Abstract
Background
Prostate-specific membrane antigen (PSMA) PET is standard for newly diagnosed high-risk and biochemically recurrent (BCR) prostate cancer. Although studies suggest high specificity of 2-(3-{1-carboxy-5-[(6-[(18)F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (DCFPyL) for targeting PSMA, false-positive findings have been identified and most studies lack histologic confirmation of malignancy.
Purpose
To estimate the positive predictive value (PPV) of DCFPyL PET/CT by providing histopathologic proof for DCFPyL-avid lesions suspected of being distant metastases at initial diagnosis and recurrence in BCR prostate cancer.
Materials and Methods
In this prospective trial, men with newly diagnosed high-risk prostate cancer (sample 1) or BCR prostate cancer and negative findings at conventional CT and/or bone scanning (sample 2) were enrolled between January and December 2021. All men underwent DCFPyL PET/CT. Suspected distant metastases and/or recurrences were biopsied. PPV was calculated.
Results
A total of 92 men with newly diagnosed prostate cancer (median age, 70 years; IQR, 64–75 years) (sample 1) and 92 men with BCR prostate cancer (median age, 71 years; IQR, 66–75 years) (sample 2) were enrolled. In sample 1, 25 of the 92 men (27%) demonstrated DCFPyL-avid lesions suspicious for distant metastases. Biopsy was performed in 23 of the 25 men (92%), with 17 of the 23 (74%) biopsies positive for malignancy and six (26%) benign. Of the six benign biopsies, three were solitary rib foci and three were solitary pelvic bone foci. In sample 2, 57 of the 92 men (62%) demonstrated DCFPyL-avid lesions suspicious for recurrence. Biopsy was performed in 37 of the 57 men (65%), with 33 of the 37 (89%) biopsies positive for malignancy and four (11%) benign. Of the four benign biopsies, two were subcentimeter pelvic nodes and/or nodules, one was a rib, and one was a pelvic bone focus.
Conclusion
PET/CT with 2-(3-{1-carboxy-5-[(6-[(18)F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (DCFPyL) had a high biopsy-proven positive predictive value for distant metastases in newly diagnosed prostate cancer (74%) and for recurrence sites in men with biochemical recurrence (89%). However, there were DCFPyL-avid false-positive findings (particularly in ribs and pelvic bones). Solitary DCFPyL avidity in these locations should not be presumed as malignant. Biopsy may still be needed prior to therapy decisions.
ClinicalTrials.gov registration no. NCT04700332
© RSNA, 2022
See also the editorial by Zukotynski and Kuo in this issue.
Summary
This prospective clinical trial demonstrated with histologic examination the high positive predictive value of 2-(3-{1-carboxy-5-[(6-[(18)F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid PET/CT for prostate cancer distant metastases and recurrences and identified sites of false-positive findings.
Key Results
■ In a prospective trial of 2-(3-{1-carboxy-5-[(6-[(18)F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (DCFPyL) in 184 patients with initially diagnosed and biochemically recurrent prostate cancer, 50 of 60 biopsied DCFPyL-avid lesion suspicious for distant metastases or recurrences (83%) were malignant.
■ The biopsy-proved positive predictive value of DCFPyL PET/CT for distant metastases was 74% in newly diagnosed high-risk prostate cancer and 89% in sites of recurrence in men with biochemical recurrence.
■ Nonmalignant DCFPyL-avid areas at biopsy were most commonly ribs and pelvic bones.
Introduction
Prostate-specific membrane antigen (PSMA) is a transmembrane glutamate-preferring carboxypeptidase known to be overexpressed in the majority of prostate cancer cells of higher grade (1). PSMA-targeting imaging agents have been developed as valuable diagnostic agents for patients with prostate cancer (2). A PSMA-targeting tracer, 2-(3-{1-carboxy-5-[(6-[(18)F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (DCFPyL), was developed at Johns Hopkins by Dr Martin Pomper’s group (3) and became a leading PSMA-targeting imaging agent given its high positron yield, low positron energy, and longer half-life than gallium 68, allowing high-quality images, high tumor-to-background ratios, and the ability of fluorine 18 (18F) agents to be centrally produced and distributed to end users (4,5). DCFPyL was successful in enabling disease detection in several early trials (4–14). Subsequent prospective multicenter studies of DCFPyL in patients with high-risk primary and biochemically recurrent (BCR) prostate cancer, the OSPREY (Study of 18F-DCFPyL PET/CT Imaging in Patients with Prostate Cancer) (15) and CONDOR (Study of 18F-DCFPyL PET/CT Imaging in Patients with Suspected Recurrence of Prostate Cancer) (16) trials, respectively, led to U.S. Food and Drug Administration approval of DCFPyL for these groups of patients in 2021 (17). Of note, although local nodal disease in cohort A of the OSPREY trial had histopathologic findings as the standard of proof, no pathologic confirmation was reported for suspected distant metastases, which have the potential to substantially alter therapeutic management. All patients in OSPREY cohort B were suspected of having regional or distant disease before the DCFPyL scan and thus represent a different set of patients with more advanced disease. In CONDOR, a composite standard of proof including pathologic examination, correlative imaging, and prostate-specific antigen (PSA) response was used in patients with BCR prostate cancer, but only 23% of patients had the preferred standard, pathologic findings. Indeed, among prospective trials of DCFPyL and other PSMA-targeted imaging agents, pathologic validation of suspicious distant metastases at the initial diagnosis and at sites of recurrence in BCR prostate cancer is either not reported or is reported in a minority of men (18–22); thus, subsequent treatment is often initiated without histologic validation. This omission is concerning because, despite its name, PSMA is not specific for prostate cancer (23,24). Multiple false-positive findings and tumor mimics have been identified with DCFPyL PET and other PSMA PET imaging agents (23–27). The presumption that PSMA-avid lesions at PET are malignant can lead to suboptimal patient management. This prospective clinical trial was designed to estimate the positive predictive value (PPV) of DCFPyL PET/CT by providing histopathologic proof for DCFPyL-avid lesions suspected of distant metastases at initial diagnosis and sites of recurrence in BCR prostate cancer.
Materials and Methods
Lantheus provided the DCFPyL used in this investigator-initiated trial. The authors had control of the data and the information submitted for publication. None of the authors are employees of Lantheus.
Participants
This prospective clinical trial (ClinicalTrials.gov identifier, NCT04700332) was performed under a U.S. Food and Drug Administration Investigational New Drug application (IND 152057). The study was approved by the institutional review board (IRB no. 2020000596), and written informed consent was obtained from all participants. The trial began in January 2021, prior to Food and Drug Administration approval of DCFPyL as piflufolastat 18F in May 2021. Consecutive men were enrolled and completed the trial at the Hoag Family Cancer Institute between January and December 2021. A total of 184 men were accrued, 92 men in each of the two sample groups. Inclusion criteria were men 18 years or older with biopsy-proven adenocarcinoma of the prostate and an Eastern Cooperative Oncology Group performance score of 0–2. Inclusion criteria for sample 1 added men with newly diagnosed high-risk prostate cancer (defined as PSA level >10 ng/mL and Gleason score of 8–10) who were scheduled to undergo prostatectomy or radiation therapy. Patients in sample 1 had not received prior therapy, including androgen deprivation therapy. Inclusion criteria for sample 2 added BCR prostate cancer (defined as PSA level ≥0.2 ng/mL following prostatectomy or PSA level ≥2.0 ng/mL above nadir following radiation therapy) (28,29) and no evidence of disease on CT and/or bone scans within 3 months of trial enrollment. Exclusion criteria were lack of consent or inability to tolerate PET/CT. Demographic and clinical data, including age, PSA level, Gleason score of the primary malignancy, and prior therapy (for men in sample 2), were recorded.
DCFPyL PET/CT
DCFPyL was obtained on demand under a production agreement with Lantheus and was synthesized according to good manufacturing practices. There were no specific participant preparation guidelines other than the recommendation to remain well hydrated. A dose of 333 MBq (9 mCi) ±10% DCFPyL was administered intravenously. Men voided before imaging. PET/CT was performed 60 minutes after tracer administration by using a Siemens Biograph mCT Flow PET/CT scanner. Images were obtained from the midthigh to the skull apex and reconstructed into multiplanar PET, CT, and fused PET/CT images. CT was used for attenuation correction of PET images. Images were reviewed with a Siemens SyngoVia Workstation by the principal investigator (G.A.U., with 17 years of experience in PET imaging). Sites of DCFPyL avidity that were not physiologic and were suspicious for malignancy were recorded. Physiologic sites of DCFPyL avidity include the lacrimal and salivary glands, liver, spleen, kidneys, ureters, bladder, and bowel. CT was performed without intravenous contrast material. Transaxial section thickness was 4 mm, with an interval of 3 mm. The median CT dose index was 6.6 mGy, and the median dose-length product was 821 mGy · cm. Participants were contacted on the day following DCFPyL PET/CT to record adverse events.
Biopsy of Sites Suspicious for Distant Metastases or Recurrent Disease
The goal of our trial was to obtain pathologic confirmation of DCFPyL-avid suspected distant metastases (sample 1) or suspected recurrence (sample 2) whenever possible. DCFPyL PET/CT images were reviewed by the principal investigator and an interventional radiologist (T.P. or T.T., with 14 and 10 years of experience, respectively) to determine consensus for an accessible and safe lesion for CT- or US-guided biopsy. Although it was common for multiple DCFPyL-avid foci to be seen on a scan, only one site of distant metastasis or suspected recurrence was targeted per participant. If lesions were not appropriate or safe for biopsy, for example owing to lack of a safe biopsy window or patient-deferred biopsy, then no biopsy was performed. If lesions were deemed safe for biopsy, then the safest representative lesion was biopsied per protocol within 14 days of DCFPyL PET/CT. Histopathologic examination was performed by board-certified pathologists.
Statistical Analysis
The primary objective of this study was to prospectively estimate the true-positive rate of DCFPyL PET/CT, or the PPV, defined as the number of scans positive at DCFPyL PET/CT and biopsy positive divided by the total number of scans positive at DCFPyL PET/CT. It was estimated that if 50% of the men had at least one pathology-confirmed distant metastasis, a sample size of 92 men per cohort would allow us to estimate the PPV with a precision of ±14%. The PPV is given with exact 95% CIs. Medians and ranges are used to summarize continuous variables and frequencies, and percentages are used to summarize categorical variables. A post hoc analysis to define results according to PSA group was performed after completion of the trial. Statistical analyses were performed by an author (A.M., with 15 years of experience in biostatistics) using R version 4.1.1 (Vienna, Austria).
Results
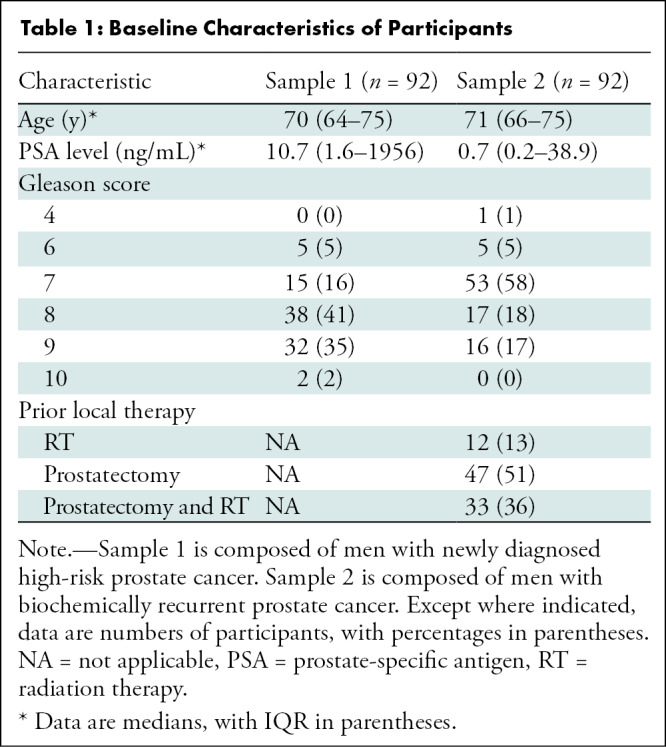
Participant Characteristics
The Standards for Reporting of Diagnostic Accuracy flow diagrams for both samples are shown in Figure 1. A total of 201 patients were screened, and 184 patients were enrolled in the trial. In sample 1 (newly diagnosed high-risk prostate cancer), 98 men met inclusion criteria. Six men were excluded because they were unwilling to consent to the clinical trial, leaving 92 patients. In sample 2 (BCR prostate cancer), 103 men met inclusion criteria. Eleven men were excluded because they were unwilling to consent to a clinical trial, leaving 92 participants. The enrollment goal was met for both samples. In sample 1, the median age was 70 years (range, 43–92 years; IQR, 64–75 years) and the median PSA level was 10.7 ng/mL (range, 1.6–1956 ng/mL). In sample 2, the median age was 71 years (range, 58–89 years; IQR, 66–75 years) and the median PSA level was 0.7 ng/mL (range, 0.2–38.9 ng/mL). The participant characteristics are summarized in Table 1. There were no reported adverse events from DCFPyL PET/CT.
Figure 1:
(A) Standards for Reporting of Diagnostic Accuracy, or STARD, diagram of sample 1, men with newly diagnosed high-risk prostate cancer. (B) Standards for Reporting of Diagnostic Accuracy diagram of sample 2, men with biochemically recurrent prostate cancer. DCFPyL = 2-(3-{1-carboxy-5-[(6-[(18)F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid.
Table 1:
Baseline Characteristics of Participants
DCFPyL PET/CT and Biopsy in Sample 1
Of the 92 men in sample 1, 25 (27%) demonstrated DCFPyL foci suspicious for distant metastases and 67 (73%) did not (Fig 1A). Of the 25 men with DCFPyL foci suspicious for distant metastases, 23 (92%) underwent biopsy. Seventeen of the 23 biopsy samples (74%) demonstrated malignancy, including 13 osseous lesions (three rib, seven pelvic bone, two spine, and one femur), three distant nodal metastases (two retroperitoneal and one left supraclavicular), and one lung nodule. The lung nodule was proven to be a renal cell carcinoma metastasis at biopsy. All other positive biopsy samples demonstrated metastatic prostate adenocarcinoma. Six of 23 biopsies (26%) were benign, and all were solitary osseous lesions (three in ribs and three in pelvic bones). For sample 1, the estimated PPV of DCFPyL PET/CT for distant metastases was 74% (17 of 23 men; 95% CI: 52, 90).
The two men with DCFPyL foci suspicious for distant metastases that were not sampled for biopsy received clinical follow-up, with one demonstrating sclerosis of osseous lesions following therapy and the other demonstrating decreased size of distant nodal lesions following therapy, suggesting that both men had distant metastases; however, these men were not included in calculations of biopsy-proven sites.
For 91 of the 92 men in sample 1 (99%), there was a prostate focus consistent with the known primary prostate cancer. For 21 of the 92 men (23%), there were pelvic nodal foci suspicious for local pelvic nodal metastases. However, neither the primary prostate focus nor the local pelvic nodal foci were the focus of this study, and correct localization was not confirmed pathologically.
DCFPyL PET/CT and Biopsy in Sample 2
Of the 92 men in sample 2, 57 (62%) demonstrated DCFPyL foci suspicious for sites of recurrence and 35 (38%) did not (Fig 1B). Of the 57 men with DCFPyL foci suspicious for sites of recurrence, 37 (65%) underwent biopsy. Thirty-three of the 37 biopsies (89%) demonstrated malignancy, including two prostate foci, three prostate bed foci, eight osseous lesions (one rib, three pelvic bones, three spine, and one scapula), 15 nodal metastases (seven pelvic, six abdominal, and two left supraclavicular), and five other lesions (abdominal muscle, penile, lung, pleural, and breast). The breast lesion was a biopsy-proven male intraductal breast carcinoma; all other biopsied malignancies were prostate cancer recurrences. Only four of the 37 biopsies (11%) were benign, including two solitary osseous lesions (one rib and one pelvic bone) and two solitary subcentimeter pelvic nodal and/or soft-tissue lesions. For sample 2, the estimated PPV of DCFPyL PET/CT for sites of recurrence in men with biochemical recurrence was 89% (95% CI: 75, 97).
Of the 20 men in sample 2 with DCFPyL foci suspicious for sites of recurrence that were not biopsied, two underwent follow-up prostate MRI that suggested a true-positive prostate DCFPyL focus and three underwent follow-up prostate or pelvic MRI that did not demonstrate an anatomic correlate to a DCFPyL focus. The latter patients may have had benign DCFPyL foci, as no lesion was apparent for biopsy. These five men with imaging follow-up were not included in calculations of biopsy-proven results. In 15 other men with DCFPyL foci suspicious for sites of recurrence, the focus was interpreted as not amenable to image-guided biopsy (n = 6), the lesion was within a previously radiated field and pathologic proof would not affect further management (n = 2), the participant had a pacemaker or stimulator preventing further MRI evaluation of prostate and/or prostate bed foci (n = 2), and the participant deferred biopsy (n = 5). Examples of DCFPyL PET/CT foci that were confirmed to be malignant and benign at biopsy are provided in Figures 2 and 3, respectively.
Figure 2:
Examples of biopsy-proven distant metastases and sites of biochemical recurrence. Red arrows highlight the biopsy-proven malignant lesions. (A–D) A 2-(3-{1-carboxy-5-[(6-[(18)F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (DCFPyL) maximum intensity projection image (A), axial CT scan (B), axial fused DCFPyL PET/CT scan (C), and CT scan obtained during image-guided biopsy (D) of a DCFPyL-avid (standardized uptake value, 5.0) biopsy-proven right fourth rib metastasis in a 76-year-old man with initially diagnosed prostate cancer (Gleason score, 8; prostate-specific antigen [PSA] level of 5.4 ng/mL). There were also DCFPyL-avid pelvic nodes that were not biopsied. (E–H) DCFPyL maximum intensity projection image (E), axial CT scan (F), axial fused DCFPyL PET/CT scan (G), and CT scan obtained during image-guided biopsy (H) of a DCFPyL-avid (standardized uptake value, 11.2) biopsy-proven subcentimeter left pelvic sidewall node in a 65-year-old man with prostate cancer after prostatectomy and salvage radiation. PSA level was 0.6 ng/mL. (I–L) DCFPyL maximum intensity projection image (I), axial CT scan (J), axial fused DCFPyL PET/CT scan (K), and US scan obtained during image-guided biopsy (L) of a DCFPyL-avid (standardized uptake value, 9.6) biopsy-proven subcentimeter right anterior abdominal wall metastasis in a 72-year-old man with prostate cancer after prostatectomy and salvage radiation. PSA level was 2.7 ng/mL. There are four anterior abdominal wall foci seen on the maximum intensity projection image.
Figure 3:
Examples of 2-(3-{1-carboxy-5-[(6-[(18)F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (DCFPyL)–avid lesions with benign pathologic findings at biopsy. Red arrows highlight the lesions with benign biopsy results. (A–D) DCFPyL maximum intensity projection image (A), axial CT scan (B), axial fused DCFPyL PET/CT scan (C), and CT scan obtained during image-guided biopsy (D) of a DCFPyL-avid (standardized uptake value, 6.9) right second rib sclerosis in a 68-year-old man with prostate cancer after prostatectomy and salvage radiation. Prostate-specific antigen (PSA) level was 1.0 ng/mL. (E–H) DCFPyL maximum intensity projection image (E), axial CT scan (F), axial fused DCFPyL PET/CT scan (G), and CT scan obtained during image-guided biopsy (H) of DCFPyL-avid (standardized uptake value, 12.7) left inferior pubic ramus sclerosis in a 59-year-old man with newly diagnosed prostate cancer (Gleason score, 8; PSA level, 19.7 ng/mL). (I–L) DCFPyL maximum intensity projection image (I), axial CT scan (J), axial fused DCFPyL PET/CT scan (K), and CT scan obtained during image-guided biopsy (L) of a DCFPyL-avid (standardized uptake value, 4.7) subcentimeter right external iliac node in a 61-year-old man with prostate cancer after prostatectomy. PSA level was 0.4 ng/mL. In each of these men, the biopsy results were benign.
Post Hoc Statistical Analysis
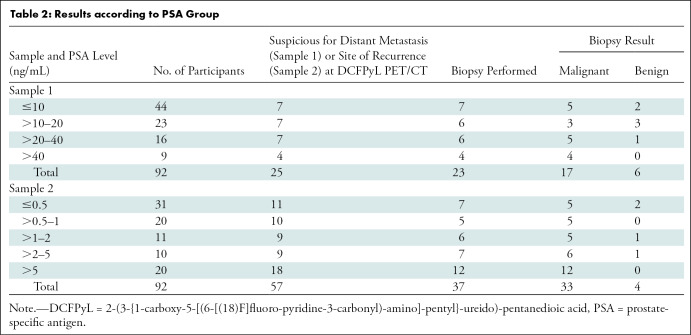
After protocol completion, the data were reviewed to determine the results by retrospectively defining the PSA groups (Table 2). In sample 1, for men with a PSA level of 20 ng/mL or lower, 14 of 67 (21%) had a site suspicious for distant metastasis at DCFPyL PET/CT. Thirteen of the 14 men underwent biopsy, with eight of 13 (62%) demonstrating malignancy. For men with a PSA level greater than 20 ng/mL, 11 of 25 (44%) had a site suspicious for distant metastasis at DCFPyL PET/CT. Ten of the 11 men underwent biopsy, with nine of 10 (90%) demonstrating malignancy. In sample 2, for men with a PSA level of 1 ng/mL or less, 21 of 51 (41%) had a site suspicious for recurrence at DCFPyL PET/CT. Twelve of the 21 men underwent biopsy, with 10 of 12 (83%) demonstrating malignancy. For men with a PSA level greater than 1 ng/mL, 36 of 41 (88%) had a site suspicious for recurrence at DCFPyL PET/CT. Twenty-five of the 36 men underwent biopsy, with 23 of the 25 (92%) demonstrating malignancy.
Table 2:
Results according to PSA Group
There were 21 biopsy-proven sites of bone metastases in the two samples. The median PSA level for these men was 12.0 ng/mL (range, 0.4–1956 ng/mL). The median PSA level for the 15 men in sample 1 was 28.2 ng/mL (range, 5.1–1956 ng/mL), and the median PSA level for the six men in sample 2 men was 1.9 ng/mL (range, 0.4–6.7 ng/mL). Four of these osseous metastases were in men in sample 2 with a PSA level less than 2.0 ng/mL (rib metastasis, PSA level of 0.4 ng/mL; spine metastasis, PSA level of 0.9 ng/mL; spine metastasis, PSA level of 1.8 ng/mL; and scapula metastasis, PSA level of 1.9 ng/mL).
Discussion
Prostate-specific membrane antigen PET is becoming a standard method of evaluating patients with newly diagnosed high-risk and biochemically recurrent prostate cancer. Although studies have suggested high specificity of 2-(3-{1-carboxy-5-[(6-[(18)F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (DCFPyL), several false-positive findings and tumor mimics have been identified, and most studies to date do not include a large proportion of patients for whom histologic confirmation was obtained for distant metastases or recurrence (4–16). In this prospective clinical trial, pathologic confirmation of DCFPyL-avid sites suspicious for distant metastases and recurrence was obtained to determine the positive predictive value (PPV) of DCFPyL-avid lesions. Although DCFPyL PET/CT in our trial demonstrated a biopsy-proven PPV of 74% for suspected distant metastases at initial diagnosis and 89% for recurrence, there were several anatomic sites with DCFPyL avidity yielding benign biopsy results, namely, solitary foci in ribs, in pelvic bones, and along pelvic nodal chains.
The benefits of DCFPyL PET/CT for staging were readily apparent in our trial. DCFPyL helped detect suspicious osseous, nodal, and soft-tissue lesions that were not identified at CT and bone scanning. This included sites previously overlooked, such as the left supraclavicular nodes and CT-occult osseous, muscle, and penile lesions. Tiny lesions identified on DCFPyL scans, which were not considered suspicious at traditional anatomic imaging, proved to be sites of active malignancy upon biopsy. Malignant osseous foci were identified in men with PSA values as low as 0.4 ng/mL, even though it is often believed that high PSA values are required for osseous metastases to be present.
Despite the apparent benefits of DCFPyL PET/CT, several anatomic sites were prone to false-positive results. Solitary rib foci on DCFPyL scans have been previously reported to be a source of false-positive findings (30). In our prospective trial, four of eight (50%) biopsied rib foci were false-positive findings. Rib lesions considered benign based on CT criteria, such as thin sclerotic margins associated with enchondromas (23), were deemed benign in our trial and not biopsied. However, even DCFPyL-avid rib foci without CT criteria defining them as benign were often benign at biopsy. Similarly, four of 14 (29%) biopsied pelvic osseous foci were false-positive findings. The benign pelvic osseous foci in our trial were all solitary. Small pelvic nodal and/or nodule foci were another source of false-positive findings, although most pelvic foci in men in our trial, as well as in participants of the OSPREY trial, were true-positive findings. The majority of benign biopsy results were in men with lower PSA values (≤20 ng/mL in sample 1 and ≤0.5 ng/mL in sample 2). These benign findings at pathologic examination should give pause in the interpretation of some DCFPyL foci, particularly when not supported by additional foci confirming malignancy. Solitary DCFPyL foci in the ribs and pelvic bones and small nodes and/or nodules should not be presumed to be malignant, and tissue sampling may still be needed.
As has previously been reported, DCFPyL uptake may be seen in malignancies other than prostate cancer (23,25,27). In our trial, of 50 biopsy-proven sites of malignancy, two (4%) were metastases from nonprostate malignancies. The OSPREY trial (15) provided outstanding data with histopathologic comparison on the detection of local nodal disease. Our trial did not contain an evaluation of local nodal disease to this level of precision. Similarly, our trial did not contain a thorough confirmation and localization of primary prostate malignancy in sample 1. The advantages of our trial include its prospective design and use of histopathologic examination as the standard of proof.
Several limitations deserve mention. First, only one site per participant was biopsied. This is due to ethical and logistical issues with multiple biopsies per participant. Second, the possibility of false-negative biopsy findings must be considered, particularly in the case of small lesions. Third, in some men, biopsy was not performed because the DCFPyL-avid foci were deemed not amenable to biopsy. These men were not included in the calculations of biopsy-proven results. Fourth, as there were no biopsy specimens available for men without suspected distant metastases or sites of recurrence, the negative predictive value of DCFPyL PET/CT could not be evaluated. Fifth, DCFPyL scans underwent single reader assessment. Sixth, the results of this study may only be applicable to a population with similar prevalence of disease as ours. Finally, the analysis of results according to PSA groups was not prespecified in the trial, but rather a post hoc analysis.
In conclusion, in this prospective trial of men with newly diagnosed and biochemically recurrent (BCR) prostate cancer, 2-(3-{1-carboxy-5-[(6-[(18)F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (DCFPyL) PET/CT demonstrated biopsy-proven positive predictive values of 74% for distant metastases at initial diagnosis and 89% at sites of recurrent malignancy in BCR prostate cancer. However, there were areas of DCFPyL avidity with a higher propensity for false-positive results (ribs, pelvic bones, and small nodal foci). Thus, foci should not be presumed to always represent malignancy. Caution should be exercised, particularly when encountering solitary lesions in ribs, pelvic bones, and small nodal foci. Tissue sampling may still be needed before selecting optimal therapies. Both clinical application and future trials of DCFPyL PET may benefit from increased pathologic correlation of imaging findings.
G.A.U. supported in part by the Hoag Hospital Foundation, James & Pamela Muzzy Endowed Chair, and Lantheus.
Data sharing: Data generated or analyzed during the study are available from the corresponding author by request.
Disclosures of conflicts of interest: G.A.U. Grants from National Institutes of Health and Department of Defense; grants, speaker fees, and consulting fees from GE Healthcare; speakers fees and consulting fees from Lantheus; President of the American College of Nuclear Medicine in 2022; member of the Radiology editorial board. B.T. No relevant relationships. J.B. No relevant relationships. R.T. No relevant relationships. C.C. No relevant relationships. K.L. No relevant relationships. T.P. No relevant relationships. T.T. No relevant relationships. A.M. Grant from National Cancer Institute (CA008748). S.P.R. Grants from Lantheus Pharmaceuticals, FutureChem USA, Precision Molecular, and PlenaryAI; royalties or licenses from Precision Molecular and PlenaryAI; consulting fees from Lantheus Pharmaceuticals, Precision Molecular, and PlenaryAI; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Lantheus Pharmaceuticals; patents planned, issues, or pending from Precision Molecular and PlenaryAI; stock or stock options in Precision Molecular and PlenaryAI. L.L. Director for American Board of Nuclear Medicine, unpaid board member for PET Center for Excellence with the Society of Nuclear Medicine, and Molecular Imaging (unpaid). E.M. No relevant relationships. P.C. No relevant relationships. J.Y. No relevant relationships.
Abbreviations:
- BCR
- biochemically recurrent
- DCFPyL
- 2-(3-{1-carboxy-5-[(6-[(18)F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid
- PPV
- positive predictive value
- PSA
- prostate-specific antigen
- PSMA
- prostate-specific membrane antigen
References
- 1. Evans JD , Jethwa KR , Ost P , et al. Prostate cancer-specific PET radiotracers: A review on the clinical utility in recurrent disease. Pract Radiat Oncol 2018;8(1):28–39. [DOI] [PubMed] [Google Scholar]
- 2. Lawhn-Heath C , Salavati A , Behr SC , et al. Prostate-specific Membrane Antigen PET in Prostate Cancer. Radiology 2021;299(2):248–260. [DOI] [PubMed] [Google Scholar]
- 3. Chen Y , Pullambhatla M , Foss CA , et al. 2-(3-{1-Carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid, [18F]DCFPyL, a PSMA-based PET imaging agent for prostate cancer. Clin Cancer Res 2011;17(24):7645–7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Szabo Z , Mena E , Rowe SP , et al. Initial Evaluation of [(18)F]DCFPyL for Prostate-Specific Membrane Antigen (PSMA)-Targeted PET Imaging of Prostate Cancer. Mol Imaging Biol 2015;17(4):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rowe SP , Macura KJ , Mena E , et al. PSMA-Based [(18)F]DCFPyL PET/CT Is Superior to Conventional Imaging for Lesion Detection in Patients with Metastatic Prostate Cancer. Mol Imaging Biol 2016;18(3):411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gorin MA , Rowe SP , Patel HD , et al. Prostate Specific Membrane Antigen Targeted 18F-DCFPyL Positron Emission Tomography/Computerized Tomography for the Preoperative Staging of High Risk Prostate Cancer: Results of a Prospective, Phase II, Single Center Study. J Urol 2018;199(1):126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rousseau E , Wilson D , Lacroix-Poisson F , et al. A Prospective Study on 18F-DCFPyL PSMA PET/CT Imaging in Biochemical Recurrence of Prostate Cancer. J Nucl Med 2019;60(11):1587–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wondergem M , Jansen BHE , van der Zant FM , et al. Early lesion detection with 18F-DCFPyL PET/CT in 248 patients with biochemically recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2019;46(9):1911–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rowe SP , Campbell SP , Mana-Ay M , et al. Prospective Evaluation of PSMA-Targeted 18F-DCFPyL PET/CT in Men with Biochemical Failure After Radical Prostatectomy for Prostate Cancer. J Nucl Med 2020;61(1):58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rowe SP , Li X , Trock BJ , et al. Prospective Comparison of PET Imaging with PSMA-Targeted 18F-DCFPyL Versus Na18F for Bone Lesion Detection in Patients with Metastatic Prostate Cancer. J Nucl Med 2020;61(2):183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Song H , Harrison C , Duan H , et al. Prospective Evaluation of 18F-DCFPyL PET/CT in Biochemically Recurrent Prostate Cancer in an Academic Center: A Focus on Disease Localization and Changes in Management. J Nucl Med 2020;61(4):546–551. [DOI] [PubMed] [Google Scholar]
- 12. Liu W , Zukotynski K , Emmett L , et al. A Prospective Study of 18F-DCFPyL PSMA PET/CT Restaging in Recurrent Prostate Cancer following Primary External Beam Radiotherapy or Brachytherapy. Int J Radiat Oncol Biol Phys 2020;106(3):546–555. [Published correction appears in Int J Radiat Oncol Biol Phys 2020;107(2):390.] [DOI] [PubMed] [Google Scholar]
- 13. Glicksman RM , Metser U , Valliant J , et al. [18F]DCFPyLPET-MRI/CT for unveiling a molecularly defined oligorecurrent prostate cancer state amenable for curative-intent ablative therapy: study protocol for a phase II trial. BMJ Open 2020;10(4):e035959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mena E , Lindenberg ML , Turkbey IB , et al. 18F-DCFPyL PET/CT Imaging in Patients with Biochemically Recurrent Prostate Cancer After Primary Local Therapy. J Nucl Med 2020;61(6):881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pienta KJ , Gorin MA , Rowe SP , et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in Prostate Cancer Patients (OSPREY). J Urol 2021;206(1):52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morris MJ , Rowe SP , Gorin MA , et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin Cancer Res 2021;27(13):3674–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.FDA approves second PSMA-targeted PET imaging drug for men with prostate cancer. U.S. Food & Drug Administration. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-second-psma-targeted-pet-imaging-drug-men-prostate-cancer. FDA approves second PSMA-targeted PET imaging drug for men with prostate cancer. Published 2021. Accessed January 14, 2022. [Google Scholar]
- 18. Hofman MS , Lawrentschuk N , Francis RJ , et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 2020;395(10231):1208–1216. [DOI] [PubMed] [Google Scholar]
- 19. Wondergem M , van der Zant FM , Broos WAM , et al. 18F-DCFPyL PET/CT for primary staging in 160 high-risk prostate cancer patients; metastasis detection rate, influence on clinical management and preliminary results of treatment efficacy. Eur J Nucl Med Mol Imaging 2021;48(2):521–531. [DOI] [PubMed] [Google Scholar]
- 20. Mena E , Rowe SP , Shih JH , et al. Predictors of 18F-DCFPyL-PET/CT Positivity in Patients with Biochemical Recurrence of Prostate Cancer After Local Therapy. J Nucl Med 2021. 10.2967/jnumed.121.262347. Published online December 16, 2021. [DOI] [PMC free article] [PubMed]
- 21. Szigeti F , Schweighofer-Zwink G , Meissnitzer M , et al. Incremental Impact of [68 Ga]Ga-PSMA-11 PET/CT in Primary N and M Staging of Prostate Cancer Prior to Curative-Intent Surgery: a Prospective Clinical Trial in Comparison with mpMRI. Mol Imaging Biol 2022;24(1):50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kesler M , Kerzhner K , Druckmann I , et al. Staging 68 Ga-PSMA PET/CT in 963 consecutive patients with newly diagnosed prostate cancer: incidence and characterization of skeletal involvement. Eur J Nucl Med Mol Imaging 2022;49(6):2077–2085. [DOI] [PubMed] [Google Scholar]
- 23. Sheikhbahaei S , Afshar-Oromieh A , Eiber M , et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur J Nucl Med Mol Imaging 2017;44(12):2117–2136. [DOI] [PubMed] [Google Scholar]
- 24. Sheikhbahaei S , Werner RA , Solnes LB , et al. Prostate-Specific Membrane Antigen (PSMA)-Targeted PET Imaging of Prostate Cancer: An Update on Important Pitfalls. Semin Nucl Med 2019;49(4):255–270. [DOI] [PubMed] [Google Scholar]
- 25. Hofman MS , Hicks RJ , Maurer T , Eiber M . Prostate-specific Membrane Antigen PET: Clinical Utility in Prostate Cancer, Normal Patterns, Pearls, and Pitfalls. RadioGraphics 2018;38(1):200–217. [DOI] [PubMed] [Google Scholar]
- 26. Barbosa FG , Queiroz MA , Nunes RF , et al. Revisiting Prostate Cancer Recurrence with PSMA PET: Atlas of Typical and Atypical Patterns of Spread. RadioGraphics 2019;39(1):186–212. [DOI] [PubMed] [Google Scholar]
- 27. de Galiza Barbosa F , Queiroz MA , Nunes RF , et al. Nonprostatic diseases on PSMA PET imaging: a spectrum of benign and malignant findings. Cancer Imaging 2020;20(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cookson MS , Aus G , Burnett AL , et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol 2007;177(2):540–545. [DOI] [PubMed] [Google Scholar]
- 29. Abramowitz MC , Li T , Buyyounouski MK , et al. The Phoenix definition of biochemical failure predicts for overall survival in patients with prostate cancer. Cancer 2008;112(1):55–60. [DOI] [PubMed] [Google Scholar]
- 30. Chen MY , Franklin A , Yaxley J , et al. Solitary rib lesions showing prostate-specific membrane antigen (PSMA) uptake in pre-treatment staging 68 Ga-PSMA-11 positron emission tomography scans for men with prostate cancer: benign or malignant? BJU Int 2020;126(3):396–401. [DOI] [PubMed] [Google Scholar]



![(A) Standards for Reporting of Diagnostic Accuracy, or STARD, diagram of sample 1, men with newly diagnosed high-risk prostate cancer. (B) Standards for Reporting of Diagnostic Accuracy diagram of sample 2, men with biochemically recurrent prostate cancer. DCFPyL = 2-(3-{1-carboxy-5-[(6-[(18)F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/148e/9619197/6bc605450c6d/radiol.220218.fig1.jpg)
![Examples of biopsy-proven distant metastases and sites of biochemical recurrence. Red arrows highlight the biopsy-proven malignant lesions. (A–D) A 2-(3-{1-carboxy-5-[(6-[(18)F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (DCFPyL) maximum intensity projection image (A), axial CT scan (B), axial fused DCFPyL PET/CT scan (C), and CT scan obtained during image-guided biopsy (D) of a DCFPyL-avid (standardized uptake value, 5.0) biopsy-proven right fourth rib metastasis in a 76-year-old man with initially diagnosed prostate cancer (Gleason score, 8; prostate-specific antigen [PSA] level of 5.4 ng/mL). There were also DCFPyL-avid pelvic nodes that were not biopsied. (E–H) DCFPyL maximum intensity projection image (E), axial CT scan (F), axial fused DCFPyL PET/CT scan (G), and CT scan obtained during image-guided biopsy (H) of a DCFPyL-avid (standardized uptake value, 11.2) biopsy-proven subcentimeter left pelvic sidewall node in a 65-year-old man with prostate cancer after prostatectomy and salvage radiation. PSA level was 0.6 ng/mL. (I–L) DCFPyL maximum intensity projection image (I), axial CT scan (J), axial fused DCFPyL PET/CT scan (K), and US scan obtained during image-guided biopsy (L) of a DCFPyL-avid (standardized uptake value, 9.6) biopsy-proven subcentimeter right anterior abdominal wall metastasis in a 72-year-old man with prostate cancer after prostatectomy and salvage radiation. PSA level was 2.7 ng/mL. There are four anterior abdominal wall foci seen on the maximum intensity projection image.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/148e/9619197/f7aee76b34ca/radiol.220218.fig2.jpg)
![Examples of 2-(3-{1-carboxy-5-[(6-[(18)F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (DCFPyL)–avid lesions with benign pathologic findings at biopsy. Red arrows highlight the lesions with benign biopsy results. (A–D) DCFPyL maximum intensity projection image (A), axial CT scan (B), axial fused DCFPyL PET/CT scan (C), and CT scan obtained during image-guided biopsy (D) of a DCFPyL-avid (standardized uptake value, 6.9) right second rib sclerosis in a 68-year-old man with prostate cancer after prostatectomy and salvage radiation. Prostate-specific antigen (PSA) level was 1.0 ng/mL. (E–H) DCFPyL maximum intensity projection image (E), axial CT scan (F), axial fused DCFPyL PET/CT scan (G), and CT scan obtained during image-guided biopsy (H) of DCFPyL-avid (standardized uptake value, 12.7) left inferior pubic ramus sclerosis in a 59-year-old man with newly diagnosed prostate cancer (Gleason score, 8; PSA level, 19.7 ng/mL). (I–L) DCFPyL maximum intensity projection image (I), axial CT scan (J), axial fused DCFPyL PET/CT scan (K), and CT scan obtained during image-guided biopsy (L) of a DCFPyL-avid (standardized uptake value, 4.7) subcentimeter right external iliac node in a 61-year-old man with prostate cancer after prostatectomy. PSA level was 0.4 ng/mL. In each of these men, the biopsy results were benign.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/148e/9619197/b24d2cebf5c7/radiol.220218.fig3.jpg)