Abstract
Juvenile psammomatoid ossifying fibroma (JPOF) is a rare, benign type of ossifying fibroma. JPOFs predominantly present as rapidly growing masses with a high recurrence rate. We report a 40-year-old male patient who suffered from a large tumor with multiple invasions into the paranasal sinuses. Total excision was performed, and significant relief of clinical symptoms was recorded after 4 months of follow-up. Multi-departmental management involving radiologists, neurology surgeons, craniofacial surgeons, pathologists, and otolaryngologists is vital for JPOF treatment. First-line treatment options include total or partial resection, depending on the patient's condition.
Keywords: Juvenile psammomatoid ossifying fibroma, Paranasal sinuses, Computed tomography, Magnetic resonance imaging
Introduction
Ossifying fibroma (OF) is a rare benign fibrous lesion first described by Menzel in 1872 [1]. Typical OFs are characterized by various amounts of calcified tissue, such as bone or cementum, on the fibrous stroma. According to the 2017 World Health Organization (WHO) classification of head and neck tumors, OFs can be categorized as cemento-OFs, juvenile trabecular OFs (JTOFs), and juvenile psammomatoid OFs (JPOFs) [2].
JPOFs are commonly diagnosed in individuals younger than 15 years, with an incidence greater than 79%, but JPOFs can also occur in adults. The vast majority of these neoplasms are encountered in the paranasal sinuses and the maxillary, mandibular, orbital, and frontal bones [3]. A definitive diagnosis of JPOF is often based on clinical manifestations, histological examinations, and imaging features. Although most JPOFs are asymptomatic, rapidly growing JPOFs can induce asymmetry in the face or dental symptoms, particularly in cases of lower jaw lesions [4]. Histopathologically, JPOFs are documented as hyperdense masses with fibrous proliferation and psammoma bodies. Lesion locations and density can be identified using computed tomography (CT), which can also provide information on adjacent bone injuries. Surgery is the first-line option for JPOF treatment due to the invasive nature of these neoplasms, but minimally invasive surgery can be applied in some situations. We describe a case of JPOF identified in the paranasal sinuses.
Case presentation
A 40-year-old male patient with a history of tumor in his left orbit (first identified 20 years prior) complained of a persistent headache within the past month. On physical examination, the patient was conscious, with left eye exophthalmos and blurred vision. No motor paralysis, sensory disorder, or infective signs were documented. Complete blood count, electrolytes, and blood biochemistry were all within normal limits.
Pre- and post-contrast CT images (Fig. 1) revealed a 76 × 60 × 57 mm mass that originated in the left maxillary sinus and spread upward into the ethmoid and bilateral frontal sinuses. This lesion was characterized by a thick capsule mass with several “ground-glass” nodules, central fluid intensity, and no post-contrast enhancement. No invading signs were detected, but nasal septal deviation, left frontal compression, and 5-mm midline displacement were reported due to a large mass effect.
Fig. 1.
Axial pre- and post-contrast computed tomography (CT) images. Pre-contrast CT scans on the bone window (A) and the soft-tissue window (C) demonstrated a paranasal lesion with a thick capsule and multiple internal “ground-glass” nodules (arrows). Central hypodensity (*) and a well-defined margin were noticed (open arrows). Post-contrast CT scans on the bone window (B) and the soft tissue window (D) showed no enhancement.
On magnetic resonance imaging (MRI; Fig. 2), the central fluid area was homogenously hyperintense on T2-weighted (T2W) and fluid-attenuated inversion recovery (FLAIR) sequences, with no restriction on diffusion and multiple internal septa. The cortex displayed an isointense or hypointense signal in the T1-weighted (T1W) sequence and hypointense signals in the T2W and T2* sequences. Post-contrast imaging showed vivid septal and ring-like enhancement.
Fig. 2.
Pre- and post-gadolinium magnetic resonance imaging (MRI). (A) Coronal T2-weighted (T2W), (C) sagittal T1-weighted (T1W), and (E) axial fluid-attenuated inversion recovery sequences demonstrated a paranasal mass with a hypointense cortical area (arrows) and a fluidized, hyperintense center area (*). A well-defined margin with an adjacent bone structure and soft tissue were also noted. (B) Post-gadolinium coronal T1W, (D) post-gadolinium sagittal T1W, and (F) post-gadolinium axial T1W sequences demonstrated ring-like and septal enhancement. G) Axial diffusion-weighted imaging and (H) axial apparent diffusion coefficient showed no restricted diffusion.
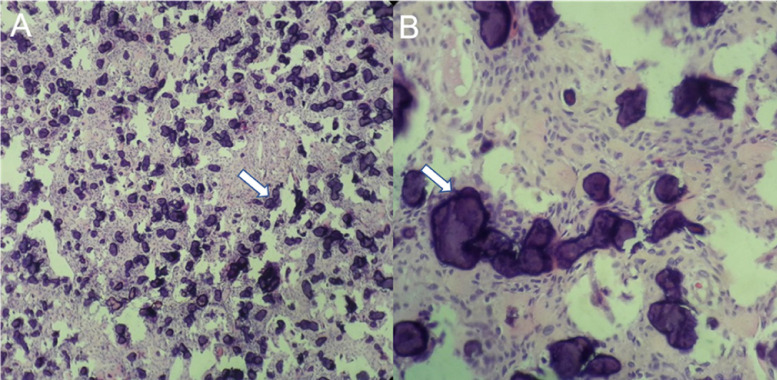
The patient underwent brain surgery for tumor resection. Macroscopic examination showed a large angiogenic cyst, which was highly suggestive of an inflammatory lesion. On microscopic examination (Fig. 3), this neoplasm consisted of large cytoplasmic spindle cells arranged in rafts with interspersed psammomatoid bodies. Fibrous and collagen stroma without tumoral necrosis was documented.
Fig. 3.
Microscopic histopathologic images. (A) A 10× magnification image showed large cytoplasmic spindle cells arranged in rafts with interspersed psammomatoid bodies interspersed (arrows). (B) A 40× magnification image showing psammomatoid bodies (arrows).
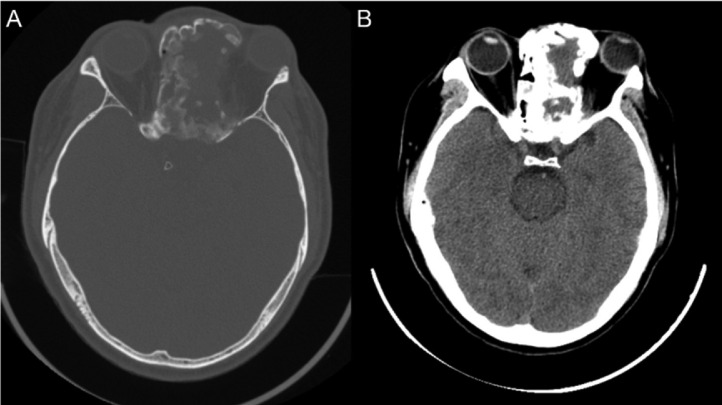
Four months after surgery, mild headache and left visual disturbance were noted, with no motor paralysis or sensory disorder. Post-surgical CT imaging (Fig. 4) revealed “ground-glass” in the lesion cortex, suggesting residual lesions after surgery.
Fig. 4.
Post-surgical computed tomography (CT) images. (A) Bone window and (B) soft tissue window showed significant size reductions, correlated with clinical symptom relief.
Discussion
According to the 2017 WHO classification of head and neck tumors, OFs are typically detected as rapidly growing masses most often encountered in young children (5-10 years) but can sometimes occur in adults [5]. JPOFs are considered a separate entity from other OFs due to their aggressive characteristics, the likelihood of encountering these lesions in children, and an equal distribution between sexes [6]. In the majority of JPOF cases, the neoplasms originate from the paranasal sinuses or the maxilla, mandible, orbit, or frontal bone [3]. Patients diagnosed with JPOF generally complain of exophthalmos, visual disturbances, headache, nasal congestion, recurrent sinusitis, tooth loss, and massive facial masses, particularly during later stages [3]. In this case, the patient suffered from exophthalmos for 20 years but only came to the hospital when left visual disturbance occurred.
JPOFs share several overlapping features with other fibrotic neoplasms; therefore, a definitive JPOF diagnosis should rely on a concrete triad, including clinical symptoms, radiologic characteristics, and pathological evidence. Macroscopically, JPOFs are characterized as firm or hard masses with a brownish-yellow color and a well-defined border relative to the surrounding bone. Microscopically, JPOFs consist of psammoma bodies, which are concentric or lamellar ossicles. Osteoblasts can also be observed in these lesions [7].
Radiologic modalities, such as MRI and CT, are helpful for identifying tumor invasion and extension. JPOFs predominantly behave as solitary, lobulated masses with well-defined borders and no soft tissue invasion. Owosho et al. [8] described JPOF CT features, including a thick capsule with a hypointense center, a solitary ground-glass nodule, or an isointense solid region [9]. The psammoma bodies within these lesions can also present with a ground-glass appearance. The findings for this case were similar to the findings described in previous reports.
On MRI, the bony shell was isointense on T1W sequences and hypointense on T2W sequences. The enhancing characteristics of this bony shell suggested tumor tissue rather than hyperostosis [10]. Solid tumor components usually appear as isointense on T1W sequences and as isointense or hypointense with a central hyperintense region on T2W sequences [[11], [12]]. Post-contrast imaging typically reveals homogeneous enhancement in solid areas and the peripheral rim, in addition to septal enhancement in cystic regions [11].
JPOFs with secondary aneurysmal bone cysts (ABCs) have been rarely reported in the literature, associated with 14.8% of JPOFs detected in the maxilla and mandible. Most ABCs have been associated with recurrent JPOFs and not with primary lesions [12]. Secondary ABCs may originate from intra-lesional alternative stroma development or intra-lesional vascular malformation, resulting in the formation of expanding bone cysts. The presentation of secondary ABCs indicates a poor prognosis, as ABCs are highly aggressive with a high likelihood of recurrence [12]. In this case, the neoplasm consisted of a primary JPOF with no evidence of secondary ABC, which may suggest a low probability of recurrence.
In general, JPOFs should be distinguished from other expanding bony lesions, including fibrous dysplasia (FD), JTOF, mucocele, osseous dysplasia, and other OFs. The long list of differential diagnoses can be excluded based on the identification of peripheral margins, ground-glass lesions, and tumor location [13]. However, a ground-glass appearance may also indicate an FD lesion, and the differential diagnosis between FD and JPOF should rely on other features. For example, FD tends to expand with ill-defined margins, whereas JPOF is well-circumscribed. Furthermore, FDs tend to preserve the overall configuration of the involved bone, whereas JPOFs develop a spherical configuration. In JTOFs, the ground-glass appearance is typically absent, and the internal structure is commonly radiolucent with scattered calcifications [8]. Therefore, the identification of a ground-glass appearance can be used to rule out JTOF, other OFs, and mucocele. Mucoceles present as large, well-defined lesions that expand into the surrounding soft tissues. Lesion location can also be helpful for distinguishing between entities. JTOFs typically arise from the maxilla or mandible, whereas osseous dysplasias are often found in the periapical region, and mucoceles originate in the nasal sinuses [14]. Osseous dysplasias and OF have mixed structures, and psammomatous bodies may not be found in JTOF [8]. JPOFs tend to occur in children, requiring consideration of other aggressive bone tumors identified in children, such as chondrosarcoma and Ewing sarcoma [13], particularly as these conditions require different treatment from JPOFs.
In this case, CT imaging identified a nasal sinus mass, and ground-glass appearances were detected in both central and peripheral regions, with no signs of bony invasion. MRI highlighted the capsule and septal post-contrast enhancement without fluid–fluid levels indicative of secondary ABCs. These findings were indicative of a low-risk JPOF case. Due to the potential for rapid growth and recurrence, total resection is the preferred first-line treatment method for JPOFs [15]. However, total resection may be challenging for both cosmetic and functional reasons. Depending on the lesion characteristics, surgery can minimally be performed endoscopically, which can preserve several crucial surrounding structures, especially in children. However, repeat surgery may be necessary in cases of recurrence [15].
No consensus recommendations exist regarding first-line surgical methods or the timing of postoperative defect reconstruction, but autologous bone grafts are considered the current gold standard reconstruction option. Autologous bone grafts provide stabilizing structures, can be used to reconstruct bony defects, and facilitate bony healing. A combination of osteoconductive, osteoinductive, and osteogenic effects has been identified with the use of autologous bone grafts [15].
The postoperative recurrence rate of JPOFs ranges from 30% to 56%. No aggressive complications have been published in the medical literature. Despite the low recurrence rate for JPOFs, postoperative recurrence was confirmed in our case.
Because of several overlapping clinical symptoms, X-ray features, and histopathological behaviors between JPOFs and other benign fibrosis lesions, a thorough understanding of JPOFs is important for facilitating diagnostic accuracy, establishing an effective treatment plan, and improving prognosis.
Conclusion
JPOFs are typically identified at a young age and characterized as rapidly growing masses with a high recurrence rate and intranasal expansion. Important imaging features include the identification of calcifying areas and cystic necrosis. Microscopic examination reveals dense fibroblastic stroma interspersed with areas of ossification (psammoma bodies). The differential diagnosis includes FD, JTOF, mucocele, and other types of OFs. Multi-departmental management is vital, including radiologists, neurology surgeons, craniofacial surgeons, pathologists, and otolaryngologists. The first-line treatment options include total or partial resection, depending on the condition of the patient. Prognosis is generally good in the majority of cases. No metastasis has been documented in the literature.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Authors’ contributions
Nguyen NT and Nguyen DH contributed equally to this article as co-first authors. All authors read and approved final version of this manuscript.
Ethics approval
Not applicable.
Patient consent
Written informed consent was obtained from the patient for the publication of patient information in this article.
Footnotes
Funding: No funding was received.
Competing Interests: The authors do not report any conflicts of interest.
References
- 1.Mohsenifar Z, Nouhi S, Abbas FM, Farhadi S, Abedin B. Ossifying fibroma of the ethmoid sinus: report of a rare case and review of literature. J Res Med Sci. 2011;16(6):841–847. [PMC free article] [PubMed] [Google Scholar]
- 2.El-Naggar A.K., Chan J.K.C., Grandis J.R., Takata T., Slootweg P.J. 4th ed. 2017. WHO classification of head and neck tumours; pp. 251–253. [Google Scholar]
- 3.Diniz JA, Siqueira ADS, Araújo GM, Faro TF, Torres LHS, Oliveira E Silva ED, et al. Intraoral Approach for surgical treatment of psammomatoid juvenile ossifying fibroma. J Craniofac Surg. 2020;31(3):e306–e309. doi: 10.1097/SCS.0000000000006171. [DOI] [PubMed] [Google Scholar]
- 4.Gantala R, Vemula AY, Kubbi JR, Sekhar MM, Jhawar D. Psammomatoid juvenile ossifying fibroma involving upper jaw: a rare case report. J Clin Diagn Res JCDR. 2015;9(7):ZD17–ZD19. doi: 10.7860/JCDR/2015/14603.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keles B, Duran M, Uyar Y, Azimov A, Demirkan A, Esen HH. Juvenile ossifying fibroma of the mandible: a case report. J Oral Maxillofac Res. 2010;1(2) doi: 10.5037/jomr.2010.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pace C, Crosher R, Holt D, Pace A. An estimate of the rate of growth of a juvenile aggressive ossifying fibroma in a 15 year old child. J Oral Sci. 2010;52(2):329–332. doi: 10.2334/josnusd.52.329. [DOI] [PubMed] [Google Scholar]
- 7.Wenig BM, Vinh TN, Smirniotopoulos JG, Fowler CB, Houston GD, Heffner DK. Aggressive psammomatoid ossifying fibromas of the sinonasal region: a clinicopathologic study of a distinct group of fibro-osseous lesions. Cancer. 1995;76(7):1155–1165. doi: 10.1002/1097-0142(19951001)76:7<1155::aid-cncr2820760710>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 8.Owosho AA, Hughes MA, Prasad JL, Potluri A, Branstetter B. Psammomatoid and trabecular juvenile ossifying fibroma: two distinct radiologic entities. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118(6):732–738. doi: 10.1016/j.oooo.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen S, Hamel MA, Chénard-Roy J, Corriveau MN, Nadeau S. Juvenile psammomatoid ossifying fibroma: a radiolucent lesion to suspect preoperatively. Radiol Case Rep. 2019;14(8):1014–1020. doi: 10.1016/j.radcr.2019.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han MH, Chang KH, Lee CH, Seo JW, Han MC, Kim CW, et al. Sinonasal psammomatoid ossifying fibromas: CT and MR manifestations. AJNR Am J Neuroradiol. 1991;12(1):25–30. [PMC free article] [PubMed] [Google Scholar]
- 11.Chang HJ, Donahue JE, Sciandra KT, Evangelista PT. Juvenile ossifying fibroma of the calvaria. Radiographics. 2009;29(4):1195–1199. doi: 10.1148/rg.294085240. [DOI] [PubMed] [Google Scholar]
- 12.Semus RL, Zielinski E, Foster WC. Juvenile psammomatoid ossifying fibroma of the calcaneus. BMJ Case Rep. 2020;13(8) doi: 10.1136/bcr-2020-234555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarode SC, Sarode GS, Waknis P, Patil A, Jashika M. Juvenile psammomatoid ossifying fibroma: a review. Oral Oncol. 2011;47(12):1110–1116. doi: 10.1016/j.oraloncology.2011.06.513. [DOI] [PubMed] [Google Scholar]
- 14.Zhang ZY, Min MP, Liu Y, Jiang HQ, Zhang J. A large psammomatoid ossifying fibroma with proptosis: a case report. Mol Clin Oncol. 2017;6(2):167–169. doi: 10.3892/mco.2016.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DY, Lee OH, Choi GC, Cho JH. A case of juvenile psammomatoid ossifying fibroma on skull base. J Craniofac Surg. 2018;29(5):e497–e499. doi: 10.1097/SCS.0000000000004510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.