Abstract
Background
Patient-specific instrumentation (PSI) has been suggested to reduce improper component positioning, though the effectiveness of PSI in total hip arthroplasty (THA) remains inconclusive. The purpose of this study was to evaluate the radiographic parameters and clinical outcomes comparing PSI and standard instrumentation (SI).
Methods
This systematic review and meta-analysis was conducted in accordance with the 2020 PRISMA statement and was registered on PROSPERO. PubMed, Embase, Scopus, Google Scholar, and ClinicalTrials.gov were searched for relevant studies pertaining to the use of PSI in THA. Inclusion criteria included PSI used in THA, PSI was directly compared to SI, and publication in English. Exclusion criteria included non-primary THA, review articles, abstracts, book chapters, and animal models.
Results
2,458 studies were initially identified, with 13 studies (677 THAs: 338 controls, 339 PSI) meeting all criteria. PSI was favored for the deviation from the preoperative plan for acetabular cup position for anteversion (p = 0.04) and inclination (p = 0.0002); risk of acetabular cup positioning outside the Lewinnek safe zone for anteversion (p = 0.005) and inclination (p < 0.0001); and postoperative Harris Hip Score (p = 0.0002). No significant differences were found for the deviation from the preoperative plan for femoral stem position for anteversion (p = 0.74) or varus/valgus (p = 0.15); intraoperative time (p = 0.55); or intraoperative blood loss (p = 0.62).
Conclusion
The use of PSI in THA is effective in improving acetabular component positioning and postoperative functional outcomes, without increasing intraoperative time or blood loss, compared to SI.
Keywords: Total hip arthroplasty (THA), Patient specific instrumentation (PSI), Joint arthroplasty, Acetabular cup position, Lewinnek safe zone
1. Introduction
Total hip arthroplasty (THA) has been one of the most frequently performed orthopaedic procedures in the United States with an estimated 2.5 million Americans currently living with a prosthetic hip.1,2 As the population of the United States continues to age, the rates of THA are expected to grow, highlighting the importance of effective management of these procedures.1,3,4 Although there have been many improvements to preoperative planning, surgical techniques, and rehabilitation, acetabular loosening due to malposition of the prosthesis is still reported in many patients.3,5,6 Furthermore, upwards of 3% of THA patients dislocate their new hip within one year.7
Improper component positioning has been a major surgical factor attributed to acetabular loosening and dislocation.8 This has prompted increased interest in the use of patient-specific instrumentation (PSI) and other innovative technologies in the preoperative and intraoperative planning of THA.9, 10, 11 The role of the PSI is to help guide the physical positioning of the components during hip arthroplasty.9,12 PSI uses a preoperative CT or MRI to create a 3D patient model that can then be used to make a patient-specific guide for prosthesis placement.13,14 It has been reported that the implementation of PSI during THA has increased surgical accuracy of component positioning leading to improved patient outcomes.15 However, current literature on the topic has been limited to small cohort studies and qualitative synthesis.15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 Due to the small sample sizes, these studies could be underpowered to detect differences between PSI and SI, necessitating a quantitative synthesis of the currently available literature. As the incidence of THA continues to rise28 so does the importance of evaluating the efficacy of PSI versus standard instrumentation in THA.
The primary aim of this systematic review and meta-analysis was to determine whether there is a significant improvement in prosthesis positioning during THA with the use of PSI compared to SI. Secondary measures included functional outcomes, intraoperative blood loss, intraoperative time, cost of PSI, and time necessary to obtain PSI.
2. Methods
2.1. Protocol registration
The Cochrane Handbook for Systematic Reviews of Interventions29 and the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement30 were used as guides to conduct this systematic review and meta-analysis. The study was registered with PROSPERO (ID number: CRD42022323011).
2.2. Search strategy
An electronic query was initiated in April 2022 using Pubmed, Embase, Scopus, and Google Scholar. ClinicalTrials.gov was additionally used to aid in the identification of pertinent ongoing clinical trials. The goal of the query was to discover all studies which compared the use of PSI and SI in total hip arthroplasty. The exact search query can be found in Appendix A. The query included search terms such as total hip arthroplasty OR hip arthroplasty OR total hip replacement OR hip replacement AND patient specific OR custom made OR custom fit OR guide OR three-dimensional.
2.3. Eligibility criteria
The PICO model was followed: P – patients who had undergone total hip arthroplasty; I – patient-specific instrumentation; C – standard instrumentation; O - acetabular cup position, femoral stem position, clinical outcomes, intraoperative effects, cost-effectiveness. Inclusion criteria for the articles included PSI used to assist with THA and a direct comparison to a SI control group. One or more of the outcomes chosen for analysis was also required to have been present. Outcomes analyzed included absolute mean deviation from the preoperative plan for the acetabulum in anteversion and inclination, the number of cups positioned outside the Lewinnek safe zone, intraoperative time, intraoperative blood loss, Harris Hip Score, and absolute mean deviation from the preoperative plan for the femoral stem in anteversion and varus/valgus. Lastly, publication in the English language was required. Studies that used PSI on living patients, cadavers, and physical models were included. Both randomized controlled trials and non-randomized studies, including non-randomized controlled trials and prospective and retrospective cohort studies, were included. Exclusion criteria for the articles used were non-primary THA, any joints other than the hip, review articles, abstracts without full manuscripts available, book chapters, and animal models. Studies in which necessary data could not be obtained after attempting to contact the authors were also excluded from this analysis.
2.4. Screening and data extraction
All articles were imported into Covidence and screened by two independent reviewers (J.C. and A.D.), with a third (J.W.) resolving all conflicts. Papers were screened against inclusion and exclusion criteria first by title and abstract and then by full text when appropriate. During the full-text review process, the references of each paper were evaluated to identify any additional relevant articles fitting the inclusion and exclusion criteria that were not discovered in the search query.
After all relevant articles were identified and screened, two independent reviewers (J.C. and J.W.) performed data extraction using a standardized form. Once again, all conflicts were resolved by a third reviewer (A.D.). Extracted data included publication information, study characteristics, patient demographics, surgical techniques, imaging and software used for preoperative planning and postoperative evaluation, PSI design and costs, measurements of acetabular and femoral component placement accuracy, Harris Hip Score, intraoperative times, intraoperative blood loss, and number of complications or revisions required.
2.5. Risk of bias assessment
The risk of bias was conducted in accordance with the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions.29 Risk of bias was assessed for each article by two independent reviewers (J.C. and J.W.), with a third reviewer (A.D.) resolving conflicts. Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2)31 was used to evaluate randomized studies, and the Risk Of Bias In Non-Randomized Studies – of Interventions (ROBINS-I) tool32 was used to evaluate non-randomized studies including non-randomized control trials and cohort studies.
2.6. Statistical analysis
The statistical analysis was conducted in accordance with the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions.29 Meta-analysis was performed using ReviewManager (version 5.4.1, The Cochrane Collaboration, 2020) to compare the PSI and SI groups with regard to the primary and secondary outcomes. The primary outcomes of this analysis were absolute mean deviation from the preoperative surgical plan of acetabular cup position measured in the postoperative angles of anteversion and inclination (both in degrees) and the accuracy of acetabular cup position measured by the number of cups positioned outside the Lewinnek safe zone for anteversion and inclination.33 The secondary outcomes of this analysis were intraoperative time (in minutes), intraoperative blood loss (in milliliters), Harris Hip Score, and absolute mean deviation from the preoperative surgical plan of femoral stem position measured in the postoperative angles of anteversion and varus/valgus (both in degrees). Data were summarized as mean difference or relative risk. For included studies that were missing standard deviations, it was calculated from the reported p-values. For other relevant missing data, the authors of this study contacted the corresponding authors of the study in question for additional information.
Due to anticipated heterogeneity of the included studies, random-effects models were used. The I2 statistic was used to measure study heterogeneity. The primary and secondary outcomes were reported with 95% confidence intervals and values were compiled in forest plots. The authors intended to investigate publication bias by performing tests for funnel plot asymmetry in accordance with Cochrane guidelines. Sensitivity analysis was performed to evaluate the impact of the effects model (fixed versus random) and the impact of imputed values for missing data. Statistical significance was detected at p < 0.05.
2.7. GRADE assessment of evidence
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was utilized by two independent reviewers (A.D. and J.W.) to assess quality of evidence. A third reviewer (J.C.) resolved all conflicts. Data from RevMan was imported into the GRADEpro web tool (GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime, 2022) to create a “Summary of findings” (SoF) table, which includes information on the overall quality of evidence for each outcome measured in the study.
3. Results
3.1. Study selection
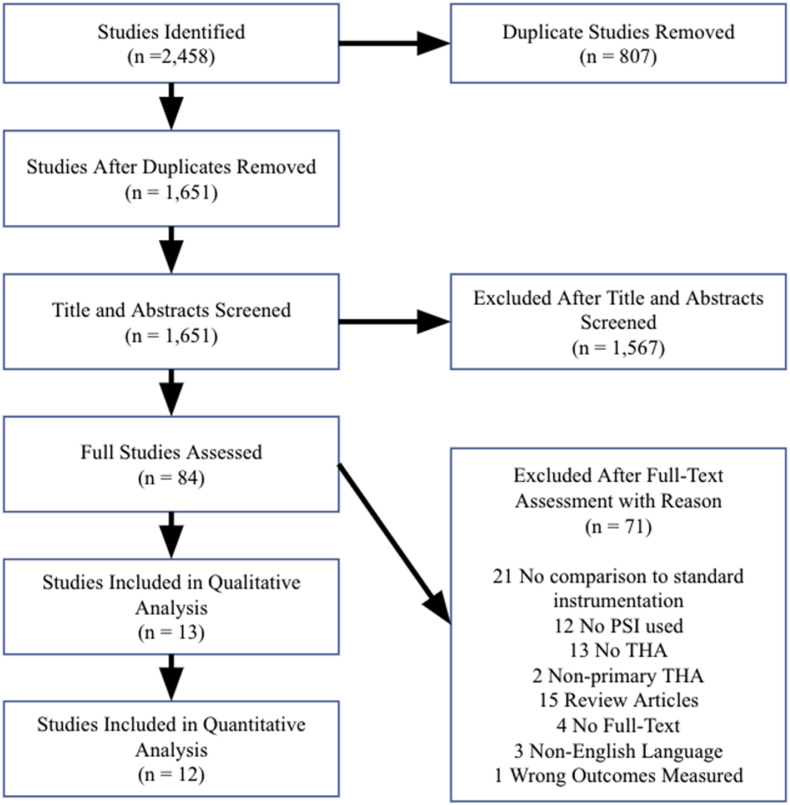
The search resulted in 2,458 studies, of which 2,445 were excluded. 807 duplicate papers were removed, 1,567 papers were removed after title and abstract screening, and 71 papers were removed after full-text screening. Of the 71 papers removed after full-text screening, 21 were removed due to a lack of comparison between PSI and SI, 12 were removed due to a lack of PSI used, 13 were removed due to a lack of THA, 2 were removed due to the inclusion of non-primary THA, 15 were removed as they were review articles, 4 were removed due to availability of only abstracts without full-text manuscripts, 3 were removed due to non-English language, and 1 was removed due to no reported outcomes measured in this study. A summary of the literature review is displayed in a PRISMA flowchart (Fig. 1).
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart with explanations for the removal and exclusion of studies.
3.2. Study characteristics
13 papers with a total of 677 THAs (338 controls and 339 PSI) met all inclusion and exclusion criteria.15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 7 of the 13 papers included were randomized control trials (RCTs). The remaining 6 studies included 3 non-randomized control trials and 3 cohort studies. Out of the 13 included studies, 12 were able to be included in the quantitative analysis.15, 16, 17, 18, 19, 20,22, 23, 24, 25, 26, 27 Further study characteristics are displayed in Appendices B.1 and B.2. Indications for surgery included osteoarthritis, femoral neck fracture, osteonecrosis of the femoral head, rheumatoid arthritis, and developmental dysplasia of the hip.
3.3. Accuracy of PSI for acetabular cup position
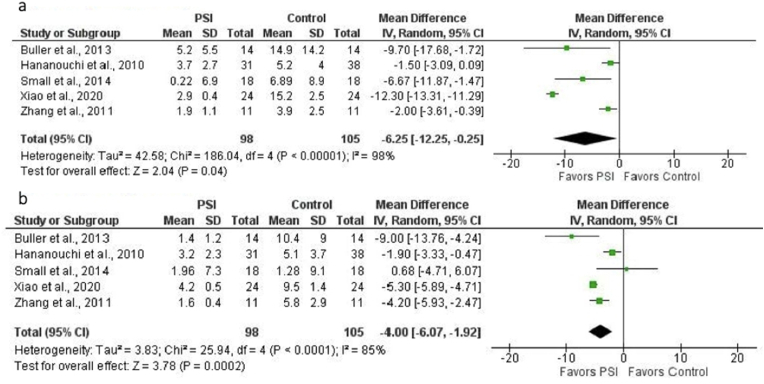
Five studies (203 THAs: 105 controls, 98 PSI) could be included in the statistical analysis of the comparison of absolute mean deviation from the preoperative surgical plan of acetabular cup position with regard to anteversion and inclination in PSI and SI (control) groups.15,16,19,24,27 The mean difference favored PSI for both anteversion (−6.25° [95% CI, −12.25° to −0.25°]; p = 0.04) (Fig. 2a) and inclination (−4.00° [95% CI, −6.07° to −1.92°]; p = 0.0002) (Fig. 2b). There was considerable heterogeneity among studies for both anteversion (I2 = 98%; p < 0.00001) and inclination (I2 = 85%; p < 0.0001).
Fig. 2.
a. Forest plot of the mean difference in acetabular cup anteversion error (in degrees) for PSI versus SI (control). Mean difference favored PSI (MD = −6.25, p = 0.04). There was considerable heterogeneity among studies (I2 = 98%; p < 0.00001). b. Forest plot of the mean difference in acetabular cup inclination error (in degrees) for PSI versus SI (control). Mean difference favored PSI (MD = −4.00, p = 0.0002). There was considerable heterogeneity among studies (I2 = 85%; p < 0.0001).
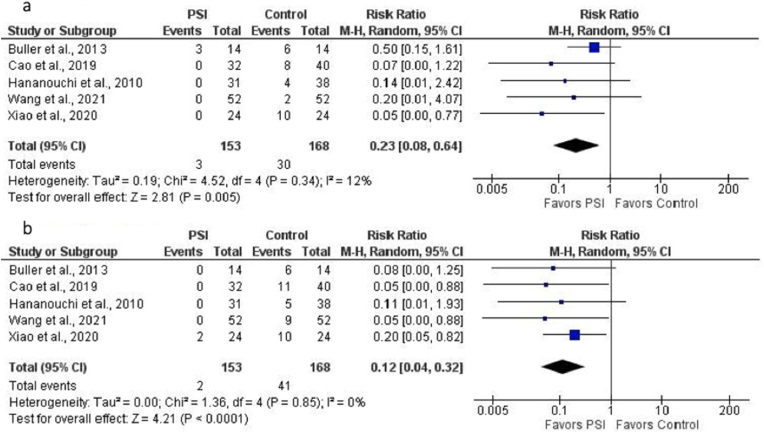
Five studies (321 THAs: 168 controls, 153 PSI) were included in the analysis of the accuracy of acetabular cup placement measured by the number of cups positioned outside the Lewinnek safe zone for anteversion and inclination.16,17,19,23,24 PSI was associated with a lower relative risk of acetabular cup malposition with regard to both anteversion (0.23 [95% CI, 0.08 to 0.64]; p = 0.005) (Fig. 3a) and inclination (0.12 [95% CI, 0.04 to 0.32]; p < 0.0001) (Fig. 3b).
Fig. 3.
a. Forest plot of the relative risk (RR) of acetabular cup malposition with regard to anteversion for PSI versus SI (control). Malposition was defined as cup placement outside Lewinnek safe zone for anteversion (15° ± 10°). PSI was associated with lower relative risk of malposition (RR = 0.23, p = 0.005). b. Forest plot of the relative risk (RR) of acetabular cup malposition with regard to inclination for PSI versus SI (control). Malposition was defined as cup placement outside Lewinnek safe zone for inclination (40° ± 10°). PSI was associated with lower relative risk of malposition (RR = 0.12, p < 0.0001).
3.4. Intraoperative variables
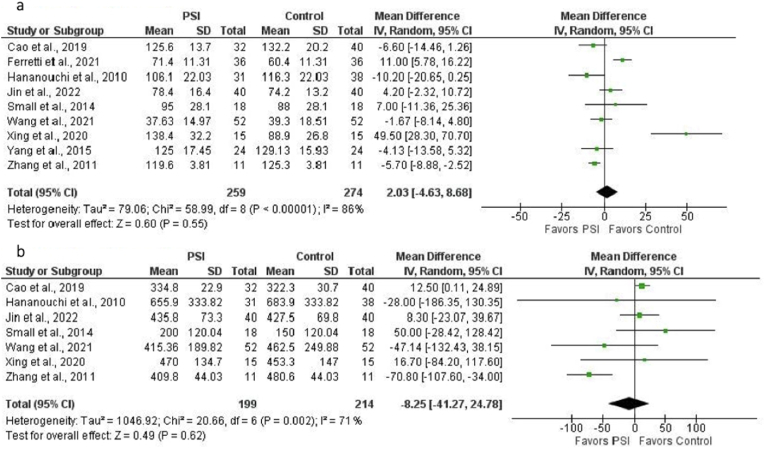
For analysis of intraoperative time, nine studies (533 THAs: 274 controls, 259 PSI) were included.15,17, 18, 19, 20,23,25, 26, 27 No significant difference was found (mean difference, 2.03 min [95% CI, −4.63 to 8.68 min]; p = 0.55) (Fig. 4a). There was considerable heterogeneity among studies (I2 = 86%; p < 0.00001). Seven studies (413 THAs: 214 controls, 199 PSI) were included for analysis of intraoperative blood loss.15,17,19,20,23,25,27 No significant difference was found (mean difference, −8.25 mL [95% CI, −41.27 to 24.78 mL]; p = 0.62) (Fig. 4b). There was substantial heterogeneity among studies (I2 = 71%; p = 0.002).
Fig. 4.
a. Forest plot of the mean difference in intraoperative time (in minutes) for PSI versus SI (control). No significant difference was found (MD = 2.03, p = 0.55). There was considerable heterogeneity among studies (I2 = 86%, p < 0.00001). b. Forest plot of the mean difference in intraoperative blood loss (in mL) for PSI versus SI (control). No significant difference was found (MD = −8.25, p = 0.62). There was substantial heterogeneity among studies (I2 = 71%, p = 0.002).
3.5. Clinical outcomes
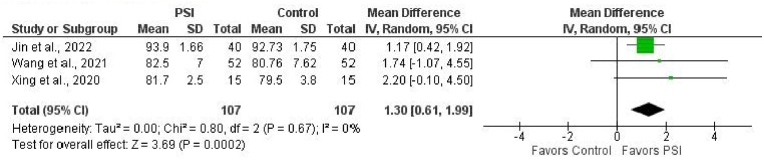
Three studies (214 THAs: 107 controls, 107 PSI) could be included in the analysis of the postoperative Harris Hip Score at 3 months follow-up.20,23,25 The mean difference favored PSI (1.30 [95% CI, 0.61 to 1.99]; p = 0.0002) (Fig. 5).
Fig. 5.
Forest plot of the mean difference in Harris Hip Score at 3-month follow-up for PSI versus SI (control). Mean difference favored PSI (MD = 1.30, p = 0.0002).
3.6. Accuracy of PSI for femoral stem position
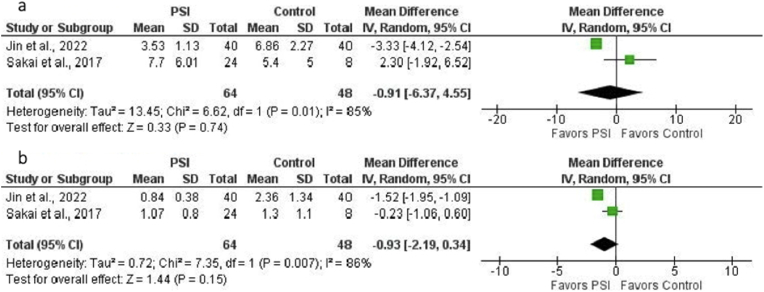
Two studies (112 THAs: 48 controls, 64 PSI) could be included in the analysis of the comparison of absolute mean deviation from the preoperative plan of femoral stem position with regard to anteversion and varus/valgus in PSI and SI (control) groups.20,22 No significant difference was found for anteversion (mean difference, −0.91° [95% CI, −6.37°–4.55°]; p = 0.74) (Fig. 6a) or varus/valgus (mean difference, −0.93° [95% CI, −2.19°–0.34°]; p = 0.15) (Fig. 6b). There was considerable heterogeneity among studies for both anteversion (I2 = 85%; p = 0.01) and varus/valgus (I2 = 86%; p = 0.007).
Fig. 6.
a. Forest plot of the mean difference in femoral stem anteversion error (in degrees) for PSI versus SI (control). No significant difference was found (MD = −0.91, p = 0.74). There was considerable heterogeneity among studies (I2 = 85%, p = 0.01). b. Forest plot of the mean difference in femoral stem varus/valgus error (in degrees) for PSI versus SI (control). No significant difference was found (MD = −0.93, p = 0.15). There was considerable heterogeneity among studies (I2 = 86%, p = 0.007).
3.7. Sensitivity analysis
The statistical analyses were repeated with fixed-effect models (not shown). PSI was favored for femoral stem anteversion (mean difference, −3.14° [95% CI, −3.91° to −2.37°]; p < 0.00001) and varus/valgus (mean difference, −1.24° [95% CI, −1.63° to −0.86°]; p < 0.00001). The findings for all other outcomes analyzed with fixed-effects models were not substantially altered. Analysis with the exclusion of imputed data did not substantially alter the findings of the study (not shown).
3.8. Risk of bias
Regarding risk of bias, for the 7 RCTs, 3 studies were deemed “low” and 4 scored “some concerns” according to the RoB 2 methodology. All non-randomized studies scored “moderate” according to the ROBINS-I methodology. Risk of bias scores for each study are shown in Appendix B.1.
3.9. Quality of evidence
Quality of evidence was assessed using GRADE for RCTs and non-randomized studies. Evidence from this study suggests that PSI used in THA may hold benefits when compared to standard procedures, though the quality of evidence was deemed low quality. The main reasons for downgrading the quality of evidence across outcomes were risk of bias, heterogeneity, low number of events or low sample size, and imprecise 95% confidence intervals. Appendix C summarizes the GRADE and quality of evidence for outcomes assessed in the study.
4. Discussion
This systematic review and meta-analysis analyzed the effect of PSI on the accuracy of implant positioning in THA. Previous literature suggested that in THA, component positioning improves with the use of PSI,15,16,19,24,27 but large-scale analysis had yet to be done in a quantitative manner. This study demonstrated a significant increase in implant positioning accuracy with regard to both anteversion (Fig. 2a) and inclination (Fig. 2b) of the acetabular component with the use of PSI when compared to standard non-PSI controls. Increases in Harris Hip Score were also shown to be of significance (Fig. 5) indicating improved short-term clinical outcomes with use of PSI in THA.
This analysis demonstrated that overall, there was an improvement in surgical and functional outcomes across a wide variety of surgical indications; however, patients with more severe injury might especially benefit from PSI. In one included study23 adult patients with developmental dysplasia of the hip were separated into four groups based on Crowe classification (I, II, III, or IV), with the higher number indicating a more severe diagnosis.34,35 When compared to standard instrumentation in Crowe groups I and II, PSI showed no significant difference. Interestingly, when compared to standard instrumentation in Crowe groups III and IV, PSI did show a significant benefit. As previously noted, this suggests that the use of PSI could be especially useful in more difficult and complex patient cases.23
The Lewinnek safe zone of 15° ± 10° of anteversion and 40° ± 10° of inclination has been used as a target for THA, as hips located within this zone have significantly less dislocations.33 The findings from this analysis suggest that the use of PSI lowers the risk of acetabular cup placement outside of the Lewinnek safe zone when compared to standard instrumentation. Although the Lewinnek safe zone has been used historically, it has come under question in recent years36, 37, 38 and some have proposed a slightly different zone.37 With the current study's findings that PSI significantly reduces the mean absolute error in acetabular cup positioning for both anteversion and inclination when compared to standard instrumentation, even if these suggested changes to the safe zone are warranted, PSI is still superior to standard instrumentation in THA in this regard.
Intraoperative blood loss is an important consideration with regard to the use of PSI compared to standard instrumentation in THA.39,40 An increase in intraoperative blood loss could lead to a substantial increase in costs, as one unit of red blood cells has been reported to cost hospitals $210 and patients $343.41 Additionally, although relatively low, in any transfusion there is a risk of transmission of an infectious process, transfusion-related acute lung injury (TRALI), or other complications.42,43 It is relevant then, that this quantitative analysis did not show any significant differences in intraoperative blood loss between PSI and standard instrumentation in THA.
Operating room time is often scarce and extremely expensive,44 with estimates placing the cost per minute at $37.45 As a result, even small increases in intraoperative time could add up to significant cost increases.46 Importantly, this analysis showed no significant difference between PSI and standard instrumentation with regard to intraoperative time in THA. Thus, concerns regarding increases in intraoperative cost from increased intraoperative time are not warranted.
Factors which the authors were not able to quantitatively analyze due to differences in methodology and reporting included the added cost associated with the creation of the PSI, as well as the time necessary to do so. Qualitative cost analysis revealed a wide range of cost for PSI among included studies, with reports ranging from $4 – $500.21,22,27 There remains a great degree of ambiguity with respect to what was included in these reported costs. Further research is needed to effectively address whether there is a significant increase in cost, and, if so, whether improvements in implant positioning are worth the cost. Similar to the cost of PSI in THA, the details regarding the time needed to manufacture and obtain PSI were limited. One included study suggested that design of PSI takes 60–120 min, with another 90–120 min for manufacturing.19 Another included study suggested that manufacturing by a service provider could take 16 h, not including design or other logistics.27 Although there is preoperative planning associated with production of the PSI, this is not reflected by any increased time in the operating room.
Regarding the possible role of surgeon experience in the efficacy of PSI in THA, there have been studies demonstrating the efficacy of the use of PSI in THA for both inexperienced24 and experienced surgeons.15,27 One study directly compared the effect of surgeon experience on the efficacy of PSI and found no significant differences.16 These results suggest that PSI can be an important addition for surgeons of any experience level looking to increase the accuracy of their acetabular component positioning in THA.
Clinically, these results suggest that surgeons can rely on the use of PSI to yield more accurate component positioning which could be especially useful in cases with challenging anatomy. PSI may serve as an effective tool to allow surgeons to perform cases which they would otherwise be uncomfortable undertaking. As PSI serves as a tailored guide for surgeons which is unique to each patient, the authors speculate that the improvements in component positions are due to the reduction in anatomical ambiguity present. The findings of this study are important for surgeons and policymakers alike, as PSI is a lower cost solution relative to others, such as robotic-assisted surgery,47,48 to improve implant component positioning and should be considered as a viable option for those seeking to provide advancements in patient care.
4.1. Limitations
Even after a comprehensive literature review only 13 studies were identified which directly compared PSI to SI in total hip arthroplasty. While 7 of the studies included were randomized controlled trials, there were also studies with lower levels of evidence as there were 3 non-randomized controlled trials and 3 cohort studies included. The studies which did include relevant data and a direct comparison included differences in methodology, including the characteristics of the PSI, surgical approach used in the THA, and specimen type (patient, cadaveric, or sawbones model). The lack of data regarding the long-term clinical outcomes of patients and the long-term implant survival rates are also limitations of this study. In addition, several of the included studies had small sample sizes, reducing the statistical power of the analysis of outcomes. Studies which were not available in English were also excluded from this study, potentially creating a selection bias. Since all of the analyzed outcomes included fewer than 10 studies, tests for funnel plot asymmetry were not performed to assess publication bias. The small number of included studies for each outcome would have decreased the power of these tests to differentiate real asymmetry from chance, thus reducing their reliability.29,49,50 Although action, like the inclusion of a “gray” literature search, was taken to reduce publication bias, this bias cannot be ruled out entirely. Finally, there was considerable heterogeneity between studies for some of the outcomes. Thus, conclusions from this study should be made with caution.
4.2. Future research
The overall quality of evidence defined by the GRADE methodology was low. Future studies to corroborate the effects of patient-specific instrumentation in THA are warranted, as further research is very likely to have an important impact on and change the estimate of effect. Future research may also further explore the true cost of PSI, considering all preoperative time spent in the planning and development phases for each patient. Studies may additionally examine whether PSI is particularly effective in certain patient populations such as men versus women or patients with a particular BMI, as well as in certain surgical approaches. Finally, research is warranted into any potential long-term effects on functional outcomes and implant survival.
5. Conclusion
The use of patient-specific instrumentation in total hip arthroplasty is effective in improving acetabular component positioning and postoperative functional outcomes, without increasing intraoperative time or blood loss, when compared to standard instrumentation.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRedit author statement
David S. Constantinescu: conceptualization, methodology, writing – original draft, writing – review and editing, visualization, supervision, project administration. Joseph P. Costello II, Anil D. Dalling, Jaxon D. Wagner: conceptualization, methodology, validation, formal analysis, investigation, writing – original draft, writing – review and editing, visualization, supervision, project administration. Waleed Al-Hardan: writing – original draft, writing – review and editing, supervision. Jaime A. Carvajal: writing – original draft, writing – review and editing, supervision, project administration.
Declaration of competing interest
None.
Acknowledgments
The authors would like to thank Thilani Samarakoon, Ph.D. and John Reynolds, M.S. of the University of Miami Calder Library for their guidance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jor.2022.10.001.
APPENDICES.
Appendix A. Detailed Search Criteria for Each Electronic Database
PubMed
((“Arthroplasty, Replacement, Hip” [Mesh]) OR (“arthroplast∗” [All Fields] AND “replace∗" [All Fields] AND “hip"” [All Fields]) OR (“total hip arthroplast∗” [All Fields]) OR (“total” [All Fields] AND “hip” [All Fields] AND “arthroplast∗” [All Fields]) OR (“total hip replace∗” [All Fields]) OR (“hip replacement arthroplast∗” [All Fields]) OR (“hip arthroplast∗” [All Fields]) OR (“hip replace∗” [All Fields])) AND ((“Patient-Specific Modeling” [Mesh]) OR (“patient-specific instrument∗" [All Fields]) OR (“patient specific instrument∗" [All Fields]) OR (“guide" [All Fields]) OR (“implant∗" [All Fields]) OR (“3D” [All Fields]) OR (“3-D” [All Fields]) OR (“three-dimension∗” [All Fields]) OR (“three dimension∗” [All Fields]) OR (“position∗” [All Fields])) AND ((patient-specific [Title/Abstract]) OR (patient specific [Title/Abstract]) OR (custom-fit [Title/Abstract]) OR (custom fit [Title/Abstract]) OR (custom-made [Title/Abstract]) OR (custom made [Title/Abstract]) OR (patient-matched [Title/Abstract]) OR (patient matched [Title/Abstract]))
Embase
(‘arthroplast∗’ AND ‘replace∗’ AND ‘hip’ OR ‘total hip arthroplast∗’ OR (‘total’ AND ‘hip’ AND ‘arthroplast∗’) OR ‘total hip replace∗’ OR ‘replacement arthroplas∗’ OR ‘hip arthroplast∗’ OR ‘hip replace∗’) AND (‘patient-specific model∗’ OR ‘guide∗’ OR ‘implant∗’ OR ‘3d′ OR ‘3-d’ OR ‘three-dimension∗’ OR ‘three dimension∗’ OR ‘position∗’ OR ‘patient-specific instrument∗’ OR ‘patient specific instrument∗’) AND (‘patient-specific’:ti,ab OR ‘patient specific’:ti,ab OR ‘custom-fit’:ti,ab OR ‘custom fit’:ti,ab OR ‘custom made’:ti,ab OR ‘patient matched’:ti,ab).
Scopus
ALL (({total hip arthroplasty}) OR ({total hip replacement}) OR ({hip replacement arthroplasty}) OR ({hip arthroplasty}) OR {hip replacement}) AND ALL (({patient-specific modeling}) OR ({guide}) OR ({implant}) OR ({3D}) OR ({3-D}) OR ({three-dimension}) OR ({three-dimensional}) OR ({three dimension}) OR ({three dimensional}) OR ({position}) OR ({positioning}) OR ({patient-specific instrument}) OR ({patient specific instrument})) AND TITLE-ABS ((patient-specific) OR ({patient specific}) OR (custom-fit) OR ({custom fit}) OR (custom-made) OR ({custom made}) OR (patient-matched) OR ({patient matched})).
Google Scholar
TI (“ (“total hip arthroplasty” OR “total hip replacement” OR “hip replacement arthroplasty” OR “hip arthroplasty” OR “hip replacement”) AND (“patient-specific” OR “patient specific” OR “custom” OR “custom-made” OR “custom made” OR “custom fit” OR “custom-fit” OR “guide” OR “implant” OR “3D” OR “3-D″ OR “three-dimension”)”)
ClinicalTrials.gov
(“total hip arthroplasty” OR “total hip replacement” OR “hip replacement arthroplasty” OR “hip arthroplasty” OR “hip replacement”) AND (“patient-specific” OR “patient specific” OR “custom” OR “custom-made” OR “custom made” OR “custom fit” OR “custom-fit” OR “guide” OR “implant” OR “3D” OR “3-D″ OR “three-dimension” OR “three-dimensional” OR “three dimension” OR “three dimensional")
Appendix B.1. Study Characteristics and Risk of Bias Assessment
| Study | Study Design | # Control | # PSI | Patient, Cadaver or Model | Surgical Approach | RoB 2 Score | ROBINS-1 Score |
|---|---|---|---|---|---|---|---|
| Small et al. (2014) ❖⍉▼ | Randomized Controlled Trial | 18 | 18 | Patient | Direct Lateral or Posterior | Low | n/a |
| Xiao et al. (2020) ❖✕ | Randomized Controlled Trial | 24 | 24 | Model | Posterolateral | Some Concerns | n/a |
| Zhang et al. (2011) ❖⍉▼ | Randomized Controlled Trial | 11 | 11 | Patient | Posterior | Low | n/a |
| Wang et al. (2021) ✕●⍉▼ | Randomized Controlled Trial | 52 | 52 | Patient | Direct Posterolateral | Some Concerns | n/a |
| Jin et al. (2022) ●⍉▼✢ | Randomized Controlled Trial | 40 | 40 | Patient | Posterolateral | Low | n/a |
| Yang et al. (2015) ⍉ | Randomized Controlled Trial | 24 | 24 | Patient | Posterolateral | Some Concerns | n/a |
| Mishra et al. (2020) △ | Randomized Controlled Trial | 18 | 18 | Patient | Posterolateral | Some Concerns | n/a |
| Sakai et al. (2017) ✢ | Non-randomized Controlled Trial | 8 | 24 | Cadaver | Anterolateral | n/a | Moderate |
| Buller et al. (2013) ❖✕ | Non-randomized Controlled Trial | 14 | 14 | Model | Not Reported | n/a | Moderate |
| Cao et al. (2019) ✕⍉▼ | Cohort | 40 | 32 | Patient | Direct Lateral | n/a | Moderate |
| Hananouchi et al. (2010) ❖✕⍉▼ | Non-randomized Controlled Trial | 38 | 31 | Patient | Modified Posterolateral | n/a | Moderate |
| Ferretti et al. (2021) ⍉ | Cohort | 36 | 36 | Patient | Direct Lateral | n/a | Moderate |
| Xing et al. (2020) ●⍉▼ | Cohort | 15 | 15 | Patient | Posterolateral | n/a | Moderate |
| ❖ = included in acetabular cup analysis | ● = included in Harris Hip Score analysis | ⍉ = included in intraoperative time analysis | △ = included only in qualitative analysis | ||||
| ✕ = included in risk of cup malposition analysis | ✢ = included in femoral stem analysis | ▼ = included in intraoperative blood loss analysis |
Includes characteristics of all papers that were included in the analysis. Characteristics include study design, number of hips in each cohort, source of hips, surgical approach, and risk of bias associated with each study.
Appendix B.2. Hip Demographics
| Control (n = 338) | PSI (n = 339) | |
|---|---|---|
| Patient Hips (n, %) | 292 (86.39%) | 277 (81.71%) |
| Model Hips (n, %) | 38 (11.24%) | 38 (11.21%) |
| Cadaveric Hips (n, %) |
8 (2.37%) |
24 (7.08%) |
| Control (n = 238) | PSI (n = 259) | |
| Sex (n, %) | ||
| Male | 76 (31.93%) | 92 (35.52%) |
| Female |
162 (68.07%) |
167 (64.47%) |
| Control (n = 238) | PSI (n = 259) | |
| Age (mean years) |
56.93 |
59.87 |
| Control (n = 136) | PSI (n = 121) | |
| BMI (mean) | 25.08 | 25.32 |
Demographic data of the hips analyzed, including hip type, sex, mean age, and BMI of patients.
Appendix C. GRADE Summary of Findings (SoF) Table
| Summary of findings: | ||||||
|---|---|---|---|---|---|---|
| The Efficacy of Patient-Specific Instrumentation (PSI) in Total Hip Arthroplasty (THA): a Systematic Review and Meta-Analysis | ||||||
|
Patient or population: Primary total hip arthroplasty patients Setting: Orthopaedic surgery Intervention: PSI Comparison: Standard instrumentation | ||||||
| Outcomes | Anticipated absolute effects∗ (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard instrumentation | Risk with PSI | |||||
| Acetabular Cup Anteversion Error | The mean acetabular Cup Anteversion Error ranged from 3.9–15.2 degrees | MD 6.25° lower(12.25 lower to 0.25 lower) | – | 203 (5 studies) | ⨁⨁◯◯ Lowa,b |
3 RCTs, 2 non-randomized studies |
| Acetabular Cup Inclination Error | The mean acetabular Cup Inclination Error ranged from 1.28–10.4 degrees | MD 4° lower(6.07 lower to 1.92 lower) | – | 203 (5 studies) | ⨁⨁◯◯ Lowb,c |
3 RCTs, 2 non-randomized studies |
| Risk of Acetabular Cup Malposition for Anteversion | 179 per 1,000 | 41 per 1,000(14 to 114) | RR 0.23(0.08 to 0.64) | 321 (5 studies) | ⨁⨁◯◯ Lowd,e |
2 RCTs, 3 non-randomized studies |
| Risk of Acetabular Cup Malposition for Inclination | 244 per 1,000 | 29 per 1,000(10 to 78) | RR 0.12(0.04 to 0.32) | 321 (5 studies) | ⨁⨁◯◯ Lowe,f |
2 RCTs, 3 non-randomized studies |
| Intraoperative Time | The mean intraoperative Time ranged from 39.3–132.2 min | MD 2.03 min higher(4.63 lower to 8.68 higher) | – | 533 (9 studies) | ⨁◯◯◯ Very lowf,g,h |
5 RCTs, 4 non-randomized studies |
| Intraoperative Blood Loss | The mean intraoperative Blood Loss ranged from 150–683.9 mL | MD 8.25 mL lower(41.27 lower to 24.78 higher) | – | 413 (7 studies) | ⨁◯◯◯ Very lowh,i,j |
4 RCTs, 3 non-randomized studies |
| Postoperative Harris Hip Score | The mean postoperative Harris Hip Score ranged from 79.5–92.73 | MD 1.3 higher(0.61 higher to 1.99 higher) | – | 214 (3 studies) | ⨁⨁⨁◯ Moderatek |
2 RCTs, 1 non-randomized study |
| Femoral Stem Anteversion Error | The mean femoral Stem Anteversion Error ranged from 5.4–6.86 degrees | MD 0.91° lower(6.37 lower to 4.55 higher) | – | 112 (2 studies) | ⨁⨁◯◯ Lowl,m |
1 RCT, 1 non-randomized study |
| Femoral Stem Varus/Valgus Error | The mean femoral Stem Varus/Valgus Error ranged from 1.3–2.36 degrees | MD 0.93° lower(2.19 lower to 0.34 higher) | – | 112 (2 studies) | ⨁⨁◯◯ Lowm,n |
1 RCT, 1 non-randomized study |
∗The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: confidence interval; MD: mean difference; RR: risk ratio.
GRADE Working Group grades of evidenceHigh certainty: we are very confident that the true effect lies close to that of the estimate of the effect.Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Explanations.
a. Downgraded one level for inconsistency: I2 = 98%; this inconsistency is not related to the direction of effect but rather to the magnitude of the effect favoring PSI.
b. Downgraded one level for imprecision: There is a wide 95% CI with wide range of benefit of PSI and low sample size.
c. Downgraded one level for inconsistency: I2 = 85%; Small et al. contributes to this inconsistency, but mostly it is not related to the direction of effect but rather to the magnitude of the effect favoring PSI.
d. Downgraded one level for risk of bias: Non-randomized studies contributed to the majority of pooled estimates. Most information is from studies with “moderate” or “some concerns” risk of bias, and limitations are likely to lower confidence in effect.
e. Downgraded one level for imprecision: Optimal information size (OIS) criteria not met.
f. Downgraded one level for risk of bias: Most information is from studies with “moderate” or “some concerns” risk of bias, and limitations are likely to lower confidence in effect.
g. Downgraded one level for inconsistency: I2 = 86%; Xing et al. is an outlier that contributed to this inconsistency. The inconsistency is related to the direction of the effect.
h. Downgraded one level for imprecision: There is a wide 95% CI that overlaps no effect.
i. Downgraded one level for risk of bias: Most information is from studies with “low” or “moderate” risk of bias, and limitations are likely to lower confidence in effect.
j. Downgraded one level for inconsistency: I2 = 71%; the inconsistency is related to the direction of the effect.
k. Downgraded one level for imprecision: Low sample size.
l. Downgraded one level for inconsistency: I2 = 85%; this is related to the direction of effect.
m. Downgraded one level for imprecision: There is a wide 95% CI that overlaps no effect and low sample size.
n. Downgraded one level for inconsistency: I2 = 86%; this is related to the magnitude of effect rather than the direction of effect.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Maradit Kremers H., Larson D.R., Crowson C.S., et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386–1397. doi: 10.2106/JBJS.N.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fingar K.R., Stocks C., Weiss A.J., Steiner C.A. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); Rockville (MD): 2006. Most frequent operating room procedures performed in U.S. Hospitals, 2003–2012. [Google Scholar]
- 3.Callanan M.C., Jarrett B., Bragdon C.R., et al. The John Charnley Award: risk factors for cup malpositioning: quality improvement through a joint registry at a tertiary hospital. Clin Orthop Relat Res. 2011;469:319–329. doi: 10.1007/s11999-010-1487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurtz S., Ong K., Lau E., Mowat F., Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 5.Free M.D., Barnes I., Hutchinson M., Harvie P. Preoperative radiographs to predict component malposition in direct anterior approach total hip arthroplasty. Hip Int. 2021 doi: 10.1177/11207000211037596. [DOI] [PubMed] [Google Scholar]
- 6.Mazoochian F., Pietschmann M.F., Hocke S., Fottner A., C VS-P. Jansson V. [Hip dislocation following THA] Orthopä. 2007;36:935–938. doi: 10.1007/s00132-007-1143-y. 940, 942-933. [DOI] [PubMed] [Google Scholar]
- 7.Brown M.L., Ezzet K.A. Relaxed hip precautions do not increase early dislocation rate following total hip arthroplasty. J Am Acad Orthop Surg. 2020;28:e440–e447. doi: 10.5435/JAAOS-D-19-00261. [DOI] [PubMed] [Google Scholar]
- 8.Scheerlinck T. Cup positioning in total hip arthroplasty. Acta Orthop Belg. 2014;80:336–347. [PubMed] [Google Scholar]
- 9.Fontalis A., Epinette J.A., Thaler M., Zagra L., Khanduja V., Haddad F.S. Advances and innovations in total hip arthroplasty. Sicot j. 2021;7:26. doi: 10.1051/sicotj/2021025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kayani B., Konan S., Ayuob A., Ayyad S., Haddad F.S. The current role of robotics in total hip arthroplasty. EFORT Open Rev. 2019;4:618–625. doi: 10.1302/2058-5241.4.180088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett W., Hoeffel D., Dalury D., Mason J.B., Murphy J., Himden S. In-vivo alignment comparing patient specific instrumentation with both conventional and computer assisted surgery (CAS) instrumentation in total knee arthroplasty. J Arthroplasty. 2014;29:343–347. doi: 10.1016/j.arth.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 12.Virk M.S., Steinmann S.P., Romeo A.A., Zuckerman J.D. Managing glenoid deformity in shoulder arthroplasty: role of new technology (Computer-Assisted navigation and patient-specific instrumentation) Instr Course Lect. 2020;69:583–594. [PubMed] [Google Scholar]
- 13.Mattei L., Pellegrino P., Calò M., Bistolfi A., Castoldi F. Patient specific instrumentation in total knee arthroplasty: a state of the art. Ann Transl Med. 2016;4:126. doi: 10.21037/atm.2016.03.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ast M.P., Nam D., Haas S.B. Patient-specific instrumentation for total knee arthroplasty: a review. Orthop Clin N Am. 2012;43:e17–e22. doi: 10.1016/j.ocl.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Small T., Krebs V., Molloy R., Bryan J., Klika A.K., Barsoum W.K. Comparison of acetabular shell position using patient specific instruments vs. standard surgical instruments: a randomized clinical trial. J Arthroplasty. 2014;29:1030–1037. doi: 10.1016/j.arth.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Buller L., Smith T., Bryan J., Klika A., Barsoum W., Iannotti J.P. The use of patient-specific instrumentation improves the accuracy of acetabular component placement. J Arthroplasty. 2013;28:631–636. doi: 10.1016/j.arth.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Cao L., Wang Y., Zou S., Cheng H. A novel positioner for accurately sitting the acetabular component: a retrospective comparative study. J Orthop Surg Res. 2019;14:279. doi: 10.1186/s13018-019-1331-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferretti A., Iannotti F., Proietti L., et al. The accuracy of patient-specific instrumentation with laser guidance in a dynamic total hip arthroplasty: a radiological evaluation. Sensors. 2021;21 doi: 10.3390/s21124232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hananouchi T., Saito M., Koyama T., Sugano N., Yoshikawa H. Tailor-made surgical guide reduces incidence of outliers of cup placement. Clin Orthop Relat Res. 2010;468:1088–1095. doi: 10.1007/s11999-009-0994-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin X., Chen M., Cheema A.N., Liu X., Yang S., Xu W. Effectiveness of a patient-specific guide for femoral stem implantation in primary total hip arthroplasty: a randomized control trial. Int Orthop. 2022;46:805–814. doi: 10.1007/s00264-021-05287-9. [DOI] [PubMed] [Google Scholar]
- 21.Mishra A., Verma T., Rajkumar, Agarwal G., Sharma A., Maini L. 3D printed patient-specific acetabular jig for cup placement in total hip arthroplasty. Indian J Orthop. 2020;54:174–180. doi: 10.1007/s43465-020-00061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakai T., Hamada H., Takao M., Murase T., Yoshikawa H., Sugano N. Validation of patient-specific surgical guides for femoral neck cutting in total hip arthroplasty through the anterolateral approach. Int J Med Robot. 2017;13 doi: 10.1002/rcs.1830. [DOI] [PubMed] [Google Scholar]
- 23.Wang C., Li Y., Hu Y., et al. Patient-specific total hip arthroplasty is superior to conventional methods for Crowe III and IV adult developmental hip dysplasia: a randomized controlled trial. Ann Transl Med. 2021;9:212. doi: 10.21037/atm-20-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao H., Wang C., Zhong D., Lei P., Hu Y., Su S. Effect of patient-specific instrument on lowering threshold for junior physicians to perform total hip arthroplasty on developmental dysplasia of the hip patients. Int Orthop. 2020;44:1281–1286. doi: 10.1007/s00264-020-04599-6. [DOI] [PubMed] [Google Scholar]
- 25.Xing Q.Q., Zhong D., Pan Y.X., et al. A comparative study of patients' subjective feelings toward total hip arthroplasty with patient-specific instruments and traditional total hip arthroplasty. Orthop Surg. 2020;12:269–276. doi: 10.1111/os.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L., Zheng Z., Chen W., Wang J., Zhang Y. Femoral neck osteotomy guide for total hip arthroplasty. BMC Surg. 2015;15:29. doi: 10.1186/s12893-015-0015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y.Z., Chen B., Lu S., et al. Preliminary application of computer-assisted patient-specific acetabular navigational template for total hip arthroplasty in adult single development dysplasia of the hip. Int J Med Robot. 2011;7:469–474. doi: 10.1002/rcs.423. [DOI] [PubMed] [Google Scholar]
- 28.Sloan M., Premkumar A., Sheth N.P. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100:1455–1460. doi: 10.2106/JBJS.17.01617. [DOI] [PubMed] [Google Scholar]
- 29.Higgins J.P.T., Thomas J., Chandler J., et al., editors. Cochrane Handbook for systematic reviews of interventions. 2022. (updated February 2022). Cochrane. [Google Scholar]
- 30.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 32.Sterne J.A., Hernán M.A., Reeves B.C., et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewinnek G.E., Lewis J.L., Tarr R., Compere C.L., Zimmerman J.R. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60:217–220. [PubMed] [Google Scholar]
- 34.Shi X.T., Li C.F., Han Y., Song Y., Li S.X., Liu J.G. Total hip arthroplasty for Crowe type IV hip dysplasia: surgical techniques and postoperative complications. Orthop Surg. 2019;11:966–973. doi: 10.1111/os.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng R., Zhang H., Kernkamp W.A., et al. Relations between the Crowe classification and the 3D femoral head displacement in patients with developmental dysplasia of the hip. BMC Muscoskel Disord. 2019;20:530. doi: 10.1186/s12891-019-2838-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tezuka T., Heckmann N.D., Bodner R.J., Dorr L.D. Functional safe zone is superior to the Lewinnek safe zone for total hip arthroplasty: why the Lewinnek safe zone is not always predictive of stability. J Arthroplasty. 2019;34:3–8. doi: 10.1016/j.arth.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 37.Yoon Y.S., Hodgson A.J., Tonetti J., Masri B.A., Duncan C.P. Resolving inconsistencies in defining the target orientation for the acetabular cup angles in total hip arthroplasty. Clin Biomech. 2008;23:253–259. doi: 10.1016/j.clinbiomech.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Dorr L.D., Callaghan J.J. Death of the Lewinnek "safe zone. J Arthroplasty. 2019;34:1–2. doi: 10.1016/j.arth.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 39.Vles G.F., Corten K., Driesen R., van Elst C., Ghijselings S.G. Hidden blood loss in direct anterior total hip arthroplasty: a prospective, double blind, randomized controlled trial on topical versus intravenous tranexamic acid. Musculoskelet Surg. 2021;105:267–273. doi: 10.1007/s12306-020-00652-0. [DOI] [PubMed] [Google Scholar]
- 40.Pan P., Song K., Yao Y., Jiang T., Jiang Q. The impact of intraoperative hypothermia on blood loss and allogenic blood transfusion in total knee and hip arthroplasty: a retrospective study. BioMed Res Int. 2020;2020 doi: 10.1155/2020/1096743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toner R.W., Pizzi L., Leas B., Ballas S.K., Quigley A., Goldfarb N.I. Costs to hospitals of acquiring and processing blood in the US: a survey of hospital-based blood banks and transfusion services. Appl Health Econ Health Pol. 2011;9:29–37. doi: 10.2165/11530740-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 42.Malone D.L., Dunne J., Tracy J.K., Putnam A.T., Scalea T.M., Napolitano L.M. Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. J Trauma. 2003;54:898–905. doi: 10.1097/01.TA.0000060261.10597.5C. discussion 905-897. [DOI] [PubMed] [Google Scholar]
- 43.Rawn J. The silent risks of blood transfusion. Curr Opin Anaesthesiol. 2008;21:664–668. doi: 10.1097/ACO.0b013e32830f1fd1. [DOI] [PubMed] [Google Scholar]
- 44.Rudy M.D., Bentley J., Ahuja N., Rohatgi N. Determinants of cost variation in total hip and knee arthroplasty: implications for alternative payment models. J Am Acad Orthop Surg. 2020;28:e245–e254. doi: 10.5435/JAAOS-D-18-00718. [DOI] [PubMed] [Google Scholar]
- 45.Childers C.P., Maggard-Gibbons M. Understanding costs of care in the operating room. JAMA Surg. 2018;153 doi: 10.1001/jamasurg.2017.6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Husted H., Kristensen B.B., Andreasen S.E., Skovgaard Nielsen C., Troelsen A., Gromov K. Time-driven activity-based cost of outpatient total hip and knee arthroplasty in different set-ups. Acta Orthop. 2018;89:515–521. doi: 10.1080/17453674.2018.1496309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vigdorchik J.M., Sharma A.K., Aggarwal V.K., Carroll K.M., Jerabek S.A. The use of robotic-assisted total hip arthroplasty in developmental dysplasia of the hip. Arthroplast Today. 2020;6:770–776. doi: 10.1016/j.artd.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Subramanian P., Wainwright T.W., Bahadori S., Middleton R.G. A review of the evolution of robotic-assisted total hip arthroplasty. Hip Int. 2019;29:232–238. doi: 10.1177/1120700019828286. [DOI] [PubMed] [Google Scholar]
- 49.Sterne J.A., Sutton A.J., Ioannidis J.P., et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Br Med J. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 50.Dalton J.E., Bolen S.D., Mascha E.J. Publication bias: the elephant in the review. Anesth Analg. 2016;123:812–813. doi: 10.1213/ANE.0000000000001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.