Abstract
As vascular networks form, endothelial cells (ECs) undergo cell fate decisions that determine whether they become tip or stalk cells of the developing vascular plexus or mature into arterial, venous, or lymphatic endothelium. EC fate decisions are coordinated with neighboring cells to initiate sprouting, maintain endothelial barrier, or ensure appropriate specialization of vessels. We describe mechanisms that control EC fate at specific steps in these processes, with an emphasis on the role of the Notch signaling pathway. Specific EC fate determination steps that are highlighted are tip/stalk selection during sprouting angiogenesis, venous-arterial specification, arteriogenesis, lymphatic vessel specification, and lymphatic valve formation.
Control of vascular growth is critical for every organ of the body (Ramasamy et al. 2015). Endothelial cells (ECs) take on a variety of specialized functions during prenatal and postnatal development of the vasculature. However, ECs retain plasticity to adjust to conditions in adults, like hypoxia, wounding, and inflammation. Vascular disruptions contribute to a broad range of diseases, including cancer, hypertension, and dementia.
Notch signaling controls numerous endothelial phenotypes and multiple EC fate decisions (Tung et al. 2012). Notch proteins are single-pass transmembrane receptors that, upon activation by ligand binding, are cleaved to release the intracellular domain, which then translocates to the nucleus and complexes with transcriptional cofactors to directly regulate target gene expression. Human and mouse ECs primarily express the Notch proteins NOTCH1 and NOTCH4 and their ligands DLL4 and JAG1 (Uyttendaele et al. 1996; Xue et al. 1999; Krebs et al. 2000, 2004; Vanlandewijck et al. 2018). Notch ligands are transmembrane proteins and generally function to activate Notch in association with cell–cell contact. The tensile force between Notch ligand–presenting cells and receptor-presenting cells is required to expose the S2 proteolytic cleavage site on the receptor that leads to full receptor activation (Wang and Ha 2013; Gordon et al. 2015; Luca et al. 2017). In many contexts, activating Notch in a cell down-regulates Notch ligand expression, creating a regulatory feedback loop where similar cells with stochastic variation in Notch expression will sort themselves into distinct high-Notch and high-ligand-expressing fates, a process known as lateral inhibition (Lai 2004; Greenwald 2012). These features of Notch signaling regulation are elements of vascular biology; to preserve vascular integrity, ECs must maintain direct contacts with adjacent EC and mural cells, and individual ECs must acquire new fates while neighboring cells preserve their original states. In this review, we provide an overview of how Notch functions to control cell fate decisions of ECs, whether it be to maintain current status or pivot to new vascular fates.
TIP CELLS AND STALK CELLS IN SPROUTING ANGIOGENESIS
Upon specification of EC fate and formation of the vascular plexus, expansion of the vascular network is achieved primarily by sprouting angiogenesis, in which new blood vessels grow from the existing vasculature. During sprouting angiogenesis, ECs respond dynamically to microenvironmental cues as well as to cell-intrinsic programs and signals from neighboring ECs to guide appropriate patterning and integrity of the vascular network (Marcelo et al. 2013). A critical aspect of sprouting angiogenesis is specification of tip and stalk ECs (Fig. 1). Tip cells lie at the leading edge of the nascent vascular sprout, extending filopodia to the surrounding microenvironment and guiding migration of the vascular sprout in the direction of angiogenic stimuli. Stalk cells, which make up the bulk of the developing vessel, lie behind the tip cell and proliferate to extend the vasculature (Gerhardt et al. 2003). Although initially thought to be a relatively static process, in which tip or stalk cell identity was specified early and maintained throughout angiogenesis, it is now recognized to be highly dynamic, with ECs competing for and switching between tip/stalk cell identity, ensuring the formation of a robust vascular network (Jakobsson et al. 2010; Arima et al. 2011; Stepanova et al. 2021).
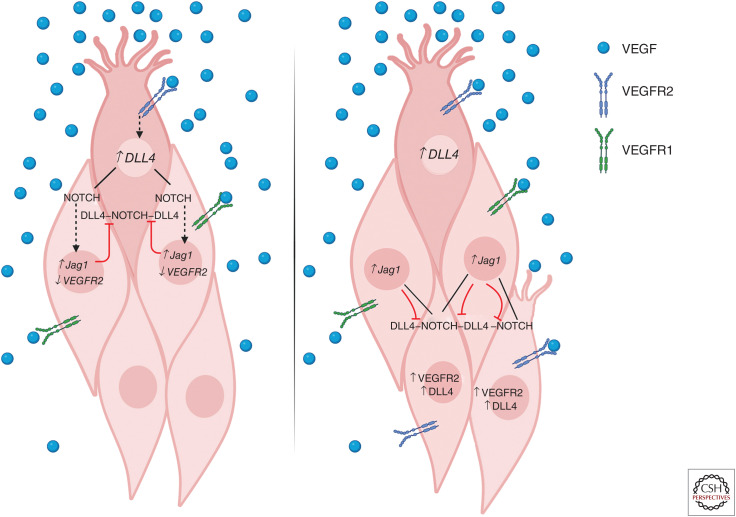
Figure 1.
Tip cell/stalk cell determination and shuffling in sprouting angiogenesis. (Left) Upon initiation of tip cell differentiation in response to vascular endothelial growth factor (VEGF)-VEGFR2 signaling, expression of Notch ligand DLL4 is increased. DLL4 signaling via Notch receptors in adjacent cells represses DLL4 and VEGFR2 expression and up-regulates VEGFR1 and Jagged1 expression, enforcing the stalk cell identity of neighboring cells and preventing unregulated tip cell sprouting. Jagged1 expression in neighboring stalk cells inhibits DLL4-Notch signaling in the tip cell, further stabilizing tip/stalk identity in the local area. (Right) Distal to the leading tip cell, stalk cells that receive lower levels of DLL4-Notch signaling and highly express VEGFR2 retain the ability to respond to VEGF stimulation and adopt tip cell identity. Upon VEGF stimulation, these cells may compete with the previously specified tip cell for position at the leading edge of the vascular front.
NOTCH CONTROLS CELL FATES DURING ANGIOGENIC SPROUTING
Angiogenic sprouting is initiated when an EC, typically at the periphery of the vascular plexus, encounters an angiogenic stimulus such as extracellular vascular endothelial growth factor A (VEGF-A), which binds to its cognate receptors VEGF receptor 1 (VEGFR1) and VEGF receptor 2 (VEGFR2), leading the responsive EC to differentiate from neighboring stalk cells and adopt a tip cell phenotype, extending filopodia to sense and migrate in the direction of the angiogenic stimulus (Fig. 1; Gerhardt et al. 2003). VEGFR1 and VEGFR2 are the most well-characterized VEGF receptors involved in the regulation of sprouting angiogenesis, with VEGF having the highest affinity to VEGFR1 and interacting more weakly with VEGFR2. However, VEGFR1 exhibits weak kinase activity and acts primarily as a decoy receptor, thus making VEGFR2 the primary mediator of VEGF signaling in the context of sprouting angiogenesis (Park et al. 1994; Olsson et al. 2006). Although restricted to the lymphatic endothelium in the adult, during sprouting angiogenesis, VEGFR3 is highly expressed by blood ECs and contributes to regulation of proper vascular network formation (Tammela et al. 2008; Zarkada et al. 2015).
Stalk cells are considered essential in the regulation of proper sprouting and vessel maturation and the Notch signaling axis guides these processes, permitting or restricting angiogenic sprouting and endothelial responsiveness to VEGF. Not all ECs that encounter VEGF will become tip cells, and tip cell selection is highly dependent on Delta-like 4 (DLL4)-Notch signaling.
As demonstrated in mouse embryoid bodies in vitro and the developing retina in vivo, adoption of tip cell identity is favored in cells that express relatively high VEGFR2 and low VEGFR1; however, rather than being strictly VEGFR dosage-dependent, the presence of an intact endothelial Notch signaling axis is required to maintain this preference, suggesting that sprouting is regulated not only by VEGFR expression, but also by proper regulation of Notch signaling levels (Jakobsson et al. 2010). Further, Notch signaling in angiogenic endothelium is essential to determination of VEGFR expression, with high Notch signaling leading to low VEGFR2 and high VEGFR1 expression, while low Notch signaling in potential tip cells restricts VEGFR1 expression and permits VEGFR2 expression and responsiveness to VEGF; these relative expression levels will determine whether a stalk EC differentiates to tip cell identity in response to VEGF (Harrington et al. 2008; Moya et al. 2012). In the embryonic hindbrain, Notch signaling in early stalk cells is potentiated by the formation of a heteromer between Notch target HES1 and the Id family of helix-loop-helix proteins, leading to down-regulation of tip cell markers such as Vegfrs and Dll4 and robust reinforcement of the stalk cell phenotype; ECs with low or deficient Id protein were favored to adopt tip cell identity (Moya et al. 2012).
Upon adoption of tip cell identity, VEGFR2 signaling leads to increased expression of the Notch ligand DLL4 in tip cells, which reinforces Notch signaling in adjacent stalk cells and ensures low VEGFR2 expression. Concurrently, the Notch ligand Jagged1 (JAG1) is highly expressed in stalk cells, potentially blocking DLL4-Notch signaling to tip cells and reinforcing their tip cell identity (Hellström et al. 2007; Lobov et al. 2007; Benedito et al. 2009). Thus, neighboring tip and stalk cells engage in an intercellular feedback loop that stabilizes tip/stalk identity in the local area and ensures proper regulation of angiogenic sprouting. While the VEGF-VEGFR-DLL4-Notch-VEGFR feedback loop may appear to stably reinforce tip/stalk patterning, live imaging has shown that stalk cells dynamically migrate and replace tip cells throughout angiogenesis, termed tip-stalk shuffling (Jakobsson et al. 2010). The tip/stalk feedback loop results in stabilization of tip/stalk identity in the cells immediately surrounding the newly specified tip cell, where high Notch signaling in stalk cells prevents the VEGF responsiveness that induces a tip cell phenotype (Pontes-Quero et al. 2019). However, as Notch signaling decreases in stalk cells distal to DLL4-expressing tip cells, stalk cells are permitted to differentiate, migrate, and compete for a tip cell position in response to VEGF stimulation (Jakobsson et al. 2010; Arima et al. 2011).
While ECs are engaged in competition during tip/stalk shuffling and stalk cells are proliferating to support the elongation of the new sprout, mural cells also proliferate prior to recruitment to the nascent vasculature. For a sprout to develop into a new, functional blood vessel, the ECs making up that sprout must recruit mural cells such as pericytes or vascular smooth muscle cells (vSMCs) to reinforce and provide the cues required for that vessel to mature and lumenize. While vSMC are generally classified as associating with large and medium caliber vessels, and pericytes with capillaries, these cell types should be characterized along a continuum, exhibiting a gradual change in morphology as the vasculature progressively branches into smaller vessels (Sinha and Santoro 2018; Vanlandewijck et al. 2018). Mural cells are thought to derive primarily from mesenchymal and neural crest cells, but there may be heterogeneity in their origin, with some pericytes reported to derive from differentiated macrophages in the embryonic brain (Yamamoto et al. 2017). During angiogenesis, pericytes are recruited to the developing vessel by endothelial tip cell secretion of platelet-derived growth factor B (PDGF-B), initially localize adjacent to the tip cell, and keep pace with the sprout as it extends and migrates, contributing to the regulation of angiogenic sprouting (Chang et al. 2013; Payne et al. 2019).
Following pericyte recruitment to the immature vasculature, pericytes and ECs engage directly via receptor-ligand binding that facilitates their endothelial–pericyte cross talk and coordination. Pericyte association with angiogenic vasculature helps to regulate proper blood vessel patterning by contributing to the control of vessel regression and pruning via endosialin, and coverage of blood vessels by pericytes marks the end of the window of blood vessel remodeling, leading to stabilization of the vessels and EC quiescence (Benjamin et al. 1998; Simonavicius et al. 2012). Pericyte-derived ANG1 is involved in the stabilization of capillaries and promotes their quiescence as well as limiting permeability by signaling through endothelial TIE2 (Thurston et al. 1999; Wakui et al. 2006; Falcón et al. 2009). Intriguingly, JAG1, which is expressed by stalk cells, likely interacts with pericyte-expressed NOTCH3, promoting pericyte differentiation and contributing to the proangiogenic program induced by pericytes (Liu et al. 2009). The recruitment and retention of pericytes mediated by endothelial–pericyte signaling contributes to the stabilization of the structure as the vascular tube undergoes lumenization and begins to transport blood by contributing elements of the vascular basement membrane, stimulating endothelial junction formation, and regulating appropriate vessel diameter and tone (Hellström et al. 2001; Stratman et al. 2009; Armulik et al. 2010; Daneman et al. 2010; Zhao and Chappell 2019).
ARTERIAL-VENOUS SPECIFICATION AND DEVELOPMENT OF THE MATURE VASCULAR TREE
In many organs, the initial phase of sprouting angiogenesis leads to a primary vascular plexus consisting of numerous small vessels of similar diameter formed into a mesh-like network with relatively uniform spacing of vessel branches (Udan et al. 2013). For optimal blood flow and tissue oxygenation, this plexus must be remodeled into a mature vascular tree consisting of arteries to deliver blood from the heart to distal tissues, veins to return blood to the heart, and an intervening capillary bed that provides diffusion of gases and nutrients. Each of these vessel types are characterized by ECs with distinct morphology, transcriptomes, and behaviors (dela Paz and D'Amore 2009). In arteries, where blood flow creates strong and pulsatile shear stress, ECs respond by elongating, polarizing against the direction of flow, and recruiting vSMCs for structural support. Capillary ECs flatten to allow increased permeability and recruit pericytes, a specialized mural cell type that interacts tightly with endothelium to regulate quiescence and vessel diameter. In veins, ECs are relatively unpolarized but form specialized valve structures to prevent backward blood flow. Depending on the organ, a wide variety of other specialized vessel structures may form, including postcapillary venules, which permit immune cell extravasation, the blood–brain barrier, which protects neural tissues, liver sinusoids, which participate in blood detoxification, and bone marrow sinusoids, which support hematopoietic development (Daneman and Engelhardt 2017; Watson and Adams 2018; Koch et al. 2021; Vella et al. 2021).
In mature vasculature, Notch signaling is high in arteries and much lower in veins and is required for mature arterial endothelial behavior, including polarization and cell-cycle suppression (Villa et al. 2001; Roca and Adams 2007). Notch restriction to arterial vessels is thought to be enforced by a reciprocal negative feedback loop with COUPTFII, a transcription factor that is highly expressed in veins (Swift and Weinstein 2009; Chen et al. 2012; Fang and Hirschi 2019). While this feedback loop is self-reinforcing, it also retains a degree of plasticity. For example, in response to increased blood flow, venous ECs activate Notch signaling and assume increasingly arterial transcriptional and morphological characteristics (Fang et al. 2017; Mack et al. 2017). Ectopic up-regulation or down-regulation of Notch signaling throughout the vasculature leads to increased arterial or venous specification of capillaries, respectively, resulting in formation of arteriovenous malformations, where capillaries are transformed into abnormal larger-caliber vessels that shunt blood directly between arteries and veins (Uyttendaele et al. 2001; Krebs et al. 2010; Murphy et al. 2014; Nielsen et al. 2014).
Prior to the formation of the mature vascular tree, Notch signaling is central to distinct steps of EC fate decisions. During mouse embryogenesis, loss of both of the Notch proteins expressed in ECs, NOTCH1 and NOTCH4, results in formation of a rudimentary and reduced vasculature—ECs form into primitive cords, but fail to properly form lumens or mature into patent vessels (Krebs et al. 2000). Similarly, complete loss of the Notch ligand Dll4 or Notch transcriptional cofactor Rbpj leads to reduced and abnormal vascular formation (Duarte et al. 2004; Krebs et al. 2004). When Notch signaling is partially reduced by removing one copy of Dll4 or Rbpj in mouse embryos or expressing a dominant-negative form of Rbpj in zebrafish embryos, ECs are capable of forming vessels, but arterial differentiation is lost (Lawson et al. 2001; Duarte et al. 2004; Gale et al. 2004; Krebs et al. 2004), confirming the critical role for Notch signaling in arterial development. Arteriovenous malformations are common in these embryos, indicating that embryonic capillaries become respecified to a more venous fate in the absence of Notch signaling.
Closer examination of Notch signaling in the angiogenic plexus has uncovered potential mechanisms by which Notch contributes to venous/arterial EC fate decisions. As a primitive vascular plexus remodels, arteries and veins develop via different cellular behaviors. Live imaging in zebrafish and lineage tracing in the mouse retina have revealed that veins grow via mitosis of resident ECs, while arteries are largely nonmitotic (Fig. 2; Xu et al. 2014). Growth of arteries is largely driven by addition of plexus ECs, which migrate against the direction of flow and incorporate into the developing artery, extending it. When either DLL4-Notch signaling or all Notch signaling is blocked in individual ECs, tip cells fail to up-regulate the migratory gene Cxcr4a or incorporate into developing arteries, and instead accumulate in the plexus (Hasan et al. 2017; Pitulescu et al. 2017; Luo et al. 2021). Conversely, loss of Jag1 from individual ECs results in depletion of those cells from the entire vascular bed (Pitulescu et al. 2017).
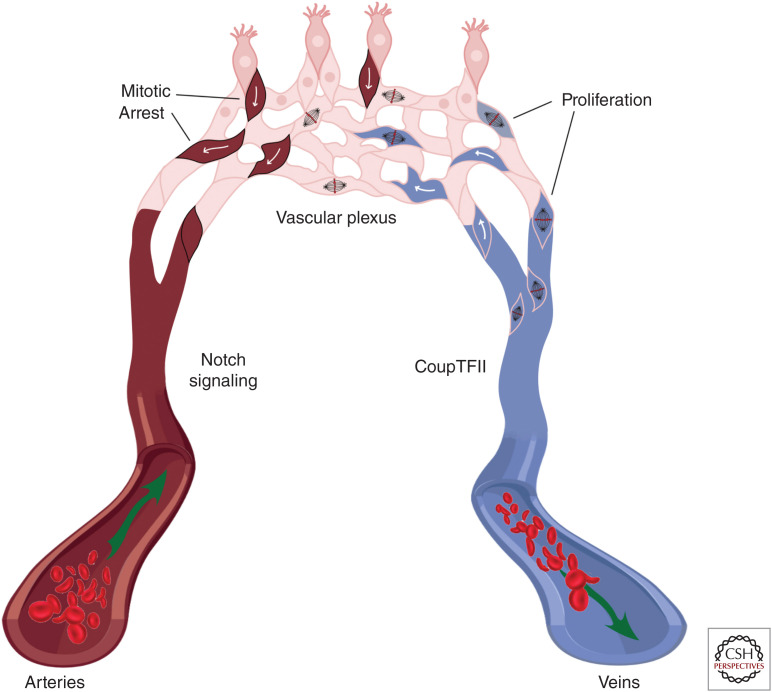
Figure 2.
Arterial/venous differentiation from the vascular plexus. Arterial endothelial cells (ECs) (left) express Notch proteins, while venous ECs (right) have low Notch expression and high CoupTFII expression. CoupTFII drives endothelial proliferation in venous endothelium, extending the vein and contributing cells to the growing vascular plexus (top). Stalk cells in the vascular plexus experience high levels of Notch signaling, which causes cell-cycle arrest. High Notch signaling and cell-cycle arrest leads the cell to assume arterial fate, migrate against the direction of blood flow, and incorporate into the artery.
While the specification of venous and arterial cell fates is perhaps best described when originating from an immature plexus, some differentiated veins retain the ability to restart the endothelial developmental program and acquire nonvenous fates (Red-Horse et al. 2010). Single-cell characterization of the developing heart vasculature reveals that the sinus venous, which was presumed to have fully venous specification, contains a subpopulation of resident ECs that sprout into a new vascular plexus that redifferentiates to form the coronary arteries and capillaries (Su et al. 2018). This prespecified endothelial population expresses Dll4 and their plexus formation could be blocked by CoupTFII, suggesting that these cells retain a Notch signaling status more typical of arterial endothelium.
Recent work has shown that a major mechanism by which Notch signaling induces arterial fate in plexus ECs is by controlling the cell cycle (Fig. 2). In cultured ECs, Notch signaling suppresses proliferation (Sainson et al. 2005). Analysis of Notch in retinal vasculature has revealed highly dosage-dependent functions: very high Notch signaling leads to rapid cell-cycle arrest, very low Notch signaling leads a brief period of mitosis followed by cell-cycle arrest, and only intermediate levels of Notch signaling permitted ongoing endothelial proliferation (Pontes-Quero et al. 2019). Individual high-Notch cells in an otherwise normal context were biased against venous cell fates, as predicted by the Notch/CoupTFII feedback loop described above, but those cells that incorporated into veins showed appropriate expression of venous morphology and markers, including the up-regulation of CoupTFII despite high Notch levels (Luo et al. 2021). In the forming vasculature, Notch signaling functioned to suppress Myc expression and activate cell-cycle inhibitor CDKN1B, leading to loss of proliferative capacity typical of arterial cells, and loss of Myc or reexpression of CDKN1B was sufficient to drive ECs toward arterial fates (Fang et al. 2017; Luo et al. 2021). Conversely, overexpression of CoupTFII in the developing plexus increases the expression of cell-cycle genes and prevents arterial differentiation, but this blockade does not reduce Notch signaling and arterial specification can be restored by treatment with a cell-cycle inhibitor (Su et al. 2018). These data suggest that Notch-induced cell-cycle arrest induces previously undifferentiated ECs to assume arterial fates, and the Notch/CoupTFII transcriptional feedback loop becomes established after that initial differentiation. These studies highlight the importance of proper dosage and spatial and temporal regulation of Notch signaling in the regulation of angiogenesis.
LYMPHATIC VASCULAR CELL FATE STEPS CONTROLLED BY NOTCH SIGNALING
Proper function of the lymphatic vasculature is critical for survival, as the lymphatic system functions in tissue fluid homeostasis, in the absorption of lipids from the digestive tract, and to recover fluid from tissues and return it to the blood circulatory system. Pathophysiological functions of the lymphatic vasculature include wound resolution, inflammation, and metastasis of tumor cells. Lymphatic vascular development and lymphangiogenesis requires cell fate decisions. The Notch signaling pathway plays essential regulatory roles in determining cell fates in multiple lymphatic cell fate decisions (Harvey and Oliver 2004; Oliver and Srinivasan 2010), in particular, the differentiation of lymphatic ECs (LECs) from venous endothelium during lymphatic specification, during LEC stalk versus tip cell differentiation, during sprouting lymphangiogenesis, and lymphatic duct versus valve specification during lymphatic maturation.
DEVELOPMENT OF THE LYMPHATIC VASCULATURE
The lymphatic system was initially hypothesized to derive from venous endothelium through observations made by Florence Sabin (Sabin 1902) and its venous origin has been confirmed through lineage-tracing studies (Srinivasan et al. 2007). The lymphatic regulator Prox1 is initially expressed in select venous ECs in the anterior cardinal vein, committing these cells to the LEC fate in murine embryonic development soon after the arterial-venous system forms (Wigle and Oliver 1999; François et al. 2008; Srinivasan et al. 2010). Prox1 expression is dependent upon the transcription factors COUPTFII and SOX18 (Wigle and Oliver 1999; François et al. 2008; Srinivasan et al. 2010). In mouse embryos from embryonic day (E)11.5–12.5, the LEC progenitor cells migrate dorsolaterally from the cardinal vein to form primitive lymph sac structures (Fig. 3; Oliver 2004; Maby-El Hajjami and Petrova 2008; Oliver and Srinivasan 2010; Tammela and Alitalo 2010; Schulte-Merker et al. 2011). The sprouting of the nascent LECs requires VEGFR3 expression by the LECs and expression of the VEGFR3 ligand, VEGFC, by mesenchymal cells (Karkkainen et al. 2004). From E12.5 to E14.5, lymph sacs initiate sprouting lymphangiogenesis and form a lymphatic plexus (Oliver 2004; Maby-El Hajjami and Petrova 2008; Oliver and Srinivasan 2010; Tammela and Alitalo 2010; Schulte-Merker et al. 2011). The initial lymphatic plexus undergoes further remodeling and maturation into smaller lymphatic capillaries, intermediate precollecting vessels, and larger collecting vessels (Oliver 2004; Maby-El Hajjami and Petrova 2008; Oliver and Srinivasan 2010; Tammela and Alitalo 2010; Schulte-Merker et al. 2011). SMCs are recruited to stabilize collecting vessels, and specialized valves begin to form in growing collecting vessels late during murine embryonic development. The periodically placed lymphatic valves are essential to maintain a unidirectional flow of lymph (Oliver 2004; Maby-El Hajjami and Petrova 2008; Tammela and Alitalo 2010; Schulte-Merker et al. 2011). Thus, the differentiated lymphatic vascular system is functional at time of birth and continues to expand along with tissue growth postnatally.
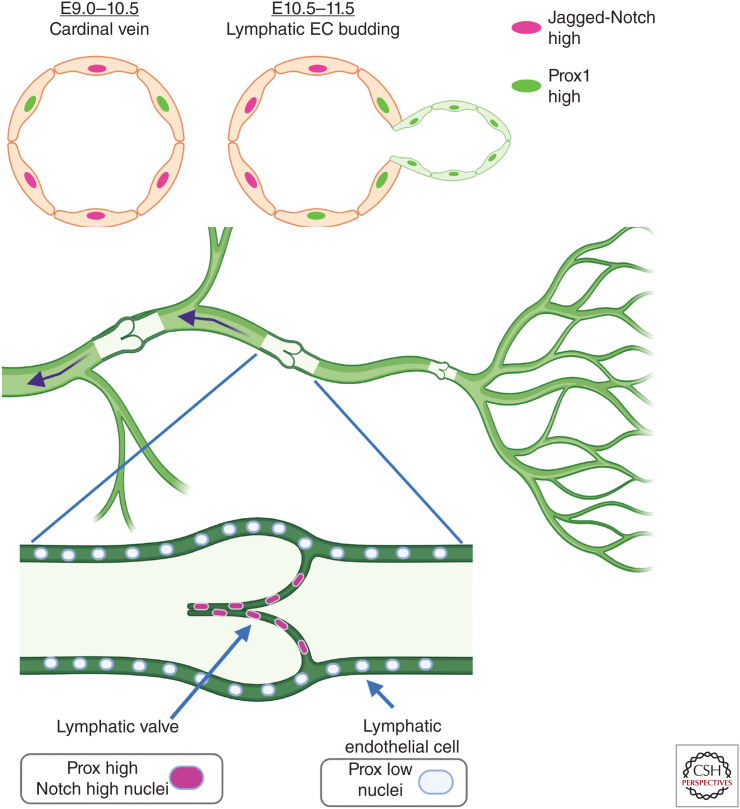
Figure 3.
Acquisition of lymphatic endothelial and lymphatic valve cell fates. (Top) During murine embryonic development, Prox1-expressing cells form in the cardinal vein (right side on schematized vein) and bud to form a sprout that initiates lymphatic development. Notch1 and Jagged1-expressing cells are located at the opposite side of the vein from Prox1-expressing cells and loss-of-function experiments demonstrate that Notch suppresses Prox1 expression, assuring proper lymphatic development. (Bottom) Lymphatic vasculature consists of capillaries that collect fluid, which is directed through lymphatic ducts containing valves that assure unidirectional flow. During initial development of lymphatic valves, lymphatic endothelial cells (ECs) express high levels of Prox1 and have high Notch signaling, and loss of Notch1 or canonical Notch signaling prevents proper lymphatic valve development.
TRANSCRIPTIONAL REGULATION OF LYMPHATIC SPECIFICATION BY NOTCH SIGNALING
Lymphatic specification in venous ECs begins at E9.5–E10, marked by expression of the lymphatic master regulator Prox1 in veins in a polarized fashion (Wigle and Oliver 1999). Transcription factors SOX18, COUPTFII, and NOTCH1 have been shown to regulate Prox1 expression during lymphatic specification (François et al. 2008; Kang et al. 2010; Srinivasan et al. 2010; Murtomaki et al. 2013). Sox18, a member of the SRY-related HMG domain family of transcription factors, is expressed in both venous and arterial vasculature (François et al. 2008), whereas CoupTFII, an orphan nuclear hormone receptor, is expressed in venous vasculature (Srinivasan et al. 2010). Sox18 and CoupTFII have both been shown to be necessary for induction of Prox1 expression in LEC progenitor cells in the cardinal vein (François et al. 2008; Srinivasan et al. 2010).
During development of the mouse embryo, Notch1 is expressed in a largely polarized fashion in the cardinal vein at E9.75, in a pattern opposing that of Prox1 expression (Fig. 3; Murtomaki et al. 2013). The polarized and opposing expression patterns of Notch1 and Prox1 has led to the hypothesis that Notch1 and Prox1 play opposing roles in the process of lymphatic specification. Activated Notch and COUPTFII are not coexpressed in the hemisphere of the cardinal vein where PROX1+ LEC progenitors originate (Murtomaki et al. 2013) and Notch signaling is critical to transcriptionally suppress Prox1 expression (Kang et al. 2010; Murtomaki et al. 2013). This arrangement is consistent with a mechanism in which Notch does not drive lymphatic specification but instead represses Prox1 by suppressing CoupTFII. In cultured human dermal LECs (HDLECs), Notch initially induces Hey1 and Hey2, which then mediates the suppression of Prox1 (Kang et al. 2010; Murtomaki et al. 2013). A negative feedback loop involving Notch, Prox1, and CoupTFII has been demonstrated (Kang et al. 2010). Notch, via its effectors HEY1 and HEY2, suppresses CoupTFII as well as Prox1 (Kang et al. 2010). Meanwhile, PROX1 and COUPTFII suppress VEGFR2 (Kang et al. 2010), which is a well-known inducer of DLL4/Notch signaling in blood ECs, as described above. This negative feedback loop appears to facilitate LEC fates and suggests plasticity between blood and LEC identities (Kang et al. 2010). Notch signaling does not suppress Prox1 expression in all EC types; for instance, Notch activation in cultured human umbilical venous ECs (HUVECs) modestly but significantly induced Prox1 transcript levels (Geudens et al. 2010).
Notch has a central role in the early development of both blood and lymphatic vascular systems in the model organism zebrafish. In zebrafish, partial silencing of dll4, notch-1b, or notch-6 blocked thoracic duct formation, while largely preserving blood vascular development (Geudens et al. 2010). This reduced thoracic duct development appears to be partly due to defects in parachordal lymphangioblast formation, which are LEC progenitor cells (Geudens et al. 2010). In the mouse, LEC-specific loss of Notch1 at E9.75 using a Prox1CreERT2 driver mouse or LEC-specific inhibition of all canonical Notch signaling by expressing a dominant-negative version of Mastermind-like (DN-MAML) results in an increase of PROX1+ LEC progenitor cells within the cardinal vein and defects in segregation of venous and lymphatic cells E14.5 (Murtomaki et al. 2013). In contrast, LEC-specific activation of NOTCH1 suppresses CoupTFII and Prox1 expression in the embryonic cardinal vein, resulting in fatal edema by E15.5 (Murtomaki et al. 2013).
NOTCH REGULATION OF SPROUTING LYMPHANGIOGENESIS; CONTROL OF TIP-STALK FATE IN LYMPHATICS
After migration of LEC progenitors out of the cardinal vein to form the primitive lymph sacs, sprouting lymphangiogenesis drives the formation of the initial lymphatic plexus (Ma and Oliver 2017). Lymphatic sprouting is controlled by signaling mediated by VEGFR3 expressed by LECs and in response to VEGFC in mesenchymal cells. VEGFR3 expression is also observed in the blood vasculature during early development (Dumont et al. 1998), but is critical during development at sites of active sprouting lymphangiogenesis (Jakobsson et al. 2009; Thomas et al. 2013). VEGFR3 expression becomes prominent in the lymphatic vasculature later in development and in adult animals (Jakobsson et al. 2009; Thomas et al. 2013).
As discussed above, VEGFR2 and Notch function in a feedback loop that underlies the process of sprouting angiogenesis (Hellström et al. 2007; Lobov et al. 2007; Suchting et al. 2007; Jakobsson et al. 2009; Thomas et al. 2013), and considering the similarities between blood and lymphatic sprouting angiogenesis, it can be hypothesized that VEGFR3 and Notch may be involved in a similar feedback loop during sprouting lymphangiogenesis. Notch and VEGFR3 cross-regulation in the blood and lymphatic vasculature has been demonstrated in a variety of settings, but the regulation appears to be context dependent (Jakobsson et al. 2009; Thomas et al. 2013). In the blood vasculature of zebrafish and mice, Notch suppresses Vegfr3 (Siekmann and Lawson 2007; Tammela et al. 2008; Hogan et al. 2009; Benedito et al. 2012) expression, but it has also been reported that Notch induces Vegfr3 (Shawber et al. 2007; Napp et al. 2012) in other studies. Thus, Notch regulates sprouting lymphangiogenesis but may play both prolymphangiogenic (Geudens et al. 2010; Niessen et al. 2011) or antilymphangiogenic roles, depending on the model system used and the context of analysis. Some have reported that Notch activation induces Vegfr3 transcription (Geudens et al. 2010; Murtomaki et al. 2013), whereas others found no change in VEGFR3 levels upon Notch activation (Niessen et al. 2011; Zheng et al. 2011). Studies in which Notch effectors HEY1 and HEY2 were ectopically expressed, however, have consistently shown reduced Vegfr3 transcripts (Kang et al. 2010; Murtomaki et al. 2013). We assume that context can control the level of negative feedback but the conditions for this difference are not well understood. We note that the methods in these divergent studies differ in use of Notch inhibitors; for example, neutralizing antibodies were used in the studies reported by Niessen et al. (2011), whereas a DLL4 decoy was used by Zheng et al. (2011). Thus, further study is needed to better understand the regulatory relationship between Notch signaling and VEGFR3 signaling.
Studies of the relationship between Notch and VEGFR3 in cultured HDLECs revealed that Notch and its downstream effectors HEY1/HEY2 transcriptionally regulate Vegfr3 in opposing manners (Murtomaki et al. 2013). This suggests that Notch directly induces Vegfr3 as a rapid transcriptional response, while also elevating Hey1 and Hey2 levels, direct transcriptional targets of Notch signaling (Murtomaki et al. 2013). As HEY1 and HEY2 levels increase in response to Notch activation, Vegfr3, a target of HEYs, is subsequently suppressed (Murtomaki et al. 2013). The dynamic transcriptional response of Vegfr3 may be central to regulating sprouting lymphangiogenesis by defining lymphatic tip and stalk cells (Murtomaki et al. 2013).
A ROLE FOR NOTCH IN LYMPHATIC MATURATION BY REGULATION OF LYMPHATIC VALVE CELL FATE DECISIONS
The maturation and remodeling of the lymphatic system lead to the formation of a fully functional hierarchy of small lymphatic capillaries, intermediate lymphatic precollectors, and large lymphatic collecting ducts. Critical to a functioning lymphatic system is formation of lymphatic valves (Bazigou et al. 2009; Sabine et al. 2012; Murtomaki et al. 2014). Initial lymphatic valve formation begins at E15.5 during murine embryonic development with clusters of high PROX1 (Prox1high) and FOXC2 expressing LEC in lymphatic vessels at sites where lymphatic valves will form. Soon after, there is local deposition of the matrix proteins laminin α5 and fibronectin-EIIIA (FN-EIIIA) at the clusters of Prox1high cells. VEGFR3, matrix adhesion protein integrin α9, the gap junction protein connexin 37, and calcineurin are also highly expressed and thought to be critical for valve maturation (Bazigou et al. 2009; Sabine et al. 2012; Murtomaki et al. 2014).
Analysis of NOTCH1 expression during lymphatic valve formation and maturation shows that NOTCH1 is highly expressed in lymphatic valves by E18.5, colocalizing with other valve markers such as VEGFR3 and integrin α9 (Murtomaki et al. 2014). Examination of Notch signaling using a transgenic Notch signaling reporter mouse demonstrates high levels of Notch signaling in lymphatic valve ECs when VEGFR3 and integrin α9 are up-regulated in forming valves. These lymphatic valve drivers may themselves be regulated by Notch, as Notch activation in HDLECs transcriptionally induces integrin α9, FN-EIIIA, and connexin37, while FOXC2 and calcineurin transcripts are not significantly changed (Murtomaki et al. 2014).
To determine the functional role of NOTCH1 and Notch signaling in lymphatic valve formation, LEC-specific loss of Notch1 (dependent on Prox1CreERT2) or LEC-specific inhibition of all canonical Notch signaling (DN-MAML) between E13.5 and E15.5 was used. The genetic loss of either Notch1 or canonical Notch signaling results in an increase of LECs with high Prox1high expression in lymphatic vessels during the time when lymphatic valves are forming. Analysis of the nascent lymphatic valves reveals defective reorientation and clustering of Prox1high LECs at putative valve sites. Loss of Notch signaling leads to decreased expression of valve markers, or fewer prospective lymphatic valve cells (Murtomaki et al. 2014). In conclusion, Notch functions as a positive regulator of lymphatic valve formation, highlighting an additional key cell fate step in lymphatic vascular development driven by Notch signaling.
ACKNOWLEDGMENTS
This work was supported by NHLBI Award 1R01 HL112626 (J.K.K.). Figures were created with or adapted from BioRender.com.
Footnotes
Editors: Diane R. Bielenberg and Patricia A. D'Amore
Additional Perspectives on Angiogenesis: Biology and Pathology available at www.perspectivesinmedicine.org
REFERENCES
- Arima S, Nishiyama K, Ko T, Arima Y, Hakozaki Y, Sugihara K, Koseki H, Uchijima Y, Kurihara Y, Kurihara H. 2011. Angiogenic morphogenesis driven by dynamic and heterogeneous collective endothelial cell movement. Development 138: 4763–4776. 10.1242/dev.068023 [DOI] [PubMed] [Google Scholar]
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. 2010. Pericytes regulate the blood–brain barrier. Nature 468: 557–561. 10.1038/nature09522 [DOI] [PubMed] [Google Scholar]
- Bazigou E, Xie S, Chen C, Weston A, Miura N, Sorokin L, Adams R, Muro AF, Sheppard D, Makinen T. 2009. Integrin-α9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell 17: 175–186. 10.1016/j.devcel.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedito R, Roca C, Sörensen I, Adams S, Gossler A, Fruttiger M, Adams RH. 2009. The Notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137: 1124–1135. 10.1016/j.cell.2009.03.025 [DOI] [PubMed] [Google Scholar]
- Benedito R, Rocha SF, Woeste M, Zamykal M, Radtke F, Casanovas O, Duarte A, Pytowski B, Adams RH. 2012. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature 484: 110–114. 10.1038/nature10908 [DOI] [PubMed] [Google Scholar]
- Benjamin LE, Hemo I, Keshet E. 1998. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 125: 1591–1598. 10.1242/dev.125.9.1591 [DOI] [PubMed] [Google Scholar]
- Chang WG, Andrejecsk JW, Kluger MS, Saltzman WM, Pober JS. 2013. Pericytes modulate endothelial sprouting. Cardiovasc Res 100: 492–500. 10.1093/cvr/cvt215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Qin J, Cheng CM, Tsai MJ, Tsai SY. 2012. COUP-TFII is a major regulator of cell cycle and Notch signaling pathways. Mol Endocrinol 26: 1268–1277. 10.1210/me.2011-1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Engelhardt B. 2017. Brain barriers in health and disease. Neurobiol Dis 107: 1–3. 10.1016/j.nbd.2017.05.008 [DOI] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. 2010. Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature 468: 562–566. 10.1038/nature09513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- dela Paz NG, D'Amore PA. 2009. Arterial versus venous endothelial cells. Cell Tissue Res 335: 5–16. 10.1007/s00441-008-0706-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, Bekman E, Costa L, Henrique D, Rossant J. 2004. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev 18: 2474–2478. 10.1101/gad.1239004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K. 1998. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science 282: 946–949. 10.1126/science.282.5390.946 [DOI] [PubMed] [Google Scholar]
- Falcón BL, Hashizume H, Koumoutsakos P, Chou J, Bready JV, Coxon A, Oliner JD, McDonald DM. 2009. Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am J Pathol 175: 2159–2170. 10.2353/ajpath.2009.090391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Hirschi K. 2019. Molecular regulation of arteriovenous endothelial cell specification. F1000Res 8: 1208. 10.12688/f1000research.16701.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang JS, Coon BG, Gillis N, Chen Z, Qiu J, Chittenden TW, Burt JM, Schwartz MA, Hirschi KK. 2017. Shear-induced Notch-Cx37-p27 axis arrests endothelial cell cycle to enable arterial specification. Nat Commun 8: 2149. 10.1038/s41467-017-01742-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- François M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M, et al. 2008. Sox18 induces development of the lymphatic vasculature in mice. Nature 456: 643–647. 10.1038/nature07391 [DOI] [PubMed] [Google Scholar]
- Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, et al. 2004. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci 101: 15949–15954. 10.1073/pnas.0407290101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, et al. 2003. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161: 1163–1177. 10.1083/jcb.200302047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geudens I, Herpers R, Hermans K, Segura I, Ruiz de Almodovar C, Bussmann J, De Smet F, Vandevelde W, Hogan BM, Siekmann A, et al. 2010. Role of delta-like-4/Notch in the formation and wiring of the lymphatic network in zebrafish. Arterioscler Thromb Vasc Biol 30: 1695–1702. 10.1161/ATVBAHA.110.203034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon WR, Zimmerman B, He L, Miles LJ, Huang J, Tiyanont K, McArthur DG, Aster JC, Perrimon N, Loparo JJ, et al. 2015. Mechanical allostery: evidence for a force requirement in the proteolytic activation of Notch. Dev Cell 33: 729–736. 10.1016/j.devcel.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald I. 2012. Notch and the awesome power of genetics. Genetics 191: 655–669. 10.1534/genetics.112.141812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LS, Sainson RC, Williams CK, Taylor JM, Shi W, Li JL, Harris AL. 2008. Regulation of multiple angiogenic pathways by Dll4 and Notch in human umbilical vein endothelial cells. Microvasc Res 75: 144–154. 10.1016/j.mvr.2007.06.006 [DOI] [PubMed] [Google Scholar]
- Harvey NL, Oliver G. 2004. Choose your fate: artery, vein or lymphatic vessel? Curr Opin Genet Dev 14: 499–505. 10.1016/j.gde.2004.07.005 [DOI] [PubMed] [Google Scholar]
- Hasan SS, Tsaryk R, Lange M, Wisniewski L, Moore JC, Lawson ND, Wojciechowska K, Schnittler H, Siekmann AF. 2017. Endothelial Notch signalling limits angiogenesis via control of artery formation. Nat Cell Biol 19: 928–940. 10.1038/ncb3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström M, Gerhardt H, Kalén M, Li X, Eriksson U, Wolburg H, Betsholtz C. 2001. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol 153: 543–554. 10.1083/jcb.153.3.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, et al. 2007. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445: 776–780. 10.1038/nature05571 [DOI] [PubMed] [Google Scholar]
- Hogan BM, Herpers R, Witte M, Heloterä H, Alitalo K, Duckers HJ, Schulte-Merker S. 2009. Vegfc/Flt4 signalling is suppressed by Dll4 in developing zebrafish intersegmental arteries. Development 136: 4001–4009. 10.1242/dev.039990 [DOI] [PubMed] [Google Scholar]
- Jakobsson L, Bentley K, Gerhardt H. 2009. VEGFRs and Notch: a dynamic collaboration in vascular patterning. Biochem Soc Trans 37: 1233–1236. 10.1042/BST0371233 [DOI] [PubMed] [Google Scholar]
- Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, et al. 2010. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol 12: 943–953. 10.1038/ncb2103 [DOI] [PubMed] [Google Scholar]
- Kang J, Yoo J, Lee S, Tang W, Aguilar B, Ramu S, Choi I, Otu HH, Shin JW, Dotto GP, et al. 2010. An exquisite cross-control mechanism among endothelial cell fate regulators directs the plasticity and heterogeneity of lymphatic endothelial cells. Blood 116: 140–150. 10.1182/blood-2009-11-252270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, et al. 2004. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 5: 74–80. 10.1038/ni1013 [DOI] [PubMed] [Google Scholar]
- Koch PS, Lee KH, Goerdt S, Augustin HG. 2021. Angiodiversity and organotypic functions of sinusoidal endothelial cells. Angiogenesis 24: 289–310. 10.1007/s10456-021-09780-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, et al. 2000. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev 14: 1343–1352. 10.1101/gad.14.11.1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. 2004. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev 18: 2469–2473. 10.1101/gad.1239204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Starling C, Chervonsky AV, Gridley T. 2010. Notch1 activation in mice causes arteriovenous malformations phenocopied by ephrinB2 and EphB4 mutants. Genesis 48: 146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai EC. 2004. Notch signaling: control of cell communication and cell fate. Development 131: 965–973. 10.1242/dev.01074 [DOI] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. 2001. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128: 3675–3683. 10.1242/dev.128.19.3675 [DOI] [PubMed] [Google Scholar]
- Liu H, Kennard S, Lilly B. 2009. NOTCH3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed JAGGED1. Circ Res 104: 466–475. 10.1161/CIRCRESAHA.108.184846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. 2007. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci 104: 3219–3224. 10.1073/pnas.0611206104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca VC, Kim BC, Ge C, Kakuda S, Wu D, Roein-Peikar M, Haltiwanger RS, Zhu C, Ha T, Garcia KC. 2017. Notch-Jagged complex structure implicates a catch bond in tuning ligand sensitivity. Science 355: 1320–1324. 10.1126/science.aaf9739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Garcia-Gonzalez I, Fernández-Chacón M, Casquero-Garcia V, Sanchez-Muñoz MS, Mühleder S, Garcia-Ortega L, Andrade J, Potente M, Benedito R. 2021. Arterialization requires the timely suppression of cell growth. Nature 589: 437–441. 10.1038/s41586-020-3018-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Oliver G. 2017. Lymphatic endothelial cell plasticity in development and disease. Physiology (Bethesda) 32: 444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maby-El Hajjami H, Petrova TV. 2008. Developmental and pathological lymphangiogenesis: from models to human disease. Histochem Cell Biol 130: 1063–1078. 10.1007/s00418-008-0525-5 [DOI] [PubMed] [Google Scholar]
- Mack JJ, Mosqueiro TS, Archer BJ, Jones WM, Sunshine H, Faas GC, Briot A, Aragón RL, Su T, Romay MC, et al. 2017. NOTCH1 is a mechanosensor in adult arteries. Nat Commun 8: 1620. 10.1038/s41467-017-01741-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelo KL, Goldie LC, Hirschi KK. 2013. Regulation of endothelial cell differentiation and specification. Circ Res 112: 1272–1287. 10.1161/CIRCRESAHA.113.300506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya IM, Umans L, Maas E, Pereira PN, Beets K, Francis A, Sents W, Robertson EJ, Mummery CL, Huylebroeck D, et al. 2012. Stalk cell phenotype depends on integration of Notch and Smad1/5 signaling cascades. Dev Cell 22: 501–514. 10.1016/j.devcel.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PA, Kim TN, Huang L, Nielsen CM, Lawton MT, Adams RH, Schaffer CB, Wang RA. 2014. Constitutively active Notch4 receptor elicits brain arteriovenous malformations through enlargement of capillary-like vessels. Proc Natl Acad Sci 111: 18007–18012. 10.1073/pnas.1415316111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtomaki A, Uh MK, Choi YK, Kitajewski C, Borisenko V, Kitajewski J, Shawber CJ. 2013. Notch1 functions as a negative regulator of lymphatic endothelial cell differentiation in the venous endothelium. Development 140: 2365–2376. 10.1242/dev.083865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtomaki A, Uh MK, Kitajewski C, Zhao J, Nagasaki T, Shawber CJ, Kitajewski J. 2014. Notch signaling functions in lymphatic valve formation. Development 141: 2446–2451. 10.1242/dev.101188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napp LC, Augustynik M, Paesler F, Krishnasamy K, Woiterski J, Limbourg A, Bauersachs J, Drexler H, Le Noble F, Limbourg FP. 2012. Extrinsic Notch ligand Delta-like 1 regulates tip cell selection and vascular branching morphogenesis. Circ Res 110: 530–535. 10.1161/CIRCRESAHA.111.263319 [DOI] [PubMed] [Google Scholar]
- Nielsen CM, Cuervo H, Ding VW, Kong Y, Huang EJ, Wang RA. 2014. Deletion of Rbpj from postnatal endothelium leads to abnormal arteriovenous shunting in mice. Development 141: 3782–3792. 10.1242/dev.108951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen K, Zhang G, Ridgway JB, Chen H, Kolumam G, Siebel CW, Yan M. 2011. The Notch1-Dll4 signaling pathway regulates mouse postnatal lymphatic development. Blood 118: 1989–1997. 10.1182/blood-2010-11-319129 [DOI] [PubMed] [Google Scholar]
- Oliver G. 2004. Lymphatic vasculature development. Nat Rev Immunol 4: 35–45. 10.1038/nri1258 [DOI] [PubMed] [Google Scholar]
- Oliver G, Srinivasan RS. 2010. Endothelial cell plasticity: how to become and remain a lymphatic endothelial cell. Development 137: 363–372. 10.1242/dev.035360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. 2006. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol 7: 359–371. 10.1038/nrm1911 [DOI] [PubMed] [Google Scholar]
- Park JE, Chen HH, Winer J, Houck KA, Ferrara N. 1994. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem 269: 25646–25654. 10.1016/S0021-9258(18)47298-5 [DOI] [PubMed] [Google Scholar]
- Payne LB, Zhao H, James CC, Darden J, McGuire D, Taylor S, Smyth JW, Chappell JC. 2019. The pericyte microenvironment during vascular development. Microcirculation 26: e12554. 10.1111/micc.12554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitulescu ME, Schmidt I, Giaimo BD, Antoine T, Berkenfeld F, Ferrante F, Park H, Ehling M, Biljes D, Rocha SF, et al. 2017. Dll4 and Notch signalling couples sprouting angiogenesis and artery formation. Nat Cell Biol 19: 915–927. 10.1038/ncb3555 [DOI] [PubMed] [Google Scholar]
- Pontes-Quero S, Fernández-Chacón M, Luo W, Lunella FF, Casquero-Garcia V, Garcia-Gonzalez I, Hermoso A, Rocha SF, Bansal M, Benedito R. 2019. High mitogenic stimulation arrests angiogenesis. Nat Commun 10: 2016. 10.1038/s41467-019-09875-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy SK, Kusumbe AP, Adams RH. 2015. Regulation of tissue morphogenesis by endothelial cell-derived signals. Trends Cell Biol 25: 148–157. 10.1016/j.tcb.2014.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Red-Horse K, Ueno H, Weissman IL, Krasnow MA. 2010. Coronary arteries form by developmental reprogramming of venous cells. Nature 464: 549–553. 10.1038/nature08873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca C, Adams RH. 2007. Regulation of vascular morphogenesis by Notch signaling. Genes Dev 21: 2511–2524. 10.1101/gad.1589207 [DOI] [PubMed] [Google Scholar]
- Sabin F. 1902. On the origin of the lymphatic system from the veins, and the development of the lymph hearts and thoracic duct in the pig. Am J Anat 1: 367–389. 10.1002/aja.1000010310 [DOI] [Google Scholar]
- Sabine A, Agalarov Y, Maby-El Hajjami H, Jaquet M, Hägerling R, Pollmann C, Bebber D, Pfenniger A, Miura N, Dormond O, et al. 2012. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev Cell 22: 430–445. 10.1016/j.devcel.2011.12.020 [DOI] [PubMed] [Google Scholar]
- Sainson RC, Aoto J, Nakatsu MN, Holderfield M, Conn E, Koller E, Hughes CC. 2005. Cell-autonomous Notch signaling regulates endothelial cell branching and proliferation during vascular tubulogenesis. FASEB J 19: 1027–1029. 10.1096/fj.04-3172fje [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Sabine A, Petrova TV. 2011. Lymphatic vascular morphogenesis in development, physiology, and disease. J Cell Biol 193: 607–618. 10.1083/jcb.201012094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawber CJ, Funahashi Y, Francisco E, Vorontchikhina M, Kitamura Y, Stowell SA, Borisenko V, Feirt N, Podgrabinska S, Shiraishi K, et al. 2007. Notch alters VEGF responsiveness in human and murine endothelial cells by direct regulation of VEGFR-3 expression. J Clin Invest 117: 3369–3382. 10.1172/JCI24311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekmann AF, Lawson ND. 2007. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 445: 781–784. 10.1038/nature05577 [DOI] [PubMed] [Google Scholar]
- Simonavicius N, Ashenden M, van Weverwijk A, Lax S, Huso DL, Buckley CD, Huijbers IJ, Yarwood H, Isacke CM. 2012. Pericytes promote selective vessel regression to regulate vascular patterning. Blood 120: 1516–1527. 10.1182/blood-2011-01-332338 [DOI] [PubMed] [Google Scholar]
- Sinha S, Santoro MM. 2018. New models to study vascular mural cell embryonic origin: implications in vascular diseases. Cardiovasc Res 114: 481–491. 10.1093/cvr/cvy005 [DOI] [PubMed] [Google Scholar]
- Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, Oliver G. 2007. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev 21: 2422–2432. 10.1101/gad.1588407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan RS, Geng X, Yang Y, Wang Y, Mukatira S, Studer M, Porto MP, Lagutin O, Oliver G. 2010. The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev 24: 696–707. 10.1101/gad.1859310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova D, Byrne HM, Maini PK, Alarcón T. 2021. A multiscale model of complex endothelial cell dynamics in early angiogenesis. PLoS Comput Biol 17: e1008055. 10.1371/journal.pcbi.1008055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. 2009. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 114: 5091–5101. 10.1182/blood-2009-05-222364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T, Stanley G, Sinha R, D'Amato G, Das S, Rhee S, Chang AH, Poduri A, Raftrey B, Dinh TT, et al. 2018. Single-cell analysis of early progenitor cells that build coronary arteries. Nature 559: 356–362. 10.1038/s41586-018-0288-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, Eichmann A. 2007. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci 104: 3225–3230. 10.1073/pnas.0611177104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift MR, Weinstein BM. 2009. Arterial-venous specification during development. Circ Res 104: 576–588. 10.1161/CIRCRESAHA.108.188805 [DOI] [PubMed] [Google Scholar]
- Tammela T, Alitalo K. 2010. Lymphangiogenesis: molecular mechanisms and future promise. Cell 140: 460–476. 10.1016/j.cell.2010.01.045 [DOI] [PubMed] [Google Scholar]
- Tammela T, Zarkada G, Wallgard E, Murtomäki A, Suchting S, Wirzenius M, Waltari M, Hellström M, Schomber T, Peltonen R, et al. 2008. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 454: 656–660. 10.1038/nature07083 [DOI] [PubMed] [Google Scholar]
- Thomas JL, Baker K, Han J, Calvo C, Nurmi H, Eichmann AC, Alitalo K. 2013. Interactions between VEGFR and Notch signaling pathways in endothelial and neural cells. Cell Mol Life Sci 70: 1779–1792. 10.1007/s00018-013-1312-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. 1999. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 286: 2511–2514. 10.1126/science.286.5449.2511 [DOI] [PubMed] [Google Scholar]
- Tung JJ, Tattersall IW, Kitajewski J. 2012. Tips, stalks, tubes: Notch-mediated cell fate determination and mechanisms of tubulogenesis during angiogenesis. Cold Spring Harb Perspect Med 2: a006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udan RS, Culver JC, Dickinson ME. 2013. Understanding vascular development. Wiley Interdiscip Rev Dev Biol 2: 327–346. 10.1002/wdev.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttendaele H, Marazzi G, Wu G, Yan Q, Sassoon D, Kitajewski J. 1996. Notch4/int-3, a mammary proto-oncogene, is an endothelial cell-specific mammalian Notch gene. Development 122: 2251–2259. 10.1242/dev.122.7.2251 [DOI] [PubMed] [Google Scholar]
- Uyttendaele H, Ho J, Rossant J, Kitajewski J. 2001. Vascular patterning defects associated with expression of activated Notch4 in embryonic endothelium. Proc Natl Acad Sci 98: 5643–5648. 10.1073/pnas.091584598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Laviña B, Gouveia L, et al. 2018. A molecular atlas of cell types and zonation in the brain vasculature. Nature 554: 475–480. 10.1038/nature25739 [DOI] [PubMed] [Google Scholar]
- Vella G, Guelfi S, Bergers G. 2021. High endothelial venules: a vascular perspective on tertiary lymphoid structures in cancer. Front Immunol 12: 736670. 10.3389/fimmu.2021.736670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa N, Walker L, Lindsell CE, Gasson J, Iruela-Arispe ML, Weinmaster G. 2001. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev 108: 161–164. 10.1016/S0925-4773(01)00469-5 [DOI] [PubMed] [Google Scholar]
- Wakui S, Yokoo K, Muto T, Suzuki Y, Takahashi H, Furusato M, Hano H, Endou H, Kanai Y. 2006. Localization of Ang-1, -2, Tie-2, and VEGF expression at endothelial-pericyte interdigitation in rat angiogenesis. Lab Invest 86: 1172–1184. 10.1038/labinvest.3700476 [DOI] [PubMed] [Google Scholar]
- Wang X, Ha T. 2013. Defining single molecular forces required to activate integrin and Notch signaling. Science 340: 991–994. 10.1126/science.1231041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson EC, Adams RH. 2018. Biology of bone: the vasculature of the skeletal system. Cold Spring Harb Perspect Med 8: a031559. 10.1101/cshperspect.a031559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle JT, Oliver G. 1999. Prox1 function is required for the development of the murine lymphatic system. Cell 98: 769–778. 10.1016/S0092-8674(00)81511-1 [DOI] [PubMed] [Google Scholar]
- Xu C, Hasan SS, Schmidt I, Rocha SF, Pitulescu ME, Bussmann J, Meyen D, Raz E, Adams RH, Siekmann AF. 2014. Arteries are formed by vein-derived endothelial tip cells. Nat Commun 5: 5758. 10.1038/ncomms6758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Gao X, Lindsell CE, Norton CR, Chang B, Hicks C, Gendron-Maguire M, Rand EB, Weinmaster G, Gridley T. 1999. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet 8: 723–730. 10.1093/hmg/8.5.723 [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Muramatsu M, Azuma E, Ikutani M, Nagai Y, Sagara H, Koo BN, Kita S, O'Donnell E, Osawa T, et al. 2017. A subset of cerebrovascular pericytes originates from mature macrophages in the very early phase of vascular development in CNS. Sci Rep 7: 3855. 10.1038/s41598-017-03994-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkada G, Heinolainen K, Makinen T, Kubota Y, Alitalo K. 2015. VEGFR3 does not sustain retinal angiogenesis without VEGFR2. Proc Natl Acad Sci 112: 761–766. 10.1073/pnas.1423278112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Chappell JC. 2019. Microvascular bioengineering: a focus on pericytes. J Biol Eng 13: 26. 10.1186/s13036-019-0158-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Tammela T, Yamamoto M, Anisimov A, Holopainen T, Kaijalainen S, Karpanen T, Lehti K, Ylä-Herttuala S, Alitalo K. 2011. Notch restricts lymphatic vessel sprouting induced by vascular endothelial growth factor. Blood 118: 1154–1162. 10.1182/blood-2010-11-317800 [DOI] [PubMed] [Google Scholar]