Abstract
Using confocal microscopy, MEST-1-positive immunofluorescence was observed within various Trypanosoma cruzi forms, except in cell-derived trypomastigotes. Glycosylinositol phosphorylceramides were identified by thin-layer chromatography immunostaining as the antigens recognized by MEST-1 in these parasites. In epimastigotes, labeling of MEST-1 coincided with acidic vesicles, indicating an internal localization of these glycoconjugates.
Glycoconjugates containing galactofuranose (Gal-furanose) residues have been described in fungi, bacteria, and trypanosomatides such as Trypanosoma cruzi and Leishmania (4, 15–17). Although the biological role of Gal-furanose residues is still unclear, the postulated absence of Gal-furanose and galactofuranosidases in mammalian species suggests the intriguing hypothesis that terminal Gal-furanose residues play a central role in survival of fungi and parasites by preventing the action of the host's glycosidases on their glycoconjugates. If this hypothesis is correct, Gal-furanose residues are potentially useful as specific target molecules for therapy of parasitic and fungal diseases.
We recently characterized the mouse monoclonal antibody (MAb) MEST-1 (immunoglobulin G3 [IgG3]) (16), which recognizes glycosylinositol phosphorylceramides (GIPCs) containing terminal residues of β-d-Gal-furanose, in either the linkage β1-6, present in the Pb-1 antigen of Paracoccidioides brasiliensis (6), or the linkage β1–3, present in GIPL-1 of Leishmania major (8).
In the present study, we analyzed the reactivity of MEST-1 with different forms of T. cruzi. In the infected host, the intracellular dividing amastigotes give rise to trypomastigotes that lyse the cell and spread the infection. Trypomastigotes may be ingested by the triatomine vector and differentiate into dividing epimastigotes and then metacyclic trypomastigotes, the forms that are infectious to mammals. A lipopeptidophosphoglycan currently termed GIPC has been described as the major glycoconjugate of the T. cruzi epimastigote surface (3). It is a GIPC with a well-known carbohydrate structure containing one or two terminal Gal-furanose residues (5, 10). We analyzed the reactivity of MEST-1 with different forms of T. cruzi, and with GIPCs purified from the parasites, by indirect immunofluorescence (IFI), solid-phase radioimmunoassay (RIA), and high-performance thin-layer chromatography (HPTLC) immunostaining.
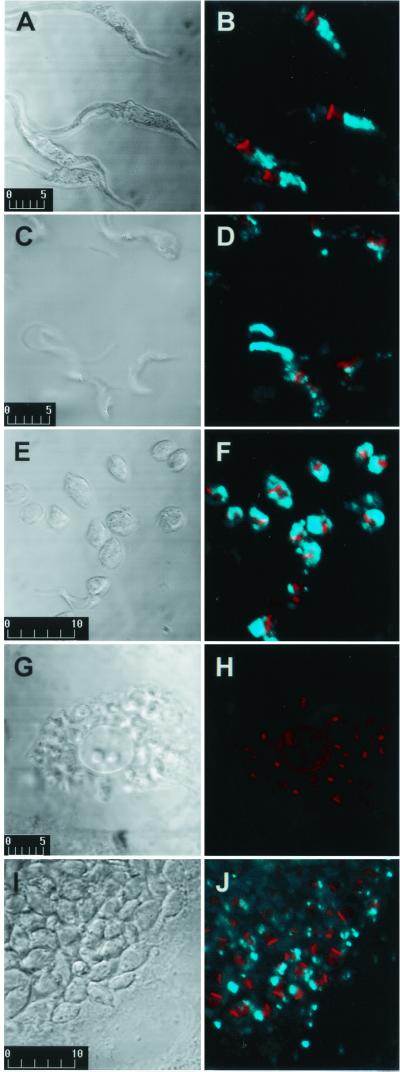
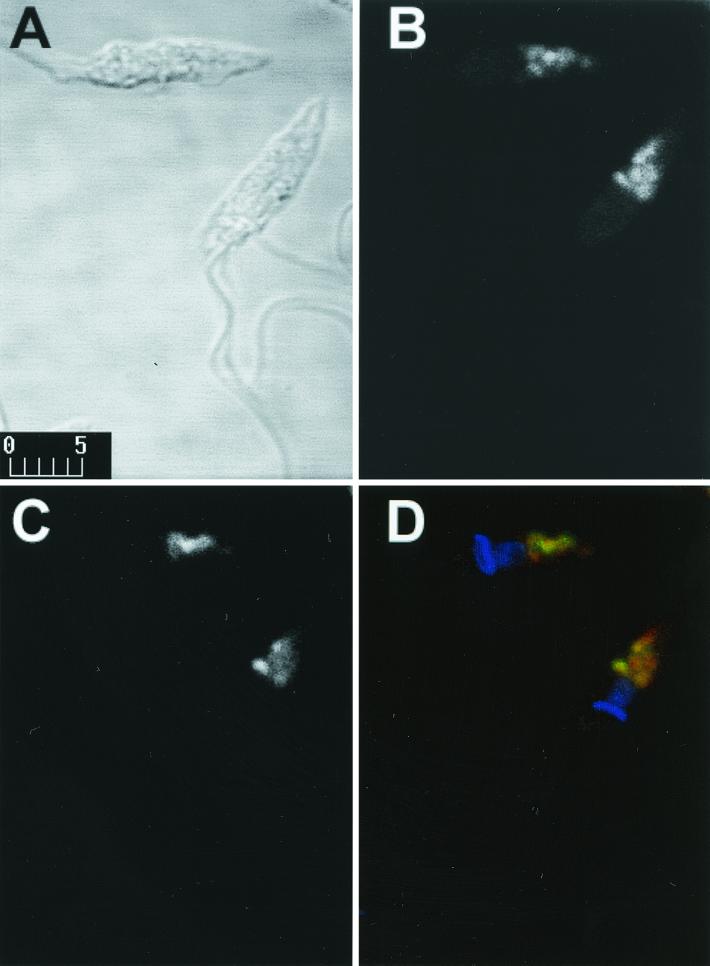
Epimastigotes were grown in liver infusion tryptose medium with 10% fetal calf serum at 27°C (2). Trypomastigotes and extracellular amastigotes were isolated from culture supernatants after 4 to 5 days infection of Vero cells with metacyclic trypomastigotes (1). Amastigote preparation contained less than 10% trypomastigotes, and trypomastigote preparation contained less than 5% amastigotes. For IFI, formaldehyde-fixed parasites were incubated on coverslips treated with 0.1% polylysine (19). The adsorbed parasites were blocked with 5% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) and incubated with MAb culture supernatant and 1% BSA containing 0.01 mM 4,6-diamidino-2-phenylindole (DAPI) and anti-mouse IgG conjugated to fluorescein isothiocyanate. The coverslips were examined on a Bio-Rad 1024-UV confocal microscope (1). Controls performed using an irrelevant MAb (IgG3) showed no fluorescence. Strong fluorescence was observed in epimastigotes (Fig. 1B) and extracellular amastigotes (Fig. 1F), and to a lesser extent in metacyclic trypomastigotes (Fig. 1D) and intracellular amastigotes (Fig. 1J). Epimastigotes showed strong labeling of vesicle-like components in the posterior region of T. cruzi. In view of previous reports of acidic vesicles, termed reservosomes, in the posterior region of T. cruzi epimastigotes (9, 12), we analyzed the relationship between MEST-1 labeling and these vesicles. As a first step, epimastigotes were incubated with Lysotracker Red, a specific label of acidic vesicles in eukaryotic cells (Handbook of Fluorscent Probes and Research Chemicals, Molecular Probes, Inc., Eugene, Oreg.), fixed with 1% formaldehyde, and incubated with MEST-1. Colocalization of Lysotracker Red and MEST-1 was confirmed (Fig. 2).
FIG. 1.
MEST-1 reactivity with various T. cruzi forms by confocal IFI. (A and B) Epimastigotes; (C and D) metacyclic trypomastigotes; (E and F) extracellular amastigotes; (G and H) Vero cells infected with trypomastigotes; (I and J) Vero cells infected with amastigotes. (A, C, E, G, and I) Differential interference contrast (bars show the scale in micrometers); (B, D, F, H, and J) fluorescence. Blue color, MEST-1 reactivity; red color, nucleus and kinetoplast stained with DAPI.
FIG. 2.
Colocalization of MEST-1 with acidic vesicles. Confocal immunofluorescence microscopy images of T. cruzi epimastigotes labeled with MEST-1 and Lysotracker Red are shown. (A) Phase contrast (bar, 5 μm); (B) Lysotracker Red fluorescence; (C) MEST-1 fluorescence; (D) overlaid image of DAPI (blue), Lysotracker Red (red), and MEST-1 (green) fluorescence.
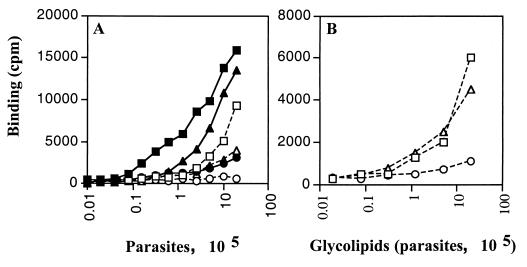
In contrast to our results, a study by Golgher et al. (3) using anti-GIPC serum showed a homogeneous label on the epimastigote surface, suggesting the predominant presence of GIPCs. We therefore characterized the T. cruzi antigen recognized by MEST-1 and analyzed the glycolipid fractions. Epimastigotes, culture-derived trypomastigotes, and extracellular amastigotes were added to 96-well plates (precoated with 0.1% poly-l-lysine; 2 × 106 parasites in the first well), doubly diluted in subsequent wells, and fixed for 15 min with 0.5% glutaraldehyde in cold PBS. MEST-1 reactivity was analyzed by solid-phase RIA (14). Fixed parasites were delipidated (or not) with a mixture of isopropanol-hexane-water (IHW) (55:20:25 [vol/vol/vol], upper phase discarded). Plates were then washed with PBS and used for solid-phase RIA (13). MEST-1 showed high reactivity with epimastigotes and extracellular amastigotes and weak reactivity with cell-derived trypomastigotes (Fig. 3A), similar to results from IFI. Delipidation of parasites abolished MEST-1 reactivity, and, as expected, most of the antigenicity was recovered in the organic extract (Fig. 3B) when the extracts were adsorbed onto 96-well plates and MEST-1 binding was detected as described above.
FIG. 3.
Reactivity of MAb MEST-1 with different T. cruzi forms. (A) Parasites (2 × 106) were serially diluted and adsorbed onto 96-well plates. Plates were treated with IHW (55:20:25, vol/vol/vol) (□, ○, and ▵) or left untreated (■, ●, and ▴) and incubated with MEST-1. (B) After treatment of parasites with IHW, the solvent was transferred to another 96-well plate and evaporated, and adsorbed glycolipid fractions were incubated with MEST-1. □ and ■, epimastigotes; ○ and ●, cell-derived trypomastigotes; ▵ and ▴, extracellular amastigotes.
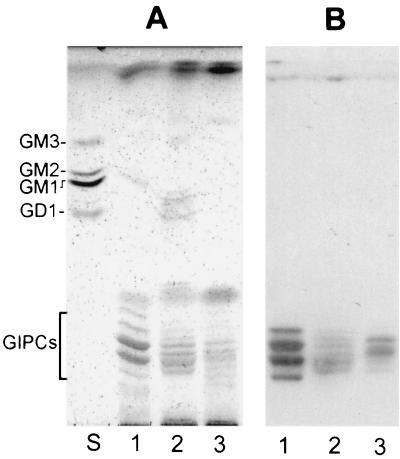
To confirm that MEST-1 recognizes all GIPC molecules and not only a subpopulation present in acidic vesicles (which could explain the lack of epimastigote surface labeling with MEST-1), GIPCs were extracted from epimastigotes, extracellular amastigotes, and cell-derived trypomastigotes by homogenizing the parasites (3 × 108) with 10 ml of isopropanol-hexane-water. The extracts were evaporated and dialyzed, and carbohydrate content was determined after densitometry of HPTLC stained with orcinol-H2SO4 (17). The GIPC fraction of epimastigotes contained 2 to 3 times more carbohydrate than that of amastigotes and 7.5 times more than that of trypomastigotes (data not shown). Aliquots containing about 3 μg of carbohydrate of the various GIPC fractions were analyzed by HPTLC on silica gel 60 plates (Whatman, Inc., Clifton, N.J.), using as solvents chloroform-methanol-H2O at 60:40:8 and 25:21:7 (vol/vol/vol). Upon staining with orcinol-H2SO4 (Fig. 4A), at least five glycolipid components with migration expected for GIPCs were visualized for epimastigotes, and six components were visualized for cell-derived trypomastigotes and amastigotes. For HPTLC immunostaining, plates were soaked in 0.5% polymethacrylate in hexane, dried, blocked for 2 h with 1% BSA in PBS, incubated overnight with MAb MEST-1, and incubated with rabbit anti-mouse IgG and 125I-labeled protein A (2 × 107 cpm per 50 ml of BSA-PBS). GIPCs were reactive with MEST-1 (Fig. 4B), indicating that even though GIPC levels vary among different T. cruzi forms (epimastigotes > amastigotes > trypomastigotes), all GIPCs express terminal Gal-furanose residues that are recognized by MEST-1. In order to better understand the role of GIPCs in the T. cruzi life cycle, we are currently investigating GIPC metabolic pathways. The relationship between GIPCs and epimastigote reservosomes is still unclear. Soares and DeSouza (11) showed by cytochemical studies that the main components of the reservosome matrix are proteins and lipid inclusions. The data presented here suggest that these inclusions contain GIPCs, among other lipids.
FIG. 4.
HPTLC of glycolipids from different forms of T. cruzi. (A) Staining with orcinol-H2SO4; (B) immunostaining with MAb MEST-1. Lane S, ganglioside standard mixture containing GM3, GM2, GM1, and GD1; lane 1, glycolipids from epimastigote forms; lane 2, amastigotes; lane 3, cell-derived trypomastigotes.
Acknowledgments
We thank Stephen Anderson for editing of the manuscript.
This work was supported by FAPESP, CNPq, and PRONEX.
REFERENCES
- 1.Barros H C, Verbisck N V, Da Silva S, Araguth M F, Mortara R A. Distribution of epitopes of Trypanosoma cruzi amastigotes during the intracellular life cycle within mammalian cells. J Euk Microbiol. 1997;44:332–344. doi: 10.1111/j.1550-7408.1997.tb05675.x. [DOI] [PubMed] [Google Scholar]
- 2.Camargo E P. Growth and differentiation in Trypanosoma cruzi: origin of metacyclic trypomastigotes in liquid media. Rev Inst Med Trop Sao Paulo. 1964;6:93–100. [PubMed] [Google Scholar]
- 3.Golgher D B, Colli W, Souto-Padron T, Zingales B. Galactofuranose-containing glycoconjugates of epimastigote and trypomastigote forms of Trypanosoma cruzi. Mol Biochem Parasitol. 1993;60:249–264. doi: 10.1016/0166-6851(93)90136-l. [DOI] [PubMed] [Google Scholar]
- 4.Lederkremer R M, Colli W. Galactofuranose-containing glycoconjugates in trypanosomatids. Glycobiology. 1995;5:547–552. doi: 10.1093/glycob/5.6.547. [DOI] [PubMed] [Google Scholar]
- 5.Lederkremer R M, Lima C, Ramirez M I, Ferguson M A J, Homans S W, Thomas-Oates J. Complete structure of the glycan of lipopeptidophosphoglycan from Trypanosoma cruzi epimastigotes. J Biol Chem. 1991;266:23670–23675. [PubMed] [Google Scholar]
- 6.Levery S B, Toledo M S, Straus A H, Takahashi H K. Structure elucidation of sphingolipids from the mycopathogen Paracoccidioides brasiliensis: an immunodominant β-galactofuranose residue is carried by a novel glycosylinositol phosphoceramide antigen. Biochemistry. 1998;37:8764–8775. doi: 10.1021/bi9730083. [DOI] [PubMed] [Google Scholar]
- 7.Magnani J L, Smith D F, Ginsburg V. Detection of gangliosides that bind toxin: direct binding of 125I-labeled toxin to thin-layer chromatography. Anal Biochem. 1990;109:399–402. doi: 10.1016/0003-2697(80)90667-3. [DOI] [PubMed] [Google Scholar]
- 8.McConville M J, Ferguson M A J. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J. 1993;294:305–324. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porto-Carreiro I, Attias M, Miranda K, De Souza W, Cunha-e-Silva N. Trypanosoma cruzi epimastigote endocytic pathway: cargo enters the cytostome and passes through an early endosomal network before storage in reservosomes. Eur J Cell Biol. 2000;79:858–869. doi: 10.1078/0171-9335-00112. [DOI] [PubMed] [Google Scholar]
- 10.Previato J O, Gorin P A J, Mazurek M, Xavier M T, Fournet B, Wieruszesk J M, Mendonça-Previato L. Primary structure of the oligosaccharide chain of lipopeptidophosphoglycan of epimastigote forms of Trypanosoma cruzi. J Biol Chem. 1990;265:2518–2526. [PubMed] [Google Scholar]
- 11.Soares M J, De Souza W. Cytoplasmic organelles of trypanosomatids: a cytochemical and stereological study. J Submicrosc Cytol Pathol. 1988;20:349–361. [PubMed] [Google Scholar]
- 12.Soares M J, Souto-Padrón T, De Souza W. Identification of a large pre-lysosomal compartment in the pathogenic protozoon Trypanosoma cruzi. J Cell Sci. 1992;102:157–167. doi: 10.1242/jcs.102.1.157. [DOI] [PubMed] [Google Scholar]
- 13.Straus A H, Travassos L R, Takahashi H K. A monoclonal antibody (ST-1) directed to native heparin chain. Anal Biochem. 1992;201:1–8. doi: 10.1016/0003-2697(92)90167-6. [DOI] [PubMed] [Google Scholar]
- 14.Straus A H, Levery S B, Jasiulionis M G, Salyan M E K, Steele S J, Travassos L R, Hakomori S, Takahashi H K. Stage-specific glycosphingolipids from amastigote forms of Leishmania (L.) amazonensis. Immunogenicity and role in parasite binding and invasion of macrophages. J Biol Chem. 1993;268:13723–13730. [PubMed] [Google Scholar]
- 15.Straus A H, Suzuki E, Toledo M S, Takizawa C M, Takahashi H K. Immunochemical characterization of carbohydrate antigens from fungi, protozoa and mammal by monoclonal antibodies directed to glycan epitope. Braz J Med Biol Res. 1995;28:919–923. [PubMed] [Google Scholar]
- 16.Suzuki E, Toledo M S, Takahashi H K, Straus A H. A monoclonal antibody directed to terminal residue of β-galactofuranose of a glycolipid antigen isolated from Paracoccidioides brasiliensis: cross-reactivity with Leishmania major and Trypanosoma cruzi. Glycobiology. 1997;7:463–468. doi: 10.1093/glycob/7.4.463. [DOI] [PubMed] [Google Scholar]
- 17.Toledo M S, Suzuki E, Straus A H, Takahashi H K. Glycolipids from Paracoccidioides brasiliensis. Isolation of a galactofuranose-containing glycolipid reactive with sera of patients with paracoccidioidomycosis. J Med Vet Mycol. 1995;33:247–251. [Google Scholar]
- 18.Toledo M S, Levery S B, Glushka J, Straus A H, Takahashi H K. Structure elucidation of sphingolipids from the mycopathogen Sporothrix schenkii: identification of novel glycosylinositol phosphorylceramides with core Manβ1-6Ins linkage. Biochem Biophys Res Commun. 2001;280:9–24. doi: 10.1006/bbrc.2000.4091. [DOI] [PubMed] [Google Scholar]
- 19.Toledo M S, Suzuki E, Levery S B, Straus A H, Takahashi H K. Characterization of monoclonal antibody MEST-2 specific to glucosylceramide of fungi and plants. Glycobiology. 2001;11:105–112. doi: 10.1093/glycob/11.2.105. [DOI] [PubMed] [Google Scholar]