Abstract
Background:
Living kidney donation is considered generally safe in healthy individuals; however, there is a need to better understand the long-term effects of donation on blood pressure and kidney function.
Objectives:
To determine the risk of hypertension in healthy, normotensive adults who donate a kidney compared with healthy, normotensive non-donors with similar indicators of baseline health. We will also compare the 2 groups on the rate of decline in kidney function, the risk of albuminuria, and changes in health-related quality of life.
Design, Participants, and Setting:
Prospective cohort study of 1042 living kidney donors recruited before surgery from 17 transplant centers (12 in Canada and 5 in Australia) between 2004 and 2014. Non-donor participants (n = 396) included relatives or friends of the donor, or donor candidates who were ineligible to donate due to blood group or cross-match incompatibility. Follow-up will continue until 2021, and the main analysis will be performed in 2022. The anticipated median (25th, 75th percentile, maximum) follow-up time after donation is 7 years (6, 8, 15).
Measurements:
Donors and non-donors completed the same schedule of measurements at baseline and follow-up (non-donors were assigned a simulated nephrectomy date). Annual measurements were obtained for blood pressure, estimated glomerular filtration rate (eGFR), albuminuria, patient-reported health-related quality of life, and general health.
Outcomes:
Incident hypertension (a systolic/diastolic blood pressure ≥ 140/90 mm Hg or receipt of anti-hypertensive medication) will be adjudicated by a physician blinded to the participant’s donation status. We will assess the rate of change in eGFR starting from 12 months after the nephrectomy date and the proportion who develop an albumin-to-creatinine ratio ≥3 mg/mmol (≥30 mg/g) in follow-up. Health-related quality of life will be assessed using the 36-item RAND health survey and the Beck Anxiety and Depression inventories.
Limitations:
Donation-attributable hypertension may not manifest until decades after donation.
Conclusion:
This prospective cohort study will estimate the attributable risk of hypertension and other health outcomes after living kidney donation.
Keywords: blood pressure, glomerular filtration rate, hypertension, kidney transplantation, living kidney donation, unilateral nephrectomy
Abrégé
Contexte:
Chez les personnes en bonne santé, faire don d’un rein est généralement considéré comme sûr. Il convient toutefois de mieux comprendre les effets à long terme de ce don sur la pression artérielle et la fonction rénale.
Objectifs:
Déterminer le risque d’hypertension chez les adultes sains et normotendus qui donnent un rein par rapport à des non-donneurs sains et normotendus ayant des indicateurs de santé de base similaires. Nous comparerons également le taux de réduction de la fonction rénale, le risque d’albuminurie et les changements dans la qualité de vie liée à la santé entre les deux groupes.
Cadre, type d’étude et participants:
Étude de cohorte rétrospective menée sur 1 042 donneurs de rein vivants, recrutés avant la chirurgie dans 17 centres de transplantation (12 au Canada et 5 en Australie) entre 2004 et 2014. Le groupe des non-donneurs (n=396) était constitué de parents ou amis du donneur, ou de candidats donneurs non admissibles à faire un don en raison d’une incompatibilité de groupe sanguin ou lors du test de compatibilité croisée. Le suivi s’est poursuivi jusqu’en 2021 et l’analyse principale sera effectuée en 2022. Le temps de suivi médian prévu (25e percentile, 75e percentile, maximum) après le don est de 7 ans (6, 8, 15 ans).
Mesures:
Les donneurs et les non-donneurs ont complété le même calendrier de mesures à l’inclusion et pendant le suivi (une date simulée de néphrectomie a été attribuée aux non-donneurs). Des mesures annuelles de pression artérielle, de débit de filtration glomérulaire estimé (DFGe), d’albuminurie, de qualité de vie liée à la santé autodéclarée et de santé générale ont été obtenues.
Issues principales:
L’hypertension incidente (pression artérielle systolique/diastolique ≥ 140/90 mm Hg ou prise d’un médicament antihypertenseur) sera jugée par un médecin aveugle au statut de don du participant. Nous évaluerons le taux de variation du DFGe à partir de 12 mois après la date de la néphrectomie et la proportion de participants qui développeront un rapport albumine/créatinine ≥ 3 mg/mmol (≥ 30 mg/g) pendant le suivi. La qualité de vie liée à la santé sera évaluée à l’aide du questionnaire de santé RAND de 36 questions et de l’Inventaire d’anxiété et de dépression de Beck.
Limites:
L’hypertension attribuable au don pourrait ne pas se manifester avant des décennies après le don.
Conclusion:
Cette étude de cohorte prospective permettra d’estimer le risque d’hypertension attribuable au don et d’autres effets sur la santé du donneur après un don de rein.
What was known before
Living kidney donation is considered safe in carefully selected candidates. However, the longer term effects of donation on blood pressure and kidney function remain uncertain, and several prior studies have been criticized for their lack of methodological rigor.
What this adds
This study will assess the attributable risk of living kidney donation using study techniques that meet modern criteria for high methodological quality. Non-donors will have similar indicators of baseline health as donors and will complete the same schedule of follow-up assessments.
Background
Each year, more than 27 000 people worldwide become living kidney donors, undergoing major surgery to donate a kidney to a patient with kidney failure.1 Living kidney donors generally have similar long-term health outcomes as the general population2-5; however, recent studies do show donors have a higher risk of developing kidney failure when compared with non-donors of similar baseline health, recognizing the 20-year risk in absolute terms remains less than 1%.6-9 Hypertension and albuminuria may be more prevalent among donors in the years following nephrectomy (studies summarized in eTable 1).5,10-18 Whether these outcomes are a direct result of nephrectomy, hereditary factors, or a combination of these and other factors remains unclear.19-24 In 2 recent systematic reviews, prior studies were reported to have poor quality.5,25 Specifically, the quality of evidence as assessed in 1 review for blood pressure, kidney function, and psychosocial outcomes after donation was reported to be very low.25
A better understanding of post-donation risk and the timing of new disease onset is critical for donor selection, informed consent, and follow-up. Donor candidates need to know how donation might affect their future health, and health professionals need to know what to expect when they monitor donors after nephrectomy to maintain long-term good health.26 Furthermore, while most donors report feelings of well-being after donation (studies summarized in eTable 2),27-31 some may experience negative psychological effects.32 Whether these effects are sustained over time remains unknown.
Estimating the attributable risk of donation requires a non-donor comparison group with a similar baseline risk as donors; however, in many studies, non-donors have not had an adequate assessment to demonstrate they have the same health as donors.13,14,16,17,33 As described in 1 recent systematic review, interpretation of the evidence has been complicated by diverse selection criteria for non-donor control groups (eg, general population vs based on donation criteria), follow-up durations, and analytic approaches (eg, different matching criteria or adjustment for potential confounders).5
In 2004, we launched a prospective cohort study of living kidney donors and non-donors in Canada and Australia. We designed this prospective study to meet high standards of methodological quality; for example, non-donors were assessed to confirm their similar baseline health as donors, and then undertook the same schedule of follow-up assessments. This protocol describes our study objectives, study design, and analytic plan.
Objectives
The objective of this study was to determine the risk of hypertension in healthy, normotensive adults who donate a kidney compared with healthy, normotensive non-donors. We will also compare the 2 groups on the rate of decline in kidney function, the risk of albuminuria, and changes in patient-reported health-related quality of life.
Methods
Design and Setting
The Living Kidney Donor Safety Study is a multicentre prospective cohort study examining the medical, financial, and psychological implications of living kidney donation (ClinicalTrials.gov Identifier: NCT00936078). The methods employed in this study were developed with guidance from an external advisory board and refined during a pilot phase from 2004 to 2008. In total, we enrolled 1042 living kidney donors (before surgery) and 396 healthy non-donors from 17 centers (12 in Canada and 5 in Australia) between 2004 and 2014 who met criteria for study inclusion as described in Table 1. Follow-up data collection continued until November 2021. All participants provided written, informed consent at the time of enrollment. Ethics approval was obtained from Western University’s Health Sciences Research Ethics Board (REB approval 6056) and all other recruiting centers. The conduct and reporting of this study follows recommended guidelines for strengthening the reporting of observational studies (eTable 3).34
Table 1.
Study Eligibility Criteria for Non-Donors and the Screening Criteria Used to Define Standard-Criteria Living Kidney Donors.
| Inclusion criteria | |
| Age | Age between 18 and 70 years. |
| Blood pressure | Average systolic/diastolic blood pressure <140/90 mm Hg based on an average of at least 3 blood pressure measurements taken during the recruitment interview. If the average of these blood pressure measurements was elevated, the participant was still eligible for participation if the average of an additional 12 home blood pressure readings was <140/90 mm Hg. All participants need to successfully record at least 12 home blood pressure readings using the self-monitoring device to be eligible. |
| Kidney function | Serum creatinine <115 μmol/L in men or <90 μmol/L in women35 or a Cockcroft-Gault estimated glomerular filtration rate >80 mL/min. |
| No urine protein | Negative urine dipstick for protein, or if trace or 0.3 g/L, a random urine albumin-to-creatinine ratio <8 mg/mmol. |
| No hematuria | Negative urine dipstick for hematuria. Those with non-persistent hematuria are eligible to participate; those with initial evidence of dipstick hematuria may have a second assessment, and for women, this should not occur during the time of menses. Individuals with hematuria that resolves after treatment of a urinary tract infection are eligible for study participation.36 |
| Non-obese body mass index | Body mass index <35 kg/m2. |
| Language | Ability to speak and read English or French. |
| Exclusion criteria | |
| Anti-hypertensive medication | Taking anti-hypertensive medication on a daily basis for any reason. |
| Kidney stones | Symptoms or evidence of kidney stones in the past 3 years. |
| History of kidney failure | Recipient of a kidney transplant; ever received dialysis. |
| Elevated plasma glucose or history of gestational diabetes | Plasma glucose ≥ 7.0 mmol/L after a 6-hour fast (if available); 2-hour oral glucose ≥11.1 mmol/L (if available). History of gestational diabetes. |
| Comorbidities | History of kidney disease; cancer, other than cured non-melanoma skin cancer; diabetes; cardiovascular disease; or pulmonary disease. |
| Contraindications to living kidney donation, general anesthesia, or surgery | Has a medical condition that would prevent them from becoming a living kidney donor or a known contraindication to general anesthesia or surgery. |
| Pregnancy | Currently pregnant or pregnant in the last month. |
| Other study participation | Participating in a clinical trial or another study that could influence the outcomes of this study. |
Donor candidates were automatically eligible to participate in the study if they were approved for donation by their local transplant center, were able to speak and read English and/or French, and were not participating in a clinical study that would affect the outcome of this study. Prospective donors who were approved for donation but who did not meet these screening criteria were deemed expanded-criteria donors (safety outcomes in expanded-criteria donors will be specified in a different protocol and examined in a separate analysis). To be eligible to participate in this study, non-donors had to meet the same screening criteria as standard-criteria donors.
Study Population
Prospective donor candidates were first asked about their interest in the study by their nephrologist or a living-donor coordinator at the transplant evaluation center; interested individuals were put in contact with a study research assistant who explained the study in greater detail, assessed eligibility, and obtained written informed consent. Non-donors included family members and friends of prospective donors, as well as some individuals who came forward for donation but did not proceed with donation despite being eligible, or who were ineligible due to blood group or cross-match incompatibility. As detailed below, prospective donors and potential non-donor participants completed a screening assessment, which involved a standardized health questionnaire, a blood pressure assessment (including up to 18 at-home blood pressure measurements), and lab testing for serum creatinine, urine protein, and hematuria.
Study eligibility
Prospective donors who were approved for donation but who did not meet the pre-specified screening criteria in Table 1, such as those with pre-donation hypertension, were deemed expanded-criteria donors and will be examined in a separate protocol and analysis.
To be eligible to participate in this study, non-donors had to pass the same screening criteria as standard-criteria donors (listed in Table 1), including the following: age 18 to 70 years, body mass index <35 kg/m2, systolic/diastolic blood pressure (SBP/DBP) <140/90 mm Hg, serum creatinine <115 μmol/L in men or <90 μmol/L in women35 (or a Cockcroft-Gault estimated glomerular filtration rate [eGFR] >80 mL/min), a negative urine dipstick for protein or a random urine albumin-to-creatinine ratio <8 mg/mmol (<70.8 mg/g), a negative urine dipstick for hematuria, and no comorbidities that would contraindicate donation, such as a history of kidney failure. These criteria reflect recommendations from the Amsterdam Forum on the Care of the Live Kidney Donor, a guidance document available when the study was being designed.37 We acknowledge that some of these criteria specify relatively high thresholds for risk according to contemporary criteria (eg, an albumin-to-creatinine ratio >8 mg/mmol vs ≥3 mg/mmol). However, when we report baseline characteristics, we expect most participants to have values far below the thresholds used for study eligibility.
Data Collection
Donors and non-donors were assigned the same schedule of baseline and follow-up measurements (summarized in eTable 4).
Baseline (pre-donation) assessment
All participants completed a standardized health questionnaire, which included questions on health-related quality of life, smoking, and alcohol consumption; participants had their blood pressure, height, and weight assessed and completed lab testing for serum creatinine (using isotope dilution mass spectrometry), urine protein, and hematuria. Participants completed a median of 14 at-home blood pressure measurements following a standardized protocol using the Omron Automatic Blood Pressure machine (HEM-705CP or HEM-711ACCAN), a self-monitoring device capable of storing 28 measurements. The device was fitted with an appropriately sized cuff for each participant’s arm circumference. Participants were taught how to use the device at the time of enrollment (seated with their feet flat on the floor, arm resting on a flat surface and cuff at level of the heart with no tight clothing on upper arm [detailed in eAppendix 1]). Participants were instructed not to share their device with others. Donor candidates were instructed to take their readings at least 1 week before nephrectomy to minimize any elevations due to anxiety.
Nephrectomy date
The donors’ nephrectomy date was obtained from hospital records, and non-donors were assigned a simulated nephrectomy date after they completed their baseline assessment.
Post-donation assessment
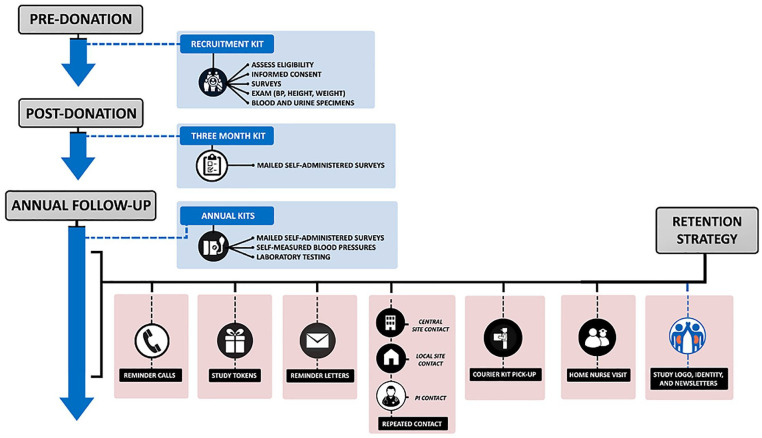
Twelve months after the nephrectomy date (simulated for non-donors), donors and non-donors were asked to complete 12 to 18 at-home blood pressure measurements as described above and were directed to nearby labs to provide blood and urine samples. These measurements were then repeated annually (from the date of nephrectomy) until 2019. Participants also completed an annual mailed health questionnaire at 3 and 12 months after donation, and then annually until 2019 (some psychosocial questionnaires were only completed 5 times in total). Multiple techniques were used to reduce participant loss to follow-up, including multiple contacts (or contact attempts) by phone, mail, or e-mail about any missing or discrepant data (Figure 1). If data on blood pressure, hypertension status, or lab tests were missing in 2019, we attempted to obtain this data until 2021.
Figure 1.
Recruitment, follow-up, and retention of participants in the living kidney donor safety study.
Outcomes
Medical outcomes
Hypertension
Incident hypertension will be adjudicated by a physician who is blinded to the participant’s donation status. A schematic of the decision process used to define hypertension is shown in eFigure 1. Adjudication will occur if a participant meets the following criteria in follow-up: (1) the participant reports a physician diagnosis of hypertension, (2) the participant reports taking medication for hypertension, or (3) the participant has an SBP ≥140 or a DBP ≥90 mmHg based on the average BP measurements at any follow-up visit (we expect participants will take at least 10 home blood pressure measurements over a median of 7 days at each annual follow-up visit). These blood pressure thresholds align with previous studies of living kidney donors and guidelines that were in effect at the study’s inception.5,38 The adjudication process will include a blinded review of medical chart data and/or follow-up with the participant in cases of missing data or discrepancies. As shown in eFigure 1, on its own, a participant-reported physician diagnosis of hypertension is insufficient to define hypertension but will prompt additional follow-up with the participant and/or a review of medical chart data to corroborate the diagnosis. In an additional analysis, we will define hypertension according to more recent guidelines, where stage 1 hypertension is defined as SBP/DBP 130 to 139/80 to 89 mm Hg39,40; the 294 participants whose blood pressure was in this range at the pre-donation baseline assessment will be excluded from this additional analysis. We will also assess the average change in SBP and DBP over time accounting for the use of anti-hypertensive medications.
Kidney function
Although an abrupt drop in eGFR after nephrectomy is normal, an ongoing accelerated loss of eGFR would be concerning.41-45 We will assess the annualized change in eGFR over time (in mL/min per 1.73 m2 per year) in donors and non-donors using all available eGFR measurements, setting the starting eGFR value to be the one obtained (1) 1 year after the nephrectomy date (or 1 year after the assigned nephrectomy date for non-donors), (2) 3 years after the nephrectomy date, and (3) at baseline (pre-donation).46 We will use the new creatinine-based Chronic Kidney Disease-Epidemiology Collaboration Equation to estimate GFR without race.47 We will also examine the proportion of participants whose eGFR fell below 60 mL/min per 1.73 m2 in follow-up, the proportion whose eGFR fell below 45 mL/min per 1.73 m2, and the proportion whose eGFR fell below 30 mL/min per 1.73 m2, acknowledging the proportions for the latter 2 categories are expected to be small.
Albuminuria
We will compare the geometric mean albumin-to-creatinine ratio in donors versus non-donors at the final follow-up visit, adjusted for the baseline (pre-donation) value. Values that are too low to measure will be recoded as 0.2 mg/mmol. We will also examine the proportion of participants who have an albumin-to-creatinine ratio ≥3 mg/mmol (≥30 mg/g) or >30 mg/mmol (>300 mg/g) at any time in follow-up, acknowledging the event rates for these variables are likely to be small.
Hypertension, an eGFR<60, and/or albuminuria
We will examine the proportion of participants who develop hypertension (as defined above), an eGFR <60 mL/min per 1.73 m2, or an albumin-to-creatinine ratio ≥3 mg/mmol. This outcome will be assessed as a composite, with death (expected to be rare during the follow-up period) treated as a competing event. We will also report the proportions of participants who develop (1) 2 or 3 of these components and (2) all 3 of these components.
Death, kidney failure, and major cardiovascular events
Death, kidney failure (ie, a persistent eGFR less than 15 mL/min per 1.73 m2, receipt of dialysis for any duration, or receipt of a kidney transplant), and major cardiovascular events (ie, myocardial infarction, stroke, or a cardiovascular procedure such as coronary angioplasty or coronary bypass surgery) will be assessed from the annual survey and from medical records, with adjudication conducted by a physician blinded to donation status.13,48 These outcomes will be assessed individually and as a composite.
Patient-reported health-related quality of life
Health-related quality of life will be assessed using the 36-item RAND Health Survey, which measures self-reported quality of life over the past 4 weeks across 8 scales: general health, physical functioning, energy/vitality, bodily pain, role limitations due to physical health, role limitations due to emotional health, social functioning, and mental health. The scales will be scored according to documented procedures using Canadian and Australian normative data as appropriate,49-52 and aggregated to form a physical component summary score and a mental component summary score.5,53-57 Higher scores indicate better health-related quality of life. Depression and anxiety will be assessed using the Beck Depression Inventory and the Beck Anxiety Inventory, respectively51,58; each inventory has 21 items and assesses symptoms experienced over the past week and past month, respectively. Scores range from 0 (no depression or anxiety) to 63 (severe depression or anxiety).59,60
Tracer outcome
Tinnitus (the perception of chronic ringing or noise in the ears) will be examined as a tracer outcome (ie, a marker of self-report bias) because it is expected to be similar in donors and non-donors. Donors and non-donors with a history of tinnitus at enrollment will be excluded from this analysis.
Study Size
For a living kidney donation to proceed, the donor candidate, the recipient, and the transplant team must all accept potential risks to the donor. The acceptable magnitude of risk varies among these groups. Before undertaking the present study, we conducted a survey to better understand how these groups defined acceptable thresholds of donor risk. Our results showed that potential donors were significantly more willing to accept greater long-term donor risks than transplant professionals, and potential recipients were the most averse to donor risk.61 For the outcome of hypertension, we found that 47% of potential donors were willing to accept a 10-year risk of hypertension that was 3 times higher than the estimated risk of 15% in non-donors, whereas only 12% of potential recipients and 24% of transplant professionals believed this level of risk was acceptable.
We originally designed this study to rule out a 2-fold higher risk of hypertension in donors compared with non-donors. With a median follow-up time of 7.5 years, a sample size of 900 standard-criteria donors and 390 non-donors will provide >80% power to detect a hazard ratio of 1.6 of hypertension in donors versus non-donors, assuming an overall event rate of 15% and 5% loss to follow-up (2-sided α = 0.05).62
Ratio of donors to non-donors
The ratio of donors to non-donors in this study is ~2:1. Although less statistically efficient than a 1:1 ratio, we learned during the pilot phase that a 2:1 ratio was more cost-efficient and feasible.
Statistical Analysis
The data integrity and analysis of this study is overseen by an external advisory board. We will conduct all analyses using SAS software Version 9.4. Missing data will be imputed using fully conditional specification multiple imputation.63 Additional analyses will be performed to confirm that conclusions are not sensitive to assumptions about the reasons for missing data. Regression diagnostics will be used to examine model fit, identify influential observations, and assess model assumptions.
Based on our pilot study, we anticipate donors and non-donors will be similar on all baseline characteristics except for a family history of kidney failure and hypertension, which will be more common in donors versus non-donors. To account for potential baseline imbalance between groups, we will use inverse probability of treatment weighting, where the weight is based on an estimated propensity score. This propensity score is defined as the probability of donating a kidney (vs not donating a kidney), conditional on pre-donation (baseline) characteristics, including age, sex, pre-donation SBP and DBP, pre-donation body mass index, a family history of kidney failure, and a family history of hypertension.63 The average treatment effect in the treated (ATT) will be estimated, where “treatment” refers to donation; methods to account for the weighting will be used.64 Covariate adjustment will be used in sensitivity analyses. Between-group differences in outcomes will be expressed in absolute and relative terms, with 95% confidence intervals (CIs) presented for all comparisons. The weighted analysis for each type of outcome is described below.
Time-to-event outcomes
Cause-specific weighted hazard ratios for time-to-event outcomes (ie, hypertension, albuminuria, kidney failure, major cardiovascular events, death, and the pre-specified composite outcomes) will be estimated using Cox proportional hazards regression. This model handles events and censoring for loss to follow-up in the same time interval.65 We will assess the proportionality assumption for each variable using the time-interaction test, and by graphing the weighted log (–log [survival functions]) versus log time.66 In the presence of significant violations of the proportional hazards assumption (eg, if the relative hazards of hypertension in donors vs non-donors narrows over time), an extended Cox regression model with time-dependent variables will be considered. The cumulative incidence function of each outcome at 5, 10, and 15 years will be displayed as a graph. Weights based on the estimated propensity score will be used to estimate the cumulative incidence function curves.67 Finally, weighted risk ratios for hypertension, reduced kidney function, and albuminuria will be examined as binary outcomes using modified Poisson regression and weighted absolute risk differences using predictive margins.
Continuous outcomes
The between-group difference in the change in continuous outcomes (SBP, DBP, eGFR, and psychosocial health scales measured annually) will be examined using weighted linear mixed-effect models accounting for repeated measures within the same individual.68 Models will include random, individual-specific intercepts and slopes with an unstructured covariance between the random effects to account for correlation among measurements within the same individual. Changes over time will be graphed using splines for any non-linear changes.
For the analyses of SBP and DBP analyzed separately, the initiation of anti-hypertensive medication will be accounted for in 2 ways: (1) the last available BP measurement before the medication start date will be retained in the model, excluding the remaining BP measures and (2) an SBP of 140 and a DBP of 90 will be imputed for the first visit after the medication start date, censoring the remaining follow-up time. We will compare the final follow-up mean SBP and DBP values between donors and non-donors, adjusted for the pre-donation mean SBP and DBP values.
The weighted annualized change in eGFR will be analyzed with different start times as defined above. eGFR measurements and their interaction with time will be included in the models as fixed effects, or using linear splines to allow the eGFR slope to change over time. In addition, we will examine the weighted between-group differences in the absolute change and the percentage change in eGFR, comparing the 1-year eGFR with the baseline (pre-donation) eGFR, the final follow-up eGFR with the baseline (pre-donation) eGFR, and the final follow-up eGFR with the 1-year eGFR.
The weighted geometric mean ratio of the albumin-to-creatinine ratio (mg/mmol) in donors versus non-donors will be estimated using linear regression, where the outcome is the log-transformed value of the final albumin-to-creatinine ratio adjusted for the group indicator variable, and the log-transformed value of the baseline albumin-to-creatinine ratio.68
The weighted between-group difference in the health-related quality of life variables in donors versus non-donors will be examined at 3 months, 1 year, and 5 years after nephrectomy (or the simulated nephrectomy date for non-donors) using separate linear regression models, where the outcome is the follow-up value adjusted for the group indicator variable and the pre-donation value.68
Subgroup analyses
We will conduct exploratory subgroup analyses of medical outcomes by family history of kidney failure because donors are often related to their recipient and are therefore more likely than non-donors to have a family history of these outcomes.3,24,69-71 We will similarly conduct exploratory subgroup analyses by sex and by baseline (pre-donation) age, SBP, DBP, and eGFR.
Interpretation of results
We have decided not to perform any significance testing (P value reporting) in this study. Rather, point estimates of differences between donors and non-donors will be accompanied by 95% CIs with no adjustment for multiple comparisons. We will interpret results cautiously and consider the range of estimated effect sizes and their clinical importance, with the recognition we are assessing harm rather than efficacy. In this interpretation, we will consider the perspectives of potential donors, prior donors, transplant recipients, and physicians. We will describe the width of CIs in plain language (a simplified and hypothetical example would be: our data are compatible with a decrease in risk of 3% and an increase in risk as high as 48%).72 We will report the consistency of our results in sensitivity analyses and how our findings align with prior evidence.
Assessment of study quality
Assuming less than 10% of participants will be lost to follow-up, the design and anticipated reporting of this study is expected to achieve a rating of 9/9 on the Newcastle Ottawa Scale (eTable 5; as used in a recent systematic review, where 9 is the highest quality),5 and a low risk of bias according to the Research Triangle Institute Bank (a quality assessment scale used in the other recent systematic review).25 As shown in eTable 6, the donors in this study have similar pre-donation characteristics as all donors who underwent nephrectomy at participating centers in Ontario during the recruiting period, suggesting study participants are representative of donors in routine care.
Discussion
Living kidney donation is practiced with the understanding that the minimal risk of short-term and long-term medical harm realized by the donor is outweighed by the clear advantage to the recipient. While donors have a similar life expectancy as healthy non-donors, the long-term effects of donation on blood pressure and kidney function remain uncertain. We are conducting a prospective cohort study to determine if healthy normotensive adults who donate a kidney have a higher risk of hypertension compared with similar healthy non-donors. We will also compare donors and non-donors on standard measures of kidney health and psychological well-being.
Kidney function and blood pressure are inextricably linked, and it is biologically plausible that the loss of kidney function that occurs after nephrectomy could lead to an increase in blood pressure over time, beyond what occurs naturally with aging. Acknowledging the methodological limitations of prior studies, in a previous meta-analysis, we found that 5 to 10 years after nephrectomy, donors’ mean arterial pressure was 5 mm Hg higher than non-donor controls.11 An increased risk of hypertension in donors relative to non-donors has been reported in several studies.11,13-17,33 The true risk for hypertension remains unclear, however, because studies have not defined hypertension uniformly, non-donors have not undergone the same health screening as donors, and follow-up in donors and non-donors has been dissimilar and incomplete. A better understanding of this risk is central to donor selection and consent. In addition, it could guide policies that reimburse donors for their expenses including the costs of anti-hypertensive medications.
After nephrectomy, donors experience an abrupt drop in kidney function—on average, eGFR will fall from ~95 to 65 mL/min per 1.73 m2, and in 10% to 40% of donors, eGFR will fall below 60 mL/min per 1.73 m2.10,42 While an eGFR <60 is associated with a higher risk of cardiovascular disease and mortality in the presence of diabetes, vascular diseases, and other pathologies, these conditions are rarely present in donors, whose low eGFR is due to the nephrectomy. Two retrospective studies, however, have reported a higher risk of kidney failure in donors 15 to 25 years after donation, although the absolute risks at this follow-up time were <0.5%.7-9 It is likely that some of this increased risk is explained by genetic and environmental risk factors shared between related donors and recipients.23,24,69-71,73 As well, because donors have less renal reserve, a progressive loss of kidney function (related or unrelated to donation) may hasten the development of kidney failure in donors relative to non-donors.74 Examining the rates of eGFR decline in donors is clearly important. Small differences in the early trajectory of eGFR decline may translate to large, long-term differences and a shorter time to developing kidney failure. To account for the large drop in eGFR that occurs after nephrectomy, we will conduct separate analyses, setting the starting eGFR value to be the one obtained (1) 1 year after the nephrectomy date (or 1 year after the simulated nephrectomy date for non-donors), (2) 3 years after the nephrectomy date, and (3) at baseline (pre-donation).
Most donors experience improved psychosocial well-being after donating a kidney, often to a family member or friend, and most confirm they would make a similar decision if given the choice again.27,31,75 While reassuring, some prospective evaluations and qualitative studies have reported that some donors experience elevated depression and anxiety, particularly if their recipient has a poor outcome.75 Some donors may also develop concerns about their own health, and may worry that their remaining kidney might fail.76 Our prospective study will help clarify the psychological effects of living kidney donation.
We have designed this study to address limitations and challenges common to previous studies of living kidney donors, including retrospective data collection, small sample sizes, biased comparisons with non-donors who do not have the same baseline risk as donors, surveillance bias, and high loss to follow-up.77,78 As described, our study is expected to generate estimates with a low risk of bias. Specifically, we are conducting a multicentre prospective cohort study, which is the strongest study design for the research questions posed given randomization is not possible. While our focus is on reporting CIs for estimates of effect, this study does have adequate power to detect a clinically relevant 60% higher risk of hypertension after donation. Non-donors must pass eligibility criteria to have a similar baseline risk for the study outcomes as donors. To minimize surveillance bias, both donors and non-donors will have the same annual schedule of blood pressure assessment during follow-up, and key medical outcomes will be assessed by central adjudicators who are blinded to donation status. Finally, we are employing numerous retention efforts to maximize sample retention, including regular contact with participants through newsletters, cards, and reminder post cards; follow-up calls for missing survey responses and discrepant data; and home visits and assistance with transportation if needed. This is being done so that we minimize the number of participants lost to long-term follow-up.
Our study has some limitations. Given the limited number of events, the CIs for the estimated risk of death, kidney failure, and major cardiovascular events,13 and for the assessment of effect modification by family history of kidney failure, will be imprecise and wide. Similarly, if the CI for our estimate of hypertension contains a value of 1.0, smaller but clinically meaningful higher risks of hypertension will likely be within the upper bound of the 95% CI (eg, an increase in risk of 25% as seen in some other recent studies).14,33 As well, the relative risk of hypertension and other outcomes may not be linear over time and may only be apparent with follow-up times longer than the 8-year median expected in this study. For future studies, we have integrated a number of strategies to facilitate extended follow-up of our cohort beyond 2021, including consent to link participant data to administrative health care databases.
Conclusion
Living kidney donation is practiced with the expectation that risks of minimal donor harm are outweighed by psychological benefits of altruism to the donor and improved recipient health. We designed this study in the early 2000s to meet modern epidemiologic standards for proper health risk assessment. Our multicentre prospective cohort study of living kidney donors will inform the practice and safety of living kidney donation, including transplant center medical policies on donor selection, patient counseling, informed consent, and long-term patient follow-up and care.
Supplemental Material
Supplemental material, sj-docx-1-cjk-10.1177_20543581221129442 for The Living Kidney Donor Safety Study: Protocol of a Prospective Cohort Study by Amit X. Garg, Jennifer B. Arnold, Meaghan Cuerden, Christine Dipchand, Liane S. Feldman, John S. Gill, Martin Karpinski, Scott Klarenbach, Greg A. Knoll, Charmaine Lok, Matthew Miller, Mauricio Monroy-Cuadros, Christopher Nguan, G. V. Ramesh Prasad, Jessica M. Sontrop, Leroy Storsley and Neil Boudville in Canadian Journal of Kidney Health and Disease
Supplemental material, sj-docx-2-cjk-10.1177_20543581221129442 for The Living Kidney Donor Safety Study: Protocol of a Prospective Cohort Study by Amit X. Garg, Jennifer B. Arnold, Meaghan Cuerden, Christine Dipchand, Liane S. Feldman, John S. Gill, Martin Karpinski, Scott Klarenbach, Greg A. Knoll, Charmaine Lok, Matthew Miller, Mauricio Monroy-Cuadros, Christopher Nguan, G. V. Ramesh Prasad, Jessica M. Sontrop, Leroy Storsley and Neil Boudville in Canadian Journal of Kidney Health and Disease
Acknowledgments
We are grateful to members of the external advisory committee for ensuring the integrity of this study: Dr Stephen Walter, Dr Sheldon Tobe, and the late Dr David Sackett. We are also grateful to the following individuals: Mary Amanda Dew, Christina Frederiksen, Dariusz Gozdzik, Kim Guastadisegni, Melodie Jansen, Ngan N Lam, Michelle Nash, Lindita Rapi, Steven Tang, Darin Treleaven, Ann Young, and Weiqiu Yuan.
Footnotes
Author Contributions: A.X.G. took primary responsibility for organizing the team, obtaining grant funding, and supervising the data coordinating center. All authors contributed to the study design, methodology, data collection, and analysis plan. A.X.G., M.C., and J.M.S. drafted the manuscript. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate: Ethics approval was obtained from Western University’s Research Ethics Board and from all participating centers. Written informed consent was obtained from all the participants before enrollment.
Consent for Publication: All authors have consented to publication.
Availability of Data and Materials: Data and materials cannot be made publicly available due to restrictions on its disclosure and use.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: During the study period, Dr Garg was supported by the Dr Adam Linton Chair in Kidney Health Analytics and a Clinician Investigator Salary Award from the CIHR, and he was the joint Ontario Renal Network-Trillium Gift of Life Network Provincial Medical Lead for Access to Kidney Transplantation. Dr Boudville was supported by grants from the National Health and Medical Research Council and the Raine Foundation; he also received unrestricted educational grants and consulting fees from Baxter, Amgen, Astra Zeneca, Otsuka, and Vifor. Dr Feldman was supported by a grant from Theator and received speaker fees from Abbott and Merck. Dr Gill was supported by a Foundation Award from the CIHR and he received grant support from Astellas Canada and Canadian Blood Services, and he served as a consultant for Veloxis, Takeda. Dr Klarenbach was supported by a Kidney Health Research Chair and the Division of Nephrology at the University of Alberta, and he was the Director of the Real World Evidence Consortium.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Canadian Institutes of Health Research (CIHR) provided operating grant support. Astellas Canada and Novartis provided partnership funding for the CIHR-funded grant. The funders/sponsors had no role in the study design or data collection and will have no role in the analysis or interpretation of data, the writing of the final report, or the decision to publish the final manuscript.
ORCID iDs: Amit X. Garg  https://orcid.org/0000-0003-3398-3114
https://orcid.org/0000-0003-3398-3114
Jessica M. Sontrop  https://orcid.org/0000-0001-7784-2028
https://orcid.org/0000-0001-7784-2028
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Horvat LD, Shariff SZ, Garg AX; Donor Nephrectomy Outcomes Research Network. Global trends in the rates of living kidney donation. Kidney Int. 2009;75(10):1088-1098. [DOI] [PubMed] [Google Scholar]
- 2. Ibrahim HN, Foley R, Tan L, et al. Long-term consequences of kidney donation. N Engl J Med. 2009;360:459-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fournier C, Pallet N, Cherqaoui Z, et al. Very long-term follow-up of living kidney donors. Transpl Int. 2012;25:385-390. [DOI] [PubMed] [Google Scholar]
- 4. Mjøen G, Reisaeter A, Hallan S, et al. Overall and cardiovascular mortality in Norwegian kidney donors compared to the background population. Nephrol Dial Transplant. 2012;27:443-447. [DOI] [PubMed] [Google Scholar]
- 5. O’Keeffe LM, Ramond A, Oliver-Williams C, et al. Mid- and long-term health risks in living kidney donors. Ann Intern Med. 2018;168:276-284. [DOI] [PubMed] [Google Scholar]
- 6. Wainwright J. Researchers Explore the 25-Year Risk of ESKD Among Living Kidney Donors. Boston, MA: American Transplant Congress; 2022. [Google Scholar]
- 7. Mjøen G, Hallan S, Hartmann A, et al. Long-term risks for kidney donors. Kidney Int. 2014;86:162-167. [DOI] [PubMed] [Google Scholar]
- 8. Muzaale AD, Massie AB, Wang M-C, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311:579-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Massie AB, Holscher CM, Henderson ML, et al. Association of early postdonation renal function with subsequent risk of end-stage renal disease in living kidney donors. JAMA Surg. 2020;155:e195472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garg AX, Muirhead N, Knoll G, et al. Proteinuria and reduced kidney function in living kidney donors: a systematic review, meta-analysis, and meta-regression. Kidney Int. 2006;70(10):1801-1810. [DOI] [PubMed] [Google Scholar]
- 11. Boudville N, Prasad GVR, Knoll G, et al. Meta-analysis: risk for hypertension in living kidney donors. Ann Intern Med. 2006;145:185-196. [DOI] [PubMed] [Google Scholar]
- 12. Garg AX, Nevis IF, McArthur E, et al. Gestational hypertension and preeclampsia in living kidney donors. N Engl J Med. 2015;372:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chaudry M, Gislason GH, Fosbøl EL, et al. Hypertension, cardiovascular disease and cause of death in Danish living kidney donors: matched cohort study. BMJ Open. 2020;10:e041122. doi: 10.1136/bmjopen-2020-041122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haugen AJ, Hallan S, Langberg NE, et al. Increased long-term risk for hypertension in kidney donors—a retrospective cohort study. Transpl Int. 2020;33(5):536-543. [DOI] [PubMed] [Google Scholar]
- 15. Doshi MD, Goggins MO, Li L, Garg AX. Medical outcomes in African American live kidney donors: a matched cohort study. Am J Transplant. 2013;13(1):111-118. [DOI] [PubMed] [Google Scholar]
- 16. Lentine KL, Schnitzler MA, Xiao H, et al. Racial variation in medical outcomes among living kidney donors. N Engl J Med. 2010;363:724-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garg AX, Prasad GVR, Thiessen-Philbrook HR, et al. Cardiovascular disease and hypertension risk in living kidney donors: an analysis of health administrative data in Ontario, Canada. Transplantation. 2008;86:399-406. [DOI] [PubMed] [Google Scholar]
- 18. Holscher CM, Bae S, Thomas AG, et al. Early hypertension and diabetes after living kidney donation. Transplantation. 2019;103(6):1216-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matas AJ, Vock DM, Ibrahim HN. GFR ≤25 years postdonation in living kidney donors with (vs. without) a first-degree relative with ESRD. Am J Transplant. 2018;18(3):625-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matas AJ, Hays RE, Ibrahim HN. A case-based analysis of whether living related donors listed for transplant share ESRD causes with their recipients. Clin J Am Soc Nephrol. 2017;12:663-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matas AJ, Berglund DM, Vock DM, Ibrahim HN. Causes and timing of end-stage renal disease after living kidney donation. Am J Transplant. 2018;18(5):1140-1150. [DOI] [PubMed] [Google Scholar]
- 22. Steiner RW. “You can’t get there from here”: critical obstacles to current estimates of the ESRD risks of young living kidney donors. Am J Transplant. 2019;19:32-36. doi: 10.1111/ajt.15089. [DOI] [PubMed] [Google Scholar]
- 23. Wainright JL, Robinson AM, Wilk AR, Klassen DK, Cherikh WS, Stewart DE. Risk of ESRD in prior living kidney donors. Am J Transplant. 2018;18(5):1129-1139. [DOI] [PubMed] [Google Scholar]
- 24. Muzaale AD, Massie AB, Al Ammary F, et al. Donor-recipient relationship and risk of ESKD in live kidney donors of varied racial groups. Am J Kidney Dis. 2020;75(3):333-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Slinin Y, Brasure M, Eidman K, et al. Long-term outcomes of living kidney donation. Transplantation. 2016;100:1371-1386. [DOI] [PubMed] [Google Scholar]
- 26. Kasiske BL, Asrani SK, Dew MA, et al. The living donor collective: a scientific registry for living donors. Am J Transplant. 2017;17(12):3040-3048. [DOI] [PubMed] [Google Scholar]
- 27. Fehrman-Ekholm I, Brink B, Ericsson C, et al. Kidney donors don’t regret: follow-up of 370 donors in Stockholm since 1964. Transplantation. 2000;69:2067-2071. [DOI] [PubMed] [Google Scholar]
- 28. Franklin PM, Crombie AK. Live related renal transplantation: psychological, social, and cultural issues. Transplantation. 2003;76:1247-1252. [DOI] [PubMed] [Google Scholar]
- 29. Pawłowski M, Fila-Witecka K, Rymaszewska JE, et al. Quality of life, depression and anxiety in living donor kidney transplantation. Transplant Rev (Orlando). 2020;34:100572. doi: 10.1016/j.trre.2020.100572. [DOI] [PubMed] [Google Scholar]
- 30. Rodrigue JR, Schold JD, Morrissey P, et al. Mood, body image, fear of kidney failure, life satisfaction, and decisional stability following living kidney donation: findings from the KDOC study. Am J Transplant. 2018;18(6):1397-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gross CR, Messersmith EE, Hong BA, et al. Health-related quality of life in kidney donors from the last five decades: results from the RELIVE study. Am J Transplant. 2013;13(11):2924-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Menjivar A, Torres X, Manyalich M, et al. Psychosocial risk factors for impaired health-related quality of life in living kidney donors: results from the ELIPSY prospective study. Sci Rep. 2020;10:21343. doi: 10.1038/s41598-020-78032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Holscher CM, Haugen CE, Jackson KR, et al. Self-reported incident hypertension and long-term kidney function in living kidney donors compared with healthy nondonors. Clin J Am Soc Nephrol. 2019;14:1493-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vandenbroucke JP, von EE, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147:W163-W194. [DOI] [PubMed] [Google Scholar]
- 35. Couchoud C, Pozet N, Labeeuw M, Pouteil-Noble C. Screening early renal failure: cut-off values for serum creatinine as an indicator of renal impairment. Kidney Int. 1999;55(5):1878-1884. [DOI] [PubMed] [Google Scholar]
- 36. Koushik R, Garvey C, Manivel JC, Matas AJ, Kasiske BL. Persistent, asymptomatic, microscopic hematuria in prospective kidney donors. Transplantation. 2005;80:1425-1429. [DOI] [PubMed] [Google Scholar]
- 37. Delmonico F. A report of the Amsterdam forum on the care of the live kidney donor: data and medical guidelines. Transplantation. 2005;79:S53-S66. [PubMed] [Google Scholar]
- 38. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206-1252. [DOI] [PubMed] [Google Scholar]
- 39. Cifu AS, Davis AM. Prevention, detection, evaluation, and management of high blood pressure in adults. JAMA. 2017;318:2132-2134. [DOI] [PubMed] [Google Scholar]
- 40. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. J Am Coll Cardiol. 2018;71:e127-e248. [DOI] [PubMed] [Google Scholar]
- 41. Barri Y, Parker T, III, Kaplan B, Glassock R. Primum non Nocere: is chronic kidney disease staging appropriate in living kidney transplant donors? Am J Transplant. 2009;9(4):657-660. [DOI] [PubMed] [Google Scholar]
- 42. Barri YM, Parker T, Daoud Y, et al. Definition of chronic kidney disease after uninephrectomy in living donors: what are the implications? Transplantation. 2010;90:575-580. [DOI] [PubMed] [Google Scholar]
- 43. Kido R, Shibagaki Y, Iwadoh K, et al. Very low but stable glomerular filtration rate after living kidney donation: is the concept of “chronic kidney disease” applicable to kidney donors? Clin Exp Nephrol. 2010;14:356-362. [DOI] [PubMed] [Google Scholar]
- 44. Kovesdy CP. Rate of kidney function decline associates with increased risk of death. J Am Soc Nephrol. 2010;21(11):1814-1816. [DOI] [PubMed] [Google Scholar]
- 45. Matsushita K, Selvin E, Bash LD, Franceschini N, Astor BC, Coresh J. Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol. 2009;20(12):2617-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lam NN, Lloyd A, Lentine KL, et al. Changes in kidney function follow living donor nephrectomy. Kidney Int. 2020;98(1):176-186. [DOI] [PubMed] [Google Scholar]
- 47. Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385:1737-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mahaffey KW, Harrington RA, Akkerhuis M, et al. Systematic adjudication of myocardial infarction end-points in an international clinical trial. Curr Control Trials Cardiovasc Med. 2001;2:180-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ware J. SF-36 health survey update. Spine. 2000;25:3130-3139. [DOI] [PubMed] [Google Scholar]
- 50. Beck A, Steer R, Brown G. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 51. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893-897. [DOI] [PubMed] [Google Scholar]
- 52. Hopman W, Towheed T, Anastassiades T, et al. Canadian normative data for the SF-36 health survey. CMAJ. 2000;163:265-267. [PMC free article] [PubMed] [Google Scholar]
- 53. Ware JE, Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Razek A. Comparison of methods for the scoring and statistical analysis of the SF-36 health profile and summary measures: results from the Medical Outcomes Study. Med Care. 1995;33:AS264-AS279. [PubMed] [Google Scholar]
- 54. Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483. [PubMed] [Google Scholar]
- 55. Ware JE, Jr, Snow KK, Kosinski MA, Gandek BG. SF-36 Health Survey Manual and Interpretation Guide. Boston, MA: The Health Institute; 1993. [Google Scholar]
- 56. Ware JE, Jr, Kosinski MA, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: The Health Institute; 1994. [Google Scholar]
- 57. Tong A, Chapman JR, Wong G, et al. Screening and follow-up of living kidney donors: a systematic review of clinical practice guidelines. Transplantation. 2011;92:962-972. [DOI] [PubMed] [Google Scholar]
- 58. Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561-571. [DOI] [PubMed] [Google Scholar]
- 59. Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77-100. [Google Scholar]
- 60. Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res (Hoboken). 2011;63(suppl 11):S467-S472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Young A, Karpinski M, Treleaven D, et al. Differences in tolerance for health risk to the living donor among potential donors, recipients, and transplant professionals. Kidney Int. 2008;73(10):1159-1166. [DOI] [PubMed] [Google Scholar]
- 62. Hsieh FY, Lavori PW. Sample-size calculations for the Cox proportional hazards regression model with nonbinary covariates. Control Clin Trials. 2000;21(6):552-560. [DOI] [PubMed] [Google Scholar]
- 63. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219-242. [DOI] [PubMed] [Google Scholar]
- 64. Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35:5642-5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Klein J, Moeschberger M. Survival Analysis: Techniques for Censored and Truncated Data. 2nd ed. New York, NY: Springer; 2003. [Google Scholar]
- 66. Kleinbaum DG, Klein M. Evaluating the proportional hazards assumption. In: Gail M, Samet JM. (eds) Survival Analysis. Statistics for Biology and Health. New York, NY: Springer; 2011:131-171. [Google Scholar]
- 67. Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75(1):45-49. [DOI] [PubMed] [Google Scholar]
- 68. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Skrunes R, Svarstad E, Reisæter AV, et al. Familial clustering of ESRD in the Norwegian population. Clin J Am Soc Nephrol. 2014;9:1692-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. O’Dea DF, Murphy SW, Hefferton D, Parfrey PS. Higher risk for renal failure in first-degree relatives of white patients with end-stage renal disease: a population-based study. Am J Kidney Dis. 1998;32(5):794-801. [DOI] [PubMed] [Google Scholar]
- 71. Lei HH, Perneger TV, Klag MJ, Whelton PK, Coresh J. Familial aggregation of renal disease in a population-based case-control study. J Am Soc Nephrol. 1998;9(7):1270-1276. [DOI] [PubMed] [Google Scholar]
- 72. Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature. 2019;567:305-307. [DOI] [PubMed] [Google Scholar]
- 73. Massie AB, Muzaale AD, Luo X, et al. Quantifying postdonation risk of ESRD in living kidney donors. J Am Soc Nephrol. 2017;28(9):2749-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gill JS, Tonelli M. Understanding rare adverse outcomes following living kidney donation. JAMA. 2014;311:577-579. [DOI] [PubMed] [Google Scholar]
- 75. Clemens KK, Thiessen-Philbrook H, Parikh CR, et al. Psychosocial health of living kidney donors: a systematic review. Am J Transplant. 2006;6(12):2965-2977. [DOI] [PubMed] [Google Scholar]
- 76. Tanriverdi N, Ozçürümez G, Colak T, et al. Quality of life and mood in renal transplantation recipients, donors, and controls: preliminary report. Transplant Proc. 2004;36(1):117-119. [DOI] [PubMed] [Google Scholar]
- 77. Ommen ES, Winston JA, Murphy B. Medical risks in living kidney donors: absence of proof is not proof of absence. CJASN. 2006;1:885-895. [DOI] [PubMed] [Google Scholar]
- 78. Ommen ES, LaPointe Rudow D, Medapalli RK, et al. When good intentions are not enough: obtaining follow-up data in living kidney donors. Am J Transplant. 2011;11:2575-2581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cjk-10.1177_20543581221129442 for The Living Kidney Donor Safety Study: Protocol of a Prospective Cohort Study by Amit X. Garg, Jennifer B. Arnold, Meaghan Cuerden, Christine Dipchand, Liane S. Feldman, John S. Gill, Martin Karpinski, Scott Klarenbach, Greg A. Knoll, Charmaine Lok, Matthew Miller, Mauricio Monroy-Cuadros, Christopher Nguan, G. V. Ramesh Prasad, Jessica M. Sontrop, Leroy Storsley and Neil Boudville in Canadian Journal of Kidney Health and Disease
Supplemental material, sj-docx-2-cjk-10.1177_20543581221129442 for The Living Kidney Donor Safety Study: Protocol of a Prospective Cohort Study by Amit X. Garg, Jennifer B. Arnold, Meaghan Cuerden, Christine Dipchand, Liane S. Feldman, John S. Gill, Martin Karpinski, Scott Klarenbach, Greg A. Knoll, Charmaine Lok, Matthew Miller, Mauricio Monroy-Cuadros, Christopher Nguan, G. V. Ramesh Prasad, Jessica M. Sontrop, Leroy Storsley and Neil Boudville in Canadian Journal of Kidney Health and Disease