Herpes zoster (HZ) is a common infection in immunocompromised patients with incidence rates up to 8–95 cases per 1000 person years (PY), depending on the immunosuppressive condition or immunosuppressive therapy.1,2 Patients with myeloproliferative neoplasms (MPN) did not have a higher risk to develop HZ, but this has changed since the introduction of treatment with ruxolitinib, a Janus kinase (JAK) 1 and JAK 2 inhibitor.3 Registration studies of ruxolitinib in MPN patients reveal incidence rates of HZ between 35 and 58 per 1000 PY.4–6 This has been confirmed recently by 2 meta-analyses showing that patients with MPN on ruxolitinib have a significantly higher risk to develop HZ in comparison to MPN patients not receiving ruxolitinib.7,8 In response to the results of the high HZ incidence in registration studies it is advised to consider varicella zoster virus (VZV) prophylaxis.4,9 However, if the incidence rates in real-life cohorts are confirmed to be equal or even outnumber those in registration studies, it is worth considering whether antiviral prophylaxis by vaccination or antivirals should be recommended more stringently to MPN patients using ruxolitinib. Therefore, the aim of this study was to determine the incidence of HZ in ruxolitinib-treated MPN patients in a real-life setting to give recommendations on antiviral prophylaxis.
We performed a retrospective cohort study. Patients started with ruxolitinib between July 2011 and September 2021 in the University Medical Center Utrecht for PV or MF were included in this study. Patients receiving ruxolitinib for another indication than MPN were excluded. We extracted data from patient files on demographics, MPN type, and ruxolitinib treatment (duration, age at the start, daily doses, and duration of treatment until developing HZ). HZ was diagnosed based on physical examination and/or a PCR test. Ethical approval for this study was waived by the medical research ethics committee of the UMC Utrecht.
The incidence was determined as the percentage of patients who developed HZ in this specific population, and per 1000 PY in order to compare with population risk. In total, 133 patients were eligible for inclusion. Five patients specifically objected to use of their medical data for research, and were therefore excluded, leaving 128 patients for analysis.
Table 1 shows the patient characteristics of the cohort. Median age for all patients was 63 years (interquartile range 54–68.8) and 52.3% of patients (n = 67) were male. The median treatment duration was 32 months and median daily ruxolitinib dose 29.9 mg. Types of MPN were polycythemia vera (41.4%) and myelofibrosis (58.6%). Two patients had a history of HZ before starting ruxolitinib.
Table 1.
Description of the Cohort
| Total n = 128 | Herpes Zoster During Ruxolitinib Treatment | ||
|---|---|---|---|
| Yes, n = 25 | No, n = 103 | ||
| Age at start ruxolitinib treatment (median, IQR) | 63 (54.0–68.8) | 60 (54.5–69.0) | 64 (53.0–69.0) |
| Male (n, %) | 67 (52.3%) | 13 (52%) | 54 (52.4%) |
| MPN diagnosis at start of treatment (n, %) | |||
| PV | 53 (41.4%) | 9 (36%) | 44 (42.7%) |
| MF | 75 (58.6%) | 16 (64%) | 59 (57.3%) |
| Duration of ruxolitinib treatment in months (median, IQR) | 32.0 (14.0–56.0) | 56 (36.0–75.5) | 26 (11.0–50.0) |
| Daily dose ruxolitinib in milligrams (median, IQR) History of HZ before starting ruxolitinib (n, %) |
29.9 (20.0–37.5) 2 (1.6%) |
30.9 (20.2–39.6) 0 (0.0%) |
29.2 (20.0–36.7) 2 (1.9%) |
IQR = interquartile range, MPN = myeloproliferative neoplasm, PV = polycythemia vera, MF = myelofibrosis
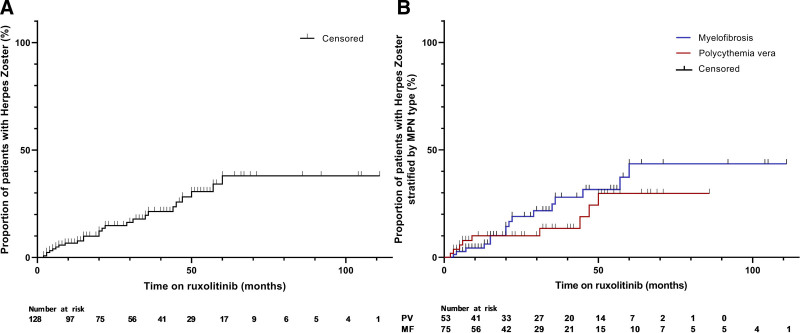
In the present study, 25 of 128 patients (19,5%) developed HZ during ruxolitinib treatment, in an average follow-up time of 37 months. Two patients had a second episode of HZ while on ruxolitinib resulting in 27 HZ cases in 25 patients. With a total treatment duration in all patients of 393.5 years, the incidence rate is 68.6 of 1000 PY. The risk to develop HZ is more or less constant over the first 5 years (Figure 1). Nine out of these 25 patients received secondary prophylaxis. The 2 patients with a second episode of HZ did not receive secondary VZV prophylaxis after the first episode. All patients who developed HZ continued ruxolitinib treatment after their HZ episode. Finally, 2 patients developed a first episode of HZ after having stopped ruxolitinib, specifically after 10 and 13 months. Both patients had undergone an allogeneic stem cell transplantation.
Figure 1.
Percentage of ruxolitinib-treated patients with herpes zoster in the total study group (A) and stratified by MPN subtype (B). MPN = myeloproliferative neoplasm.
Cox proportional hazard analyses showed that age and ruxolitinib doses were no risk factors in developing HZ (hazard ratio [HR] 1.0; 95% confidence interval [CI] 0.97–1.04; P 0.987 and HR 1.0; 95% CI 0.96–1.07; P 0.675, respectively). Whether the type of MPN is a risk factor could not be investigated as the assumption of proportionality was violated for this covariate.
According to these results the incidence of HZ in patient with MPN on ruxolitinib is more than twice as high as compared to patients with a hematological malignancy in general (68.6/1000 PY versus 31.0 per 1000 PY) and almost 7 times as high as compared to immunocompetent patients aged 50 years or older (9.9/1000 PY)2,10 We also found a much higher incidence in real life as compared to the ruxolitinib registration studies, reporting HZ incidence rates of 35–58/1000 PY in patients with comparable age and ruxolitinib dosage.4–6 Only a few studies have been performed on the safety of ruxolitinib in myelofibrosis in a real-life setting. A postauthorization safety surveillance of ruxolitinib in myelofibrosis showed comparable incidence rates of HZ with the registration studies.11 However, in this report, 56% of patient were prevalent users of ruxolitinib and HZ episodes before participation in the surveillance may have been missed. Two more studies showed lower incidence rates but had a short follow-up time.12,13 Whereas our study shows that HZ not only occurs shortly after starting ruxolitinib but also on the long-term. Noteworthy, previous studies show that MPN itself was not associated with an increased risk for HZ.3
Primary VZV prophylaxis for patients undergoing ruxolitinib treatment is currently not recommended in Dutch and international guidelines. Remarkably, in other hematological conditions with comparable risk for HZ, antiviral prophylaxis for VZV is recommended in guidelines and generally adapted in clinical practice, for example, for hematopoietic stem cell transplantation or treatment with proteasome inhibitors. The incidence rate of HZ in hematopoietic stem cell transplants is 43 of 1000 PY, and the incidence of HZ for myeloma patients on bortezomib is 13%.1,14 Based on these incidences, antiviral prophylaxis for MPN patients treated with ruxolitinib should be strongly recommend. The need of antiviral prophylaxis in these patients is also suggested by previous reports.15
Interestingly most studies on HZ incidence are performed before the recombinant zoster vaccine (RZV) was available. In 2017, the FDA approved RZV for use in immunocompetent persons aged ≥50 years and in 2021, for use in immunocompromised adults. Dagnew et al16 studied the immunogenicity of RZV in patients with hematological malignancies and reported response rates 1 month after completing vaccination series of 65.4% and 83.7% for humoral and cell-mediated immunity, respectively. After 22 months, response rates were, respectively, 52.1% and 66.7%. Post-hoc analysis revealed a vaccine efficacy of 87.2%. Noteworthy, little to no MPN patients were included in this study. Studies investigating the effect of ruxolitinib on vaccination responses are scarce, but there are some data on SARS-COV-2 vaccination. SARS-COV-2 vaccine induced antibody responses in ruxolitinib-treated patients were between 42% and 60% and hereby impaired as compared to controls.17–19 Studies in rheumatoid arthritis patients treated with JAK inhibitors tofacitinib or baricitinib confirm these results and also show impaired vaccine immune responses.20,21
Given the high incidence of HZ in ruxolitinib-treated MPN patients, we advocate for prophylaxis in all these patients. Since the incidence of HZ does not decrease over time with consequently an indication for prolonged valacyclovir prophylaxis, we prefer vaccination with RZV over valacyclovir prophylaxis awaiting the results of the pending studies on the effect of RZV in patients treated with JAK inhibitors. However, because it is uncertain whether RZV is effective enough, it should be considered to give valacyclovir prophylaxis in patients with additional risk factors, that is, a previous history of HZ.
In conclusion, patients with MPN treated with ruxolitinib have a high risk to develop HZ, and this risk remains constant during the course of treatment. Prophylaxis with vaccination and antivirals against VZV should therefore be adapted in clinical practice.
ACKNOWLEDGMENTS
We would like to thank epidemiologist Paco M. J. Welsing, PhD, for his statistical advice to this manuscript.
AUTHOR CONTRIBUTIONS
A.B. conceptualized the study; L.B. participated in data collection; E.L. performed the statistical analyses; E.L., L.D., M.W. and A.B. interpreted the data; E.L. wrote the first draft of the manuscript. All authors revised and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria.
DISCLOSURES
The authors have no conflicts of interest to disclose.
Footnotes
The dataset of this study is available upon request.
REFERENCES
- 1.Chen SY, Suaya JA, Li Q, et al. Incidence of herpes zoster in patients with altered immune function. Infection. 2014;42:325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKay SL, Guo A, Pergam SA, et al. Herpes zoster risk in immunocompromised adults in the United States: a systematic review. Clin Infect Dis. 2020;71:e125–e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Titmarsh GJ, McMullin MF, McShane CM, et al. Community-acquired infections and their association with myeloid malignancies. Cancer Epidemiol. 2014;38:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison CN, Vannucchi AM, Kiladjian JJ, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2016;30:1701–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verstovsek S, Mesa RA, Gotlib J, et al. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J Hematol Oncol. 2017;10:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica. 2016;101:821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo Q, Xiao Z, Peng L. Effects of ruxolitinib on infection in patients with myeloproliferative neoplasm: a meta-analysis. Hematology. 2021;26:663–669. [DOI] [PubMed] [Google Scholar]
- 8.Lussana F, Cattaneo M, Rambaldi A, et al. Ruxolitinib-associated infections: A systematic review and meta-analysis. Am J Hematol. 2018;93:339–347. [DOI] [PubMed] [Google Scholar]
- 9.Al-Ali HK, Griesshammer M, le Coutre P, et al. Safety and efficacy of ruxolitinib in an open-label, multicenter, single-arm phase 3b expanded-access study in patients with myelofibrosis: a snapshot of 1144 patients in the JUMP trial. Haematologica. 2016;101:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tseng HF, Bruxvoort K, Ackerson B, et al. The epidemiology of herpes zoster in immunocompetent, unvaccinated adults >/=50 years old: incidence, complications, hospitalization, mortality, and recurrence. J Infect Dis. 2020;222:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barraco F, Greil R, Herbrecht R, et al. Real-world non-interventional long-term post-authorisation safety study of ruxolitinib in myelofibrosis. Br J Haematol. 2020;191:764–774. [DOI] [PubMed] [Google Scholar]
- 12.Breccia M, Andriani A, Montanaro M, et al. Ruxolitinib in clinical practice for primary and secondary myelofibrosis: an analysis of safety and efficacy of Gruppo Laziale of Ph-negative MPN. Ann Hematol. 2017;96:387–391. [DOI] [PubMed] [Google Scholar]
- 13.Komatsu N, Kirito K, Shimoda K, et al. Assessing the safety and efficacy of ruxolitinib in a multicenter, open-label study in Japanese patients with myelofibrosis. Int J Hematol. 2017;105:309–317. [DOI] [PubMed] [Google Scholar]
- 14.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. [DOI] [PubMed] [Google Scholar]
- 15.Heine A, Brossart P, Wolf D. Ruxolitinib is a potent immunosuppressive compound: is it time for anti-infective prophylaxis? Blood. 2013;122:3843–3844. [DOI] [PubMed] [Google Scholar]
- 16.Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis. 2019;19:988–1000. [DOI] [PubMed] [Google Scholar]
- 17.Caocci G, Mulas O, Mantovani D, et al. Ruxolitinib does not impair humoral immune response to COVID-19 vaccination with BNT162b2 mRNA COVID-19 vaccine in patients with myelofibrosis. Ann Hematol. 2022;101:929–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gagelmann N, Passamonti F, Wolschke C, et al. Antibody response after vaccination against SARS-CoV-2 in adults with haematological malignancies: a systematic review and meta-analysis. Haematologica. 2022;107:1840–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herzog Tzarfati K, Gutwein O, Apel A, et al. BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies. Am J Hematol. 2021;96:1195–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winthrop KL, Bingham CO, 3rd, Komocsar WJ, et al. Evaluation of pneumococcal and tetanus vaccine responses in patients with rheumatoid arthritis receiving baricitinib: results from a long-term extension trial substudy. Arthritis Res Ther. 2019;21:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winthrop KL, Silverfield J, Racewicz A, et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis. 2016;75:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]