Highlights
-
•
Treatment options for pediatric SRSE beyond continuous IV infusions of anesthetics are limited; novel therapeutic options are warranted to decrease morbidity and mortality.
-
•
Ganaxolone has a unique mechanism acting on BOTH intrasynaptic AND extrasynaptic GABAA receptors promoting tonic inhibition of neuronal signaling.
-
•
Adjunctive ganaxolone appeared effective in terminating SRSE in two of our patients, permitting IV anesthetics to be weaned and ultimate discharge.
Abbreviations: SE, status epilepticus; SRSE, super-refractory status epilepticus; E-IND, emergency investigational new drug; GABAAR, GABAA receptors; GNX, ganaxolone; ASMs, anti-seizure medications; NORSE, new onset refractory status epilepticus; FIRES, fever-induced refractory epilepsy syndrome; NMDA, N-methyl-d-aspartate; AMPA, alpha-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid; KA, kainate
Keywords: Status epilepticus, Super-refractory status epilepticus, Pediatric, Neurosteroids
Abstract
Synaptic GABAA receptor (GABAAR) internalization contributes to the drug resistant nature of super-refractory status epilepticus (SRSE). Ganaxolone is a 3β-methylated synthetic analog of the endogenous neuroactive steroid, allopregnanolone, that has positive allosteric modulatory activity on synaptic and extrasynaptic GABAA receptors. Ganaxolone is currently in clinical trials to treat rare pediatric seizure disorders and established and refractory SE.
Two pediatric patients with SRSE (age 17 and age 7) were treated under emergency investigational new drug (E-IND) applications with intravenous (IV) ganaxolone administered as an initial bolus and a maintenance infusion for up to 4.5 days with intermittent IV boluses as-needed followed by taper on day 5 and transitioned to chronic treatment using ganaxolone suspension.
Adjunctive ganaxolone was effective in terminating SRSE in both patients, safely permitting IV anesthetics to be weaned. Seizure control has been maintained after transitioning to enteric ganaxolone. Further investigation of ganaxolone as a safe and effective treatment for SRSE is warranted.
1. Introduction
Super-refractory status epilepticus (SRSE) is a life-threatening neurological emergency and carries a risk for major morbidity and mortality [1], [2], [3]. Because of the lack of high-quality comparative clinical studies of SRSE treatment strategies, therapeutic decisions mainly rely on case series or expert opinion [1], [4]. In addition to seizure control, the treatment objectives in SRSE include minimization of neuronal injury as well as other organ injury often observed during prolonged treatments with intravenous (IV) anesthesia [5]. Prolonged seizures as well as prolonged GABA stimulation are known to cause internalization of synaptic GABAA receptors (GABAAR). This may play a role in the development of pharmaco-resistance [5], [6]. By contrast, extrasynaptic GABAAR have been found to be preserved in SE and identified as a potential target for therapeutic intervention for drug resistant SE [6].
The investigational drug ganaxolone (GNX) is a neuroactive steroid and a positive allosteric modulator of GABAAR that targets a unique binding site distinct from benzodiazepines or barbiturates [7], [8], [9], [10]. Ganaxolone is a synthetic derivative of an endogenous neuroactive steroid, allopregnanolone (a metabolite of progesterone), and both exert very similar pharmacological properties [8]. GNX acts on both synaptic and extrasynaptic GABAAR to maximize inhibitory signaling as well as maintaining activity when synaptic receptors are downregulated. Intravenous GNX is currently in clinical trials to treat established and refractory SE.
Here we summarize a single institutional experience in treating two pediatric patients with SRSE with IV ganaxolone under emergency investigational new drug (E-IND) applications.
2. Case studies
2.1. Patient #1
A 17-year-old female with a remote history of sporadic seizures in early childhood was transferred to our pediatric hospital for inpatient rehabilitation after 7-month hospitalization for recurrent refractory SE (as defined by ILAE criteria) [11] at an outside facility. Over the course of 7 months at the initial tertiary hospital, she had 6 separate episodes of SE and required intubation 4 times with medically induced coma for seizure suppression. She was discharged to our inpatient rehabilitation unit on 5 maintenance antiseizure medications (ASMs): cannabidiol, perampanel, phenobarbital, lacosamide, and lorazepam; along with pyridoxine, a ketogenic diet, anakinra, and menstrual suppression. A thorough infectious, metabolic, genetic, vascular, and autoimmune evaluation was nondiagnostic (Table 1a).
Table 1a.
Diagnostic studies and evaluations for Patient #1.
| Infectious | Autoimmune/inflammatory | Metabolic |
|---|---|---|
| B cell panel: negative HLH gene panel: negative HIV and hepatitis: negative COVID Ag testing ×3: negative |
CSF 4/2/20: 1 cell, 57 protein, 71 glucose Autoimmune encephalopathy panel: negative Pelvic US: negative for teratoma Oligoclonal band panel: negative NMO: negative |
Serum carbohydrate deficient transferrin: normal and inconsistent with a CDG Serum biotinidase level, 13.8 U/L (within normal limits) Homocysteine: low @ 3.5 Urine purine and pyrimidine panel: normal Urine sulfocysteine: normal Urine mucopolysaccharides screen: with elevated heparan sulfate, 0.33 Serum carbohydrate deficient transferrin: inconsistent with CDG Acylcarnitine profile: normal Free and total L-carnitine: plasma level normal Urine organic acids: normal Plasma amino acids: normal CSF metabolic studies: negative |
| Imaging | Genetic |
|---|---|
| Normal unenhanced and enhanced MRI of the brain (×5) Negative MRA of the head |
Epilepsy panela with 5 VUS: PCDH19, POLG, DOCK7, NRXN1, and PLCB1 c.39-5T>G DOCK7 gene: ✓ father; X mother c.2299A>G p.Met767Val NRXN1 gene: ✓ mother; X father c.199A>C p.Ser67Arg PLCB1 gene: ✓ father; X mother c.3131T>C p.Val1044Ala POLG gene: ✓ father; X mother All are responsible for autosomal recessive disorders, and a second change was not found c.545G>C pGly182Ala PCDH19 gene: ✓ mother; X father Mother asymptomatic; uncertain significance Whole exome sequencing with mitochondrial genome (TRIO): negative |
Ag, antigen; CDG, congenital disorders of glycosylation; COVID, coronavirus disease; CSF, cerebrospinal fluid; HLH, hemophagocytic lymphohistiocytosis; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; NMO, neuromyelitis optica; US, ultrasound.
GeneDx, Inc., Gaithersburg, MD.
While in the inpatient rehabilitation unit, the patient developed a fever of 41.7 °C, and a respiratory viral panel was positive for parainfluenza. Recurrence of SE required transfer to the intensive care unit for midazolam and pentobarbital infusions. In addition to the prior antiseizure medications and therapies, enteric felbamate was introduced at this time. Video-electroencephalogram (vEEG) monitoring throughout the course of status epilepticus demonstrated multifocal seizure onset. She was seizure-free for 2 days after infusions were weaned, until seizures returned, prompting placement of a vagus nerve stimulator and a prolonged 2-week course of IV pentobarbital titrated to EEG burst suppression. Repeat magnetic resonance imaging demonstrated progression of diffuse global parenchymal atrophy.
After two failed attempts to wean pentobarbital while remaining in ketosis and on the same antiseizure medication regimen, she was treated with IV-to-enteric GNX approved under E-IND. On a third attempt to wean pentobarbital seizures returned, and GNX was administered via protocol using an IV bolus, followed by infusion over 4 days (with GNX boluses as needed for breakthrough seizures) followed by a taper on day 5 (Fig. 1a). On day 1, pentobarbital was discontinued; by day 3, clinical and electrographic seizures stopped (Fig. 2). The patient tolerated the protocol well. As pentobarbital was discontinued and GNX treatment initiated, she became more alert and interactive. On day 5 she was transitioned from IV to enteric GNX (maximal adult dose of 1800 mg/day divided three times a day) without recurrent seizures. During the introduction of ganaxolone she was maintained on the same antiseizure regimen and ketogenic diet though was weaned off phenobarbital.
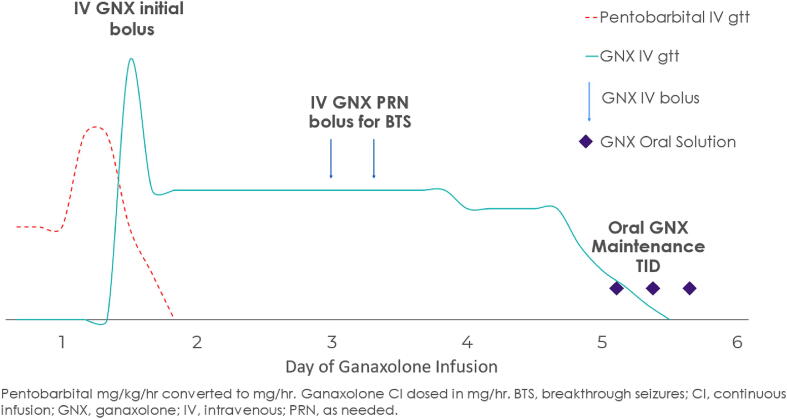
Fig. 1a.
Graphical representation of IV to enteric ganaxolone for Patient #1. For our first patient, GNX was administered via protocol using an IV bolus, followed by infusion over 4 days (with GNX boluses as needed for breakthrough seizures) followed by a taper on day 5 and transition to enteric maintenance GNX. On day 1, pentobarbital was discontinued; by day 3, clinical and electrographic seizures stopped.
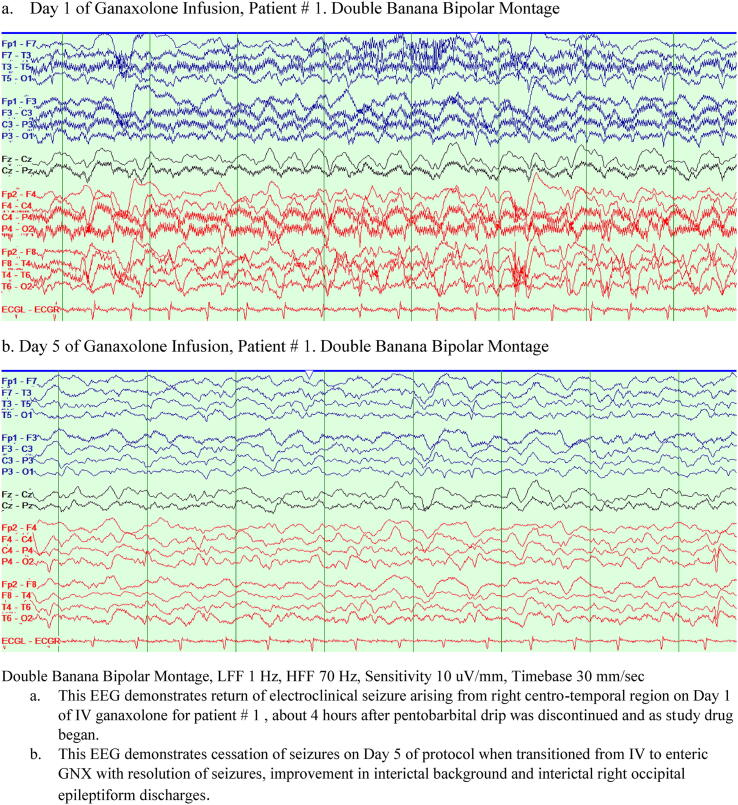
Fig. 2.
EEG during ganaxolone infusion for Patient # 1. Day 1 of Ganaxolone Infusion, Patient # 1. Anterior-posterior longitudinal bipolar montage (a.k.a. “Double Banana”) b. Day 5 of Ganaxolone Infusion, Patient # 1. Double Banana montage.
2.2. Patient #1 disposition and update
One month after GNX initiation, the patient was transferred to inpatient rehabilitation service and was discharged home one month later. While in the hospital she was weaned off the ketogenic diet and anakinra, and as an outpatient she has been weaned off felbamate with minimal breakthrough clinical seizures (primarily in the setting of intercurrent illness or decreasing ASMs). More than eighteen months later, the patient’s seizure control has been maintained as she continues to be on four ASMs (perampanel, brivaracetam, lorazepam and valproic acid-the latter two at lower doses) and GNX. To note, the patient remains with significant morbidity, with gastrostomy tube and tracheostomy dependence, requiring assistance with all activities of daily living.
2.3. Patient #2
A 7-year-old, previously healthy female presented with abdominal pain, encephalopathy and fever and developed SRSE concordant with febrile infection-related epilepsy syndrome (FIRES). The patient was admitted to PICU and placed on vEEG which demonstrated multifocal independent electrographic seizures arising from posterior head regions. Upon admission, MRI demonstrated FLAIR changes in bilateral temporal lobes consistent with prolonged seizures. A thorough infectious, metabolic, genetic, vascular, and autoimmune evaluation was nondiagnostic (Table 1b). SE (as defined by ILAE criteria) [11] persisted despite multiple ASMs (levetiracetam, valproic acid, perampanel, lacosamide, zonisamide), acetazolamide, attempts to initiate ketogenic diet, IV anesthetics including midazolam and pentobarbital, and subsequent ketamine infusions. Immunomodulatory therapies with IV methylprednisolone and IVIG were initiated concomitantly in the ICU. After two failed attempts to wean IV anesthetics, she was treated with IV-to-enteric GNX approved under E-IND.
Table 1b.
Diagnostic studies and evaluations for Patient # 2.
| Infectious | Autoimmune/inflammatory | Metabolic |
|---|---|---|
| CSF and Blood cultures: negative HSV PCR CSF: negative CSF and Serum Arbovirus CSF EBV, VZV PCR: negative CSF RPR and VDRL (CSF) negative Bartonella serology and PCR: Negative Mycoplasma: Negative COVID Ag and Ab testing negative |
CSF 12/30/20: 4 WBC, 49 protein, 71 glucose Autoimmune encephalopathy panel: negative (serum and CSF) Pelvic US: negative for teratoma CSF oligoclonal bands: negative NMO antibodies: negative ANA, dsDNA, ACE Lysozyme CSF cytokines showed no elevation and serum cytokines showed elevation of IL-6. |
Vit. B12, TSH/FT4, folate, copper, ceruloplasmin: Normal Acylcarnitine profile: normal Urine organic acids: normal Plasma amino acids: normal Heavy metals: normal Porphyria: negative Very long chain fatty acids: normal |
| Imaging | Genetic |
|---|---|
| MRI Brain: Abnormal (*) Negative MRA/MRV of the head and neck |
Epilepsy panel: negative Whole exome sequencing (TRIO): negative |
MRI brain demonstrated FLAIR changes in bilateral temporal lobes consistent with prolonged seizures.
As anesthesia was weaned on a third attempt and seizures returned while on the same antiseizure medication regimen, GNX was initiated on hospital day 17 using an IV bolus, followed by infusion over 4.5 days (with boluses as needed for breakthrough seizures followed by a taper and transition to enteric maintenance on day 5 (Fig. 1b). Midazolam was discontinued on day 2 of IV GNX protocol; ketamine was discontinued by day 8 with the assistance of phenobarbital loads and maintenance for breakthrough seizures. Anakinra was initiated on day 2 of IV GNX protocol, hospital day 19. Seizure cessation was gradual and achieved by day 8 (Fig. 3).
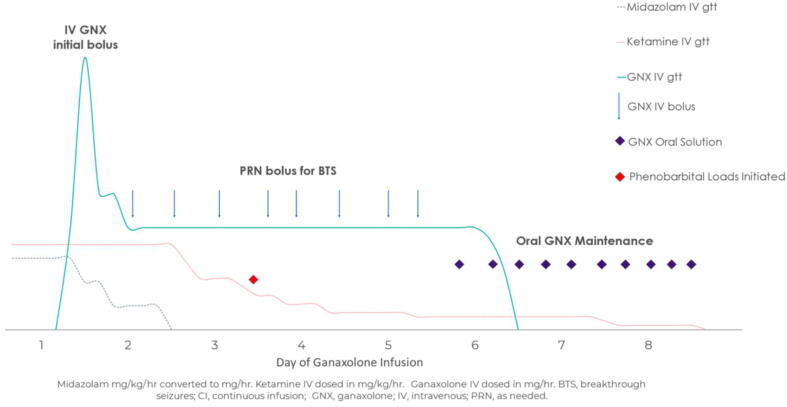
Fig. 1b.
Graphical representation of IV to enteric ganaxolone for Patient #2. For our second patient GNX was initiated while she remained on two continuous IV infusions: midazolam and ketamine. GNX was initiated on hospital day 17 using an IV bolus, followed by infusion over 4.5 days (with boluses as needed for breakthrough seizures followed by a taper and transition to enteric maintenance on day 5. Midazolam was discontinued by day 2 of IV GNX protocol; ketamine was discontinued by day 8 with the assistance of phenobarbital loads and maintenance for breakthrough seizures.
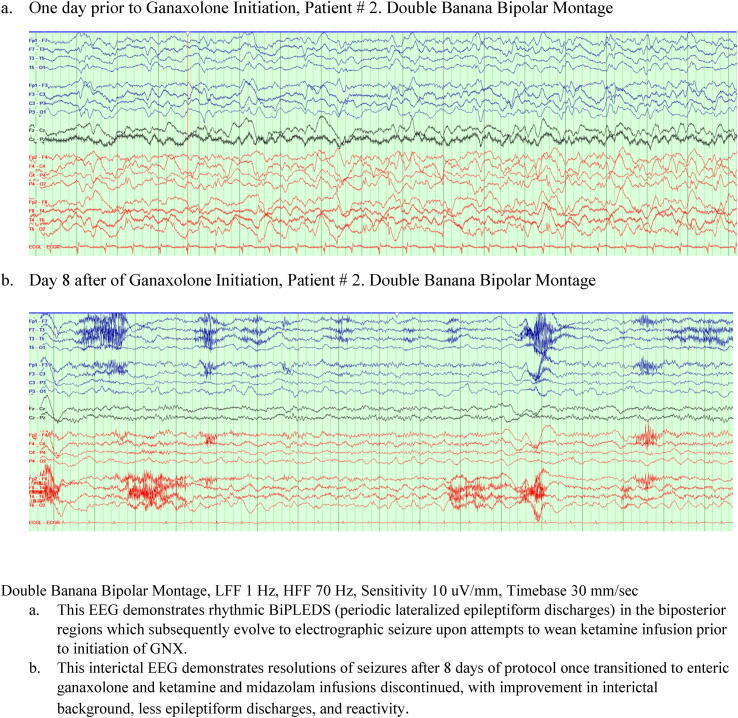
Fig. 3.
EEG prior to and after ganaxolone initiation for Patient # 2. One day prior to Ganaxolone Initiation, Patient # 2. Anterior-posterior longitudinal bipolar montage (a.k.a. “Double Banana”) Day 8 after Ganaxolone initiation, Patient # 2. Double Banana Bipolar Montage.
By 4 weeks after admission, the patient was more responsive; at 6 weeks, she was transferred from ICU to the floor, as she became increasingly alert, conversant, and ambulatory. Her acute hospitalization was further complicated by bruxism, movement disorder, vagus nerve stimulator infection, neuropathic pain, and sporadic breakthrough clinical seizures.
2.4. Patient #2 disposition and update
At discharge, the patient remained on several ASMs (levetiracetam, lacosamide, perampanel, phenobarbital, lorazepam) ganaxolone (63 mg/kg/day divided three times a day), anakinra, and tetrabenazine. Note, tetrabenazine was introduced for her transient hyperkinetic movement disorder which became more apparent as she became more active and awake. MRI throughout the inpatient course demonstrated FLAIR changes in the basal ganglia explaining these symptoms. By 8 months from discharge, she was weaned off perampanel and tetrabenazine as the movement disorder resolved. More than one year after initial presentation, she continues on levetiracetam, lacosamide, phenobarbital, lorazepam, anakinra (the latter both at lower doses) and ganaxolone in addition to iron and pregabalin with rare breakthrough seizures in the setting of intercurrent illness or attempts at medication weans. Cognition remains age-appropriate without any physical limitations. She also attends school with modifications.
3. Discussion
3.1. Pathophysiology
The International League Against Epilepsy (ILAE) has defined SE as a condition of prolonged seizure activity resulting from either the failure of mechanisms responsible for seizure termination (mainly through GABAA receptors) or the initiation of mechanisms which lead to enhanced excitation (mainly through NMDA/AMPA/KA receptors) [11]. Refractory SE is defined as SE resistant to treatment with first-line ASM (benzodiazepines) and one second-line IV ASM such as fosphenytoin/phenytoin, valproic acid or levetiracetam [12], [13]. SRSE is defined as SE that fails to respond or recurs after a trial of a third-line agent (IV anesthesia). Overall, one-third of patients with SRSE die and another one-third recover with chronic neurologic deficits [14], [15].
3.2. Neurosteroids: targeting extrasynaptic GABAA receptors
Neurosteroids increase tonic inhibition via their allosteric modulation of both synaptic and extrasynaptic GABAAR [16]. While synaptic GABAAR (a target for benzodiazepines and barbiturates) are progressively internalized with prolonged seizures leading to pharmacoresistance [6], the function of extrasynaptic GABAAR is preserved [16].
A recent randomized controlled trial in adults and children failed to demonstrate the efficacy of IV allopregnanolone (brexanolone) compared to placebo in the resolution of SRSE, when it was added to the standard care [17]. However, novel dosing regimen with ganaxolone described here offers a new opportunity to these highly refractory and medically complex patients. Ganaxolone is a proprietary IV solution solubilized by Captisol® (betadex suflobutyl ether sodium). The dosing targets concentrations of ganaxolone that are expected to demonstrate anticonvulsant properties [18]. In preclinical models, GNX has exhibited broad-spectrum antiseizure properties, including when benzodiazepine resistance developed [8], [10]. In an open-label, Phase 2 study with IV ganaxolone in refractory SE, ganaxolone achieved rapid and durable seizure control in patients with RSE, and showed acceptable safety and tolerability [9]. This study provided the rationale for specific IV dosing paradigm for SRSE [19], [20] as well as for further investigation of ganaxolone in an ongoing, phase 3 trial in refractory SE (NCT04391569). Specifically, infusion parameters were optimized to allow for rapid loading of ganaxolone (plasma concentration ∼900 ng/ml) designed to abort SE (median time to SE cessation following ganaxolone initiation was 5 min), followed by maintenance doses aimed at sustaining seizure control (no patient required escalation to third-line IV anesthetics during the 24-h period following ganaxolone initiation) (for details see [19]). Of note, target serum ganaxolone levels achieved by the current IV dosing paradigm were 5–10 times higher as compared to serum brexanolone levels in the STATUS trial [9], [21]. Ganaxolone’s properties and mechanism of action along with the availability of both IV and oral formulations allows reaching adult and pediatric patients in acute and chronic care settings [22].
3.3. Patient-specific discussion
Although both patients in our series developed SRSE, their prior histories and courses were quite different.
Our first patient had a history of isolated early childhood seizures but was off daily antiseizure medication for more than one decade when she initially developed SRSE as a teenager. Although her course was similar to those who develop NORSE (New-onset refractory status epilepticus), she did not meet the traditional criterion or definition of de novo onset of seizures. In contrary our second patient had no history of seizures, and her presentation was most consistent with FIRES(a subset of NORSE) with the clinical presentation characterized by de novo onset of RSE or SRSE without a clearly identifiable acute or active structural, toxic, or metabolic cause [23], [24]. Regardless of cause or etiology, mortality estimates in pediatric RSE range from 14 to 44 % [3].
In SRSE, traditional antiseizure management has poor success rates, especially during the acute phase [5], [23], [24]. The majority of patients require anesthetics during the acute phase and prolonged burst-suppression coma is often necessary. At least one-third of patients require multiple anesthetics drugs to achieve seizure control. The challenge is that seizures may recur upon attempts to wean off the continuous infusions. Exact pathophysiology remains unclear, though emerging evidence suggests a role for a postinfectious or inflammatory cytokine-mediated mechanism [25]. Though treatment protocols vary among institutions, most often utilize other available nonpharmacologic therapies: immunomodulatory therapy, ketogenic diet, therapeutic hypothermia, electroconvulsive therapy, vagus nerve stimulator, each of which has varying efficacy, though without substantial change in the overall morbidity or mortality [5], [25]. The treatments for SRSE are guided by preclinical studies and retrospective case reviews as there are no published randomized control studies to guide clinicians.
Over the recent years both the ketogenic diet and novel immunomodulatory therapies are often utilized in unknown or presumed autoimmune causes of SRSE [5]. The evidence to use these agents to treat SRSE is based on preclinical and observational studies. Modulation of proinflammatory cytokines with anakinra (IL-1 receptor antagonist) or tocilizumab (IL-6 receptor antagonist) is being increasingly utilized in treatment of RSE, SRSE and FIRES [5], [26], [27]. Ketogenic diet has also gained significant attention in recent years for the treatment of SE as an adjunctive therapy due to its anti-inflammatory and anti- seizure properties and has been effective in FIRES [28], [29]. To note, our first patient relapsed back into SRSE while on therapeutic doses of anakinra and the ketogenic diet. Our second patient was initiated on the ketogenic diet briefly but did not ever achieve ketosis beyond trace ketones in the acute ICU stay and thus was weaned off at parents’ request once seizures became under control.
Although the exact cause of SE in each of our patients remains unknown despite comprehensive diagnostic evaluations ruling out acute and remote symptomatic causes, such is often the case in pediatric SRSE. Time to achieve seizure cessation for each patient varied again reflecting the heterogenous nature and course of patients with SRSE: Patient 1 was on a single continuous IV infusion and achieved seizure control after 5 days of Ganaxolone initiation. However, Patient 2 was on two continuous IV infusions and took longer to achieve seizure control as both infusions had to be weaned (8 days). Each patient had an extremely complicated course with multiple concurrent medication changes that could be confounding. Despite that, ganaxolone’s mechanism of action and preclinical data was suggestive of its potential role in treatment of SRSE in our patients.
4. Conclusions
Despite recent advances in novel therapies for the treatment of pediatric SRSE beyond IV anesthetics, morbidity and mortality especially in the acute stages remains high. The patient's experience in this series reflects the heterogenous nature of various treatment approaches for SRSE which often employs a trial-and-error manner until treatment response is achieved [5]. Ganaxolone appeared to contribute to termination of SRSE in two pediatric patients at our institution, permitting IV anesthetics to be weaned and ultimately discharge from the hospital. Long term seizure control has been maintained in both patients after transitioning to adjunctive oral ganaxolone excepting rare breakthrough seizures.
Disclosure of funding
Marinus Pharmaceuticals, Inc. provided IV and oral ganaxolone. No monetary support was provided.
Ethical statement
Each patients E-IND trial was approved by the FDA and was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Atrium Health System.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Rani K. Singh – Reports research funding from the Pediatric Epilepsy Research Foundation, has served on the advisory board for Zogenix, Prasco, and AKPharma, and is a member of the executive board of the Western Region of the Epilepsy foundation of North Carolina. Joseph Hulihan, Heather van Heusen, Henrikas Vaitkevicius, Maciej Gasior are all employees of Marinus Pharmaceuticals, Inc.
Acknowledgements
Informed Consent Statement: Patient consent was obtained as part of the clinical trial.
References
- 1.Shorvon S., Ferlisi M. The treatment of super-refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain. 2011;134:2802–2818. doi: 10.1093/brain/awr215. [DOI] [PubMed] [Google Scholar]
- 2.Sahin M., Menache C.C., Holmes G.L., Riviello J.J. Outcome of severe refractory status epilepticus in children. Epilepsia. 2001;42:1461–1467. doi: 10.1046/j.1528-1157.2001.21301.x. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert D.L., Gartside P.S., Glauser T.A. Efficacy and mortality in treatment of refractory generalized convulsive status epilepticus in children: a meta-analysis. J Child Neurol. 1999;14:602–609. doi: 10.1177/088307389901400909. [DOI] [PubMed] [Google Scholar]
- 4.Kravljanac R., Djuric M., Jankovic B., Pekmezovic T. Etiology, clinical course, and response to treatment of status epilepticus in children: A 16-year single center experience based on 6–2 episodes of status epilepticus. Eur J Paediatr Neurol. 2015;19:584–590. doi: 10.1016/j.ejpn.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Vasquez A., Moeller R.F., Tatum W. Pediatric refractory and super-refractory status epilepticus. Seizure. 2019;68:62–71. doi: 10.1016/j.seizure.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Goodkin H.P., Joshi S., Mtchedlishvili Z., Brar J., Kapur J. Subunit-specific trafficking of GABA(A) receptors during status epilepticus. J Neurosci. 2008;28:2527–2538. doi: 10.1523/JNEUROSCI.3426-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saporito M.S., Gruner J.A., DiCamillo A., Hinchliffe R., Barker-Haliski M., White H.S. Intravenously administered ganaxolone blocks diazepam-resisitant lithium- pilocarpine-induced status epilepticus in rats: comparison with allopregnanolone. J Pharmacol Exp Ther. 2019;368:326–337. doi: 10.1124/jpet.118.252155. [DOI] [PubMed] [Google Scholar]
- 8.Nohria V., Giller E. Ganaxolone. Neurotherapeutics. 2007;4:102–105. doi: 10.1016/j.nurt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaitkevicius H, Ramsay RE, Swisher CB, Husain AM, Aimetti A, Gasior M. Intravenous ganaxolone for the treatment of refractory status epilepticus: Results from an open-label, dose-finding, phase 2 trial. Epilepsia. [published online ahead of print, 2022 Jun 24]. [DOI] [PMC free article] [PubMed]
- 10.Bialer M., Johannessen S.I., Koepp M.J., Levy R.H., Perucca E., Perucca P., et al. Progress report on new antiepileptic drugs: A summary of the Fifteenth Eilat Conference on New Antiepileptic Drugs and Devices (EILAT XV). II. Drugs in more advanced clinical development. Epilepsia. 2020;61:2365–2385. doi: 10.1111/epi.16726. [DOI] [PubMed] [Google Scholar]
- 11.Trinka E., Cock H., Hesdorffer D., Rossetti A.O., Scheffer I.E., Shinnar S., et al. A definition and classification of status epilepticus-Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56:1515–1523. doi: 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- 12.Mayer S., Claassen J., Lokin J., Mendelsohn F., Dennis L., Fitzsimmons B.F. Refractory status epilepticus: frequency, risk factors, and impact on outcome. Arch Neurol. 2002;59:205–210. doi: 10.1001/archneur.59.2.205. [DOI] [PubMed] [Google Scholar]
- 13.Rossetti A., Logroscino G., Bromfield E. Refractory status epilepticus: effect of treatment aggressiveness on prognosis. Arch Neurol. 2005;62:1698–1702. doi: 10.1001/archneur.62.11.1698. [DOI] [PubMed] [Google Scholar]
- 14.Neligan A., Shorvon S. Frequency and prognosis of convulsive status epilepticus of different causes: a systematic review. Arch Neurol. 2010;67:931–940. doi: 10.1001/archneurol.2010.169. [DOI] [PubMed] [Google Scholar]
- 15.Sutter R., Kaplan P.W., Rüegg S. Outcome predictors for status epilepticus-what really counts. Nature Rev Neurol. 2013;9:525–534. doi: 10.1038/nrneurol.2013.154. [DOI] [PubMed] [Google Scholar]
- 16.Naylor D.E., Liu H., Wasterlain C.G. Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci. 2005;25:7724–7733. doi: 10.1523/JNEUROSCI.4944-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.A study with SAGE-547 for super-refractory status epilepticus [Internet].2019 May [cited 2021 Nov 1]. https://www.clinicaltrials.gov/ct2/show/results/NCT02477618.
- 18.Ganaxolone Investigator’s Brochure. (January 2020). Radnor, PA: Marinus Pharmaceuticals, Inc.
- 19.Merical B., Curran C., Fehnel C. The use of high dose ganaxolone as a late adjunctive therapy in super refractory status epilepticus (4203) Neurology. 2021;96(15 Suppl):4203. [Google Scholar]
- 20.Chez M, Hulihan J, Gasior M. Treatment of super refractory status epilepticus using intravenous ganaxolone in a patient with Lennox-Gastaut syndrome and Angelman syndrome. American Epilepsy Society Annual Meeting, Dec 04-08, 2020.
- 21.Rosenthal E.S., Claassen J., Wainwright M.S., Husain A.M., Vaitkevicius H., Raines S., et al. Brexanolone as adjunctive therapy in super-refractory status epilepticus. Annal Neurol. 2017;82:342–352. doi: 10.1002/ana.25008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lattanzi S., Riva A., Striano P. Ganaxolone treatment for epilepsy patients: from pharmacology to place in therapy. Expert Rev Neurother. 2021;21:1317–1332. doi: 10.1080/14737175.2021.1904895. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch L.J., Gaspard N., van Baalen A., Nabbout R., Demeret S., Loddenkemper T., et al. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia. 2018;59:739–744. doi: 10.1111/epi.14016. [DOI] [PubMed] [Google Scholar]
- 24.Gaspard N., Hirsch L.J., Sculier C., Loddenkemper T., van Baalen A., Lancrenon J., et al. New-onset refractory status epilepticus (NORSE) and febrile infection–related epilepsy syndrome (FIRES): state of the art and perspectives. Epilepsia. 2018;59:745–752. doi: 10.1111/epi.14022. [DOI] [PubMed] [Google Scholar]
- 25.Specchio N., Pietrafusa N. New-onset refractory status epilepticus and febrile infection-related epilepsy syndrome. Dev Med Child Neurol. 2020;62:897–905. doi: 10.1111/dmcn.14553. [DOI] [PubMed] [Google Scholar]
- 26.Dube C., Vezzani A., Behrens M., Bartfai T., Baram T.Z. Interleukin-1beta contributes to the generation of experimental febrile seizures. Ann Neurol. 2005;57:152–155. doi: 10.1002/ana.20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai Y.-C., Muscal E., Wells E., Shukla N., Eschbach K., Hyeong Lee K.i., et al. Anakinra usage in febrile infection related epilepsy syndrome: an international cohort. Ann Clin Transl Neurol. 2020;7:2467–2474. doi: 10.1002/acn3.51229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kossoff E.H., Nabbout R. Use of dietary therapy for status epilepticus. J Child Neurol. 2013;28:1049–1051. doi: 10.1177/0883073813487601. [DOI] [PubMed] [Google Scholar]
- 29.Nabbout R., Mazzuca M., Hubert P., Peudennier S., Allaire C., Flurin V., et al. Efficacy of ketogenic diet in severe refractory status epilepticus initiating fever induced refractory epileptic encephalopathy in school age children (FIRES) Epilepsia. 2010;51:2033–2037. doi: 10.1111/j.1528-1167.2010.02703.x. [DOI] [PubMed] [Google Scholar]