Abstract
Background:
Cerebral palsy (CP) is a prevalent group of neuromotor disorders caused by early injury to brain regions or pathways that control movement. Patients with CP exhibit a range of functional motor disabilities and pathologic gait patterns. Crouch gait, characterized by increased knee flexion throughout stance, is a common gait pattern in CP that increases energy costs of walking and contributes to ambulatory decline. Our aim was to perform the first systematic literature review on the effectiveness of interventions utilized to ameliorate crouch gait in CP.
Methods:
Comprehensive searches of five medical databases yielded 38 papers with 30 focused on orthopaedic management.
Results:
Evidence supports the use of initial hamstring lengthenings and rectus femoris transfers, where indicated, for improving objective gait measures with limited data on improving gait speed or gross motor function. In contrast, evidence argues against hamstring transfers and revision hamstring lengthening, with recent interest in more technically demanding corrective procedures. Only eight studies evaluated alternatives to surgery, specifically strength training, botulinum toxin or orthoses, with inconsistent and/or short-lived results.
Conclusions:
Although crouch in CP is recognized clinically as a complex multi-joint, multi-planar gait disorder, this review largely failed to identify interventions beyond those which directly address sagittal plane knee motion, indicating a major knowledge gap. Quality of existing data was notably weak, with few studies properly controlled or adequately sized. Outcomes from specific procedures are confounded by multilevel surgeries. Successful longer term strategies to prevent worsening of crouch and subsequent functional decline are needed.
Level of evidence:
Systematic review.
Keywords: Cerebral palsy, Crouch gait, Hamstring lengthening, Pediatric orthopedics, Knee flexion
1. Introduction
Cerebral palsy (CP) is a group of motor disorders caused by non-progressive insults to the brain during early development. The resulting neuronal damage leads to various types and severity of motor involvement, and a range of primary symptoms such as spasticity, paresis, and poor motor control along with other secondary symptoms [1]. Pathological gait patterns such as crouch gait (also referred to as crouched or flexed knee gait) are common [2]. While definitions vary among experts and studies, crouch is generally characterized by an overly flexed knee during the stance phase of gait. A crouched posture reduces the capacity of muscles to extend the knee and hip [3], leading to generation of higher than normal muscle forces during gait [4], and is significantly less efficient [5]. Higher muscle forces lead to higher joint reaction forces [6], which may contribute over time to higher rates of joint pain and degenerative arthritis [7,8]. Left untreated or inadequately addressed, children with CP will experience progressive gait deterioration, leading to even greater functional disability [9–12]. Multiple potential causes of crouch have been proposed including hamstrings spasticity and contracture [13–16], gastrosoleus insufficiency [17], psoas spasticity and contracture [15,18,19], and quadriceps weakness [20], among other causes. Various treatments have been utilized to ameliorate crouch gait. While earlier reviews have investigated gait outcomes of interventions in CP [21,22], no systematic review specifically investigating treatments for crouch gait has been published. Our specific research question for this review was as follows: What is the universe of treatments reported in the scientific literature for the treatment of crouch gait in CP and what is the evidence for their effectiveness or efficacy? The purpose here was to comprehensively identify and evaluate outcomes of interventions in those with CP and crouch gait to inform clinical practice and guide future research.
2. Methods
2.1. Inclusion and exclusion criteria
Our goal was to identify all full length, peer reviewed, English language studies on interventions in CP aimed at correcting excessive knee flexion that provided objective quantification of changes in temporal-spatial aspects of gait and sagittal plane knee joint kinematics using 3-dimensional gait analysis as a result of a clinical intervention. Crouch gait may be referred to by other terms in the scientific literature such as “flexed knee” or “crouched gait,” and as such these were included in the relevant database searches. Additionally, studies that may not have called this specific gait pattern any of the above terms but described outcomes of treatments aimed at correcting excessive knee flexion during stance were included. Studies that investigated an intervention and its effect on multiple gait abnormalities must have stratified results by gait pattern to allow for analysis of that intervention’s effects on crouch. Included studies must also have reported 3-dimensional gait analysis (3DGA) data, specifically on sagittal plane knee kinematics, before and after intervention. Any nonhuman or modeling studies were excluded. Any case studies or studies without statistical analyses were likewise excluded.
2.2. Search methods
Databases searched were Pubmed, EMBASE (www.embase.com ), Scopus (www.scopus.com), Web of Science (www.webofknowledge.com), and CINAHL up until August 13, 2015. The search term strategy, allowing for syntactical differences between databases, was as follows: “Cerebral Palsy” AND “crouch” OR “crouched” OR “flexed knee” OR “flex knee” OR “stiff knee” OR “knee flexion” OR “hamstring” OR “hamstrings” OR “knee” OR “gait disorders” OR “gait.” All terms were designated to be searched within both titles and abstracts.
2.3. Reference review
After identifying all citations from each database, duplicates were removed. Titles of remaining citations were independently reviewed by two authors to exclude any that were clearly not investigations of interventions for improving crouch in CP. Remaining abstracts were then reviewed by three authors and inclusion/exclusion criteria applied with disagreements resolved by discussion. Reference lists of included citations were reviewed to further ensure all relevant studies were identified. Manuscripts for each citation were obtained and reviewed independently by three authors.
2.4. Data extraction and quality appraisal
Study type, design, sample size, intervention type and follow-up times were extracted from each manuscript. Extracted patient demographic information included gender, age, GMFCS level, and CP subtype, where available. Collected outcomes focused primarily on knee kinematics (all measured in angles) and included pre and post treatment knee flexion at initial contact (KFIC), maximum knee extension in stance (MKESt), maximum knee flexion in swing (MKFSw), total knee excursion (TKE), and mean pelvic tilt (MPT). Additional data extracted included popliteal angle, cadence, walking speed, and stride length as well as any functional outcomes.
The strength of each study design was assessed using Sackett’s Levels I–V of evidence [23]. Since it was immediately apparent that few studies would be Level I–II, the Methodological Index for Non-Randomized Studies (MINORS) was applied to assess scientific rigor of each study [24]. Three authors performed each of these determinations with disagreements resolved by discussion. This review was constructed in accordance with the PRISMA statement and guidelines, a set of evidence-based criteria for reporting in systematic reviews [25].
3. Results and discussion
3.1. Search results
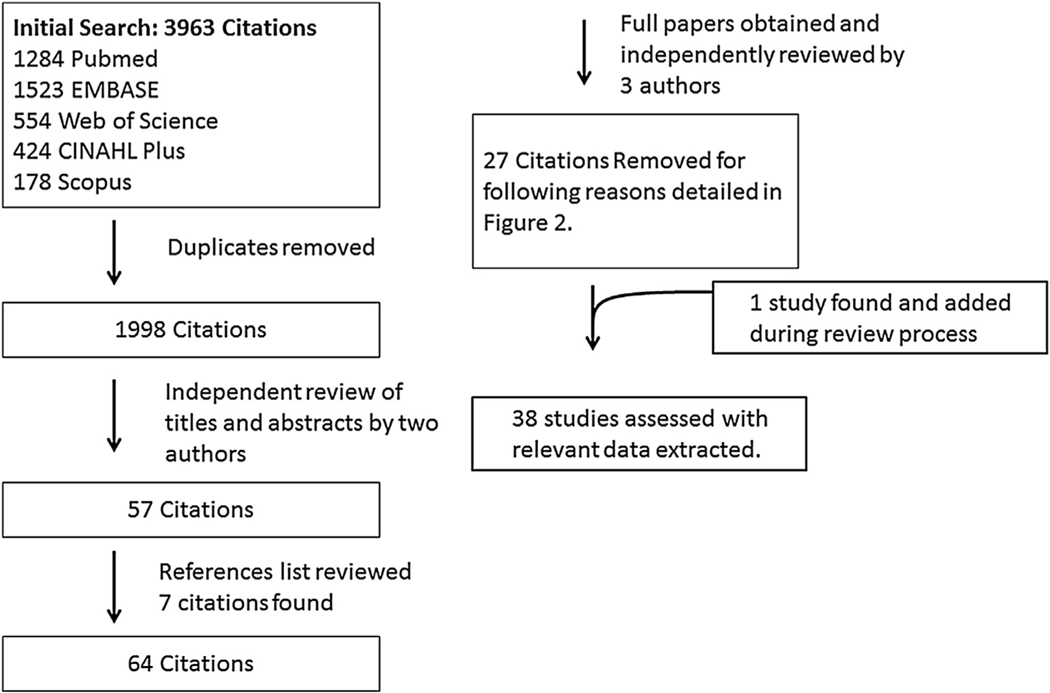
The search strategy yielded 1998 unique citations from five databases. After title and abstract review, 57 remained for full text review. Review of references from these identified 7 additional papers, leaving 64 papers for full text review [13,14,19–21,26–85]. One additional paper was identified and added during the manuscript review process which recommended that we search for additional terms to ensure that our review had not failed to capture the outcomes of interventions that addressed lower limb alignment on crouch. These additional terms included: lever” OR “anteversion” OR “torsion” OR “malalignment”. Of note, this additional search did not yield any new unique citations. The one new paper that emerged had been eliminated previously because the title and abstract did not mention crouch or flexed knee gait, but was selected this time for full text review because it appeared under multiple search terms and was an intervention study that included kinematics. Of 65 full papers evaluated, 27 were excluded for not meeting eligibility criteria and 38 papers (including this new paper) comprised the final selection (Fig. 1, Table 1). Relevant data from each were extracted as detailed above (Tables 2 and 3).
Fig. 1.
Systematic search and review strategy and results.
Table 1.
Reasons for exclusion for 27 of 65 publications after full text review. 3DGA – Three dimensional gait analysis. ST-Spatio-temporal.
| Reasons for Exclusion | |
|---|---|
| Baumann [28] | No 3DGA or ST measures |
| Bozinovski [29] | No 3DGA or ST measures |
| Das [35] | No 3DGA or ST measures |
| Drummond [41] | No 3DGA or ST measures |
| Eek [42] | Not specific to crouch |
| Gage[44] | Treatment of stiff knee gait, not crouch gait |
| Ganjwala [45] | No 3DGA |
| Gannoti [78] | Interventions not specific to crouch gait |
| Gough [75] | No aggregate group data |
| Haumont [48] | Not all patients had preop data |
| Hesse [49] | Not specific to crouch |
| Hsu [50] | No 3DGA or ST measures |
| Joseph [51] | No 3DGA or ST measures |
| Kadhim [52] | Some patients had normal knee flexion, not stratified |
| Lucarelli [79] | Inadequate statistical analysis/reporting |
| McGinley [21] | Interventions not specific to crouch |
| Morton [58] | Intervention not targeted at crouch, no 3DGA |
| Park [61] | Treatment of jump-knee gait, not crouch gait |
| Patritti [84] | Case reports with no statistical analysis |
| Rethlefsen [62] | Not limited to CP population |
| Roosth [66] | No 3DGA or ST measures |
| Scholtes [80] | No 3DGA or ST measures |
| Sutherland [71] | No 3DGA or ST measures |
| Svhelik 2011 [81] | Inadequate 3DGA reporting |
| Thometz 1989 [72] | Inadequate statistical analysis/reporting |
| Unger 2006 [82] | Not specific to crouch |
| Wesdock 2003 [83] | Not a gait study |
Table 2.
Extracted data from the 24 non-comparative studies reviewed. All data in parentheses is either SD of mean value or range of values. RC – Retrospective cohort, PC – Prospective cohort.
| Characteristic and Outcomes of Non-Comparative Studies | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||
| Study | Intervention | Study | MINORS Score | # of patients | # of males | Average Age (yrs) | Type of CP | GMFCS Level | Mean #of procedures | Mean follow up (mo) | KFIC (°) | MKES t(°) | MKFSw (°) | TKE (°) | Cadence (steps/min) | Walking Speed (m/s) | Stride Length (m) | Mean Pelvic Tilt (°) | Popliteal Angle (°) | |
| Adolfsen [26] | HSL, RFT, and GSL | RC | 8 | 15 | 8.5 (2) | 3 | 23 | Pre Post |
37 (7) 24 (10) |
24 (5) 16 (11) |
NS NS |
NS NS |
57 (10) 42 (10) |
|||||||
| Carney [30] | HSL and RFT, SEMLS | RC | 8 | 23 | 14 | 11.75 (2.5) | I-III | 3.2 | > 12 | Pre Post |
26 (13) 12 (14) |
57 (9) 50 (12) |
31 (11) 67 (14) |
|||||||
| Carney [13] | HSL alone | RC | 8 | 16 | 8 | 12 (8–16) | I-III | 1 | >12 | Pre Post |
33 (10) 10 (13) |
64 (9) 48 (11) |
31 (12) 39 (12) |
|||||||
| Corry [31] | Botox injection of Hamstrings | PC | 13 | 10 | 3 | 7.2 (4–11) | 1 | 3 | Pre Post |
NS NS |
NS NS |
NS NS |
NS NS |
NS NS |
NS NS |
20 (6.5) 25 (7.9) |
64 (12.8) 47 (7.3) |
|||
| Damiano [34] | Extensor Strengthening | PC | 15 | 8 | 3 | 7.78 (2.6) | SD | I-III | 2 | Pre Post |
NS NS |
NS NS |
NS NS |
NS NS |
||||||
| Damiano [33] | HSL in SEMLS | PC | 19 | 13 | 9.46 (3.2) | 9 | Pre Post |
30.9 39 |
NS NS |
NS NS |
0.71 0.84 |
|||||||||
| Damiano [20] | Quadriceps Strengthening | PC | 12 | 14 | 10 | 9.1 (2.5) | SD | 1.2 | Pre Post |
32 (10) 27 (15) |
NS NS |
NS NS |
NS NS |
NS NS |
0.74 (0.2) 0.82 (0.15) |
|||||
| de Morais [38] | DFEO in SEMLS | RC | 7 | 12 | 5 | 13.1 (9.1–19.9) | SD | II-IV | 5.2 | 27 | Pre Post |
43.56 () 22.29 () |
12 21.9 |
|||||||
| Gordon [46] | Percutaneous medial HSL | RC | 8 | 48 | 9.45 | SD, SH, SQ | I-III | 33.8 | Pre Post |
26.6 (9.9) 20.1 (12.3) |
NS NS |
NS NS |
0.9 (0.34) 1.0 (0.27) |
0.86 (0.34) 0.99 (0.2) |
NS NS |
49.6 (11.9) 41.5 (17) |
||||
| Hadley [47] | RFT and HSL in SEMLS | RC | 7 | 24 | 11 (5–18) | SD, SH | >3 | Pre Post |
23.2 3.2 |
NS NS |
30.7 46.1 |
|||||||||
| Koca [54] | RFT and HSL in SEMLS | RC | 8 | 19 | 9 | 12.1 (11–27) | SD | 5.2 | 6.3 | Pre Post |
43.8 (4.1) 20.1 (2.1) |
40.5 (NR) 18 (7) |
NS NS |
14 (2.1) 32 (1.4) |
112 87 |
0.42 (0.1) 0.56 (0.2) |
0.41 (0.06) 0.57 (0.08) |
61 (5.2) 22 (3.8) |
||
| Lovejoy [55] | HSL during SEMLS (Med and Lat) | RC | 9 | 38 | 25 | 12.33 (4.6–19.6) | SD | 21.6 | Pre Post |
43.9 (12.8) 26.8 (10.8) |
51.5 (12) 39 (10.6) |
41.2 (10.5) 26.6 (9.9) |
57.8 (11.1) 37.2 (10.3) |
|||||||
| Ma [56] | HST/HSL | RC | 9 | 19 | 10 | 9 (5–14) | SD, SQ | III-IV | 6.7 | 25.2 | Pre Post |
39 (8) 22 (7) |
39 (8) 22 (7) |
70 (9.2) 42.6 (9.5) |
||||||
| Metaxiotis [57] | CBM (RFT, HST, GST) | PC | 14 | 20 | 13 | 11.5 (5.6–17.0) | SD | 12.05 | 37.2 | Pre Post |
41.5 (15.4) 19.1 (12.8) |
28.7 (23.4) 6.2 (14.5) |
58.3 (12.9) 51.4 (8.1) |
31.9 (15.2) 45.2 (13.9) |
118 (25) 103 (24) |
NS NS |
0.6 (0.14) 0.67 (0.13) |
53.6 (13.6) 25 (12.6) |
||
| Ounpuu [59] | HSL, GSL, RFT in SEMLS | RC | 12 | 22 | 11 | 8 (2.7) | I-III | 4.7 | 132 | Pre Post |
35 (9) 24 (9) |
NS NS |
NS NS |
139 (23) 115 (15) |
NS NS |
0.79 (0.22) 0.98 (0.23) |
NS NS |
|||
| Papadonikolakis [60] | Botox injection of hamstrings | PC | 20 | 14 | 11 (217) | SH, SQ, SD | 49 | Pre Post |
NS NS |
14 (9) 10 (9) |
NS NS |
NS NS |
NS NS |
NS NS |
L - 0.70 (.2) L - 0.76 (.2) |
|||||
| Rodda [64] | SEMLS for severe crouch | RC | 11 | 10 | 7 | 12 (7.9–16.2) | SD | II-III | 7 | 60 | Pre Post |
52 (7) 26 (10) |
44 (9) 17 (11) |
14 (12) 24 (9) |
NS NS |
|||||
| Rogozinski [65] | FRAFO | PC | 9 | 27 | 18 | 12.4 (2.4) |
SD, ST, SQ | II-III | Bare AFO |
29 (14.3) 18 (14.4) |
NS NS |
.72 (.25) .83 (.19) |
.84 (.19) 1.00 (.17) |
|||||||
| Sossai [67] | PTS in SEMLS | RC | 15 | 24 | 16 | 16.1 (5.8) | SD | II-III | 4.6 | 21.8 | Pre Post |
51.1 (18.2) 32.4 (10.8) |
32.4 (22.8) 20.5 (13.9) |
71.9 (16.5) 57.8 (10) |
55 (19) 37.6 (12.2) |
14.1 (6.8) 21.6 (6.6) |
||||
| Steele [69] | Strength Training | MA | 12 | 30 | 13.7 (3.8) | I-III | 2 | Pre Post |
NS NS |
NS NS |
||||||||||
| Sung [70] | HSL in SEMLS | RC | 10 | 29 | 18 | 8.3 (5.1–16.3) | SD | I-III | 9.1 | 141.6 | Pre Post |
31.1 (12.7) 23.6 (8.1) |
NS NS |
31.4 (12.7) 23 (7.8) |
NS NS |
NS NS |
0.71 (.25) 0.87 (.23) |
NS NS |
||
| Thompson [19] | Botox injection of hamstrings | PC | 11 | 10 | 7.2 (4–12) | SQ, SD | 0.5 | Pre Post |
NS NS |
26 12.42 |
NS NS |
|||||||||
| van der Linden [73] | HSL during SEMLS (Med and Lat) | RC | 8 | 18 | 11 (6–20) | SH, SD | 13 | Pre Post |
42 (12) 30 (12) |
32 (13) 20 (14) |
63 (12) 54 (10) |
30 (9) 34 (11) |
60 (9) 56 (10) |
NS NS |
NS NS |
NS NS |
||||
| Westwell [74] | HSL in SEMLS | RC | 9 | 21 | 9 | 18.9 (14.2–29.3) | SH, SD | I-IV | 13.2 | Pre Post |
32 (15) 22 (13) |
36 (12) 42 (16) |
58 (12) 31 (18) |
|||||||
Table 3.
Extracted data from the 13 comparative studies reviewed. All data in parentheses is either SD of mean value or range of values. With the exception of Abd El-Kafy 2014, an RCT, and Dreher 2013, a prospective cohort study, all studies in this figure were retrospective cohort studies.
| Characteristic and Outcomes of Comparative Studies | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||
| Study | Group Description | MINORS Score | Number of patients | Average Age (yrs) | Type of CP | GMFCS Level | Mean follow up (mo) | KFIC (°) | MKESt (°) | MKFSw (°) | TKE (°) | Cadence (steps/min) | Walking Speed (m/s) | Stride Length (m) | Mean Pelvic Tilt (°) | Popliteal Angle (°) | |
| Abd El-Kafy [85] | PT | 20 | 19 | 7.5 (1.2) | SD | I-II | 3 | Pre | 35.4 (4.0) | 64.5 (3.3) | 0.74 (.06) | .69 (.03) | |||||
| Post | 31.5 (4.2) | 68.6 (4.4) | 0.80 (.06) | .77 (.04) | |||||||||||||
| PT + TheraTogs | 19 | 7.3 (1.6) | Pre | 36.9 (3.2) | 63.4 (4.4) | 0.76 (.06) | .68 (.05) | ||||||||||
| Post | 30.9 (2.6) | 69.3 (3.5) | 0.81(.05) | .83 (.04) | |||||||||||||
| PT + TheraTogs + GRAFO | 19 | 7.1 (1.5) | Pre | 36.3 (3.2) | 62.5 (4.4) | 0.74 (.05) | .71 (.03) | ||||||||||
| Post | 24.4 (3.9) | 73.4 (3.9) | 0.86 (.06) | .89 (.04) | |||||||||||||
| Aiona [27] | HSL w/Delayed RFT | 9 | 26 | 11.6 (3.2) | I-III | 12 | Pre | 26.5 (13.7) | 55.7 (11.2) | 29.2 (10.5) | |||||||
| Post HSL |
5.5 (12.7) |
41.5 (10.5) |
35.9 (13.8) |
||||||||||||||
| Post RFT |
8.8 (11.6) |
51 (7.5) | 42.2 (11.5) |
||||||||||||||
| HSL with RFT | 57 | I-III | 12 | Pre | 27.1 (14.7) |
NS | 28.6 (13.3) |
||||||||||
| Post | 9.8 (11.5) |
NS | 44 (12.6) |
||||||||||||||
| HSL only | 28 | I-III | 12 | Pre | 22.8 (7.1) |
56.8 (11.7) |
34 (13.2) |
||||||||||
| Post | 6.7 (8.2) |
47.7 (8.4) |
41 (9.6) |
||||||||||||||
| Chang [32] | Primary HSL | 14 | 49 | 8.7 (4.3) | SD, SH, SQ, SM | 14.6 | Pre | 39.1 (11.1) |
42.1 (15.8) |
21.3 (5.6) | 52 (12) | ||||||
| Post | 28.0 (11.4) |
48.6 (14.5) |
23.3 (6.9) | 41 (16) | |||||||||||||
| Repeat HSL | 12 | 12.7 (2.9) | 16.4 | Pre | 35.4 (11.9) |
NS | 20 (9.2) | NS | |||||||||
| Post | 25.0 (8.6) | NS | 25.9 (8) | NS | |||||||||||||
| DeLuca [36] | HSL (Med) | 15 | 37 | 8.9 (4.3) | SD | 12 | Pre | 30 (11) | 11 (14) | NS | 42 (17) | ||||||
| Post | 17 (12) | 3 (12) | NS | 30 (17) | |||||||||||||
| HSL (Med and Lat) | 12 | 14.4 (5.8) | SD | 12 | Pre | 37 (11) | 27 (18) | NS | 59 (12) | ||||||||
| Post | 19 (8) | 9 (9) | NS | 38 (11) | |||||||||||||
| HSL (Med) and PL | 9 | 10.6 (4.3) | SD | 12 | Pre | 33 (9) | 11 (9) | NS | NS | ||||||||
| Post | 21 (9) | 1 (11) | NS | NS | |||||||||||||
| HSL (Med and Lat) and PL | 15 | 10.5 (3.6) | SD | 12 | Pre | 32 (10) | 16 (17) | NS | 55 (11) | ||||||||
| Post | 21 (6) | 7 (13) | NS | 32 (18) | |||||||||||||
| De Mattos [37] | HSL | 13 | 18 | 11.6 (3.4) | SD, ST, SQ, SH | I-III | 51.6 | Pre | 22.4 (13.3) | NS | 58.9 (14.4) | ||||||
| Post | 10.8 (12.1) | NS | 51.1 (12.3) | ||||||||||||||
| HST | 32 | 10.5 (2.9) | I-III | 54 | Pre | 14.1 (7) | 57.7 (10.7) | ||||||||||
| SD, ST, SQ, SH | 24.9 (11.9) | ||||||||||||||||
| Post | 12.5 (14.3) | 19.3 (7.9) | 49.4 (10.1) | ||||||||||||||
| Dreher [39] | HSL (Med) | 10 | 30 | 10.2 (3.5) | SD | I-III | 97.2 | Pre | 36(17) | NS | NS | NS | .82(.21) | NS | NS | ||
| Post | 23(10) | NS | NS | NS | .99(.20) | NS | NS | ||||||||||
| HSL (Med and Lat) | 9 | Pre | 45(14) | 35(23) | NS | NS | NS | NS | NS | ||||||||
| Post | 23(10) | 12(16) | NS | NS | NS | NS | NS | ||||||||||
| Dreher [40] | CBM (HST, RFT, GST) | 16 | 21 | 11.3 (3.1) | I-III | 110.4 | Pre | 41 (14) | 28 (20) | 58 (12) | 30 (13) | NS | NS | NS | NS | NS | |
| Post | 24 (8) | 13(11) | 52 (9) | 39 (12) | NS | NS | NS | NS | NS | ||||||||
| HSL | 21 | 11.1 (5.4) | I-III | 109.2 | Pre | 41 (16) | 28 (21) | 61 (13) | 33 (13) | NS | NS | NS | 14 (8) | NS | |||
| Post | 27 (8) | 16 (10) | 56 (7) | 40 (11) | NS | NS | NS | 17 (7) | NS | ||||||||
| Feng [43] | HSL | 14 | 20 | 11.9 (3) | SD, SH | I-III | 13.1 | Pre | 31.7 (11.5) | NS | 61.4 (13.5) | ||||||
| Post | 23.2 (11.6) | NS | 46.2 (9.9) | ||||||||||||||
| HST | 18 | 10.1 (3.4) | SD, ST, SQ | I-III | 12.8 | Pre | 32.6 (11) | 15.7 (6.1) | 57.8 (11.7) | ||||||||
| Post | 18.9 (9.8) | 22.2 (6.9) | 45.3 (14.7) | ||||||||||||||
| Kay [53] | HSL (Med and Lat) | 13 | 21 | 11.2 (4.5) | SH,SD | 17 | Pre | 27 (15) | 53(11) | ||||||||
| Post | 12 (16) | 33 (13) | |||||||||||||||
| HSL (Med) | 16 | 9.2 (4.8) | SH, SD | 18 | Pre | 20 (12) | 51 (12) | ||||||||||
| Post | 16(17) | 41 (11) | |||||||||||||||
| Laracca [14] | HSL (Med and Lat) | 13 | 15 | 14 (11–17) | SD | 27 | Pre | 44.5 (14.5) | NS | NS | NS | NS | 49.7 (13.9) | ||||
| Post | 31.7 (14.9) | NS | NS | NS | NS | 42 (14.6) | |||||||||||
| No HSL during SEMLS | 15 | 12.6 (10–14) | SD | 21 | Pre | NS | NS | 0.92 (0.32) | .46 (.08) | NS | NS | ||||||
| Post | NS | NS | 0.68 (0.35) | .40 (.12) | NS | NS | |||||||||||
| Rethlefsen [63] | Primary HSL | 15 | 21 | 9.2 (4.3) | II-IV | 19.2 | Pre | 45 (12) | 30 (16) | 55 (12) | |||||||
| Post | 28 (9) | 12 (15) | 37 (12) | ||||||||||||||
| Repeat HSL | 18 | 10.8 (3.7) | II-IV | 24 | Pre | NS | NS | NS | |||||||||
| Post | NS | NS | NS | ||||||||||||||
| Stout [68] | DFEO | 12 | 16 | 14.8 (6) | I-IV | 13.2 | Pre | 43 (18) | 40 (17) | NS | NS | NS | 19 (9) | ||||
| Post | 34 (16) | 31 (16) | NS | NS | NS | 5(11) | |||||||||||
| DFEO and PTA | 33 | 14.1 (2.6) | I-IV | 14.4 | Pre | 42 (12) | 38 (15) | 25 (15) |
NS | 15(9) | 15 (8) | ||||||
| Post | 26 (11) | 9 (10) | 47 (15) | NS | 21(7) | −1 (5) | |||||||||||
| PTA Only | 24 | 12.9 (2.5) | I-IV | 14.4 | Pre | 35 (9) | 27 (11) | 32 (16) | NS | 16(6) | 3 (7) | ||||||
| Post | 24 (9) | 10 (12) | 46 (16) | NS | 22(8) | −1 (5) | |||||||||||
| Yngve [76] | Ind. Amb. (DRFT and HSL) | 9 | 39 | 11 | SD,SQ | 13.6 | Pre | 20 (15) | NS | 31 (13) | 131 (20) | NS | 0.9 (0.18) | ||||
| Post | 7 (13) | NS | 43 (14) | 126 (20) | NS | 0.94 (0.19) | |||||||||||
| Dep. Amb. (DRFT and HSL) | 39 | 9.8 | SD,SQ | 13.6 | Pre | 26 (23) | 54 (16) | 28 (14) | 107 (26) | NS | 0.63 (0.18) | ||||||
| Post | 7 (16) | 47 (12) | 39 (16) | 99 (27) | NS | 0.72 (0.18) | |||||||||||
| 21 | 9.8 | SD,SQ | 13.6 | Pre | 31 (18) | 56 (12) | NS | 0.27 (0.11) | 0.4 (0.15) | ||||||||
| Household Amb. (DRFT and HSL) | 25 (13) | ||||||||||||||||
| Post | 12 (13) | 45 (9) | 33 (15) | NS | 0.4 (0.18) | 0.55 (0.17) | |||||||||||
| Zwick [77] | DRFT + HSL + Psoas tenotomy | 18 | 8 | 11.3 (2.7) | SD | 28.8 | Pre | 31.8 (4.4) | 11.4 (5.6) | NS | NS | 0.91 (0.16) | 17.8 (7) | 52.5 (1.6) | |||
| Post | 25.6 (9.8) | 1.3 (6) | NS | NS | 1.05 (0.30) | 29.5 (6.8) | 20.5 (9.3) | ||||||||||
| DRFT + HSL | 9 | 11.2 (2.7) | SD | 28.8 | Pre Post |
NS NS |
NS NS |
NS NS |
NS NS |
0.96 (0.19) 1.15 (0.13) |
NS NS |
52.5 (8.7) 30 (8.2) |
|||||
3.2. Study type, design, and size
Of the 38 papers, 28 were retrospective cohort studies, 8 were prospective cohort studies 1 was a meta-analysis of three prospective cohort studies, and 1 was a randomized controlled trial (Table 2 and 3). Thirteen studies were comparative or included a control (Table 3). Results from approximately 1250 unique patients with CP across 38 studies were reviewed. Sample sizes ranged from 8 to 111 patients.
3.3. Participant demographics
The mean age across studies was 10.5 years. Of the 28 studies reporting gender, 56.7% of the studied patients were male. CP types varied across studies and included spastic diplegia, hemiplegia, and quadriplegia. Reported GMFCS levels ranged from I–IV.
3.4. Interventions
Orthopaedic surgical interventions were the focus of 30/38 papers with 27 of those addressing effects of hamstring lengthening (HSL) or transfer (HST) with or without associated procedures such as rectus femoris transfers (RFT) or psoas lengthenings. The remaining 3 surgical studies investigated procedures such as distal femoral extension osteotomy (DFEO) and patellar tendon advancement/shortening (PTA/PTS) [38,67,68]. The remaining 8 papers studied the effects of strengthening [20,34,69] botulinum toxin injections [19,31,60], and orthoses [65,85] on crouch gait.
3.5. Follow up and outcome measures
Follow up periods for surgical studies ranged from 6 months [54] to 11.8 years [70] with 7 reporting outcomes at least three years post-surgery. Outcomes included a wide array of clinical, kinematic, spatiotemporal and functional measures. All publications reported at least one knee kinematic outcome of interest obtained via three dimensional gait analysis (3DGA) since that was an inclusion criterion, and 20 included spatiotemporal measures and only 4 reported functional measures (Tables 2 and 3).
3.6. Level of evidence and study quality
Sackett’s levels of evidence ranged from level II (n = 1) to level IV (n = 26), with 11 studies of level III [23]. The average MINORs score was 11.9 out of 24 possible points. Most received low scores for being retrospective studies without adequate control or comparative groups (Tables 2 and 3).
3.7. Outcomes and discussion
Crouch gait is a common problem in a condition prevalent in the developed and developing world [2,86]. This review indicates that orthopaedic surgery remains the dominant intervention to treat crouch in CP with hamstring lengthening still the most common approach, as has been the case for decades. Scientific evidence supports some general surgical recommendations, but does not definitively state which procedures among those studied are most effective for improving biomechanical outcomes in specific patients and provides little data on expected functional changes or long term effects. Despite the recognition that crouch in CP often has a complex multijoint, multiplanar and multisymptom etiology, this review failed to yield knee kinematic outcomes data from surgical procedures directed at other joints and planes commonly utilized in clinical practice to improve lower limb alignment. The literature also largely failed to support successful alternatives to surgery.
An apparent issue in CP literature is a lack of consensus on crouch definitions which varied across studies in the considered joints, planes of motion, and phases of gait [32,47,56,67]. In this review, we allowed for a more inclusive view of crouch gait by including all interventional studies that stated they were addressing crouch or flexed knee gait and/or had 3D kinematic data showing that the knee was excessively flexed at initial contact and/or later in stance. Ankle and hip position or rotational abnormalities were neither an inclusion or exclusion criteria for article selection which depended solely on whether the paper presented pre-post intervention kinematic data on a group or subgroup of subjects all of whom had excessive knee flexion in stance. If we had only included papers that explicitly stated they were addressing crouch, 15 papers would have been reviewed. Of those, 10 provided definitions of crouch, with only 3 using the Rodda et al. definition of crouch gait as both increased knee flexion and ankle dorsiflexion throughout stance [54,64,65,87]. The rest had variable definitions, some of which defined crouch as increased KFIC (n = 3) [20,32,34], and others as increased knee flexion throughout stance (n = 4) [38,47,54,69]. We also included studies that addressed “flexed knee gait” without mention of crouch (n = 7) [13,27,38– 40,43,67] because these satisfied the same knee kinematic criterion, although with similar ambiguity in the precise definition of when in stance the flexion occurred. Most of the remaining papers (n = 15) targeted hamstring contracture or spasticity but did not state they were addressing crouch. These were included here since hamstrings procedures are typically reserved for those with increased knee flexion and all had kinematic data supporting this. Any study explicitly stating that the entire sample had jump-knee gait was excluded, even though KFIC was increased. However, given our more inclusive selection criteria, we acknowledge that some subjects across studies may have had jump-knee gait, especially in those studies that used KFIC to define crouch [20,32,34] or those where the sample was described as containing both crouch and jump-knee gait. In the latter case, if data were reported separately for the different gait patterns, we only extracted and reported the outcomes for the subgroup with crouch. Finally, the entire universe of treatments for crouch gait may not be fully represented here, in part because we excluded studies on crouch that did not present 3D kinematic data. This likely restricted the type and range of interventions and may have skewed the interventions that were included towards greater inclusion of surgical procedures. Future studies should aim to investigate intervention effects on specific, well defined gait patterns or stratify patient outcomes by gait patterns rather than present outcomes on heterogeneous group of patients.
Crouch gait can result from variable combinations of muscular, neurologic, and/or bony pathologic processes. Imperative to successful treatment of crouch gait is an understanding to the mechanism and time course of gait disability in children with bilateral CP. While the hamstrings are often targeted in crouch gait, other muscle or joint abnormalities can precipitate the development or progression of crouch such as planovalgus foot deformity [52] or hip flexion contractures [15,18,19]. A patient may initially walk with increased sagittal plane knee flexion due to hamstring or psoas spasticity [13–16,18,19], quadriceps or gastrocsoleus weakness [17,20], and/or lever arm dysfunction from malalignment or rotational bony deformities that develop as a result of imbalanced muscle forces across joints [52,64,88]. With time, shortening and dynamic contracture of the hamstrings may develop into a static contracture, preventing full active and passive knee extension [88]. Finally, rapid bone growth and weight gain during puberty may lead to progressive gait deterioration in multiple joints and planes and loss of mobility in adolescence and adulthood [88,89].
The multifactorial nature of crouch gait complicates the construction and interpretation of a review such as this, as the underlying deficits are likely different in patients undergoing the various interventions studied here. For example, a child with a dynamic but not yet static hamstring contracture and no bony deformity is not a candidate for muscle-tendon lengthening or femoral extension osteotomy procedures, and is more likely to be treated with braces, botulinum toxin, and physical therapy, limiting the utility or validity of a comparison of these interventions [90]. Future studies should aim to provide as much pre-intervention data on the patient population as possible, including but not limited to type and pattern of CP, functional ability, clinical exam measurements, all relevant lower limb kinematics, and radiologic findings in those with bony deformity.
Only eight studies were identified on non-surgical approaches to crouch gait. The mean age of the patients included in these studies was substantially younger than the age of patients in the surgical studies (9.7 years and 11.3 years, respectively). This trend is not surprising given the proposed mechanism of crouch gait development and progression. Strengthening, orthoses, and botulinum toxin are more likely to be deployed earlier in life before the development of static contractures which would then require surgical correction. Strengthening and orthoses may also be utilized post-operatively to optimize surgical outcomes. The reviewed alternative procedures and their outcomes in this study must then be considered relative to the degree and type of contractures as well as relative to patient size and age.
Hamstrings were lengthened or transferred in 27 of 30 surgical studies. Only 1 study had an adequate, non-hamstring lengthened control group [14], and one other reported outcomes after isolated HSL procedures [13]. Diverse surgical methods of hamstring lengthening are reported, including tenotomies, z-lengthenings, fractional lengthenings, and aponeurotic lengthenings which could differentially affect outcomes, yet no comparative studies were identified.
Guidelines for which hamstrings to lengthen are also not well-established. One noted strategy was to lengthen only medial hamstrings to prevent untethering of the pelvis, anterior pelvic tilt (APT) and genu recurvatum (GR), leaving the lateral hamstrings intact to prevent hyperextension. Another was to lengthen lateral hamstrings based on intraoperative examination after medial hamstring lengthening [36,37]. One comparative study showed 9 greater improvement in MKESt in the medial plus lateral versus the medial only group at 1.5 years. However, 30% in the medial plus lateral group developed GR versus 5% in the medial group [53]. Discrepancies in GR rates were not found in a similar study which had a 12% rate at 8 years in both groups [39]. Additional comparative evidence showed greater improvements in KFIC and MKESt in the medial and lateral versus medial group, although they had even greater flexion preoperatively [36]. Importantly, they found that medial and lateral HSL in patients with normal preoperative pelvic positioning produced significantly increased APT compared to those with increased pelvic tilt prior to surgery or who had medial HSL. More extensive medial and lateral hamstrings lengthening carries a significant risk of increasing APT, but may be necessary in those with severe static contractures to adequately increase knee extension.
One concern with HSL or HST is development of limited postoperative knee flexion in swing (stiff-knee gait) as these procedures often shift the sagittal knee kinematics curve into more extension. Multiple groups have investigated the role of rectus femoris transfer (RFT) to the medial hamstrings. Proposed benefits are two-fold: to augment the hamstrings flexion moment while diminishing excessive rectus activity limiting knee flexion in swing. While debate exists on mechanisms potentiating increased peak swing flexion [18,91], previous studies have demonstrated improved MKFSw after RFT as part of SEMLS [39,92,93]. Aiona et al. reported outcomes in 111 patients with HSL without RFT (n = 57), HSL plus RFT (n = 28), and HSL with delayed RFT (n = 26) at another surgical event. Those who had RFT plus HSL showed no decrease in mean peak knee flexion in swing, while the HSL alone group had significantly decreased mean MKFSw at 1 year. Those with later RFT showed improved MKFSw back to baseline, suggesting HSL procedures exacerbate limited knee flexion in swing and RFT may prevent this [27]. Further evidence from non-comparative studies corroborated this [26,30,47,54,76]. One study showed patients with limited knee flexion preoperatively had significantly improved TKE and MKFSw with RFT, while patients treated with RFT prophylactically before developing stiff-knee gait had 15° deterioration of MKFSw at 9 years [94]. Preoperative gait analysis may help predict the likelihood of RFT success in those with knee flexion limitations [95]. Some have cautioned that RFT may further compromise already weakened knee extensor function, increasing the risk of long term crouch recurrence [93].
Since HSL may also further weaken weak muscles [33] and resultant increased hamstring laxity may lead to excessive APT, hyperlordosis, back pain, and other gait problems, hamstrings transfers have been considered as an alternative, e.g. the Eggers procedure introduced in the 1950s which transferred all hamstrings to the femoral condyles to potentially reduce hamstring laxity. While this soon fell out of favor due to concerns about GR and loss of active knee flexion [96,97], renewed interest has surfaced in procedures that convert bi-articular to mono-articular muscles (CBM), such as HST to the femoral condyles. Besides biomechanical benefits, CBM procedures are theorized to improve neural control in CP as mono-articular muscles require less complex control strategies [98]. A retrospective cohort study reported outcomes after CBM of the gastrocsoleus, rectus femoris and semitendinosus versus outcomes after HSL and GSL. No significant group differences in spatiotemporal measures were found at 9 years. However, GR rates in the CBM group were two times higher at 1 and 9 years, with similar rates of increased anterior pelvic tilt [40]. A study comparing HST to gracilis and hamstring lengthening found no group differences except for increased APT from 15.7° to 22.2° only in the HST group [43] with another study comparing HSL and HST showing nearly identical results [37]. Interestingly, most reported rates of GR between HST and HSL groups have been similar [39,40,56,57]. Despite one study showing slightly superior APT outcomes in the CBM group compared to HSL and GSL [40], the group differences were clinically insignificant. HST fails to demonstrate any superior outcomes to justify use.
Although HSL demonstrates positive effects on knee biomechanics, multiple modeling studies postulate that short hamstrings may not be the primary cause of crouch in many patients. A short iliopsoas may instead be responsible for pulling the pelvis anteriorly and stretching the hamstrings at their origin thus making them appear too short at the knee [18,99,100]. One report on 8 patients with psoas lengthenings and 9 without during SEMLS, with similar kinematics preoperatively, showed improved MKESt for both, but increased APT only in the psoas group [77]. Another found improvements in psoas and no psoas groups in KFIC and MKESt, but instead found no significant changes in pelvic tilt at 1.5 years [36]. Reliance on passive range of motion measures alone which correlate poorly with gait analysis results and hence actual dynamic gait function [101,102] may be especially problematic when assessing true hamstring length in CP for surgical decision making [77].
Available evidence does not support the effectiveness of revision HSL. One study compared outcomes of primary versus revision HSL procedures in 39 patients, and found that primary procedures improved knee kinematics and popliteal angle at 2 years while revision HSL was ineffective [63]. A similar study in 61 patients reported improved KFIC, TKE, and popliteal angle 1 year after primary HSL with revision HSL only improving KFIC [32]. Better alternatives to revision HSL may require different surgical approaches or other less invasive strategies. There is emerging interest in correcting knee flexion deformities through distal femoral extension osteotomy and improving quadriceps extensor function through patellar tendon advance/shortening [68,103]. One retrospective study reported outcomes from DFEO alone, DFEO and PTA, and PTA alone [68]. All groups showed significant improvements in KFIC and mean improvements in MKESt of 9°, 29°, and 17°, respectively. All had increased APT, largest in the combined group. Notably, 61% of these 73 patients had prior HSLs [68]. Two other studies reported similar improvements in MKEST after DFEO during SEMLS in 12 patients [38] and PTS during SEMLS in 24 patients [67]. However, APT increased with these procedures [38,67] and knee flexion deformity recurred in 27% after DFEO [38]. Complications, including persistent pain, neuropathy, postoperative deformity, and infection, were reported in 18–19% in one study [68]. Improved surgical techniques including more advanced locking plates for internal fixation are now available with lower complication rates [68,104,105]. DFEO procedures involve the removal of a wedge of bone from the femur, and the post-operative benefits reported may be attributable to an effective lengthening of the hamstrings relative to the femur [106]. While these procedures offer a promising alternative, more investigation of positive and negative effects is needed.
Bony deformities such as pes planovalgus, femoral anteversion, and external tibial torsion may decrease the ability of multiple muscles to extend the hip and knee, referred to as lever-arm dysfunction, thereby exacerbating crouch gait [52,88,107]. Rotational osteotomies to correct femoral anteversion and external tibial torsion, were performed in many of studies included that were primarily investigating HSL, HST, and RFT in the setting of SEMLS [14,27,32,37,39,40,43,46,54,59,62,64,70,73,74,76], so the differential effects of bony surgeries on crouch were not discernible. However, examination of these procedures in isolation has previously demonstrated improved knee kinematics [108,109]. Similarly, another study not included in the review because it did not perform a separate statistical analysis on the subset of participants who had crouch, did demonstrate through correlational analyses of the entire sample that a greater improvement in the pes planovalgus deformity and excessive ankle dorsiflexion after surgery was associated with greater knee extension in stance [52].
Three studies investigated the effects of hamstrings botulinum toxin injections [19,31,60]. Modestly improved knee kinematics were seen 2 weeks post injection but disappeared by 12 weeks. Repeated injections are commonly utilized to facilitate muscle stretch during growth and purportedly delay or prevent development of static contractures [110,111]. Three studies investigated effects of short term strength training with inconsistent results. One showed decreased KFIC and improved mobility after a 6 week quadriceps strengthening program, while two others reported no change in any measures. Although modeling studies indicate that adequate strength of all lower limb extensor muscles is important to prevent crouch [4,112], strengthening hip extensor muscles may exacerbate hamstring contracture in those with spasticity [69]. Selective, longer duration strengthening may be effective in augmenting or maintaining outcomes after HSL or botulinum toxin injections if the etiology is multifactorial; however, more research is needed to support this hypothesis.
Orthoses are highly prescribed and utilized interventions in CP [113], with floor reaction ankle foot orthoses designed specifically for crouch gait [64,114]. A study by Rogozinski et al. investigated effects of already prescribed FRAFOs compared to barefoot walking in 27 patients. Patients had an 11° improvement in MKESt with FRAFOs and increased walking speed and step length as well [65]. The only RCT in our review by Abd El-Kafy investigated the effects of a strapping system and GRAFOs on the gait of CP crouch patients after a 12-week training program. Patients were randomized to receive either gait training, gait training + de-rotational strapping (Thera-Togs™), or gait training + de-rotational strapping and GRAFOs during the study. All groups underwent a rigorous 12-week gait training program with their randomized interventions. The group randomized to strapping and GRAFOS showed the largest improvement in knee kinematics from baseline, and was the only intervention group to differ from the control, gait training group, suggesting that the GRAFOs, and not de-rotational strapping devices, were responsible for the kinematic improvement [85]. Effects of AFOs that control excessive dorsiflexion on crouch remain largely unknown but improvements in upright stance seem biomechanically plausible and initial data suggests that GRAFOs do improve knee extension in stance. Conversely, a brace stretching the bi-articular gastrocnemius to achieve a more plantigrade position in mid-stance could exacerbate knee flexion.
Disappointingly, no studies were identified on other potentially effective interventions such as selective dorsal rhizotomy (SDR) or functional electrical stimulation (FES) on crouch. Studies on SDR have shown generalized improvements in functional measures and knee kinematics in CP [115–118]. FES has demonstrated effectiveness in addressing other gait abnormalities in CP so another future research direction could be exploring its role in alleviating crouch.
Another major shortcoming apparent in the current literature is the lack of functional outcomes reported in 31 of 38 studies [32,34,56,60,64,68]. While the authors acknowledge that inclusion criteria may have excluded studies from this review without kinematics that reported functional outcomes, the current imperative in the care and management of patients with CP is to improve current and future functional mobility and degree of participation in the community. While gait analysis data can measure the direct effects of interventions, kinematics have only a weak correlation with functional ability measures [119–121]. Many well-validated patient reported outcome measures of mobility and physical functioning are now available and are strongly recommended for inclusion in future trials evaluating crouch in CP such as the Functional Assessment Questionnaire (FAQ) [119], the Gross Motor Function Measure (GMFM) [122], Functional Mobility Scale (FMS) [123], the Pediatric Outcomes Data Collection Instrument [124], and the Pedi-CAT [125]. Additionally, a previous study by Kondo et al. and a study included in this review by Yngve et al. have demonstrated differential changes in both functional and objective measures of gait based on patient preoperative functional ability [76,126]. While many studies did report the range of patient GMFCS levels studied, only one stratified results by preoperative functional group or ability. Given that these studies have demonstrated strong predictive value of pre-treatment GMFCS, future studies should strongly consider stratification by functional grouping in order to better advise future individualized patient care and management.
Of those studies that did report the type and pattern of CP involvement, few reported outcomes by sub-group or provided individual data, preventing any analysis of intervention effect on patients of different CP diagnoses. Especially relevant to this review, a few studies investigated the outcomes of interventions in a heterogeneous group that included children with both unilateral and bilateral involvement. The definition and mechanism of flexed-knee gait varies between these two diagnostic groups, and crouch gait is not typically described in children with hemiplegia [7]. The varying underlying pathology of these CP involvement patterns likely also alters the effectiveness of the specific interventions between groups, warranting stricter patient selection or reporting stratification.
Nearly all studies reviewed were small, retrospective, and uncontrolled with many surgical studies having inadequate baseline patient characterization and confounding by concomitant procedures. Given its overwhelming prevalence, McGinley et al. called for more rigorous investigation of SEMLS through RCTs [21]. A subsequent RCT in 19 patients demonstrated improved kinematic outcomes for SEMLS [127] compared to strength training with the sample size too small to evaluate specific procedures. While this study demonstrated that it is possible to evaluate SEMLS with this rigorous type of design that temporarily delayed surgery in the control group, randomizing children to surgery or no surgery when they have a static contracture poses ethical challenges. Additionally, large scale RCTs require considerable resources in terms of patient and project staff time as well as cost [128]. Alternative approaches are gaining traction to document clinical outcomes including establishment of patient registries for both ongoing quality improvement and outcomes research and the implementation of large-scale prospective observational trials to compare effectiveness of surgical approaches. These pragmatic clinical trials do not require randomization and instead utilize advanced statistical methods to control for pre-operative patient differences.
This review highlights a few key issues in the current CP literature that need to be resolved to advance the current state of knowledge supporting clinical practice for those with crouch and other gait abnormalities. The joints and planes of motion and points in the gait cycle used to define crouch gait vary widely in the literature, with no clear consensus [129]. We propose here that a minimum requirement for crouch gait includes excessive knee flexion during the period after weight acceptance until terminal stance., It is also evident that in many cases, sagittal as well as other 3D deformities at the ankle-foot complex and at the hip contribute to the crouch posture as well as interfere with optimal lower limb alignment and should be carefully documented before and after any intervention that may affect muscle length or joint position. These data would inform future discussions aimed at achieving consensus on definitions and more clearly delineate different subgroups that may require different types or combinations of treatments.
This review highlights the paucity of kinematic evidence for other non-surgical interventions commonly used to address crouch including rigid AFOs, dynamic stretching devices and stretching exercises, among others. Additionally, although nearly all of the available literature investigated surgical interventions targeted at the knee joint, it is important to view crouch gait as a disorder of the entire lower limb. Patients can have deformities or limitations at the hip joint, or ankle-foot complex, or deformity of the lower-limb long bones that contribute to crouch gait. The lack of knee kinematic data on the various procedures that address those deformities is a major gap in the literature that warrants further study.
In conclusion, the only well-supported intervention for crouch in CP has long been and remains HSL even though surgical practices have evolved beyond this more simplistic approach. Regardless, even with current treatment, rates of ambulatory decline in adults with CP remain staggering [130]. Lengthening procedures also further weaken already weak muscles. Future research should focus on continued non-surgical and surgical innovations that optimally preserve strength while enhancing alignment and motion.
Acknowledgements
The authors thank Informationist Judith Welsh, NIH Library, for her collaboration on the research strategy and conducting the literature searches in support of this systematic review.
Funding
The work was funded in part by the NIH Medical Research Scholars Program and the Intramural Research Program of the NIH Clinical Center.
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to report.
References
- [1].Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B, Jacobsson B, A report: the definition and classification of cerebral palsy April 2006, Dev. Med. Child Neurol. Suppl 109 (2007) 8–14. [PubMed] [Google Scholar]
- [2].Wren TAL, Rethlefsen SP, Kay RM, Prevalence of specific gait abnormalities in children with cerebral palsy: influence of cerebral palsy subtype, age, and previous surgery, J. Pediatr. Orthop 25 (2005) 79–83. [DOI] [PubMed] [Google Scholar]
- [3].Hicks JL, Schwartz MH, Arnold AS, Delp SL, Crouched postures reduce the capacity of muscles to extend the hip and knee during the single-limb stance phase of gait, J. Biomech 41 (2008) 960–967, doi: 10.1016/j.jbiomech.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Steele KM, Seth A, Hicks JL, Schwartz MS, Delp SL, Muscle contributions to support and progression during single-limb stance in crouch gait, J. Biomech 43 (2010) 2099–2105, doi: 10.1016/j.jbiomech.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Waters RL, Mulroy S, The energy expenditure of normal and pathologic gait, Gait Posture 9 (1999) 207–231, doi: 10.1016/S0966-6362(99)00009-0. [DOI] [PubMed] [Google Scholar]
- [6].Steele KM, DeMers MS, Schwartz MS, Delp SL, Compressive tibiofemoral force during crouch gait, Gait Posture 35 (2012) 556–560, doi: 10.1016/j.gaitpost.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Graham HK, Selber P, Musculoskeletal aspects of cerebral palsy, J. Bone Jt. Surg.-Br 85 (2003) 157–166. [DOI] [PubMed] [Google Scholar]
- [8].Opheim A, Jahnsen R, Olsson E, Stanghelle JK, Walking function, pain, and fatigue in adults with cerebral palsy: a 7-year follow-up study, Dev. Med. Child Neurol 51 (2009) 381–388, doi: 10.1111/j.14698749.2008.03250.x. [DOI] [PubMed] [Google Scholar]
- [9].Bell KJMS, Ounpuu SMS, DeLuca PAMD, Romness MJMD, Natural progression of gait in children with cerebral palsy, J. Pediatr. Orthop 22 (2002) 677–682. [PubMed] [Google Scholar]
- [10].Johnson DC, Damiano DL, Abel MF, The evolution of gait in childhood and adolescent cerebral palsy, J. Pediatr. Orthop 17 (1997) 392–396. [PubMed] [Google Scholar]
- [11].Rose GE, Lightbody KA, Ferguson RG, Walsh JC, Robb JE, Natural history of flexed knee gait in diplegic cerebral palsy evaluated by gait analysis in children who have not had surgery, Gait Posture 31 (2010) 351–354, doi: 10.1016/j.gaitpost.2009.12.006. [DOI] [PubMed] [Google Scholar]
- [12].Hanna SE, Rosenbaum PL, Bartlett DJ, Palisano RJ, Walter SD, Avery L, Russell DJ, Stability and decline in gross motor function among children and youth with cerebral palsy aged 2–21 years, Dev. Med. Child Neurol 51 (2009) 295–302, doi: 10.1111/j.1469-8749.2008.03196.x. [DOI] [PubMed] [Google Scholar]
- [13].Carney BT, Oeffinger D, Meo AM, Sagittal knee kinematics after hamstring lengthening, J. Pediatr. Orthop. B 15 (2006) 348–350. [DOI] [PubMed] [Google Scholar]
- [14].Laracca E, Stewart C, Postans N, Roberts A, The effects of surgical lengthening of hamstring muscles in children with cerebral palsy – the consequences of pre-operative muscle length measurement, Gait Posture 39 (2014) 847–851, doi: 10.1016/j.gaitpost.2013.11.010. [DOI] [PubMed] [Google Scholar]
- [15].Canale ST, Beaty JH, Campbell’s Operative Orthopaedics: Expert Consult Premium Edition-Enhanced Online Features, Elsevier Health Sciences, 2012. https://books.google.com/books?hl=en&lr=&id=1Bvfn1ZRWgC&oi=fnd&pg=PT515&dq=campbell%27s+operative+orthopedics&ots=VHpj1tSd7E&sig=3nY6u0iWERtqetTh2bUJ0XN7S4Y (Accessed 29 October 2015). [Google Scholar]
- [16].Sutherland DHMD, Davids JRMD, Common gait abnormalities of the knee in cerebral palsy, Clin. Orthop 288 (1993) 139–147. [PubMed] [Google Scholar]
- [17].Vuillermin C, Rodda J, Rutz E, Shore BJ, Smith K, Graham HK, Severe crouch gait in spastic diplegia can be prevented a population-based study, J. Bone Joint Surg. Br 93-B (2011) 1670–1675, doi: 10.1302/0301-620X.93B12.27332. [DOI] [PubMed] [Google Scholar]
- [18].Delp SL, Arnold AS, Speers RA, Moore CA, Hamstrings and psoas lengths during normal and crouch gait: implications for muscle-tendon surgery, J. Orthop. Res 14 (1996) 144–151, doi: 10.1002/jor.1100140123. [DOI] [PubMed] [Google Scholar]
- [19].Thompson N, Baker R, Cosgrove A, Corry I, Graham H, Musculoskeletal modelling in determining the effect of botulinum toxin on the hamstrings of patients with crouch gait, Dev. Med. Child Neurol 40 (1998) 622–625, doi: 10.1111/j.1469-8749.1998.tb15428.x. [DOI] [PubMed] [Google Scholar]
- [20].Damiano DL, Kelly LE, Vaughn CL, Effects of quadriceps femoris muscle strengthening on crouch gait in children with spastic diplegia, Phys. Ther 75 (1995) 658–667. [DOI] [PubMed] [Google Scholar]
- [21].Mcginley JL, Dobson F, Ganeshalingam R, Shore BJ, Rutz E, Graham HK, Single-event multilevel surgery for children with cerebral palsy: a systematic review, Dev. Med. Child Neurol 54 (2012) 117–128, doi: 10.1111/j.1469-8749.2011.04143.x. [DOI] [PubMed] [Google Scholar]
- [22].Novak I, Mcintyre S, Morgan C, Campbell L, Dark L, Morton N, Stumbles E, Wilson S-A, Goldsmith S, A systematic review of interventions for children with cerebral palsy: state of the evidence, Dev. Med. Child Neurol 55 (2013) 885–910, doi: 10.1111/dmcn.12246. [DOI] [PubMed] [Google Scholar]
- [23].Sackett DL, Strause SE, Richardson WS, Rosenberg W, Haynes RB, Evidence-Based Medicine: How to Practice and Teach EBM, Churchill Livingstone, 2000.
- [24].Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J, Methodological index for non-randomized studies (MINORS):development and validation of a new instrument, ANZ J. Surg 73 (2003) 712–716, doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- [25].Moher D, Liberati A, Tetzlaff J, Altman DG, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement, Int. J. Surg 8 (2010) 336–341, doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- [26].Adolfsen SE, Ounpuu S, Bell KJ, DeLuca PA, Kinematic and kinetic outcomes after identical multilevel soft tissue surgery in children with cerebral palsy, J. Pediatr. Orthop 27 (2007) 658–667, doi: 10.1097/BPO.0b013e3180dca114. [DOI] [PubMed] [Google Scholar]
- [27].Aiona M, Do KP, Feng J, Jabur M, Comparison of rectus femoris transfer surgery done concomitant with hamstring lengthening or delayed in patients with cerebral palsy, J. Pediatr. Orthop (2015), doi: 10.1097/BPO.0000000000000596. [DOI] [PubMed]
- [28].Baumann JU, Ruetsch H, Schürmann K, Distal hamstring lengthening in cerebral palsy. An evaluation by gait analysis, Int. Orthop. 3 (1980) 305–309. [DOI] [PubMed] [Google Scholar]
- [29].Bozinovski Z, Popovski N, Operative treatment of the knee contractures in cerebral palsy patients, Med. Arch 68 (2014) 182–183, doi: 10.5455/medarh.2014.68.182-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Carney BT, Oeffinger D, Sagittal knee kinematics following combined hamstring lengthening and rectus femoris transfer, J. South. Orthop. Assoc 12 (2003) 149–153. [PubMed] [Google Scholar]
- [31].Corry IS, Cosgrove AP, Duffy CM, Taylor TC, Graham HK, Botulinum toxin A in hamstring spasticity, Gait Posture 10 (1999) 206–210, doi: 10.1016/S0966-6362(99)00037-5. [DOI] [PubMed] [Google Scholar]
- [32].Chang W-N, Tsirikos AI, Miller F, Lennon N, Schuyler J, Kerstetter L, Glutting J, Distal hamstring lengthening in ambulatory children with cerebral palsy: primary versus revision procedures, Gait Posture 19 (2004) 298–304, doi: 10.1016/S0966-6362(03)00070-5. [DOI] [PubMed] [Google Scholar]
- [33].Damiano DL, Abel MFMD, Pannunzio MMD, Romano J-PMS, Interrelationships of strength and gait before and after hamstrings lengthening. [Miscellaneous article], J. Pediatr. Orthop 19 (1999) 352–358. [PubMed] [Google Scholar]
- [34].Damiano DL, Arnold AS, Steele KM, Delp SL, Can strength training predictably improve gait kinematics? A pilot study on the effects of hip and knee extensor strengthening on lower-extremity alignment in cerebral palsy, Phys. Ther 90 (2010) 269–279, doi: 10.2522/ptj.20090062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Das SP, Pradhan S, Ganesh S, Sahu PK, Mohanty RN, Das SK, Supracondylar femoral extension osteotomy and patellar tendon advancement in the management of persistent crouch gait in cerebral palsy, Indian J. Orthop 46 (2012) 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].DeLuca PAMD, Ounpuu SMS, Davis RB, Walsh JHPMD, Effect of hamstring and psoas lengthening on pelvic tilt in patients with spastic diplegic cerebral palsy, J. Pediatr. Orthop 18 (1998) 712–718. [PubMed] [Google Scholar]
- [37].De Mattos C, Patrick Do K, Pierce R, Feng J, Aiona M, Sussman M, Comparison of hamstring transfer with hamstring lengthening in ambulatory children with cerebral palsy: further follow-up, J. Child. Orthop 8 (2014) 513–520, doi: 10.1007/s11832-014-0626-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].de Morais Filho MC, Neves DL, Abreu FP, Juliano Y, Guimarães L, Treatment of fixed knee flexion deformity and crouch gait using distal femur extension osteotomy in cerebral palsy, J. Child. Orthop 2 (2008) 37–43, doi: 10.1007/s11832-007-0073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dreher T, Vegvari D, Wolf SI, Geisbüsch A, Gantz S, Wenz W, Braatz F, Development of knee function after hamstring lengthening as a part of multilevel surgery in children with spastic diplegia, J. Bone Jt. Surg. Am 94 (2012) 121–130, doi: 10.2106/JBJS.J.00890. [DOI] [PubMed] [Google Scholar]
- [40].Dreher T, Vegvári D, Wolf SL, Klotz M, Müller S, Metaxiotis D, Wenz W, Döderlein L, Braatz F, Long-term effects after conversion of biarticular to monoarticular muscles compared with musculotendinous lengthening in children with spastic diplegia, Gait Posture 37 (2013) 430–435, doi: 10.1016/j.gaitpost.2012.08.020. [DOI] [PubMed] [Google Scholar]
- [41].Drummond DS, Rogala E, Templeton J, Cruess R, Proximal hamstring release for knee flexion and crouched posture in cerebral palsy, J. Bone Jt. Surg. Am 56 (1974) 1598–1602. [PubMed] [Google Scholar]
- [42].Eek MN, Tranberg R, Zügner R, Alkema K, Beckung E, Muscle strength training to improve gait function in children with cerebral palsy, Dev. Med. Child Neurol 50 (2008) 759–764, doi: 10.1111/j.14698749.2008.03045.x. [DOI] [PubMed] [Google Scholar]
- [43].Feng L, Patrick Do K, Aiona M, Feng J, Pierce R, Sussman M, Comparison of hamstring lengthening with hamstring lengthening plus transfer for the treatment of flexed knee gait in ambulatory patients with cerebral palsy, J. Child. Orthop 6 (2012) 229–235, doi: 10.1007/s11832-0120405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gage JR, Perry J, Hicks RR, Koop S, Werntz JR, Rectus femoris transfer to improve knee function of children with cerebral palsy, Dev. Med. Child Neurol 29 (1987) 159–166, doi: 10.1111/j.1469-8749.1987.tb02131.x. [DOI] [PubMed] [Google Scholar]
- [45].Ganjwala D, Multilevel orthopedic surgery for crouch gait in cerebral palsy: an evaluation using functional mobility and energy cost, Indian J. Orthop 45 (2011) 314, doi: 10.4103/0019-5413.82334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gordon AB, Baird GO, McMulkin ML, Caskey PM, Ferguson RL, Gait analysis outcomes of percutaneous medial hamstring tenotomies in children with cerebral palsy, J. Pediatr. Orthop 28 (2008) 324–329, doi: 10.1097/BPO.0b013e318168d1c0. [DOI] [PubMed] [Google Scholar]
- [47].Hadley NMD, Chambers CPT, Scarborough NPT, Cain TMD, Rossi DMS, Knee motion following multiple soft-tissue releases in ambulatory patients with cerebral palsy, J. Pediatr. Orthop 12 (1992) 324–328. [DOI] [PubMed] [Google Scholar]
- [48].Haumont T, Church C, Hager S, Cornes MJ, Poljak D, Lennon N, Henley J, Taylor D, Niiler T, Miller F, Flexed-knee gait in children with cerebral palsy: a 10-year follow-up study, J. Child. Orthop 7 (2013) 435–443, doi: 10.1007/s11832-013-0505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hesse S, Brandl-Hesse B, Seidel U, Doll B, Gregoric M, Lower limb muscle activity in ambulatory children with cerebral palsy before and after the treatment with Botulinum toxin A, Restor. Neurol. Neurosci 17 (2000) 1–8. [PubMed] [Google Scholar]
- [50].Hsu LC, Li HS, Distal hamstring elongation in the management of spastic cerebral palsy, J. Pediatr. Orthop 10 (1990) 378–381. [DOI] [PubMed] [Google Scholar]
- [51].Joseph B, Reddy K, Varghese RA, Shah H, Doddabasappa SN, Management of severe crouch gait in children and adolescents with cerebral palsy, J. Pediatr. Orthop 30 (2010) 832–839, doi: 10.1097/BPO.0b013e3181fbfd0e. [DOI] [PubMed] [Google Scholar]
- [52].Kadhim M, Miller F, Crouch gait changes after planovalgus foot deformity correction in ambulatory children with cerebral palsy, Gait Posture 39 (2014) 793–798, doi: 10.1016/j.gaitpost.2013.10.020. [DOI] [PubMed] [Google Scholar]
- [53].Kay RMMD, Rethlefsen SAPT, Skaggs DMD, Leet AMD, Outcome of medial versus combined medial and lateral hamstring lengthening surgery in cerebral palsy, J. Pediatr. Orthop 22 (2002) 169–172. [PubMed] [Google Scholar]
- [54].Koca K, Yildiz C, Yurttaş Y, Bïlgïç S, Ozkan H, Kürklü M, Balaban B, Haznecï B, Başbozkurt M, Outcomes of combined hamstring release and rectus transfer in children with crouch gait, Ortop. Traumatol. Rehabil 11 (2009) 333–338. [PubMed] [Google Scholar]
- [55].Lovejoy SA, Tylkowski C, Oeffinger D, Sander L, The effects of hamstring lengthening on hip rotation, J. Pediatr. Orthop 27 (2007) 142–146, doi: 10.1097/01.bpb.0000248568.43251.b0. [DOI] [PubMed] [Google Scholar]
- [56].Ma FYP, Selber P, Nattrass GR, Harvey AR, Wolfe R, Graham HK, Lengthening and transfer of hamstrings for a flexion deformity of the knee in children with bilateral cerebral palsy. Technique and preliminary results, J. Bone Jt. Surg. Br 88-B (2006) 248–254, doi: 10.1302/0301620x.88b2.16797. [DOI] [PubMed] [Google Scholar]
- [57].Metaxiotis D, Wolf S, Doederlein L, Conversion of biarticular to monoarticular muscles as a component of multilevel surgery in spastic diplegia, J. Bone Jt. Surg. Br 86-B (2004) 102–109, doi: 10.1302/0301-620X.86B1.13689. [DOI] [PubMed] [Google Scholar]
- [58].Morton JF, Brownlee M, McFadyen AK, The effects of progressive resistance training for children with cerebral palsy, Clin. Rehabil 19 (2005) 283–289. [DOI] [PubMed] [Google Scholar]
- [59].Õunpuu S, Solomito M, Bell K, DeLuca P, Pierz K, Long-term outcomes after multilevel surgery including rectus femoris, hamstring and gastrocnemius procedures in children with cerebral palsy, Gait Posture 42 (2015) 365–372. [DOI] [PubMed] [Google Scholar]
- [60].Papadonikolakis A, Vekris M, Korompilias A, Kostas J, Ristanis S, Soucacos P, Botulinum A toxin for treatment of lower limb spasticity in cerebral palsyGait analysis in 49 patients, Acta Orthop. Scand 74 (2003) 749–755, doi: 10.1080/00016470310018315. [DOI] [PubMed] [Google Scholar]
- [61].Park MS, Chung CY, Lee SH, Choi IH, Cho T-J, Yoo WJ, Park BSMY, Lee KM, Effects of distal hamstring lengthening on sagittal motion in patients with diplegia: hamstring length and its clinical use, Gait Posture 30 (2009) 487–491, doi: 10.1016/j.gaitpost.2009.07.115. [DOI] [PubMed] [Google Scholar]
- [62].Rethlefsen SPT, Tolo VTMD, Reynolds RAKMD, Kay RMD, Outcome of hamstring lengthening and distal rectus femoris transfer surgery, J. Pediatr. Orthop. B 8 (1999) 75–88. [PubMed] [Google Scholar]
- [63].Rethlefsen SA, Yasmeh S, Wren TAL, Kay RM, Repeat hamstring lengthening for crouch gait in children with cerebral palsy, J. Pediatr. Orthop 33 (2013) 501–504, doi: 10.1097/BPO.0b013e318288b3e7. [DOI] [PubMed] [Google Scholar]
- [64].Rodda JM, Graham HK, Nattrass GR, Galea MP, Baker R, Wolfe R, Correction of severe crouch gait in patients with spastic diplegia with use of multilevel orthopaedic surgery, J. Bone Jt. Surg 88 (2006) 2653–2664, doi: 10.2106/JBJS.E.00993. [DOI] [PubMed] [Google Scholar]
- [65].Rogozinski BM, Davids JR, Davis RB, Jameson GG, Blackhurst DW, The efficacy of the floor-reaction ankle-foot orthosis in children with cerebral palsy, J. Bone Jt. Surg. Am 91 (2009) 2440–2447, doi: 10.2106/JBJS.H.00965. [DOI] [PubMed] [Google Scholar]
- [66].Roosth HP, Flexion deformity of the hip and knee in spatic cerebral palsy: treatment by early release of spastic hip-flexor muscles, J. Bone Jt. Surg. Am 53 (1971) 1489–1510. [PubMed] [Google Scholar]
- [67].Sossai R, Vavken P, Brunner R, Camathias C, Graham HK, Rutz E, Patellar tendon shortening for flexed knee gait in spastic diplegia, Gait Posture 41 (2015) 658–665, doi: 10.1016/j.gaitpost.2015.01.018. [DOI] [PubMed] [Google Scholar]
- [68].Stout JL, Gage JR, Schwartz MH, Novacheck TF, Distal femoral extension osteotomy and patellar tendon advancement to treat persistent crouch gait in cerebral palsy, J. Bone Jt. Surg 90 (2008) 2470–2484, doi: 10.2106/JBJS.G.00327. [DOI] [PubMed] [Google Scholar]
- [69].Steele K, Damiano D, Eek M, Unger M, Delp S, Characteristics associated with improved knee extension after strength training for individuals with cerebral palsy and crouch gait, J. Pediatr. Rehabil. Med 5 (2012) 99–106, doi: 10.3233/PRM-2012-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sung KH, Chung CY, Lee KM, Akhmedov B, Lee SY, Choi IH, Cho T-J, Yoo WJ, Park MS, Long term outcome of single event multilevel surgery in spastic diplegia with flexed knee gait, Gait Posture 37 (2013) 536–541, doi: 10.1016/j.gaitpost.2012.09.011. [DOI] [PubMed] [Google Scholar]
- [71].Sutherland DH, Larsen LJ, Mann R, Rectus femoris release in selected patients with cerebral palsy: a preliminary report, Dev. Med. Child Neurol 17 (1975) 26–34, doi: 10.1111/j.1469-8749.1975.tb04953.x. [DOI] [PubMed] [Google Scholar]
- [72].Thometz J, Simon S, Rosenthal R, The effect on gait of lengthening of the medial hamstrings in cerebral palsy, J. Bone Jt. Surg. Am 71 (1989) 345–353. [PubMed] [Google Scholar]
- [73].van der Linden ML, Aitchison AM, Hazlewood ME, Hillman SJ, Robb JE, Effects of surgical lengthening of the hamstrings without a concomitant distal rectus femoris transfer in ambulant patients with cerebral palsy, J. Pediatr. Orthop 23 (2003) 308–313. [PubMed] [Google Scholar]
- [74].Westwell M, Õunpuu S, DeLuca P, Effects of orthopedic intervention in adolescents and young adults with cerebral palsy, Gait Posture 30 (2009) 201–206, doi: 10.1016/j.gaitpost.2009.04.012. [DOI] [PubMed] [Google Scholar]
- [75].Gough M, Eve LC, Robinson RO, Shortland AP, Short-term outcome of multilevel surgical intervention in spastic diplegic cerebral palsy compared with the natural history, Dev. Med. Child Neurol 46 (2004) 91–97, doi: 10.1111/j.1469-8749.2004.tb00457.x. [DOI] [PubMed] [Google Scholar]
- [76].Yngve DAMD, Scarborough NPT, Goode BMS, Haynes RMD, Rectus and hamstring surgery in cerebral palsy: a gait analysis study of results by functional ambulation level. [Miscellaneous article], J. Pediatr. Orthop 22 (2002) 672–676. [PubMed] [Google Scholar]
- [77].Zwick EB, Saraph V, Zwick G, Steinwender C, Linhart WE, Steinwender G, Medial hamstring lengthening in the presence of hip flexor tightness in spastic diplegia, Gait Posture 16 (2002) 288–296, doi: 10.1016/S0966-6362(02)00022-X. [DOI] [PubMed] [Google Scholar]
- [78].Gannotti ME, Gorton GE, Nahorniak MT, Masso PD, Landry B, Lyman J, Sawicki R, Hagedorn K, Ross E, Warner J, Postoperative gait velocity and mean knee flexion in stance of ambulatory children with spastic diplegia four years or more after multilevel surgery, J. Pediatr. Orthop 27 (2007) 451–456, doi: 10.1097/01.bpb.0000271327.79481.e3. [DOI] [PubMed] [Google Scholar]
- [79].Lucareli PRG, de M, Lima O, de JG, Lucarelli A, Lima FPS, Changes in joint kinematics in children with cerebral palsy while walking with and without a floor reaction ankle-foot orthosis, Clinics 62 (2007) 63–68, doi: 10.1590/S1807-59322007000100010. [DOI] [PubMed] [Google Scholar]
- [80].Scholtes VA, Dallmeijer AJ, Knol DL, Speth LA, Maathuis CG, Jongerius PH, Becher JG, Effect of multilevel botulinum toxin a and comprehensive rehabilitation on gait in cerebral palsy, Pediatr. Neurol 36 (2007) 30–39, doi: 10.1016/j.pediatrneurol.2006.09.010. [DOI] [PubMed] [Google Scholar]
- [81].Svehlík M, Steinwender G, Kraus T, Saraph V, Lehmann T, Linhart WE, Zwick EB, The influence of age at single-event multilevel surgery on outcome in children with cerebral palsy who walk with flexed knee gait, Dev. Med. Child Neurol 53 (2011) 730–735, doi: 10.1111/j.14698749.2011.03995.x. [DOI] [PubMed] [Google Scholar]
- [82].Unger M, Faure M, Frieg A, Strength training in adolescent learners with cerebral palsy: a randomized controlled trial, Clin. Rehabil 20 (2006) 469–477, doi: 10.1191/0269215506cr961oa. [DOI] [PubMed] [Google Scholar]
- [83].Wesdock KA, Edge AM, Effects of wedged shoes and ankle-foot orthoses on standing balance and knee extension in children with cerebral palsy who crouch, Pediatr. Phys. Ther. Off. Publ. Sect. Pediatr. Am. Phys. Ther. Assoc 15 (2003) 221–231, doi: 10.1097/01.PEP.0000096383.80789. A4. [DOI] [PubMed] [Google Scholar]
- [84].Patritti BL, Sicari M, Deming LC, Romaguera F, Pelliccio MM, Kasi P, Benedetti MG, Nimec DL, Bonato P, The role of augmented feedback in pediatric robotic-assisted gait training: a case series, Technol. Disabil 22 (2010) 215–227. [Google Scholar]
- [85].Abd El-Kafy EM, The clinical impact of orthotic correction of lower limb rotational deformities in children with cerebral palsy: a randomized controlled trial, Clin. Rehabil 28 (2014) 1004–1014, doi: 10.1177/0269215514533710. [DOI] [PubMed] [Google Scholar]
- [86].Odding E, Roebroeck ME, Stam HJ, The epidemiology of cerebral palsy: incidence, impairments and risk factors, Disabil. Rehabil 28 (2006) 183–191, doi: 10.1080/09638280500158422. [DOI] [PubMed] [Google Scholar]
- [87].Rodda JM, Graham HK, Carson L, Galea MP, Wolfe R, Sagittal gait patterns in spastic diplegia, J. Bone Jt. Surg. Br 86-B (2004) 251–258, doi: 10.1302/0301-620x.86b2.13878. [DOI] [PubMed] [Google Scholar]
- [88].Herring JA, Tachdjian’s Pediatric Orthopaedics: From the Texas Scottish Rite Hospital for Children, Elsevier Health Sciences, 2013. [Google Scholar]
- [89].Davids JR, The foot and ankle in cerebral palsy, Orthop. Clin 41 (2010) 579–593, doi: 10.1016/j.ocl.2010.06.002. [DOI] [PubMed] [Google Scholar]
- [90].Damiano DL, Rehabilitative therapies in cerebral palsy: the good, the not as good, and the possible, J. Child Neurol 24 (2009) 1200–1204, doi: 10.1177/0883073809337919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Riewald SA, Delp SL, The action of the rectus femoris muscle following distal tendon transfer: does it generate knee flexion moment? Dev. Med. Child Neurol 39 (1997) 99–105, doi: 10.1111/j.14698749.1997.tb07391.x. [DOI] [PubMed] [Google Scholar]
- [92].Moreau N, Tinsley S, Li L, Progression of knee joint kinematics in children with cerebral palsy with and without rectus femoris transfers: a long-term follow up, Gait Posture 22 (2005) 132–137, doi: 10.1016/j.gaitpost.2004.08.003. [DOI] [PubMed] [Google Scholar]
- [93].Saw A, Smith PA, Sirirungruangsarn Y, Chen S, Hassani S, Harris G, Kuo KN, Rectus femoris transfer for children with cerebral palsy: long-term outcome, J. Pediatr. Orthop 23 (2003) 672–678. [DOI] [PubMed] [Google Scholar]
- [94].Dreher T, Wolf SI, Maier M, Hagmann S, Vegvari D, Gantz S, Heitzmann D, Wenz W, Braatz F, Long-term results after distal rectus femoris transfer as a part of multilevel surgery for the correction of stiff-Knee gait in spastic diplegic cerebral palsy, J. Bone Jt. Surg. Am 94 (2012) e142, doi: 10.2106/JBJS.K.01300. [DOI] [PubMed] [Google Scholar]
- [95].Reinbolt JA, Fox MD, Schwartz MH, Delp SL, Predicting outcomes of rectus femoris transfer surgery, Gait Posture 30 (2009) 100–105, doi: 10.1016/j.gaitpost.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Pollock GA, Surgical treatment of cerebral palsy, J. Bone Jt. Surg. Br 44 (1962) 68–81. [DOI] [PubMed] [Google Scholar]
- [97].Gage JRMD, Surgical treatment of knee dysfunction in cerebral palsy, Clin. Orthop 253 (1990) 45–54. [PubMed] [Google Scholar]
- [98].Prilutsky BI, Coordination of two- and one-joint muscles: functional consequences and implications for motor control, Motor Control. 4 (2000) 1–44. [DOI] [PubMed] [Google Scholar]
- [99].Hoffinger SAMD, Rab GTMD, Abou-Ghaida HMS, Hamstrings in cerebral palsy crouch gait, J. Pediatr. Orthop 13 (1993) 722–726. [DOI] [PubMed] [Google Scholar]
- [100].Rhie T-Y, Sung KH, Park MS, Lee KM, Chung CY, Hamstring and psoas length of crouch gait in cerebral palsy: a comparison with induced crouch gait in age- and sex-matched controls, J. NeuroEng. Rehabil 10 (2013) 10, doi: 10.1186/1743-0003-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Desloovere K, Molenaers G, Feys H, Huenaerts C, Callewaert B, de Walle PV, Do dynamic and static clinical measurements correlate with gait analysis parameters in children with cerebral palsy? Gait Posture 24 (2006) 302–313, doi: 10.1016/j.gaitpost.2005.10.008. [DOI] [PubMed] [Google Scholar]
- [102].Lee KM, Chung CY, Kwon DG, Han HS, Choi IH, Park MS, Reliability of physical examination in the measurement of hip flexion contracture and correlation with gait parameters in cerebral palsy, J. Bone Jt. Surg 93 (2011) 150–158, doi: 10.2106/JBJS.J.00252. [DOI] [PubMed] [Google Scholar]
- [103].Beals RK, Treatment of knee contracture in cerebral palsy by hamstring lengthening, posterior capsulotomy, and quadriceps mechanism shortening, Dev. Med. Child Neurol 43 (2001) 802–805, doi: 10.1111/j.1469-8749.2001.tb00166.x. [DOI] [PubMed] [Google Scholar]
- [104].Novacheck TF, Stout JL, Gage JR, Schwartz MH, Distal femoral extension osteotomy and patellar tendon advancement to treat persistent crouch gait in cerebral palsy, J. Bone Jt. Surg. Am 91 (2009) 271–286, doi: 10.2106/JBJS.I.00316. [DOI] [PubMed] [Google Scholar]
- [105].Rutz E, Gaston MS, Camathias C, Brunner R, Distal femoral osteotomy using the LCP pediatric condylar 90-degree plate in patients with neuromuscular disorders, J. Pediatr. Orthop 32 (2012) 295–300, doi: 10.1097/BPO.0b013e31824b29d7. [DOI] [PubMed] [Google Scholar]
- [106].Healy MT, Schwartz MH, Stout JL, Gage JR, Novacheck TF, Is simultaneous hamstring lengthening necessary when performing distal femoral extension osteotomy and patellar tendon advancement? Gait Posture 33 (2011) 1–5, doi: 10.1016/j.gaitpost.2010.08.014. [DOI] [PubMed] [Google Scholar]
- [107].Hicks J, Arnold A, Anderson F, Schwartz M, Delp S, The effect of excessive tibial torsion on the capacity of muscles to extend the hip and knee during single-limb stance, Gait Posture 26 (2007) 546–552, doi: 10.1016/j.gaitpost.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Stefko RM, De Swart RJ, Dodgin DA, Wyatt MP, Kaufman KR, Sutherland DH, Chambers HG, Kinematic and kinetic analysis of distal derotational osteotomy of the leg in children with cerebral palsy, J. Pediatr. Orthop 18 (1998) 81–87. [PubMed] [Google Scholar]
- [109].Õunpuu S, DeLuca P, Davis R, Romness M, Long-term effects of femoral derotation osteotomies: an evaluation using three-dimensional gait analysis, J. Pediatr. Orthop 22 (2002) 139–145. [PubMed] [Google Scholar]
- [110].Heinen F, Desloovere K, Schroeder AS, Berweck S, Borggraefe I, van Campenhout A, Andersen GL, Aydin R, Becher JG, Bernert G, Caballero IM, Carr L, Valayer EC, Desiato MT, Fairhurst C, Filipetti P, Hassink R-I, Hustedt U, Jozwiak M, Kocer SI, Kolanowski E, Krägeloh-Mann I, Kutlay Ş, Mäenpää H, Mall V, McArthur P, Morel E, Papavassiliou A, Pascual-Pascual I, Pedersen SA, Plasschaert FS, van der Ploeg I, Remy-Neris O, Renders A, Di Rosa G, Steinlin M, Tedroff K, Valls JV, Viehweger E, Molenaers G, The updated European Consensus 2009 on the use of Botulinum toxin for children with cerebral palsy, Eur. J. Paediatr. Neurol 14 (2010) 45–66, doi: 10.1016/j.ejpn.2009.09.005. [DOI] [PubMed] [Google Scholar]
- [111].Fortuna R, Horisberger M, Vaz MA, Herzog W, Do skeletal muscle properties recover following repeat onabotulinum toxin A injections? J. Biomech 46 (2013) 2426–2433, doi: 10.1016/j.jbiomech.2013.07.028. [DOI] [PubMed] [Google Scholar]
- [112].Steele KM, van der Krogt MM, Schwartz MH, Delp SL, How much muscle strength is required to walk in a crouch gait? J. Biomech 45 (2012) 2564–2569, doi: 10.1016/j.jbiomech.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Wingstrand M, Hägglund G, Rodby-Bousquet E, Ankle-foot orthoses in children with cerebral palsy: a cross sectional population based study of 2200 children, BMC Musculoskelet. Disord 15 (327) (2014), doi: 10.1186/1471-2474-15-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Gage JRMD, Novacheck TFMD, An update on the treatment of gait problems in cerebral palsy, J. Pediatr. Orthop. B 10 (2001) 265–274. [PubMed] [Google Scholar]
- [115].Grunt S, Henneman WJP, Bakker MJ, Harlaar J, van der Ouwerkerk WJR, van Schie P, Reeuwijk A, Becher JG, Vermeulen RJ, Effect of selective dorsal rhizotomy on gait in children with bilateral spastic paresis: kinematic and EMG-pattern changes, Neuropediatrics 41 (2010) 209–216, doi: 10.1055/s-0030-1267983. [DOI] [PubMed] [Google Scholar]
- [116].Nordmark E, Josenby AL, Lagergren J, Andersson G, Strömblad L-G, Westbom L, Long-term outcomes five years after selective dorsal rhizotomy, BMC Pediatr. 8 (2008) 54, doi: 10.1186/1471-2431-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Thomas SS, Aiona MD, Buckon CE, Piatt JH, Does gait continue to improve 2 years after selective dorsal rhizotomy? J. Pediatr. Orthop 17 (1997) 387–391. [PubMed] [Google Scholar]
- [118].Engsberg JR, Ross SA, Collins DR, Park TS, Effect of selective dorsal rhizotomy in the treatment of children with cerebral palsy, J. Neurosurg 105 (2006) 8–15, doi: 10.3171/ped.2006.105.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Novacheck TFMD, Stout JLMS, Tervo RMD, Reliability and validity of the gillette functional assessment questionnaire as an outcome measure in children with walking disabilities, J. Pediatr. Orthop 20 (2000). [PubMed] [Google Scholar]
- [120].Abel MF, Damiano DL, Blanco JS, Conaway M, Miller F, Dabney K, Sutherland D, Chambers H, Dias L, Sarwark J, et al. , Relationships among musculoskeletal impairments and functional health status in ambulatory cerebral palsy, J. Pediatr. Orthop 23 (2003) 535–541. [PubMed] [Google Scholar]
- [121].Tervo RC, Azuma S, Stout J, Novacheck T, Correlation between physical functioning and gait measures in children with cerebral palsy, Dev. Med. Child Neurol 44 (2002) 185–190, doi: 10.1111/j.14698749.2002.tb00784.x. [DOI] [PubMed] [Google Scholar]
- [122].Russell DJ, Rosenbaum PL, Cadman DT, Gowland C, Hardy S, Jarvis S, The gross motor function measure: a means to evaluate the effects of physical therapy, Dev. Med. Child Neurol 31 (1989) 341–352, doi: 10.1111/j.1469-8749.1989.tb04003.x. [DOI] [PubMed] [Google Scholar]
- [123].Graham HK, Harvey A, Rodda J, Nattrass GR, Pirpiris M, The functional mobility scale (FMS), J. Pediatr. Orthop 24 (2004) 514–520. [DOI] [PubMed] [Google Scholar]
- [124].McMulkin ML, Baird GO, Gordon AB, Caskey PM, Ferguson RL, The pediatric outcomes data collection instrument detects improvements for children with ambulatory cerebral palsy after orthopaedic intervention, J. Pediatr. Orthop 27 (2007) 1–6, doi: 10.1097/01.bpo.0000242442.34553.c7. [DOI] [PubMed] [Google Scholar]
- [125].Dumas HM, Fragala-Pinkham MA, Concurrent validity and reliability of the pediatric evaluation of disability inventory-computer adaptive test mobility domain, Pediatr. Phys. Ther 24 (2012) 171–176, doi: 10.1097/PEP.0b013e31824c94ca. [DOI] [PubMed] [Google Scholar]
- [126].Kondo I, Hosokawa K, Iwata M, Oda A, Nomura T, Ikeda K, Asagai Y, Kohzaki T, Nishimura H, Effectiveness of selective muscle-release surgery for children with cerebral palsy: longitudinal and stratified analysis, Dev. Med. Child Neurol 46 (2004) 540–547, doi: 10.1111/j.14698749.2004.tb01012.x. [DOI] [PubMed] [Google Scholar]
- [127].Thomason P, Baker R, Dodd K, Taylor N, Selber P, Wolfe R, Graham HK, Single-event multilevel surgery in children with spastic diplegia, J. Bone Jt. Surg. Am 93 (2011) 451–460, doi: 10.2106/JBJS.J.00410. [DOI] [PubMed] [Google Scholar]
- [128].Graham HK, The trials of trials, Dev. Med. Child Neurol 49 (2007), doi: 10.1111/j.1469-8749.2007.00163.x(163-163). [DOI] [PubMed] [Google Scholar]
- [129].Dobson F, Morris ME, Baker R, Graham HK, Gait classification in children with cerebral palsy: a systematic review, Gait Posture 25 (2007) 140–152, doi: 10.1016/j.gaitpost.2006.01.003. [DOI] [PubMed] [Google Scholar]
- [130].Bottos M, Gericke C, Ambulatory capacity in cerebral palsy: prognostic criteria and consequences for intervention, Dev. Med. Child Neurol 45 (2003) 786–790, doi: 10.1111/j.1469-8749.2003.tb00890.x. [DOI] [PubMed] [Google Scholar]