Abstract
Background:
Obstetrical brachial plexus palsy is a common birth injury to nerves passing through the brachial plexus that may result in structural and functional abnormalities. Individual joint trajectories from kinematic analyses have been used to evaluate the source and extent of abnormalities. Here, two summary measures of limb kinematics were utilized: 1) the Arm Profile Score summarizing upper limb joint kinematic abnormalities from a typical pattern across a task, and 2) the recently developed Multi-joint Coordination Measure using principal component analysis to characterize typical coordination of multiple joints throughout a task and compute deviations in time and space. Our aim was to compare these kinematic measures in persons with and without injury and relate these to clinical and functional scales.
Methods:
3D kinematic data from 10 upper limb joints were collected on 15 children and adolescents with obstetrical brachial plexus palsy and 21 controls during a reach-to-grasp task in both limbs. The two kinematic measures were computed and correlated with each other and the Mallet and ABILIHAND-Kids.
Findings:
Both measures revealed that joint angles primarily contributing to shoulder and wrist motion were most prominently affected in the non-dominant limb in obstetrical brachial plexus palsy, with the Multi-joint Coordination Measure additionally indicating when in the motion coordination worsens. These were moderately interrelated but neither correlated with other scales.
Interpretation:
The Multi-joint Coordination Measure, while related to the Arm Profile Score, may have additional utility for individualized treatment planning and evaluation of any motor task due to the unique spatial-temporal information provided.
Keywords: Brachial plexus palsy, Arm Profile Score, Multi-joint Coordination Measure, Upper limb, Pediatric, Shoulder
1. Introduction
Obstetrical brachial plexus palsy (OBPP) is a peripheral nerve injury that occurs in approximately 1 to 4 of 1000 live births (Chauhan et al., 2014; Hoeksma et al., 2004) and is coincident to complications in utero or childbirth, e.g., macrosomia, shoulder dystocia, prolonged labor, and complicated deliveries (Dodds and Wolfe, 2000). OBPP may be sub-classified according to injury level; e.g., Erb’s palsy affecting C5-C6, extended Erb’s palsy affecting C5-C7, Klumpke’s palsy affecting C8-T1, and complete palsy affecting C5-T1, where each level innervates different muscle groups of the upper limbs and has different afferent input. While many infants with OBPP have transient injuries, at least 20% sustain permanent injuries leading to movement limitations and strength imbalances of the affected limb (Brochard et al., 2014) that may hinder activities of daily living such as grooming, dressing, feeding and other tasks (Dodds and Wolfe, 2000). Persistent neurological deficits may lead to secondary musculoskeletal impairments, such as scapular dysplasia, abnormal glenohumeral morphology, and eventual posterior shoulder dislocation, which further restrict upper limb coordination and function (Dodds and Wolfe, 2000; Pearl and Edgerton, 1998; Price et al., 2000; van Gelein Vitringa et al., 2013; Waters et al., 1998; Waters et al., 2009). Motor habilitation therapies are often prescribed in OBPP. However, determining the most effective interventions is often challenging due to variable recovery patterns that produce multi-joint and multi-planar impairments that disrupt functional movements in different ways across individuals.
Current clinical scales developed to systematically evaluate children with OBPP are useful in identifying the basic patterns and extent of involvement but provide limited information on specific joint kinematic contributions to a given movement. Measures such as the Narakas classification (Narakas, 1987) and Mallet score (Mallet, 1972), have been developed and validated mainly to evaluate passive and active range of motion of the impaired limb. As suggested by Mosqueda et al. (2004), 3-dimensional kinematic analysis enables clinicians to measure multi-planar functional limitations in the upper limbs and may provide valuable information for pre- and post-clinical evaluations in OBPP. Accordingly, previous kinematic studies of children with OBPP have evaluated individual joint trajectories, demonstrated a loss of active shoulder motion in the impaired limb (Mosqueda et al., 2004) due to abnormal scapulothoracic (ST) and glenohumeral (GH) joint contributions during movement when compared to their unimpaired dominant limb (Duff et al., 2007) or either limb in typically developing children (Russo et al., 2014). In addition, children with OBPP have shown increased variability in arm movements and inter-limb differences in arm resting position, which may relate to observed compensatory strategies (Duff et al., 2007; Mosqueda et al., 2004; Russo et al., 2014). However, these analyses focused on single joint trajectories rather than focusing on the coordination of multiple joint angles. Since activities of daily living depend on concurrent movement of many joints, novel kinematic methods that take into account multiple joint angles in their analysis may have greater clinical utility.
The Arm Profile Score (APS) (Jaspers et al., 2011) was developed to summarize multiple upper limb joint kinematic abnormalities averaged over the duration of the task of interest. While this measures the extent of involvement in individuals at a single point in time or before and after intervention, the APS does not provide information on when during the task that movement patterns deviate from a typical kinematic pattern. Recently, the Multi-joint Coordination Measure (MJCM) (Kukke et al., 2015) described by Kukke et al. was created to quantify abnormalities in time-varying multi-joint coordination patterns at each 1% of task duration. Additionally, it provides a visual as well as a quantitative depiction of which joints deviate the most from the typical pattern during different phases of the task for a single individual or averaged across a group, while providing a summary score of kinematic abnormalities across joints and time similar to the APS.
Our objective was to quantify multi-joint upper limb kinematics during a functional reach task in children and adolescents with unilateral OBPP at an individual and group level when compared to the uninvolved limb and to a group of participants within the same age range with no upper limb involvement. The reach task was selected because of its importance for performing activities of daily living (Butler et al., 2010). Both the APS and the MJCM, initially used in individuals with child onset brain injuries, were utilized and compared for the first time in this population, and their summary scores were related to other clinical and functional scales in OBPP. We hypothesized that individuals with OBPP would have significantly greater joint kinematic abnormalities as indicated by both the APS and the MJCM in the impaired limb compared to their contralateral limb and to both limbs in typically developing children and adolescents (TDCA). Moreover, because the same kinematic data were applied to the APS and MJCM, we hypothesized that these would be moderately or highly correlated. We also expected that the APS and MJCM would be related to the clinical and functional scales used in our study, based on the strong positive relationships found between the MJCM and functional scales in earlier work from our laboratory on individuals with child-onset brain injuries. Lastly, in terms of treatment planning or outcome assessment for an individual patient with a complex multi-joint injury, we hoped to demonstrate the greater utility of the MJCM since it provides detailed quantitative information across task time and joint space that is not available with the APS.
2. Methods
2.1. Participants
Fifteen children and adolescents with unilateral OBPP (10 male, 5 female) with a mean age of 11.5 years (SD 3.5) completed comprehensive evaluations of muscle and joint integrity and function. Inclusion criteria were: age 5–18 years inclusive, unilateral OBPP from birth, and ability to flex and abduct the impaired arm at least 30°. Exclusions were any other significant neurological or orthopedic impairment, surgery in the past year, botulinum toxin injections to upper limbs within 6 months, inability to follow verbal directions or too small to fit reliably and comfortably in the standardized testing set-up. A comparison group of 21 TDCA (10 male, 11 female) with a mean age of 11.8 (SD 2.7) with no history of musculoskeletal or neurological problems also participated. Written informed consent was obtained from parents of children under 18 years. Children 7 years or older also provided written assent. One participant who was 18 years old provided written consent. The protocol was approved by the institutional review board at the National Institutes of Health, Bethesda, MD.
2.2. Procedures
Medical histories were reviewed to confirm eligibility. A pediatric physiatrist performed a physical examination of joint passive range of motion in both upper limbs. The Mallet Scale and ABILHAND-Kids measure were conducted on the impaired (non-dominant) limb in OBPP to assess shoulder function and manual ability of the non-dominant limb, respectively.
The Mallet scoring system (Mallet, 1972) assesses shoulder abduction/external rotation limitations by evaluating active range of motion in shoulder abduction and external rotation, for tasks such as hand to top of head, hand to mouth, and back of hand to lower spine. Performance for each of the 5 motions was assigned a score from 1 (no motion) to 5 (normal range of motion), with a maximum total of 25. Lower scores (5) indicate more limited range of motion.
The ABILHAND-Kids (Arnould et al., 2004), a global measure of manual ability, was utilized to assess the level of disability and to relate kinematic impairments to functional limitations. It is a questionnaire, comprised of 21 manual activities. The parents of individuals with OBPP were ask to report perceptions of their child’s ability to perform the tasks on a 3-level rating scale: impossible (0), difficult (1), or easy (2). The participant aged 18 years reported perceptions of their own ability. Raw scores were used with higher scores (maximum = 44) indicating greater function, but these can also be converted to logits using a Rasch analysis.
For the reach-to-grasp, all participants were seated comfortably in an armless chair with their feet on the ground with ankle in neutral and the hips and knees flexed 90°. A cylindrical rod (6 in. height × 2 in. diameter) was placed on a table at elbow height and at a distance near but less than maximum reach for each individual. A reflective marker was placed on top of the rod to track its location. Joint coordinate system definitions and the placement of thirty-four reflective markers on the upper limbs and trunk were based on ISB-recommendations (Wu et al., 2005) with the exclusion of a clavicle marker. Additionally, we included a 4-marker cluster on the humerus for upper arm tracking, a 3-marker cluster on the acromion for scapular tracking, markers on the ulna distal to the olecranon and on the styloids for forearm tracking, and used functional calibration (Schwartz and Rozumalski, 2005) to estimate the shoulder joint center.
Three-dimensional motion of the markers was captured using a 10-camera, Vicon MX system (Vicon Motion Systems, Oxford, UK) at a rate of 100 Hz. The reach task began with the arm hanging freely at the participant’s side with the contralateral hand resting on the lap. After being instructed to begin, the subject reached towards the rod at a self-selected pace with the suspended hand, and the task ended when the hand grasped the rod. Three trials were collected per arm.
2.3. Data processing
Visual3D software (C-Motion, Inc., Germantown, MD, USA) was used to compute joint angles from marker locations. Due to computational guidelines (Streiner, 1994) and relevance to both our study population and the reach task, 10 joint angles were selected for application of both the APS and the MJCM: ST protraction/retraction, ST lateral/medial rotation, ST anterior/posterior tilt, GH elevation, GH plane of elevation, GH axial rotation, elbow flexion/extension, elbow pronation/supination, wrist flexion/extension, and wrist ulnar/radial deviation. A detailed illustration of ST and GH joint angles is depicted in Fig. 1. It is important to note that the shoulder model is often simplified to analysis of the humerus relative to trunk, but modeling the ST and GH joints provides a more accurate representation, e.g. ST lateral/medial rotation and GH elevation more precisely describe what is reported in the former model as humerothoracic abduction/adduction (Senk and Chèze, 2006).
Fig. 1.
Depiction of scapulothoracic (ST) (above) and glenohumeral (GH) (below) joint angles used for kinematic analysis. The columns represent the shoulder movements of (a) internal/external rotation, (b) abduction/adduction, and (c) flexion/extension that are primarily involved by the particular ST and GH joints in that column.
For each trial, the start of reach was defined as the time when the magnitude of the radial styloid marker velocity exceeded 0.05 m/s. Rod contact time was defined as the time when the minimum velocity (<0.05 m/s) of the radial styloid marker occurred. Video recordings of all trials were further reviewed to verify timing of events.
Kinematic data from the same 10 joint angles were used to compute the APS and MJCM. For the APS (Jaspers et al., 2011), kinematic data for all trials were normalized in time to allow comparison of task durations of different lengths. Time normalization was accomplished by re-sampling all kinematic data for each trial at 100 points spaced evenly between the start time and the time of rod contact. For each trial, the root mean square error between the point-by-point comparison of each joint angle in the dominant and non-dominant arms in all participants (OBPP and TDCA) with the mean of that same joint angle in the comparison database (data only from both limbs in TDCA) was calculated to create an Arm Variable Score for each joint in all subjects. The Arm Variable Score yields an index of kinematic abnormalities for a single joint angle and an APS is calculated as the mean of the 10 Arm Variable Scores for each trial. The results from each trial were then averaged to create a global APS for each participant which can be further be averaged across groups, as were done for this study.
For the MJCM (Kukke et al., 2015), kinematic data were normalized in time and amplitude. Time normalization was achieved in the same manner as with the APS. Amplitude normalization was accomplished by first subtracting the minimum value in each trajectory to reduce variability due to differences in initial postures between subjects. A typical movement filter was defined by applying a principal component analysis at each 1% of reach time for the 10 joint angles on all data from the dominant arm reach of the TDCA (3 trials for each of the 21 TDCA resulting in 63 total trials). The first 4 principal components accounted for approximately 87% of the total variance at every time point in the kinematic data and were used to summarize the time-varying typical movement pattern. Subsequently, all data from dominant and non-dominant limbs of all participants were transformed into this 4-dimensional principal component space and then projected back into the 10-dimensional kinematic space. The deviation at each 1% of reach time between the original kinematics and the filtered kinematics was computed for each joint angle, representing the kinematic error for that joint angle from the typical pattern. A threshold range per joint angle, calculated as the mean error plus or minus 3 standard deviations of the error in the dominant arms in TDCA, was used to determine whether a subject’s movement was atypical (outside the threshold range) or not (within the threshold range) at every 1% of the reach. Instances of atypical kinematics for each joint angle were then summed to create a score for each trial (atypical kinematics score) that ranged from 0 to 1, where 0 represents no abnormality at any 1% of reach time in any joint angle, and 1 represents abnormalities at every 1% of reach time in every joint angle. This was done for each participant’s limb and then all trials were averaged to create a global atypical kinematics score for each subject which can further be averaged across participant groups. Similarly, these global scores were used for this study. Ten-fold cross validation was performed and confirmed that the typical movement filter was not sensitive to the particular data set used to compute it.
2.4. Statistics
Data from the APS and MJCM were analyzed using SPSS 19 software (IBM Corporation, Armonk, NY, USA). A Mann-Whitney U test was used to compare the APS between groups (OBPP vs. TDCA), and a Wilcoxon signed rank test was used to compare the APS between arms within the group with OBPP. The same comparisons were performed for the MJCM. Bonferroni corrections were used for multiple comparisons with the level of significance set at P < 0.008. Correlation between the kinematic measures using data from dominant and non-dominant arms in OBPP and TDCA was performed using a Spearman rho coefficient (rs). The APS and the MJCM were correlated (Spearman’s rho) with the Mallet and ABILHAND-Kids in the group with OBPP only to explore the relationships between clinical and functional impairments and kinematic deficits during a reach task.
3. Results
In this convenience sample of individuals with Erb’s and Extended Erb’s palsy, all were able to complete the Mallet protocol (mean 15.1, SD 3.1), and had a parent rate their manual ability using the ABILHAND-Kids, except for the participant aged 18 years (mean 35.2, SD 5.1). Clinical testing was not performed for TDCA. Demographic and clinical data are shown in Table 1.
Table 1.
Characteristics of 15 children with obstetrical brachial plexus palsy (OBPP) and a summary of typically developing children and adolescents (TDCA).
| Subject number | Age (years) | Sex | Dom limb | Narakas classification | Mallet scale | ABILHAND Kids | Surgery group | Diagnosis |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| OBPP 1 | 13.99 | M | R | 3 | 19 | 42 | 1 | Erb’s |
| OBPP 2 | 11.89 | M | L | 3 | 18 | 38 | - | Erb’s |
| OBPP 3 | 13.3 | M | L | 3 | 15 | 36 | 1, 2 | Extended |
| OBPP 4 | 18.78 | M | L | 3 | 20 | 41 | - | Erb’s |
| OBPP 5 | 9.23 | M | R | 3 | 18 | 37 | - | Extended |
| OBPP 6 | 5.39 | F | R | 4 | 12 | 29 | - | Extended |
| OBPP 7 | 9.23 | F | L | 3 | 16 | 34 | 1, 2 | Extended |
| OBPP 8 | 13.62 | M | L | 2 | 16 | 31 | - | Erb’s |
| OBPP 9 | 6.69 | F | L | 3 | 15 | 31 | - | Erb’s |
| OBPP 10 | 12.97 | M | R | 3 | 14 | 36 | 1, 2, 3 | Extended |
| OBPP 11 | 13.37 | M | L | 3 | 14 | 36 | 1, 2 | Extended |
| OBPP 12 | 8.1 | M | L | 3 | 11 | 32 | - | Extended |
| OBPP 13 | 8.94 | F | L | 2 | 17 | 24 | - | Erb’s |
| OBPP 14 | 11.48 | F | R | 2 | 13 | 42 | - | Erb’s |
| OBPP 15 | 15.22 | M | R | 4 | 9 | 39 | 1, 2 | Extended |
| OBPP (n = 15) | 11.5(SD 3.5) | 10M, 5F | 6R, 9L | 2.9(SD 0.6) | 15.1(SD 3.1) | 35.2(SD 5.1) | - | - |
| TDCA (n = 21) | 11.5(SD 3.5) | 10M, 11F | 18R, 4L | - | - | - | - | - |
1 = lengthening or release; 2 = muscle transfers; 3 = acromioplasty.
All participants were able to complete the reach-to-grasp task, but a small number of trials had to be discarded from analysis due to marker occlusion during movement. Fig. 2 shows the original trajectories of the 10 joint angles in the upper limb for the dominant and non-dominant sides prior to application of both the APS and MJCM. This illustrates that trajectories of the dominant limb in children with OBPP generally appear similar to those of both limbs in TDCA while the non-dominant limb trajectories in OBPP deviated from their own dominant limb and both limbs in TDCA to a larger extent.
Fig. 2.
Original kinematic trajectories of 10 upper limb joint angles in dominant and non-dominant limbs during reach prior to the application of the APS and MJCM. Mean (and standard deviation) kinematics of TDCA are represented by gray shaded area. Mean trajectories of children with OBPP are shown by solid lines.
3.1. Intra-limb coordination during reach-to-grasp
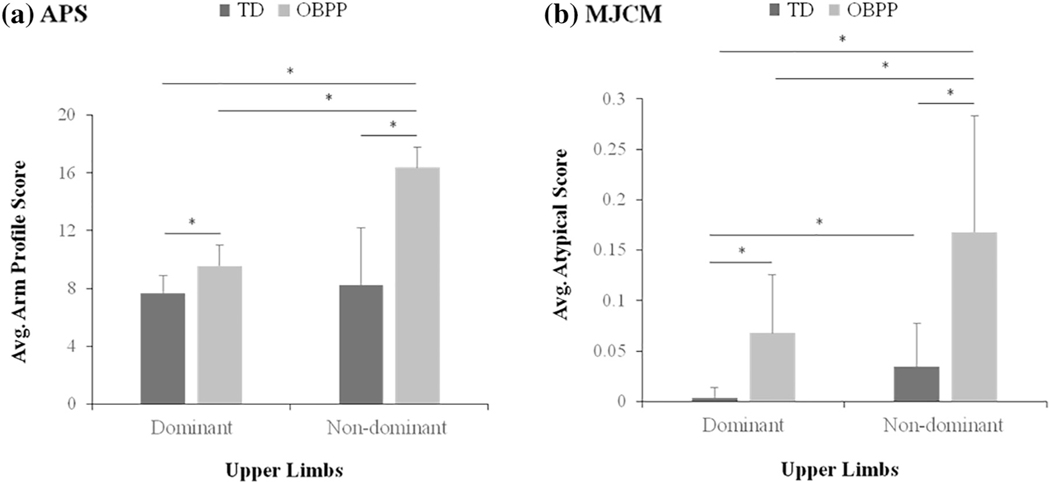
Group comparisons of the APS (a) and MJCM (b) are shown in Fig. 3. For the APS, the non-dominant limb in OBPP was significantly different than both arms of TDCA (P < 0.001) and their contralateral limb (P = 0.001). For the dominant arm in OBPP, a significant difference was found between the dominant arm in TDCA (P < 0.001) but slightly missed the threshold for significance in the non-dominant arm in TDCA (P = 0.008). Dominant and non-dominant arms in TDCA demonstrated no significant difference (P = 0.12).
Fig. 3.
Group comparisons of the APS (a) and the MJCM (b) for the reach-to-grasp task with the dominant and non-dominant arms.
For the MJCM, the non-dominant limb in OBPP is significantly different from both arms of TDCA (P < 0.001) and their contralateral limb (P = 0.006). The dominant arm in OBPP differed from the dominant arm in TDCA (P < 0.001) but not from the non-dominant arm in TDCA (P = 0.10). However, in contrast to the APS, there was a significant difference between the dominant and non-dominant arms in TDCA (P = 0.003).
A positive correlation was found between the APS and MJCM, in the dominant arms (rs = 0.43, P = 0.008) and non-dominant arms (rs = 0.53, P = 0.001) in the whole data set (OBPP and TDCA). Because the non-dominant arm in OBPP is used for the Mallet and ABILHAND-Kids, the APS and MJCM for the non-dominant arm in OBPP was used for these correlations. None were found between either kinematic measures or clinical and functional scales.
3.2. Timing and occurrence of abnormalities at group and individual levels
The MJCM differs from the APS in that it provides an indication of abnormal coordination at each 1% of time during an entire movement that can be visually as well as quantitatively interpreted. Fig. 4 presents the percentage of subjects in each cohort with atypical kinematics for each joint angle at every 1% of the reach-to-grasp task for both arms at a group level. These data represent the error calculated between the original kinematics and the filtered data, which indicates when a particular degree of freedom diverges from the typical reaching pattern. On the non-dominant side in OBPP, divergence from the typical pattern was most prominent in the ST lateral/medial rotation and GH plane of elevation joint trajectories in about 75% of individuals with OBPP, occurring during approximately the last 20% and 5% of the reach, respectively. Abnormalities were also prevalent in ST anterior/posterior tilt during 25–30% of the reach in about 60% of those with OBPP. Other abnormalities were found in wrist flexion/extension and scapulothoracic protraction/retraction in about 40–50% of individuals with OBPP during mid-reach.
Fig. 4.
Percentage of subjects with atypical kinematics at every 1% of the reach-to-grasp task for each joint angle. ST Pro; M-L; A-P = scapulothoracic protraction/retraction, scapulothoracic lateral/medical rotation, and scapulothoracic anterior/posterior tilt. GH Elev; PoE; Rot = glenohumeral elevation, glenohumeral plane of elevation, and glenohumeral axial rotation. Elb Flex; Pro = elbow flexion/extension and elbow pronation/supination. Wrist Flex; Dev = wrist flexion/extension and wrist ulnar/radial deviation.
Data from the MJCM can also be used to derive atypical kinematic scores and profiles for individuals with OBPP, as shown in Fig. 5 in order of decreasing atypical kinematic scores with higher values corresponding to greater divergence from the typical movement pattern.
Fig. 5.
Increasing to decreasing atypical kinematic scores (a) and kinematic profiles (b) for individual children with OBPP. Yellow indicates atypical kinematics. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
This study evaluated kinematic profiles of the dominant and non-dominant upper limbs in individuals with OBPP and TDCA, using the APS and the MJCM to assess intra-limb kinematic abnormalities and inter-joint coordination. Initially used in individuals with unilateral child-onset brain injuries, these methods were similarly effective in distinguishing individuals with peripheral injuries from TDCA. Kinematic findings from this study confirm the clinical observation that children with OBPP display notably more atypical kinematic patterns in their impaired limb than their contralateral limb and either limb in TDCA. These may be the result of both the initial injury as well as secondary musculoskeletal deformities related to OBPP. It has been proposed that atrophy and non-physiologic imbalance of muscles observed in OBPP alter shoulder stability (Duff et al., 2007), leading to possible obligatory resting postures and habitual use patterns that may contribute to the progression of skeletal deformities (Poyhia et al., 2005; Waters, 1997).
Results from the MJCM further indicated that there was no mean kinematic difference between the dominant limb in individuals with OBPP and the non-dominant limb in TDCA. It has been previously reported that a shift in arm dominance may occur in this population after injury (Anand and Birch, 2002). Approximately, 90% of the general population prefer the use of their right limb (Yang et al., 2005). Interestingly, our sample showed that 81% of controls preferred their right limb, while only 40% of those with OBPP were right dominant, and in all cases, the self-reported dominant side was the non-impaired side. It has also been reported that more infants are affected on their right side due to more frequent obstetrical presentation of the left occiput anteriorly, increasing the risk of brachial plexus injuries on the right (Zafeiriou and Psychogiou, 2008). It is unknown which limb would have been dominant if injury had not occurred, but based on previous studies it is likely that some of our subjects shifted limb dominance to the non-injured hand and most likely in the case of right arm injury due to the greater prevalence of right-handedness.
Our results also supported the hypothesis that the APS and the MJCM would be correlated with one another. The fair to moderate correlation observed between the APS and MJCM is most likely due to the fact that the same upper limb kinematics were inputs for both measures. However, their methodologies are fundamentally different and may have reduced the strength of the correlation. As described previously, the APS assesses single joint kinematic abnormalities by comparing these joints to the average for that same joint in a control group and then averaging all single joint abnormalities to create a global score over time. On the other hand, the MJCM is unique in that divergence of each degree of freedom from a time-varying pattern across all joint trajectories is quantified. Also, some differences may be due to the fact that the MJCM was computed based on only 87% of the variance explained whereas the APS included 100% of the kinematic data.
The MJCM and the APS are sensitive to different aspects of movement, and may be complementary tools. The MJCM provides an indication of multi-joint coordination that is not apparent with a joint-by-joint kinematic analysis such as the APS. This may account for the lack of difference in APS between the dominant and non-dominant arms of the TDCA, while the MJCM indicated there was a difference (Fig. 3). The MJCM uniquely focuses on the temporal-spatial relationships of all of the joints considered together during task performance. Therefore, the choice of measurement should be based whether the research or clinical question is related more to coordination (MJCM) or joint angle kinematic abnormalities (APS).
Unexpectedly, our results did not support the hypothesis that the APS and the MJCM would be correlated with the Mallet and ABILHAND-Kids measures. Although functional reach is essential to performing activities of daily living, the clinical and functional scales utilized in this study interestingly do not incorporate reach tasks within their assessments. The Mallet assesses the performance of 5 tasks which do not include reach-to-grasp, and the ABILHAND-Kids examines the performance of 21 tasks, which are primarily comprised of fine motor activities. Because the APS and the MJCM can be used for any motion, it is possible that a relationship could have been observed if similar tasks were performed in this study as used in the Mallet or ABILHAND-Kids.
The group level kinematic findings from the MJCM support the typical clinical presentation of Erb’s and extended Erb’s Palsy, which according to Dodds and Wolfe (Dodds and Wolfe, 2000), commonly affect the external rotators and abductors of the shoulder, elbow flexors, forearm supinators, and extensors of the wrist. Similarly, our results indicate that our group with OBPP was affected in the same joint angles except for elbow flexion and supination. According to our protocol, the arm was to freely hang at the side prior to reaching, a position that does not require appreciable forearm pronation or supination. Interestingly, many participants with OBPP appeared not to flex their elbow during the task with their impaired limb and as seen on video review, for most of the task the elbow remained extended as it was in the rest position. When initiating movement most subjects used shoulder abduction to move around the side of the table to bring the hand into position to grasp the rod. The group with OBPP also employed greater scapular motion in their impaired arm as previously suggested (Duff et al., 2007) to complete the task. The group level analysis performed here was to determine whether the MJCM could capture a typical clinical picture across subjects with a similar type of peripheral nerve injury.
In this study, the kinematic analyses at an individual basis was more of interest due to the variability seen in recovery patterns across individuals, which may require different treatments. As shown by Fig. 5, the unique spatial and temporal information provided by the MJCM kinematic profiles, not available with the APS, draws attention to the time that specific degrees of freedom become uncoupled from the other degrees of freedom which may help to direct intervention. For example, the child labeled “BPP10” presumably has functional impairments during shoulder flexion/extension due to the abnormal pattern in ST anterior/posterior tilt for the majority of the task. This subject may benefit from a rehabilitation program aimed primarily to improve the contribution of this degree of freedom to the reach-to-grasp movement. In addition, in future studies or clinical evaluations, these kinematic profiles could serve as outcome measures to evaluate the effects of specific interventions or to track changes over time. As seen in the most abnormal kinematic profile from our sample, labeled “BPP12”, nearly every degree of freedom during the latter half of the reaching task is controlled in an atypical manner. Therefore, it may be difficult to determine a specific target for the rehabilitation program for this individual; however, whatever approach was chosen, this analysis could capture the change in coordination after treatment to observe if and where improvements may have occurred.
From these data and analysis methods, it is not clear to what extent the kinematic abnormalities quantified are due to the primary impairments or to secondary changes in musculoskeletal integrity such as contracture or to compensatory movements or the extent to which kinematic abnormalities were no longer evident because of surgical or other interventions. In studies evaluating the result of surgical correction for elbow (Senes et al., 2016) and shoulder (Ozben et al., 2011) contractures, improved function in elbow flexion, shoulder abduction and external rotation were achieved. In our sample with OBPP, 6 of 15 participants had prior surgery which may have influenced the group summary results. However, this does not diminish the utility of the APS or MJCM for quantifying kinematic deficits across groups or individuals since these measures do not define the disorder but rather quantify the current motor performance of individuals with OBPP regardless of intervention. A limitation here was that subgroup (surgical versus non-surgical) sizes were too small to evaluate separately given the methodological constraints, but this could be done in future studies if these group comparisons are of major interest. Another limitation of the methods used is that abnormalities found in the kinematic profiles are absolute deviations from typical patterns, so to identify the direction of error; e.g., excess elbow flexion or extension, the unfiltered trajectory (elbow flexion/extension) would have to be verified on the individual joint kinematic plots. Other recommendations for future work would be to expand task selection, perhaps including classic tasks from the Mallet or specific activities from the ABILHAND-Kids, which may show greater relationships with kinematic measures.
5. Conclusions
The APS and the MJCM demonstrated novel utility here in a peripheral nerve injury, validating their use in different upper limb pathologies. Both could discriminate individuals with OBPP from TDCA, with perhaps slightly greater sensitivity in the MJCM since it further differentiated the dominant and non-dominant arms of TDCA. The APS or MJCM could be useful for assessing the functional impact or outcomes of invasive interventions or therapy, with the MJCM additionally able to assess inter-joint coordination and timing. The MJCM may provide valuable information for lower limb pathologies due to the measure’s unique ability to evaluate the coordination of any type and number of joints during any functional task.
Acknowledgment
This work was funded by the Intramural Research Program of the National Institutes of Health, Clinical Center, Bethesda, MD, USA (Protocol 90-CC-0168) and the Undergraduate Scholarship Program of the Office of Intramural Training and Education at the National Institutes of Health. Sylvain Brochard was also funded by the University Hospital of Brest the French Society of Physical Medicine and Rehabilitation (SOFMER) and the French Society of Research in Children with Disabilities (SFERHE). In addition, we would like to thank Laurie Ohlrich and Cristiane Zampieri-Gallagher for their help in data collection and Frances Sheehan for her 3D illustrations of the shoulder joints to describe the rotations.
References
- Anand P, Birch R, 2002. Restoration of sensory function and lack of long-term chronic pain syndromes after brachial plexus injury in human neonates. Brain 125 (Pt 1), 113–122 (Jan). [DOI] [PubMed] [Google Scholar]
- Arnould C, Penta M, Renders A, Thonnard JL, 2004. ABILHAND-Kids: a measure of manual ability in children with cerebral palsy. Neurology 63 (6), 1045–1052 (Sep 28). [DOI] [PubMed] [Google Scholar]
- Brochard S, Alter K, Damiano D, 2014. Shoulder strength profiles in children with and without brachial PLEXUS PALSY. Muscle Nerve 50 (1), 60–66 (Jul). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EE, Ladd AL, Lamont LE, Rose J, 2010. Jul. Temporal-spatial parameters of the upper limb during a Reach & Grasp Cycle for children. Gait Posture 32 (3), 301–306. [DOI] [PubMed] [Google Scholar]
- Chauhan SP, Blackwell SB, Ananth CV, 2014. Neonatal brachial plexus palsy: incidence, prevalence, and temporal trends. Semin. Perinatol 38 (4), 210–218 (Jun). [DOI] [PubMed] [Google Scholar]
- Dodds SD, Wolfe SW, 2000. Perinatal brachial plexus palsy. Curr. Opin. Pediatr 12 (1), 40–47 (Feb). [DOI] [PubMed] [Google Scholar]
- Duff SV, Dayanidhi S, Kozin SH, 2007. Jul. Asymmetrical shoulder kinematics in children with brachial plexus birth palsy. Clin. Biomech 22 (6), 630–638. [DOI] [PubMed] [Google Scholar]
- van Gelein Vitringa VM, van Royen BJ, van der Sluijs JA, 2013. Scapular deformity in obstetric brachial plexus palsy and the Hueter-Volkmann law; a retrospective study. BMC Musculoskelet. Disord 14, 107 (Mar 22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeksma AF, ter Steeg AM, Nelissen RG, van Ouwerkerk WJ, Lankhorst GJ, de Jong BA, 2004. Neurological recovery in obstetric brachial plexus injuries: an historical cohort study. Dev. Med. Child Neurol 46 (2), 76–83 (Feb). [DOI] [PubMed] [Google Scholar]
- Jaspers E, Feys H, Bruyninckx H, Klingels K, Molenaers G, Desloovere K, 2011. Jun. The Arm Profile Score: a new summary index to assess upper limb movement pathology. Gait Posture 34 (2), 227–233. [DOI] [PubMed] [Google Scholar]
- Kukke S, Curatalo L, de Campos A, Hallett M, Alter K, Damiano D, 2015. Coordination of reach-to-grasp kinematics in individuals with childhood-onset dystonia due to hemiplegic cerebral palsy. IEEE Trans. Neural Syst. Rehabil. Eng 24, 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet J, 1972. Obstetrical paralysis of the brachial plexus. II. Therapeutics. Treatment of sequelae. Priority for the treatment of the shoulder. Rev. Chir. Orthop. Reparatrice Appar. Mot 58 (Suppl. 1), 166–168 French. [PubMed] [Google Scholar]
- Mosqueda T, James MA, Petuskey K, Bagley A, Abdala E, Rab G, 2004. Kinematic assessment of the upper extremity in brachial plexus birth palsy. J. Pediatr. Orthop 24 (6), 695–699 (Nov–Dec). [DOI] [PubMed] [Google Scholar]
- Narakas AO, 1987. Obstetrical brachial plexus injuries. In: Lamb DW (Ed.), The Paralysed Hand. Churchill Livingstone, Edinburgh, pp. 116–135. [Google Scholar]
- Ozben H, Atalar AC, Bilsel K, Demirhan M, 2011. Transfer of latissmus dorsi and teres major tendons without subscapularis release for the treatment of obstetrical brachial plexus palsy sequela. J. Shoulder Elb. Surg 20 (8), 1265–1267 (Dec). [DOI] [PubMed] [Google Scholar]
- Pearl ML, Edgerton BW, 1998. Glenoid deformity secondary to brachial plexus birth palsy. J. Bone Joint Surg. Am 80 (5), 659–667 (May) Erratum in: J. Bone Joint Surg. Am. 1998 Oct; 80(10):1555–9. [DOI] [PubMed] [Google Scholar]
- Poyhia TH, Nietosvaara YA, Remes VM, Kirjavainen MO, Peltonen JI, Lamminen AE, 2005. MRI of rotator cuff muscle atrophy in relation to glenohumeral joint incongruence in brachial plexus birth injury. Pediatr. Radiol 35 (4), 402–409. [DOI] [PubMed] [Google Scholar]
- Price A, Tidwell M, Grossman JA, 2000. Improving shoulder and elbow function in children with Erb’s palsy. Semin. Pediatr. Neurol 7 (1), 44–51 (Mar). [DOI] [PubMed] [Google Scholar]
- Russo SA, Kozin SH, Zlotolow DA, Thomas KF, Hulbert RL, Mattson JM, Rowley KM, Richards JG, 2014. Mar. Scapulothoracic and glenohumeral contributions to motion in children with brachial plexus birth palsy. J. Shoulder Elb. Surg 23 (3), 327–338. [DOI] [PubMed] [Google Scholar]
- Schwartz MH, Rozumalski A, 2005. Jan. A new method for estimating joint parameters from motion data. J. Biomech 38 (1), 107–116. [DOI] [PubMed] [Google Scholar]
- Senes FM, Catena N, Dapelo E, Senes J, 2016. Correction of elbow flexion contracture by means of olecranon resection and anterior arthrolysis in obstetrical brachial plexus palsy sequelae. J. Pediatr. Orthop B 7 (Apr). [DOI] [PubMed] [Google Scholar]
- Senk M, Chèze L, 2006. Rotation sequence as an important factor in shoulder kinematics. Clin. Biomech (Bristol, Avon)(Suppl. 21) 1:S3–8. [DOI] [PubMed] [Google Scholar]
- Streiner DL, 1994. Figuring out factors: the use and misuse of factor analysis. Can. J. Psychiatr 39 (3), 135–140 (Apr). [DOI] [PubMed] [Google Scholar]
- Waters PM, 1997. Obstetric brachial plexus injuries: evaluation and management. J.Am. Acad. Orthop. Surg 5, 205–214. [DOI] [PubMed] [Google Scholar]
- Waters PM, Smith GR, Jaramillo D, 1998. Glenohumeral deformity secondary to brachial plexus birth palsy. J. Bone Joint Surg. Am 80 (5), 668–677 (May). [DOI] [PubMed] [Google Scholar]
- Waters PM, Monica JT, Earp BE, Zurakowski D, Bae DS, 2009. Correlation of radiographic muscle cross-sectional area with glenohumeral deformity in children with brachial plexus birth palsy. J. Bone Joint Surg. Am 91 (10), 2367–2375 (Oct). [DOI] [PubMed] [Google Scholar]
- Wu G, van der Helm FC, Veeger HE, Makhsous M, Van Roy P, Anglin C, Nagels J, Karduna AR, McQuade K, Wang X, Werner FW, Buchholz B, International Society of Biomechanics, 2005. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion—part II: shoulder, elbow, wrist and hand. J. Biomech 38 (5), 981–992 (May). [DOI] [PubMed] [Google Scholar]
- Yang LJ, Anand P, Birch R, 2005. Limb preference in children with obstetric brachial plexus palsy. Pediatr. Neurol 33 (1), 46–49 (Jul). [DOI] [PubMed] [Google Scholar]
- Zafeiriou DI, Psychogiou K, 2008. Apr. Obstetrical brachial plexus palsy. Pediatr. Neurol 38 (4), 235–242. [DOI] [PubMed] [Google Scholar]