Abstract
In classical Hodgkin lymphoma (cHL), the malignant cells represent only a small fraction of the tumor. Yet, they orchestrate a lymphocyte-dominated tumor microenvironment (TME) that supports their survival and growth. The systemic effects of this local immunomodulation are not fully elucidated. Here, we aimed at characterizing circulating lymphocytes and plasma proteins in relation to clinical parameters and treatment effect. Peripheral blood (PB) samples were obtained from 48 consecutive patients at diagnosis and at 2 time points after successful primary treatment. Single-cell suspensions were prepared from lymph node (LN) biopsies obtained for routine diagnostic purposes. Twenty healthy individuals were included as controls. Cells from PB and LN were analyzed by flow cytometry, and plasma proteins by Proximity Extension Assay. We found that the frequencies of T and B cells positively correlated between the LN and the PB compartments. Compared to controls, cHL patients had higher frequencies of proliferating T cells as well as higher expression of programmed death (PD)-1 and cytotoxic T lymphocyte antigen (CTLA)-4 in circulating T cells, and lower naive T-cell frequencies. Advanced-stage patients had fewer NK cells with a functionally impaired phenotype. Differences in the immune profile were observed in patients with a high tumor burden and with high inflammation, respectively. Most of these deviations disappeared after standard first-line treatment. Patients who received radiotherapy involving the mediastinum had low T-cell counts for a prolonged period. Our findings suggest that the immunomodulation of lymphocytes in the TME of cHL might affect immune biomarkers in the PB.
INTRODUCTION
Classical Hodgkin lymphoma (cHL) is one of the most common lymphomas and derives from B cells of the germinal center. It has a peculiar histology with pathognomonic Hodgkin and Reed-Sternberg (HRS) cells, which usually only represent around 1% of the cells in the tumor tissue, in an environment of polymorphous infiltration of immune cells.1
The most common frontline treatment regimens for cHL are doxorubicin-bleomycin-vinblastine-dacarbazine (ABVD) with or without radiotherapy (RT) in limited-stage cHL,2 and escalated-dose bleomycin-etoposide-doxorubicin-cyclophosphamide-vincristine-procarbazine-prednisone (escalated BEACOPP) in advanced-stage cHL.3 Over the course of several decades, treatment for cHL has evolved and the disease is nowadays often curable; 80%–90% are cured after first-line treatment.4,5
Brentuximab vedotin and anti-programmed death (PD)-1 therapy are used in patients with relapsed or refractory (R/R) disease and are currently being tested as monotherapy and in combination with chemotherapy as primary treatment.
Besides their intrinsic malignant characteristics, HRS cells attract and reprogram other immune cells to support their growth and survival. These include T cells, natural killer (NK) cells, B cells, plasma cells, mast cells, granulocytes, and macrophages.1 CD4+ T cells are the most abundant in cHL lymph nodes (LNs) and HRS cells preferentially recruit T helper (Th)2 and regulatory T (Treg) cells by secreting C-C motif chemokine ligand (CCL)17 (also known as thymus and activation-related chemokine; TARC), CCL5 (also known as regulated upon activation, normal T cell expressed and presumably secreted; RANTES), interleukin (IL)-4, IL-5, IL-10, and IL-13.6–8 CD8+ T cells are less frequent and functionally impaired by IL-10 and transforming growth factor (TGF)-β.9,10 HRS cells also recruit macrophages by secreting macrophage colony-stimulating factor (M-CSF, also known as CSF1); the presence of macrophages is an adverse prognostic factor.11 Fibroblasts are attracted by HRS cell-produced fibroblast growth factor and IL-13,12,13 eosinophils by IL-5, CCL5, CCL11 and CCL28,14,15 mast cells by CCL5,8 and neutrophils by IL-8.14 B cells are most likely present as remnants of the original LN structure.16 HRS cells constitutively express PD ligand (PD-L)1 due to genetic amplification or copy number gain of chromosome 9p24.1.17 Tumor-associated macrophages (TAM) are major contributors to PD-L1 overexpression in the cHL microenvironment as well.18 Such overexpression on the one hand suppresses the function of effector T cells and therefore hampers the antitumor immune response, and on the other supports the survival of HRS cells. Indeed, cHL patients have significantly higher levels of both membrane-bound and soluble PD-1, which can induce reverse PD-L1 signaling in HRS cells, increasing cell proliferation and reducing apoptosis.19
This may explain the great efficacy of anti-PD-1 therapy in the R/R setting.20
The fact that immune cells are profoundly manipulated by HRS cells in the tumor microenvironment (TME), where their presence and characteristics have vast prognostic implications,21,22 and that many interactions are cytokine-mediated, suggests that even circulating immune cells may be affected in patients with cHL. Indeed, the peripheral blood (PB) levels of CCL17/TARC can be used to predict treatment response.23 Furthermore, CCL17/TARC levels positively correlate to the metabolic tumor volume.23,24 Soluble CD163, a TAM marker, also reflects disease activity, yet not as accurately as CCL17/TARC.25 Patients with cHL also have higher percentages of Tregs,26 functionally suppressed Th1 cells,27,28 and a higher percentage of PD-1+ T cells, in their PB.29
Extensive knowledge is available on the local interactions of immune cells with tumor cells in cHL LNs. However, the systemic immune suppressive effects of HRS cells and their TME are less well studied. In this study, we aimed at characterizing circulating lymphocytes and plasma proteins in cHL patients in relation to their frequency in LNs, tumor load, and the effect of standard first-line treatment.
METHODS
Patients and controls
Forty-eight consecutive patients with newly diagnosed cHL who started treatment at the Hematology Department at Karolinska University Hospital between the years 2015 and 2018 were included in the study. Patients with a high viral load of Epstein-Barr virus (EBV) or human immunodeficiency virus (HIV) were excluded to avoid the immune response against the virus being a confounding factor. Since the aim of the study was to evaluate whether treatment-related changes reverted after successful treatment, patients who were not in complete remission after standard first-line treatment were excluded from the follow-up analysis. Specifically, 6 patients were excluded for the following reasons: refractory disease (n = 2), discontinued treatment (n = 1), and atypical treatment strategy (n = 3). After a median follow-up of 42 months (range, 23–71) all the patients were still in complete remission.
Baseline characteristics are shown in Table 1. This study was approved by the regional ethics committee and conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all study participants.
Table 1.
Baseline Characteristics
| Limited-stage (I–IIA) (n = 28) | Advanced-stage (IIB–IV) (n = 20) | |
|---|---|---|
| Age (y), median (range) | 35 (18–73) | 36.5 (18–84) |
| Male | 15 (54) | 13 (65) |
| Female | 13 (46) | 7 (35) |
| Histological subtype | ||
| Nodular sclerosis | 18 (64) | 16 (80) |
| Mixed cellularity | 5 (18) | 2 (10) |
| Lymphocyte-rich | 1 (4) | 1 (5) |
| Lymphocyte-depleted | 0 | 0 |
| Unclassified | 4 (14) | 1 (5) |
| Ann Arbor stage | ||
| I | 6 (21) | - |
| II | 22 (79) | 5 (25) |
| III | - | 4 (11) |
| IV | - | 11 (55) |
| B-symptoms | 0 (0) | 14 (70) |
| Bulky disease | 7 (25) | 1 (5) |
| >2 nodal sites | 15 (54) | 19 (95) |
| Erythrocyte sedimentation rate ≥50 mm | 1 (4) | 13 (65) |
| Age >45 y | 8 (29) | 9 (45) |
| Hemoglobin <10.5 g/dL | 0 (0) | 6 (30) |
| White blood cell count >15 × 109/L) | 1 (4) | 3 (15) |
| Absolute lymphocyte count <8% or <0.6 × 109/L | 1 (4) | 1 (5) |
| Serum albumin <40 g/L | 17 (61) | 19 (95) |
All data are presented as number (percentage) unless stated otherwise.
The patients were treated according to the contemporary Swedish national treatment guidelines (Suppl. Materials and Methods). PB samples were obtained before the start of any treatment (including pre-chemotherapy steroids), right at the end of treatment, and at follow-up 6 months. Clinical chemistry parameters were determined by the hospital’s routine laboratories and collected from the patients’ medical records.
Twenty age- and sex-matched healthy individuals were used as controls. The absolute lymphocyte counts (ALCs) in their PB samples were measured with an Automated Hematology Analyzer pocH-100i (Sysmex, Kobe, Japan).
Flow cytometric analysis of immune cells in the PB and LNs
Heparinized whole blood samples were stained with antibodies (Suppl. Table S1), exposed to red blood cell lysis buffer, and washed before flow cytometry (Suppl. Materials and Methods). PB mononuclear cells (PBMCs) were purified from heparinized whole blood by density gradient centrifugation using a Ficoll-Hypaque gradient, washed immediately, stained with antibodies (Suppl. Table S2), and washed again before flow cytometry. See Suppl. Materials and Methods, and Suppl. Table S3 and S4, for more details and intracellular staining methods. Both excisional and needle core biopsies from LNs were performed for routine diagnostic purposes. Single-cell suspensions were stained with antibodies (Suppl. Table S5) and analyzed by flow cytometry according to standard protocols for clinical samples.
PB data were acquired on a 6-color FACSCanto II Flow Cytometer, while LN data were acquired on an 8-color FACSCanto Clinical Flow Cytometry System. Analysis was performed using FACSDiva software version 6.1.3 (BD Biosciences). Absolute numbers of various cell subsets in the PB were calculated using a dual-platform method (Suppl. Materials and Methods). The different immune cell populations were defined according to the surface phenotype specified in the Suppl. Table S6.
Measurement of protein biomarker levels in plasma
Proximity Extension Assays (PEA) were performed on thawed plasma samples using the Target 96 Immuno-Oncology panel (Olink Bioscience, Uppsala, Sweden)30 to analyze 92 protein biomarkers (Suppl. Table S7). Measurements were done in duplicates using 1 µl of plasma. Normalized protein expression values were row-normalized for display. Row normalization, statistical tests, and data visualization were performed using R 3.6.0 (R Foundation).
See Suppl. Materials and Methods, for details on ELISA of CCL17/TARC.
Statistical analyses
Statistics were performed using GraphPad Prism 9.2.0 (GraphPad Software, La Jolla, CA) or R 3.6.0 (R Foundation). Comparisons of numerical variables between groups were performed with nonparametric Mann-Whitney tests and with Wilcoxon signed-rank tests in case of repeated measures. The Benjamini-Hochberg procedure was applied to correct for multiple testing. Multiple Kruskal-Wallis tests with Dun’s post-hoc test were used when appropriate. Pearson correlation coefficients were computed only if both data sets had a confirmed Gaussian distribution (Shapiro-Wilk normality test), otherwise, the nonparametric Spearman R correlation coefficient was used. A 2-tailed P-value of <0.05 was considered statistically significant across all tests.
RESULTS
Advanced-stage patients have normal lymphocyte counts, yet few NK cells
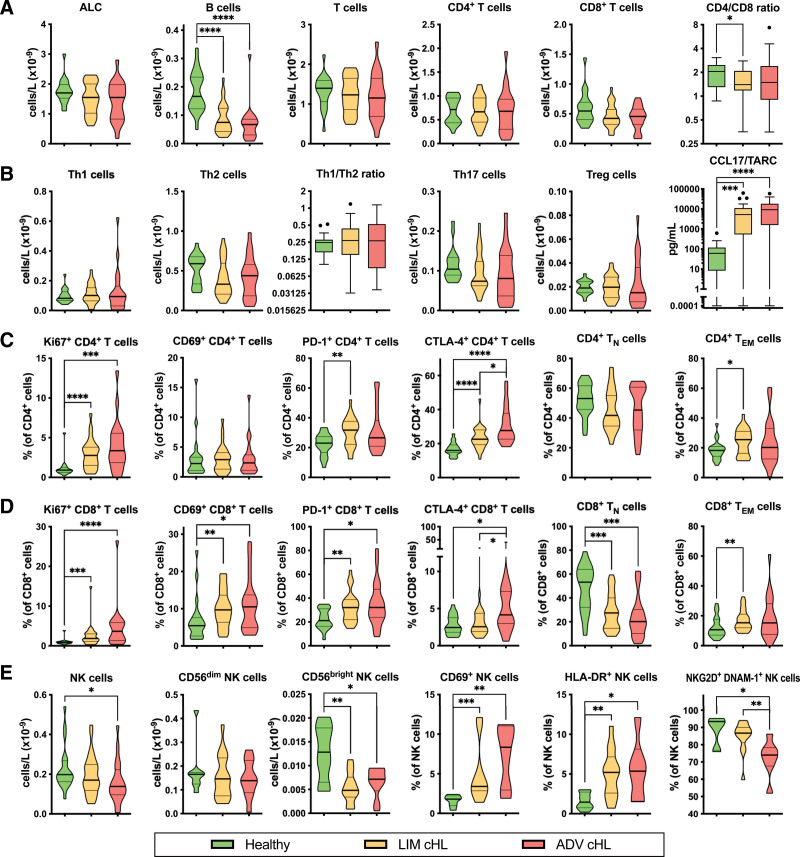
Although the ALCs of the patients at baseline were normal, we observed lower numbers of B cells in both limited- and advanced-stage cHL compared to controls (median 0.075 [P < 0.0001] and 0.067 [P < 0.0001] versus 0.17 × 109 cells/L, respectively) (Figure 1A). The absolute numbers of T cells, CD4+ and CD8+ T cells in cHL patients were normal. However, advanced cHL patients had lower numbers of NK cells than controls (median 0.14 versus 0.20 × 109 cells/L, respectively, P = 0.0135) (Figure 1E).
Figure 1.
Absolute numbers of lymphocytes and their subsets in the PB of limited-stage and advanced-stage cHL patients before primary treatment. Comparing healthy controls (n = 20) to limited-stage (n = 28) and advanced-stage (n = 20) cHL patients. (A) Absolute lymphocyte count (ALC) and absolute numbers of major lymphocyte populations (B cells, T cells, CD4+ T cells, CD8+ T cells, and CD4/CD8 ratio). (B) Absolute numbers of T helper cells (Th1 cells, Th2 cells, Th1/Th2 ratio, Th17 cells, and Tregs) and concentrations of CCL17/TARC in plasma. (C) Percentages of CD4+ T cells that are expressing functional markers (Ki67, CD69, PD-1, and CTLA-4) and absolute numbers of CD4+ TN and TEM cells. (D) Percentages of CD8+ T cells that are expressing functional markers (Ki67, CD69, PD-1, and CTLA-4) and absolute numbers of CD8+ TN and TEM cells. (E) Absolute numbers of NK cells, CD56dim NK cells, CD56bright NK cells, and percentages of NK cells that are expressing functional markers (CD69, HLA-DR, NKG2D, and DNAM-1). The CD4/CD8 ratio, Th1/Th2 ratio, and CCL17/TARC data are depicted as box and whiskers plots in the style of Tukey. All other data are depicted as violin plots with a thick line at the median and thin lines at the quartiles. Stars indicate the P-value of a Mann-Whitney test comparing the indicated groups. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. ADV = advanced-stage; cHL = classical Hodgkin lymphoma; CTLA = cytotoxic T lymphocyte antigen; DNAM = DNAX accessory molecule; LIM = limited-stage; NK = natural killer; NKG2D = NK group 2 member D; PB = peripheral blood.
We did not observe any differences in the numbers of Th1, Th2, Th17, and Tregs, nor in the Th1/Th2 ratio, between cHL patients and controls. However, the plasma concentrations of CCL17 (TARC) were elevated in both limited- and advanced-stage cHL compared to controls (median 5256 [P = 0.0002] and 9165 [P < 0.0001] versus 61.03 pg/mL, respectively) (Figure 1B).
PB T cells have an activated and antigen-experienced phenotype
We observed that both limited-stage and advanced-stage cHL patients had higher frequencies of intracellular Ki67 expression than controls among CD4+ (median 2.77 [P < 0.0001] and 3.36 [P = 0.0004] versus 0.92%, respectively) and CD8+ (median 1.85 [P = 0.0009] and 3.66 [P < 0.0001] versus 0.91%, respectively) T cells at baseline. The expression of CD69 was not aberrant on CD4+ T cells, but it was more frequent on CD8+ T cells in limited-stage and advanced-stage cHL than on those in controls (median 9.70 [P = 0.0077] and 10.50 [P = 0.0318] versus 5.45%, respectively). The PD-1 expressing fraction among CD4+ T cells was higher in limited-stage cHL than in controls (median 31.75% versus 23.05%, respectively, P = 0.0030), while on CD8+ T cells it was higher in both limited-stage and advanced-stage cHL (median 32.05 [P = 0.0041] and 32.20 [P = 0.0135] versus 21.15%, respectively). Both limited-stage and advanced-stage cHL patients had higher proportions of intracellular cytotoxic T lymphocyte antigen (CTLA)-4 expression among CD4+ T cells than controls (median 22.55 [P < 0.0001] and 27.65 [P < 0.0001] versus 15.95%, respectively), while only advanced-stage cHL patients had a higher CTLA-4+ fraction among CD8+ T cells (median 4.15 versus 2.45%, respectively, P = 0.0170). Interestingly, the frequency of PD-1 and TIM-3 double-positive T cells was higher in both limited- (n = 13) and advanced-stage (n = 6) cHL than in controls (n = 7) (median 2.40 [P < 0.0001] and 2.85 [P = 0.0012] versus 0.80%, respectively) (Suppl. Figure S1).
While we observed no difference in the percentages of naive T cells (TN), defined as C-C motif chemokine receptor (CCR)7+CD45RA+, within the CD4+ population, the frequency of TN cells within the CD8+ population was lower in limited- and advanced-stage cHL than in controls (median 27.40 [P = 0.0005] and 20.15 [P = 0.0003] versus 53.25%, respectively). Furthermore, effector memory T cells (TEM), defined as CCR7−CD45RA−, were more frequent among CD4+ (median 25.50 versus 18.20%, respectively, P = 0.0267) and CD8+ (median 15.30 versus 10.65%, respectively, P = 0.0074) T cells in limited-stage cHL (Figure 1C and D).
Circulating NK cells are activated, but have a functionally impaired phenotype
When looking into the NK-cell subsets, we found that the numbers of CD56bright NK cells were lower in both limited- and advanced-stage cHL at baseline compared to controls (median 4.81 [P = 0.0024] and 7.16 [P = 0.0320] versus 12.83 × 106 cells/L, respectively).
Compared to controls (n = 8), both limited- (n = 13) and advanced-stage (n = 6) cHL patients had higher percentages of NK cells that express CD69 (median 1.80 versus 3.40 [P = 0.0004] and 8.35% [P = 0.0043], respectively) and HLA-DR (median 1.45 versus 5.20 [P = 0.0056] and 5.35% [P = 0.0426], respectively). Notably, though, a lower percentage of NK cells in advanced-stage cHL (n = 7) was double-positive for NK group 2 member D (NKG2D) and DNAX accessory molecule (DNAM)-1 compared to controls (n = 7) (median 74.0 versus 93.4%, respectively, P = 0.0105) and even compared to limited-stage cHL (n = 13) (median 74.0 versus 86.7%, respectively, P = 0.0085) (Figure 1E).
Disease-related changes in the levels of plasma protein biomarkers are displayed in Suppl. Figure S2.
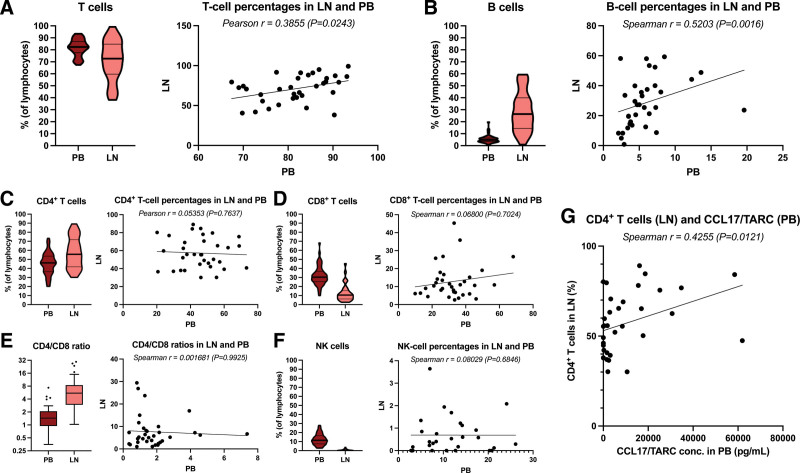
T- and B-cell frequencies are correlated between PB and LNs
When analyzing the flow-cytometry data from paired baseline PB and LN samples of cHL patients, we observed that the frequencies of T cells (median 82.35% and 72.64%, respectively) and B cells (median 4.85% and 26.37%, respectively) positively correlated (Pearson r = 0.3855 [P = 0.0243] and Spearman r = 0.5203 [P = 0.0016], respectively) between the 2 compartments (Figure 2A and B). However, there was no such correlation for CD4+ T cells, CD8+ T cells, CD4/CD8 ratio, and NK cells (Figure 2C–F). Interestingly, though, the percentage of CD4+ T cells in the LNs correlated positively with the concentration of CCL17/TARC in the PB (Spearman r = 0.4255, P = 0.0121) (Figure 2E).
Figure 2.
Lymphocyte subset frequencies in PB and LN and correlations between the 2 compartments in cHL patients before primary treatment. (A) The percentage of T cells in PB and LNs and the correlation between these. (B) The percentage of B cells in PB and LNs and the correlation between these. (C) The percentage of CD4+ T cells in PB and LNs and the correlation between these. (D) The percentage of CD8+ T cells in PB and LNs and the correlation between these. (E) The CD4/CD8 ratio in PB and LNs and the correlation between these. (F) The percentage of NK cells in PB and LNs and the correlation between these. (G) Correlation between the concentration of CCL17/TARC in the PB and the percentage of CD4+ T cells in the LNs. The CD4/CD8 ratio data are depicted as box and whiskers plots in the style of Tukey. Percentages are depicted as violin plots with a thick line at the median and thin lines at the quartiles. Correlations are visualized by scatter plots with a simple linear regression line. cHL = classical Hodgkin lymphoma; LN = lymph node; NK = natural killer; PB = peripheral blood.
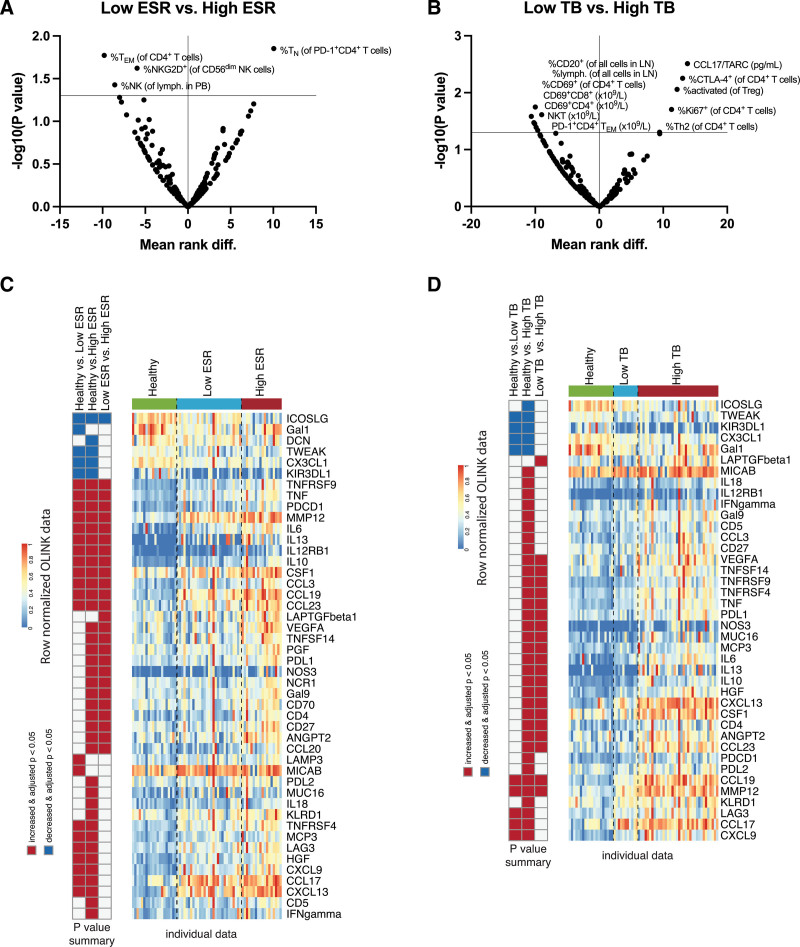
Patients with high inflammation and high tumor burden, respectively, have distinct immune profiles
To assess possible differences in the immune profiles of patients with an inflammatory status compared to those who had not, we divided the patients into 2 groups, with a low erythrocyte sedimentation rate (ESR) (n = 29) and high (≥50 mm) ESR (n = 19), respectively. We observed that patients with a high ESR had higher percentages of PD-1+CD4+ TN cells and lower percentages of CD4+ TEM cells, NKG2D+ CD56dim NK cells, and total NK cells in their PB (Figure 3A). When we looked at the plasma protein biomarker profiles, we observed that patients with a high ESR have lower levels of inducible T cell costimulator ligand (ICOSLG) and higher levels of multiple chemoattractants, PD-1 and PD-L1, among others (Figure 3C).
Figure 3.
Immune profiles of cHL patients with high ESR and high tumor burden before primary treatment. High erythrocyte sedimentation rate (ESR) was defined as ≥50 mm. High tumor burden (TB) was defined as any of the following: bulky disease, >2 nodal sites, and stage IV. Multiple comparisons between patients with (A) low (n = 29) vs high (n = 19) ESR, and (B) low (n = 11) vs high (n = 37) TB. Data are depicted as volcano plots of multiple Mann-Whitney tests with a P-value threshold of 0.05 and without correction for multiple comparisons. Y-axes depict the negative log10-transformed P values, so dots that are plotted above the horizontal line have a P-value that is <0.05. X-axes show the mean rank difference between the 2 indicated groups. A positive X-value means that the difference is in favor of the latter group in the graph title. (C, D) The left columns show the results of multiple Kruskal-Wallis tests with Dun’s post-hoc test, between the respective groups. The middle columns depict the median row-normalized normalized protein expression (NPX) values of the respective groups. The right columns show the individual row-normalized NPX values of the healthy individuals and patients clustered by group. Since MIC-A and MIC-B were measured with the same probe it was regarded as one unique protein. cHL = classical Hodgkin lymphoma.
To determine possible differences in the immune profile of patients with a high tumor burden compared to those who had not, we divided the patients into 2 groups, with a low (n = 11) and high (n = 37) tumor burden (defined as bulky tumor or >2 nodal sites involved or stage IV), respectively, and performed the same analysis. Patients with a high tumor burden had higher plasma levels of CCL17/TARC, higher percentages of CTLA-4+ CD4+ T cells, activated Tregs, Ki67+ CD4+ T cells, and Th2 cells in their PB. They also had higher levels of TGF-β, PD-L1, IL-10, and M-CSF (also known as CSF1) among others, in their plasma than patients with a low tumor burden (Figure 3D). Furthermore, patients with a high tumor burden have lower percentages of B cells and lymphocytes in their LNs, lower percentages of CD69+ CD4+ T cells, and lower absolute numbers of CD69+CD4+ T cells, CD69+CD8+ T cells, NKT cells, and PD-1+CD4+ TEM cells in their PB (Figure 3B). When comparing different combinations of low/high ESR and low/high tumor burden, these differences were still consistent (Supp. Figure S3).
Most immunological changes are reverted after successful standard primary treatment
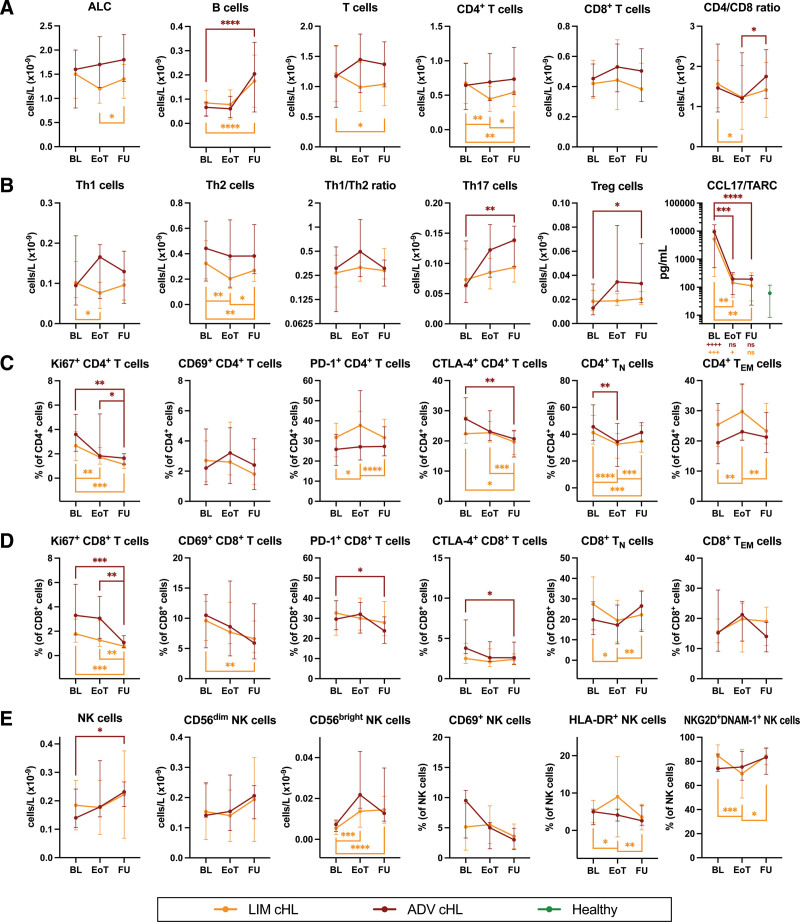
To examine the effect of treatment on the cHL-related immunological changes, we performed the same analyses after treatment. Compared to baseline, the numbers of B cells increased at follow-up in limited-stage (median 0.075 versus 0.138 × 109 cells/L, respectively, P < 0.0001) and advanced-stage (median 0.204 versus 0.069 × 109 cells/L, respectively, P = 0.0002) cHL (Figure 4A). As expected, CCL17/TARC concentrations dropped back to control levels at end of treatment and follow-up (Figure 4B).
Figure 4.
Follow-up of lymphocyte numbers, subset frequencies, and functional characteristics in cHL patients at the end of standard primary treatment and 6 months later. Comparing limited-stage cHL patients at baseline (n = 27) to EoT (n = 25) and FU (n = 25), and advanced-stage cHL patients at baseline (n = 15) to EoT (n = 12) and FU (n = 14). (A) Absolute lymphocyte count (ALC) and absolute numbers of major lymphocyte populations (B cells, T cells, CD4+ T cells, CD8+ T cells, and CD4/CD8 ratio before and after treatment). (B) Percentages of CD4+ T cells that are expressing functional markers (Ki67, CD69, PD-1, and CTLA-4) before and after treatment. (C) The concentration of CCL17/TARC in plasma before and after treatment. Data are depicted as connected dots that indicate the median with error bars that indicate the interquartile range. Plus signs illustrate the P-value of Mann-Whitney tests comparing the respective time points with controls. (D) Percentages of CD8+ T cells that are expressing functional markers (Ki67, CD69, PD-1, and CTLA-4) before and after treatment. (E) Absolute numbers of NK cells, CD56bright NK cells, CD56dim NK cells, and NK cells that are expressing functional markers (CD69, HLA-DR, NKG2D, and DNAM-1) before and after treatment. Paired data are depicted as connected dots that indicate the median with error bars that indicate the interquartile range. Stars indicate the P-value of a Wilcoxon test comparing the indicated time points. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. ADV = advanced-stage; BL = baseline; cHL = classical Hodgkin lymphoma; CTLA = cytotoxic T lymphocyte antigen; DNAM = DNAX accessory molecule; EoT = end of treatment; FU = follow-up 6 months after EoT; LIM = limited-stage; NK = natural killer; NKG2D = NK group 2 member D.
Furthermore, comparing follow-up to baseline, the Ki67+ fraction of CD4+ T cells decreased in limited-stage (median 1.15 versus 2.56%, respectively, P = 0.0002) and advanced-stage (median 1.15 versus 2.44%, respectively, P = 0.0031) cHL. Likewise, the percentage of Ki67+ CD8+ T cells went down in limited-stage (median 0.78 versus 1.85%, respectively, P = 0.0002) and advanced-stage (median 1.07 versus 3.71%, respectively, P = 0.0006) cHL. The frequencies of CD69+ CD8+ T cells in limited-stage cHL, PD-1+ CD8+ T cells in advanced-stage cHL, CTLA-4+ CD4+ T cells in both patient groups and CD8+ T cells in advanced-stage cHL decreased as well. Fractions of TN cells decreased, and TEM cells increased among CD4+ T cells in limited-stage cHL at end of treatment, then changed in the opposite direction at follow-up. A similar trend was observed in the CD8+ T-cell compartment (Figure 4C and D).
The numbers of NK cells in advanced-stage cHL increased at follow-up compared to baseline (median 0.231 versus 0.143 × 109 cells/L, respectively, P = 0.0353). Likewise, CD56bright NK-cell numbers went up in limited-stage cHL (median 0.0132 versus 0.0049 × 109 cells/L, respectively, P < 0.0001). However, the numbers of CD69+, HLA-DR+, and NKG2D+DNAM-1+ did not change lastingly (Figure 4E).
Most disease-related changes in plasma protein biomarkers were also normalized after standard first-line treatment (Suppl. Figure S4).
Plasma protein biomarker profiles are aberrant in cHL and normalize after treatment
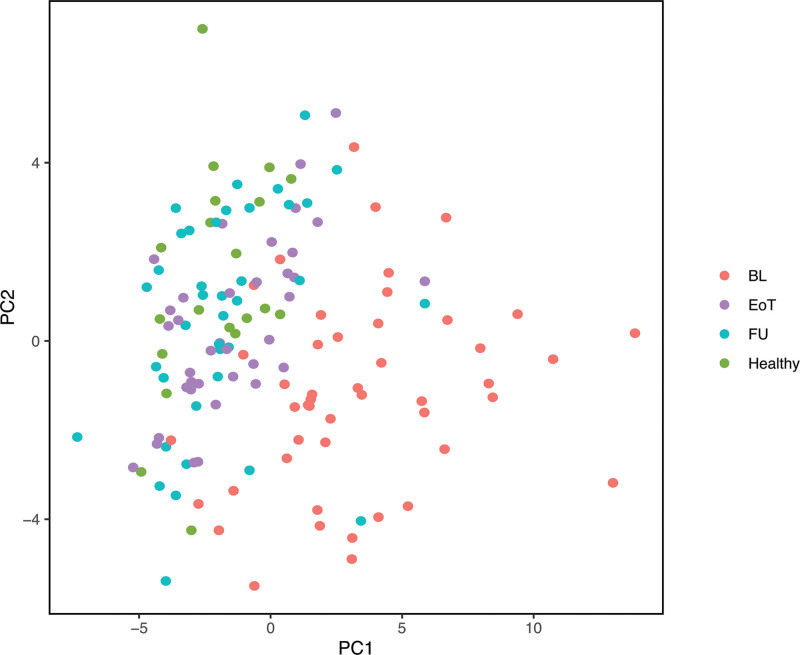
To characterize the general profile of the analyzed plasma protein biomarkers, we performed a principal component analysis of these data. We observed a distinct clustering with a low level of overlap of healthy individuals and cHL patients at baseline. Interestingly, the plasma protein profiles of patients at end of treatment and follow-up were almost identical to those of controls (Figure 5).
Figure 5.
Principal component analysis of plasma protein biomarker profiles of healthy individuals and cHL patients before and after treatment. The plasma protein biomarker profiles of all healthy individuals and patients at various time points are depicted. Data points are computationally clustered based on covariance. BL = baseline; cHL = classical Hodgkin lymphoma; EoT = end of treatment; FU = follow-up 6 months after EoT; Healthy = healthy individuals; PC = principal component.
Patients that undergo RT involving the mediastinum have a prolonged T-cell deficit
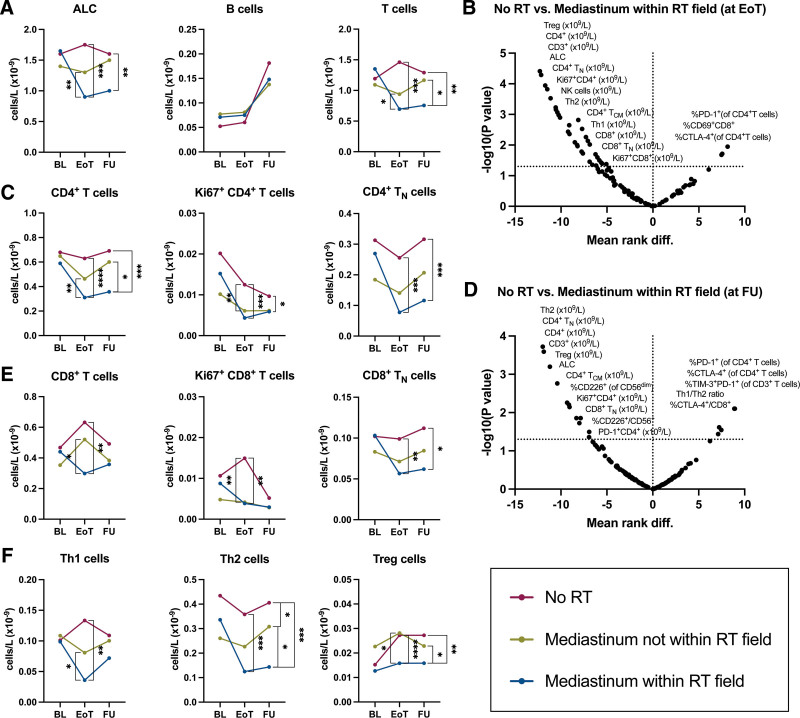
Patients treated with RT (n = 20), especially if the mediastinum was within the RT field (n = 12), had a lower ALC at end of treatment and follow-up than patients who were not treated with RT (n = 22). In particular, patients who underwent RT involving the mediastinum had lower T-cell numbers compared to patients who had not received RT at end of treatment (median 0.70 versus 1.46 × 109 cells/L, respectively, P = 0.00011) and follow-up (median 0.76 versus 1.29 × 109 cells/L, respectively, P = 0.00173), respectively, while no differences were observed in B-cell numbers (Figure 6A). When we performed multiple comparisons to check which subpopulations of T cells were mainly affected, we observed that the numbers of CD4+ and specifically Th2 cells, CD4+ TN cells, Tregs, and Ki67+ CD4+ T cells were most significantly lower at end of treatment and follow-up, while none of these differences were present before treatment (Figure 6B–F). Of note, both CD4+ and CD8+ TN cells were still lower at follow-up in these patients compared to those who did not receive RT. The levels of soluble CD4 and CD5 in plasma were also lower at follow-up in patients who received RT than in those who did not (Suppl. Figure S5). No statistically significant differences were found in plasma protein biomarker levels comparing patients who underwent RT involving the mediastinum to those who underwent RT elsewhere.
Figure 6.
Longitudinal comparison of ALC and T-cell numbers between cHL patients treated with and without RT. Absolute numbers of lymphocyte subsets before and after treatment that did not include RT (No RT; red dots and line), treatment that included RT where the mediastinum was not within the RT field (yellow dots and line), and treatment that included RT where the mediastinum was within the RT field. (A) ALC and absolute numbers of B cells and T cells. (B) Multiple comparisons between No RT (n = 22) and Mediastinum within RT field (n = 12) at EoT. (C) Absolute numbers of CD4+ T cells and selected subpopulations. (D) Multiple comparisons between No RT (n = 22) and Mediastinum within RT field (n = 12) at FU. (E) Absolute numbers of CD8+ T cells and selected subpopulations. (F) Absolute numbers of T helper subsets (Th1, Th2, and Treg). Paired data are depicted as connected dots that indicate the median without error bars. Multiple comparisons are depicted as volcano plots of multiple Mann-Whitney tests with a P-value threshold of 0.05 and without correction for multiple comparisons. The y-axis depicts the negative log10-transformed P values, so dots that are plotted above the horizontal line have a P value that is <0.05. The x-axis show the mean rank difference between the 2 indicated groups. A positive x-value means that the difference is in favor of the latter group in the graph title. Stars indicate the P-value of a Mann-Whitney test comparing the indicated groups at the indicated time point. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. ALC = absolute lymphocyte count; BL = baseline; cHL = classical Hodgkin lymphoma; EoT = end of treatment; FU = follow-up 6 months after EoT; RT = radiotherapy.
DISCUSSION
In this study, we characterized the disease-related changes in circulating lymphocytes by measuring the baseline expression of lineage and functional markers by flow cytometry on immune cells from freshly processed whole blood and isolated PBMCs in cHL patients and controls. Furthermore, we analyzed 92 plasma protein biomarkers by PEA. This is the first extensive report of disease-related alterations in PB in a consecutive cohort of cHL patients before and after successful standard-of-care first-line treatment. Additionally, we compared lymphocyte subset frequencies between PB and LNs, and assessed the influence of inflammation and tumor burden on the PB immune profiles. Finally, we showed that these disease-related alterations reverted once the patients achieved complete remission.
Compared to controls, we found that increased frequencies of CD4+ and CD8+ T cells were proliferating, and that CD8+, but not CD4+, T cells had a higher expression of the early activation marker CD69 at baseline. Furthermore, higher percentages of CD4+ and CD8+ T cells were expressing the inhibitory molecules PD-1 and CTLA-4 in patients compared to controls, suggesting that their T cells are exhausted. The expression of CD69 on T cells was significantly higher in patients with a low tumor burden and CTLA-4 was significantly higher in patients with a high tumor burden, potentially reflecting relatively competent T-cell activity in early stages and T-cell exhaustion in later stages. Accordingly, patients with a high tumor burden had higher percentages of tumor-promoting activated Tregs and Th2 cells in the PB. All patients also had reduced frequencies of CD8+ TN cells and limited-stage cHL patients had increased fractions of CD4+ and CD8+ TEM cells, suggesting that a higher fraction of T cells was in an antigen-experienced and more differentiated state.
In contrast to our findings, Garcia-Marquez et al31 recently reported a lower relative fraction of T cells, CD4+ T cells, and Tregs and normal expression of CD69 in cryopreserved PBMCs from treatment-naive early-stage unfavorable cHL patients enrolled in the NIVAHL trial, where patients were treated either concomitantly or sequentially with anti-PD-1 therapy and AVD followed by consolidative RT. Nevertheless, in accordance with our data from freshly analyzed material, Garcia-Marquez et al, and others, also reported that the relative fraction of CD4+ TN cells was unchanged, that the percentage of CD4+ TEM cells increased and that the percentage of CD8+ TN cells decreased accompanied by an increase in the percentage of CD8+ TEM cells, compared to controls.31,32 Despite some discrepancies, all reports, including ours, point out that cHL patients have antigen-experienced, differentiated, and exhausted T cells in the PB. This is most likely a consequence of the vast immune modulation that takes place in the affected LNs33 and continuous neoantigen exposure due to the high tumor mutational burden.34
Following standard-of-care first-line treatment, the fractions of Ki67+ and CTLA-4+ CD4+ and CD8+ T cells and the fractions of CD69+ and PD-1+ CD8+ T cells decreased. So, it seems that T-cell exhaustion is reversed by standard chemo-/RT, similar to what has been reported for anti-PD-1 therapy in combination with chemotherapy in treatment-naive patients31 and anti-PD-1 monotherapy in R/R patients.32 This raises the question of whether T-cell exhaustion is simply being remedied by erasing the tumor by any means rather than by any specific treatment or drug. The patient’s plasma protein biomarker profiles were also normalized after treatment.
We generated paired data on lymphocyte frequencies in 2 compartments (PB and LNs) at diagnosis and found that T-cell frequencies in the PB correlated positively with those in LNs. Since CCL17/TARC is a well-established and clinically applicable biomarker in cHL,24,25,35 we measured it by ELISA and expectedly found elevated concentrations. CCL17/TARC selectively recruits the Th2 subtype of CD4+ T cells, which express the CCL17/TARC receptor CCR4.36 We found a positive correlation between the concentration of CCL17/TARC in plasma and the percentage of CD4+ T cells in LNs. Furthermore, we show that CCL17/TARC concentrations are significantly higher in patients with a high tumor burden than in those with a low tumor burden. CCL17/TARC levels normalized at end of treatment and remained low 6 months later. These findings are in concordance with the reported correlation between CCL17/TARC and metabolic tumor volume.24,25 Similarly, patients with a high tumor burden also had higher plasma levels of TGF-β, PD-L1, and M-CSF/CSF1 in their plasma than patients with a low tumor burden, suggesting tumor-associated recruitment of macrophages (M-CSF) and active secretion of ligands (PD-L1) and cytokines (TGF-β, IL-10) with immune modulating activity by these and other immune cells.
Additionally, we found that the absolute numbers of B cells were markedly decreased in both patient groups, which confirms previously published data.32,37 We also show that the percentages of B cells in the PB and LNs are positively correlated and that patients with a high tumor burden have a significantly lower fraction of B cells in LNs. Interestingly, the number of B cells in the PB is stably low at end of treatment but normalizes 6 months later.
Similar to earlier reports,31,32,38 we show here that the absolute numbers of NK cells are decreased in advanced-stage cHL. Further, we show that this is accompanied by a sharp decrease in the number of CD56bright NK cells and that this is associated with high inflammation in all patients. The higher CD69 expression together with decreased NKG2D and DNAM-1 expression furthermore indicate that NK cells from patients had an activated, yet functionally impaired phenotype. This is in line with previous reports that showed elevated serum levels of soluble NK-cell inhibiting factors and increased proportions of NK cells with limited killing capacity in the PB of cHL patients.39,40 However, this is in contrast with Vari et al that reported that the proportion of CD56bright NK cells is increased in the PB of cHL compared to controls.38 The reason for this discrepancy is unclear. In this study, total NK-cell and CD56bright NK-cell numbers normalized after treatment, but those that co-expressed activating receptors NKG2D and DNAM-1 did not, for unknown reasons. These data suggest that the systemic immunomodulation extends to other cytotoxic effector cells, that is, NK cells, that arguably play an important role in a microenvironment that is characterized by a decreased expression of β2-microglobulin and major histocompatibility complex class I.41
Interestingly, when we compared patients who received RT to those who did not, we noticed that the ALC at end of treatment and follow-up was significantly lower. Especially those that underwent RT involving the mediastinum had lower T cells, but not B cells. This group still had significantly lower Th2 cells, CD4+ TN cells, Tregs, Ki67+ CD4+ T cells, and CD8+ TN cells 6 months after end of treatment, compared to patients who did not receive RT. Since mainly T cells, and particularly since both CD4+ TN and CD8+ TN cells, were low in patients who had received RT involving the mediastinum, we hypothesize that thymus irradiation could contribute to this relative T-cell deficit.
Furthermore, the prolonged CD4+ T-cell paucity might have clinical implications. Indeed, low CD4+ T-cell counts put even HIV-negative immunocompromised patients at risk for Pneumocystis jirovecii pneumonia (PJP),42 as CD4+ T cells are crucial in clearing Pneumocystis infections.43 Moreover, cHL patients who receive standard first-line treatment are at risk of developing bleomycin-induced lung injury44 and radiation-induced lung injury if the lungs were within the radiation field.45 Interestingly, concurrent chemo-/RT is a risk factor for the development of PJP in patients with lung cancer.46 Prospective studies should clarify if cHL patients who received RT involving the mediastinum could benefit from prolonged PJP prophylaxis.
From this study, we conclude that cHL may have systemic effects on the lymphocytes, leading to exhausted and more antigen-experienced T cells, reduced numbers of B cells, and NK cells with a functionally impaired phenotype at the periphery. Systemic NK cell suppression seems to be most prominent in patients with high inflammation, while elevated levels of CCL17/TARC and suppressive T-cell populations seem to associate with a high tumor burden. Most of these changes were reversed by successful standard first-line treatment. Furthermore, patients who received RT involving the mediastinum had remarkably low CD4+ T-cell numbers 6 months after end of treatment.
Thus, in addition to the tight control that the neoplastic HRS cells have over local immune cells, the circulating lymphocytes might be adversely affected as well. We believe that this study provides preliminary insight into cHL biology and possible correlations between immune biomarkers and some clinical features of the disease. However, the relatively small number of patients included is a limitation and prompts further validation of the results in a larger patient cohort with differential clinical outcome, including therapy-refractory patients. This could potentially allow the identification of laboratory parameters of prognostic or predictive value which could be considered in the choice of the primary treatment or motivate treatment intensification if still persistent after the end of treatment. The presence of remarkably high levels of PD-1 ligands at diagnosis, as an example, could strengthen the indication to incorporate PD-1 blockade in the primary treatment, a therapeutic approach currently being assessed in clinical trials. Similarly, the persistence of high plasma levels of TARC in the presence of PET-negative enlarged residual nodes could motivate more intensive instrumental follow-up.
ACKNOWLEDGMENTS
We thank Barbro Näsman-Glaser and Ann Svensson for excellent technical support and Leila Relander for providing secretarial assistance.
AUTHOR CONTRIBUTIONS
TAM contributed to designing flow-cytometry panels, handling patient samples, and performing flow-cytometry analyses, performed ELISA analyses, prepared samples for PEA analyses, processed, and statistically analyzed the data, interpreted the results, created figures, and wrote the manuscript. MLA collected the clinical data, contributed to interpreting results and writing the manuscript. LPP processed, and statistically analyzed the PEA data, created figures, and contributed to writing the manuscript. KH designed the flow-cytometry panels, handled patient samples, performed flow-cytometry analyses, and analyzed the data. IX and GR collected and analyzed flow-cytometry data from LN biopsies, contributed to interpreting results, and provided feedback on the manuscript. BEW, RM, and LH contributed to interpreting results and provided feedback on the manuscript. MP designed the study, contributed to designing flow-cytometry panels, analyzing data, interpreting results, and writing the manuscript.
DISCLOSURES
MP received research funding from Takeda Pharma AB. All the other authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
This work was supported by grants from The Swedish Cancer Society, The Cancer Society in Stockholm, The Cancer and Allergy Foundation, Dr. Åke Olsson´s Foundation for Haematology research and by an unrestricted research grant from Takeda Pharma AB (MP).
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
REFERENCES
- 1.Kuppers R. The biology of Hodgkin’s lymphoma. Nat Rev Cancer. 2009;9:15–27. [DOI] [PubMed] [Google Scholar]
- 2.Fermé C, Eghbali H, Meerwaldt JH, et al. Chemotherapy plus involved-field radiation in early-stage Hodgkin’s disease. N Engl J Med. 2007;357:1916–1927. [DOI] [PubMed] [Google Scholar]
- 3.Borchmann P, Goergen H, Kobe C, et al. PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet. 2017;390:2790–2802. [DOI] [PubMed] [Google Scholar]
- 4.Engert A, Haverkamp H, Kobe C, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379:1791–1799. [DOI] [PubMed] [Google Scholar]
- 5.Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol. 2013;31:684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Berg A, Visser L, Poppema S. High expression of the CC chemokine TARC in Reed-Sternberg cells. A possible explanation for the characteristic T-cell infiltrate in Hodgkin’s lymphoma. Am J Pathol. 1999;154:1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohshima K, Tutiya T, Yamaguchi T, et al. Infiltration of Th1 and Th2 lymphocytes around Hodgkin and Reed-Sternberg (H&RS) cells in Hodgkin disease: Relation with expression of CXC and CC chemokines on H&RS cells. Int J Cancer. 2002;98:567–572. [DOI] [PubMed] [Google Scholar]
- 8.Fischer M, Juremalm M, Olsson N, et al. Expression of CCL5/RANTES by Hodgkin and Reed-Sternberg cells and its possible role in the recruitment of mast cells into lymphomatous tissue. Int J Cancer. 2003;107:197–201. [DOI] [PubMed] [Google Scholar]
- 9.Hsu SM, Lin J, Xie SS, et al. Abundant expression of transforming growth factor-beta 1 and -beta 2 by Hodgkin’s Reed-Sternberg cells and by reactive T lymphocytes in Hodgkin’s disease. Hum Pathol. 1993;24:249–255. [DOI] [PubMed] [Google Scholar]
- 10.Atayar C, Poppema S, Blokzijl T, et al. Expression of the T-cell transcription factors, GATA-3 and T-bet, in the neoplastic cells of Hodgkin lymphomas. Am J Pathol. 2005;166:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo B, Cen H, Tan X, et al. Meta-analysis of the prognostic and clinical value of tumor-associated macrophages in adult classical Hodgkin lymphoma. BMC Med. 2016;14:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skinnider BF, Elia AJ, Gascoyne RD, et al. Interleukin 13 and interleukin 13 receptor are frequently expressed by Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood. 2001;97:250–255. [DOI] [PubMed] [Google Scholar]
- 13.Khnykin D, Troen G, Berner JM, et al. The expression of fibroblast growth factors and their receptors in Hodgkin’s lymphoma. J Pathol. 2006;208:431–438. [DOI] [PubMed] [Google Scholar]
- 14.Skinnider BF, Mak TW. The role of cytokines in classical Hodgkin lymphoma. Blood. 2002;99:4283–4297. [DOI] [PubMed] [Google Scholar]
- 15.Hanamoto H, Nakayama T, Miyazato H, et al. Expression of CCL28 by Reed-Sternberg cells defines a major subtype of classical Hodgkin’s disease with frequent infiltration of eosinophils and/or plasma cells. Am J Pathol. 2004;164:997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vardhana S, Younes A. The immune microenvironment in Hodgkin lymphoma: T cells, B cells, and immune checkpoints. Haematologica. 2016;101:794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roemer MG, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol. 2016;34:2690–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carey CD, Gusenleitner D, Lipschitz M, et al. Topological analysis reveals a PD-L1-associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma. Blood. 2017;130:2420–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jalali S, Price-Troska T, Bothun C, et al. Reverse signaling via PD-L1 supports malignant cell growth and survival in classical Hodgkin lymphoma. Blood Cancer J. 2019;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schreck S, Friebel D, Buettner M, et al. Prognostic impact of tumour-infiltrating Th2 and regulatory T cells in classical Hodgkin lymphoma. Hematol Oncol. 2009;27:31–39. [DOI] [PubMed] [Google Scholar]
- 22.Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362:875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones K, Vari F, Keane C, et al. Serum CD163 and TARC as disease response biomarkers in classical Hodgkin lymphoma. Clin Cancer Res. 2013;19:731–742. [DOI] [PubMed] [Google Scholar]
- 24.Plattel WJ, van den Berg A, Visser L, et al. Plasma thymus and activation-regulated chemokine as an early response marker in classical Hodgkin’s lymphoma. Haematologica. 2012;97:410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plattel WJ, Alsada ZN, van Imhoff GW, et al. Biomarkers for evaluation of treatment response in classical Hodgkin lymphoma: comparison of sGalectin-1, sCD163 and sCD30 with TARC. Br J Haematol. 2016;175:868–875. [DOI] [PubMed] [Google Scholar]
- 26.Barath S, Aleksza M, Keresztes K, et al. Immunoregulatory T cells in the peripheral blood of patients with Hodgkin’s lymphoma. Acta Haematol. 2006;116:181–185. [DOI] [PubMed] [Google Scholar]
- 27.Mainou-Fowler T, Taylor PR, Miller S, et al. Intracellular cytokine profiles by peripheral blood CD3+ T-cells in patients with classical Hodgkin lymphoma. Leuk Lymphoma. 2003;44:1325–1331. [DOI] [PubMed] [Google Scholar]
- 28.Franzke A, Koenecke C, Geffers R, et al. Classical Hodgkin’s lymphoma: molecular evidence for specific alterations in circulating T lymphocytes. Tumour Biol. 2006;27:329–333. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto R, Nishikori M, Kitawaki T, et al. PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 2008;111:3220–3224. [DOI] [PubMed] [Google Scholar]
- 30.Assarsson E, Lundberg M, Holmquist G, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9:e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Marquez MA, Thelen M, Reinke S, et al. Reverted exhaustion phenotype of circulating lymphocytes as immune correlate of anti-PD1 first-line treatment in Hodgkin lymphoma. Leukemia. 2022;36:760–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cader FZ, Hu X, Goh WL, et al. A peripheral immune signature of responsiveness to PD-1 blockade in patients with classical Hodgkin lymphoma. Nat Med. 2020;26:1468–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cader FZ, Schackmann RCJ, Hu X, et al. Mass cytometry of Hodgkin lymphoma reveals a CD4(+) regulatory T-cell-rich and exhausted T-effector microenvironment. Blood. 2018;132:825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wienand K, Chapuy B, Stewart C, et al. Genomic analyses of flow-sorted Hodgkin Reed-Sternberg cells reveal complementary mechanisms of immune evasion. Blood Adv. 2019;3:4065–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sauer M, Plutschow A, Jachimowicz RD, et al. Baseline serum TARC levels predict therapy outcome in patients with Hodgkin lymphoma. Am J Hematol. 2013;88:113–115. [DOI] [PubMed] [Google Scholar]
- 36.Imai T, Nagira M, Takagi S, et al. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol. 1999;11:81–88. [DOI] [PubMed] [Google Scholar]
- 37.Gajl-Peczalska KJ, Hansen JA, Bloomfield CD, et al. B lymphocytes in untreated patients with malignant lymphoma and Hodgkin’s disease. J Clin Invest. 1973;52:3064–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vari F, Arpon D, Keane C, et al. Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood. 2018;131:1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reiners KS, Kessler J, Sauer M, et al. Rescue of impaired NK cell activity in Hodgkin lymphoma with bispecific antibodies in vitro and in patients. Mol Ther. 2013;21:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stannard KA, Lemoine S, Waterhouse NJ, et al. Human peripheral blood DNAM-1. Blood Adv. 2019;3:1681–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roemer MG, Advani RH, Redd RA, et al. Classical Hodgkin lymphoma with reduced beta2M/MHC class I expression is associated with inferior outcome independent of 9p24.1 status. Cancer Immunol Res. 2016;4:910–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Messiaen PE, Cuyx S, Dejagere T, et al. The role of CD4 cell count as discriminatory measure to guide chemoprophylaxis against Pneumocystis jirovecii pneumonia in human immunodeficiency virus-negative immunocompromised patients: a systematic review. Transpl Infect Dis. 2017;19:e12651. [DOI] [PubMed] [Google Scholar]
- 43.Kelly MN, Shellito JE. Current understanding of Pneumocystis immunology. Future Microbiol. 2010;5:43–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sleijfer S. Bleomycin-induced pneumonitis. Chest. 2001;120:617–624. [DOI] [PubMed] [Google Scholar]
- 45.Arroyo-Hernández M, Maldonado F, Lozano-Ruiz F, et al. Radiation-induced lung injury: current evidence. BMC Pulm Med. 2021;21:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee EH, Kim EY, Lee SH, et al. Risk factors and clinical characteristics of Pneumocystis jirovecii pneumonia in lung cancer. Sci Rep. 2019;9:2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.