Abstract
Vascular imaging with color and power Doppler is a useful tool in the assessment of various disease processes. Assessment of blood flow, from infarction and ischemia to hyperemia, in organs, neoplasms, and vessels, is used in nearly every US investigation. Recent developments in this area are sensitive to small-vessel low velocity flow without use of intravenous contrast agents, known as microvascular flow imaging (MVFI). MVFI is more sensitive in detection of small vessels than color, power, and spectral Doppler, reducing the need for follow-up contrast-enhanced US (CEUS), CT, and MRI, except when arterial and venous wash-in and washout characteristics would be helpful in diagnosis. Varying clinical applications of MVFI are reviewed in adult and pediatric populations, including its technical underpinnings. MVFI shows promise in assessment of several conditions including benign and malignant lesions in the liver and kidney, acute pathologic abnormalities in the gallbladder and testes, and superficial lymph nodes. Future potential of MVFI in different conditions (eg, endovascular repair) is discussed. Finally, clinical cases in which MVFI correlated and potentially obviated additional CEUS, CT, or MRI are shown.
© RSNA, 2022
Learning Objectives:
After completing this journal-based SA-CME activity, participants will be able to:
■ Describe the basics of improved slow flow detection with microvascular flow imaging (MVFI)
■ Identify the MVFI findings in the absence of flow to a structure
■ Identify improved determination of vascular distribution in lesions with MFI
Accreditation and Designation Statement
The RSNA is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians. The RSNA designates this journal-based SA-CME activity for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Disclosure Statement
The ACCME requires that the RSNA, as an accredited provider of CME, obtain signed disclosure statements from the authors, editors, and reviewers for this activity. For this journal-based CME activity, author disclosures are listed at the end of this article.
Summary
Microvascular flow imaging is a diagnostic tool that may provide additional value over conventional color and power Doppler imaging, potentially obviating contrast-enhanced US in certain lesions.
Essentials
■ Microvascular flow imaging (MVFI) involves the use of filters that are different than Doppler imaging, reducing artifacts from random motion and thereby resulting in a greater sensitivity to lower velocity blood flow evaluation.
■ MVFI has shown additional diagnostic value in emergent indications, assessment of neoplastic lesions, superficial organ vascular assessment, and various pediatric conditions.
■ Future applications may include pre- and posttreatment vascularity assessment, vascular indications (eg, endovascular stent repair), and musculoskeletal indications.
Introduction
US is widely used as the most effective initial diagnostic modality in the assessment of various clinical conditions in visceral, vascular, and soft tissue because of its widespread availability, ease of use, and excellent safety profile. Imaging of vascular networks with color and power Doppler is valuable in the assessment of many disease processes, ranging from hyperemia and ischemia to infarction and flow patterns in organs, neoplasms, and vessels, and in evaluation of treatment results (1).
Color Doppler US uses measurement of phase change after reflection from a moving target such as circulating blood (2). Because the frequency change is not measured, color flow imaging is not strictly color Doppler, but hereafter we will use the word Doppler because of its widespread use (3). Power Doppler imaging improves flow sensitivity compared with conventional Doppler imaging by depicting amplitude of the Doppler shift. However, it typically cannot show smaller vessels and slower capillary blood flow (1); color flow depicts flow larger than approximately 0.2 mm in diameter (4). A spectral Doppler waveform analysis is paramount to identifying venous or arterial flow, and the waveform pattern gives information on vascular abnormalities. Whereas this is a useful quantitative approach, it lacks the ability to provide full two-dimensional imaging in estimating tissue vascularity.
Perfusion is defined as blood flow per unit time (the difference between arterial inflow and venous outflow). Assessment of vascularity is of the utmost importance in inflammation, ischemia, injury, and tumor angiogenesis evaluation (5,6). Availability of diagnostic tools to adequately image vascular beds is critical to improve diagnosis, treatment, and posttreatment surveillance, and further imaging with contrast-enhanced US (CEUS), CT, or MRI is often required. Perfusion assessment with CEUS by using microbubble contrast agents substantially improves evaluation in superficial and deep organ systems (7–9). However, despite the alternative of more costly and potentially nephrotoxic intravenous CT and MRI contrast agents, and risk of radiation with CT, CEUS is limited in use, especially in emergent settings where it may not be readily available.
Recently, a number of tools were developed for assessment of smaller vessels at lower velocity flow without use of intravenous contrast agents. These tools adaptively filter random motion while preserving the detection of directional motion by flowing blood, resulting in more sensitive and higher resolution blood flow evaluation relative to color or power Doppler. Similar to power Doppler imaging, these techniques typically depict a map of blood flow on the gray-scale image but without directional information. These techniques are also prone to other limitations including angle dependency, depth dependency, and generalized poor performance in low signal-to-noise situations.
This new method for depicting blood flow in small vessels is herein termed microvascular flow imaging (MVFI), encompassing multiple proprietary manufacturers’ brand names. The purpose of this article is to review the background and physics of the emerging MVFI tool and to review current applications of MVFI in adult and pediatric populations. While acknowledging the somewhat limited available scientific data on this emerging tool, future directions of this promising technique are highlighted.
The Standard of CEUS Perfusion Imaging
CEUS is a widely used and ideal tool for MVFI. Commercially available US contrast agents consist of gas microbubbles stabilized by an outer shell (10). The mechanisms of action for contrast enhancement of US images rely on the gas core within the microbubble, creating large, backscattered signals, and the generation of nonlinear responses to the oscillating pressure wave. The contrast agents are injected intravenously (for vascular applications) and must be small enough (<6 μm) to clear the pulmonary capillary beds, allowing for passage through the capillary system (11).
Commercially available CEUS packages are available on most mid-to-high-level US scanners and provide real-time views of US contrast agent perfusion. Most commercial systems use nondestructive (mechanical index, <0.1) techniques to optimize nonlinear signals from the US contrast agent to better separate microbubble signal from those of the surrounding tissues. Whereas specific pulse sequences are manufacturer dependent, all strive to maximize nonlinear microbubble signals while suppressing tissue signals to create angiogram-like images of vasculature on “contrast-only” or “tissue-subtracted” images. Along with high signal-to-noise ratio, a unique advantage of CEUS is the ability to observe wash-in and washout of the contrast agent in a lesion or vessel, with some manufacturers allowing perfusion analysis. This includes quantitative analysis of contrast signal intensity as a function of time, which can provide an indirect measure of either blood flow, total tissue perfusion, or other perfusion kinetics. These commercial packages are now available on many scanners or as stand-alone postprocessing software packages.
Current Status of Noncontrast-enhanced US MVFI
Advanced flow detection methods have not yet achieved the same level of sensitivity to slow flow and perfusion detection as CEUS and cannot evaluate contrast agent arrival and washout kinetics. However, there has been a noticeable and clinically relevant improvement in sensitivity for detection of slow flow versus traditional color or power Doppler imaging.
Historically, color and power Doppler have been the workhorses in viewing noncontrast-enhanced microvascular flow, and they rely on reconstructing images using direction (for color Doppler) or magnitude (for power Doppler) of the Doppler shift within the received ultrasound echo (12). Advanced Doppler techniques are now commercially available on many high-end US systems (eg, Superb Microvascular Imaging SMI, Canon Medical; Slow Flow, Siemens Healthineers; Microvascular Imaging MVI, GE Healthcare; MicroFlow Imaging, Philips; and MV-flow, Samsung) (13). Whereas the exact algorithms behind these modes vary and are proprietary, all rely on a combination of approaches to reduce noise while improving sensitivity to slower flow Doppler signals. These continually evolving approaches include flash and motion suppression and other artifact reduction techniques, and adaptive filtering approaches (such as singular value decomposition) to remove clutter (5,14). Display modes vary by manufacturer but generally mimic presentation of color or power Doppler modes, providing flow information overlayed on B-mode data using a variety of color hues. However, MVFI information can also be displayed using dual (side-by-side) imaging or as a stand-alone imaging tool to appreciate smaller vessels. It should be noted that because these approaches rely on detection of Doppler shifts, they remain angle dependent.
Diagnostic use of CEUS includes evaluation of the arterial characteristics of a lesion and the degree of flow in a vessel or organ. In this review article, we detail the ability of MVFI to alleviate the use of CEUS in multiple organ and vascular clinical applications.
Clinical Applications of US MVFI
Liver
US is the most common diagnostic modality in detection and assessment of various liver conditions (7). The liver receives a unique dual supply from both the hepatic artery and portal vein, with vascular assessment typically by color Doppler imaging (15). CEUS is commonly used in assessment of hepatic vessels and focal liver lesions. The unique dual blood supply of the liver renders unique CEUS characteristics for focal liver lesions, observed in real time and captured in a cine clip. The arterial pattern is often paramount to the diagnosis. If the arterial pattern within a liver lesion can be reliably detected by using noncontrast microvascular flow techniques, CEUS imaging may not be necessary.
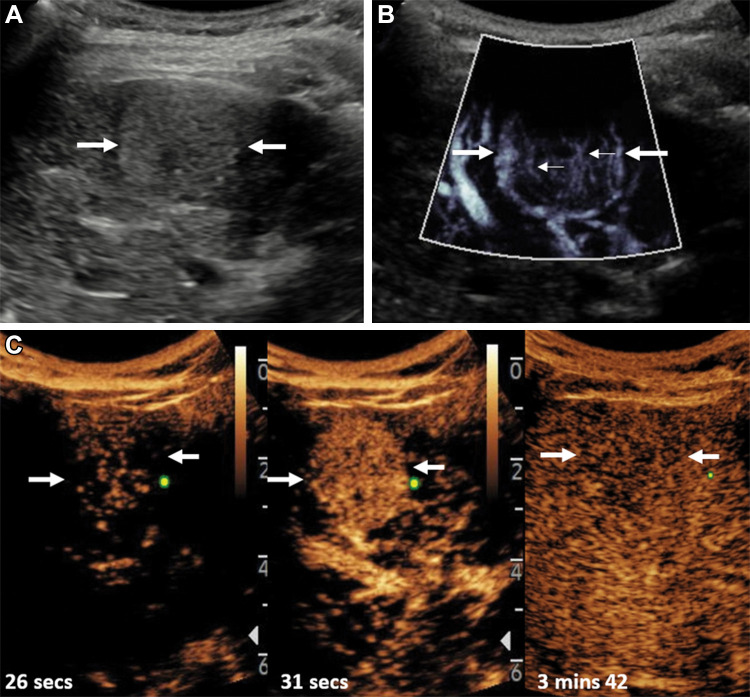
Hepatocellular carcinoma is the most common primary liver cancer and one of the most common causes of mortality in cirrhosis. US is used to screen patients with a high risk of developing hepatocellular carcinoma, with a decrease in mortality rates demonstrated with screening programs (16). The diagnosis and treatment of hepatocellular carcinoma is highly dependent on contrast-enhanced imaging, including US, CT, and MRI by using the American College of Radiology Liver Imaging and Radiology Assessment Data System, with CEUS equivalent in its diagnostic accuracy to CT and MRI. At noncontrast-enhanced MVFI, tumor vascularity, graded from absent (grade 0) to more than 50% of the lesion (grade 4), was shown in 58% of patients compared with 14% with color and power Doppler imaging (17); however, there was limited evaluation in lesions greater than 4 cm in depth. Other studies corroborated this finding by depicting increased microvessel density of hepatic lesions with MVFI compared with conventional US imaging, yielding improved depiction of some features associated with hepatocellular carcinoma (18–20) before assessment at contrast-enhanced imaging (Fig 1). Factors such as patient body habitus and motion may limit applicability of this technique.
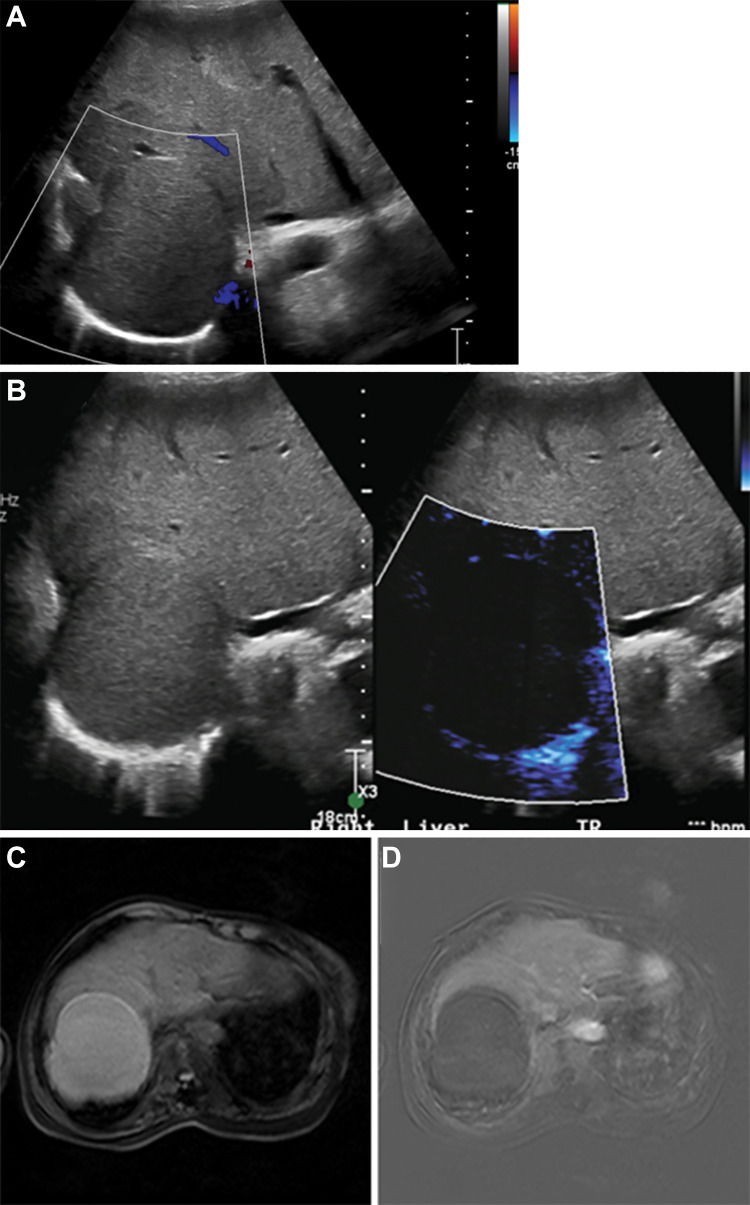
Figure 1:
Images of hepatocellular carcinoma in a 16-year-old girl with cryptogenic cirrhosis who was found to have a focal liver lesion increasing in size on serial US images. (A, B) Longitudinal gray-scale image through the right liver lobe. (A) On baseline US image, the lesion (arrows) was slightly hyperechoic with a hypoechoic halo. (B) Microvascular flow imaging showed hypertrophic peripheral vessels (thick arrows) with abnormal central vessels in a haphazard distribution (thin arrows). (C) Contrast-enhanced US of the focal liver lesion (arrows) showed early arterial enhancement at 26 seconds after administration of contrast agent, with more avid arterial enhancement at 31 seconds and subsequent late washout at 3 minutes 42 seconds.
MVFI has shown improved value in assessment of nonhepatocellular carcinoma tumors. Hemangioma is the most common benign tumor of the liver, often incidentally detected at US, CT, or MRI. At US, hemangiomas typically appear as echogenic lesions without definite color or power Doppler flow. At CEUS, hemangiomas show discontinuous peripheral nodular enhancement with gradual fill-in. It is estimated that up to 90% of hemangiomas have a nodular rim or spotty dot-like vascular pattern with perfusion imaging (21–23) (Fig 2).
Figure 2:
Longitudinal images through the left liver lobe of hemangioma in a 39-year-old man referred for investigation of microscopic hematuria, noted to have an incidental solitary liver lesion at US. (A) B-mode scan showed an isoechoic lesion (long arrows) with an eccentric hypoechoic component (short arrow). (B) At microvascular flow imaging, there were peripheral globular vessels observed in the periphery (arrows) of the focal liver lesion. (C) A contrast-enhanced US examination depicted globular enhancement at 18 seconds (short arrows), centripetal filling of the lesion (long arrows), with incomplete filling attributed to either a thrombus or scar and hyalinization (short arrow) at 39 seconds of contrast agent administration.
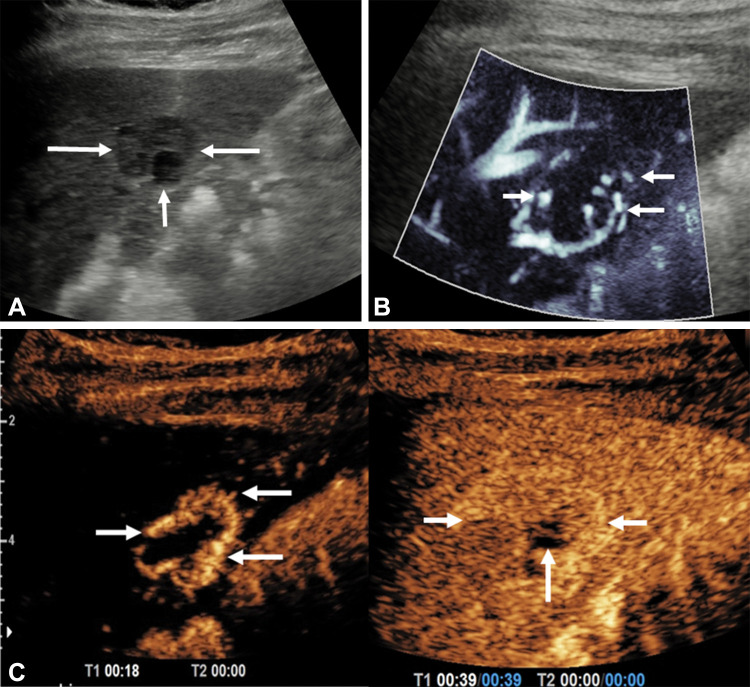
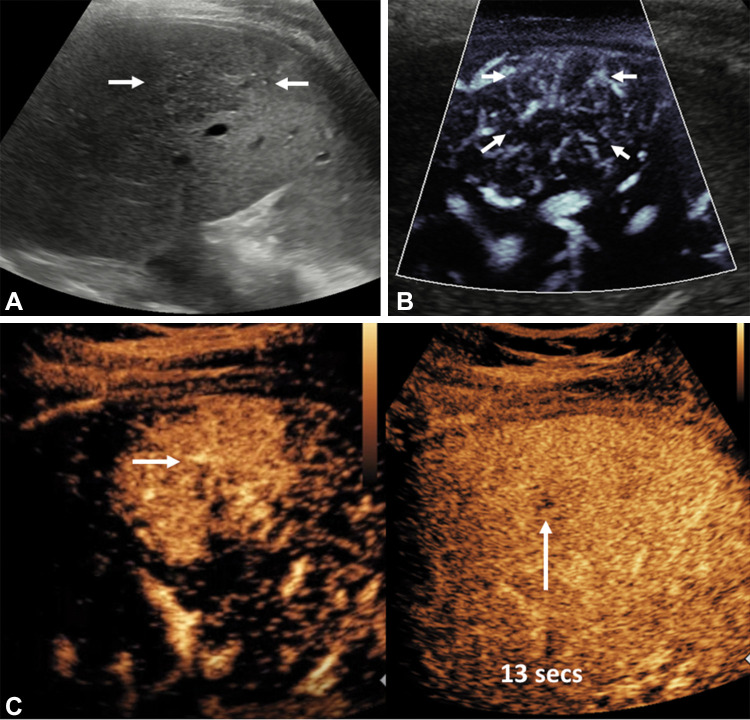
Focal nodular hyperplasia is the second most common hepatic benign neoplasm, with a spoke-wheel pattern of vascularity identified in the majority of patients by using perfusion techniques (23,24). This spoke-wheel pattern is a consequence of lobulated nodules divided by radiating scar tissue extending to the periphery (25) (Fig 3). Other focal liver lesions (hepatic adenomas, cholangiocarcinoma, and hepatic lymphoma) have also been characterized with a varying degree of success (18,19,23,26).
Figure 3:
Longitudinal image through the right liver lobe in a 14-year-old girl with a background fatty liver and an incidental focal liver lesion. (A) B-mode US showed a focal liver lesion (arrows) of low reflectivity compared with the background liver parenchyma, measuring 58 × 47 × 36 mm. (B) Microvascular flow image of the lesion showed a so-called spoke wheel distribution of vessels (arrows). (C) Contrast-enhanced US performed at the arterial phase confirmed this pattern of spoke wheel vascularization (horizontal arrow), with central vessels at 13 seconds after contrast agent injection and hyperenhancement of the lesion (vertical arrow) at 38 seconds after contrast agent administration. The lesion had complete fill-in and no washout, with no observed central scar. A subsequent MRI scan verified the findings at US of a focal nodular hyperplasia (not shown).
MVFI serves as an additional, useful tool for gray-scale, color, and spectral Doppler evaluation of an incidentally detected liver lesion, improving diagnostic confidence (Fig 4). Anecdotally, we frequently use MVFI clinically to aid in finding and assessing slow flow versus thrombus in the liver vasculature, particularly in patients with cirrhosis and the posttransplant liver, reducing the need for supplementary imaging with CT or MRI (Fig 5). MVFI may be useful for tumor thrombus detection if it shows vascularity within the clot, which is then confirmed by showing arterial flow on pulsed Doppler. However, there is lack of data establishing efficacy of MVFI against the more established contrast-enhanced imaging techniques, emphasizing the need for careful application and result interpretation.
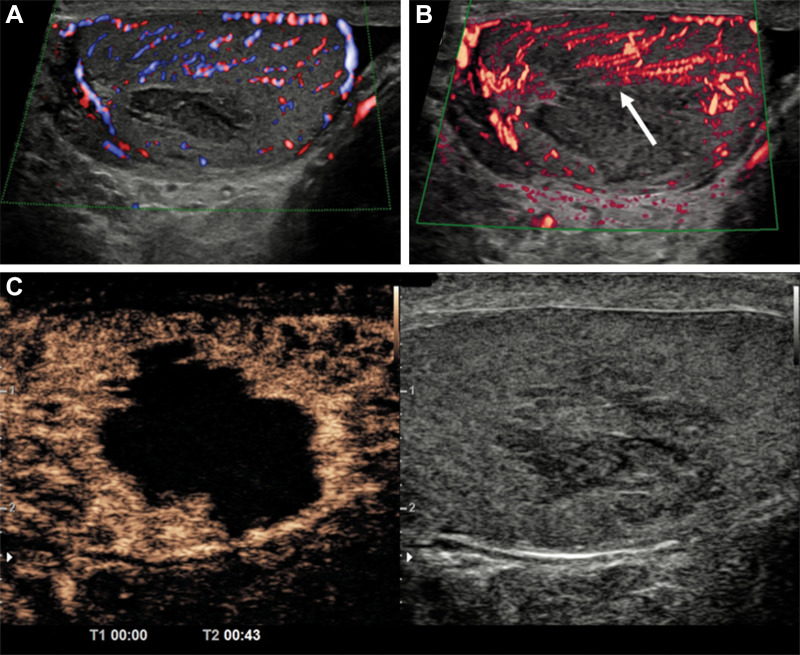
Figure 4:
Hemorrhagic hepatic cyst in a 55-year-old man with hepatic mass. (A) Gray-scale image with color Doppler showed a solid mass with isoechoic echotexture in the liver without color flow at Doppler. (B) Corresponding microvascular flow imaging (MVFI) did not show any internal vascularity; however, some of the posterior aspects were considered too deep for definitive characterization. (C) The lesion was hyperintense at contrast-enhanced T1-weighted MRI. (D) No enhancement was identified on the subtraction image, confirming the assessment at MVFI.
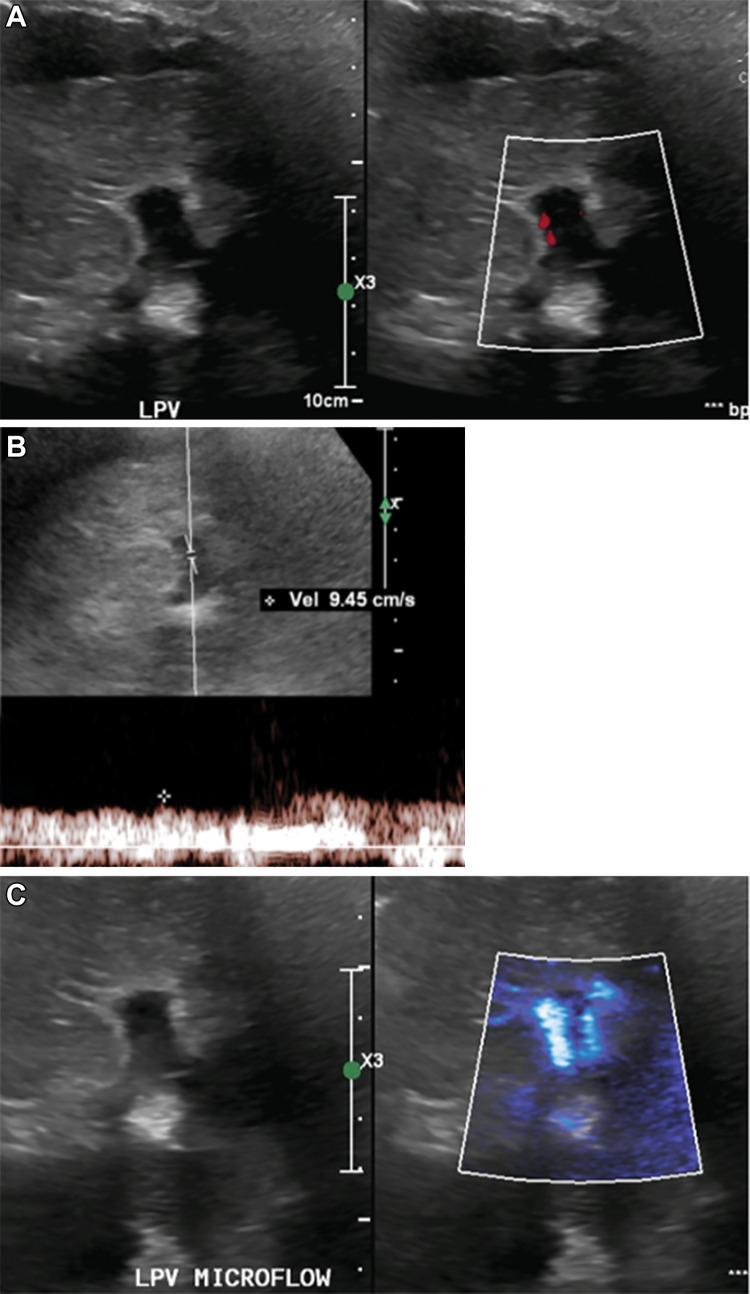
Figure 5:
(A) Gray-scale and color Doppler US images in an adult patient with elevated liver function tests without clinically significant color flow within the left portal vein (LPV). (B) Spectral Doppler image showed slow velocity flow in the left portal vein. (C) Microvascular flow imaging depicted internal vascularity, which confirmed patency and obviated further investigations.
Gallbladder
US is the initial diagnostic modality used for evaluation of the gallbladder and right upper quadrant pain (27). Gallbladder inflammation is a common cause of abdominal pain with serious sequelae such as gallbladder perforation, overlapping with simple acute inflammation, and potentially leads to diagnostic uncertainty, increased morbidity, and mortality (28,29). Distention and thickening of the gallbladder wall and surrounding pericholecystic fluid may be observed with acute cholecystitis and perforation. The application of MVFI to the gallbladder wall in inflammation has been shown to improve detection of blood flow compared with conventional color and power Doppler US (30). Similar findings were observed in patients suspected of having cholecystitis, yielding increased confidence in the diagnosis of perforation, which was confirmed at surgery or interventional radiology (31). We use MVFI routinely in all patients who are suspected of having gallbladder perforation to help view wall microvascular flow and to increase confidence in diagnosing or excluding gallbladder perforation (Fig 6).
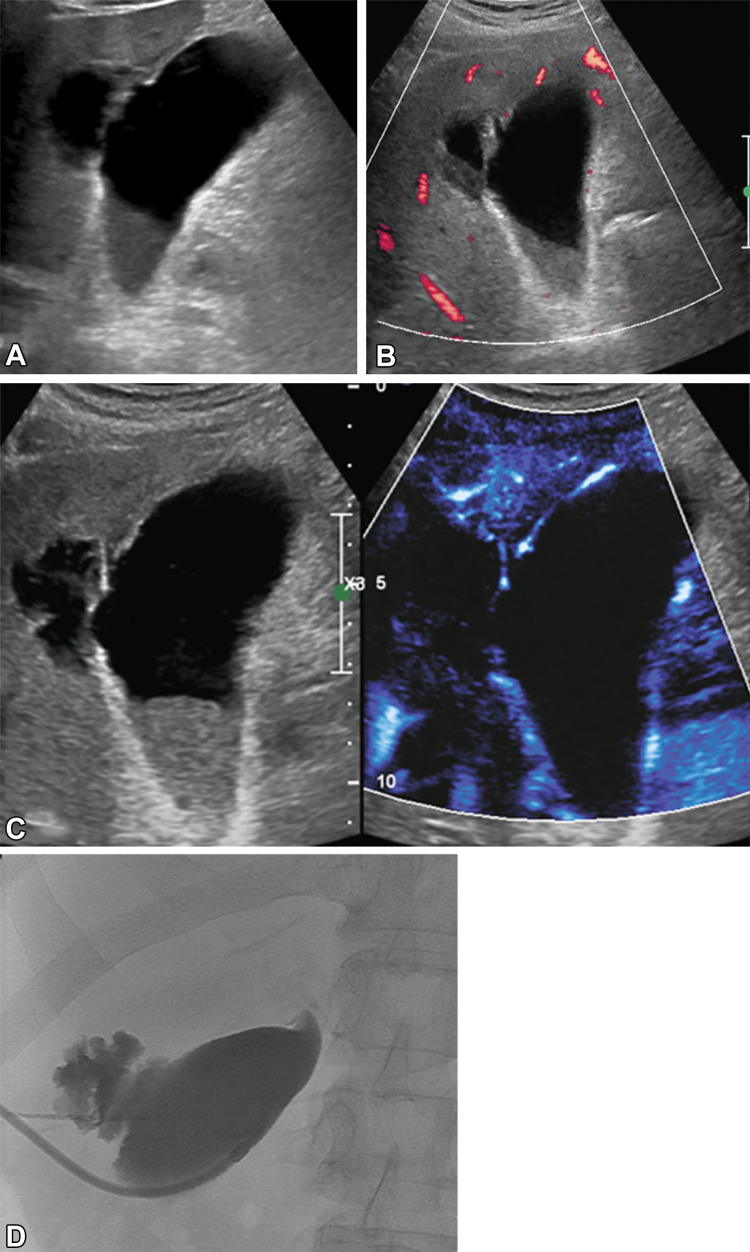
Figure 6:
(A) Gray-scale US image shows irregular gallbladder wall in a 65-year-old female patient with upper abdominal pain. There is a fluid collection in the liver adjacent to the gallbladder, as seen. (B) Power Doppler mode shows only limited vascularity in the most superficial portion of the gallbladder wall. (C) Microvascular flow image and gray-scale image depicted diffuse vascularity in the gallbladder wall, without definite blood flow in the irregular portion of the gallbladder wall of concern. This increased confidence in diagnosis of gallbladder wall perforation, with communication with the adjacent intrahepatic collection. (D) Spot image from cholecystostomy tube placement confirmed focal perforation of the gallbladder with contrast leak into the adjacent collection.
Gallbladder cancer is an aggressive and fatal malignancy; diagnosis results in curative resection and the 3-year survival rate of less than 20% (32,33). MVFI showed efficacy in evaluation of gallbladder lesions with assessment of vascularity pattern (eg, morphologic structure, branching, and caliber change), showing higher presence of tortuous microvessels or abrupt caliber change in malignancy (34). US performance was higher with contrast agent administration, increasing accuracy from 65% to approximately 84%, particularly with lesions resulting in wall thickening (35). This finding is contrasted with lack of flow at MVFI in tumefactive sludge, an example of MVFI application avoiding need for further testing (Fig 7). The specular reflection artifact from gallstones that can be observed at MVFI can be differentiated from blood flow because artifacts will not have motion, whereas blood vessels will show pulsation.
Figure 7:
Images in a 67-year-old man who was positive for COVID-19 and who presented with fever and abdominal pain. (A) Gray-scale US image showed a large isoechoic mass in the gallbladder lumen, and gallbladder tumor was in the differential. (B) Limited color Doppler was observed in the gallbladder wall; therefore, confidence was low that absence of flow in the gallbladder mass indicated sludge versus tumor. (C) Increased depiction of vascularity of the gallbladder wall and adjacent liver on microvascular flow image with no internal vascularity in the gallbladder mass improved confidence in a diagnosis of tumefactive sludge. (D) Follow-up CT confirmed lack of contrast-enhanced mass in the gallbladder.
Kidney
Benign and malignant solid and cystic renal lesions are common. US is routinely the initial diagnostic tool in renal imaging and in further assessment of indeterminate renal masses incidentally found at CT and MRI. Some patients may have a degree of acutely reduced renal function or may develop contrast agent–induced renal injury, thereby limiting intravenous nephrotoxic contrast agent administration (36). The available US contrast agents do not have a renal excretion phase and are not nephrotoxic; this is ideal for patients with renal impairment. The ability to use MVFI in evaluation at the initial US examination would be faster than placing an intravenous line and administering a contrast agent.
MVFI has been used in evaluation of accuracy of renal cystic lesion classification. It performs better than color Doppler alone and may be a viable tool in more accurate differentiation of benign and malignant cystic renal masses (37,38). Renal cysts frequently have septations at US examination, not observed at CT or MRI and attributable to the higher spatial resolution of US. Reliable depiction at US of blood flow in solid and cystic lesions with thicker septations or nodules could help stratify renal lesions that require further assessment at contrast-enhanced CT, MRI, or US (Fig 8).
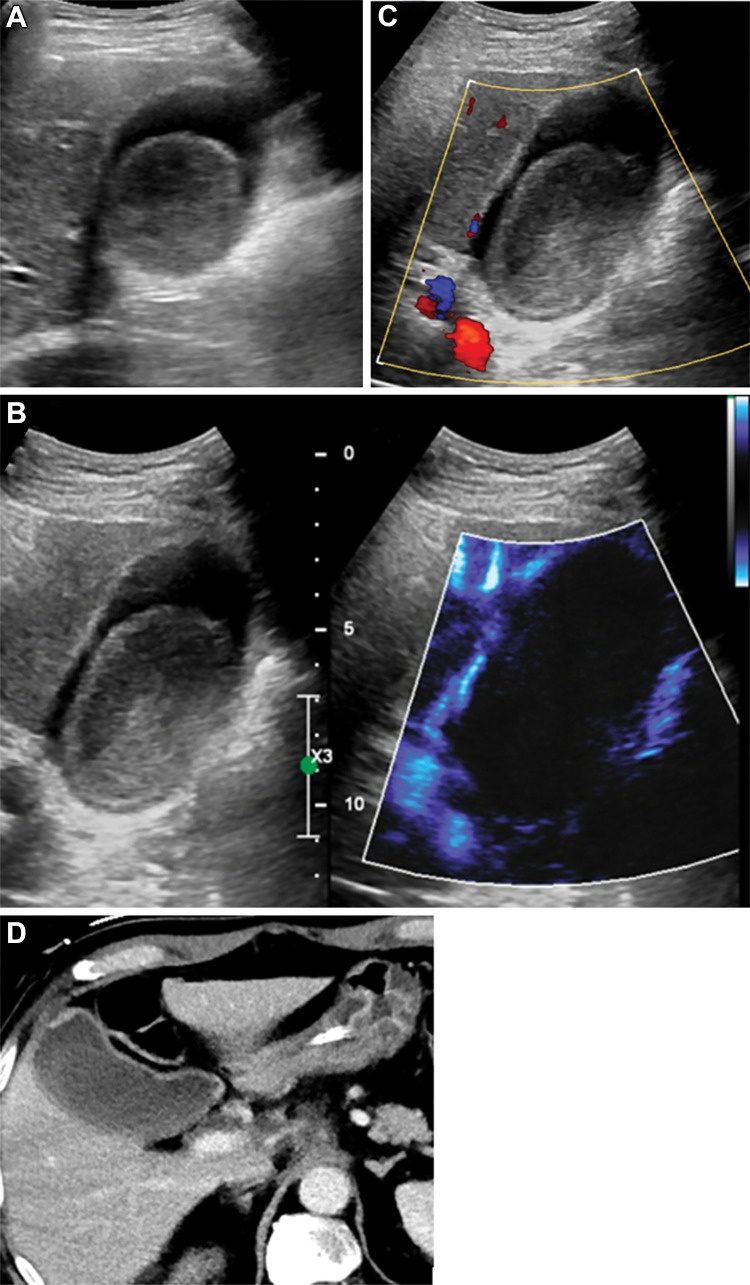
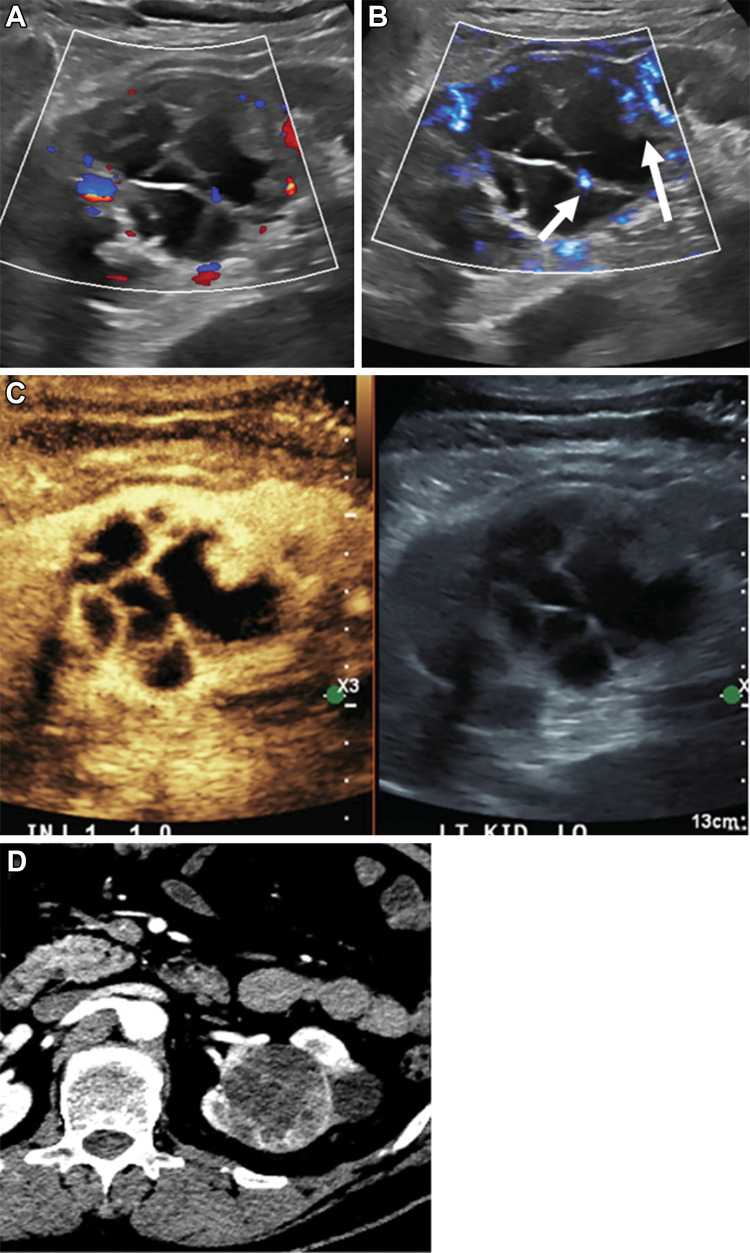
Figure 8:
Incidentally detected renal cystic lesion in a 53-year-old male patient (Bosniak type IV). (A) A mixed cystic and solid lesion with little internal vascularity on color Doppler images. (B) At microvascular flow imaging, greater discrete internal vascularity (arrows) was observed within the solid component of the mass and septations, increasing diagnostic confidence in diagnosis of Bosniak type IV renal cell carcinoma. (C) Subsequent contrast-enhanced US imaging depicted vascular septations and mural nodules, consistent with cystic renal cell carcinoma. (D) Subsequent CT confirmed presence of cystic renal cell carcinoma.
Renal cell cancer is one of the most common malignancies in the urinary tract and is frequently an incidental finding at US. Evaluation is limited with color Doppler alone, with MVFI sensitivity approaching 82% in detection of flow in solid renal lesions compared with 42% and 47% with color and power Doppler imaging, respectively (39). MVFI is also useful in helping to differentiate between benign and malignant solid neoplasms; higher-grade vascularity is observed in malignant lesions (40,41), defined as absent (grade 0) to marked (grade 3).
Application of MVFI in renal microvascular flow in screening for small cancers in patients with prehemodialysis has been reported (42). In one study (43), MVFI showed improved renal cortical vascularity compared with color and power Doppler imaging. This implies potential applications for assessment of possible renal infarcts and may also have value in evaluations before and after transplant (Fig 9). For example, we frequently use MVFI to differentiate slow flow from thrombus in the central and hilar renal vessels and to evaluate global renal microvascular flow in the transplant kidney.
Figure 9:
Images in a 28-year-old man with history of trauma. (A) US Doppler image depicted a heterogeneous area in the right kidney with relative paucity of flow in the mid and lower pole. Hypoechoic area (white arrow) within infarcted zone represented laceration with hemorrhage and fluid, which was observed at CT. (B) Noncontrast microvascular flow image clearly demarcated the area without vascularity that was confirmed on the (C) subsequent contrast-enhanced CT image.
Superficial Organs
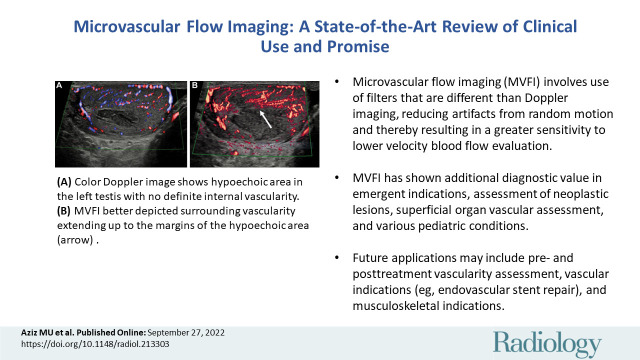
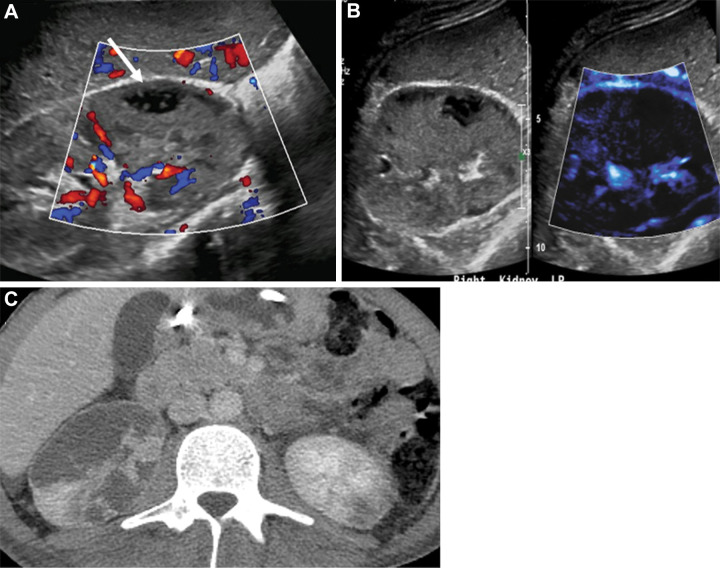
Testes.—The degree of testicular flow is clinically significant in a number of benign and malignant conditions and in emergent settings. Testicular vascularity was assessed by Durmaz and Sivri (44) comparing color and power Doppler and MVFI techniques, with MVFI performance best in depicting testicular flows and volumes in all age groups. MVFI was also helpful in evaluating reduced microcirculation in testes with associated varicocele (45). We found the imaging technique clinically useful in improving diagnostic confidence of testicular infarction (Fig 10).
Figure 10:
Images in a 50-year-old man with no predisposing factors and sudden onset of pain in the left testis. (A) Color Doppler image shows hypoechoic area in the left testis with no definite internal vascularity. (B) Microvascular flow image better depicted surrounding vascularity extending up to the margins of the hypoechoic area (arrow) compared with the color Doppler image, which increased its diagnostic value. (C) Contrast-enhanced US (CEUS) showed no internal vascularity consistent with segmental infarction. The CEUS image gave the best definition of the vascularized borders. Contraction and resolution were identified over 6 months of serial US.
Lymph nodes.—Nodal evaluation at US is standard of care to differentiate between benign inflammatory versus malignant adenopathy, using both gray-scale and color Doppler findings. Vascularity is an important feature in metastatic lymph nodes, considered to have more vascularity overall, rather than only hilar vascularity observed in normal lymph nodes. Few studies have examined the diagnostic value of MVFI in differentiation between malignant and benign lymph nodes. Statistically significant improvement was observed in detecting internal vascularity in malignant or metastatic nodes compared with tuberculous lymphadenitis, with a sensitivity of approximately 89%, and in differentiation of malignant from benign nodes (sensitivity, 87%) compared with power Doppler imaging (sensitivity, 54%) in a study of 147 patients (46,47). Other studies (48,49) showed similar findings.
Papillary thyroid carcinoma usually spreads to local-regional lymph nodes, and accurate detection of these nodes is important in staging and prognosis, surgical decisions, and potential ablation dosage. In a recent study (50), MVFI showed a high specificity of nearly 95% and accuracy of 86% in correctly identifying these nodes as suspicious for metastasis on the basis of increased abnormal vascularity. Of the 80 lymph nodes categorized as indeterminate at power Doppler imaging, 34 were reclassified as suspicious for malignancy at MVFI in this study (malignancy risk, 94%). These findings point to the high performance of MVFI for these indications, which are likely to help accurately prioritize patients for further evaluation, including tissue sampling or PET/CT, although further study is needed.
Vessel Assessment for Disease
Microcirculation is one of the most important components of cardiovascular system. Microcirculatory disorders are associated with a higher mortality despite an otherwise optimized larger vessel flow (51,52). Therefore, a well-regulated and well-functioning microcirculatory system may be associated with improved outcomes. Assessment of perfusion is limited with conventional modalities like catheter angiography, CT, MRI, and conventional US techniques. MVFI may be useful in multiple different indications such as carotid plaque vascularity and characterization of postoperative complications. We use MVFI clinically to depict slow flow and to differentiate slow flow from thrombus in the peripheral upper and lower extremity vessels, abdomen pelvis vasculature and organs, and superficial soft-tissue masses.
Carotid artery plaques commonly result in arterial embolization, with plaques that are prone to rupture containing microvessels from the vasa vasorum of the adventitia. This finding is not observed in a stable calcified plaque. Recent studies showed excellent views of neovessels in noncalcified carotid plaques, confirmed in histologic specimens (53), with a specificity of 100% compared with contrast-enhanced US (54). Another study showed correlation of microvascularity at MVFI with histologic neovascularity, inflammation, and plaque heterogeneity, which indicates plaque vulnerability (55). Carotid endarterectomy has been associated with intracranial microembolism during and after the procedure. At MVFI, flow within the plaque was manifest in 94% of patients with microemboli compared with 57% of patients without microemboli (56), again indicating value of MVFI in helping to predict adverse events during and after the procedure.
Other inflammatory etiologic causes such as large vessel vasculitis demonstrate involvement of the inner third of the media in inflammation, with wall vascularity helping to diagnose active inflammation. MVFI was used to depict microvessels extending into the media, consistent with active Takayasu arteritis, which resolved within 6 months of steroid use (57). This was confirmed by further work that showed a 100% specificity and 97% sensitivity of MVFI compared with fluorodeoxyglucose PET uptake (53), albeit in a small group of patients.
Pediatric Applications
Previously, microvascular flow US has been applied to a variety of pediatric indications (58–62), and authors described its usefulness in helping to detect and delineate microvascular anatomy of the neonatal brain (63), microvascular flow of undescended testes and ovarian pathologic abnormalities as an indicator of tissue viability (64,65), increased vascularity in thyroiditis and hyperthyroidism (66), and areas of hypoperfusion in acute pyelonephritis (67). Furthermore, MVFI may have an expanding role in depicting normal and high-grade vesicoureteral reflux with the advantage of avoiding invasive catheter procedures, as shown in a study that used MVFI to sensitively help identify jets in the bladder and kidneys (68).
Because it depicts small vessels, the role of MVFI can be expanded to a broad variety of other clinical scenarios in pediatric practice; its use in the characterization of focal liver lesions was described previously in the adult population (21,69) and can be similarly applied to children. MVFI can in fact readily depict the characteristic “spoke wheel” pattern of vascular distribution in focal nodular hyperplasia, associated with a feeding vessel, and central vascular nidus (Fig 3), allowing for a confident diagnosis without the need for US contrast agent administration. Likewise, detection of the typical and predominantly peripheral globular vascularity can help differentiate between hemangiomas (Fig 2) and malignant liver lesions, such as hepatoblastomas or hepatocellular carcinomas, where neoangiogenesis results in haphazard distribution of vessels within the lesion (Fig 1) but a CEUS examination would still be required to document the so-called washout typical of malignancy.
Another promising new application for MVFI is follow-up for solid organ injury because it can depict small arterial pseudoaneurysms, either as initial screening before US contrast agent administration, or even as a stand-alone technique. In the event a pseudoaneurysm is detected and managed conservatively, MVFI could prove to be an effective, safe, and inexpensive modality that can be performed at regular intervals to ensure complete resolution and/or self-thrombosis of the pseudoaneurysm (Fig 11). An additional benefit is that image degradation resulting in limited evaluation because of lesion depth is rarely an issue in pediatric imaging, and therefore there may be fewer challenges in use of MVFI, particularly in the abdomen and pelvis.
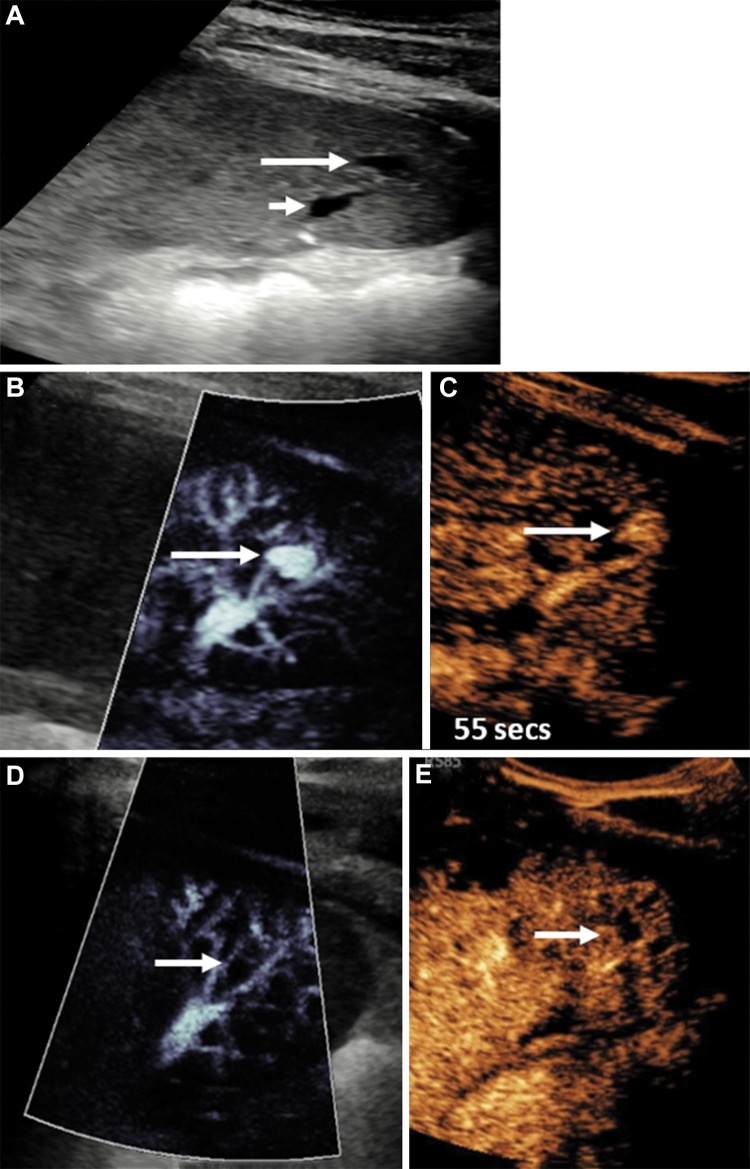
Figure 11:
Longitudinal US image in the spleen in a 14-year-old boy who fell on his elbow while playing soccer, sustaining a grade 4 splenic laceration. (A) Day 5 postinjury US image showed a small anechoic lesion within the splenic fracture plane (long arrow) adjacent to splenic vessels (short arrow). (B) Microvascular flow image showed a pseudoaneurysm of the splenic artery (arrow), confirmed on a (C) contrast-enhanced US (CEUS) image (arrow) at 55 seconds after contrast agent administration. A follow-up examination 3 days later, with both microvascular flow imaging (D) and CEUS (E), showed that the pseudoaneurysm (arrows in D and E) had spontaneously thrombosed without any intervention.
Future Directions and Potential Applications
MVFI is a technique that was developed relatively recently. Its use is small but growing. Difficulties in adoption are similar to the introduction of power Doppler in the 1990s (70), which also does not provide directional information. However, in our experience and with the case examples provided, MVFI is a valuable addition to color Doppler imaging, likely reducing or eliminating the need for power Doppler imaging. The choice of power Doppler or MVFI use after color Doppler to improve sensitivity in slower flow detection may be based on scanner capability and patient factors such as depth. If present and if clinically indicated, patients can be triaged to receive further dedicated imaging such as in the case of equivocal renal lesions.
Considering the ability of MVFI in detection and characterization of benign and malignant lesions in different organ systems as described, MVFI may also be useful in assessing treatment response. Preliminary studies have evaluated increased vascularity in endometrial and prostate cancer (71,72). MVFI may be of use in determining residual versus recurrent disease in pre- and posttreatment (eg, transplant, ablation) vascularity assessment (73), although CEUS, CT, and MRI are likely needed for follow-up imaging (74–76).
Other potential applications may include evaluation of complications from vascular interventions such as endovascular stent repair. MVFI was used in assessing postprocedural complications from previous aortic endovascular stent repair in a study by Cantisani et al (77) along with CT angiography, CEUS, and conventional US. In detecting endoleaks, MVFI showed an improved sensitivity of 75% versus 63% and specificity of 98% versus 96%, respectively, compared with color Doppler (78). We use MVFI routinely to assess aortic endostents when an endoleak is not observed on color and power Doppler images, although CEUS is still helpful in determining the origin of the leak.
Limitations
MVFI is expected to be a relatively cost-effective method due to potential avoidance of additional investigations including contrast studies in selected cases. Whereas emerging evidence supports its use in viewing microvascularity, there is a paucity of literature with histopathologic confirmation. Moreover, to our knowledge, there are currently no guidelines or recommendations for optimal classification of various vascularity patterns. MVFI is unable to provide directional flow and quantitative assessment; however, quantitative imaging is possible using contrast perfusion imaging, although with differing values based on equipment and manufacturer. MVFI is also limited in deeper organs, and it can be clinically difficult to assess whether lack of flow detection is because of depth limitations or decreased perfusion, requiring further investigation. Similarly, because of signal amplification that uses many adaptive filtering approaches, artifacts from specular reflectors or unsuppressed motion may create the appearance of flow, particularly at deeper imaging. The key to differentiation of actual flow versus artifact is to observe whether there is pulsation detected while observing the area, similar to what would be observed with blood flow. This technique also requires dedicated software and/or upgrades that may not be available on all transducers and in all centers. Additionally, like all US techniques, MVFI is also affected by motion, especially from breathing and cardiac pulsatility.
Although we have tried to consolidate MVFI techniques under one umbrella, each manufacturer takes slightly different approaches to data collection, processing, and display. There is a dearth of head-to-head comparisons between manufacturers to show that the performance of these techniques is comparable between systems because techniques are rapidly evolving.
Conclusion
Color and power Doppler imaging and contrast-enhanced US (CEUS) imaging have become increasingly important in the complete sonographic depiction of disease in vessels and organs. The development of increasingly sensitive microvascular flow imaging US techniques extends the ability of noncontrast-enhanced US to help confidently diagnose and exclude pathologic abnormalities, and it may obviate further CEUS, contrast-enhanced CT, and contrast-enhanced MRI. Further research and experience will inform future applications, including differentiation of benign and malignant conditions, rapid vessel vascularity assessment, and pre- and posttreatment indications.
Acknowledgments
Acknowledgments
We thank Dr Brian Fowlkes and Dr Paul Carson from the University of Michigan for their input.
J.R.E. supported by the National Institutes of Health.
Disclosures of conflicts of interest: M.U.A. No relevant relationships. J.R.E. Royalties from Elsevier; reviewer honorarium from NIH; support for meeting attendance from International Contrast Ultrasound Society; data safety monitoring board at Thomas Jefferson University; chair of High Frequency and Preclinical US Community at AIUM; receipt of equipment and grant support from GE Healthcare, Siemens Healthcare, Canon, Lantheus Medical Imaging. A.D. No relevant relationships. M.Z. No relevant relationships. K.S. No relevant relationships. P.S. Equipment loan from Samsung; consulting fees from ITREAS; speaker fees from Siemens Heathcare, Bracco, Samsung, Philips Healthcare; treasurer of WFUMB; member of Radiology editorial board. M.L.R. New equipment evaluation and contract from Philips Ultrasound; payment or honoraria from Philips Ultrasound; member of Radiology editorial board.
Abbreviations:
- CEUS
- contrast-enhanced US
- MVFI
- microvascular flow imaging
References
- 1. Martinoli C , Derchi LE , Rizzatto G , Solbiati L . Power Doppler sonography: general principles, clinical applications, and future prospects . Eur Radiol 1998. ; 8 ( 7 ): 1224 – 1235 . [DOI] [PubMed] [Google Scholar]
- 2. Cosgrove D , Lassau N . Imaging of perfusion using ultrasound . Eur J Nucl Med Mol Imaging 2010. ; 37 ( Suppl 1 ): S65 – S85 . [DOI] [PubMed] [Google Scholar]
- 3. Evans DH , Jensen JA , Nielsen MB . Ultrasonic colour Doppler imaging . Interface Focus 2011. ; 1 ( 4 ): 490 – 502 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ma Y , Li G , Li J , Ren WD . The Diagnostic Value of Superb Microvascular Imaging (SMI) in Detecting Blood Flow Signals of Breast Lesions: A Preliminary Study Comparing SMI to Color Doppler Flow Imaging . Medicine (Baltimore) 2015. ; 94 ( 36 ): e1502 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tierney J , Walsh K , Griffith H , Baker J , Brown DB , Byram B . Combining Slow Flow Techniques With Adaptive Demodulation for Improved Perfusion Ultrasound Imaging Without Contrast . IEEE Trans Ultrason Ferroelectr Freq Control 2019. ; 66 ( 5 ): 834 – 848 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fu Z , Zhang J , Lu Y , et al . Clinical Applications of Superb Microvascular Imaging in the Superficial Tissues and Organs: A Systematic Review . Acad Radiol 2021. ; 28 ( 5 ): 694 – 703 . [DOI] [PubMed] [Google Scholar]
- 7. Postema M , Gilja OH . Contrast-enhanced and targeted ultrasound . World J Gastroenterol 2011. ; 17 ( 1 ): 28 – 41 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sboros V , Tang MX . The assessment of microvascular flow and tissue perfusion using ultrasound imaging . Proc Inst Mech Eng H 2010. ; 224 ( 2 ): 273 – 290 . [DOI] [PubMed] [Google Scholar]
- 9. Guo YF , Li FH , Xie SW , Xia JG , Fang H , Li HL . Value of contrast-enhanced sonographic micro flow imaging for prostate cancer detection with t-PSA level of 4-10 ng/mL . Eur J Radiol 2012. ; 81 ( 11 ): 3067 – 3071 . [DOI] [PubMed] [Google Scholar]
- 10. Lyshchik A . Speciality Imaging: Fundamentals of CEUS . Philadelphia, Pa: : Elsevier; , 2019. . [Google Scholar]
- 11. Eisenbrey JR , Forsberg F . Contrast-enhanced ultrasound for molecular imaging of angiogenesis . Eur J Nucl Med Mol Imaging 2010. ; 37 ( Suppl 1 ): S138 – S146 . [DOI] [PubMed] [Google Scholar]
- 12. Szabo T . Diagnostic ultrasound imaging: inside out . 2nd ed. Amsterdam, the Netherlands: : Elsevier/Academic Press; , 2014. ; 122 – 160 . [Google Scholar]
- 13. Malho AS , Ximenes R , Ferri A , Bravo-Valenzuela NJ , Araujo Júnior E . MV-Flow and LumiFlow: new Doppler tools for the visualization of fetal blood vessels . Radiol Bras 2021. ; 54 ( 4 ): 277 – 278 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tierney J , Coolbaugh C , Towse T , Byram B . Adaptive Clutter Demodulation for Non-Contrast Ultrasound Perfusion Imaging . IEEE Trans Med Imaging 2017. ; 36 ( 9 ): 1979 – 1991 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tanaka S , Kitamura T , Fujita M , Nakanishi K , Okuda S . Color Doppler flow imaging of liver tumors . AJR Am J Roentgenol 1990. ; 154 ( 3 ): 509 – 514 . [DOI] [PubMed] [Google Scholar]
- 16. Zhang BH , Yang BH , Tang ZY . Randomized controlled trial of screening for hepatocellular carcinoma . J Cancer Res Clin Oncol 2004. ; 130 ( 7 ): 417 – 422 . [DOI] [PubMed] [Google Scholar]
- 17. Bae JS , Lee JM , Jeon SK , Jang S . Comparison of MicroFlow Imaging with color and power Doppler imaging for detecting and characterizing blood flow signals in hepatocellular carcinoma . Ultrasonography 2020. ; 39 ( 1 ): 85 – 93 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han H , Ji Z , Ding H , Zhang W , Zhang R , Wang W . Assessment of blood flow in the hepatic tumors using non-contrast micro flow imaging: Initial experience . Clin Hemorheol Microcirc 2019. ; 73 ( 2 ): 307 – 316 . [DOI] [PubMed] [Google Scholar]
- 19. Han H , Ding H , Ji Z , Zhang W , Wang Q , Wang W . Primary Application of Micro-Flow Imaging Technology in the Diagnosis of Hepatic Tumors . Ultrasound Med Biol 2019. ; 45 ( 2 ): 395 – 401 . [DOI] [PubMed] [Google Scholar]
- 20. Li W , Wang W , Liu GJ , et al . Differentiation of Atypical Hepatocellular Carcinoma from Focal Nodular Hyperplasia: Diagnostic Performance of Contrast-enhanced US and Microflow Imaging . Radiology 2015. ; 275 ( 3 ): 870 – 879 . [DOI] [PubMed] [Google Scholar]
- 21. Lee DH , Lee JY , Han JK . Superb microvascular imaging technology for ultrasound examinations: Initial experiences for hepatic tumors . Eur J Radiol 2016. ; 85 ( 11 ): 2090 – 2095 . [DOI] [PubMed] [Google Scholar]
- 22. Jeon SK , Lee JY . Superb microvascular imaging technology of ultrasound examinations for hepatic hemangiomas: A preliminary result . Ultrasound Med Biol 2019. ; 45 : S97 . [Google Scholar]
- 23. Yang F , Zhao J , Liu C , et al . Superb microvascular imaging technique in depicting vascularity in focal liver lesions: more hypervascular supply patterns were depicted in hepatocellular carcinoma . Cancer Imaging 2019. ; 19 ( 1 ): 92 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bonacchi G , Becciolini M , Seghieri M . Superb microvascular imaging: a potential tool in the detection of FNH . J Ultrasound 2017. ; 20 ( 2 ): 179 – 180 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hussain SM , Terkivatan T , Zondervan PE , et al . Focal nodular hyperplasia: findings at state-of-the-art MR imaging, US, CT, and pathologic analysis . RadioGraphics 2004. ; 24 ( 1 ): 3 – 17 ; discussion 18–19 . [DOI] [PubMed] [Google Scholar]
- 26. Artul S , Nseir W , Armaly Z , Soudack M . Superb Microvascular Imaging: Added Value and Novel Applications . J Clin Imaging Sci 2017. ; 7 : 45 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Expert Panel on Gastrointestinal Imaging ; Peterson CM , McNamara MM , et al . ACR Appropriateness Criteria® Right Upper Quadrant Pain . J Am Coll Radiol 2019. ; 16 ( 5 5S ): S235 – S243 . [DOI] [PubMed] [Google Scholar]
- 28. Chiapponi C , Wirth S , Siebeck M . Acute gallbladder perforation with gallstones spillage in a cirrhotic patient . World J Emerg Surg 2010. ; 5 ( 1 ): 11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Menakuru SR , Kaman L , Behera A , Singh R , Katariya RN . Current management of gall bladder perforations . ANZ J Surg 2004. ; 74 ( 10 ): 843 – 846 . [DOI] [PubMed] [Google Scholar]
- 30. Ra JC , Lee ES , Park HJ , et al . Efficacy of Superb Microvascular Imaging for Diagnosing Acute Cholecystitis: Comparison with Conventional Ultrasonography . Ultrasound Med Biol 2018. ; 44 ( 9 ): 1968 – 1977 . [DOI] [PubMed] [Google Scholar]
- 31. Aziz MU , Robbin ML . Improved Detection of Gallbladder Perforation Using Ultrasound Small Vessel Slow Flow “Perfusion” Imaging . J Ultrasound Med 2022. ; 41 ( 2 ): 511 – 518 . [DOI] [PubMed] [Google Scholar]
- 32. D’Hondt M , Lapointe R , Benamira Z , et al . Carcinoma of the gallbladder: patterns of presentation, prognostic factors and survival rate. An 11-year single centre experience . Eur J Surg Oncol 2013. ; 39 ( 6 ): 548 – 553 . [DOI] [PubMed] [Google Scholar]
- 33. He C , Cai Z , Zhang Y , Lin X . Prognostic Model to Predict Cancer-Specific Survival for Patients With Gallbladder Carcinoma After Surgery: A Population-Based Analysis . Front Oncol 2019. ; 9 : 1329 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kin T , Nagai K , Hayashi T , Takahashi K , Katanuma A . Efficacy of superb microvascular imaging of ultrasound for diagnosis of gallbladder lesion . J Hepatobiliary Pancreat Sci 2020. ; 27 ( 12 ): 977 – 983 . [DOI] [PubMed] [Google Scholar]
- 35. Kong WT , Shen HY , Qiu YD , Han H , Wen BJ , Wu M . Application of contrast enhanced ultrasound in gallbladder lesion: is it helpful to improve the diagnostic capabilities? Med Ultrason 2018. ; 20 ( 4 ): 420 – 426 . [DOI] [PubMed] [Google Scholar]
- 36. Davenport MS , Perazella MA , Yee J , et al . Use of Intravenous Iodinated Contrast Media in Patients with Kidney Disease: Consensus Statements from the American College of Radiology and the National Kidney Foundation . Radiology 2020. ; 294 ( 3 ): 660 – 668 . [DOI] [PubMed] [Google Scholar]
- 37. Mu J , Mao Y , Li F , Xin X , Zhang S . Superb microvascular imaging is a rational choice for accurate Bosniak classification of renal cystic masses . Br J Radiol 2019. ; 92 ( 1099 ): 20181038 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leong JY , Wessner CE , Kramer MR , et al . Superb Microvascular Imaging Improves Detection of Vascularity in Indeterminate Renal Masses . J Ultrasound Med 2020. ; 39 ( 10 ): 1947 – 1955 . [DOI] [PubMed] [Google Scholar]
- 39. Chen M , Fu X , Shen Y . Evaluation of Multimode Color Doppler Flow Imaging in the Diagnosis of Solid Renal Tumor . Contrast Media Mol Imaging 2021. ; 2021 : 6656877 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mao Y , Mu J , Zhao J , Zhao L , Xin X . The value of superb microvascular imaging in differentiating benign renal mass from malignant renal tumor: a retrospective study . Br J Radiol 2018. ; 91 ( 1082 ): 20170601 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hwang SI , Lee HJ , Ahn HW , Kim YG . Comparison of ultrasonographic MicroFlow Imaging with contrast enhanced CT in the evaluation of renal mass . Ultrasound Med Biol 2019. ; 45 : S41 . [Google Scholar]
- 42. Barr RG , Wilson SR , Lyshchik A , et al . Contrast-Enhanced Ultrasound: State of the Art in North America . Ultrasound Q 2020. ; 36 ( 3 ): 206 – 217 . [DOI] [PubMed] [Google Scholar]
- 43. Gao J , Thai A , Erpelding T . Comparison of superb microvascular imaging to conventional color Doppler ultrasonography in depicting renal cortical microvasculature . Clin Imaging 2019. ; 58 : 90 – 95 . [DOI] [PubMed] [Google Scholar]
- 44. Durmaz MS , Sivri M . Comparison of superb micro-vascular imaging (SMI) and conventional Doppler imaging techniques for evaluating testicular blood flow . J Med Ultrason (2001) 2018. ; 45 ( 3 ): 443 – 452 . [DOI] [PubMed] [Google Scholar]
- 45. Ates F , Durmaz MS , Sara HI , Kara T . Comparison of testicular vascularity via superb microvascular imaging in varicocele patients with contralateral normal testis and healthy volunteers . J Ultrasound 2022. ; 25 ( 1 ): 59 – 65 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sim JK , Lee JY , Hong HS . Differentiation Between Malignant and Benign Lymph Nodes: Role of Superb Microvascular Imaging in the Evaluation of Cervical Lymph Nodes . J Ultrasound Med 2019. ; 38 ( 11 ): 3025 – 3036 . [DOI] [PubMed] [Google Scholar]
- 47. Ryoo I , Suh S , You SH , Seol HY . Usefulness of Microvascular Ultrasonography in Differentiating Metastatic Lymphadenopathy from Tuberculous Lymphadenitis . Ultrasound Med Biol 2016. ; 42 ( 9 ): 2189 – 2195 . [DOI] [PubMed] [Google Scholar]
- 48. Shen MJ , Chen HW , Bi K , Zhang Y , Cong Y , Wang Y . Application value of superb micro-vascular imaging in classification of cervical tuberculous lymphadenitis . Chin J Antituberc 2019. ; 41 ( 8 ): 816 – 821 . [Google Scholar]
- 49. Lei Y , Jiang H , Tang H , Wu C . Application of superb microvascular imaging in differential diagnosis of cervical lymph node lesions . Chin J Med Imaging Technol 2016. ; 32 ( 5 ): 655 – 658 . [Google Scholar]
- 50. Lee S , Lee JY , Yoon RG , Kim JH , Hong HS . The Value of Microvascular Imaging for Triaging Indeterminate Cervical Lymph Nodes in Patients with Papillary Thyroid Carcinoma . Cancers (Basel) 2020. ; 12 ( 10 ): 2839 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. De Backer D , Donadello K , Sakr Y , et al . Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome . Crit Care Med 2013. ; 41 ( 3 ): 791 – 799 . [DOI] [PubMed] [Google Scholar]
- 52. Sakr Y , Dubois MJ , De Backer D , Creteur J , Vincent JL . Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock . Crit Care Med 2004. ; 32 ( 9 ): 1825 – 1831 . [DOI] [PubMed] [Google Scholar]
- 53. Sato W , Suto Y , Yamanaka T , Watanabe H . An advanced ultrasound application used to assess peripheral vascular diseases: superb microvascular imaging . J Echocardiogr 2021. ; 19 ( 3 ): 150 – 157 . [DOI] [PubMed] [Google Scholar]
- 54. Oura K , Kato T , Ohba H , Terayama Y . Evaluation of Intraplaque Neovascularization Using Superb Microvascular Imaging and Contrast-Enhanced Ultrasonography . J Stroke Cerebrovasc Dis 2018. ; 27 ( 9 ): 2348 – 2353 . [DOI] [PubMed] [Google Scholar]
- 55. Zamani M , Skagen K , Scott H , Lindberg B , Russell D , Skjelland M . Carotid Plaque Neovascularization Detected With Superb Microvascular Imaging Ultrasound Without Using Contrast Media . Stroke 2019. ; 50 ( 11 ): 3121 – 3127 . [DOI] [PubMed] [Google Scholar]
- 56. Chiba T , Fujiwara S , Oura K , et al . Superb Microvascular Imaging Ultrasound for Cervical Carotid Artery Stenosis for Prediction of the Development of Microembolic Signals on Transcranial Doppler during Carotid Exposure in Endarterectomy . Cerebrovasc Dis Extra 2021. ; 11 ( 2 ): 61 – 68 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sato W , Sato T , Iino T , Seki K , Watanabe H . Visualization of arterial wall vascularization using superb microvascular imaging in active-stage Takayasu arteritis . Eur Heart J Cardiovasc Imaging 2019. ; 20 ( 6 ): 719 . [DOI] [PubMed] [Google Scholar]
- 58. Jacob J , Deganello A , Sellars ME , Hadzic N , Sidhu PS . Contrast enhanced ultrasound (CEUS) characterization of grey-scale sonographic indeterminate focal liver lesions in pediatric practice . Ultraschall Med 2013. ; 34 ( 6 ): 529 – 540 . [DOI] [PubMed] [Google Scholar]
- 59. Fang C , Bernardo S , Sellars ME , Deganello A , Sidhu PS . Contrast-enhanced ultrasound in the diagnosis of pediatric focal nodular hyperplasia and hepatic adenoma: interobserver reliability . Pediatr Radiol 2019. ; 49 ( 1 ): 82 – 90 . [DOI] [PubMed] [Google Scholar]
- 60. Rafailidis V , Deganello A , Watson T , Sidhu PS , Sellars ME . Enhancing the role of paediatric ultrasound with microbubbles: a review of intravenous applications . Br J Radiol 2017. ; 90 ( 1069 ): 20160556 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sidhu PS , Cantisani V , Deganello A , et al . Role of Contrast-Enhanced Ultrasound (CEUS) in Paediatric Practice: An EFSUMB Position Statement . Ultraschall Med 2017. ; 38 ( 1 ): 33 – 43 . [DOI] [PubMed] [Google Scholar]
- 62. Dietrich CF , Augustiniene R , Batko T , et al . European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB): An Update on the Pediatric CEUS Registry on Behalf of the “EFSUMB Pediatric CEUS Registry Working Group” . Ultraschall Med 2021. ; 42 ( 3 ): 270 – 277 . [DOI] [PubMed] [Google Scholar]
- 63. Goeral K , Hojreh A , Kasprian G , et al . Microvessel ultrasound of neonatal brain parenchyma: feasibility, reproducibility, and normal imaging features by superb microvascular imaging (SMI) . Eur Radiol 2019. ; 29 ( 4 ): 2127 – 2136 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lee YS , Kim MJ , Han SW , et al . Superb microvascular imaging for the detection of parenchymal perfusion in normal and undescended testes in young children . Eur J Radiol 2016. ; 85 ( 3 ): 649 – 656 . [DOI] [PubMed] [Google Scholar]
- 65. Ayaz E , Aslan A , İnan İ , Yıkılmaz A . Evaluation of Ovarian Vascularity in Children by Using the “Superb Microvascular Imaging” Ultrasound Technique in Comparison With Conventional Doppler Ultrasound Techniques . J Ultrasound Med 2019. ; 38 ( 10 ): 2751 – 2760 . [DOI] [PubMed] [Google Scholar]
- 66. Bayramoglu Z , Kandemirli SG , Caliskan E , et al . Assessment of paediatric Hashimoto’s thyroiditis using superb microvascular imaging . Clin Radiol 2018. ; 73 ( 12 ): 1059.e9 – 1059.e15 . [DOI] [PubMed] [Google Scholar]
- 67. Yoo J , Je BK , Choo JY . Ultrasonographic Demonstration of the Tissue Microvasculature in Children: Microvascular Ultrasonography Versus Conventional Color Doppler Ultrasonography . Korean J Radiol 2020. ; 21 ( 2 ): 146 – 158 [Published correction appears in Korean J Radiol 2020;21(4):509.] . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kim HK , O’Hara S , Je BK , Kraus SJ , Horn P . Feasibility of superb microvascular imaging to detect high-grade vesicoureteral reflux in children with urinary tract infection . Eur Radiol 2018. ; 28 ( 1 ): 66 – 73 . [DOI] [PubMed] [Google Scholar]
- 69. He MN , Lv K , Jiang YX , Jiang TA . Application of superb microvascular imaging in focal liver lesions . World J Gastroenterol 2017. ; 23 ( 43 ): 7765 – 7775 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rubin JM , Bude RO , Carson PL , Bree RL , Adler RS . Power Doppler US: a potentially useful alternative to mean frequency-based color Doppler US . Radiology 1994. ; 190 ( 3 ): 853 – 856 . [DOI] [PubMed] [Google Scholar]
- 71. Shen TT , Xue JL . Impact of a novel ultrasound microvascular imaging and elastography on prostate cancer classification . Transl Androl Urol 2019. ; 8 ( 6 ): 696 – 702 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xie SW , Li FH , Li HL , et al . Value of contrast-enhanced sonography with micro flow imaging in the diagnosis of prostate cancer . J Clin Ultrasound 2011. ; 39 ( 7 ): 371 – 377 . [DOI] [PubMed] [Google Scholar]
- 73. Tokodai K , Miyagi S , Nakanishi C , et al . The utility of superb microvascular imaging for monitoring low-velocity venous flow following pancreas transplantation: report of a case . J Med Ultrason (2001) 2018. ; 45 ( 1 ): 171 – 174 . [DOI] [PubMed] [Google Scholar]
- 74. Lekht I , Gulati M , Nayyar M , et al . Role of contrast-enhanced ultrasound (CEUS) in evaluation of thermal ablation zone . Abdom Radiol (NY) 2016. ; 41 ( 8 ): 1511 – 1521 . [DOI] [PubMed] [Google Scholar]
- 75. Lekht I , Nayyar M , Luu B , et al . Intra-arterial contrast-enhanced ultrasound (IA CEUS) for localization of hepatocellular carcinoma (HCC) supply during transarterial chemoembolization (TACE): a case series . Abdom Radiol (NY) 2017. ; 42 ( 5 ): 1400 – 1407 . [DOI] [PubMed] [Google Scholar]
- 76. Coutinho C , Werner H , Lopes FP , Zelaquett M , Marchiori E , Araujo E . Cutting-edge application of ultrasound elastography and superb microvascular imaging in radiofrequency ablation of uterine fibroids . J Ultrason 2021. ; 21 ( 84 ): 80 – 81 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cantisani V , David E , Ferrari D , et al . Color Doppler Ultrasound with Superb Microvascular Imaging Compared to Contrast-enhanced Ultrasound and Computed Tomography Angiography to Identify and Classify Endoleaks in Patients Undergoing EVAR . Ann Vasc Surg 2017. ; 40 : 136 – 145 . [DOI] [PubMed] [Google Scholar]
- 78. Gabriel M , Tomczak J , Snoch-Ziółkiewicz M , et al . Superb Micro-vascular Imaging (SMI): a Doppler ultrasound technique with potential to identify, classify, and follow up endoleaks in patients after Endovascular Aneurysm Repair (EVAR) . Abdom Radiol (NY) 2018. ; 43 ( 12 ): 3479 – 3486 . [DOI] [PMC free article] [PubMed] [Google Scholar]