Abstract
Background:
Individuals with ischemic stroke or transient ischemic attack (TIA) have a high early risk of ischemic stroke despite dual antiplatelet therapy. The risk of ischemic stroke, and associated disability, represents a significant unmet clinical need. Genetic variants resulting in reduced factor XI levels are associated with reduced risk for ischemic stroke but are not associated with increased intracranial bleeding. Milvexian is an oral small-molecule inhibitor of FXIa that binds activated factor XI with high affinity and selectivity and may reduce the risk of stroke when added to antiplatelet drugs without significant bleeding. We aimed to evaluate the dose-response relationship of milvexian in participants treated with dual antiplate-lets.
Methods:
We began a phase II, double-blinded, randomized, placebo-controlled trial at 367 sites in 2019. Participants (N = 2366) with ischemic stroke (National Institutes of Health Stroke Scale score ≤7) or high-risk TIA (ABCD2 score ≥6) were randomized to 1 of 5 doses of milvexian or placebo for 90 days. Participants also received clopidogrel 75 mg daily for the first 21 days and aspirin 100 mg for 90 days. The efficacy endpoint was the composite of ischemic stroke or incident infarct on magnetic resonance imaging. Major bleeding, defined as type 3 or 5 bleeding according to the Bleeding Academic Research Consortium, was the safety endpoint. Participant follow-up will end in 2022.
Conclusion:
The AXIOMATIC-SSP trial will evaluate the dose-response of milvexian for ischemic stroke occurrence in participants with ischemic stroke or TIA.
Keywords: Stroke—Stroke, prevention—Milvexian—Factor, XI—MRI—Randomized trial
Study rationale
Early stroke occurrence after ischemic stroke or transient ischemic attack (TIA) remains a significant risk despite advances in secondary prevention. Ischemic stroke is largely a thromboembolic disease, and major efforts to reduce the early risk of recurrent stroke in patients with non-cardioembolic stroke have focused on antiplatelet strategies.1 Improvements in outcome have been associated with novel antiplatelet strategies, but significant residual risk of ischemic stroke and the potential for major bleeding, including intracranial hemorrhage, limit the effectiveness of these options. In a recent trial, patients with minor ischemic stroke and high-risk TIA had a risk of stroke within 90 days of 6.3% when treated with aspirin and 4.6% when treated with the combination of aspirin and clopidogrel with an increase in major bleeding.1 Clopidogrel is a prodrug that requires activation through the cytochrome P450 2C19 (CYP2C19) system and has reduced efficacy in those with loss of function alleles.2, 3 Ticagrelor does not require conversion to an active metabolite and is unaffected by the presence of loss of function alleles.4 In individuals with loss of function alleles, ticagrelor combined with aspirin reduces stroke occurrence better than the combination of clopidogrel combined with aspirin.3 Cilostazol-based dual antiplatelet therapy seems to be effective in chronic stroke, but the effect against early recurrence has been uncertain.5, 6 There is an unmet need for an antithrombotic strategy that reduces the risk of ischemic stroke with a low potential to cause serious bleeding.
Emboli that cause stroke may arise from multiple sources in the cardiac and arterial beds.7 Thrombus formation at the source of embolization may be triggered or amplified by platelet activation and fibrin deposition. These pathways interact, and resulting thrombi are a combination of platelets and fibrin, suggesting that optimization of antithrombotic strategies can be achieved by targeting both pathways—the dual pathway approach.8 This hypothesis has been tested in acute and stable arterial disease using a combination of aspirin and the factor Xa (FXa) inhibitor rivaroxaban. In patients with acute coronary syndromes, rivaroxaban added to standard antiplatelet therapy reduced the combined endpoint of stroke, myocardial infarction (MI), and death over standard therapy alone, with an increase in major bleeding and intracranial hemorrhage.9
The risk of stroke, MI, and death was reduced by the combination of rivaroxaban and aspirin over aspirin alone in patients with stable coronary or peripheral artery disease.10 Of note, there was a reduction of 42% in the hazard of stroke (hazard ratio [HR], 0.58; 95% confidence interval [CI], 0.44–0.76), and the absolute benefit was greatest in those with a prior history of stroke.11 The reduction was consistently noted across different stroke subtypes, suggesting that the dual pathway approach was effective in preventing thromboembolism in multiple stroke subtypes.12 However, combined therapy with rivaroxaban and aspirin was also associated with an increase in major bleeding compared with aspirin alone (HR, 1.70; 95% CI, 1.40–2.05).10
Factor XI (FXI) is the precursor of the coagulation protease factor XIa (FXIa). While FXIa makes relatively modest contributions to hemostasis, it is involved in thrombus growth and stabilization13 (Fig. 1). Epidemiologic and Bayesian randomization studies in individuals born with FXI deficiency suggest that FXI(a) inhibition is a promising strategy for secondary stroke prevention.14, 15 Patients with FXI deficiency have a significantly lower risk for stroke than matched controls with normal FXI levels, whereas patients with high FXI levels have an increased risk for recurrent stroke.16 Spontaneous hemorrhage in FXI-deficient patients is rare. Bleeding most commonly occurs with trauma to tissues with high intrinsic fibrinolytic activity, such as the nose and genitourinary tract.17 Studies of deep venous thrombosis prophylaxis have demonstrated a reduction in venography-defined thrombosis and favorable bleeding profiles with reduction in FXI levels or inhibition of FXIa.18–20 The potential for a therapeutic effect with low risk of bleeding makes FXI and FXIa promising targets for secondary stroke prevention.
Fig. 1. Coagulation pathway.
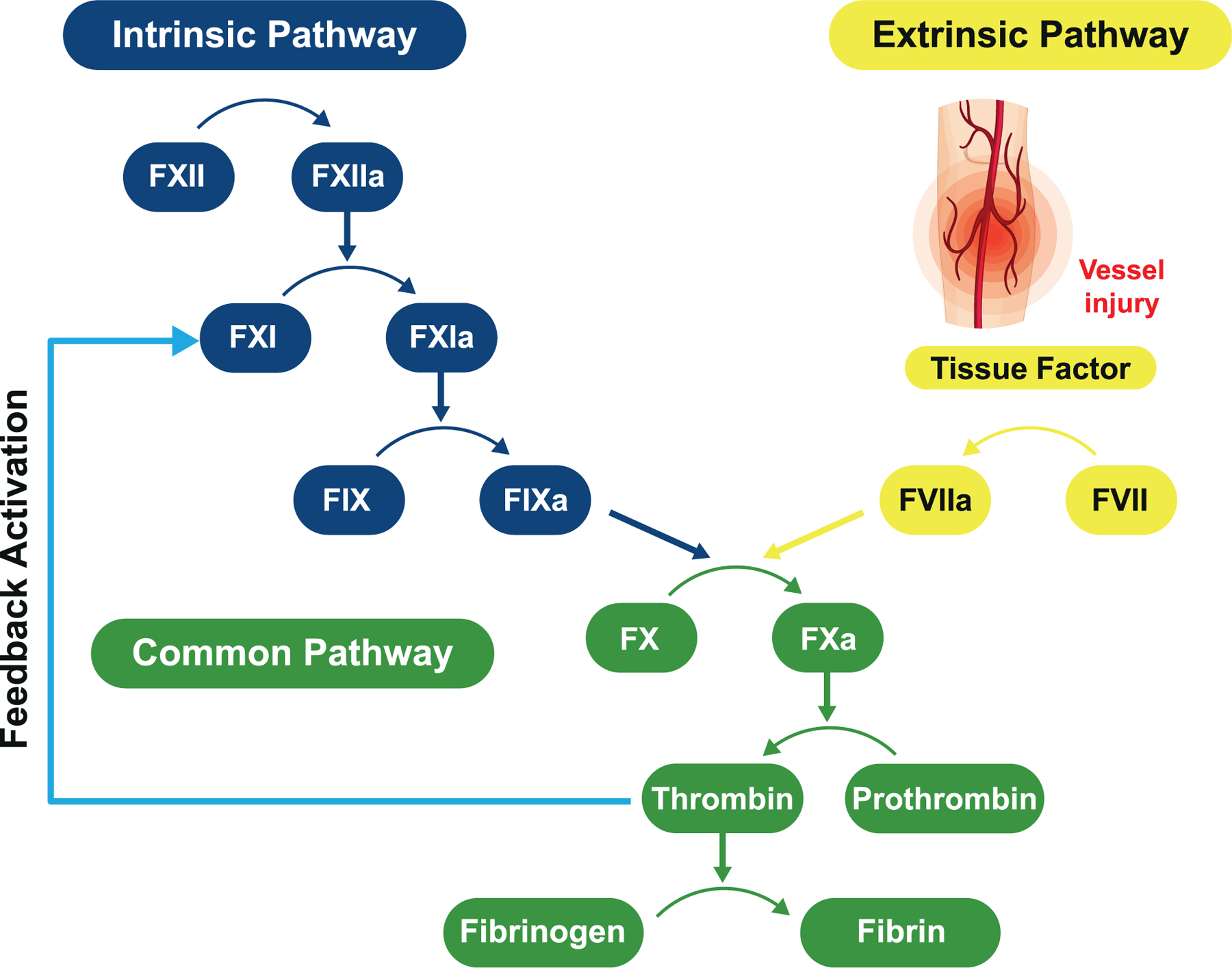
Thrombin also activates FV and FVIII which amplify the generation of thrombin and are unaffected by inhibition of FXIa.
FXII, factor XII; FXIIa, activated factor XII; FXI, factor XI; FXIa, activated factor XI; FIX, factor IX; FIXa, activated factor IX; FVIIa, activated factor VII; FVII, factor VII; FX, factor X; FXa, activated factor X.
Reprinted with permission from Quan ML, Pinto DJP, Smallheer JM, et al. Factor XIa inhibitors as new anticoagulants. J Med Chem. 2018;61 (17):7425–7447. Copyright 2018 American Chemical Society.
Milvexian (BMS-986177/JNJ-70033093) is a direct-acting, high-affinity inhibitor of human coagulation FXIa.21 Milvexian binds rapidly and reversibly to FXIa and exhibits high selectivity and specificity for FXIa over other proteases of the blood coagulation cascade and anticoagulant systems. Fig. 2 shows the x-ray crystal structure of milvexian bound to the FXIa protease domain.21 As opposed to thrombin and FXa inhibitors which act on the common coagulation pathway required for hemostasis, milvexian acts on FXIa, reducing the contribution of this feedback loop to thrombin generation without affecting thrombin activation of FV and FVIII. Activation of FV and FVIII also amplify the generation of thrombin (Fig. 1). Milvexian is predicted to have limited effects on hemostasis and could provide the first opportunity to determine whether clinically effective antithrombic activity can occur without significant increases in bleeding risk. Pre-clinical studies and human trials have assessed its safety, pharmacokinetic (PK) and pharmacodynamic (PD) effects. At doses that preserved hemostasis, milvexian exhibited robust antithrombotic efficacy in the prevention and treatment of arterial thrombosis in a rabbit electrically induced carotid artery thrombosis (ECAT) model.22 Administration of milvexian also resulted in dose-dependent inhibition of thrombosis in a rabbit arterio-venous shunt model.23
Fig. 2. X-ray crystal structure of milvexian bound to factor XIa (Protein Data Bank ID: 7MBO).
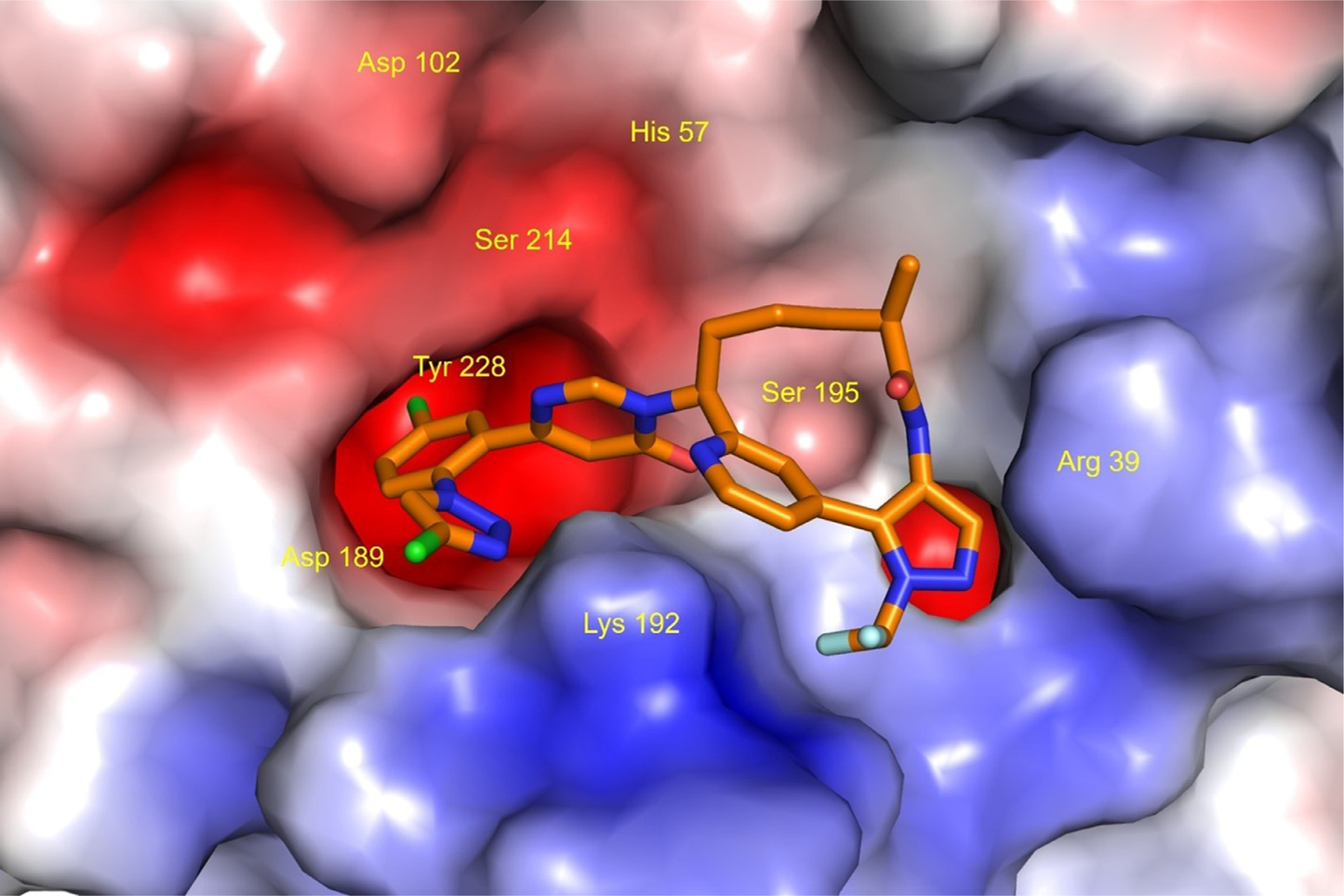
Milvexian is represented as a stick model (atom colors: C, orange; O, red; N, blue; Cl, green; F, light blue; H, not shown). The molecular surface of Factor XIa is displayed colored by electrostatic potential (blue: positive; red: negative; white: neutral). Amino acid labels are displayed for the catalytic triad residues (His57, Asp102, Ser195) and selected residues (Arg39, Asp189, Lys192, Tyr228) that are within a distance of 3.5 angstroms from milvexian. This illustration was created using the PyMOL Molecular Graphics System, version 1.7.6.0, Schrödinger, LLC, New York.
Phase I studies of milvexian demonstrated that it was safe and well tolerated at doses up to 200 mg twice daily (BID) and 500 mg once daily (QD).24 The drug is rapidly absorbed after oral administration, with a time to peak of 2 to 4 hours. The primary elimination routes are through cytochrome P450 enzyme (CYP) metabolism, specifically CYP3A, with relatively low renal excretion (<20%) and the dose range of 20 – 200 mg is generally dose proportional.25–27 The drug has a terminal elimination half-life of 11 to 18 hours, supporting either BID or QD dosing.24 Milvexian increases the activated partial thromboplastin time (aPTT) in a concentration-dependent manner.24 Milvexian is well tolerated in participants with mild or moderate hepatic impairment and demonstrated a similar PK profile as in healthy subjects.28 After accounting for protein-binding, the exposure-response relationship with the aPTT overlapped with that observed in healthy study participants, indicating that the biologic effect of milvexian was unchanged in hepatic impairment.28 In participants with severe renal impairment, milvexian had a modest increase in drug exposure compared to those with normal renal function.27 Based on preclinical data, milvexian has a low potential for interactions as a perpetrator (i.e., a drug that causes an effect on the substrate drug) or substrate. Studies with moderate and strong CYP3A4 inhibitors indicated relatively small and modest milvexian plasma exposure increases, respectively, due to changes in the half-life as expected based on the metabolic profile of milvexian.26 Strong CYP3A4 induction with rifampin resulted in modest decreases in milvexian concentration.25 The dose range of 25 mg QD to 200 mg BID was selected based on pre-clinical blood concentration targets from the rabbit ECAT model and the phase I human study.22, 24 The goal of the dose range selected was to enable an assessment of the exposure-response in stroke prevention and enable an evaluation of the FXIa mechanism of action.
Post-operative FXIa inhibition with milvexian in participants undergoing total knee arthroplasty was effective for preventing venous thromboembolism and was associated with a low risk of clinically relevant bleeding compared with enoxaparin in a phase II trial.20 Following administration of milvexian at daily doses from 25 to 400 mg, a significant dose-response was noted for the incidence of venous thromboembolism after elective knee arthroplasty with BID and QD regimens. The incidence of venous thromboembolism was significantly lower with daily milvexian doses of 100 mg or more compared to enoxaparin 40 mg QD. Bleeding of any severity occurred in 4% of milvexian-treated patients and 4% of enoxaparin-treated patients. Major or clinically relevant non-major bleeding occurred in fewer milvexian patients than those treated with enoxaparin (1% vs 2%). No major bleeding was observed with the use of milvexian.
Evidence to date suggests that inhibition of FXIa is a promising target for antithrombotic therapy for ischemic stroke, and has the potential to reduce thrombin generation enough to prevent thrombotic vascular occlusion or embolization without impairing hemostasis.
Design
Overview
Antithrombotic treatment with factor XIa inhibition to Optimize Management of Acute Thromboembolic events for Secondary Stroke Prevention (AXIOMATIC-SSP [ClinicalTrials.gov Identifier: NCT03766581]) is an international, phase II, randomized, double-blind, placebo-controlled, dose-ranging clinical trial examining the safety and efficacy of milvexian in patients with acute ischemic stroke or high-risk TIA. The main objective is to determine the dose-response relationship of milvexian in participants with acute ischemic stroke or TIA who are treated with aspirin and clopidogrel. The main secondary objective is to assess the rate of major bleeding in this population compared with placebo. Between January 2019 and December 2021, we randomized 2,366 participants at 367 sites in 27 countries. The main results will be available in 2022.
Study population
Key inclusion criteria are listed in Table 1. Individuals ≥40 years of age who have had an ischemic stroke or high-risk TIA were recruited and randomized within 48 hours of the onset of symptoms. Qualifying strokes were defined as symptomatic non-lacunar acute brain infarcts visible on neuroimaging (computed tomography [CT] or magnetic resonance imaging [MRI]) and relevant to the clinical symptoms. Participants were required to have a National Institutes of Health Stroke Scale (NIHSS) score of ≤7 at the time of randomization (scores range from 0–42, with higher scores indicating greater stroke severity). Petechial hemorrhagic transformation of the index infarct and cerebral microbleeds were permitted. Qualifying TIA was defined as acute onset of neurologic deficits attributable to brain ischemia with complete resolution of deficit and no brain infarction on neuroimaging and with an ABCD2 score ≥6 (scores range from 0–7, with higher scores indicating a greater risk of stroke) or motor symptoms.29 All participants were required to have visible intracranial or extracranial atherosclerotic plaque of any degree, but not complete occlusion on an imaging study, and a premorbid modified Rankin score (mRS) of ≤3. After approximately 450 patients had been randomized and the data monitoring committee (DMC) had reviewed the safety and efficacy data, enrollment of participants who had received thrombolytic therapy and/or mechanical thrombectomy for the treatment of the index stroke was permitted. Additional requirements were that 24 hours had passed between the acute recanalization therapy and the first dose of study medication, provided that neuroimaging excluded hemorrhagic transformation of the acute infarct and all other study criteria were met.
Table 1.
Key inclusion criteria.
| Participant and target disease characteristics | |
|---|---|
|
Ischemic stroke: a neurological deficit attributable to a non-lacunar acute brain infarction detected by neuroimaging (CT or MRI) and relevant to the clinical symptoms AND NIHSS score ≤7 at time of randomization AND Evidence of relevant intracranial or cervical arterial atherosclerotic plaque, ulceration, or thrombus in a feeding artery documented by imaging (either Doppler ultrasound or CTA or MRA or catheter angiography) AND mRS ≤3 before the index event (pre-morbid) |
TIA: acute onset neurological deficit attributable to focal ischemia of the brain by history or examination, with complete resolution of the deficit and no brain infarction on neuroimaging (CT or MRI) AND ABCD2 score ≥6 or motor symptoms AND Evidence of relevant intracranial or cervical arterial atherosclerotic plaque, ulceration, or thrombus in a feeding artery documented by imaging (either Doppler ultrasound or CTA or MRA or catheter angiography) AND mRS ≤3 before the index event (pre-morbid) |
Additional criteria
| |
CT, computed tomography; MRI, magnetic resonance imaging; NIHSS, National Institutes of Health Stroke Scale; CTA, computed tomography angiography; MRA, magnetic resonance angiography; mRS, modified Rankin score; TIA, transient ischemic attack; IV, intravenous; INR, international normalized ratio; aPTT, activated partial thromboplastin time; ULN, upper limit of normal.
Key exclusion criteria are summarized in Table 2. Potential participants were excluded if there was evidence of large vessel dissection, intracranial tumor (other than meningioma), or arteriovenous malformation that could explain the symptoms. Also excluded were those with a history of hemorrhage in the brain or spinal cord, and those with conditions that, in the opinion of the investigator, would contradict anticoagulant therapy. A requirement for dual antiplatelet therapy beyond 21 days or planned use of anticoagulants, with the exception of heparin or low molecular weight heparin for maintaining the patency of indwelling catheters, were exclusions. Known SARS-CoV-2 infection within 4 weeks prior to screening was also an exclusion.
Table 2.
Key exclusion criteria.
|
AVM, arteriovenous malformation; CT, computed tomography; MRI, magnetic resonance imaging; NVAF, non-valvular atrial fibrillation; DVT, deep vein thrombosis; PE, pulmonary embolism; DAPT, dual antiplatelet therapy; CMB, cerebral microbleed; HI1, hemorrhagic infarction type 1; aPTT, activated partial thromboplastin time; ULN, upper limit of normal; INR, international normalized ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ESRD, end-stage renal disease; eGFR, estimated glomerular filtration rate; LMWH, low molecular weight heparin; NSAID, non-steroidal anti-inflammatory drug; COX-2, cyclo-oxygenase 2; P-gp, P-glycoprotein; CYP3A4, cytochrome P450 3A4; PPI, proton pump inhibitor; ECG, electrocardiogram; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Study treatment and visits
Following informed consent, participants were randomized to milvexian or matching placebo plus open-label uncoated aspirin 100 mg and clopidogrel (loading dose of 300–600 mg, followed by 75 mg/day). Clopidogrel was administered for 21 days, while aspirin and milvexian or matching placebo were continued until Day 90 (Fig. 3). Randomization was initially to milvexian in QD doses of 25 mg and BID doses of 25 mg, 50 mg, 100 mg, and, after review of safety and efficacy data by the independent DMC, to 200 mg BID also. Despite the delayed start of randomization to the 200-mg BID dose, the study targeted at 2:1 randomization between placebo and each milvexian dose to occur by the end of enrollment. No dose adjustments were made to milvexian, aspirin, or clopidogrel after randomization. A baseline MRI brain scan was performed according to a standardized study-specific protocol (Supplementary Table 1) and was recommended prior to randomization. Milvexian dosing was started within 6 hours of the baseline scan. In instances where the baseline MRI could not be performed within 48 hours of symptom onset, it could be acquired post-randomization, but no later than 72 hours from symptom onset. Study visits occurred at 21 and 90 days, with a telephone contact at 60 days. In approximately 450 patients, central laboratory tests were also obtained at 60 days. An end-of-study MRI brain scan was obtained at 90 days, with a telephone contact at 97 days. The NIHSS, the Montreal Cognitive Assessment (MoCA), and the Digit Symbol Substitution Test (DSST) were collected at the Day 1, Day 21, and Day 90 visits and following any new clinical stroke. Blood samples for safety markers were collected at Day 1 (baseline), Day 21, Day 60, and Day 90 visits while PK, PD, and biomarker samples were collected between Day 1 to Day 4, Day 21, and Day 90 visits (Appendix 1).
Fig. 3. AXIOMATIC-SSP study design.
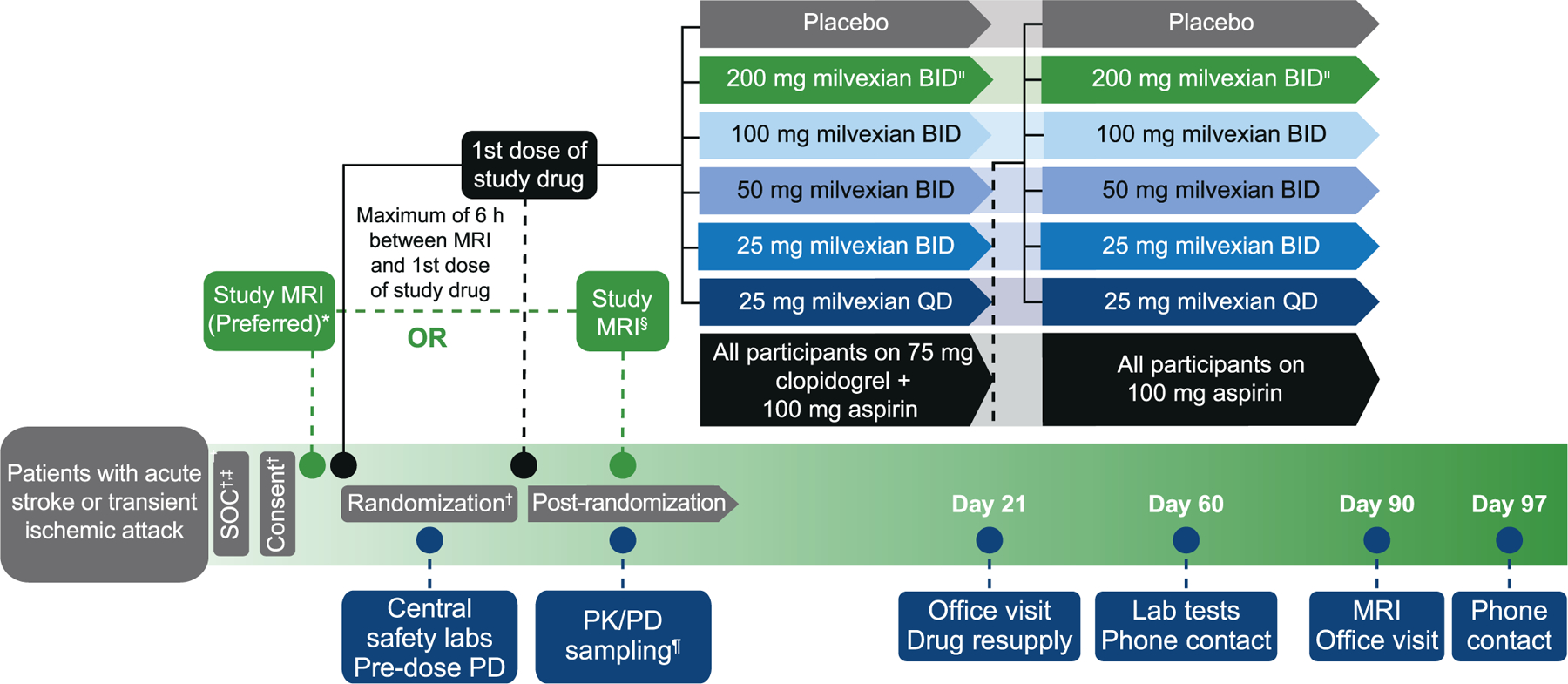
BID, twice daily; MRI, magnetic resonance imaging; QD, once daily; SOC, standard of care; PD, pharmacodynamic; PK, pharmacokinetic; LD, loading dose.
*Within 48 hours of index event and prior to randomization.
†300 mg clopidogrel LD + 100 mg aspirin.
‡600 mg clopidogrel LD permitted.
§Within 72 hours of index event and up to 24 hours after randomization; clinical neuroimaging is recommended for eligibility assessment if MRI is done post-randomization (to minimize protocol deviation in case of negative imaging findings).
¶Relative to the 1st or 2nd dose of study drug.
|The 200-mg BID cohort was added after 450 participants from the lower doses completed the day 21 visit.
Study objectives and endpoints
The primary efficacy endpoint was the composite of incident ischemic stroke during the treatment period or new covert brain infarction detected by the comparison of 90-day and baseline MRIs. All images were read independently by 2 blinded neuroradiologists, and disagreements were resolved by consensus. A new infarct was determined if the 90-day MRI demonstrated a new lesion on the diffusion weighted sequences or the fluid attenuated inversion recovery sequence consistent with an ischemic infarct. Clinically overt strokes were determined by site investigators following standardized definitions. Briefly, to qualify as an outcome event, a clinical diagnosis of stroke required a sustained increase in the NIHSS score of ≥3 points that was not explained by other factors, such as infection, or was confirmed by imaging as a new infarct.
The secondary endpoint of major bleeding was determined by the occurrence of types 3 and 5 bleeding, according to the Bleeding Academic Research Consortium (BARC) classification system (Appendix 2).30 Secondary endpoints also included the volume and number of new infarcts detected by MRI imaging and the rate of clinical bleeding of any type with milvexian compared to placebo. Bleeding was additionally evaluated utilizing International Society on Thrombosis and Haemostasis (ISTH) and PLATelet inhibition and patient Outcomes (PLATO) criteria. Central laboratory samples were used to determine the dose-response relationship to PD markers as well as the PK of milvexian.
Study organization
AXIOMATIC-SSP was sponsored by Bristol Myers Squibb, in collaboration with Janssen Pharmaceuticals, Inc. A steering committee, chaired by the principal investigator and composed of national leaders, experts in stroke and thrombosis, and sponsor representatives, supervised the conduct of the study to ensure standards of high quality were met and was responsible for the scientific aspects of the trial. An independent DMC, whose membership includes experts in stroke, clinical trials, thrombosis, and statistics, had access to unblinded data and was responsible for the safety of participants in the trial. No formal interim analyses were performed. An administrative interim analysis was performed by the sponsor using personnel who had no other connection to the study when approximately 1600 participants had been randomized to facilitate subsequent program development. The principal investigator, steering committee, and all study personnel involved with the study conduct, sites, and vendors were blinded to the results of this analysis.
Statistical considerations
Sample size calculations for the primary endpoint were performed for detecting a dose-response effect, with generalized Multiple Comparisons and Modeling (gMCP-Mod) using simulations with the Dose-Finding package in R statistical analysis software.31, 32 Simulations of 2500 clinical trials were performed, assuming a true incidence for placebo of 15%, a plateau-shaped dose-response relationship with maximum relative risk reduction of 32% for milvexian 100 or 200 mg BID relative to placebo, and 25 mg QD as the lowest dose added to the ordered BID dose regimens.33, 34 Candidate models included an Emax model, a logistic model, and an exponential model.
Using these assumptions, a total of 2100 participants allocated in a 2:1:1:1:1:1 ratio to the placebo and milvexian 25 mg QD, 25 mg BID, 50 mg BID, 100 mg BID, and 200 mg BID groups would provide approximately 80% power to demonstrate a dose-response relationship, with a 1-sided type I error of 0.049. Accounting for up to 10% of participants with missing images at day 90, a sample size of approximately 2350 patients was targeted.
All randomized participants will be included in the analysis of the primary endpoint if they have either a symptomatic ischemic stroke event by day 90 or an evaluable MRI at the day 90 visit. Because of the COVID-19 pandemic, all day 90 visit MRIs will be included in the primary analysis, regardless of timing. All other efficacy analyses will be performed on a randomized (intent-to-treat [ITT]) population up to day 90.
If a dose-response relationship exists, then fitted estimates for the incidence of the primary endpoint will be calculated in each of the treatment groups using the weighted average of fitted models, with weights determined from the Akaike information criteria (AIC) for each model. The observed incidence of the primary endpoint will be summarized by treatment group using the fitted model from the gMCP-Mod procedure. The gMCP-Mod will incorporate the event proportion at each dose level, and then the logit scale will be used during the rest of the gMCP-Mod procedure.
To assess the robustness of the primary efficacy analysis, additional sensitivity analyses will be carried out using the following approaches:
A sensitivity analysis will repeat the primary analysis, but include MRIs obtained up to Day 106.
The primary analysis will be repeated using all participants randomized, counting any event-free participants without a Day 90 MRI as having no event (analysis #1) or as having an event (analysis #2).
The primary analysis will be repeated excluding participants with a protocol deviation meeting predefined criteria having the potential to affect interpretation of major safety or efficacy endpoint analyses, if ≥5% of participants have such a deviation.
Key subgroup analyses will be performed for demographic variables, index event type (ischemic stroke or TIA), time from onset to first dose, location of atherosclerosis, baseline NIHSS score, prior ischemic stroke or TIA, hypertension, thrombolysis/thrombectomy, prior antiplatelet therapy, and prior statin therapy.
For other events, incidence within each treatment group and the associated relative risk reduction between each milvexian arm and placebo will be presented. NIHSS, mRS, MoCA, and DSST (subtest of WAIS-IV) will be summarized at baseline, on Days 21 and 90, and at the time of a new stroke event.
Analysis of all safety data will be performed on participants treated with ≥1 dose of double-blind drug. The proportion of participants with major bleeding events (BARC types 3 and 5), as well as BARC-, ISTH-, and PLATO-defined criteria, will be summarized by treatment group. Additional summaries will include characteristics of cerebral microbleeds, hemorrhagic transformation of ischemic stroke, and asymptomatic intracranial bleeding. Adverse event and laboratory data, as well as PK and PD data, will be summarized.
Study conduct during the COVID-19 pandemic
Our trial was conducted during the COVID-19 pandemic resulting in a number of operational and logistical challenges. Innovative changes were instituted to overcome challenges imposed by the pandemic. Remote site initiation visits and remote source data verification were permitted for experienced sites, provided on-site monitoring would occur as soon as travel restrictions were removed. For patients who were unable to attend follow-up study visits in-person, qualified health care professionals visited them at home for safety and endpoint assessments. Direct shipment of investigational products from study centers to participants, via courier with proper temperature control, was arranged. AXIOMATIC-SSP study-specific guidance along with the sponsor’s general guidance for the conduct of clinical research during the pandemic were provided to study centers to ensure the safety and welfare of patients, sites, and study members while assuring study compliance.
Discussion
The dual pathway approach, combining an anticoagulant with antiplatelet therapy for stroke prevention, holds promise but requires careful dose-finding to ensure adequate inhibition of thrombosis without increasing the risk for major bleeding. FXIa inhibition with milvexian offers the potential to reduce thrombosis without impeding hemostasis, a significant advantage over previous generations of anticoagulants. AXIOMATIC-SSP is, to our knowledge, the largest dose-finding trial for secondary stroke prevention with the dual pathway approach conducted to date. We combined imaging evidence of new infarctions with clinical stroke events to achieve a placebo event rate sufficient to allow completion of the trial within a reasonable time-frame. This model of arterial thromboembolism has significant advantages in assessing efficacy and safety over previous approaches that developed antithrombotic doses for stroke prevention in a population subject to venous thromboembolism. The results will provide dosing data for a larger phase III trial.
Supplementary Material
Acknowledgment:
The authors would like to acknowledge Donald J. P. Pinto, PhD, and Stanley R. Krystek, PhD, of Bristol Myers Squibb, for their work on the milvexian chemical structure image.
Funding
This study is sponsored by Bristol Myers Squibb and Janssen Research & Development, LLC.
Editorial support was provided by Dana Tabor, PhD, of Cello Health Communications/MedErgy, and was funded by Bristol Myers Squibb and Janssen Global Services, LLC. Drafting and revision of the manuscript was completed solely by the authors.
Glossary
- CT
computed tomography
- MRI
magnetic resonance imaging
- NIHSS
National Institutes of Health Stroke Scale
- CTA
computed tomography angiography
- MRA
magnetic resonance angiography
- mRS
modified Rankin score
- TIA
transient ischemic attack
- IV
intravenous
- INR
international normalized ratio
- aPTT
activated partial thromboplastin time
- ULN
upper limit of normal
Footnotes
Competing interests
Dr Sharma reports receiving research grants from Bristol Myers Squibb; consulting fees from Janssen, HLS Therapeutics, and Bayer; and is on the board of the Canadian Stroke Consortium. Dr Molina reports receiving consulting fees from Bristol Myers Squibb. Dr Toyoda reports receiving payment or honoraria from Bristol Myers Squibb, Daiichi-Sankyo, Novartis, Otsuka, Bayer, and Abbot Medical; and is an advisory committee member of the CHARM trial for Biogen. Prof Bereczki is a member of the AXIOMATIC-SSP Steering Committee; and reports receiving grant support, consulting fees, and meeting support from Bristol Myers Squibb. Dr Kasner reports receiving grant support from Genentech, Medtronic, and WL Gore; royalties or licenses from UpToDate; and consulting fees from Bristol Myers Squibb; and was on a Data Safety Monitoring Board for AstraZeneca. Dr Lutsep reports receiving honoraria from serving on the Editorial Board of Medscape Neurology; and participated in Data Safety Monitoring Boards or Advisory Boards and as a National Leader and Steering Committee member for Bristol Myers Squibb, NINDs, the Mayo Clinic, and Coherex Medical. Dr Tsivgoulis reports participation on a Data Safety Monitoring Board or Advisory Board for Bristol Myers Squibb. Dr Ntaios reports receiving payment or honoraria from Abbott Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Elpen, Ferrer, and Sanofi. Prof Czlonkowska is a member of the AXIOMATIC-SSP Steering Committee, country leader of AXIOMATIC-SSP, and is a hospital PI for AXIOMATIC-SSP; reports receiving consulting fees from Bristol Myers Squibb, Vivet Therapeutics, and Alexion. Dr Shuaib reports receiving grants and consulting fees from Bristol Myers Squibb. Dr. Amarenco reports receiving writing support from Bristol Myers Squibb and Janssen; grant support from Sanofi, AstraZeneca, Pfizer, Amgen, and the French government; consulting fees from Amgen; payment or honoraria from Sanofi, AstraZeneca, and Viatris; and has participated on Data Safety Monitoring Boards or Advisory Boards for AstraZeneca and Kowa. Dr Endres is a member of the AXIOMATIC-SSP Steering Committee and National Leader for Germany; he reports receiving grants and consulting fees from Bayer; and payment or honoraria from Abbott, Boehringer Ingelheim, Pfizer, Amgen, GSK, Sanofi, and Novartis; participated on Data Safety Monitoring Boards or Advisory Boards for Bristol Myers Squibb, Bayer, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Amgen, and Covidien; had a leadership or fiduciary role in EAN, DGN, ISCBFM, AHA/ASA, ESO, WSO, German Center of Cardiovascular Research (DZHK), and German Center for Neurodegenerative Diseases (DZNE); and received materials from Amgen. Dr Diener reports receiving writing support from Bristol Myers Squib; grant support from Boehringer Ingelheim and Alexion; payment or honoraria from Abbott, Boehringer Ingelheim, and Novo Nordisk; and participating on Data Safety Monitoring Boards or Advisory Boards for DSMB Swift Prime and DSMB CLOSURE-AF. Dr Gailani reports receiving grant support from National Heart, Lung and Blood Institute, and National Institute of Allergy and Infectious Disease; royalties from Washington University; consulting fees from Anthos Therapeutics, Bayer, Bristol Myers Squibb, Ionis Pharmaceuticals, and Janssen; payment or honoraria from Anthos Therapeutics, Bayer, Bristol Myers Squibb, and Janssen; holds patents for factor XI inhibitor and factor XII inhibitor; and has participated on Data Safety Monitoring Boards or Advisory Boards for Bristol Myers Squibb and Janssen. Dr Kahl reports receiving writing support from Bristol Myers Squibb and owns stock in Bristol Myers Squibb. Dr Donovan is an employee and shareholder of Bristol Myers Squibb. Dr Perera is an employee and shareholder of Bristol Myers Squibb; an Associate Editor of BMC Pharmacology and Toxicology; and holds patents for factor XIa inhibitor treatment methods and methods of treating acute respiratory disorders. Dr Li is an employee and shareholder of Bristol Myers Squibb; and has a patent with Bristol Myers Squibb. Prof Hankey is a member of the AXIOMATIC-SSP Steering Committee and National Leader for Australia; he reports receiving consulting fees from Bayer and Janssen; payment or honoraria from Bristol Myers Squibb and Medscape; and is a co-chief investigator in the Antiplatelet Secondary Prevention International Randomized Trial After INtracerebral haemorrhaGe (ASPIRING)-Pilot Phase clinical trial (ClinicalTrials.gov Identifier: NCT04522102).
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jstrokecerebrovasdis.2022.106742.
References
- 1.Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med 2018;379(3):215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu J, Wang A, Wangqin R, et al. Efficacy of clopidogrel for stroke depends on CYP2C19 genotype and risk profile. Ann Neurol 2019;86(3):419–426. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Meng X, Wang A, et al. Ticagrelor versus clopidogrel in CYP2C19 loss-of-function carriers with stroke or TIA. N Engl J Med 2021;385(27):2520–2530. [DOI] [PubMed] [Google Scholar]
- 4.Johnston SC, Amarenco P, Denison H, et al. Ticagrelor and aspirin or aspirin alone in acute ischemic stroke or TIA. N Engl J Med 2020;383(3):207–217. [DOI] [PubMed] [Google Scholar]
- 5.Toyoda K, Uchiyama S, Yamaguchi T, et al. Dual antiplatelet therapy using cilostazol for secondary prevention in patients with high-risk ischaemic stroke in Japan: a multicentre, open-label, randomised controlled trial. Lancet Neurol 2019;18(6):539–548. [DOI] [PubMed] [Google Scholar]
- 6.Tan CH, Wu AG, Sia CH, et al. Cilostazol for secondary stroke prevention: systematic review and meta-analysis. Stroke Vasc Neurol 2021;6(3):410–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014;13(4):429–438. [DOI] [PubMed] [Google Scholar]
- 8.Capodanno D, Bhatt DL, Eikelboom JW, et al. Dual-pathway inhibition for secondary and tertiary antithrombotic prevention in cardiovascular disease. Nat Rev Cardiol 2020;17(4):242–257. [DOI] [PubMed] [Google Scholar]
- 9.Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012;366(1):9–19. [DOI] [PubMed] [Google Scholar]
- 10.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377(14):1319–1330. [DOI] [PubMed] [Google Scholar]
- 11.Sharma M, Hart RG, Connolly SJ, et al. Stroke outcomes in the COMPASS trial. Circulation 2019;139(9):1134–1145. [DOI] [PubMed] [Google Scholar]
- 12.Perera KS, Ng KKH, Nayar S, et al. Association between low-dose rivaroxaban with or without aspirin and ischemic stroke subtypes: a secondary analysis of the COMPASS trial. JAMA Neurol 2020;77(1):43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredenburgh JC, Weitz JI. Factor XI as a target for new anticoagulants. Hamostaseologie 2021;41(2):104–110. [DOI] [PubMed] [Google Scholar]
- 14.Gill D, Georgakis MK, Laffan M, et al. Genetically determined FXI (Factor XI) levels and risk of stroke. Stroke 2018;49(11):2761–2763. [DOI] [PubMed] [Google Scholar]
- 15.Salomon O, Steinberg DM, Koren-Morag N, Tanne D, Seligsohn U. Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood 2008;111 (8):4113–4117. [DOI] [PubMed] [Google Scholar]
- 16.Rohmann JL, Huo S, Sperber PS, et al. Coagulation factor XII, XI, and VIII activity levels and secondary events after first ischemic stroke. J Thromb Haemost 2020;18(12):3316–3324. [DOI] [PubMed] [Google Scholar]
- 17.Duga S, Salomon O. Congenital factor XI deficiency: an update. Semin Thromb Hemost 2013;39(6):621–631. [DOI] [PubMed] [Google Scholar]
- 18.Buller HR, Bethune C, Bhanot S, et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med 2015;372(3):232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weitz JI, Bauersachs R, Becker B, et al. Effect of osocimab in preventing venous thromboembolism among patients undergoing knee arthroplasty: the FOXTROT randomized clinical trial. JAMA 2020;323(2):130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weitz JI, Strony J, Ageno W, et al. Milvexian for the prevention of venous thromboembolism. N Engl J Med 2021;385(23):2161–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dilger AK, Pabbisetty KB, Corte JR, et al. Discovery of milvexian, a high-affinity, orally bioavailable inhibitor of factor XIa in clinical studies for antithrombotic therapy. J Med Chem 2022;65(3):1770–1785. [DOI] [PubMed] [Google Scholar]
- 22.Wong PC, Crain E, Dilger A, et al. Small-molecule factor XIa inhibitor, BMS-986177/JNJ70033093, Prevents and treats arterial thrombosis in rabbits at doses that preserve hemostatic. Res Pract Thromb Haemost 2020;4(Suppl 1):52. [Google Scholar]
- 23.Wang X, Qiu L, Du F, Shukla N, Nawrocki A, Chintala M. Antithrombotic effects of a novel small molecule FXIa inhibitor BMS-986177/JNJ-70033093 in a rabbit AV-shunt model of thrombosis In: Poster PB0179. Presented at the International Society on Thrombosis and Haemostasis (ISTH) 2020 Virtual Congress; July 12–14; 2020. p. 2020. [Google Scholar]
- 24.Perera V, Wang Z, Luettgen J, et al. First-in-human study of milvexian, an oral, direct, small molecule factor XIa inhibitor. Clin Transl Sci 2021;15(2):330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perera V, Wang Z, Lubin S, et al. Pharmacokinetic and pharmacodynamic effects of coadministration of rifampin and BMS-986177/JNJ-70033093, an oral, direct, reversible, small molecule factor XIa inhibitor. Clinic Pharmacol Therap 2021;109(Supplement 1):S73. [Google Scholar]
- 26.Perera V, Wang Z, Lubin S, et al. Effects of itraconazole and diltiazem on the pharmacokinetics and pharmacodynamics of milvexian, a factor XIa inhibitor. Cardiol Ther 2022. 10.1007/s40119-022-00266-6. [DOI] [PMC free article] [PubMed]
- 27.Perera V, Abelian G, Li D, et al. Single-dose pharmacokinetics of milvexian in participants with normal renal function and participants with moderate or severe renal impairment. Clin Pharmacokinet 2022. 10.1007/s40262-022-01150-1. [DOI] [PMC free article] [PubMed]
- 28.Perera V, Abelian G, Li D, et al. Single-dose pharmacokinetics of milvexian in participants with mild or moderate hepatic impairment compared with healthy participants. Clin Pharmacokinet 2022;61(6):857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007;369(9558):283–292. [DOI] [PubMed] [Google Scholar]
- 30.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011;123(23):2736–2747. [DOI] [PubMed] [Google Scholar]
- 31.Bornkamp B, Pinheiro J, Bretz F. R: DoseFinding package, ver 0.9–16. Comprehensive R Archive Network 2018.
- 32.Cran.r-project. The Comprehensive R Archive Network https://cran.r-project.org/
- 33.Bretz F, Pinheiro JC, Branson M. Combining multiple comparisons and modeling techniques in dose-response studies. Biometrics 2005;61(3):738–748. [DOI] [PubMed] [Google Scholar]
- 34.Pinheiro J, Bornkamp B, Glimm E, Bretz F. Model-based dose finding under model uncertainty using general parametric models. Stat Med 2014;33(10):1646–1661. [DOI] [PubMed] [Google Scholar]
- 35.von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke 2015;46(10):2981–2986. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


