Summary
Lung cancer is the leading cause of cancer-related death. Intriguingly, males with non-small cell lung cancer (NSCLC) have a higher mortality rate than females. Here, we investigated the role of serine metabolism as a predictive marker for sensitivity to the antifolate pemetrexed in male and female NSCLC cell lines. Using [13C6] glucose tracing in NSCLC cell lines, we found that a subset of male cells generated significantly more serine from glucose than female cells. Higher serine biosynthesis was further correlated with increased sensitivity to pemetrexed in male cells only. Concordant sex differences in metabolic gene expression were evident in NSCLC and pan-cancer transcriptome datasets, suggesting a potential mechanism with wide-reaching applicability. These data were further validated by integrating antifolate drug cytotoxicity and metabolic pathway transcriptome data from pan-cancer cell lines. Together, these findings highlight the importance of considering sex differences in cancer metabolism to improve treatment for all patients.
Subject areas: Cellular physiology, Cell biology, Cancer
Graphical abstract

Highlights
-
•
Male NSCLC cells utilize more glucose for serine biosynthesis
-
•
High serine biosynthesis predicts sensitivity to antifolates in male NSCLC cells
-
•
Serine biosynthesis correlates with sensitivity to antifolates in male cancer cells
Cellular physiology; Cell biology; Cancer
Introduction
Lung cancer is the second most common cancer and the leading cause of cancer-related death in the U.S. and worldwide (Siegel et al., 2021; Sung et al., 2021). Non-small cell lung cancer (NSCLC) is the most common subtype, accounting for approximately 80%–85% of all lung cancers (Howlader et al., 2021). Most patients with NSCLC are diagnosed at advanced stages and generally have a poor prognosis with a median survival of 8–10 months (Shih et al., 2020; Siegel et al., 2021). One of the front-line chemotherapeutics for patients with non-squamous NSCLC is pemetrexed (Ettinger et al., 2021), an antifolate chemotherapeutic. Although the addition of pemetrexed, either as a first-line treatment in combination with carboplatin or cisplatin or as a monotherapy for second-line treatment, has improved survival and overall response rate in men and women, about 50% of patients with advanced NSCLC show no response to treatment (Ettinger, 2002; Jo et al., 2018; Park et al., 2015; Shih et al., 2020). Thus, better biomarkers for treatment response are needed to improve survival for all patients with NSCLC.
As in most cancers, males with NSCLC exhibit a higher mortality rate compared to females (de Perrot et al., 2000; Dong et al., 2020; Moore et al., 2004; Siegel et al., 2021; Wainer et al., 2018). This sex difference in mortality may be, at least partially, driven by sex differences in response to the current treatment. Indeed, multiple phase 3 clinical trials have shown that female patients with NSCLC respond better to certain treatment regimens, including pemetrexed-based treatment combinations (Paz-Ares et al., 2012; Wang et al., 2017). Pemetrexed inhibits multiple folate cycle enzymes, thus inhibiting DNA synthesis and cell replication (Adjei, 2004). The folate cycle is a metabolic nexus of multiple metabolic and cellular processes, such as providing purines and pyrimidines for DNA synthesis, serine and glycine metabolism, thymine and methionine synthesis, and redox homeostasis (Yang et al., 2021). Whether the activity of these metabolic pathways could be utilized to predict sensitivity to antifolates has yet to be determined.
Most metabolic pathways exhibit sex differences across many species from fertilization to adulthood (Bermejo-Álvarez et al., 2008; Bredbacka and Bredbacka, 1996; Gutiérrez-Adán et al., 2001, 2006; Hedrington and Davis, 2015; Krumsiek et al., 2015; Pergament et al., 1994; Ray et al., 1995; Rubin et al., 2020; Tagirov and Rutkowska, 2014; Tiffin et al., 1991; Van Blerkom, 2008). Thus, we hypothesized that sensitivity to pemetrexed may be driven by different metabolic pathways in male and female NSCLC. Previously, we provided support for this idea when we identified glycolytic transcriptome and metabolome signatures that selectively stratified male, but not female patients with lower grade gliomas (Ippolito et al., 2017). These data suggest that sex-specific analyses of predictive metabolic biomarkers may help stratify patients for treatment approaches.
Here, we provide further support for the presence of sex differences in cancer cell metabolism and its impact on predicting treatment response in male and female cancer cell lines. Using [13C6] glucose tracing datasets of over 80 NSCLC cell lines (Chen et al., 2019), we demonstrate that a subset of male cell lines have increased de novo serine and glycine biosynthesis from glucose compared to female cell lines. Higher serine biosynthesis correlated with increased sensitivity to pemetrexed in male NSCLC cell lines only. Concordant sex differences in metabolic gene expression were evident in NSCLC and pan-cancer transcriptome datasets, showing increased transcript enrichment of this pathway in male tumors. These findings were also validated with drug cytotoxicity and gene expression analyses of pan-cancer cell lines obtained from The Cancer Cell Line Encyclopedia (TCLE). In summary, we discovered sex differences in the role of de novo serine biosynthesis in male and female NSCLC cell lines, which may be leveraged to better predict sex-specific treatment response to antifolates in patients with NSCLC. Thus, our data highlight the importance of considering sex, even in established cancer cell lines, as understanding sex differences in cancer metabolism and its impact on therapeutic response will lead to the development of new diagnostic and therapeutic approaches to improve cancer outcomes for both men and women.
Results
Male NSCLC cell lines utilize more glucose for de novo serine biosynthesis
Serine is a non-essential amino acid that can fuel the folate cycle by donating a one-carbon unit to the synthesis of glycine. It can be either taken up by the cell or synthesized de novo from glycolytic intermediates (Locasale, 2013). Upon its uptake into the cell, most glucose is converted to pyruvate via the glycolytic pathway, which can then be converted into acetyl-CoA to replenish the TCA cycle. However, glycolysis also provides 3P-glycerate, the substrate for serine biosynthesis, via the phosphoserine pathway (Figure 1A). To determine whether male and female NSCLCs may differ in their requirement for de novo serine biosynthesis, we first investigated whether male and female NSCLC cell lines utilize glucose differently. We assessed published stable isotope nutrient tracing data of 57 male and 30 female NSCLC cell lines (for cell line characteristics, see Table S1) cultured in [13C6] glucose for either 6 or 24 h (Chen et al., 2019). While total label enrichment was similar in male and female cell lines in TCA cycle metabolites (Table S2), we found that male cell lines incorporated more glucose into serine and glycine biosynthesis than female cell lines (Figures 1B and 1C). Interestingly, density plots revealed an almost bimodal distribution in the male cell lines (particularly for serine), suggesting that the male population consists of a high and a low de novo serine and glycine biosynthesis population. The bimodal distribution in female cell lines was almost absent, indicating that the female population is primarily comprised of a low de novo serine and glycine biosynthesis population. To exclude the possibility that differences in mutational status rather than sex drive these differences in de novo serine biosynthesis, we assessed whether EGFR, KEAP1, KRAS, NFE2L2 (NRF2), or STK11 mutations had a significant effect on glucose label enrichment in serine and glycine. Importantly, mutational status had no significant effect on label enrichment, while the effect of sex remained significant (Table S3). NRF2 is an important regulator of de novo serine biosynthesis, and abnormalities in NRF2 expression have been linked to heterogeneity in de novo serine biosynthesis in NSCLC cell lines (DeNicola et al., 2015). Using published data from 39 male and 21 female NSCLC cell lines (DeNicola et al., 2015), we compared NRF2 protein expression levels in males and females and found no significant difference (Figure S1A). Next, we grouped male and female NSCLC cells into NRF2-high and NRF2-low groups based on the same method described in the study by DeNicola et al. (2015). We compared glucose label incorporation into serine in NRF2-high and NRF2-low male and female cells and found that NRF2-high male cells had significantly greater label incorporation than NRF2-low male cells (Figure S1B). However, there was no difference in female cells (Figure S1B), suggesting that NRF2 levels may impact de novo serine biosynthesis in male but not female NSCLC cells. Together, these data suggest that male NSCLC cells may have a greater requirement for glucose-dependent de novo serine and glycine biosynthesis.
Figure 1.
Male NSCLC cell lines utilize more glucose for de novo serine biosynthesis
(A) Schematic of representative isotopologues generated from [13C6] glucose-based labeling.
(B) Density plots showing total [13C6] glucose enrichment in serine (M+1, M+2, M+3) and glycine (M+1, M+2) after 6 and 24 h in male (blue; n = 49) and female (red; n = 28) non-small-cell lung cancer (NSCLC) cell lines.
(C) Violin plots showing isotopologue enrichment of [13C6] glucose into serine and glycine after 6 and 24 h in male (n = 49) and female (n = 28) NSCLC cell lines. All possible isotopologues are shown. Serine: M+0, M+1, M+2, M+3; glycine: M+0, M+1, M+2. ∗q < 0.05, ∗∗q < 0.01; Mann-Whitney test, q = FDR-adjusted p-values. Abbreviations: ser, serine; gly, glycine; G, glucose. See also Figure S1 and Tables S1–S3.
De novo serine biosynthesis from glucose is associated with sensitivity to antifolate drugs in male NSCLC cell lines
Serine is an important one-carbon donor to the folate cycle and therefore contributes to nucleotide synthesis, NADPH generation, and methylation reactions (Locasale, 2013; Minton et al., 2018; Mullarky et al., 2016; Singh et al., 2021; Tanaka et al., 2021; Tedeschi et al., 2013; Ye et al., 2014). The folate cycle is a well-studied target of multiple antifolate therapeutics. In fact, the antifolate pemetrexed is a front-line chemotherapeutic for patients with non-squamous NSCLC (Ettinger et al., 2021).
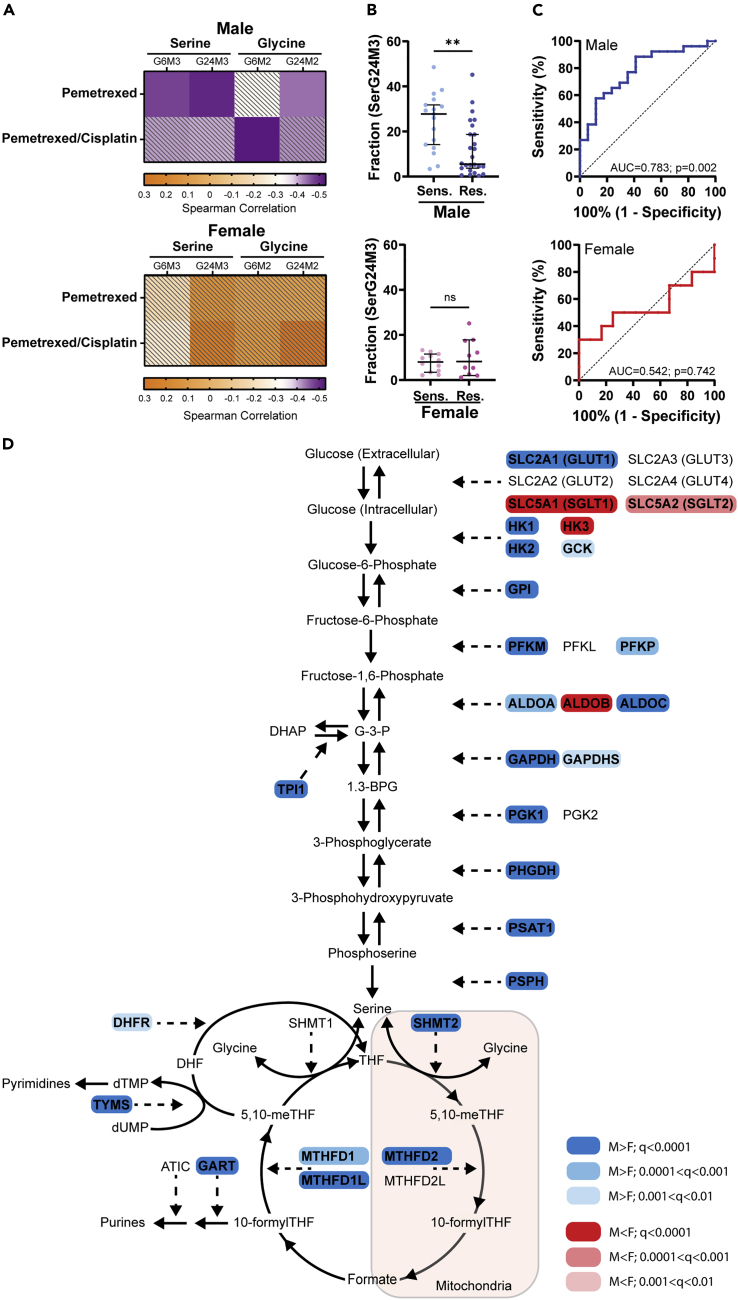
As the serine M+3 isotopologue had the highest correlation with pemetrexed when these cell lines were evaluated as a whole previously (Chen et al., 2019), we correlated label enrichment of serine M+3 and glycine M+2 with pemetrexed IC50 values in male and female cell lines separately. We found that sensitivity to pemetrexed and the combination of pemetrexed and the alkylating agent cisplatin, another front-line chemotherapeutic given with pemetrexed for NSCLC (Ettinger et al., 2021), positively correlated with label enrichment in serine and glycine in male, but not female, NSCLC cell lines (Figure 2A). Interestingly, no other chemotherapeutic agents tested showed a significant correlation in either the male or female cell lines (Table S4). To exclude the possibility that differences in mutational status rather than sex drive these differences, we assessed whether the effect of mutational status of EGFR, KEAP1, KRAS, NFE2L2 (NRF2), or STK11 had a significant effect on the correlation between label enrichment in serine and glycine and the sensitivity to pemetrexed. Importantly, mutational status had no significant effect on the correlation between label enrichment in serine and glycine and the sensitivity to pemetrexed, while the effect of sex remained significant (Table S3).
Figure 2.
De novo serine biosynthesis from glucose is associated with sensitivity to antifolate drugs in male NSCLC cell lines
(A) Heatmaps representing spearman correlation coefficients of [13C6] glucose incorporation into serine (M+3) and glycine (M+2) isotopologues and sensitivity to pemetrexed or pemetrexed/cisplatin in male (n = 43) and female (n = 22) non-small-cell lung cancer (NSCLC) cell lines. Colored squares reached FDRadjusted p-value cut-off of 0.05.
(B) Isotopologue enrichment (M+3) of [13C6] glucose into serine after 24 h in male pemetrexed-sensitive (n = 17) and male pemetrexed-resistant (n = 26), and female pemetrexed-sensitive (n = 12) and female pemetrexed-resistant (n = 10) NSCLC cell lines. ∗∗p < 0.01; Mann-Whitney test.
(C) Receiver operating characteristic (ROC) curve analyses of male pemetrexed-sensitive (n = 17) and male pemetrexed-resistant (n = 26), and female pemetrexed-sensitive (n = 12) and female pemetrexed-resistant (n = 10) NSCLC cell lines. ∗∗p < 0.01; Mann-Whitney test.
(D) Pathway schematic of enzymes involved in the de novo biosynthesis of serine from glucose and folate metabolism demonstrating mRNA expression of male (n = 592) and female (n = 400) NSCLC tumor samples with a male (blue) or female (red) bias. Color shade represents grade of significance. ∗∗q < 0.01, ∗∗∗q < 0.001, ∗∗∗∗q < 0.0001; Mann-Whitney test, FDR-adjusted p-values (q<0.01). Abbreviations: ser, serine; gly, glycine; G, glucose; sens, sensitive; res, resistant; AUC, area under the curve. See also Figures S2–S4 and Tables S3 and S4.
Next, we divided male and female cell lines into pemetrexed-resistant and pemetrexed-sensitive cell lines (Chen et al., 2019) to assess whether male or female pemetrexed-sensitive cell lines exhibit significantly greater glucose label enrichment in serine. Concordant with our correlation data, label enrichment in serine was greater in male pemetrexed-sensitive cell lines than male pemetrexed-resistant cell lines (Figure 2B). There was no significant difference in the female cells (Figure 2B). Although pemetrexed-sensitive male cell lines had significantly greater label enrichment than pemetrexed-resistant male cell lines, there was overlap between sensitive and resistant cell lines, suggesting that pemetrexed sensitivity in males may also be driven by additional factors. Furthermore, we performed receiver operating characteristic curve analyses of male and female pemetrexed-sensitive and pemetrexed-resistant cell lines to assess how efficiently glucose label enrichment in serine predicts pemetrexed sensitivity in male and female NSCLC cell lines. As expected, label enrichment was efficient in predicting pemetrexed sensitivity in male NSCLC cells (AUC = 0.783; p = 0.002) but failed to predict pemetrexed sensitivity in female NSCLC cell lines (AUC = 0.542, p = 0.742) (Figure 2C). These data indicate that increased de novo serine and glycine biosynthesis in male NSCLC cell lines can predict increased sensitivity to pemetrexed. However, not every pemetrexed-sensitive male cell line is characterized by increased de novo serine biosynthesis. Thus, additional factors may drive pemetrexed sensitivity in males.
Next, we examined mRNA expression levels of 35 genes involved in the de novo biosynthesis of serine from glucose and the folate cycle in male (n = 592) and female (n = 400) NSCLC samples from the TCGA Pan-Cancer dataset. The majority of the genes were expressed at higher levels in male vs female patient samples (Figures 2D and S2A), including phosphoglycerate dehydrogenase (PHGDH), the rate-limiting enzyme of the serine biosynthesis pathway. This suggests that de novo serine biosynthesis and the folate cycle may be more important in male NSCLCs. Because pemetrexed has greater efficacy in adenocarcinomas, we assessed mRNA expression levels of the same genes in patients with adenocarcinoma and squamous cell carcinoma separately. Notably, there were fewer significantly different genes that may be due to sample size (Figures S3 and S4). However, of the genes that were significant, most genes were expressed at significantly greater levels in males, paralleling the NSCLC mRNA expression data. Interestingly, three folate cycle genes that include thymidylate synthase (TYMS; the primary target of pemetrexed) (Adjei, 2004; Curtin and Hughes, 2001), methylene tetrahydrofolate reductase (MTHFD2), and 5-Aminoimidazole-4-Carboxamide Ribonucleotide Formyltransferase/IMP Cyclohydrolase (ATIC) were enriched only in male adenocarcinomas (Figure S3A). Together, these data suggest that male NSCLCs may depend more on de novo serine biosynthesis than female NSCLCs to supply the folate cycle.
De novo serine biosynthesis is associated with sensitivity to antifolate drugs in male pan-cancer cell lines
Next, we wanted to test if the sex-specific associations of pemetrexed cytotoxicity with de novo serine biosynthesis were generalizable to other cancers. We correlated mRNA expression levels of the genes involved in the de novo biosynthesis of serine from glucose and the folate cycle with drugs that inhibit the folate cycle and downstream pathways (i.e., methotrexate and 5-fluorouracil) on a cohort of pan-cancer cell lines obtained from TCLE using both area under the curve (AUC) and IC50 parameters derived from dose-response curves for cytotoxicity. Significant negative correlations between folate cycle gene expression and IC50 and AUC values (i.e., higher gene expression corresponded to lower drug dose needed for cytotoxicity) indicated that male and female pan-cancer cell lines with high folate cycle gene expression are sensitive to antifolate drugs (Figure 3A). While there was no significant difference in the number of correlations between the folate cycle genes and antifolate drug cytotoxicity in male and female cell lines, (25 significant correlations in males, 24 significant correlations in females; p = 1.0, Fisher Exact Test), only male cell lines exhibited a significant enrichment of de novo serine synthetic genes whose expression correlated negatively with antifolate drug IC50 and AUC values (18 significant correlations in males, 5 significant correlations in females; p = 0.007, Fisher Exact Test). This indicates that increased gene expression of de novo serine biosynthesis enzymes predicts sensitivity to antifolates in male pan-cancer cell lines only. The de novo serine synthetic genes PHGDH and phosphoserine aminotransferase 1 (PSAT1) as well as other glycolytic genes such as hexokinase 2 (HK2), phosphofructokinase L (PFKL), and aldolase C (ALDOC) showed significant negative correlations between gene expression and antifolate drug IC50 and AUC values in male pan-cancer cell lines (Figure 3A). Also of note, there were significant positive correlations between GLUT1 (SLC2A1) and hexokinase 1 (HK1) gene expression and antifolate drug cytotoxicity in both males and females, suggesting a potential mechanism for antifolate resistance. Together, these data indicate that, similar to NSCLC cell lines, de novo serine biosynthesis may be a predictive marker for antifolate drug sensitivity in male, but not female, pan-cancer cell lines.
Figure 3.
De novo serine biosynthesis is associated with sensitivity to antifolate drugs in male pan-cancer
(A) Heatmaps representing spearman correlation coefficients of mRNA expression levels and cytotoxicity to antifolate drugs in male (n = 325) and female (n = 276) pan-cancer cell lines. Colored squares reached FDR-adjusted p-value cut-off of 0.05.
(B) Pathway schematic of enzymes involved in the de novo biosynthesis of serine from glucose and the folate cycle demonstrating mRNA expression of 4,076 male and 2,782 female pan-cancer tumor samples with a male (blue) or female (red) bias. Color shade represents grade of significance. ∗∗q < 0.01, ∗∗∗q < 0.001, ∗∗∗∗q < 0.0001; Mann-Whitney test, FDR-adjusted p-values (q<0.01). Abbreviations: AUC, area under the curve. See also Figure S2 and Table S5.
Next, we examined mRNA expression levels of the same genes in male (n = 4,076) and female (n = 2,782) tumors from the TCGA Pan-Cancer dataset. Similar to NSCLC samples, most of the genes were expressed at higher levels in male tumors (Figures 3B and S2B), suggesting that sex differences in de novo serine biosynthesis may extend to other cancers beyond NSCLC.
Discussion
Targeting metabolic adaptations of cancer cells is a promising therapeutic approach. However, to this date, sex differences in cancer metabolism have not been taken much into account. Here, we report for the first time that male and female NSCLC cells exhibit different metabolic features that predict sex-biased sensitivity to cytotoxic reagents. We found that male NSCLC cell lines exhibited increased de novo serine and glycine biosynthesis from glucose compared to female cell lines. Notably, the increased de novo serine biosynthesis in males was driven by a subset of cell lines, as shown by the bimodal distribution of percent total carbon label in serine and glycine (Figure 1B). This indicates that only a subset of male NSCLC tumors exhibit increased de novo serine biosynthesis. The mechanisms underlying this metabolic heterogeneity in male NSCLC cells remain to be determined.
Furthermore, we showed that de novo serine biosynthesis predicts sensitivity to pemetrexed in male, but not female, NSCLC cell lines, suggesting that de novo serine biosynthesis may be an important key player in male NSCLC. We validated these findings using transcriptome analyses of human NSCLC and pan-cancer tumors from TCGA, establishing that male tumors had increased transcript enrichment of the de novo serine biosynthesis pathway and the folate cycle. In addition, we showed that increased expression of serine biosynthesis genes predicted increased antifolate cytotoxicity in male, but not female, pan-cancer cell lines from TCLE.
The antifolate pemetrexed is a front-line chemotherapeutic for patients with non-squamous NSCLC (Ettinger et al., 2021). Although pemetrexed-based treatment regimens showed improved survival and improved overall response rate in males and females, about 50% of patients with NSCLC continue to show no response to treatment (Ettinger, 2002; Jo et al., 2018; Park et al., 2015; Shih et al., 2020). Thus, better methods to predict therapeutic responses to pemetrexed in patients with NSCLC are needed. Here, we show for the first time that stable isotope labeling studies with glucose can predict sensitivity to antifolates on a sex-specific basis in vitro. Thus, these data provide the basis for future research to determine whether the phosphoserine pathway may be a sex-specific biomarker for antifolate treatment response in NSCLC and other cancers. NRF2 is a known regulator of serine metabolism and differences in NRF2 expression have been linked to the heterogeneity of de novo serine biosynthesis in NSCLC cell lines (DeNicola et al., 2015). Although we did not see sex differences in NRF2 protein expression, we found that, in males but not females, NRF2-high cell lines had significantly greater glucose label incorporation into serine than NRF2-low cell lines. Thus, increased NRF2 signaling may lead to increased de novo serine biosynthesis in male, but not female, cell lines. In addition to a larger sample size, additional experiments are necessary to fully understand the mechanisms that underlie the observed sex differences in de novo serine biosynthesis. Future experiments would include sex-specific analyses of NRF2 binding sites in the genome and metabolic analyses of male and female NSCLC cell lines depleted of NRF2. In addition, assessing whether NRF2 signaling may be a sex-specific predictive marker for pemetrexed sensitivity may be beneficial. This could be an additional avenue for the development of sex-specific biomarkers for antifolate treatment response.
Multiple publications support that the folate cycle may differ between males and females. Certain polymorphisms in the 5,10-methylenetetrahydrofolate reductase gene, an enzyme of the folate cycle, are associated with increased or decreased risk of lung cancer in females, but not males (Shi et al., 2005), which further underlines the importance of considering sex in antifolate targeting approaches. Although the mechanisms for this clinical finding are unclear, published data indicate the presence of sex differences in the function of this pathway. In addition, multiple reports have identified sex differences in folate input into one-carbon metabolism. Males typically have lower folate levels, requiring increased supplementation (Cohen et al., 2021; Winkels et al., 2008). Additional data identify that abnormal folate metabolism results in sex differences in fetal development and the presence of neural tube defects (Padmanabhan et al., 2017; Poletta et al., 2018). To support these findings further, there are sex differences in the activity of one-carbon metabolism where females have higher methionine flux compared to males (Sadre-Marandi et al., 2018; Witham et al., 2013). These findings suggest a potential avenue for advancement in predicting female therapeutic response. Because de novo biosynthesis of serine and glycine from glucose is associated with sensitivity to antifolates only in male cell lines, female sensitivity to antifolates may be dependent upon input of other nutrients into one-carbon metabolism. However, this needs to be established. While the data we provided here suggest that altered serine biosynthesis may sensitize male NSCLC to pemetrexed and may therefore function as a predictive marker for response to pemetrexed treatment in males, this does not necessarily suggest that male patients with NSCLC respond better to pemetrexed than female patients. In fact, clinical data have shown that while pemetrexed improved survival and overall response rate in men and women (Jo et al., 2018; Park et al., 2015; Shih et al., 2020), female patients with NSCLC respond better to pemetrexed-based treatment combinations (Paz-Ares et al., 2012; Wang et al., 2017). This may be confounded further by the presence of sex-specific metabolic subgroups. In this case, the larger population may mask the response of high-serine male patients with NSCLC. Currently, there are no routine clinical assays to identify these high-serine male patients. Thus, additional research is required to better understand the sex-biased outcome in male and female patient populations that received pemetrexed.
Established cancer cell lines are used in almost every aspect of basic cancer research. However, little to no attention is given to the sex of these cell lines. Here, we provide evidence that established cancer cell lines exhibit sex differences in metabolism even after years of in vitro culture. In addition to sex differences in de novo serine biosynthesis in NSCLC cell lines, we recently reported that glioma cell lines exhibit sex differences in glutaminase 1 expression (Sponagel et al., 2022), suggesting that sex differences in cancer cell lines persist across multiple metabolic pathways. Not only do these findings underline the importance of correctly identifying and considering the sex of cancer cell lines in the laboratory setting but it also identifies a completely unexplored and very important scientific space. The fact that cell lines such as these have been used for years in laboratory research and still retain sex differences in fundamental metabolic pathways suggests that the selective advantage of retaining these characteristics in cell culture is significant and outweighs the selective pressures of conventional laboratory cell culture. As the majority of genes encoding enzymes involved in central carbon metabolism are not present on sex chromosomes, these findings suggest the presence of epigenetic influences on sex differences, as has been previously identified in cancers including lung (Lopes-Ramos et al., 2018, 2020; Vaissière et al., 2009; Wu et al., 2008).
Limitations of the study
The most important limitation of this study is that it is retrospective and limited to a subset of metabolites and nutrient utilization pathways in central carbon metabolism. Specifically, the stable isotope-labeled nutrient analyzed in this study was [13C6] glucose and its analyses were restricted to serine, glycine, and selected glycolytic and TCA cycle metabolites. Emerging data from our group also showed sex differences in anaplerosis from glutamine in GBM and that these sex differences may not come from carbons as part of the TCA cycle, but rather nitrogens for transamination and amino acid synthesis (Sponagel et al., 2022). Therefore, follow-up studies would need to be performed to confirm whether similar sex differences exist in NSCLC. Notably, independent validation of the sex differences in NSCLC metabolism and pemetrexed sensitivity described here is mandatory before we can begin to dissect the mechanisms underlying these differences.
Multiple confounding variables other than sex may impact de novo serine biosynthesis. While we showed that mutational status of EGFR, KEAP1, KRAS, NFE2L2 (NRF2), or STK11 does not impact de novo serine biosynthesis, other confounding variables, such as smoking history, could not be reported or controlled for as these data were not available.
Although the use of TCLE cell lines further supports the presence of sex differences in cell line and tumor metabolism and its impact on chemotherapeutic sensitivity, this is also retrospective, and more work needs to be done to characterize these sex-specific metabolic-therapeutic connections on a cancer-by-cancer basis. Furthermore, additional important steps are necessary to confirm that de novo serine biosynthesis may be a reliable biomarker for pemetrexed sensitivity in male NSCLCs. While our data provide strong in vitro evidence that altered de novo serine biosynthesis predicts sensitivity in male NSCLC and pan-cancer cell lines, in vivo studies in patient xenograft models should be conducted to confirm both, sex differences in de novo serine biosynthesis from glucose and the male-specific correlation between serine synthesis and pemetrexed sensitivity. In addition, clinical data are required to provide evidence that altered de novo serine biosynthesis can stratify male, but not female, patients into pemetrexed-responsive and pemetrexed-non-responsive patient cohorts. These studies will require a patient cohort of male and female patients with NSCLC who received pemetrexed and the development of a reliable measurement of de novo serine biosynthesis in patients, such as stable isotope glucose labeling performed in vivo (Hensley et al., 2016) or in ex vivo surgically resected tumor specimens (Fan et al., 2016) which could be used to measure de novo serine biosynthesis, and thus pemetrexed sensitivity.
Nonetheless, the combination of human cell line and tumor tissue data provide strong evidence to further support the emerging paradigm of factoring sex and metabolism into cancer therapeutic response. As mentioned above, the presence of sex differences in normal metabolism and the persistent sex disparity in incidence and mortality suggest that these findings may be applied to additional other cancers.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| Original code | GitHub |
https://github.com/rosyluo/Sex-Differential-Metabolite-in-Lung-Cancer/releases/tag/V1.0.0 https://doi.org/10.5281/zenodo.7121214 |
| GraphPad Prism v9 | GraphPad | https://www.graphpad.com/scientific-software/prism/; RRID:SCR_002798 |
| R v4.1.1 | R Core Team (2021) | https://www.r-project.org/ |
Resource availability
Lead contact
Further information and request for resources and reagents should be directed to and will be fulfilled by the lead contact Joseph E. Ippolito (ippolitoj@wustl.edu).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
This paper analyzes existing, publicly available data. No experimental models were used.
Method details
Cell line stable isotope labeling datasets
[13C6] glucose labeling data for 87 NSCLC (n = 57 male and n = 30 female) cell lines were downloaded as supplemental information from Chen et al. (2019) and used for analyses in Figures 1 and 2. NRF2 protein expression data, NRF2-high and -low expression groups, and [13C6] glucose labeling data for 60 NSCLC (n = 39 male and n = 21 female) cell lines were downloaded as supplemental information from DeNicola et al. (2015). The sex of each of the cell lines was verified with the Cellosaurus database (https://web.expasy.org/cellosaurus/) (Bairoch, 2018). Isotopologue data were converted into total labeled metabolite data by summing up the percentage abundance of each of the labeled isotopologues (i.e., M+1 … M + n) for each metabolite. Total labeled metabolites were used in subsequent sex-based comparisons.
Sex-based correlations of drug cytotoxicity and labeled metabolites in NSCLC cell lines
Drug cytotoxicity data for the 87 NSCLC cell lines and 20 drugs were downloaded as supplemental information from the publication (Chen et al., 2019). Nonparametric Spearman correlation coefficient was calculated between each drug IC50 value and the total label of serine and glycine, and linear regression was fit to model the linear relationship between the IC50 value of pemetrexed and total label of serine and glycine. Correlations with an FDR- adjusted p-value<0.05 were considered statistically significant.
TCGA gene expression analyses
All gene expression data were obtained through cBioPortal (http://cbioportal.org). Batch normalized RNASeq RSEM data of male and female NSCLC patient samples (n = 510 adenocarcinoma and n = 482 squamous cell carcinomas) corresponding to 35 genes involved in de novo serine biosynthesis and the folate cycle were downloaded from the TCGA Pan-Cancer dataset. Adenocarcinomas included 236 male and 274 female samples and squamous cell carcinomas included 356 males and 126 females. Batch normalized RNASeq RSEM data of male and female pan-cancer patient samples corresponding to 35 genes involved in de novo serine biosynthesis and the folate cycle were downloaded from the TCGA Pan-Cancer dataset. It included 4,076 male and 2,782 female samples. Sex-specific cancers (i.e., breast, ovarian, endometrial, cervical, prostate, and testicular cancers) were excluded.
The Cancer Cell Line Encyclopedia (TCLE) gene expression analyses
All TCLE data (Ghandi et al., 2019; Nusinow et al., 2020) were obtained through cBioPortal (http://cbioportal.org). The sex of each cell line was obtained from TCLE data and confirmed using the Cellosaurus database (Bairoch, 2018). Only cell lines that had the same sex assigned in both databases were included (Table S5). Any cell line that was flagged by Cellosaurus as “problematic” because of contamination was excluded. This dataset included 487 males and 318 females. Cytotoxicity data for the chemotherapeutic drugs methotrexate and 5-fluorouracil, which target the folate cycle and downstream pathways, were obtained as well as the RPKM expression analyses for 35 genes involved in de novo serine biosynthesis and the folate cycle. The final dataset included 325 male and 267 female cell lines. Note that pyrimethamine data were excluded, as the cytotoxicity data were absent for 65% of the cell lines. The drug sensitivity measurements were IC50 and “area under the curve” (AUC) values which are both derived from a sigmoidal curve by fitting a mixed-effects model (Vis et al., 2016). AUC values may be used to compare the effect of a drug across multiple cell populations in a more robust fashion than IC50 (Fallahi-Sichani et al., 2013). Nonparametric Spearman correlations of each drug cytotoxicity parameter were made against the expression of each of the 35 genes. Following correction of p-values for FDR (q<0.05), only those genes that remained significant were plotted on the heatmaps. A Fisher exact test was performed to assess if there were significantly different numbers of correlations between male and female cell lines in either de novo serine biosynthesis or the folate cycle.
Quantification and statistical analysis
All data except for Figure 1B were graphed and analyzed using GraphPad Prism software version 9.0. Data for Figure 1B were graphed using R (R Core Team, 2021) (http::/cran.r-project.org; version 4.1.1) ggplot2 package. All violin plots show the median and quartiles. For statistical analysis of two groups, test for normality distribution was first performed, using the D'Agostino-Pearson omnibus K2 test. If samples had a normal distribution an unpaired, two-tailed, parametric t-test was performed. If samples did not have a normal distribution an unpaired, two-tailed, nonparametric Mann-Whitney test was performed. For correlation analyses, nonparametric Spearman correlation coefficient was calculated, and linear regression was fit to model the linear relationship between two markers. For gene mutation analyses, a linear model was fit to each metabolite and a two-way ANOVA was performed to assess the effects of sex and gene mutation. Unless noted otherwise, an FDR-adjusted p-value (termed q-value) or raw p-value<0.05 as noted was considered statistically significant. Statistical details of experiments can be found in the figure legends.
Acknowledgments
This work was supported by the Cancer Biology Pathway Molecular Oncology Training Grant NIH T32CA113275 (J.S.), the NIH grants K99/R00 CA218869 (JEI), R21 CA242221 (J.E.I.), R01 CA174737-06 (J.B.R.), the Alvin J. Siteman Cancer Center Investment Program/The Foundation for Barnes-Jewish Hospital (J.B.R., J.E.I.) and the Barnard Research Fund (J.B.R., J.E.I.), and Joshua’s Great Things (J.B.R.). The authors gratefully acknowledge the Transdisciplinary Research in Energetics and Cancer (TREC) Training Workshop R25CA203650 (PI: Melinda Irwin). The results shown here are in part based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga. This paper is dedicated to the memory of Karen Ippolito.
Author contributions
Conceptualization: J.S., S.D., and J.E.I. Investigation and formal analysis: J.S., J.L., and J.E.I. performed and analyzed experiments; J.S., J.L., and J.E.I. helped with data collection and analysis; Data curation: J.L. Supervision: S.D. and J.E.I. Visualization: J.S., J.L., and J.E.I. Writing – original and revised draft: J.S. and J.E.I. Writing – review & editing: All authors. Funding acquisition: J.B.R. and J.E.I.
Declaration of interests
S.D. serves on the advisory board of Genentech.
Inclusion and diversity
We worked to ensure diversity in experimental samples through the selection of the cell lines. We worked to ensure diversity in experimental samples through the selection of the genomic datasets.
Published: November 18, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.105339.
Supplemental information
Data and code availability
This paper analyzes existing, publicly available data. Original data for Figures 1 and 2 in the paper are available in Chen et al. (2019). Original data for Figure S1 in the paper are available in DeNicola et al. (2015).
All original code has been deposited at GitHub and is publicly available as of the date of publication. DOIs are listed in the key resources table.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Adjei A.A. Pharmacology and mechanism of action of pemetrexed. Clin. Lung Cancer. 2004;5:S51–S55. doi: 10.3816/CLC.2004.s.003. [DOI] [PubMed] [Google Scholar]
- Bairoch A. The cellosaurus, a cell-line knowledge resource. J. Biomol. Tech. 2018;29:25–38. doi: 10.7171/jbt.18-2902-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo-Álvarez P., Rizos D., Rath D., Lonergan P., Gutierrez-Adan A. Epigenetic differences between male and female bovine blastocysts produced in vitro. Physiol. Genomics. 2008;32:264–272. doi: 10.1152/physiolgenomics.00234.2007. [DOI] [PubMed] [Google Scholar]
- Bredbacka K., Bredbacka P. Glucose controls sex-related growth rate differences of bovine embryos produced in vitro. J. Reprod. Fertil. 1996;106:169–172. doi: 10.1530/jrf.0.1060169. [DOI] [PubMed] [Google Scholar]
- Chen P.-H., Cai L., Huffman K., Yang C., Kim J., Faubert B., Boroughs L., Ko B., Sudderth J., McMillan E.A., et al. Metabolic diversity in human non-small cell lung cancer cells. Mol. Cell. 2019;76:838–851.e5. doi: 10.1016/j.molcel.2019.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E., Margalit I., Shochat T., Goldberg E., Krause I. Sex differences in folate levels: a cross sectional study of a large cohort from Israel. Isr. Med. Assoc. J. 2021;23:17–22. [PubMed] [Google Scholar]
- Curtin N.J., Hughes A.N. Pemetrexed disodium, a novel antifolate with multiple targets. Lancet Oncol. 2001;2:298–306. doi: 10.1016/S1470-2045(00)00325-9. [DOI] [PubMed] [Google Scholar]
- de Perrot M., Licker M., Bouchardy C., Usel M., Robert J., Spiliopoulos A. Sex differences in presentation, management, and prognosis of patients with non–small cell lung carcinoma. J. Thorac. Cardiovasc. Surg. 2000;119:21–26. doi: 10.1016/S0022-5223(00)70213-3. [DOI] [PubMed] [Google Scholar]
- DeNicola G.M., Chen P.-H., Mullarky E., Sudderth J.A., Hu Z., Wu D., Tang H., Xie Y., Asara J.M., Huffman K.E., et al. NRF2 regulates serine biosynthesis in non–small cell lung cancer. Nat. Genet. 2015;47:1475–1481. doi: 10.1038/ng.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M., Cioffi G., Wang J., Waite K.A., Ostrom Q.T., Kruchko C., Lathia J.D., Rubin J.B., Berens M.E., Connor J., Barnholtz-Sloan J.S. Sex differences in cancer incidence and survival: a pan-cancer analysis. Cancer Epidemiol. Biomarkers Prev. 2020;29:1389–1397. doi: 10.1158/1055-9965.EPI-20-0036. [DOI] [PubMed] [Google Scholar]
- Ettinger D.S. Pemetrexed (Alimta®): a new antifolate for non-small-cell lung cancer. Clin. Lung Cancer. 2002;3:22–25. doi: 10.3816/CLC.2002.s.004. [DOI] [PubMed] [Google Scholar]
- Ettinger D.S., Wood D.E., Aisner D.L., Akerley W., Bauman J.R., Bharat A., Bruno D.S., Chang J.Y., Chirieac L.R., D’Amico T.A., et al. Non-small cell lung cancer, Version 2.2021 featured updates to the NCCN guidelines. J. Natl. Compr. Canc. Netw. 2021;19:254–266. doi: 10.6004/jnccn.2021.0013. [DOI] [PubMed] [Google Scholar]
- Fallahi-Sichani M., Honarnejad S., Heiser L.M., Gray J.W., Sorger P.K. Metrics other than potency reveal systematic variation in responses to cancer drugs. Nat. Chem. Biol. 2013;9:708–714. doi: 10.1038/nchembio.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T.W.-M., Lane A.N., Higashi R.M. Stable isotope resolved metabolomics studies in ex vivo TIssue slices. Bio. Protoc. 2016;6:e1730. doi: 10.21769/bioprotoc.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghandi M., Huang F.W., Jané-Valbuena J., Kryukov G.V., Lo C.C., McDonald E.R., Barretina J., Gelfand E.T., Bielski C.M., Li H., et al. Next-generation characterization of the cancer cell line Encyclopedia. Nature. 2019;569:503–508. doi: 10.1038/s41586-019-1186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Adán A., Granados J., Pintado B., De La Fuente J. Influence of glucose on the sex ratio of bovine IVM/IVF embryos cultured in vitro. Reprod. Fertil. Dev. 2001;13:361–365. doi: 10.1071/RD00039. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Adán A., Perez-Crespo M., Fernandez-Gonzalez R., Ramirez M.A., Moreira P., Pintado B., Lonergan P., Rizos D. Developmental consequences of sexual dimorphism during pre-implantation embryonic development. Reprod. Domest. Anim. 2006;41:54–62. doi: 10.1111/j.1439-0531.2006.00769.x. [DOI] [PubMed] [Google Scholar]
- Hedrington M.S., Davis S.N. Sexual dimorphism in glucose and lipid metabolism during fasting, hypoglycemia, and exercise. Front. Endocrinol. 2015;6:61. doi: 10.3389/fendo.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley C.T., Faubert B., Yuan Q., Lev-Cohain N., Jin E., Kim J., Jiang L., Ko B., Skelton R., Loudat L., et al. Metabolic heterogeneity in human lung tumors. Cell. 2016;164:681–694. doi: 10.1016/j.cell.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader N., Noone A.M., Krapcho M., Miller D., Brest A., Yu M., Ruhl J., Tatalovich Z., Mariotto A., Lewis D.R., et al., editors. SEER Cancer Statistics Review, 1975-2018. National Cancer Institute; 2021. https://seer.cancer.gov/csr/1975_2018/ [Google Scholar]
- Ippolito J.E., Yim A.K.Y., Luo J., Chinnaiyan P., Rubin J.B. Sexual dimorphism in glioma glycolysis underlies sex differences in survival. JCI insight. 2017;2:92142. doi: 10.1172/jci.insight.92142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J., Kim S.H., Kim Y.J., Lee J., Kim M., Keam B., Kim T.M., Kim D.-W., Heo D.S., Chung J.-H., et al. Efficacy of pemetrexed-based chemotherapy in comparison to non-pemetrexed-based chemotherapy in advanced, ALK+ non-small cell lung cancer. Yonsei Med. J. 2018;59:202–210. doi: 10.3349/ymj.2018.59.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumsiek J., Mittelstrass K., Do K.T., Stückler F., Ried J., Adamski J., Peters A., Illig T., Kronenberg F., Friedrich N., et al. Gender-specific pathway differences in the human serum metabolome. Metabolomics. 2015;11:1815–1833. doi: 10.1007/s11306-015-0829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale J.W. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer. 2013;13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes-Ramos C.M., Kuijjer M.L., Ogino S., Fuchs C.S., DeMeo D.L., Glass K., Quackenbush J. Gene regulatory network analysis identifies sex-linked differences in colon cancer drug metabolism. Cancer Res. 2018;78:5538–5547. doi: 10.1158/0008-5472.CAN-18-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes-Ramos C.M., Quackenbush J., DeMeo D.L. Genome-wide sex and gender differences in cancer. Front. Oncol. 2020;10:597788. doi: 10.3389/fonc.2020.597788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton D.R., Nam M., McLaughlin D.J., Shin J., Bayraktar E.C., Alvarez S.W., Sviderskiy V.O., Papagiannakopoulos T., Sabatini D.M., Birsoy K., Possemato R. Serine catabolism by SHMT2 is required for proper mitochondrial translation initiation and maintenance of formylmethionyl-tRNAs. Mol. Cell. 2018;69:610–621.e5. doi: 10.1016/j.molcel.2018.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R., Doherty D., Chamberlain R., Khuri F. Sex differences in survival in non-small cell lung cancer patients 1974-1998. Acta Oncol. 2004;43:57–64. doi: 10.1080/02841860310017973. [DOI] [PubMed] [Google Scholar]
- Mullarky E., Lucki N.C., Zavareh R.B., Anglin J.L., Gomes A.P., Nicolay B.N., Wong J.C.Y., Christen S., Takahashi H., Singh P.K., et al. Erratum: identification of a small molecule inhibitor of 3-phosphoglycerate dehydrogenase to target serine biosynthesis in cancers. Proc. Natl. Acad. Sci. USA. 2016;113:E1585. doi: 10.1073/pnas.1602228113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusinow D.P., Szpyt J., Ghandi M., Rose C.M., McDonald E.R., Kalocsay M., Jané-Valbuena J., Gelfand E., Schweppe D.K., Jedrychowski M., et al. Quantitative proteomics of the cancer cell line encyclopedia. Cell. 2020;180:387–402.e16. doi: 10.1016/j.cell.2019.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan N., Rakoczy J., Kondratowicz M., Menelaou K., Blake G.E.T., Watson E.D. Multigenerational analysis of sex-specific phenotypic differences at midgestation caused by abnormal folate metabolism. Environ. Epigenet. 2017;3:dvx014. doi: 10.1093/eep/dvx014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Park T.S., Choi C.-M., Lee D.H., Kim S.-W., Lee J.-S., Kim W.S., Song J.S., Lee J.C. Survival benefit of pemetrexed in lung adenocarcinoma patients with anaplastic lymphoma kinase gene rearrangements. Clin. Lung Cancer. 2015;16:e83–e89. doi: 10.1016/j.cllc.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Paz-Ares L., de Marinis F., Dediu M., Thomas M., Pujol J.L., Bidoli P., Molinier O., Sahoo T.P., Laack E., Reck M., et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, random. Lancet Oncol. 2012;13:247–255. doi: 10.1016/S1470-2045(12)70063-3. [DOI] [PubMed] [Google Scholar]
- Pergament E., Fiddler M., Cho N., Johnson D., Holmgren W.J. Sexual differentiation and preimplantation cell growth. Hum. Reprod. 1994;9:1730–1732. doi: 10.1093/oxfordjournals.humrep.a138783. [DOI] [PubMed] [Google Scholar]
- Poletta F.A., Rittler M., Saleme C., Campaña H., Gili J.A., Pawluk M.S., Gimenez L.G., Cosentino V.R., Castilla E.E., López-Camelo J.S. Neural tube defects: sex ratio changes after fortification with folic acid. PLoS One. 2018;13:e0193127. doi: 10.1371/journal.pone.0193127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . 2021. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Ray P.F., Conaghan J., Winston R.M., Handyside A.H. Increased number of cells and metabolic activity in male human preimplantation embryos following in vitro fertilization. J. Reprod. Fertil. 1995;104:165–171. doi: 10.1530/jrf.0.1040165. [DOI] [PubMed] [Google Scholar]
- Rubin J.B., Lagas J.S., Broestl L., Sponagel J., Rockwell N., Rhee G., Rosen S.F., Chen S., Klein R.S., Imoukhuede P., Luo J. Sex differences in cancer mechanisms. Biol. Sex Differ. 2020;11:17. doi: 10.1186/s13293-020-00291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadre-Marandi F., Dahdoul T., Reed M.C., Nijhout H.F. Sex differences in hepatic one-carbon metabolism. BMC Syst. Biol. 2018;12:89. doi: 10.1186/s12918-018-0621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q., Zhang Z., Li G., Pillow P.C., Hernandez L.M., Spitz M.R., Wei Q. Sex differences in risk of lung cancer associated with methylene- tetrahydrofolate reductase polymorphisms. Cancer Epidemiol. Biomarkers Prev. 2005;14:1477–1484. doi: 10.1158/1055-9965.EPI-04-0905. [DOI] [PubMed] [Google Scholar]
- Shih J.Y., Inoue A., Cheng R., Varea R., Kim S.W. Does pemetrexed work in targetable, nonsquamous non-small-cell lung cancer? A narrative review. Cancers. 2020;12:2658. doi: 10.3390/cancers12092658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA. Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- Singh R.K., Kumar D., Gourinath S. Phosphoserine aminotransferase has conserved active site from microbes to higher eukaryotes with minor deviations. Protein Pept. Lett. 2021;28:996–1008. doi: 10.2174/0929866528666210215140231. [DOI] [PubMed] [Google Scholar]
- Sponagel J., Jones J.K., Frankfater C., Zhang S., Tung O., Cho K., Tinkum K.L., Gass H., Nunez E., Spitz D.R., et al. Sex differences in brain tumor glutamine metabolism reveal sex-specific vulnerabilities to treatment. Med. 2022 doi: 10.1016/j.medj.2022.08.005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Tagirov M., Rutkowska J. Sexual dimorphism in the early embryogenesis in zebra finches. PLoS One. 2014;9:e114625. doi: 10.1371/journal.pone.0114625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Sasayama T., Nagashima H., Irino Y., Takahashi M., Izumi Y., Uno T., Satoh N., Kitta A., Kyotani K., et al. Glioma cells require one-carbon metabolism to survive glutamine starvation. Acta Neuropathol. Commun. 2021;9:16. doi: 10.1186/s40478-020-01114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi P.M., Markert E.K., Gounder M., Lin H., Dvorzhinski D., Dolfi S.C., Chan L.L.Y., Qiu J., DiPaola R.S., Hirshfield K.M., et al. Contribution of serine, folate and glycine metabolism to the ATP, NADPH and purine requirements of cancer cells. Cell Death Dis. 2013;4:e877. doi: 10.1038/cddis.2013.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffin G.J., Rieger D., Betteridge K.J., Yadav B.R., King W.A. Glucose and glutamine metabolism in pre-attachment cattle embryos in relation to sex and stage of development. J. Reprod. Fertil. 1991;93:125–132. doi: 10.1530/jrf.0.0930125. [DOI] [PubMed] [Google Scholar]
- Vaissière T., Hung R.J., Zaridze D., Moukeria A., Cuenin C., Fasolo V., Ferro G., Paliwal A., Hainaut P., Brennan P., et al. Quantitative analysis of DNA methylation profiles in lung cancer identifies aberrant DNA methylation of specific genes and its association with gender and cancer risk factors. Cancer Res. 2009;69:243–252. doi: 10.1158/0008-5472.CAN-08-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Blerkom J. Mitochondria as regulatory forces in oocytes, preimplantation embryos and stem cells. Reprod. Biomed. Online. 2008;16:553–569. doi: 10.1016/S1472-6483(10)60463-4. [DOI] [PubMed] [Google Scholar]
- Vis D.J., Bombardelli L., Lightfoot H., Iorio F., Garnett M.J., Wessels L.F. Multilevel models improve precision and speed of IC 50 estimates. Pharmacogenomics. 2016;17:691–700. doi: 10.2217/pgs.16.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainer Z., Wright G.M., Gough K., Daniels M.G., Russell P.A., Choong P., Conron M., Ball D., Solomon B. Sex-dependent staging in non–small-cell lung cancer; analysis of the effect of sex differences in the eighth edition of the tumor, node, metastases staging system. Clin. Lung Cancer. 2018;19:e933–e944. doi: 10.1016/j.cllc.2018.08.004. [DOI] [PubMed] [Google Scholar]
- Wang L., Cao Y., Ren M., Chen A., Cui J., Sun D., Gu W. Sex differences in hazard ratio during drug treatment of non–small-cell lung cancer in major clinical trials: a focused data review and meta-analysis. Clin. Ther. 2017;39:34–54. doi: 10.1016/j.clinthera.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Winkels R.M., Brouwer I.A., Verhoef P., Van Oort F.V.A., Durga J., Katan M.B. Gender and body size affect the response of erythrocyte folate to folic acid treatment. J. Nutr. 2008;138:1456–1461. doi: 10.1093/jn/138.8.1456. [DOI] [PubMed] [Google Scholar]
- Witham K.L., Butcher N.J., Sugamori K.S., Brenneman D., Grant D.M., Minchin R.F. 5-Methyl-Tetrahydrofolate and the S-adenosylmethionine cycle in C57BL/6J mouse tissues: gender differences and effects of arylamine N-Acetyltransferase-1 deletion. PLoS One. 2013;8:e77923. doi: 10.1371/journal.pone.0077923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.Y., Wang J., Lai J.C., Cheng Y.W., Yeh K.T., Wu T.C., Chen C.Y., Lee H. Association of O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation with p53 mutation occurrence in non-small cell lung cancer with different histology, gender, and smoking status. Ann. Surg Oncol. 2008;15:3272–3277. doi: 10.1245/s10434-008-0078-9. [DOI] [PubMed] [Google Scholar]
- Yang C., Zhang J., Liao M., Yang Y., Wang Y., Yuan Y., Ouyang L. Folate-mediated one-carbon metabolism: a targeting strategy in cancer therapy. Drug Discov. Today. 2021;26:817–825. doi: 10.1016/j.drudis.2020.12.006. [DOI] [PubMed] [Google Scholar]
- Ye J., Fan J., Venneti S., Wan Y.W., Pawel B.R., Zhang J., Finley L.W.S., Lu C., Lindsten T., Cross J.R., et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. 2014;4:1406–1417. doi: 10.1158/2159-8290.CD-14-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This paper analyzes existing, publicly available data. Original data for Figures 1 and 2 in the paper are available in Chen et al. (2019). Original data for Figure S1 in the paper are available in DeNicola et al. (2015).
All original code has been deposited at GitHub and is publicly available as of the date of publication. DOIs are listed in the key resources table.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.