Abstract
Purpose
Ophthalmia nodosa (ON) is a rare but important disease describing ocular inflammation caused by injury from insect hairs (“setae”). Type V ON occurs when there is vitreoretinal involvement. Treatment with systemic steroids are first-line, but vitrectomy is indicated in resistant cases. The purpose of this study was to illustrate how multimodal imaging can facilitate diagnosis and management of ON.
Observations
This is a single retrospective case report of a patient who presented to Bascom Palmer Eye Institute with Type V ON. Multimodal imaging in a patient with Type V ON was illustrated. A moth seta was localized to the anterior vitreous cavity. Intraocular inflammation responded to 2 weeks of high-dose oral prednisone.
Conclusions and Importance
Multimodal imaging may guide diagnosis and management of ON by documenting baseline features of ON and facilitating comparison at follow up visits. This allows for safe non-surgical management of Type V ON. Long-term follow up would be necessary to determine whether subsequent surgical intervention was needed in this case.
Keywords: Ophthalmia nodosa, Uveitis, Corticosteroids, Vitreoretinal surgery, Multimodal imaging
1. Introduction
Ophthalmia nodosa (ON) is ocular inflammation due to injury with the hairs (“setae”) of insects – overwhelmingly pine processional caterpillars, although cases related to tarantula, bat, and bristle worm hairs have been reported.1, 2, 3, 4 ON was first reported in 1861 by Schon et al. and was named by Pagenstecher in 1883 because of nodular conjunctivitis.4,5 In 1966, Watson and Sevel reported that 6 out of 15 moth species that cause urticaria also cause ON.4
Cadera et al. classified ON into 5 types (I–V): Immediate toxic reaction; mechanical keratoconjunctivitis; subconjunctival granulomas; iritis secondary to setae in the cornea, anterior chamber, or lens; and vitreoretinal involvement. Types I-II are most common and treated with topical steroids and meticulous setae removal. Surgical removal is necessary for Types III-IV. Systemic steroids are first-line for Type V disease, but vitrectomy is indicated in resistant cases to remove setae.6 Setae deposit onto the eye by wind, contaminated fabrics, or direct contact with the insect.4,5 Intraocular penetration can be propagated by setae shape and velocity, ocular movement and vascular pulsations, eyelid stress, and inflammation at the setae base.5,7 Histologically, setae stimulate granuloma formation and inflammation.4,8
Despite the existence of a classification system, the clinical diagnosis and management of ON can be challenging, particularly when culprit setae are not immediately visible. We herein illustrate a case in which multimodal imaging was useful in establishing a diagnosis of ON by localizing a single seta in the vitreous cavity.
2. Case
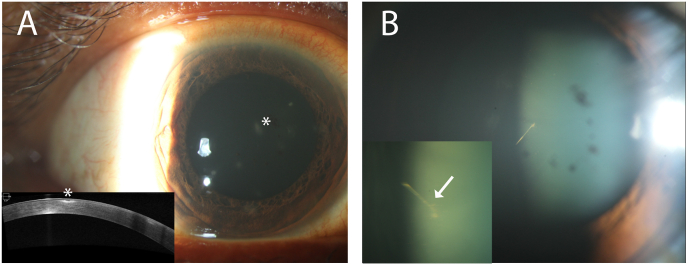
A 45 year old man was struck in the right eye by a large moth while working outdoors in the Bahamas. He saw a local ophthalmologist due to the onset of persistent pain and floaters since the injury two months prior. He was prescribed 7 days of topical prednisolone acetate drops, which he completed one day prior to presentation at our tertiary care facility. Visual acuity was 20/20 and intraocular pressures were 13 mm Hg, bilaterally. Examination of the left eye was normal. Examination of the right eye revealed no foreign body in the ocular adnexae, ocular surface, or on gonioscopy. There were multifocal corneal infiltrates with an intact corneal epithelium (Fig. 1A). The anterior chamber had flare, but no cells. There was pigment on the anterior lens capsule, but no evidence of a traumatic cataract. There were pigmented cells in the anterior vitreous and trace vitreous haze. Upon careful examination, a short gray, linear opacity was oriented vertically in the anterior vitreous cavity, consistent with a moth setae (Fig. 1B).
Fig. 1.
A. Slit lamp photograph of the right eye focused anteriorly demonstrates multifocal punctate inflammatory infiltrates in the cornea at presentation (asterisk). Optical coherence tomography (Cirrus, Carl Zeiss Meditec, Inc.) of the anterior segment (bottom left) illustrates subepithelial hyperreflectivity (asterisk) without the presence of retained intracorneal setae. B. Slit lamp photograph of the right eye focused posteriorly shows pigmentary deposits on the anterior lens capsule and a sharp linear structure consistent with a moth seta located in the anterior hyaloid face. The magnified view of the seta (bottom left) shows a different orientation indicating its dynamic positioning within the vitreous cavity. A spine of the seta is visualized (arrow).
Dilated examination showed perifoveal pigmentary changes, but no focal chorioretinal lesions, retinal vascular sheathing, or subretinal fluid. There were scattered peripheral white, focal preretinal opacities (Fig. 2A and B). Fluorescein angiography revealed peripheral small vessel leakage in zone 3 of the right eye (Fig. 2C). A B-scan ultrasound localized a single foreign body in the central vitreous cavity. No additional moth setae were identified (Fig. 3A). Optical coherence tomography (OCT) revealed perifoveal disruption of the ellipsoid zone (Fig. 3B). Routine labs for causes of uveitis, including tuberculosis, syphilis, and sarcoidosis, were unremarkable.
Fig. 2.
A. Fundus photograph (Optos®, UK) of the right eye illustrates preretinal inflammatory deposits inferiorly (arrows) at presentation. B. Fundus autofluorescence (Optos®, UK) of the right eye shows a thin hypoautofluorescent line (to the right of the asterisk), consistent with a shadow caused by the linear seta located more anteriorly in the vitreous cavity. C. Fluorescein angiography (Optos®, UK) of the right eye at presentation shows inferior pinpoint areas of hyperfluorescence, consistent with small vessel retinal vasculitis in zone 3 caused by type V ophthalmia nodosa.
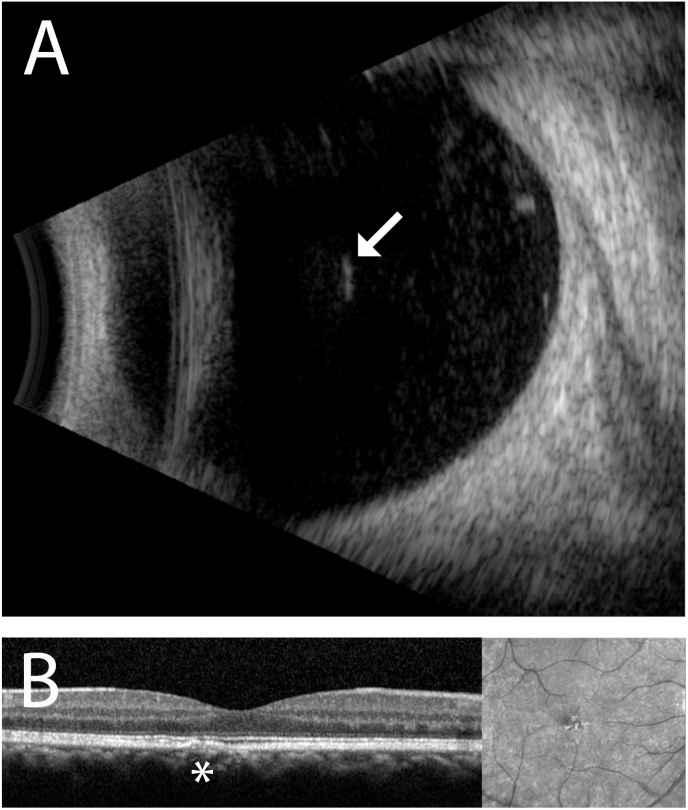
Fig. 3.
A. B-scan ultrasound (Aviso, Quantel Medical) of the right eye localizes the hyperreflective, linear intraocular seta within the anterior vitreous cavity (arrow). Adherent inflammatory deposits are visualized around it. No other intraocular setae are identified within the vitreous cavity. B. Spectral-domain optical coherence tomography (Heidelberg Spectralis, Heidelberg Engineering, Germany) shows a focal, perifoveal region of ellipsoid zone disruption of unclear significance. The finding remained unchanged over the 4 week follow up interval and visual acuity remained stable at 20/20-20/25 in the right eye.
The patient was diagnosed with ON with vitreous involvement. Vitreous opacities and corneal infiltrates improved following two weeks of high-dose oral prednisone. Prednisone was discontinued due to steroid-induced hyperglycemia. Ophthalmic examination remained stable 1 week later, and there was no indication for a pars plana vitrectomy. Visual acuity was 20/25 in the affected eye 4 weeks after initial presentation to our institution. The patient returned to the Bahamas for follow up care.
3. Discussion
The differential diagnosis for intraocular inflammation following ocular injury with an insect is broad and includes intraocular hemorrhage, traumatic or viral-mediated uveitis, endophthalmitis, and ophthalmia nodosa. The presence of retinal vasculitis and the absence of red blood cells made traumatic iritis and hemorrhage unlikely. There were no iris changes, ocular hypertension, or retinitis suggestive of viral uveitis. An insect hair in the vitreous cavity following moth-induced eye trauma was consistent with the diagnosis of Type V ON.
In the largest published series of 544 cases of caterpillar-induced ON in India, Sengupta et al. reported that 35% of eyes had Type IV/V involvement. Two risk factors for intraocular penetration were the presence of intracorneal setae or failure to remove them. The majority of Type V ON cases lacked anterior chamber setae, favoring trans-scleral penetration.9 Fraser et al. reported a case of a 15 year old male who was exposed to caterpillar setae while on a school trip. Despite removal of periocular setae over multiple sessions, setae migrated into the vitreous cavity. One seta was embedded in the retina, requiring removal by pars plana vitrectomy 1 year after presentation.10
Despite the need for surgery in some cases of Type V ON, vitreoretinal setae can be well-tolerated with conservative treatment, which is the preferred initial managment.6 Ibarra et al. reported a case of a 4 year old male who had caterpillar setae embedded in the corneal stroma and retina with minimal inflammation. The patient tolerated treatment with topical steroids alone and achieved a favorable outcome at 4 months of follow up.11 Despite a relatively short follow up duration, the patient in our case remained quiescent after only 2 weeks of high-dose oral steroids. Nonetheless, attempts to wean patients off steroids sometimes fail to achieve long-term control of intraocular inflammation, requiring surgery. Shibui et al. published a case of angle-involving ON that developed a recurrent sterile hypopyon upon each taper of oral and topical steroids. A pars plana vitrectomy at 7 months after presentation achieved disease control.12
Endophthalmitis is a rare complication of ON and should be treated with intravitreal antibiotics.4,9 Sengupta et al. reported only 1 endophthalmitis case, in a pediatric patient, in their large cohort study.9 Seasonal hyperacute panuveitis (SHAPU) is a blinding form of ON that simulates endophthalmitis. Endemic to Nepal, SHAPU has occurred mainly in the autumns of odd years since 1975.13 Its pathogenesis may be related to a strong local immune response to moth toxins, secondary endophthalmitis, herpesvirus, and anellovirus inoculation.13,14 Early surgical intervention should be considered in SHAPU, as it may improve visual outcomes.15 With the absence of significant cellular inflammation or a reported history of travel to an endemic area, both endophthalmitis and SHAPU were unlikely in the present case.
A high clinical suspicion, detailed clinical history, and meticulous physical examination were instrumental in diagnosing ON in our case, in which the culprit moth seta was not immediately noticeable. Multimodal imaging can help localize setae and determine the best immediate management. Anterior segment OCT characterized the inflammatory corneal opacities and excluded the presence of small intracorneal setae that would require meticulous removal. Timucin and Baykara similarly used Scheimpflug imaging to guide removal of an intracorneal seta and to demonstrate resolution of keratitis at 3 weeks follow up.16 In conjunction with clinical examination, B-scan ultrasound helped localize a single seta in the vitreous cavity in our case. Similarly, Agarwal et al. achieved an early diagnosis of Type V ON using high resolution ultrasound to localize multiple setae that were present beyond the anterior segment in a case in which only intracorneal setae were seen by physical examination.17
OCT of the macula in the present case showed perifoveal ellipsoid zone disruption of unclear etiology, as this has not been previously reported in ON, to the best of our knowledge. While direct mechanical injury was unlikely in the absence of a full-thickness retinal hole, resolved cystoid macular edema and/or focal toxicity from the moth setae were potential causes. However, there was no indication for surgical seta removal, as the OCT remained stable and inflammation improved with nonsurgical treatment over the follow up course of 4 weeks.
Multimodal imaging in our reported case helped to identify baseline features of ON and to compare findings at follow up. This allowed us to safely manage this patient non-surgically. Long-term follow up would be necessary to determine whether subsequent surgical intervention was needed in this case of Type V ON.
Ethical approval
This case series was conducted in accordance with the Declaration of Helsinki. The collection and evaluation of all protected patient health information was performed in a Health Insurance Portability and Accountability Act (HIPAA)–compliant manner.
Patient consent
Informed consent was obtained prior to performing the procedures, including permission for the educational use of non-identifiable information. No identifying information was included in the content of this manuscript.
Funding
This work was supported in part by a National Institutes of Health Center Grant (P30EY014801) and an unrestricted grant from Research to Prevent Blindness (GR004596) to the University of Miami.
Declaration of competing interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to acknowledge the Bascom Palmer photography and echography department for their technical support with the images included in this manuscript.
Footnotes
This manuscript has not been presented at a meeting.
The Department of Ophthalmology receives grant support from the NIH Center Core Grant P30EY014801 (Bethesda, Maryland) and the Research to Prevent Blindness Unrestricted Grant (GR004596) to the University of Miami.
References
- 1.Wiwatwongwana D., Ausayakun S., Chaidaroon W., et al. Bat attack!: an unusual cause of keratouveitis. Graefes Arch Clin Exp Ophthalmol. 2012;250:1109–1110. doi: 10.1007/s00417-011-1739-0. [DOI] [PubMed] [Google Scholar]
- 2.Kirk J., Gunn D., Wong N., Minchin E., Darcy K. Bristle worm-induced keratouveitis: a case report. Cornea. 2020;39(5):654–656. doi: 10.1097/ICO.0000000000002225. [DOI] [PubMed] [Google Scholar]
- 3.Hered R., Spaulding A.G., Sanitato J.J., Wander A.H. Ophthalmia nodosa caused by tarantula hairs. Ophthalmology. 1988;95(2):166–169. doi: 10.1016/s0161-6420(88)33191-x. [DOI] [PubMed] [Google Scholar]
- 4.Watson P., Sevel D. Ophthalmia nodosa. Br J Ophthalmol. 1966;50:209–217. doi: 10.1136/bjo.50.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sridhar M., Ramakrishnan M. Ocular lesions by capillar hairs. Eye. 2004;18:540–543. doi: 10.1038/sj.eye.6700692. [DOI] [PubMed] [Google Scholar]
- 6.Cadera W., Pachtman M.A., Fountain J.A., Ellis F.D., Wilson F.M. Ocular lesions caused by caterpillar hairs (Ophthalmia Nodosa) Can J Ophthalmol. 1984;19:40–44. [PubMed] [Google Scholar]
- 7.Ascher K. Mechanism of locomotion observed on caterpillar hairs. Br J Ophthalmol. 1968;52(2):210. doi: 10.1136/bjo.52.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haluska F., Puliafito C.A., Henriquez A., Albert D.M. Experimental gypsy moth (Lymantria dispar) ophthalmia nodosa. Arch Ophthalmol. 1983;101(5):799–801. doi: 10.1001/archopht.1983.01040010799022. [DOI] [PubMed] [Google Scholar]
- 9.Sengupta S., Reddy P.R., Gyatsho J., Ravindran R.D., et al. Risk factors for intraocular penetration of caterpillar hair in Ophthalmia Nodosa: a retrospective analysis. Indian J Ophthalmol. 2010;58:540–543. doi: 10.4103/0301-4738.71711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser S., Dowd T.C., Bosanquet R.C. Intraocular caterpillar hairs (setae): clinical course and management. Eye (Lond). 1994;8:596–598. doi: 10.1038/eye.1994.144. [DOI] [PubMed] [Google Scholar]
- 11.Ibarra M., Orlin S.E., Saran B.R., Liss R.P., Maguire A.M. Intraocular caterpillar setae without subsequent vitritis or iridocyclitis. Am J Ophthalmol. 2002;134(1):118–120. doi: 10.1016/s0002-9394(02)01497-6. [DOI] [PubMed] [Google Scholar]
- 12.Shibui H., Kawashima H., Kamata K., Sasaki H., Inoda S., Shimizu H. Vitrectomy for caterpillar seta-induced endophthalmitis. Arch Ophthalmol. 1997;115(4):555–556. doi: 10.1001/archopht.1997.01100150557023. [DOI] [PubMed] [Google Scholar]
- 13.Upadhyay M., Kharel S.R., Shrestha B., et al. Seasonal hyperacute panuveitis in Nepal: a review over 40 Years of surveillance. Ocul Immunol Inflamm. 2019;27(5):709–717. doi: 10.1080/09273948.2018.1439643. [DOI] [PubMed] [Google Scholar]
- 14.Smits S., Manandhar A., van Loenen F.B., et al. High prevalence of anelloviruses in vitreous fluid of children with seasonal hyperacute panuveitis. J Infect Dis. 2012;205(12):1877–1884. doi: 10.1093/infdis/jis284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shrestha E. A profile and treatment outcome of seasonal hyper-acute panuveitis. Nepal J Ophthalmol. 2010;2(1):35–38. doi: 10.3126/nepjoph.v2i1.3702. [DOI] [PubMed] [Google Scholar]
- 16.Timucin O., Baykara M. Role of Scheimpflug imaging in the diagnosis and management of keratitis caused by caterpillar seta. Oman J Ophthalmol. 2010;3(3):150–152. doi: 10.4103/0974-620X.71900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal M., Acharya M.C., Majumdar S., Paul L. Managing multiple caterpillar hair in the eye. Indian J Ophthalmol. 2017;65:248–250. doi: 10.4103/ijo.IJO_985_15. [DOI] [PMC free article] [PubMed] [Google Scholar]