Abstract
While evolutionary explanations for aging have been widely acknowledged, the application of evolutionary principles to the practice of aging research has, until recently, been limited. Aging research has been dominated by studies of populations in evolutionarily novel industrialized environments and by use of short-lived animal models that are distantly related to humans. In this review, I address several emerging areas of “evolutionarily relevant” aging research, which provide a valuable complement to conventional biomedical research on aging. Nonhuman primates offer particular value as both translational and comparative models due to their long life spans, shared evolutionary history with humans, and social complexity. Additionally, because the human organism evolved in a radically different environment than that in which most humans live today, studying populations living in diverse ecologies has redefined our understanding of healthy aging by revealing the contribution of industrialized human environments to age-related pathologies.
EVOLUTIONARY THEORY OF AGING
Evolutionary theory has long been at the heart of aging science as a way to explain why we age. Purely mechanical views of aging propose that like any machine, biological systems inevitably deteriorate over time. In contrast, evolutionary biologists have rooted their inquiry into how and why the negative effects of aging have been maintained in the face of natural selection, a process that is expected to eliminate detrimental traits. Early evolutionary theories of aging attempted to address why natural selection may simply be unable to counteract the aging process. For example, the Mutation Accumulation Theory explains that natural selection has a diminished capacity to eliminate genetic mutations that cause disease or dysfunction late in the life span, as these traits will be passed on through reproduction before their negative effects begin to manifest (Haldane 1941; Medawar 1946, 1952). Subsequent theories embraced this core idea but proposed that the imbalance between the force of selection early versus late in life may not just fail to remove harmful late-acting genes but may actively promote them. Williams originally formalized the concept of “antagonistic pleiotropy,” demonstrating that genes that have positive effects on reproductive success early in life will be favored even if they produce negative effects on survival (Williams 1957). Williams’ observation was central to the development of life history theory, which poses that when organisms spend energy on reproduction, they have less energy available to invest in somatic maintenance (Williams 1966; Stearns 1989).
These core ideas have evolved as the understanding of aging biology has increased. One of the most prominent contemporary evolutionary theories of aging, the Disposable Soma theory, proposes a mechanistic extension of antagonistic pleiotropy: damage accumulates over the life span because it is less advantageous to spend energy on damage repair and prevention than it is to spend the same energy on reproductive effort (Kirkwood 1977; Kirkwood and Rose 1991). Although Kirkwood's original formulation of this theory focused on the accumulation of errors during cell divisions, this essential trade-off can affect diverse aging mechanisms. The Disposable Soma theory is frequently applied to proximate trade-offs within species, whereby individuals with higher reproductive investment are expected to experience accelerated aging. However, persistence of these trade-offs over evolutionary time is predicted to set a rate of somatic repair that promotes senescence even in the absence of reproduction.
The Developmental Theory of Aging interprets the principle of antagonistic pleiotropy in a different way, by positing that aging results as a side effect of mechanisms that have been optimized by natural selection to promote successful development (de Magalhães and Church 2005). Some physiological processes calibrated for healthy development appear to exert damaging effects when maintained over time, while other types of age-related deterioration occur because processes important in development become less active over time. Together, these theories contribute to a growing explanatory framework, which, while still incomplete, can be used to generate new lines of research.
Whereas evolutionary influences on aging are widely acknowledged, evolutionarily minded approaches to aging are uncommon. The perspective that aging is a natural biological process is often juxtaposed with the medical view of aging as a “disease” that could be cured to extend human life spans (de Magalhães 2014; Bulterijs et al. 2015; Blagosklonny 2018). The emerging field of geroscience shifts this perspective in that it embraces aging as an inevitable part of our biology, but targets the extension of disease-free life, or “health span” (Burch et al. 2014; Kennedy et al. 2014). Nevertheless, research effort remains largely focused on the diseases of aging rather than on the biology of aging itself (Hayflick 2000, 2004). To a certain extent, this is necessary to target interventions at the leading causes of death and disability. Yet, by asking how the biology of aging has been shaped by natural selection, evolutionary approaches can generate unique perspectives on the origins of disease and the potential success (or harm) of interventions. Embedded in this way of thinking is that biological traits evolve in response to challenges posed by the environment across the life course. Exposure to different environments affects health directly but may also alter the salience of particular risk factors or the effects of interventions.
The evolutionary approach to aging has undergone a recent revolution, guided by the principles of evolutionary medicine, which uses knowledge about how human biology evolved to better understand the factors influencing health (Grunspan et al. 2018). Here, I highlight “evolutionarily relevant” approaches that are being used alongside conventional biomedical approaches to develop a holistic science of aging. These involve comparative biodemography to identify evolutionary trends in aging, the selection of model species with longer life spans and closer genetic relationships to humans, and the study of aging across a broader range of ecological contexts.
EVOLUTIONARY BIODEMOGRAPHY OF AGING
Evolutionary biodemographers have made important contributions toward understanding aging in the context of broader life history evolution. With this approach, the effects of aging are identified at the species or population level via the expected increase in mortality rates across adulthood (Jones et al. 2014). Broad cross-species comparisons identify patterns of evolutionary change, such as in similarities in life span or patterns of mortality shared by species with a common ancestry. However, in revealing the diversity of life history patterns possible, these studies have also challenged assumptions and identified limitations to existing evolutionary theories of aging (Baudisch 2012).
Among the most surprising revelations from evolutionary biodemographic approaches is that aging is not a universal characteristic of living things, or even of animals. Whereas mammals share a pattern of accelerated mortality with age, mortality rates of some animals remain relatively constant throughout adulthood, and some even exhibit “negative aging,” whereby mortality risks are reduced at later ages (Jones et al. 2014). Within this variation are clear phylogenetic effects, supporting the hypothesis that some attributes of aging in a given species are likely to be inherited from deep in the evolutionary past and may be subject to selective constraints. Mammals show more evidence of these constraints than do other broad taxa, such as birds. However, important variation in mortality patterns exists even among closely related taxa. Following the logic of evolutionary trade-offs, it is hypothesized that much of this variation may be linked to the effects of widely varying growth and reproductive patterns across species (Jones et al. 2014). This hypothesis is supported by the considerable sex differences observed in many species where reproductive effort of males and females differs in timing or intensity. Surprisingly, the shape of mortality profiles does not appear to be intrinsically related to life span.
Accordingly, biodemographers have found it useful to distinguish the “shape” of aging, defined as above by the relative rate at which mortality changes across the adult life span, from the “pace” of aging defined by measures like longevity or life expectancy (Baudisch 2011). Humans’ long life spans would suggest a slow rate of senescence, but when standardized for differences in life span, humans experience a steeper increase in mortality than many shorter-lived species. This pace-standardized approach also provides a way to examine “life span equality,” or how evenly ages of death are distributed across the life span (Wrycza et al. 2015).
Primates are long-lived compared to other mammals of equivalent body size (Charnov and Berrigan 1993). Within the Primate Order, human mortality patterns are not distinct, but fall along a continuum with other primates species (Bronikowski et al. 2011) Whereas life span and life span equality vary independently across mammals, they are positively correlated across primate species (Colchero et al. 2016) and are very tightly correlated across populations in any particular primate species or genus (Colchero et al. 2021). For example, variation in life expectancy across human populations experiencing diverse environments is overwhelmingly explained by differences in life span equality (Edwards and Tuljapurkar 2005; Colchero et al. 2016; Németh 2017). In other words, populations that experience higher life expectancy do so because fewer individuals die at young ages and not because they exhibit slower aging. By contrast, selection on the rate of aging explains differences in longevity observed between primate species (Colchero et al. 2021). An important implication is that although life expectancy has rapidly increased in industrialized populations, as life span equality plateaus, we may be reaching the natural limits of human life spans.
EVOLUTIONARY-RELEVANT ANIMAL MODELS
Laboratory animal models have been a critical resource for investigating the mechanisms of aging (Conn 2011). Selected species are typically small and short-lived, making it feasible to study the effects of experimental interventions across entire life spans and large samples. Many model organisms are selected because they exhibit extraordinary anti-aging mechanisms, or alternatively because they experience close analogs to human age-related diseases. However, significant physiological differences result from the great evolutionary distance that separates humans from these species (Chiou et al. 2020), yielding a high rate of translation failures from simple animal models (McGonigle and Ruggeri 2014). Indeed, even the relatively small differences between rats and mice or among different strains of these species can influence the outcomes of experiments, and the effects of artificial laboratory breeding and environments may confound the generalizability of results (Mitchell et al. 2015). Aside from the problems of taxonomic divergence, it may also be unrealistic to generalize findings from short-lived species when the evolution of longer life spans likely involved fundamental changes to the aging process.
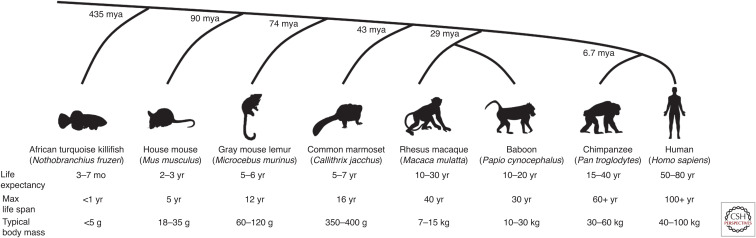
Nonhuman primate models present an attractive middle ground (Fig. 1). Their close evolutionary relationship to humans is associated with increased genetic and physiological similarities, yet life spans are often short enough to be feasible for study (Lavery 2000; Shively and Clarkson 2009; Colman 2018; Emery Thompson et al. 2020d). Where similarities to humans can be identified, they are likely to be a result of common descent, increasing the probability that these are true functional similarities. Whereas studying nonhuman primates involves stricter ethical considerations, many species can be practicably housed and managed in captivity. Most captive nonhuman primate populations also offer greater genetic diversity than is typical of other model organisms.
Figure 1.
Common vertebrate aging models: life history comparison and relatedness to humans (see Dyban et al. 1991; Altmann et al. 1993; Colman et al. 1998; Altmann and Alberts 2003; Tardif et al. 2011; Languille et al. 2012; Reuter et al. 2018; Chiou et al. 2020; Emery Thompson and Sabbi 2022). Estimated divergence time from humans in millions of years ago (mya): Timetree.org (Kumar et al. 2017).
Two primate models, mouse lemurs and callitrichids (a neotropical group including marmosets and tamarins), offer several of the advantages of conventional animal models in that they are small, short-lived, and easy to maintain in captivity. However, as primates, they live considerably longer than other mammals of the same size. For example, the gray mouse lemur, a prosimian primate, is rodent-sized but can live 12 years (Languille et al. 2012). Rather than being used explicitly as genetic or disease models, mouse lemurs have been used primarily to study natural aging processes, including changes in physiological regulation, motor function, and cognition (Languille et al. 2012), although they also show promise as models for Alzheimer's disease and related pathologies (Bons et al. 2006). Marmosets, the smallest and shortest lived of the anthropoid primates, develop many human-like aging diseases in captivity, distinguishing them from rodent models (Tardif et al. 2011; Ross 2019).
The most abundant and commonly used nonhuman primate models in captivity are macaques, which take a significant step closer to humans in terms of body size, life span, and genetic relatedness (Chiou et al. 2020). They are attractive models not because of any specific shared aging mechanisms, but because they exhibit many similarities in aging across domains, offering enhanced potential to model complex aging phenotypes. Additionally, they are particularly prone to obesity when fed processed captive diets, and develop associated metabolic diseases (Simmons 2016). Thus, macaques were instrumental in developing the paradigm that caloric restriction is associated with healthier aging (Masoro 2000; Lane et al. 2001; Mattison et al. 2017). Captive macaques have also been useful for studying interactions of diets with other risk factors in the development of atherosclerosis (Shively and Clarkson 1994; Shively et al. 2009). Whereas rhesus macaques have scarcely been studied in the wild, a large research colony of free-ranging rhesus macaques are maintained on the island of Cayo Santiago in Puerto Rico (Rawlins and Kessler 1986). Here, animals can be studied in a relatively naturalistic social and ecological context, yet it is still possible to perform some procedures that could not be done routinely in a fully wild setting, such as blood draws, veterinary examinations, and histopathology. This setting is emerging as a natural laboratory for aging research (Chiou et al. 2020).
PRIMATES AS ESSENTIAL MODELS FOR SOCIAL DETERMINANTS OF AGING
Nonhuman primates are of special significance for the study of social aging and the social determinants of health. Whereas research in human populations has established clear and compelling interactions between social relationships and health (Sapolsky 2004; Uchino 2009; Holt-Lunstad et al. 2010), progress in this area is hampered by the difficulty of deriving tractable social experience variables, particularly when influential environments may have occurred decades prior to enrollment in a research study. Many nonhuman primate species live in large, stable social groups with status hierarchies and complex differentiation of social relationships, but social networks can be more easily defined than in many human populations. Nonhuman primate social behavior can also be freely observed and objectively quantified. Several long-term research studies of free-ranging and wild primates have recorded social behavior of the same cohorts of animals on a near-daily basis for decades, and these studies have generated rigorous metrics of social relationship quality and social status.
Like humans, nonhuman primates experience significant and independent influences of social integration and social status on survival (Silk et al. 2003, 2010; McFarland and Majolo 2013; Archie et al. 2014; Brent et al. 2017; Thompson and Cords 2018; Ellis et al. 2019; Campos et al. 2020), indicating that they are not only a convenient model but an appropriate one for investigating the mechanisms by which social environments impact human aging. Studies of primates allow for investigation of social influences on health without many of the confounding factors affecting human studies, such as variable access to medical care and health information, smoking, and substance use.
Status (or dominance) hierarchies are near-universal features of primate societies and, as in humans, are associated with unequal access to resources. The complexity of social competition in many primates is also thought to lead to a relatively unique association between status and chronic psychosocial stress, compounding the direct effects of resource inequality (Sapolsky 2021). Like in humans, low social status in primates is typically associated with elevated glucocorticoids and increased mortality (Cavigelli and Caruso 2015; Shively and Day 2015; Snyder-Mackler et al. 2020), supporting the hypothesis that stress may be a common pathway by which social factors influence multiple dimensions of health (Sapolsky et al. 1987; Sapolsky 2005). Primate affiliative bonds are hypothesized to influence health through similar pathways, such as by increasing access to resources, reducing exposure to stressful events, or by buffering the stress response (Ostner and Schüelke 2018; Thompson 2019). The form and function of social relationships vary considerably within and between primate species, offering excellent potential for modeling different feature of social environments.
Researchers studying the interaction of primate social environments and health have increasingly favored naturalistic studies where individuals have greater freedom of association and experience the full range of environmental selection pressures that are likely to have shaped the evolution of both social behavior and of aging. However, there are ethical and logistical impediments to obtain detailed biomedical data. The Amboseli Baboon Research Project has conducted a continuous, longitudinal study of individually recognized wild baboons (Papio cynocephalus) in Kenya for five decades, amassing extensive ecological, behavioral, and demographic data sets (Alberts and Altmann 2012). Routine, noninvasive fecal sampling is conducted, allowing for the quantification of glucocorticoids, parasites, and genetic relatedness. Because baboons are primarily terrestrial, it is also possible to safely immobilize the baboons with tranquilizer darts, allowing for occasional sampling of skin and blood for high-quality DNA and RNA. In this wild system, females acquire rank through maternal inheritance and subsequent alliances with kin, while male ranks are continually renegotiated via aggressive competition. Whereas high status is associated with lower glucocorticoid activity in females (Levy et al. 2020), it is associated with higher glucocorticoid activity in males (Gesquiere et al. 2011). Accordingly, social status is associated with inverse effects on immune cell gene regulation in males and females (Anderson et al. 2022). While status does not directly predict longevity in females (Campos et al. 2020), the high glucocorticoid exposure typical of low rank reduces adult female survival (Campos et al. 2021), while high rank is associated with reduced survival and accelerated epigenetic aging in males (Campos et al. 2020; Anderson et al. 2021). In contrast, social bonds yield survival advantages in both sexes (Archie et al. 2014; Campos et al. 2020). While both social status and social integration affect gene expression and regulation, the specific targets of these effects are distinct (Runcie et al. 2013; Anderson et al. 2022).
An inherent limitation of wild studies is inferring causality if the relationship between social environment and health is bidirectional. However, many of the above results are supported by captive experiments on rhesus macaques, where social status can be reliably manipulated by removing females from established groups and introducing them sequentially to a newly created group. Both in baseline and post-manipulation groups, status is associated with widespread effects on the immune system, where the effects of low status closely resemble those of senescence (Tung et al. 2012; Snyder-Mackler et al. 2014). Status affects glucocorticoid regulation, proliferation of immune cells, gene expression and regulation, and chromatin accessibility (Kohn et al. 2016; Snyder-Mackler et al. 2016, 2019; Debray et al. 2019; Sanchez-Rosado et al. 2021). These differences reflect a more proinflammatory phenotype and greater glucocorticoid resistance in low-ranking individuals and a stronger antiviral phenotype in high-status individuals. The effects of status on health are mediated by differential rates of harassment and degree of social integration (Snyder-Mackler et al. 2016), although as in baboons, social integration appears to have independent positive effects on health, such as improved mtDNA regulation in immune cells (Debray et al. 2019).
COMPARATIVE PRIMATE AGING MODELS
While nonhuman primates offer significant promise as experimental and translational models of aging, evolutionary approaches to aging also emphasize the value of primates as comparative models to trace how recent evolutionary history has shaped human aging biology. By identifying shared ancestry of particular facets of aging biology, we are better equipped to evaluate how unique features of human aging are linked to other recently evolved features of our species, as well as to our unusual longevity and changes in vulnerability to age-related diseases.
While many aging processes are broadly shared across primates, even relatively small evolutionary distances between species are associated with significant differences. For example, aging humans experience physiological dysregulation, an emergent aging phenomenon marked by more frequent or extreme departures from homeostasis and increased risk of disease and mortality (Arbeev et al. 2019). This phenomenon is detectable in multivariate biomarker data sets from other primates, but more distantly related species show increasingly divergent patterns (Dansereau et al. 2019). Only chimpanzees, the species most closely related to humans, exhibit a pattern that strongly correlates with that of humans. Similar findings are beginning to emerge for CpG methylation, a highly conserved aging feature across primates (Horvath et al. 2020). While it is possible to construct a broadly applicable primate “molecular clock,” species differ markedly in the specific sites and rates of methylation (Horvath et al. 2020). A human-derived reference, adjusted for differences in longevity, can predict rhesus macaque ages (R2∼0.5), but with considerable error (Chiou et al. 2020), while performing substantially better when predicting chimpanzee ages (R2∼0.9) (Guevara et al. 2020).
Humans are a part of the hominid family (the “great apes”), which also includes seven extant species of orangutans, gorillas, chimpanzees, and bonobos. As a group, the hominids are characterized by extended life histories compared with other primates (Emery Thompson and Sabbi 2022). All great apes can live over 40 years in the wild, and chimpanzees can survive into their 60s. Comparative data on the great apes is of unique value for understanding the evolution of human aging because this can allow us to reconstruct the probable features of a last common ancestor at the origins of the human lineage (Muller et al. 2017). Yet, remarkably little is known about the aging biology of these species.
Until recently, most of the aging information available for great apes derived from veterinary screening of captive chimpanzees in biomedical research laboratories. For example, aging captive chimpanzees exhibit declining liver and kidney function and increased risk of hypertension and anemia (Videan et al. 2008; Ely et al. 2013). Compared with healthy humans, captive chimpanzees maintain markedly higher levels of biomarkers typically associated with cardiovascular risk and accelerated aging, including blood pressure, cholesterol, fibrinogen, and insulin (Videan et al. 2009; Ely et al. 2013; Cole et al. 2020). Despite this, chimpanzees and other captive great apes are at relatively low risk of most age-related pathologies that plague industrialized human populations, including coronary artery disease, cancers, osteoporosis, degenerative joint disease, and Alzheimer's-related pathologies, although strokes are not uncommon (Lowenstine et al. 2015; Edler et al. 2020). Like humans, heart disease is the most common cause of death for captive chimpanzees, yet the nature of the pathology differs. Great apes develop myocardial fibrosis and aortic dissections, rare conditions for humans, while they rarely develop advanced atherosclerosis (Kenny et al. 1994; Schulman et al. 1995; Seiler et al. 2009; Varki et al. 2009). This contrast has led to novel consideration of how human endurance activities may have shaped the evolution of the cardiovascular system and vulnerability to disease (Shave et al. 2019). Additionally, comparative genetic study reveals that apolipoprotein E4 (apoE4), an allele that increases risk of cardiovascular disease in humans, is the ancestral allele found in chimpanzees and bonobos (Hanlon and Rubinsztein 1995; McIntosh et al. 2012), whereas novel polymorphisms associated with reduced risk have evolved more recently in humans.
Other apparent differences between humans and chimpanzees may be side effects of captivity. Studies of free-living chimpanzees in sanctuaries observe significantly lower levels of blood pressure, glucose, cholesterol, and triglycerides than in zoos or laboratories (Ely et al. 2013; Ronke et al. 2015; Cole et al. 2020). In contrast to most captive laboratory chimpanzees, which are relatively sedentary and are fed processed animal chows, most wild-born sanctuary chimpanzees consume fresh fruits and vegetables and free range over large, forested areas. They are accordingly less likely to be obese, a factor that raises the levels of unhealthy biomarkers in both species (Nehete et al. 2014; Obanda et al. 2014).
Valid comparisons of aging physiology between humans and closely related species thus depend on evaluating how they age in in their natural environments. One such model system has been developed in the Kibale National Park, Uganda, where wild chimpanzees have been under continuous observation for more than 30 years (Emery Thompson et al. 2020c). Because chimpanzees are endangered in the wild, this research is limited to noninvasive measures; yet a wide range of informative data are possible from urine and fecal samples in addition to observational health and activity surveys. Aging in these wild chimpanzees is associated with increased parasite loads, greater diversity of viral infection, and increased morbidity and mortality from respiratory disease, implicating immunosenescence as a driver of declining health (Emery Thompson et al. 2018; Negrey et al. 2019, 2022; Phillips et al. 2020). Chimpanzees also exhibit age-related dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, characterized by increases in glucocorticoid production and a human-like blunting of the circadian rhythm (Emery Thompson et al. 2020a). Shared patterns of immunosenescence and physiological dysregulation between humans and chimpanzees indicate dimensions of our natural aging biology that are evolutionarily ancient.
Despite shorter life spans and challenging environments characterized by resource constraints and infectious disease, wild chimpanzees age quite successfully. For example, wild chimpanzees do not show evidence of a physical frailty syndrome, despite maintaining physically demanding foraging strategies that involve climbing high into the forest canopy. They experience only moderate changes in body condition and physical activity with age, and variation in condition does not predict mortality (Emery Thompson et al. 2020b). Similarly, studies of skeletal collections indicate that, despite high rates of healed fractures and moderate bone loss with age, wild chimpanzees and mountain gorillas rarely experience advanced osteoporosis, osteoarthritis, or degenerative joint disease (Jurmain 2000; Morbeck et al. 2002; Ruff et al. 2020). In an analogous fashion, captive chimpanzees exhibit cognitive aging, moderate neuronal loss, and even lesions characteristic of Alzheimer's disease, but they do not exhibit the severity of neurodegeneration associated with dementia in humans (Finch and Austad 2015; Edler et al. 2020; Lacreuse et al. 2020).
The process of senescence for great apes is at once very human-like, with age-related functional losses in the same domains, but it is not commonly associated with the aging pathologies that are leading causes of death for humans. Chronic inflammation has been identified as a key variable distinguishing healthy and pathological aging in humans (Franceschi et al. 2007, 2018; Baylis et al. 2013). The data from free-living chimpanzees in sanctuaries predict that wild populations may resist inflammaging, but noninvasive tools for direct measurement of inflammation in wild chimpanzees are limited. With opportunistic sampling, it can be also be difficult to distinguish the effects of immunosenescence, causing older individuals to experience more frequent acute bouts of inflammation, from the effects of chronic inflammation. For example, urinary neopterin, a proinflammatory marker of cellular immune activation, increased with age in one short-term study of wild chimpanzees (Negrey et al. 2021), but not in a longitudinal study within the same population (Thompson González et al. 2020). The latter study found that increased inflammation was only detectable in the 2–3 years before the death of some older chimpanzees, suggesting the expected association with declining health but not with aging per se. In support of this, aging was not associated with elevation of oxidative stress, a signature of human inflammaging (Zuo et al. 2019), in two independent studies of wild chimpanzees (Thompson González et al. 2020; Costantini et al. 2021).
AGING IN HUMANS IN EVOLUTIONARILY RELEVANT ENVIRONMENTS
Overwhelmingly, human aging research has been conducted in postindustrial populations. It has been only ∼10,000 years since the origins of agriculture and only a few generations since the first nations underwent the industrial transition. Prior to these transitions, the vast majority of human existence was spent in a hunting and gathering (i.e., foraging) context. Thus, a major guiding principle of evolutionary medicine is that human biology has been shaped by these environments of the past (Stearns 2012). We have had little time to adapt to the radical lifestyle changes of industrial development, and this environmental mismatch may lead to novel, and even maladaptive, health outcomes (Gurven and Lieberman 2020).
Whereas medical innovations have increased longevity in industrialized populations, all available evidence suggests that the human species is naturally long-lived. For example, modal ages of death for contemporary foraging populations are ∼68–78 years when individuals survive to adulthood, closely matching demographics from pre-industrialized Europe (Gurven and Kaplan 2007).
Recent insights from studies of contemporary foragers and other small-scale subsistence populations (e.g., pastoralists, horticulturalists) have fundamentally challenged our understanding of human aging. It is important to note that foraging populations cannot be considered relics of our evolutionary past, nor is their lifestyle untouched by the market-integrated populations that surround them. Rather, the environments and lifestyle of these communities replicate key selection pressures that would have shaped the evolution of the modern human organism: resource limitations, high workload, unprocessed wild diets, small kin-based communities, and fewer barriers to infectious disease and environmental stress.
Small-scale subsistence populations appear to be models of successful aging, resisting many of the age-associated diseases that plague industrialized populations (Pontzer et al. 2018; Gurven et al. 2022). Heart disease and strokes are rare causes of death (Hill and Hurtado 1996; Gurven et al. 2007), and screening of forager and forager-horticulturalist populations find little evidence for underlying atherosclerosis, chronic inflammation, or hypertension (Lindeberg and Lundh 1993; Vasunilashorn et al. 2010; Kaplan et al. 2017; Raichlen et al. 2017). This is remarkable given that forager diets are often heavily meat-based. It is hypothesized that the relatively high balance of protein to carbohydrates, prevalence of “good” fats, low sodium, high fiber, and rich micronutrients in forager diets may counteract atherogenic effects that might typically arise from high fat content (Cordain et al. 2002). Resistance to chronic inflammation is also surprising given that subsistence populations experience relatively high rates of infection, leading to frequent acute bouts of inflammation (Gurven et al. 2008). High prevalence of helminth infections is suspected to help to resist cardiovascular disease and diabetes by increasing anti-inflammatory immune responses, consuming blood lipids, and diverting resources that might otherwise contribute to obesity or arterial plaques (Gurven et al. 2016).
Cross-cultural studies also highlight the importance of gene–environment interactions in moderating Alzheimer's disease (AD) risk. Whereas the apoE4 allele has been widely implicated in increased risk of AD in industrialized populations, and was similarly associated with increased risk of dementia in the Tsimane and Moseten forager-horticulturalists of Bolivia (Gatz et al. 2022), the allele was associated with increased cognitive performance among Tsimane with moderate-to-severe eosinophilia, characteristic of parasitic infection (Trumble et al. 2017). Tsimane with the apoE4 allele do not suffer higher mortality than others (Vasunilashorn et al. 2011).The apoE4 allele has also been implicated in improved child health and cognitive development in a Brazilian population experiencing high rates of early life mortality (Oriá et al. 2010). These findings suggest reasons why this allele, deleterious in industrialized environments, may have been conserved in humans. While the Tsimane exhibit mild cognitive impairment with age, they exhibit very low rates of dementia (∼1%) compared with industrialized populations (Gurven et al. 2017; Gatz et al. 2022). It is not yet clear how generalizable this finding is to other subsistence settings. A meta-analysis of 15 “indigenous” populations found widely varying rates of dementia (Warren et al. 2015), but the most affected populations, such as Australian aborigines, are already significantly market-integrated and experience high rates of obesity and sedentism (Radford et al. 2019).
Subsistence workloads involve considerable investment in physical activity. Rather than contributing to accelerated wear and tear, intensive workloads appear to be protective against frailty. Studies of Hadza foragers of Tanzania and Pokot pastoralists of Kenya find that strength and daily duration of physical activity declines by about half across adulthood, but rates of physical performance among the oldest adults remain remarkable remarkably high (Sayre et al. 2019, 2020). For example, the average 60-year-old Hadza or Pokot engages in ∼150 min of moderate-to-vigorous physical activity per day, while most Americans fail to meet the recommended 30 min per day. Physical inactivity and obesity are also implicated in the severity of knee osteoarthritis (Berenbaum et al. 2018; Wallace et al. 2022), which may help to explain a doubling in prevalence in the industrial era (Wallace et al. 2017).
Physical activity is likely to be a key mechanism by which subsistence populations resist chronic inflammation and its damaging consequences for health (Pontzer et al. 2018; Gurven and Lieberman 2020). Indeed, many aspects of human anatomy and physiology are optimized for the long periods of physical activity that would have characterized prehistoric lifeways, setting us apart from our close primate relatives (Bramble and Lieberman 2004; Kraft et al. 2021). These adaptations that increased fitness throughout most of our evolutionary history predispose us to disease when mismatched to sedentary environments (Shave et al. 2019; Lieberman et al. 2021). The recent “active grandparent” hypothesis goes one step further in posing that adaptation to physically active lifestyles were integral to the evolution of extended human life spans (Lieberman et al. 2021). Two mechanisms are proposed. First, activity could promote healthy aging by drawing energy away from excess fat storage and reproduction, thus reducing harmful downstream effects of inflammation, high steroid production, and insulin resistance. In addition, exercise induces mild stress, activating mechanisms of cellular repair and maintenance that combat senescence (Lieberman et al. 2021).
CONCLUDING REMARKS
Evolutionary approaches to aging research aim to move beyond the acknowledgment that aging has evolved toward reconstructing how the mechanisms of aging and disease have been shaped by our evolutionary past. There has been a conspicuous gap in our knowledge of aging biology during our recent evolutionary history that is now being filled in by anthropologists and primatologists studying our closest primate relatives and humans living in preindustrial environments. While this body of work is still new, it has already demonstrated the potential to redefine our understanding of the relationship between aging, health, and disease.
While the findings of the evolutionary aging program demonstrate that human aging biology retains many features from our evolutionary past, our capacity for healthy aging is dramatically affected by current environments. On the one hand, there is evidence that unique human adaptations may predispose us to severe degenerative disease risks not experienced by our close evolutionary relatives. On the other hand, there is mounting evidence to support the hypothesis that these risks may be limited to novel environments to which humans are not yet adapted. If so, there is even more reason to consider that natural aging and disease emerge from distinct, although interacting, processes. Diversifying the contexts for aging research will provide increased capacity to discriminate these processes and how they respond to complex social and ecological phenomena across the life course.
ACKNOWLEDGMENTS
The author is supported by a grant from the National Institute on Aging R01AG049395.
Footnotes
Editors: James L. Kirkland, S. Jay Olshansky, and George M. Martin
Additional Perspectives on Aging: Geroscience as the New Public Health Frontier available at www.cshperspectives.org
REFERENCES
- Alberts SC, Altmann J. 2012. The Amboseli baboon research project: 40 years of continuity and change. In Long-term field studies of primates, pp. 261–287. Springer, Berlin. [Google Scholar]
- Altmann J, Alberts SC. 2003. Variability in reproductive success viewed from a life-history perspective in baboons. Am J Hum Biol 15: 401–409. 10.1002/ajhb.10157 [DOI] [PubMed] [Google Scholar]
- Altmann J, Schoeller D, Altmann SA, Muruthi P, Sapolsky RM. 1993. Body size and fatness of free-living baboons reflect food availability and activity levels. Am J Primatol 30: 149–161. 10.1002/ajp.1350300207 [DOI] [PubMed] [Google Scholar]
- Anderson JA, Johnston RA, Lea AJ, Campos FA, Voyles TN, Akinyi MY, Alberts SC, Archie EA, Tung J. 2021. High social status males experience accelerated epigenetic aging in wild baboons. eLife 10: e66128. 10.7554/eLife.66128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JA, Lea AJ, Voyles TN, Akinyi MY, Nyakundi R, Ochola L, Omondi M, Nyundo F, Zhang Y, Campos FA, et al. 2022. Distinct gene regulatory signatures of dominance rank and social bond strength in wild baboons. Philos Trans R Soc Lond B Biol Sci 377: 20200441. 10.1098/rstb.2020.0441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeev KG, Ukraintseva SV, Bagley O, Zhbannikov IY, Cohen AA, Kulminski AM, Yashin AI. 2019. “Physiological dysregulation” as a promising measure of robustness and resilience in studies of aging and a new indicator of preclinical disease. J Gerontol A 74: 462–468. 10.1093/gerona/gly136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archie EA, Tung J, Clark M, Altmann J, Alberts SC. 2014. Social affiliation matters: both same-sex and opposite-sex relationships predict survival in wild female baboons. Proc Biol Sci 281: 20141261. 10.1098/rspb.2014.1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudisch A. 2011. The pace and shape of ageing. Methods Ecol Evol 2: 375–382. 10.1111/j.2041-210X.2010.00087.x [DOI] [Google Scholar]
- Baudisch A. 2012. Birds do it, bees do it, we do it: contributions of theoretical modelling to understanding the shape of ageing across the tree of life. Gerontology 58: 481–489. 10.1159/000341861 [DOI] [PubMed] [Google Scholar]
- Baylis D, Bartlett DB, Patel HP, Roberts HC. 2013. Understanding how we age: insights into inflammaging. Longev Healthspan 2: 8. 10.1186/2046-2395-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum F, Wallace IJ, Lieberman DE, Felson DT. 2018. Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat Rev Rheumatol 14: 674–681. 10.1038/s41584-018-0073-x [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. 2018. Disease or not, aging is easily treatable. Aging (Albany NY) 10: 3067–3078. 10.18632/aging.101647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bons N, Rieger F, Prudhomme D, Fisher A, Krause KH. 2006. Microcebus murinus: a useful primate model for human cerebral aging and Alzheimer's disease? Genes Brain Behav 5: 120–130. 10.1111/j.1601-183X.2005.00149.x [DOI] [PubMed] [Google Scholar]
- Bramble DM, Lieberman DE. 2004. Endurance running and the evolution of Homo. Nature 432: 345–352. 10.1038/nature03052 [DOI] [PubMed] [Google Scholar]
- Brent LJ, Ruiz-Lambides A, Platt ML. 2017. Family network size and survival across the lifespan of female macaques. Proc Biol Sci 284: 20170515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronikowski AM, Altmann J, Brockman DK, Cords M, Fedigan L, Pusey A, Stoinski T, Morris WF, Strier KB, Alberts SC. 2011. Aging in the natural world: comparative data reveal similar mortality patterns across primates. Science 331: 1325–1328. 10.1126/science.1201571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulterijs S, Hull RS, Björk VCE, Roy AG. 2015. It is time to classify biological aging as a disease. Front Genet 6: 205. 10.3389/fgene.2015.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch JB, Augustine AD, Frieden LA, Hadley E, Howcroft TK, Johnson R, Khalsa PS, Kohanski RA, Li XL, Macchiarini F, et al. 2014. Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A 69: S1–S3. 10.1093/gerona/glu041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos FA, Villavicencio F, Archie EA, Colchero F, Alberts SC. 2020. Social bonds, social status and survival in wild baboons: a tale of two sexes. Philos Trans R Soc Lond B Biol Scii 375: 20190621. 10.1098/rstb.2019.0621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos FA, Archie EA, Gesquiere LR, Tung J, Altmann J, Alberts SC. 2021. Glucocorticoid exposure predicts survival in female baboons. Sci Adv 7: eabf6759. 10.1126/sciadv.abf6759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavigelli SA, Caruso MJ. 2015. Sex, social status and physiological stress in primates: the importance of social and glucocorticoid dynamics. Philos Trans R Soc Lond B Biol Sci 370: 20140103. 10.1098/rstb.2014.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnov EL, Berrigan D. 1993. Why do female primates have such long lifespans and so few babies? Or life in the slow lane. Evol Anthropol 1: 191–194. 10.1002/evan.1360010604 [DOI] [Google Scholar]
- Chiou KL, Montague MJ, Goldman EA, Watowich MM, Sams SN, Song J, Horvath JE, Sterner KN, Ruiz-Lambides A, Martinez MI, et al. 2020. Rhesus macaques as a tractable physiological model of human ageing. Philos Trans R Soc Lond B Biol Sci 375: 20190612. 10.1098/rstb.2019.0612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colchero F, Rau R, Jones OR, Barthold JA, Conde DA, Lenart A, Nemeth L, Scheuerlein A, Schoeley J, Torres C, et al. 2016. The emergence of longevous populations. Proc Natl Acad Sci 113: E7681–E7690. 10.1073/pnas.1612191113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colchero F, Aburto J, Archie EA, Boesch C, Breuer T, Campos F, Collins A, Conde DA, Cords M, Crockford C, et al. 2021. The long lives of primates and the ‘invariant rate of ageing’ hypothesis. Nat Commun 12: 1–10. 10.1038/s41467-021-23894-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MF, Cantwell A, Rukundo J, Ajarova L, Fernandez-Navarro S, Atencia R, Rosati AG. 2020. Healthy cardiovascular biomarkers across the lifespan in wild-born chimpanzees (Pan troglodytes). Philos Trans R Soc Lond B Biol Sci 375: 20190609. 10.1098/rstb.2019.0609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ. 2018. Non-human primates as a model for aging. Biochim Biophys Acta 1864: 2733–2741. 10.1016/j.bbadis.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Roecker EB, Ramsey JJ, Kemnitz JW. 1998. The effect of dietary restriction on body composition in adult male and female rhesus macaques. Age Clin Exper Res 10: 83–92. 10.1007/BF03339642 [DOI] [PubMed] [Google Scholar]
- Conn PM. 2011. Handbook of models for human aging. Elsevier, Burlington, MA. [Google Scholar]
- Cordain L, Eaton SB, Miller JB, Mann N, Hill K. 2002. The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur J Clin Nutr 56: S42–S52. 10.1038/sj.ejcn.1601353 [DOI] [PubMed] [Google Scholar]
- Costantini D, Masi S, Rachid L, Beltrame M, Rohmer M, Krief S. 2021. Mind the food: rapid changes in antioxidant content of diet affect oxidative status of chimpanzees. Am J Physiol Regul Integr Comp Physiol 320: R728–R734. 10.1152/ajpregu.00003.2021 [DOI] [PubMed] [Google Scholar]
- Dansereau G, Wey TW, Legault V, Brunet MA, Kemnitz JW, Ferrucci L, Cohen AA. 2019. Conservation of physiological dysregulation signatures of aging across primates. Aging Cell 18: e12925. 10.1111/acel.12925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debray R, Snyder-Mackler N, Kohn JN, Wilson ME, Barreiro LB, Tung J. 2019. Social affiliation predicts mitochondrial DNA copy number in female rhesus macaques. Biol Lett 15: 20180643. 10.1098/rsbl.2018.0643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magalhães JP. 2014. The scientific quest for lasting youth: prospects for curing aging. Rejuvenation Res 17: 458–467. 10.1089/rej.2014.1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magalhães JP, Church GM. 2005. Genomes optimize reproduction: aging as a consequence of the developmental program. Physiology 20: 252–259. 10.1152/physiol.00010.2005 [DOI] [PubMed] [Google Scholar]
- Dyban AP, Puchkov VF, Samoshkina NA, Khozhai LI, Chebotar’ NA, Baranov VS. 1991. Laboratory mammals: mouse (Mus musculus), rat (Rattus norvegicus), rabbit (Oryctolagus cuniculus), and golden hamster (Cricetus auratus). In Animal species for developmental studies: vertebrates (ed. Dettlaff TA, Vassetzky SG), pp. 351–443. Springer, Boston, MA. [Google Scholar]
- Edler MK, Munger EL, Meindle RS, Hopkins WD, Ely JJ, Erwin JM, Mufson EJ, Hof PR, Sherwood CC, Raghanti MA. 2020. Neuron loss associated with age but not Alzheimer's disease pathology in the chimpanzee brain. Philos Trans R Soc Lond B Biol Sci 375: 20190619. 10.1098/rstb.2019.0619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RD, Tuljapurkar S. 2005. Inequality in life spans and a new perspective on mortality convergence across industrialized countries. Popul Dev Rev 31: 645–674. 10.1111/j.1728-4457.2005.00092.x [DOI] [Google Scholar]
- Ellis S, Snyder-Mackler N, Ruiz-Lambides A, Platt ML, Brent LJ. 2019. Deconstructing sociality: the types of social connections that predict longevity in a group-living primate. Proc Biol Sci 286: 20191991. 10.1098/rspb.2019.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely JJ, Zavaskis T, Lammey ML. 2013. Hypertension increases with aging and obesity in chimpanzees (Pan troglodytes). Zoo Biol 32: 79–87. 10.1002/zoo.21044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M, Sabbi KH. 2022. Evolutionary demography of the great apes. In Human evolutionary demography (ed. Sear R, Burger O, Lee R). Open Book Publishers, Cambridge, UK. [Google Scholar]
- Emery Thompson M, Machanda ZP, Scully EJ, Enigk DK, Otali E, Muller MN, Goldberg TG, Chapman CA, Wrangham RW. 2018. Risk factors for respiratory illness in a community of wild chimpanzees (Pan troglodytes schweinfurthii). R Soc Open Sci 5: 180840. 10.1098/rsos.180840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M, Fox SA, Berghäenel A, Sabbi K, Phillips-Garcia S, Enigk DK, Otali E, Machanda ZP, Wrangham RW, Muller MN. 2020a. Wild chimpanzees exhibit humanlike aging of glucocorticoid regulation. Proc Natl Acad Sci 117: 8424–8430. 10.1073/pnas.1920593117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M, Machanda ZP, Fox SA, Sabbi KH, Otali E, Thompson González N, Muller MN, Wrangham RW. 2020b. Evaluating the impact of physical frailty during ageing in wild chimpanzees (Pan troglodytes schweinfurthii). Philos Trans R Soc Lond B Biol Sci 375: 20190607. 10.1098/rstb.2019.0607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M, Muller MN, Machanda ZP, Otali E, Wrangham RW. 2020c. The Kibale Chimpanzee Project: over thirty years of research, conservation, and change. Biol Conserv 252: 108857. 10.1016/j.biocon.2020.108857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M, Rosati AG, Snyder-Mackler N. 2020d. Insights from evolutionarily relevant models for human ageing. Philos Trans R Soc Lond B Biol Sci 375: 20190605. 10.1098/rstb.2019.0605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Austad SN. 2015. Commentary: is Alzheimer's disease uniquely human? Neurobiol Aging 36: 553–555. 10.1016/j.neurobiolaging.2014.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, et al. 2007. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128: 92–105. 10.1016/j.mad.2006.11.016 [DOI] [PubMed] [Google Scholar]
- Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. 2018. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 14: 576–590. 10.1038/s41574-018-0059-4 [DOI] [PubMed] [Google Scholar]
- Gatz M, Mack WJ, Chui HC, Law EM, Barisano G, Sutherland ML, Sutherland JD, Eid Rodriguez D, Quispe Gutierrez R, Copajira Adrian J, et al. 2022. Prevalence of dementia and mild cognitive impairment in indigenous Bolivian forager-horticulturalists. Alzheimers Dement 10.1002/alz.12626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesquiere LR, Learn NH, Simao MCM, Onyango PO, Alberts SC, Altmann J. 2011. Life at the top: rank and stress in wild male baboons. Science 333: 357–360. 10.1126/science.1207120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunspan DZ, Nesse RM, Barnes ME, Brownell SE. 2018. Core principles of evolutionary medicine: a Delphi study. Evol Med Public Health 2018: 13–23. 10.1093/emph/eox025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara EE, Lawler RR, Staes N, White CM, Sherwood CC, Ely JJ, Hopkins WD, Bradley BJ. 2020. Age-associated epigenetic change in chimpanzees and humans. Philos Trans R Soc Lond B Biol Sci 375: 20190616. 10.1098/rstb.2019.0616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Kaplan H. 2007. Longevity among hunter-gatherers: a cross-cultural examination. Popul Dev Rev 33: 321–365. 10.1111/j.1728-4457.2007.00171.x [DOI] [Google Scholar]
- Gurven MD, Lieberman DE. 2020. WEIRD bodies: mismatch, medicine and missing diversity. Evol Hum Behav 41: 330–340. 10.1016/j.evolhumbehav.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Kaplan HS, Zelada Supa A. 2007. Mortality experience of Tsimane Amerindians of Bolivia: regional variation and temporal trends. Am J Hum Biol 19: 379–398. 10.1002/ajhb.20600 [DOI] [PubMed] [Google Scholar]
- Gurven M, Kaplan HS, Winking J, Finch C, Crimmins EM. 2008. Aging and inflammation in two epidemiological worlds. J Gerontol 63: 196–199. 10.1093/gerona/63.2.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven MD, Trumble BC, Stieglitz J, Blackwell AD, Michalik DE, Finch CE, Kaplan HS. 2016. Cardiovascular disease and type 2 diabetes in evolutionary perspective: a critical role for helminths? Evol Med Public Health 2016: 338–357. 10.1093/emph/eow028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Fuerstenberg E, Trumble B, Stieglitz J, Beheim B, Davis H, Kaplan H. 2017. Cognitive performance across the life course of Bolivian forager-farmers with limited schooling. Dev Psychol 53: 160–176. 10.1037/dev0000175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Kaplan H, Trumble B, Stieglitz J. 2022. The biodemography of human health in contemporary non-industrial populations: insights from the Tsimane Health and Life History Project. In Human evolutionary demography (ed. Sear R, Burger O, Lee R). Open Book; Publishers, Cambridge, UK. [Google Scholar]
- Haldane J. 1941. New paths in genetics. Allen & Unwin, London. [Google Scholar]
- Hanlon CS, Rubinsztein DC. 1995. Arginine residues at codons 112 and 158 in the apolipoprotein E gene correspond to the ancestral state in humans. Atherosclerosis 112: 85–90. 10.1016/0021-9150(94)05402-5 [DOI] [PubMed] [Google Scholar]
- Hayflick L. 2000. The future of ageing. Nature 408: 267–269. 10.1038/35041709 [DOI] [PubMed] [Google Scholar]
- Hayflick L. 2004. Debates: the not-so-close relationship between biological aging and age-associated pathologies in humans. J Gerontol A 59: B547–B550. 10.1093/gerona/59.6.B547 [DOI] [PubMed] [Google Scholar]
- Hill KR, Hurtado AM. 1996. Ache life history: the ecology and demography of a foraging people. Transaction, Piscataway, NJ. [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. 2010. Social relationships and mortality risk: a meta-analytic review. PLoS Med 7: e1000316. 10.1371/journal.pmed.1000316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Haghani A, Zoller J, Lu A, Ernst J, Pellegrini M, Jasinska A, Mattison J, Salmon A, Raj K, et al. 2020. Pan-primate DNA methylation clocks. bioRxiv 10.1101/2020.11.29.402891 [DOI] [Google Scholar]
- Jones OR, Scheuerlein A, Salguero-Gómez R, Camarda CG, Schaible R, Casper BB, Dahlgren JP, Ehrlén J, García MB, Menges ES, et al. 2014. Diversity of ageing across the tree of life. Nature 505: 169–173. 10.1038/nature12789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurmain R. 2000. Degenerative joint disease in African great apes: an evolutionary perspective. J Hum Evol 39: 185–203. 10.1006/jhev.2000.0413 [DOI] [PubMed] [Google Scholar]
- Kaplan H, Thompson RC, Trumble BC, Wann LS, Allam AH, Beheim B, Frohlich B, Sutherland ML, Sutherland JD, Stieglitz J, et al. 2017. Coronary atherosclerosis in indigenous south American Tsimane: a cross-sectional cohort study. Lancet 389: 1730–1739. 10.1016/S0140-6736(17)30752-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, et al. 2014. Geroscience: linking aging to chronic disease. Cell 159: 709–713. 10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny DE, Cambre RC, Alvarado TP, Prowten AW, Allchurch AF, Marks SK, Zuba JR. 1994. Aortic dissection: an important cardiovascular disease in captive gorillas (Gorilla gorilla gorilla). J Zoo Wildl Med 25: 561–568. [Google Scholar]
- Kirkwood T. 1977. Evolution of ageing. Nature 270: 301–304. 10.1038/270301a0 [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, Rose MR. 1991. Evolution of senescence: late survival sacrificed for reproduction. Philos Trans R Soc Lond B Biol Sci 332: 15–24. 10.1098/rstb.1991.0028 [DOI] [PubMed] [Google Scholar]
- Kohn JN, Snyder-Mackler N, Barreiro LB, Johnson ZP, Tung J, Wilson ME. 2016. Dominance rank causally affects personality and glucocorticoid regulation in female rhesus macaques. Psychoneuroendocrinology 74: 179–188. 10.1016/j.psyneuen.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft TS, Venkataraman VV, Wallace IJ, Crittenden AN, Holowka NB, Stieglitz J, Harris J, Raichlen DA, Wood B, Gurven M, et al. 2021. The energetics of uniquely human subsistence strategies. Science 374: eabf0130. 10.1126/science.abf0130 [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Suleski M, Hedges SB. 2017. Timetree: a resource for timelines, timetrees, and divergence times. Mol Biol Evol 34: 1812–1819. DOI: 10.1093/molbev/msx116 [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Raz N, Schmidtke D, Hopkins WD, Herndon JG. 2020. Age-related decline in executive function as a hallmark of cognitive ageing in primates: an overview of cognitive and neurobiological studies. Philos Trans R Soc Lond B Biol Sci 375: 20190618. 10.1098/rstb.2019.0618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Black A, Handy A, Tilmont EM, Ingram DK, Roth GS. 2001. Caloric restriction in primates. Ann NY Acad Sci 928: 287–295. 10.1111/j.1749-6632.2001.tb05658.x [DOI] [PubMed] [Google Scholar]
- Languille S, Blanc S, Blin O, Canale CI, Dal-Pan A, Devau G, Dhenain M, Dorieux O, Epelbaum J, Gomez D, et al. 2012. The grey mouse lemur: a non-human primate model for ageing studies. Age Res Rev 11: 150–162. 10.1016/j.arr.2011.07.001 [DOI] [PubMed] [Google Scholar]
- Lavery WL. 2000. How relevant are animal models to human ageing? J R Soc Med 93: 296–298. 10.1177/014107680009300605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy EJ, Gesquiere LR, McLean E, Franz M, Warutere JK, Sayialel SN, Mututua RS, Wango TL, Oudu VK, Altmann J, et al. 2020. Higher dominance rank is associated with lower glucocorticoids in wild female baboons: a rank metric comparison. Horm Behav 125: 104826. 10.1016/j.yhbeh.2020.104826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman DE, Kistner TM, Richard D, Lee IM, Baggish AL. 2021. The active grandparent hypothesis: physical activity and the evolution of extended human healthspans and lifespans. Proc Natl Acad Sci 118: e2107621118. 10.1073/pnas.2107621118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeberg S, Lundh B. 1993. Apparent absence of stroke and ischaemic heart disease in a traditional Melanesian island: a clinical study in Kitava. J Intern Med 233: 269–275. 10.1111/j.1365-2796.1993.tb00986.x [DOI] [PubMed] [Google Scholar]
- Lowenstine LJ, McManamon R, Terio KA. 2016. Comparative pathology of aging great apes. Vet Pathol 53: 250–276. 10.1177/0300985815612154 [DOI] [PubMed] [Google Scholar]
- Masoro EJ. 2000. Caloric restriction and aging: an update. Exper Gerontol 35: 299–305. 10.1016/S0531-5565(00)00084-X [DOI] [PubMed] [Google Scholar]
- Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, Ingram DK, Weindruch R, de Cabo R, Anderson RM. 2017. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun 8: 14063. 10.1038/ncomms14063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland R, Majolo B. 2013. Coping with the cold: predictors of survival in wild Barbary macaques, Macaca sylvanus. Bio Lett 9: 20130428. 10.1098/rsbl.2013.0428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonigle P, Ruggeri B. 2014. Animal models of human disease: challenges in enabling translation. Biochem Pharmacol 87: 162–171. 10.1016/j.bcp.2013.08.006 [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Bennett C, Dickson D, Anestis SF, Watts DP, Webster TH, Fontenot MB, Bradley BJ. 2012. The apolipoprotein E (APOE) gene appears functionally monomorphic in chimpanzees (Pan troglodytes). PLoS ONE 7: e47760. 10.1371/journal.pone.0047760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medawar PB. 1946. Old age and natural death. Modern Quarterly 1: 30–56. [Google Scholar]
- Medawar PB. 1952. An unsolved problem of biology. HK Lewis, London. [Google Scholar]
- Mitchell SJ, Scheibye-Knudsen M, Longo DL, Cabo RD. 2015. Animal models of aging research: implications for human aging and age-related diseases. Annu Rev Anim Biosci 3: 283–303. 10.1146/annurev-animal-022114-110829 [DOI] [PubMed] [Google Scholar]
- Morbeck M, Galloway A, Sumner DR. 2002. Getting old at Gombe: skeletal aging in wild-ranging chimpanzees. Interdisc Top Gerontol 31: 48–62. 10.1159/000061458 [DOI] [Google Scholar]
- Muller MN, Wrangham RW, Pilbeam DR. 2017. Chimpanzees and human evolution. Harvard University Press, Cambridge, MA. [Google Scholar]
- Negrey JD, Reddy RB, Scully EJ, Phillips-Garcia S, Owens L, Langergraber KE, Mitani J, Emery Thompson M, Wrangham RW, Muller MN, et al. 2019. Simultaneous outbreaks of respiratory disease in wild chimpanzees caused by distinct viruses of human origin. Emerg Microbes Infect 8: 139–149. 10.1080/22221751.2018.1563456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrey JD, Behringer V, Langergraber KE, Deschner T. 2021. Urinary neopterin of wild chimpanzees indicates that cell-mediated immune activity varies by age, sex, and female reproductive status. Sci Rep 11: 1–11. 10.1038/s41598-021-88401-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrey JD, Mitani JC, Wrangham RW, Otali E, Reddy RB, Pappas TE, Grindle KA, Gern JE, Machanda ZP, Muller MN. 2022. Viruses associated with ill health in wild chimpanzees. Am J Primatol 84: e23358. 10.1002/ajp.23358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehete P, Magden ER, Nehete B, Hanley PW, Abee CR. 2014. Obesity related alterations in plasma cytokines and metabolic hormones in chimpanzees. Int J Inflam 2014: 856749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Németh L. 2017. Life expectancy versus lifespan inequality: a smudge or a clear relationship? PLoS ONE 12: e0185702. 10.1371/journal.pone.0185702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obanda V, Omondi GP, Chiyo PI. 2014. The influence of body mass index, age and sex on inflammatory disease risk in semi-captive chimpanzees. PLoS ONE 9: e104602. 10.1371/journal.pone.0104602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oriá RB, Patrick PD, Oriá MOB, Lorntz B, Thompson MR, Azevedo OGR, Lobo RNB, Pinkerton RF, Guerrant RL, Lima AAM. 2010. Apoe polymorphisms and diarrheal outcomes in Brazilian shanty town children. Braz J Med Biol Res 43: 249–256. 10.1590/S0100-879X2010007500003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostner J, Schüelke O. 2018. Linking sociality to fitness in primates: a call for mechanisms. Adv Study Behav 50: 127–175. 10.1016/bs.asb.2017.12.001 [DOI] [Google Scholar]
- Phillips SR, Goldberg T, Muller M, Machanda Z, Otali E, Friant S, Carag J, Langergraber K, Mitani J, Wroblewski E, et al. 2020. Faecal parasites increase with age but not reproductive effort in wild female chimpanzees. Philos Trans R Soc Lond B Biol Sci 375: 20190614. 10.1098/rstb.2019.0614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontzer H, Wood BM, Raichlen DA. 2018. Hunter-gatherers as models in public health. Obes Rev 19: 24–35. 10.1111/obr.12785 [DOI] [PubMed] [Google Scholar]
- Radford K, Lavrencic LM, Delbaere K, Draper B, Cumming R, Daylight G, Mack HA, Chalkley S, Bennett H, Garvey G, et al. 2019. Factors associated with the high prevalence of dementia in older aboriginal Australians. J Alzheimers Dis 70: S75–S85. 10.3233/JAD-180573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichlen DA, Pontzer H, Harris JA, Mabulla AZP, Marlowe FW, Josh Snodgrass J, Eick G, Colette Berbesque J, Sancilio A, Wood BM. 2017. Physical activity patterns and biomarkers of cardiovascular disease risk in hunter-gatherers. Am J Hum Biol 29: e22919. 10.1002/ajhb.22919 [DOI] [PubMed] [Google Scholar]
- Rawlins RG, Kessler MJ. 1986. The Cayo Santiago macaques: history, behavior, and biology. SUNY Press, Albany, NY. [Google Scholar]
- Reuter H, Krug J, Singer P, Englert C. 2018. The African turquoise killifish Nothobranchius furzeri as a model for aging research. Drug Discovery Today: Disease Models 27: 15–22. 10.1016/j.ddmod.2018.12.001 [DOI] [Google Scholar]
- Ronke C, Dannemann M, Halbwax M, Fischer A, Helmschrodt C, Brügel M, André C, Atencia R, Mugisha L, Scholz M, et al. 2015. Lineage-specific changes in biomarkers in great apes and humans. PLoS ONE 10: e0134548. 10.1371/journal.pone.0134548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CN. 2019. Marmosets in aging research. In The common marmoset in captivity and biomedical research (ed. Marini R, et al. ), pp. 355–376. Academic, Cambridge, MA. [Google Scholar]
- Ruff CB, Junno JA, Eckardt W, Gilardi K, Mudakikwa A, McFarlin SC. 2020. Skeletal ageing in Virunga mountain gorillas. Philos Trans R Soc Lond B Biol Sci 375: 20190606. 10.1098/rstb.2019.0606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runcie DE, Wiedmann RT, Archie EA, Altmann J, Wray GA, Alberts SC, Tung J. 2013. Social environment influences the relationship between genotype and gene expression in wild baboons. Philos Trans R Soc Lond B Biol Sci 368: 20120345. 10.1098/rstb.2012.0345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Rosado M, Snyder-Mackler N, Higham J, Brent L, Marzan-Rivera N, Pavez-Fox M, Watowich M, Sariol CA. 2021. Effects of age and social adversity on immune cell populations in a non-human primate model of human aging. Innov Aging 5: 530–530. 10.1093/geroni/igab046.2044 [DOI] [Google Scholar]
- Sapolsky RM. 2004. Social status and health in humans and other animals. Annu Rev Anthropol 33: 393–418. 10.1146/annurev.anthro.33.070203.144000 [DOI] [Google Scholar]
- Sapolsky RM. 2005. The influence of social hierarchy on primate health. Science 308: 648–652. 10.1126/science.1106477 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. 2021. Glucocorticoids, the evolution of the stress-response, and the primate predicament. Neurobiol Stress 14: 100320. 10.1016/j.ynstr.2021.100320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R, Armanini M, Packan D, Tombaugh G. 1987. Stress and glucocorticoids in aging. Endocrinol Metab Clin North Am 16: 965–980. 10.1016/S0889-8529(18)30453-5 [DOI] [PubMed] [Google Scholar]
- Sayre MK, Pike IL, Raichlen DA. 2019. High levels of objectively measured physical activity across adolescence and adulthood among the pokot pastoralists of Kenya. Am J Hum Biol 31: e23205. 10.1002/ajhb.23205 [DOI] [PubMed] [Google Scholar]
- Sayre MK, Pontzer H, Alexander GE, Wood BM, Pike IL, Mabulla AZ, Raichlen DA. 2020. Ageing and physical function in East African foragers and pastoralists. Philos Trans R Soc Lond B Biol Sci 375: 20190608. 10.1098/rstb.2019.0608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman FY, Farb A, Virmani R, Montali RJ. 1995. Fibrosing cardiomyopathy in captive western lowland gorillas (Gorilla gorilla gorilla) in the United States: a retrospective study. J Zoo Wildl Med 26: 43–51. [Google Scholar]
- Seiler BM, Dick EJ Jr, Guardado-Mendoza R, VendeBerg JL, Williams JT, Mubiru JN, Hubbard GB. 2009. Spontaneous heart disease in the adult chimpanzee (Pan troglodytes). J Med Primatol 38: 51–58. 10.1111/j.1600-0684.2008.00307.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shave RE, Lieberman DE, Drane AL, Brown MG, Batterham AM, Worthington S, Atencia R, Feltrer Y, Neary J, Weiner RB, et al. 2019. Selection of endurance capabilities and the trade-off between pressure and volume in the evolution of the human heart. Proc Natl Acad Sci 116: 19905–19910. 10.1073/pnas.1906902116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Clarkson TB. 1994. Social status and coronary artery atherosclerosis in female monkeys. Arterioscler Thromb 14: 721–726. 10.1161/01.ATV.14.5.721 [DOI] [PubMed] [Google Scholar]
- Shively CA, Clarkson TB. 2009. The unique value of primate models in translational research. Am J Primatol 71: 715–721. 10.1002/ajp.20720 [DOI] [PubMed] [Google Scholar]
- Shively CA, Day SM. 2015. Social inequalities in health in nonhuman primates. Neurobiol Stress 1: 156–163. 10.1016/j.ynstr.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Register TC, Clarkson TB. 2009. Social stress, visceral obesity, and coronary artery atherosclerosis: product of a primate adaptation. Am J Primatol 71: 742–751. 10.1002/ajp.20706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JB, Alberts SC, Altmann J. 2003. Social bonds of female baboons enhance infant survival. Science 302: 1231–1234. 10.1126/science.1088580 [DOI] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. 2010. Strong and consistent social bonds enhance the longevity of female baboons. Curr Biol 20: 1359–1361. 10.1016/j.cub.2010.05.067 [DOI] [PubMed] [Google Scholar]
- Simmons HA. 2016. Age-associated pathology in rhesus macaques (Macaca mulatta). Vet Pathol 53: 399–416. 10.1177/0300985815620628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder-Mackler N, Somel M, Tung J. 2014. Shared signatures of social stress and aging in peripheral blood mononuclear cell gene expression profiles. Aging Cell 13: 954–957. 10.1111/acel.12239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder-Mackler N, Sanz J, Kohn JN, Brinkworth JF, Morrow S, Shaver AO, Grenier JC, Pique-Regi R, Johnson ZP, Wilson ME, et al. 2016. Social status alters immune regulation and response to infection in macaques. Science 354: 1041–1045. 10.1126/science.aah3580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder-Mackler N, Sanz J, Kohn JN, Voyles T, Pique-Regi R, Wilson ME, Barreiro LB, Tung J. 2019. Social status alters chromatin accessibility and the gene regulatory response to glucocorticoid stimulation in rhesus macaques. Proc Natl Acad Sci 116: 1219–1228. 10.1073/pnas.1811758115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder-Mackler N, Burger JR, Gaydosh L, Belsky DW, Noppert GA, Campos FA, Bartolomucci A, Yang YC, Aiello AE, O'Rand A, et al. 2020. Social determinants of health and survival in humans and other animals. Science 368: eaax9553. 10.1126/science.aax9553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC. 1989. Trade-offs in life history evolution. Funct Ecol 3: 259–268. 10.2307/2389364 [DOI] [Google Scholar]
- Stearns SC. 2012. Evolutionary medicine: its scope, interest and potential. Proc Biol Sci 279: 4305–4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif SD, Mansfield KG, Ratnam R, Ross CN, Ziegler TE. 2011. The marmoset as a model of aging and age-related diseases. ILAR J 52: 54–65. 10.1093/ilar.52.1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson NA. 2019. Understanding the links between social ties and fitness over the life cycle in primates. Behaviour 156: 859–908. 10.1163/1568539X-00003552 [DOI] [Google Scholar]
- Thompson NA, Cords M. 2018. Stronger social bonds do not always predict greater longevity in a gregarious primate. Ecol Evol 8: 1604–1614. 10.1002/ece3.3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson González NA, Otali E, Machanda ZP, Muller MN, Wrangham RW, Emery Thompson M. 2020. Urinary markers of oxidative stress respond to infection and late life in wild chimpanzees. PLoS ONE 15: e0238066. 10.1371/journal.pone.0238066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumble BC, Stieglitz J, Blackwell AD, Allayee H, Beheim B, Finch CE, Gurven M, Kaplan H. 2017. Apolipoprotein E4 is associated with improved cognitive function in Amazonian forager-horticulturalists with a high parasite burden. FASEB J 31: 1508–1515. 10.1096/fj.201601084R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J, Barreiro LB, Johnson ZP, Hansen KD, Michopoulos V, Toufexis D, Michelini K, Wilson ME, Gilad Y. 2012. Social environment is associated with gene regulatory variation in the rhesus macaque immune system. Proc Natl Acad Sci 109: 6490–6495. 10.1073/pnas.1202734109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN. 2009. Understanding the links between social support and physical health: a life-span perspective with emphasis on the separability of perceived and received support. Perspect Psychol Sci 4: 236–255. 10.1111/j.1745-6924.2009.01122.x [DOI] [PubMed] [Google Scholar]
- Varki N, Anderson D, Herndon JG, Pham T, Gregg CJ, Cheriyan M, Murphy J, Strobert E, Fritz J, Else JG, et al. 2009. Heart disease is common in humans and chimpanzees, but is caused by different pathological processes. Evol Appl 2: 101–112. 10.1111/j.1752-4571.2008.00064.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasunilashorn S, Crimmins EM, Kim JK, Winking J, Gurven M, Kaplan H, Finch CE. 2010. Blood lipids, infection, and inflammatory markers in the Tsimane of Bolivia. Am J Hum Biol 22: 731–740. 10.1002/ajhb.21074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasunilashorn S, Finch C, Crimmins EM, Vikman SA, Stielitz J, Gurven M, Kaplan HS, Allayee H. 2011. Inflammatory gene variants in the Tsimane, an indigenous Bolivian population with a high infectious load. Biodemography Soc Biol 57: 33–52. 10.1080/19485565.2011.564475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videan EN, Fritz J, Murphy J. 2008. Effects of aging on hematology and serum clinical chemistry in chimpanzees (Pan troglodytes). Am J Primatol 70: 327–338. 10.1002/ajp.20494 [DOI] [PubMed] [Google Scholar]
- Videan EN, Heward CB, Chowdhury K, Plummer J, Su Y, Cutler RG. 2009. Comparison of biomarkers of oxidative stress and cardiovascular disease in humans and chimpanzees. Comp Med 59: 287–296. [PMC free article] [PubMed] [Google Scholar]
- Wallace IJ, Worthington S, Felson DT, Jurmain RD, Wren KT, Maijanen H, Woods RJ, Lieberman DE. 2017. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci 114: 9332–9336. 10.1073/pnas.1703856114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace IJ, Riew GJ, Landau R, Bendele AM, Holowka NB, Hedrick TL, Konow N, Brooks DJ, Lieberman DE. 2022. Experimental evidence that physical activity inhibits osteoarthritis: implications for inferring activity patterns from osteoarthritis in archeological human skeletons. Am J Biol Anthropol 177: 223–231. 10.1002/ajpa.24429 [DOI] [Google Scholar]
- Warren LA, Shi Q, Young K, Borenstein A, Martiniuk A. 2015. Prevalence and incidence of dementia among indigenous populations: a systematic review. Int Psychoger 27: 1959–1970. 10.1017/S1041610215000861 [DOI] [PubMed] [Google Scholar]
- Williams GC. 1957. Pleiotropy, natural selection and the evolution of senescence. Evolution 11: 398–411. 10.1111/j.1558-5646.1957.tb02911.x [DOI] [Google Scholar]
- Williams GC. 1966. Natural selection, the costs of reproduction, and a refinement of lack's principle. Am Nat 100: 687–690. 10.1086/282461 [DOI] [Google Scholar]
- Wrycza TF, Missov TI, Baudisch A. 2015. Quantifying the shape of aging. PLoS ONE 10: e0119163. 10.1371/journal.pone.0119163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L, Prather ER, Stetskiv M, Garrison DE, Meade JR, Peace TI, Zhou T. 2019. Inflammaging and oxidative stress in human diseases: from molecular mechanisms to novel treatments. Int J Mol Sci 20: 4472. 10.3390/ijms20184472 [DOI] [PMC free article] [PubMed] [Google Scholar]