Abstract
The use of cardiopulmonary bypass (CPB) can be associated with significant hemodilution, coagulopathy and a systemic inflammatory response for infants and children undergoing cardiac surgery. Intra-operative ultrafiltration has been used for decades to ameliorate these harmful effects. The novel combination of a continuous and non-continuous form of ultrafiltration, Subzero Balance Simple Modified Ultrafiltration (SBUF-SMUF) here described, seeks to enhance recovery from pediatric cardiac surgery and CPB.
Keywords: pediatric, cardiac surgery, subzero balance, simple modified, ultrafiltration, cardiopulmonary bypass, SBUF-SMUF
Introduction
Cardiac surgery using cardiopulmonary bypass (CPB) can be associated with significant inflammation, coagulopathy, and hemodilution. The exposure to a non-endothelialized foreign circuit activates both the intrinsic coagulation and the alternative complement pathways.1 Altogether, systemic inflammatory response and resulting cardiopulmonary dysfunction can contribute to postoperative morbidity and mortality.2 Since the early 1990s, intra-operative ultrafiltration has been used during pediatric cardiac surgery to mitigate the physiologic disruptions of CPB.2 Ultrafiltration removes fluid and small molecules, <65 kDa, while preserving larger blood proteins and cell lines in the patient’s circulation.2
Non-continuous forms such as conventional ultrafiltration (CUF), modified ultrafiltration (MUF), and simple modified ultrafiltration (SMUF) have demonstrated reduced bleeding complications in the postoperative period.2–4 There is some evidence that shows continuous ultrafiltration such as zero-balance ultrafiltration (ZBUF) improve postoperative cardiopulmonary function as this method is hypothesized to be effective in removing many inflammatory cytokines during the CPB period.2,5 A similar form of continuous ultrafiltration termed subzero-balance ultrafiltration (SBUF) has been described in the adult cardiac surgery literature and targets a net-negative fluid balance rather than zero-balance as in ZBUF.6 Continuous and non-continuous techniques can be combined within the same operation, although the configuration and execution can be challenging to manage for perfusionists during a case.
A novel combination ultrafiltration method—subzero-balance simple modified ultrafiltration (SBUF-SMUF)—here described targets the unique benefits of both continuous and non-continuous forms of ultrafiltration in pediatric cardiac surgery. This technique utilizes infusion pumps to remove effluent and infuse physiologic solution to maintain a constant and exact negative balance during SBUF. Furthermore, the circuit design allows for efficient and safe transition between SBUF and SMUF without any physical alterations to the CPB manifold.
Perfusion technique
Cardiopulmonary bypass (CPB)
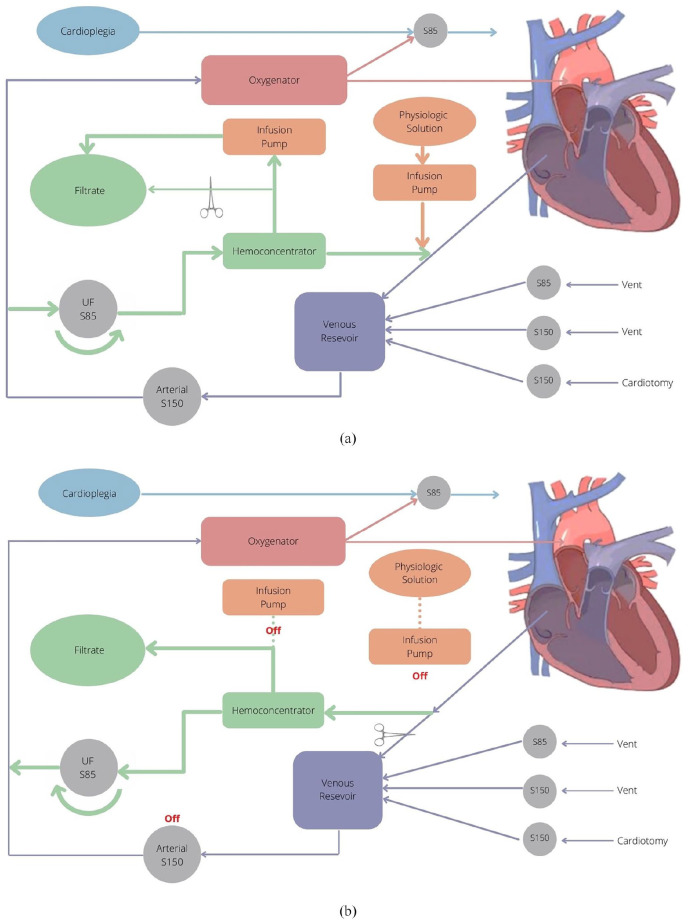
The Liva Nova S5™ CPB System (48-40-00, London, UK) is used at this institution, which includes three Liva Nova S150 roller pumps (10-80-00, London, UK) for arterial, cardiotomy and secondary vent as well as three Liva Nova S85 roller pumps (10-85-00, London, UK) used for primary vent, ultrafiltration and 1:4 blood cardioplegia (Figure 1). It features a non-miniaturized Sorin phosphorylcholine-coated polyvinyl chloride tubing system (AB2313, Saluggia, Italy) and Terumo FX05 or FX15 oxygenators (1CX*FX05RE/1CX*FX15E, Tokyo, Japan) are used for gas exchange.
Figure 1.
CPB Circuit for SBUF-SMUF: (a) SBUF. Blood flow is shunted in a veno-venous fashion by an anti-clockwise rotation of the S85 roller pump. Braun Infusomat® Space pumps accurately remove a specified ultrafiltrate effluent volume and also reinfuse a specified volume of physiologic solution to the venous reservoir. The clamp can be removed to bypass the Braun syringe pump regulation and allow for high-volume ultrafiltration as needed, (b) SMUF. After termination of CPB, the polarity of the S85 roller pump is reversed to clockwise and stop-link disengaged. Blood flow is shunted in a veno-arterial fashion through the ultrafiltration circuit. Braun Infusomat® Space pumps are deactivated during SMUF, as shown by the dotted lines. The S150 arterial pump can be activated to transfuse the patient if required.
Sanguineous prime is utilized for patients <10 kg; consisting of red blood cells, fresh frozen plasma, Plasmalyte, heparin, bicarbonate, magnesium sulfate, and mannitol. Subsequently, the Terumo Capiox® Hemoconcentrator HC05 (1CX*HC05S, Tokyo, Japan) is used to correct prime electrolyte abnormalities and reduce the circuit’s volume to avoid excessive hemodilution. This Buffer Ultrafiltration (BUF) of the prime targets normal sodium, potassium, bicarbonate and pH values along with hemoglobin (Hb) goal >100 g/L.7 BUF facilitates a good approximation of the patient’s pre-operative Hb, when initiating CPB, while always maintaining a Hb >100 g/L. For patients greater than 10 kg, a crystalloid prime is utilized and displaced with a retrograde autologous prime of arterial and venous lines.
Arterial and venous cannulation are individualized for each patient’s size and pathology. Following systemic heparinization and activated clotting time (ACT) >480 seconds, CPB is initiated. Regular monitoring and maintenance of electrolytes, oxygen saturation, carbon dioxide and anticoagulation are guided by Terumo CDI550 In-Line Blood Monitoring System (550AHCT, Tokyo, Japan) as well as point of care blood gas and ACT analysis every 30 minutes. SBUF is continuous ultrafiltration during the whole CPB time, while SMUF is used following CPB cessation.
Subzero balance simple modified ultrafiltration (SBUF-SMUF)
The hemoconcentrator is connected to the circuit between the pre-reservoir venous drainage line and the arterial pump outflow line proximal to the oxygenator; it includes a dedicated S85 roller pump stop-linked to the arterial S150 roller pump (Figure 1). Upon initiation of full-flow CPB, approximately 5% of the calculated cardiac output is directed to the hemoconcentrator by way of an S85 roller pump, in an anti-clockwise rotation, to establish SBUF in a veno-venous (V-V) fashion (Figure 1(a)). One Braun Infusomat® Space pump (8710351U, Frankfurt, Germany) is set to remove 30 mL/kg/hour of ultrafiltrate effluent while a second Braun Infusomat® Space pump infuses a physiologic solution, “Solution A” (500 mL 0.45% NaCl, 500 mL 0.9% NaCl and 35 mL 1 mEq/mL NaHCO3) or “Solution B” (500 mL Plasmalyte, 100 mL 0.45% NaCl and 15 mL 1 mEq/mL NaHCO3) into the venous reservoir at a lesser rate of 25 mL/kg/hour (Figure 1). This achieves a net fluid removal of 5 mL/kg/hour. These infusion pump rates are easily adjustable to achieve individualized goals for specific patients. Selection between Solution A and B depends largely on potassium management; Solution A used when potassium >5.5 mmol/L, and Solution B used when potassium <5.5 mmol/L. Significant volume infusions, such as cardioplegia administration or saline irrigation in the surgical field, are immediately removed from the circulation via the hemoconcentrator—by temporarily manually bypassing the infusion pump limitation—until pre-cardioplegia reservoir levels are restored (Figure 1(a)). SBUF continues until the cardiac operation is complete and the patient is fit to be weaned from CPB. Immediately before CPB weaning, both Braun Infusomat® Space pumps are deactivated, clamped out, and the ultrafiltration S85 roller pump is turned off.
Once the patient is separated from CPB, the venous line is clamped immediately proximal to the venous reservoir and there should be absolutely no other clamping of the venous line. The ultrafiltration S85 roller pump’s direction must be changed to clockwise for SMUF, versus anti-clockwise during SBUF, and the ultrafiltration S85 pump’s stop-link is disabled. SMUF is initiated in a veno-arterial (V-A) fashion as, 5% of the calculated cardiac output is directed to the hemoconcentrator (Figure 1(b)). Concentrated blood is now directed through the oxygenator, which doubles as a filter, and is returned to the patient via the arterial cannula. Effluent is directly removed without infusion pump limitation. It is important to note that should CPB be re-initiated, that the ultrafiltration S85 roller pump should be switched to anti-clockwise and stop-link re-engaged. If these changes are not made while going back on CPB, there will be an alarm due to incongruent roller head directions.
Typically, the SMUF endpoint is inadequate patient preload with depletion of the venous reservoir, an arbitrary time endpoint of 8–10 minutes or a patient hematocrit 35%–50%. If reservoir volume is depleted and further SMUF is requested, volume in the form of Solution A, Solution B or blood products is infused into the venous reservoir and transfused to the patient via the arterial pump as SMUF continues. The surgeon, anesthetist, cardiologist, and perfusionist work cooperatively to optimize the patient’s preload and hemodynamics in the immediate post-CPB period. At our institution, SMUF has been used for more than 21 years. SBUF-SMUF has been the standard for the last 5 years without any adverse perfusion-related events.
Discussion
SBUF-SMUF is an advanced intra-operative ultrafiltration technique that can be standardized in a pediatric perfusion program; it has been routinely and safely used at our institution since January 2016. There are some limitations and important considerations related to this technique. First, the Braun Infusomat® Space pumps cannot exceed 1L/hour, and therefore by, can only be effectively offered patients <30 kg when using an effluent removal rate of 30 mL/kg/hour. Second, the S85 ultrafiltration roller head has reduced linking accuracy with the arterial roller pump when arterial flows are <300 mL/minute; applicable to low-flow states or neonatal cases. In these scenarios, either 10 mL/minute or 20 mL/minute flow for the ultrafiltration pump would be selected with the true target of 5% cardiac output being 15 mL/minute. The use of 1/8″ tubing in the S85 ultrafiltration pump improves accuracy during these low flow scenarios. Third, if the venous line is inappropriately clamped proximal to the ultrafiltration circuit before or during SMUF—in the operative field for example—there could be a risk of air embolism via the hemoconcentrator. As safeguards to this, air embolism detector, bubble filter and oxygenator are all distal to the hemoconcentrator circuit. The surgeon should be fully educated on the ultrafiltration protocol to avoid this complication.
The continuous SBUF component is used during the entire CPB time and precisely targets a slight negative volume balance during the CPB time as an optimal peri-operative perfusion strategy; this method avoids inaccurate “eye-balling” of fluid balance.8 The adjustable control of SBUF volume infusion rate and ultrafiltration effluent rate by Braun Infusomat® Space pumps allows for individualized weight standardization, thus safely applied to a range of neonatal and pediatric patients. After separation from CPB, this novel ultrafiltration circuit configuration allows for efficient and effective transition from SBUF to SMUF. Physical alterations to the CPB manifold are completely avoided. In conclusion, SBUF-SMUF can be safely and efficiently implemented to optimize the inflammatory, coagulation and volume parameters for infants and children undergoing open-heart surgery with CPB.
Acknowledgments
Physiologic Solutions “A” and “B” and ultrafiltration contributions from the Clinical Perfusion experts at Texas Children’s Hospital, Houston, Texas.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The first author received funding for this project through the Clinician Investigator Program of the Ministry of Health (Health Canada).
ORCID iD: Joel Bierer  https://orcid.org/0000-0002-4579-5674
https://orcid.org/0000-0002-4579-5674
References
- 1.Bronicki RA, Hall M. Cardiopulmonary bypass-induced inflammatory response: Pathophysiology and treatment. Pediatr Crit Care Med 2016; 17(8): S272–S278. [DOI] [PubMed] [Google Scholar]
- 2.Bierer J, Stanzel R, Henderson M, et al. Ultrafiltration in pediatric cardiac surgery review. World J Pediatr Congenit Hear Surg 2019; 10(6): 778–788. [DOI] [PubMed] [Google Scholar]
- 3.Naik SK, Knight A, Elliott MJ. A successful modification of ultrafiltration for cardiopulmonary bypass in children. Perfusion 1991; 6(1): 41–50. [DOI] [PubMed] [Google Scholar]
- 4.Myers GJ, Leadon RB, Mitchell LB, et al. Simple modified ultrafiltration. Perfusion 2000; 15(5): 447–452. [DOI] [PubMed] [Google Scholar]
- 5.Journois D, Israel-Biet D, Pouard P, et al. High-volume, zero-balanced hemofiltration to reduce delayed inflammatory response to cardiopulmonary bypass in children. Anesthesiology 1996; 85: 965–976. [DOI] [PubMed] [Google Scholar]
- 6.Zhang T, Jiang SL, Gao CQ, et al. Effect of subzero-balanced ultrafiltration on lung gas exchange capacity after cardiopulmonary bypass in adult patients with heart valve disease. Heart Surg Forum 2011; 14(1): 22–27. [DOI] [PubMed] [Google Scholar]
- 7.Osthaus WA, Görler H, Sievers J, et al. Bicarbonate-buffered ultrafiltration during pediatric cardiac surgery prevents electrolyte and acid-base balance disturbances. Perfusion 2009; 24(1): 19–25. [DOI] [PubMed] [Google Scholar]
- 8.Grist G, Whittaker C, Merrigan K, et al. The correlation of fluid balance changes during cardiopulmonary bypass to mortality in pediatric and congenital heart surgery patients. J Extra Corpor Technol 2011; 43(4): 215–226. [PMC free article] [PubMed] [Google Scholar]