade with all other data for this taxon (in orange). Most deeper nodes had very low support for ML analysis.
Fig. 6.
Phylogram of Camallanidae based on 18S rDNA, with Spirocerca lupi (Rudolphi, 1809) as the outgroup. Procamallanus and Paracamallanus data from the present study are indicated in purple and orange, respectively. Nodal support presented for Bayesian inference and Maximum Likelihood approaches (BI/ML), with support lower than 0.75/75% excluded and support above 0.9/90% indicated by an asterisk (*).
3.2.2. CO1 mtDNA
A total of 30 specimens were used (five males and ten females of each species) to amplify the CO1 mtDNA gene region, for which ten Procamallanus samples (587–613 bp) and 12 Paracamallanus (585–613 bp) produced useable sequence data. The alignment of all Camallanidae data, including this study's sequences, was a total of 613 bp long with 261 conserved sites, 352 variable sites and 244 parsimony informative sites. The calculated intraspecific limit for the family is up to 9.28%, and the interspecific range 7.50%–22.22% based on COI mtDNA (supplementary file F), with a slight overlap. Resulting CO1 mtDNA data showed that all Procamallanus sequences are highly similar with two haplotypes, only varying by one bp (0.17%). Procamallanus sequences from the current study were similarly close (0%–0.33%) to MN523682 for P. (P.) pseudolaeviconchus by Svitin et al. (2019), far below the intraspecific limit. This supports the morphological identification of the present material as P. (P.) pseudolaeviconchus. There is no CO1 mtDNA genetic data available for P (P.) laeviconchus.
Variation was observed between Paracamallanus sequences. Of the 12 obtained sequences, seven haplotypes were detected belonging to two distinct lineages, designated as lineage one (LI1) and lineage two (LI2). Divergences within lineages were below the intraspecific limit (LI1 – 0%–0.49%; LI2 – 0%–1.03%), but distances between the lineages were within the interspecific range (10.09%–11.58%). Further, LI2 sequences are within intraspecific distances (1.31%) of P. cyathopharynx (MN523683) by Svitin et al. (2019), indicating that LI2 is likely conspecific to P. cyathopharynx. In comparison, LI1 sequences are 10.77%–10.93% (66–67 bp) distant to P. cyathopharynx (MN523683), falling within the interspecific range. This suggests that LI1 is genetically distinct to P. cyathopharynx.
The phylogram in Fig. 7 shows that all P. (P.) pseudolaeviconchus form a well-supported monophyletic clade (in purple). Similarly, all data for P. cyathopharynx also form a well-supported monophyletic group. However, for the latter taxon, the two lineages (LI1 in green and LI2 in orange) form well-supported sister clades in this grouping, with the sequence for this taxon by Svitin et al. (2019) grouping with LI2. The same is seen for Procamallanus (Spirocamallanus) neocaballeroi (Caballero-Deloya, 1977) which forms a strongly monophyletic group with three well-supported lineages following Santacruz et al. (2020). Some deeper nodes were not well-supported, with conspecific grouping well-supported.
Fig. 7.
Phylogram of Camallanidae based on CO1 mtDNA, with Spirocerca lupi (Rudolphi, 1809) as the outgroup. Procamallanus data and Paracamallanus lineage one (LI1) and two (LI2) from the present study are indicated in purple, orange and green, respectively. Nodal support presented for Bayesian inference and Maximum Likelihood approaches (BI/ML), with support lower than 0.75/75% excluded and support above 0.9/90% indicated by an asterisk (*).
3.3. Paracamallanuscyathopharynx morphological re-evaluation
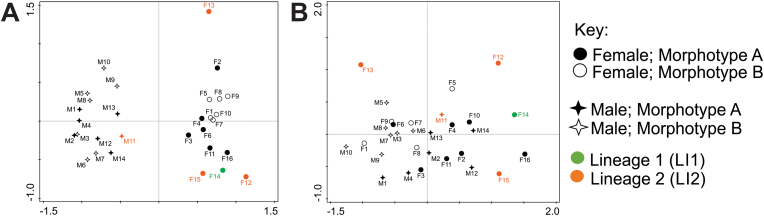
Based on the CO1 mtDNA genetic variation observed for Paracamallanus samples, their morphometry was revisited using statistical analyses to identify distinguishable traits between specimens to differentiate between the two lineages. Two suspected morphotypes were observed pertaining to the buccal capsules of Paracamallanus specimens (supplementary file G). In Fig. 8, the isolated buccal capsules of the two suspected morphotypes can be seen using both LM (brightfield and fluorescence) and SEM. The most distinct trait is the shape of the anterior and posterior capsules (Fig. 8A(iii), 8 B(iii)), which are elliptical (wider than long) in morphotype A and more rectangular (longer than wide) in morphotype B. Further, morphotype A has large tridents which extend past the anterior part of the posterior capsule, reaching the posterior part (Fig. 8A(ii)). In comparison, in morphotype B, the tridents extend approximately three-quarters down the anterior part of the posterior capsule and do not reach the posterior part (Fig. 8B(ii)). Nine ridges were observed in both morphotypes. However, the central ridge of morphotype A was less conspicuous and shorter than in morphotype B. Both morphotypes were observed in males and females. Most female specimens with morphotype A (elliptical buccal capsules) were ovigerous (subgravid) and morphotype B (rectangular buccal capsules) females were larvigerous (gravid). Similarly, the differences in buccal capsules are suspected to be related to maturity in males, but this could not be distinguished.
Fig. 8.
Lateral view of isolated buccal capsules of Paracamallanus specimens, morphotypes A and B. (i). Brightfield. (ii). Epifluorescence [Filter-set 09 (Ex. 470/40)]. (iii). SEM. 1 = anterior part of posterior capsule; 2 = posterior part of posterior capsule; t = trident; ellipse = elliptical shape of capsule; rectangle = rectangular shape of capsule.
By overlaying the PCA plot generated using all morphometric ratios with suspected buccal capsule morphotypes and CO1 lineages, definite separation is seen between males and females due to sexual dimorphism (Fig. 9A). Eigenvalues were high as the plot explains 82.55% of the total variation with 20.42% on the x-axis and 62.13% on the y-axis. However, only a few specimens could be accurately traced back to their respective lineages. There was no clear indication of a relationship between buccal capsule morphotype and CO1 lineages, with all females sharing morphotype A but representing both lineages. Notably, F13 appears to be a morphological outlier but shares the same lineage as F12 and F15. Similarly, the PCA plot in Fig. 9B, based on only buccal capsule ratios overlayed with morphotype and lineage data, does not show any consistent pattern or grouping. Unlike in the first plot, data is randomly scattered with no specific morphometric drivers. Eigenvalues were lower as the plot explains 70.83% of the total variation with 19.34% on the x-axis and 51.49% on the y-axis. Again, no clear relationship between buccal capsule morphotype and CO1 lineages was observed.
Fig. 9.
Principal Component Analyses (PCA) of Paracamallanus morphometry collected from Clarias gariepinus (Burchell, 1822). A- PCA using morphometric ratios for both males and females. B- PCA using buccal capsule ratios for both males and females. Each parasite is indicated as a dot, with the fill, shape and colour corresponding to morphotype and lineage (refer to key). F = female; M = male.
3.4. Infection biology
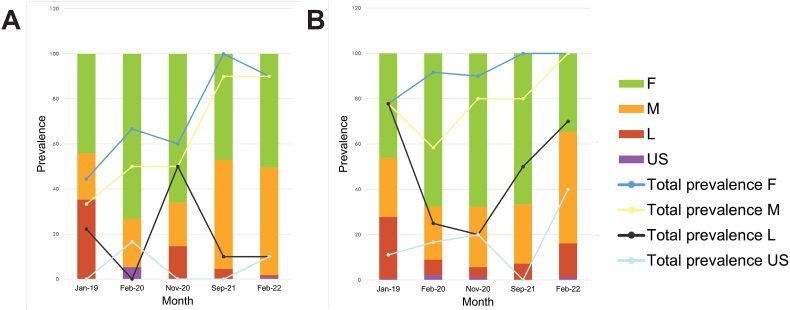
The total prevalence of P. (P.) pseudolaeviconchus was reported as 78.4% (CI = 67.1–89.7%) over all collection surveys, with this taxon recorded during all sampling surveys (supplementary file H). The highest prevalence was recorded in September 2021 (100%; CI = 100–100%). The highest mean intensity (12.3 ± 2.2) and mean abundance (11.1) recorded was in February 2022. Female P. (P.) pseudolaeviconchus had the highest prevalence in September 2021 (100%; CI = 100–100%), while males were the most prevalent in September 2021 and February 2022 (both at 90%; CI = 70.4–100%). The lowest prevalence was recorded in January 2019 (female 44.4% (CI = 12–76.9%); male 33.3% (CI = 2.5–64.1%)). For adults, the highest mean abundance was reported in February (2022) (female 5.6; male 5.3), with the highest mean intensity in February 2022 (female 6.2 ± 1.1; male 5.9 ± 1.4). Procamallanus (P.) pseudolaeviconchus larvae were most prevalent (50%; CI = 17.3–82.7%) in November 2020, with no larvae recorded in February 2020. However, the highest mean intensity (5 ± 0) of larvae was reported in September 2021 (Fig. 10A).
Fig. 10.
Bar graphs showing the ratio of life stages and sexes in each sample. A- Procamallanus (Procamallanus) pseudolaeviconchus Moravec and van As, 2015. B- Paracamallanus cyathopharynx (Baylis, 1923). Prevalence of each group in each month is given in the line graphs. F = female, M = male, L = larvae, US = unknown sex.
The total prevalence of P. cyathopharynx was reported as 94.1% (CI = 87.7–100%) over all collection surveys, with this taxon recorded during all sampling surveys (supplementary file I). The highest prevalence was recorded in September 2021 and February 2022 (both at 100%; CI = 100–100%). The highest mean intensity (61.2 ± 17.3) and mean abundance (61.2) was in February 2022. Adult females had the highest prevalence in September 2021 and February 2022 (both at 100%; CI = 100–100%), while males were most prevalent in February 2022 (100%; CI = 100–100%). Both mean intensity (female 21.2 ± 5.1; male 30.1 ± 8.3) and mean abundance (female 21.2; male 30.1) were highest in February 2022. Larvae were most prevalent (77.8%; CI = 50.6–100%) in January 2019 with the lowest prevalence (20%; CI = 0–46.1%) in November 2020. However, the highest mean intensity (13.1 ± 7.6) and mean abundance (9.2) of larvae was reported in February 2022 (Fig. 10B).
4. Discussion
4.1. Procamallanus (Procamallanus) pseudolaeviconchus
The morphometry of P. (P.) pseudolaeviconchus described in this study conforms to the description by Moravec and van As (2015a), the studies by Moravec (2019) and Svitin et al. (2019), as well as those reported as P. (P.) laeviconchus from clariid hosts (Moravec, 1975; Boomker, 1982; Barson and Avenant-Oldewage, 2006) with minor differences. Variation was noted at the caudal end of females, with either three or four digit-like processes observed on the tail tip. Barson and Avenant-Oldewage (2006) reported three digit-like processes. Similarly, Moravec and van As (2015a) reported adult females with three digit-like processes, one situated dorsally and the rest subventrally. The dorsal process appears larger and distinctly different to the subventral processes in the present material. However, Moravec (2019) described the larvae of P. (P.) pseudolaeviconchus and recorded four conical digital processes on third-stage larvae, whereas Barson and Avenant-Oldewage (2006) suggested that a specimen studied using SEM was likely a fourth-stage larva as it lacked digit-like processes. There is no mention of these structures in the females studied by Svitin et al. (2019). Therefore, the variation observed in the number of digit-like processes in the current study are most likely related to development.
Furthermore, cloacal papillae in males also varied, with Moravec (1975) and Boomker (1982) reporting eight precloacal papillae, whereas Barson and Avenant-Oldewage (2006), Moravec and van As, 2015a, Moravec and van As, 2015b and Moravec (2019) report nine to ten papillae. Also, Moravec and van As (2015a) and Moravec (2019) reported an additional pair of ventral sessile papillae. In comparison, the current study reports eight to nine precloacal papillae in males. It appears that this variation might be due to designation and counting of papillae, apart from the report by Moravec (1975). In that study, he listed three pairs adcloacal papillae and six pairs of postcloacal papillae, which is much more than other records for P. (P.) pseudolaeviconchus, but this variation is not mentioned in subsequent studies even by the same author (Moravec and van As, 2015a, Moravec and van As, 2015b; Moravec 2019).
The current study observed isolated buccal capsules for P. (P.) pseudolaeviconchus for the first time. This allowed for the confirmation of the crescent-shaped marginal elevations on the anterior margin, the distinctly smooth inner surface, and the narrow ring above the basal ring (Moravec and van As, 2015a; Svitin et al., 2019). The right and left spiculae were also isolated and observed using SEM for the first time. The right (large) spicule is more sclerotised than the left, however both are simple and similar in shape. Moravec and van As (2015a) and Svitin et al. (2019) refer to the distal tips as being ‘sharply’ pointed, however, the right spicule tip appears to be slightly rounded in the present study, not ending in a distinct apex. Additionally, the surface topography of the vulva was described using SEM for the first time. Although there was a SEM image of a Procamallanus vulva in the study of Mašová et al. (2011), the species was not identified and a first-stage larvae is being released from the vulva, obscuring the gross morphology of the vulval opening. Moravec and van As (2015a) mention the vulva having ‘elevated lips’ whereas Svitin et al. (2019) refers to a small opening situated posteriorly of a body wall projection. The current study combines these ideas as the elevated vulva forms a body wall projection with a lateral opening surrounded by lips.
4.2. Paracamallanus cyathopharynx
The morphometry of P. cyathopharynx conforms to the description by Baylis (1923), the redescription by Moravec and van As (2015b), as well as other studies (Moravec, 1974b, 2019; Svitin et al., 2019; Rindoria et al., 2020). However, in the description of P. cyathopharynx from C. anguillaris by Baylis (1923), the tridents are depicted as very small and barely extending past the anterior buccal capsule. Moravec and van As (2015b) recorded larger tridents, similar to the current and other studies, and suggest that the smaller tridents reported by Baylis (1923) were not sufficiently sclerotised. Additionally, Baylis (1923) reported ten to twelve longitudinal ridges in the female anterior buccal capsule, while Moravec (2019) reported nine to ten in females, and the current study as well as other records of this species reported nine in both males and females. This calls for a re-evaluation of the specimens studied by Baylis (1923) and females studied by Moravec (2019).
A pair of small anterior tridents was observed by Rindoria et al. (2020) who also isolated the buccal capsules of P. cyathopharynx. These small tridents were situated on either side of the oral opening, anterior to the large pair of tridents. Although the current study used the same method of soft tissue digestion to isolate the buccal capsules, these small tridents were not observed. This may either be due to the premature removal of the buccal capsule from the digestion buffer, or digestion was left too long, and the small tridents fell off or were digested.
Rindoria et al. (2020) described two lateral spines posterior to the buccal capsule in males, and a single and small deirid in females near the level of the nerve ring. Lateral spines are not recorded in previous reports of P. cyathopharynx and based on the SEM images provided by Rindoria et al. (2020), both of these structures (lateral spines and deirids) appear to be identical. These structures were also situated in the same region on males and females, therefore likely deirids instead. The present study identified deirids in both males and females conforming to Moravec and van As (2015b) and Moravec (2019). Baylis (1923) and Svitin et al. (2019) do not mention deirids, whereas Moravec (1974b) refers to these as “cervical papillae”.
Moravec (1974b) mentions two very small processes on the tail tip of males. Moravec and van As (2015b) and Moravec (2019) described an additional single, “sharply-pointed cuticular process” on the tail tip in larger male specimens. Boomker (1982) and Svitin et al. (2018) also studied P. cyathopharynx but did not mention these structures. Thereafter, Rindoria et al. (2020) reported four processes of which two are large and two very small. They acknowledge variation in their results in comparison to previous reports and suggested the possibility of intraspecific morphological variation amongst adult specimens which may be linked to sexual maturity or age. In the current study, two digit-like processes were observed. Furthermore, Moravec (1974b) mentioned that the small processes were only visible in the dorsoventral view and indistinct in young specimens. We concur with the observation regarding the orientation of the specimen.
Digit-like processes in females varied between three and four in adults and larvae, respectively. Moravec (1974b), Moravec and van As (2015b) and Svitin et al. (2019) reported three in adults, with Moravec and van As (2015b) reporting four to six digits in subgravid/ovigerous females. Following this, the specimens studied by Rindoria et al. (2020) with four (Kenyan specimens) and five (Tanzanian specimens) digits are more likely subgravid females. There is some confusion on this topic as Moravec (2019) recorded P. cyathopharynx third and fourth-stage larvae bearing three processes.
Furthermore, variation occurred in the size of the female specimens which were extremely variable throughout studies, the largest recorded by Svitin et al. (2019). Other measurements were also variable, some ranges broader than others, but these mostly overlapped. This variation is possibly due to different life cycle stages analyzed together accidently. In terms of males, the morphometry is more consistent, other than the left spicule and number of postcloacal papillae. In the current study, the left spicule was larger, possibly due to visualization, as it is inconspicuous when not isolated. In the present study, six postcloacal papillae were recorded, corresponding to Moravec and van As (2015b), Moravec (2019) and Svitin et al. (2019). However, Rindoria et al. (2020) recorded four postcloacal papillae.
The morphometry of the posterior part of the posterior buccal capsule was included for the first time, which could prove to be a useful morphometric variable to consider in future studies. Svitin et al. (2019) included morphometry for the oesophageal cup for P. cyathopharynx for the first time. However, they did not mention that the posterior buccal capsule is split into two distinct parts as was mentioned by Moravec (1974b, 2019) and Rindoria et al. (2020). This may reflect on confusion caused by inconclusive terminology used for these structures.
Both spiculae of P. cyathopharynx were isolated and observed using SEM for the first time. Moravec and van As (2015b) refer to the distal part of the right spicule as a separate part, which Rindoria et al. (2020) describes as harpoon-shaped. From the present material, the distal part is a continuation of the right spicule bearing a hook-like structure at the base. This structure was visible in both LM and SEM. It can be seen in the images by Rindoria et al. (2020), however, no mention or illustration of this structure has been provided previously.
Suspected morphotypes were found amongst the buccal capsules of adults. Morphological distinction in the buccal capsules has not been discussed previously for the species, unless comparing larval and adult stages. In Rindoria et al. (2020), both morphotypes observed in the present study are illustrated. Morphotype B is linked to a female in the figure legend, while the sex of the other is not mentioned. In the current study, adult males and females exhibited both morphotypes which were also present in both genetic lineages. In terms of the statistical analyses, there is a high degree of natural morphological variation between the specimens as seen by the random scattering of specimens in all PCA plots. Thus, the suspected morphotypes based on buccal capsule morphology are not supported by these statistical analyses or any other morphology. The most probable explanation of buccal capsule variation is intraspecific morphological variability amongst adults related to age or sexual maturity. Moravec (1974b, 2019) studied P. cyathopharynx larval stages, and did not mention or illustrate a posterior buccal capsule divided into two parts in the third or fourth-stage larvae. This could indicate that the posterior part of the posterior buccal capsule is a characteristic of adults. Following this, all specimens morphometrically analyzed in the current study are considered adult. However, it is possible that age plays a role in the buccal capsule shape. Alternatively, this could be an example of morphological plasticity, perhaps in response to the different regions of the distal intestine inhabited by the specimens.
4.3. Molecular findings
The 18S rDNA and CO1 mtDNA analyses support P. (P.) pseudolaeviconchus infecting C. gariepinus in this study, with negligible variation. This also supports that P. (P.) pseudolaeviconchus is present in sharptooth catfish in the Crocodile River system in South Africa and not P. (P.) laeviconchus. This is due to the high similarity of the present data to that of Svitin et al. (2019) for this taxon, which is the only data available for these gene regions. Additionally, all P. (P.) pseudolaeviconchus data for these gene regions form strongly monophyletic clades in all phylograms. In terms of P. (P.) laeviconchus, Svitin et al. (2019) stated that P. (P.) laeviconchus is distinct from P. (P.) pseudolaeviconchus based on 18S rDNA, with which the present study agrees. Interestingly, the 18S rDNA sequence P. laevionchus (DQ813446) published by Mwita and Nkwengulila (2010) appears distant to both P. (P.) pseudolaeviconchus by Svitin et al. (2019) and P. laeviconchus (JF803934) by Černotíková et al. (2011), but groups basal to P. (P.) pseudolaeviconchus, while P. (P.) laeviconchus (JF803934) is distantly located in the phylogram alongside Batrachocamallanus xenopodis (Baylis, 1929). This may be due to the specimens studied by Mwita and Nkwengulila (2010) being collected from a Clarias sp. However, the specimens in Mwita and Nkwengulila (2010) were collected from C. alluaudi in Tanzania and, due to the divergence from P. (P.) pseudolaeviconchus (1.18%), may represent a distinct species. Another unpublished sequence for P. (P.) laeviconchus (“Procamallanus laevionchus”) [KP274849] was removed from the alignment during trimming, but could be compared to the present data and that of P. (P.) laeviconchus (JF803934) by Černotíková et al. (2011). Sequence KP624894 was collected from C. gariepinus and thus likely represents P. (P.) pseudolaeviconchus. This is supported by the distance between KP274849 and sequence JF803934 for P. (P.) laeviconchus (2.43%; 17 bp). However, sequence KP274849 is also significantly distant to the present haplotype for P. (P.) pseudolaeviconchus (5.64%–5.91%; 35 bp to 36 bp) and does not overlap with other data for this taxon, indicating that this needs further attention.
Based on 18S rDNA data from the present study, all Paracamallanus samples infecting C. gariepinus were identical to available data for P. cyathopharynx. Similarly, there was high similarity between the CO1 mtDNA of the present material and that of available data for this taxon. However, two distinct lineages were detected within the CO1 mtDNA in the present study which was unexpected. A similar scenario was detected by Santacruz et al. (2020), who observed three lineages within CO1 mtDNA data for P. (S.) neocaballeroi, all of monophyletic origin, with two lineages highly supported. Even though the specimens in their study were from different hosts and different geographic regions, there were no associated morphological differences observed between any of the lineages. Similar to the current study, the morphological (SEM) and morphometric analyses by Santacruz et al. (2020) did not indicate any distinctions amongst the diagnostic characteristics of the species. The genetic distances between the P. (S.) neocaballeroi lineages were high and ranged from 5.12% to 6.91%. Similarly, the two P. cyathopharynx lineages in the present study were also very distant to each other and ranged from 10.09% to 11.58%. Santacruz et al. (2020) concluded that this was due to incipient genetic divergence without conspicuous trait differences and denoted P. (S.) neocaballeroi a cryptic species complex. Following this, LI1 in the current study is still considered P. cyathopharynx as all Paracamallanus specimens are from one host species, from the same sampling locality, the same site within the host intestine and a lack of distinguishing morphological characters. However, this variance may indicate a species complex or early stages of genetic divergence of P. cyathopharynx, but this can only be confirmed with more intricate morphological and molecular analyses.
The stark contrast between the variation of 18S rDNA and CO1 mtDNA data for P. cyathopharynx warrants further investigation. Due to the slower evolutionary rate of 18S rDNA, this gene region is generally more suitable for species diagnosis. This may indicate that the high variation in CO1 mtDNA might be due to other factors, which may include translocation of hosts, intermediate host availability, environmental conditions and even external pressures, like AMD, exerted on camallanid free-living stages. However, based on both gene regions used, all P. cyathopharynx data of specimens collected from C. gariepinus still form well-supported monophyletic clades, supporting conspecificity.
Within published data for 18S rDNA of Camallanidae, there appears to be some confusion. Several instances were detected of distinct species sharing identical haplotypes, for example, Camallanus lacustris (Zoega in Müller, 1776) [DQ442663] and Camallanus cotti Fujita, 1927 (EF180071); Spirocamallanus rarus Travassos, Artigas and Pereira, 1928 (DQ494195) and Procamallanus pacificus Moravec, Justine, Würtz and Sasal, 2006 (DQ442665). Furthermore, P. sigani (HM545908) is identical to Procamallanus annulatus Yamaguti, 1955 (JF803932), Procamallanus rebecae (Andrade-Salas, Pineda-López and Garcia-Magaña, 1994) [DQ442667] and Spirocamallanus philippinensis Machida and Taki, 1985 (JF934736). However, even though P. sigani (HM545908) is identical to these four sequences, they are not identical to each other indicating that the data for P. sigani likely spans a highly conserved region. All the above listed identical sequences were excluded from inter- and intraspecific range calculations. Conversely, some conspecific data appeared highly divergent likely indicating incorrect designations, for example, S. rarus sequences (DQ494195 and JF803912).
Similar to 18S rDNA data, discrepancies are also present in CO1 mtDNA. For example, the data for Procamallanus slomei Southwell and Kirshner, 1937 (MG948463) and B. xenopodis (MN523681) are identical, yet are designated to distinct taxa. Svitin et al. (2019) indicate a 3% distance between the two species using 28S rDNA, with no mention of their relationship or distances using CO1 mtDNA data. However, the CO1 mtDNA results in the current study indicated that they may represent the same species with 0% genetic distance and 0 bp differences. These species appear distinct based on 18S rDNA, thus it is possible that an error occurred when uploading the CO1 mtDNA data. Also, Spirocamallanus istiblenni Noble, 1966 sequences by Gaither et al. (2013), appear to represent two distinct taxa due to their genetic divergence and phylogenetic position. According to Gaither et al. (2013), some S. istiblenni specimens (KC517383–KC517402) were collected from Lutjanus kasmira (Forsskål, 1775) in French Polynesia and Hawaii whereas, others (KC517403–KC517405) were collected from Lutjanus fulvus (Forster, 1801) and Cephalopholis argus Schneider, 1801 in the Northern Line Islands. There appears to be some confusion whether one of these groups refers to the Spirocamallanus sp. mentioned by the authors or S. istiblenni. The divergence of S. istiblenni data is not discussed in subsequent studies (Ailán-Choke and Pereira, 2021). This further highlights the confusion within the molecular data available for the family.
4.4. Seasonal variation and habitat patterns
The present study reported a high P. (P.) pseudolaeviconchus total prevalence (78.4%; CI = 67.1–89.7%), higher than that reported by Boomker (1982) (33%; 14 of 43 infected). Similarly, other studies reported prevalence's as low as 8%–46% (Yakubu et al., 2002; Barson and Avenant-Oldewage, 2006; Moravec and van As, 2015a; Sorour and Hamouda, 2019). In terms of P. cyathopharynx, the current study reported a high total prevalence (94.1%; CI = 87.7–100%), similar to the report by Paperna (1964) (100%). These are higher in comparison to previous reports which ranged from 8% to 54% (Khalil, 1969; Boomker, 1982; Mwita and Nkwengulila, 2004; Barson et al., 2008; Moravec and van As, 2015b; Svitin et al., 2019; Sorour and Hamouda, 2019; Rindoria et al., 2020).
Regarding seasonality, the current study reported the highest prevalence and intensity of P. (P.) pseudolaeviconchus in September (spring) and February (mid-summer). Similarly, Boomker (1982) recorded the highest prevalence (33%) and intensity (8) for this species in March (summer) and Sorour and Hamouda (2019) in summer (70%). Barson and Avenant-Oldewage (2006) reported 13 specimens of P. (P.) pseudolaeviconchus in May (Autumn), lower in comparison to the current study. Other reports of this taxon reported pooled infection data or exclude intensity values. Paracamallanus cyathopharynx prevalence in the present study were highest in September (spring) and February (summer), similar to the observation by Boomker (1982) who reported the highest prevalence in February and November (summer), and September and October (spring). Interestingly, Mwita (2014) reported a high prevalence and intensity of P. cyathopharynx in the dry season (June to October) but the infection data was pooled. Most other records of P. cyathopharynx infection regarding sampling months were pooled (Khalil, 1969; Mwita and Nkwengulila, 2004; Barson et al., 2008; Moravec and van As, 2015b; Moravec and Jirků, 2017; Moravec and Scholz, 2017; Sorour and Hamouda, 2019; Svitin et al., 2019; Rindoria et al., 2020). Boomker (1982) discusses a distinct seasonal pattern for P. cyathopharynx with high infection numbers in mid-summer (February and November) and linked it to the increased occurrence of the copepod intermediate hosts.
The infection site of P. (P.) pseudolaeviconchus seems variable as Boomker (1982) reported specimens from the stomach, Svitin et al. (2019) from the intestine and Moravec and van As (2015a) and Moravec (2019) from the stomach and intestine. Barson and Avenant-Oldewage (2006) reported that specimens in their study occurred in the pyloric region of the stomach. In comparison, the present study recorded P. (P.) pseudolaeviconchus inhabiting the oesophagus, especially the oesophageal brush border, and the cardiac and fundic regions of the stomach, but rarely from the pyloric region. Importantly, no P. (P.) pseudolaeviconchus specimens were found attached to intestinal tissue. In terms of P. cyathopharynx, Sorour and Hamouda (2019) recorded specimens from the stomach and intestine, however all other reports (including the current study) recorded specimens from the intestine (mostly the distal part).
Declaration of competing interest
The authors confirm that there is no conflict of interest.
Acknowledgements
Funding: University of Johannesburg (UJ), URC and FRC funding to AAO; UJ GES and Oppenheimer memorial trust PDRF funding to QDMS; UJ GES and UJ faculty merit bursary to AP Nofal. This work was furthermore financially supported by the National Research Foundation of South Africa (South Africa/Austria joint scientific and technological cooperation; Reference number STGR180409318751, Unique Grant number 116067) to AAO. We acknowledge the use of the equipment at Spectrum central analytical facility.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2022.09.007.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Abiye T.A., Mengistu H., Masindi K., Demlie M. Surface water and groundwater interaction in the upper Crocodile River basin. S. Afr. J. Geol. 2015;118:109–118. [Google Scholar]
- Adeleke B., Robertson–Andersson D., Moodley G., Taylor S. Aquaculture in Africa: a comparative review of Egypt, Nigeria, and Uganda vis–à–vis South Africa. Rev. Fish. Sci. Aquac. 2020;29:167–197. [Google Scholar]
- Ailán-Choke L.G., Pereira F.B. Deep in the systematics of Camallanidae (Nematoda): using integrative taxonomy to better understand the phylogeny and consistency of diagnostic traits. Parasitology. 2021:1–13. doi: 10.1017/S0031182021000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinsanya B., Otubanjo O.A. Helminth parasites of Clarias gariepinus (Clariidae) in lekki lagoon, lagos, Nigeria. Rev. Biol. Trop. 2006;54:93–99. doi: 10.15517/rbt.v54i1.14003. [DOI] [PubMed] [Google Scholar]
- Atta K.P.T., Maree J.P., Onyango M.S., Mpenyana-Monyatsi L., Mujuru M. Chemical phosphate removal from Hartbeespoort Dam water, South Africa. WaterSA. 2020;46:610–614. [Google Scholar]
- Avenant-Oldewage A., Le Roux L.E., Mashego S.N., Van Vuuren B.J. Paradiplozoon ichthyoxanthon n. sp. (monogenea: diplozoidae) from Labeobarbus aeneus (cyprinidae) in the vaal river, South Africa. J. Helminthol. 2014;88:166–172. doi: 10.1017/S0022149X12000879. [DOI] [PubMed] [Google Scholar]
- Barson M., Avenant-Oldewage A. Nematode parasites of Clarias gariepinus (burchell, 1822) from the Rietvlei Dam, South Africa. Onderstepoort J. Vet. Res. 2006;73:87–94. doi: 10.4102/ojvr.v73i2.152. [DOI] [PubMed] [Google Scholar]
- Barson M., Bray R., Ollevier F., Huyse T. Taxonomy and faunistics of the helminth parasites of Clarias gariepinus (Burchell, 1822), and Oreochromis mossambicus (Peters, 1852) from temporary pans and pools in the Save-Runde River floodplain, Zimbabwe. Comp. Parasitol. 2008;75:228–240. [Google Scholar]
- Baylis H. Report on a collection of parasitic nematodes, mainly from Egypt. (Part III) Parasitology. 1923;15:24–38. [Google Scholar]
- Boomker J. Parasites of South African freshwater fish. I. Some nematodes of the catfish [Clarias gariepinus (Burchell, 1822)] from Hartbeespoort Dam. Onderstepoort J. Vet. Res. 1982;49:41–51. [PubMed] [Google Scholar]
- Boomker J. Parasites of South African freshwater fish. VI. Nematode parasites of some fish species in the Kruger National Park. Onderstepoort J. Vet. Res. 1994;61:35–43. [PubMed] [Google Scholar]
- Bouckaert R., Vaughan T.G., Barido-Sottani J., Duchêne S., Fourment M., Gavryushkina A., Joseph H., Jones G., Kühnert D., De Maio N., Matschiner M., Mendes F.K., Müller N.F., Ogilvie, Lo H.A., Drummond A.J. Beast 2.5: an advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019;15 doi: 10.1371/journal.pcbi.1006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush A.O., Lafferty K.D., Lotz J.M., Shostak A.W. Parasitology meets ecology on its own terms: margolis et al. revisited. J. Parasitol. 1997;83:575–583. [PubMed] [Google Scholar]
- Campana-Rouget Y. Nématodes de Poissons. Exploration hydrobiologique des lacs Kivu, Édouard et albert (1952–1954). Bruxelles. Résultats Sci. 1961;3:1–61. [Google Scholar]
- Campos A., Cummings M.P., Reyes J.L., Laclette J.P. Phylogenetic relationships of platyhelminthes based on 18S ribosomal gene sequences. Mol. Phylogenet. Evol. 1998;10:1–10. doi: 10.1006/mpev.1997.0483. [DOI] [PubMed] [Google Scholar]
- Černotíková E., Horák A., Moravec F. Phylogenetic relationships of some spirurine nematodes (Nematoda: chromadorea: Rhabditida: spirurina) parasitic in fishes inferred from SSU rRNA gene sequences. Folia Parasitol. 2011;58:135–148. [PubMed] [Google Scholar]
- Crafford D., Avenant-Oldewage A. Application of a fish health assessment index and associated parasite index to Clarias gariepinus (Teleostei: Clariidae) in the Vaal River system, South Africa. Afr. J. Aquat. Sci. 2009;34:261–272. [Google Scholar]
- Department of Water Affairs and Forestry, S.A . BKS (PTY) & ARCUS GIBB (PTY) LTD; 2008. The Development of a Reconciliation Strategy for the Crocodile (West) Water Supply System: Summary of Previous and Current Studies. (P WMA 03/000/00/3408) [Google Scholar]
- Dos Santos Q.M., Avenant-Oldewage A. Soft tissue digestion of Paradiplozoon vaalense for SEM of sclerites and simultaneous molecular analysis. J. Parasitol. 2015;101:94–97. doi: 10.1645/14-521.1. [DOI] [PubMed] [Google Scholar]
- Durand J. The impact of gold mining on the Witwatersrand on the rivers and karst system of Gauteng and North West Province, South Africa. J. Afr. Earth Sci. 2012;68:24–43. [Google Scholar]
- Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R., Rogers A., Lambshead P., Smith C. Nematode-specific PCR primers for the 18S small subunit rRNA gene. Mol. Ecol. 2005;5:611–612. [Google Scholar]
- Gaither M.R., Aeby G., Vignon M., Meguro Y.-i., Rigby M., Runyon C., Toonen R.J., Wood C.L., Bowen B.W. An invasive fish and the time-lagged spread of its parasite across the Hawaiian Archipelago. PLoS One. 2013;8:1–9. doi: 10.1371/journal.pone.0056940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M., Kishino H., Yano T. Dating the human-ape split by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985;22:160–174. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- Johnson M., Zaretskaya I., Raytselis Y., Merezhuk Y., McGinnis S., Madden T.L. NCBI BLAST: a better web interface. Nucleic Acids Res. 2008;36:5–9. doi: 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil L.F. Studies on the helminth parasites of freshwater fishes of the Sudan. J. Zool. London. 1969;158:143–170. [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K. Molecular evolutionary genetics analysis version 7.0. Mol. Biol. Evol. 2015;33 doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leketa K., Abiye T., Zondi S., Butler M. Assessing groundwater recharge in crystalline and karstic aquifers of the upper Crocodile River basin, Johannesburg, South Africa. Groundw. Sustain. Dev. 2019;8:31–40. [Google Scholar]
- Madanire-Moyo G., Barson M. Diversity of metazoan parasites of the African catfish Clarias gariepinus (Burchell, 1822) as indicators of pollution in a subtropical African river system. J. Helminthol. 2009;84:216–227. doi: 10.1017/S0022149X09990563. [DOI] [PubMed] [Google Scholar]
- Mašová Š., Baruš V., Moravec F. New morphological data on the first-stage larvae of two Procamallanus species (Nematoda: Camallanidae) based on SEM studies. Folia Parasitol. 2011;58:318–321. doi: 10.14411/fp.2011.032. [DOI] [PubMed] [Google Scholar]
- Møller O.S., Olesen J., Avenant-Oldewage A., Thomsen P.F., Glenner H. First maxillae suction discs in Branchiura (Crustacea): development and evolution in light of the first molecular phylogeny of Branchiura, Pentastomida, and other “Maxillopoda. Arthropod Struct. Dev. 2008;37:333–346. doi: 10.1016/j.asd.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Moravec F. On some nematodes from Egyptian fresh-water fishes. Acta Soc. Zool. Bohemoslov. 1974;38:32–51. [Google Scholar]
- Moravec F. The development of Paracamallanus cyathopharynx (Baylis, 1923) (nematoda: Camallanidae) Folia Parasitol. 1974;21:333–343. [PubMed] [Google Scholar]
- Moravec F. The development of Procamallanus laeviconchus (Wedl, 1862) (nematoda: Camallanidae). Věstík cesko slov. Společnosti Zool. 1975;39:23–28. [Google Scholar]
- Moravec F. Academia; Prague: 2019. Parasitic Nematodes of Freshwater Fishes of Africa. [Google Scholar]
- Moravec F., Jirků M. Some nematodes from freshwater fishes in central Africa. Folia Parasitol. 2017;64:1–39. doi: 10.14411/fp.2017.033. [DOI] [PubMed] [Google Scholar]
- Moravec F., Scholz T. Some nematodes, including two new species, from freshwater fishes in the Sudan and Ethiopia. Folia Parasitol. 2017;64 doi: 10.14411/fp.2017.010. [DOI] [PubMed] [Google Scholar]
- Moravec F., van As J.G. Nematodes from the squeaker fishes Synodontis nigromaculatus and S. vanderwaali from the Okavango River, Botswana, including three new species. Syst. Parasitol. 2004;59:169–187. doi: 10.1023/B:SYPA.0000048097.20483.e4. [DOI] [PubMed] [Google Scholar]
- Moravec F., van As L.L. Procamallanus (Procamallanus) spp. (nematoda: Camallanidae) in fishes of the okavango river, Botswana, including the description of P. (P.) pseudolaeviconchus n. sp. parasitic in Clarias spp. (Clariidae) from Botswana and Egypt. Syst. Parasitol. 2015;90:137–149. doi: 10.1007/s11230-014-9541-0. [DOI] [PubMed] [Google Scholar]
- Moravec F., van As L.L. Studies on some spirurids (nematoda: spirurida) from fishes of the okavango river, Botswana. Syst. Parasitol. 2015;91:119–138. doi: 10.1007/s11230-015-9565-0. [DOI] [PubMed] [Google Scholar]
- Mwita C.J. The community of parasites infecting Clarias gariepinus in the Tanzanian waters: a case of Lake Victoria. Open J. Ecol. 2014;4:873–882. [Google Scholar]
- Mwita C., Nkwengulila G. Parasites of Clarias gariepinus (burchell, 1822) (pisces: Clariidae) from the mwanza gulf, lake victoria. Tanzan. J. Sci. 2004;30:53–62. [Google Scholar]
- Mwita C.J., Nkwengulila G. Phylogenetic relationships of the metazoan parasites of the clariid fishes of Lake Victoria inferred from the partial 18S rDNA sequences. Tanzan. J. Sci. 2010;36:47–58. [Google Scholar]
- Nation J.L. A new method using hexamethyldisilazane for preparation of soft insect tissues for scanning electron microscopy. Stain Technol. 1983;58:347–351. doi: 10.3109/10520298309066811. [DOI] [PubMed] [Google Scholar]
- Nei M., Kumar S. Oxford University Press; New York: 2000. Molecular Evolution and Phylogenetics. [Google Scholar]
- Paperna I. The metazoan parasite fauna of Israel inland water fishes. Bamidgeh. 1964;16:13–20. [Google Scholar]
- Papuc T., Petrescu-Mag I.V., Gavriloaie C., Botha M., Kovacs E., Coroian C.O. Swimming in the mud – a short review of environmental parameter ranges tolerated by Clarias gariepinus. Extrem. Life, Biospeology Astrobiol. 2019;11:9–18. [Google Scholar]
- Rammah A., Hirschmann H. Morphological comparison and taxonomic utility of copulatory structures of selected nematode species. J. Nematol. 1987;19:314–31423. [PMC free article] [PubMed] [Google Scholar]
- Rindoria N.M., Dos Santos Q.M., Avenant-Oldewage A. Additional morphological features and molecular data of Paracamallanus cyathopharynx (Nematoda: Camallanidae) infecting Clarias gariepinus (Actinopterygii: Clariidae) in Kenya. Parasitology. 2020;106:157–166. [PubMed] [Google Scholar]
- Santacruz A., Ornelas-García C.P., Pérez-Ponce de León G. Incipient genetic divergence or cryptic speciation? Procamallanus (Nematoda) in freshwater fishes (Astyanax) Zool. Scripta. 2020;49:768–778. [Google Scholar]
- Satia B. An overview of the large marine ecosystem programs at work in Africa today. Environ. Dev. 2016;17 [Google Scholar]
- Scholz T., Vanhove M., Smit N., Jayasundera Z., Gelnar M. A guide to the parasites of African freshwater fishes. Abc Taxa. 2018;18:1–425. [Google Scholar]
- Shapi M., Jordaan M.A., Mbambo A.T., Davies, Theophilus Clavell Chirenje E., Mpumelelo D. Determination of potentially harmful element (PHE) distribution in water bodies in Krugersdorp, a mining city in the West Rand, Gauteng province, South Africa. Minerals. 2021;11:1133. [Google Scholar]
- Sorour S.S., Hamouda A.H. Prevalence of nematodes infestation in Clarias gariepinus from el-burullus lake and lake nasser, Egypt. Iraqi J. Vet. Sci. 2019;33:181–188. [Google Scholar]
- Svitin R., Schoeman A.L., du Preez L.H. New information on morphology and molecular data of camallanid nematodes parasitising Xenopus laevis (Anura: pipidae) in South Africa. Folia Parasitol. 2018;65 doi: 10.14411/fp.2018.003. [DOI] [PubMed] [Google Scholar]
- Svitin R., Truter M., Kudlai O., Smit N.J., Du Preez L. Novel information on the morphology, phylogeny and distribution of camallanid nematodes from marine and freshwater hosts in South Africa, including the description of Camallanus sodwanaensis n. sp. Int. J. Parasitol. Parasites Wildl. 2019;10:263–273. doi: 10.1016/j.ijppaw.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Braak C., Smilauer P. 2002. CANOCO Reference Manual and CanoDraw for Windows User's Guide: Software for Canonical Community Ordination.www.canoco.com Ithaca NY, USA. [Google Scholar]
- Windisch J., Gradwohl A., Gilbert B.M., Dos Santos Q.M., Wallner G., Avenant-Oldewage A., Jirsa F. Toxic elements in sediment and water of the Crocodile River (West) System, South Africa, following acid mine drainage. Appl. Sci. 2022;12:10531. doi: 10.3390/app122010531. [DOI] [Google Scholar]
- Yakubu D.P., Omoregie E., Wade J.W., Faringoro D.U. A comparative study of gut helminths of Tilapia zilli and Clarias gariepinus from river Uke, Plateau state, Nigeria. J. Aquat. Sci. 2002;17:137–139. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.