Abstract
OBJECTIVES:
Severe alcoholic hepatitis (sAH) confers substantial mortality, but the disease course is difficult to predict. As iron parameters are attractive outcome predictors in other liver diseases, we tested their prognostic ability in sAH.
METHODS:
Serum ferritin, transferrin, iron, transferrin saturation, nontransferrin-bound iron, soluble transferrin receptor, and hepcidin were measured in 828 patients with sAH recruited prospectively through the STOPAH trial. The cohort was randomly divided into exploratory (n = 200) and validation sets (n = 628).
RESULTS:
Patients with sAH had diminished serum transferrin but increased transferrin saturation. Among iron parameters, baseline transferrin was the best predictor of 28-day (area under the receiver operated characteristic 0.72 [95% confidence interval 0.67–0.78]) and 90-day survival (area under the receiver operated characteristic 0.65 [0.61–0.70]). Transferrin’s predictive ability was comparable with the composite scores, namely model of end-stage liver disease, Glasgow alcoholic hepatitis score, and discriminant function, and was independently associated with survival in multivariable analysis. These results were confirmed in a validation cohort. Transferrin did not correlate with markers of liver synthesis nor with non-transferrin-bound iron or soluble transferrin receptor (as markers of excess unbound iron and functional iron deficiency, respectively).
DISCUSSION:
In patients with sAH, serum transferrin predicts mortality with a performance comparable with commonly used composite scoring systems. Hence, this routinely available parameter might be a useful marker alone or as a component of prognostic models.
INTRODUCTION
Alcoholic hepatitis (AH) is a distinct clinical presentation of alcohol-related liver disease which develops in a subset of patients with prolonged, heavy alcohol misuse. It is characterized by a recent onset of jaundice coupled with signs of liver failure (1-3). Severe AH (sAH) is defined by a Maddrey’s discriminant function (DF), derived from the serum bilirubin and prothrombin time, ≥32. It is associated with 28-day mortality as high as 15–30%, while 1-year mortality is typically over 50% (4). In addition to DF, the model for end-stage liver disease (MELD) and Glasgow alcoholic hepatitis score (GAHS) are other commonly used prognostic tools (1,5). Apart from these, demographic and genetic factors, oxidative stress, development of infection, as well as the ability of patients to abstain from alcohol have been shown to affect the course of AH (4,6-8). However, the pathogenesis of AH remains incompletely understood. Recent studies in other etiologies of liver disease (9-14) led us to hypothesize that parameters of iron metabolism might also be of prognostic relevance in AH.
Iron is essential for virtually all organisms including invading microbes. Consequently, iron overload and especially “free iron” may contribute to the development of infection (15-17). In addition, unbound iron is highly reactive and causes oxidative stress (18). To prevent this, iron is coupled to transferrin in serum and sequestered by ferritin in tissues (15,18). Accordingly, transferrin is the central iron recycler facilitating iron uptake into tissues (15,18). A proportion of liver ferritin is released into serum, and its level represents an imperfect, but widely used marker of overall body iron load (19). Circulating serum iron constitutes a small and highly dynamic iron transit compartment that is rapidly altered in disease states (18,20). Increased serum iron results in elevated transferrin saturation (TSAT) (12,19,21) and may lead to emergence of highly reactive non–transferrin-bound iron (NTBI) (22,23). The liver regulates plasma iron homeostasis and total body iron because it constitutes a large iron storage compartment and a major producer of ferritin, transferrin, and hepcidin (24,25). Hepcidin is the key regulator of plasma iron and blocks the absorption of iron from the intestine and the release of iron from macrophages (15,18). Patients with chronic liver diseases often display altered serum iron parameters. Transferrin, a well-known negative acute-phase protein (26-28), is decreased in various entities of chronic liver disease and is particularly low in both acute-on-chronic liver failure and sepsis (12,14,21,29). Similarly, serum hepcidin levels are often diminished during liver injury promoting the development of parenchymal iron overload (9,11,24,25). As a consequence, serum iron may increase, and TSAT becomes elevated (12,21). Moreover, as a result of a parenchymal iron overload and hepatocellular damage, ferritin is released into the circulation, and its serum levels are increased (10,12,13,21).
Multiple studies have demonstrated that in patients with liver disease, serum parameters of iron metabolism are attractive prognostic markers. Most studies have focused on transferrin—decreased levels confer a poorer prognosis on patients with alcohol-related liver cirrhosis and acute-on-chronic liver failure (14,21). Increased serum ferritin and TSAT, as well as diminished circulating hepcidin, have also been associated with worse outcomes (10,12,13,21). However, in AH, neither serum parameters of iron metabolism nor the amount of hepatic iron load has been comprehensively analyzed.
Oxidative stress and impaired immune regulation play a crucial role in the development of AH (30). Although altered serum iron parameters were suggested to give rise to both oxidative stress and impaired immune regulation (15,16,18), the prognostic utility of serum iron parameters in AH remains unknown. To assess the association between serum iron parameters and outcome in AH, we analyzed iron parameters in patients recruited to the Steroids or Pentoxifylline for Alcoholic Hepatitis (STOPAH) clinical trial, the largest cohort of patients with AH ever studied (31).
METHODS
Study population
Patients with sAH (n = 828) were recruited through the STOPAH trial (ISRCTN88782125) as per the trial protocol (31,32). All had a history of long-standing alcohol misuse; compatible clinical, laboratory, and/or liver biopsy features of AH; no other identified causes for their liver disease; and a DF ≥32. Outcome data were collected for mortality at 28 and 90 days. Patients were randomized to treatment with prednisolone or pentoxifylline for 28 days using a double-blind, double-dummy factorial 2 × 2 design. Patients were consented for follow-up through the NHS Information Centre Data Linkage service ensuring ongoing follow-up and reliable capture of mortality data. Patients presenting with either infection or gastrointestinal hemorrhage were eligible for inclusion into the trial once the condition was deemed clinically controlled by the treating physician. A more detailed description is provided in the primary analysis of the trial data (31) and in the supplement.
Data processing and statistical analysis
To prove robust association in independent populations, exploratory and validation cohorts were defined. Each subject was assigned a random number using the “RAND()” function in Excel (Microsoft, Seattle). The 200 patients with the lowest numbers and MELD score >20 served as the exploratory population. This minimum MELD threshold was applied to ensure an adequate number of events in the smaller cohort to power statistical analyses. The remaining cases (n = 628), i.e., patients with low and high MELD scores not included in the exploratory cohort, were used as a replication cohort to validate findings.
Nominal two-sided P values were reported for all tests and were considered to be statistically significant when P < 0.05 unless mentioned otherwise. Based on the results of a normality test, continuous variables were compared by an unpaired t test or by Mann-Whitney U test. Associations between iron parameters and clinical endpoints were tested by logistic regression. To analyze the relevance of iron parameters as predictors of mortality, a threshold of P < 0.05 was used to define statistical significance. Given that gastrointestinal bleeding and the presence of infection affects the parameters of iron metabolism, both subgroups were analyzed separately. Multivariable logistic regression was used to test for independent associations to calculate odds ratios (OR), which are given with their 95% confidence interval [in brackets]. Independent variables were entered into the models based on previously described associations with outcomes in this cohort of patients. Correlations between clinical variables and serum biomarkers were tested by the Spearman rank correlation test. Owing to the large number of comparisons in correlation analyses, the Benjamini-Hochberg method was used to adjust P values to maintain a false discovery rate equivalent to 5%.
Area under the receiver operating characteristic (AUROC) analysis was used to assess the predictive performance of biomarkers and scoring systems. The predictive performance of common prognostic scores in combination with serum transferrin was tested by fitting logistic regression models with leave 1- of 10-fold cross-validation to generate AUROCs and avoid overoptimism. Statistical differences between AUROCs were tested using the DeLong method. The optimal cutoff values for transferrin as a predictor of mortality were determined using the Youden index. Kaplan-Meier curves were generated, and differences in survival were tested using the log rank test.
Statistical analyses were performed using SPSS v24 (IBM, Armonk, NY) and R (R Foundation, Vienna, Austria) using the packages pROC, OptimalCutpoints, survival, ggplot2, gridExtra, plyr, reshape2, psych, and car. Graphs were created with Prism 5 (GraphPad, La Jolla, CA).
RESULTS
Group characteristics and determination of iron indices
Baseline characteristics of the exploratory and validation group were similar and reflected the overall cohort, although the exploratory cohort displayed a somewhat higher mortality because of the selection strategy (Table 1).
Table 1.
Baseline characteristics of the exploratory and replication cohorts
| Baseline characteristic |
Exploratory population (n = 200) |
Replication population (n = 628) |
|---|---|---|
| Age (years) | 49 (42–57) | 49 (42–56) |
| Sex (male; n, %) | 123 (62%) | 400 (64%) |
| Prednisolone (n, %) | 100 (50%) | 313 (50%) |
| Infection | 21 (11%) | 86 (14%) |
| GI bleeding | 13 (7%) | 63 (10%) |
| Hemoglobin (g/L) | 104 (89–120) | 107 (95–120) |
| Platelets (×109/L) | 114 (70–171) | 111 (77–166) |
| White cell count (×106/mm3) | 8.9 (6.0–12.7) | 8.6 (6.1–12.3) |
| Neutrophils (×106/mm3) | 6.2 (4.0–10.1) | 5.9 (4.1–9.4) |
| INR | 1.9 (1.6–2.2) | 1.7 (1.5–2.0) |
| Bilirubin (μmol/L) | 317 (192–451) | 250 (154–394) |
| Albumin (g/L) | 25 (21–29) | 25 (21–30) |
| ALT (IU/L) | 42 (30–62) | 42 (29–61) |
| AST (IU/L) | 121 (84–171) | 120 (87–161) |
| Sodium (mmol/L) | 134 (129–136) | 134 (131–137) |
| Urea (mmol/L) | 3.8 (2.4–6.9) | 3.2 (2.1–4.9) |
| Creatinine (μmol/L) | 67 (53–104) | 63 (52–79) |
| Prognostic scores | ||
| DF | 60 (48–79) | 54 (42–71) |
| MELD | 24 (22–29) | 23 (21–26) |
| GAHS | 9 (8–10) | 8 (7–9) |
| Mortality | ||
| Day 28 (n, %) | 51 (26%) | 66 (11%) |
| Day 90 (n, %) | 70 (35%) | 126 (20%) |
Data are presented as median and interquartile range. Baseline serum parameters were available in 828 patients, who were randomly divided into the exploratory and replication subgroup.
ALT, alanine transaminase; AST, aspartate transaminase; DF, discriminant function; GAHS, Glasgow alcoholic hepatitis score; GI, gastrointestinal; INR, international normalized ratio; MELD, model for end-stage liver disease.
Serum iron, transferrin, ferritin, and TSAT were successfully determined in 196–199 (98–99%) patients in the exploratory and in all patients in the replication population. Serum hepcidin levels were obtained in 199 (99%) patients in the exploratory and in 563 (90%) patients in the replication cohort.
Patients with sAH displayed deranged iron parameters with elevated serum ferritin/TSAT and low serum transferrin (Table 2).
Table 2.
Baseline serum parameters of iron metabolism in the exploratory and replication cohorts
| Characteristic | Exploratory population (n = 200) |
Replication population (n = 628) |
Reference ranges |
|---|---|---|---|
| Ferritin (ng/mL) | Male: 862 (307–1,574) Female: 497 (260–853) |
Male: 827 (353–1,577) Female: 692 (275–1,310) |
Male: 30–400 Female: 13–150 |
| Transferrin (mg/dL) | 93 (72–128) | 123 (91–168) | 200–360 |
| TSAT (%) | 69 (40–86) | 58 (34–82) | 25–45% |
| Iron (μmol/L)) | 13.8 (9.6–18.3) | 16.5 (11.4–22.7) | 5.8–35 |
| Hepcidin (ng/mL) | 9.0 (3.2–22.7) | 12.4 (4.6–26.0) | NA |
Data are presented as median and interquartile range. Baseline serum parameters were available in 828 patients, who were randomly divided into the exploratory and replication subgroup.
NA, not available; TSAT, transferrin saturation.
As expected, higher ferritin values were observed in male patients, while other serum parameters did not differ substantially between the sexes (see Table 1, Supplementary Digital Content 1, http://links.lww.com/AJG/B340). There was no statistically significant interaction between serum transferrin level and sex in relation to mortality in either the exploratory or replication cohorts (P = 0.89 and P = 0.18, respectively); therefore, groups were not analyzed separately by sex.
Associations between iron indices and mortality
Serum ferritin, transferrin, hepcidin, and TSAT were significantly associated with 28-day mortality in the exploratory cohort while serum iron was not (Table 3). Serum transferrin (OR 0.98 [95% CI 0.97–0.99], P < 0.001), TSAT (OR 1.02 [1.003–1.027], P = 0.01), and ferritin (OR 1.000 [1.000–1.001], P = 0.01) but not the other parameters (iron: OR 0.98 [0.94–1.03], P = 0.46; hepcidin: OR 1.01 [0.999–1.027], P = 0.07) were also associated with 90-day mortality (Table 4).
Table 3.
Baseline serum iron parameters in survivors and nonsurvivors based on 28-day mortality
| Characteristic | Exploratory population (n = 200) | Replication population (n = 628) |
|---|---|---|
| Ferritin (ng/mL) | 526 (219–1,147) vs 1,050 (594–2003) | 723 (313–1,460) vs 1,033 (531–1,590) |
| OR 1.001, 95% CI 1.000–1.001 P = 0.001 | OR 1.000, 95% CI 1.000–1.000 P = 0.07 | |
| Transferrin (mg/dL) | 102 (78–132) vs 74 (57–99) | 126 (96–171) vs 96 (71–129) |
| OR 0.98, 95% CI 0.97–0.99 P = 0.0001 | OR 0.99, 95% CI 0.983–0.995 P = 0.0002 | |
| TSAT (%) | 65 (36–83) vs 82 (53–95) | 56 (33–81) vs 80 (41–91) |
| OR 1.02, 95% CI 1.01–1.04 P = 0.001 | OR 1.02, 95% CI 1.01–1.03 P = 0.0001 | |
| Iron (μmol/L) | 13.8 (9.3–18.6) vs 13.8 (11.4–17.4) | 16.5 (11.4–22.9) vs 15.9 (11.9–22.5) |
| OR 0.98, 95% CI 0.94–1.03 P = 0.53 | OR 0.99, 95% CI 0.96–1.02 P = 0.51 | |
| Hepcidin (ng/mL) | 7.2 (2.5–18.6) vs 17.6 (6.2–35.2) | 12.4 (4.6–26) vs 12.3 (4.8–28.4) |
| OR 1.02, 95% CI 1.01–1.04 P = 0.007 | OR 1.005, 95% CI 0.99–1.02 P = 0.50 |
Baseline serum parameters were available in 828 patients, who were randomly divided into the exploratory and replication subgroup. Data obtained from 28-day survivors vs nonsurvivors are shown as medians and interquartile ranges.
CI, confidence interval; OR, odds ratio; TSAT, transferrin saturation.
Table 4.
Association between serum iron indices and 90-day mortality
| Characteristic | Exploratory population (n = 200) | Replication population (n = 628) |
|---|---|---|
| Ferritin (ng/mL) | 526 (233–1,296) vs 857 (470–1709) | 761 (327–1,499) vs 782 (323–1,406) |
| OR 1.000, 95% CI 1.000–1.001 P = 0.01 | OR 1.000, 95% CI 1.000–1.000 P = 0.86 | |
| Transferrin (mg/dL) | 102 (78–135) vs 78 (61–104) | 129 (96–174) vs 108 (80–142) |
| OR 0.98, 95% CI 0.97–0.99 P < 0.001 | OR 0.994, 95% CI 0.990–0.997 P = 0.001 | |
| TSAT (%) | 65 (37–83) vs 76 (43–91) | 56 (33–81) vs 68 (39–87) |
| OR 1.02, 95% CI 1.003–1.027 P = 0.01 | OR 1.01, 95% CI 1.002–1.017 P = 0.01 | |
| Serum iron (μmol/L) | 13.8 (9.3–18.6) vs 14.0 (10.4–18.0) | 16.8 (11.4–23.1) vs 15.3 (11.4–21.4) |
| OR 0.98, 95% CI 0.94–1.03 P = 0.46 | OR 0.98, 95% CI 0.96–1.01 P = 0.16 | |
| Hepcidin (ng/mL) | 8.0 (2.5–18.9) vs 12.4 (5.1–29.9) | 12.6 (4.7–26.3) vs 12.0 (4.5–24.1) |
| OR 1.01, 95% CI 0.999–1.027 P = 0.07 | OR 1.000, 95% CI 0.99–1.01 P = 0.98 |
Baseline serum parameters were available in 828 patients, who were randomly divided into the exploratory and replication subgroup. Data obtained from 90-day survivors vs nonsurvivors are shown as medians and interquartile ranges.
CI, confidence interval; OR, odds ratio; TSAT, transferrin saturation.
In the replication cohort, serum transferrin (OR 0.99 [0.983–0.995], P = 0.0002) and TSAT (OR 1.02 [1.01–1.03], P = 0.0001) associated with 28-day mortality, while the association between ferritin and hepcidin with 28-day mortality could not be reproduced (OR 1.000 [1.000–1.000], P = 0.07 and OR 1.005 [0.99–1.02], P = 0.50, respectively). The lack of association of serum iron (OR 0.99 [0.96–1.02], P = 0.51) with 28-day mortality was confirmed (Table 3). The associations between serum transferrin (OR 0.994 [0.990–0.997], P = 0.001) and TSAT (OR 1.01 [1.002–1.017], P = 0.01) and 90-day mortality were also reproducible. No other significant associations between iron indices and 90-day mortality were seen (ferritin: OR 1.000 [1.000–1.000], P = 0.86; iron: OR 0.98 [0.96–1.01], P = 0.16; hepcidin: OR 1.000 [0.99–1.01], P = 0.98) (Table 4).
Notably, serum transferrin was associated with both 28-day and 90-day mortality in nearly all treatment arms including the placebo-placebo arm, indicating that there was no relevant imbalance between treatment subgroups or between the cohorts (Table 5). In line, no correlation was observed between the serum transferrin level and the Lille score in the entire cohort (rho = −0.39, P = 0.381). Moreover, Lille responders and nonresponders displayed similar median serum transferrin levels (114 [IQR 84–156] vs 108 [IQR 81–144, P = 0.273]). No significant associations were revealed when analyses were restricted to only patients treated with prednisolone. Finally, a logistic regression model revealed that there was no significant interaction between prednisolone and serum transferrin levels in relation to the Lille response, thereby demonstrating that serum transferrin levels do not predict “prednisolone responsiveness” (see Table 2, Supplementary Digital Content 1, http://links.lww.com/AJG/B340).
Table 5.
Sensitivity analysis of transferrin for predicting 28- and 90-day mortality
| Treatment arm | OR | 95% CI | P |
|---|---|---|---|
| 28-day mortality | |||
| Placebo-placebo | 0.967 | 0.952–0.982 | <0.0001 |
| Prednisolone | 0.991 | 0.982–1.000 | 0.0493 |
| Pentoxifylline | 0.988 | 0.981–0.996 | 0.00385 |
| Prednisolone and pentoxifylline | 0.983 | 0.972–0.994 | 0.00348 |
| 90-day mortality | |||
| Placebo-placebo | 0.987 | 0.979–0.996 | 0.00249 |
| Prednisolone | 0.995 | 0.989–1.000 | 0.0679 |
| Pentoxifylline | 0.990 | 0.983–0.996 | 0.00171 |
| Prednisolone and pentoxifylline | 0.990 | 0.983–0.997 | 0.00778 |
The odds ratios with their corresponding 95% confidence intervals for the different treatment arms of the STOPAH trial (2 × 2 factorial design) are presented.
Effect of gastrointestinal hemorrhage and infection at baseline on iron parameters
As anticipated, patients with gastrointestinal bleeding at baseline displayed significantly lower serum iron, hepcidin, ferritin, and TSAT levels, but had higher serum transferrin than individuals, who experienced neither infection nor bleeding (see Table 3, Supplementary Digital Content 1, http://links.lww.com/AJG/B340). Comparable, but less unequivocal findings were made, when exploratory and validation cohorts were subdivided into patients with and without bleeding at the baseline (see Table 4, Supplementary Digital Content 1, http://links.lww.com/AJG/B340). Notably, individuals with infection had somewhat lower levels of serum iron and transferrin than patients with neither infection nor bleeding (see Table 3, Supplementary Digital Content 1, http://links.lww.com/AJG/B340). Again, less distinct changes were seen when the exploratory and validation cohorts were subdivided based on their infection status at the baseline (see Table 5, Supplementary Digital Content 1, http://links.lww.com/AJG/B340). Importantly, serum transferrin was a similarly good predictor of 28-day mortality in all subgroups. The association between TSAT and 28-day mortality was seen in individuals without gastrointestinal bleeding and infection but was not significant in patients with either bleeding or infection (see Table 3, Supplementary Digital Content 1, http://links.lww.com/AJG/B340).
Multivariable analysis
In light of the independently replicated associations between serum transferrin and TSAT in relation to both 28- and 90-day mortality, multivariable logistic regression was performed to assess whether it was an independent predictor. In these analyses, both cohorts were combined. Baseline serum transferrin was associated with mortality both at 28 days (OR 0.993, 95% CI 0.987–1.000, P = 0.04) and 90 days (OR 0.995, 95% CI 0.991–1.000, P = 0.03) independently of baseline factors recognized to influence outcome (Table 6). However, TSAT was not an independent predictor of either 28- or 90-day mortality.
Table 6.
Multivariable logistic regression analysis for 28- and 90-day mortality
| Characteristic | 28-day mortality |
90-day mortality |
||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age (years) | 1.07 (1.04–1.10) | <0.0001 | 1.06 (1.04–1.08) | <0.001 |
| Sex (male) | 0.99 (0.59–1.67) | 0.98 | 0.81 (0.54–1.22) | 0.32 |
| Prednisolone | 0.54 (0.33–0.90) | 0.02 | 0.91 (0.62–1.34) | 0.63 |
| Encephalopathy (grade) | 1.59 (1.18–2.14) | 0.002 | 1.39 (1.07–1.80) | 0.01 |
| White cell count (×106/mm3) | 1.03 (0.98–1.07) | 0.21 | 1.02 (0.98–1.06) | 0.30 |
| INR | 2.36 (1.47–3.80) | <0.001 | 2.53 (1.70–3.75) | <0.001 |
| Albumin (g/L) | 0.98 (0.94–1.02) | 0.34 | 0.98 (0.95–1.01) | 0.19 |
| Bilirubin (μmol/L) | 1.002 (1.001–1.004) | <0.01 | 1.002 (1.001–1.003) | 0.01 |
| Sodium (mmol/L) | 0.96 (0.92–0.99) | 0.04 | 0.97 (0.93–1.003) | 0.07 |
| Urea (mmol/L) | 1.10 (1.04–1.16) | <0.01 | 1.06 (1.005–1.112) | 0.03 |
| Creatinine (μmol/L) | 1.000 (0.994–1.006) | 0.99 | 1.001 (0.996–1.006) | 0.76 |
| Transferrin (mg/dL) | 0.993 (0.987–1.000) | 0.04 | 0.995 (0.991–1.000) | 0.03 |
| TSAT (%) | 1.006 (0.995–1.018) | 0.30 | 0.998 (0.989–1.007) | 0.63 |
CI, confidence interval; INR, international normalized ratio; OR, odds ratio; TSAT, transferrin saturation.
Prediction of mortality
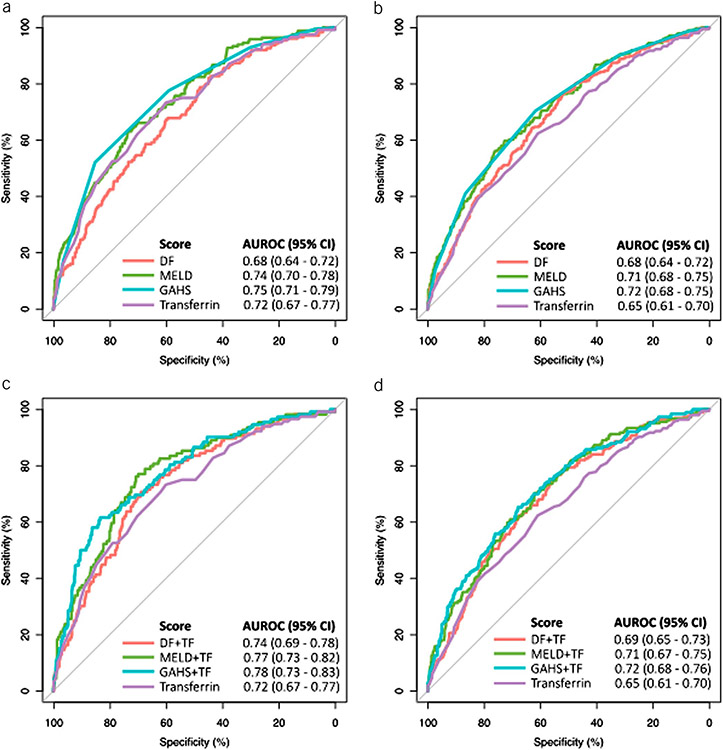
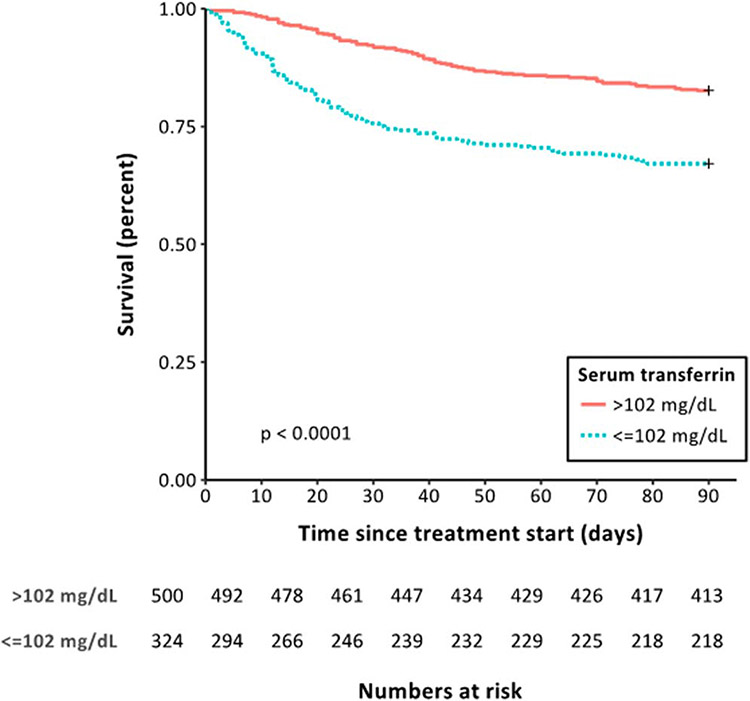
The AUC for transferrin to predict 28-day mortality (0.72 [0.67–0.77]) was similar to and not significantly different from that of the commonly used prognostic scoring systems (DF: 0.68 [0.64–0.72], P = 0.4; MELD: 0.74 [0.70–0.78], P = 0.59; and GAHS: 0.75 [0.71–0.79], P = 0.32; Figure 1a). Although discrimination in relation to 90-day mortality for transferrin (AUC: 0.65 [0.61–0.70]) was not statistically different to DF (0.68 [0.64–0.72], P = 0.25) or MELD (0.71 [0.68–0.75], P = 0.073), it was inferior to GAHS (0.71 [0.68–0.75], P = 0.016) (Figure 1b). Application of the Youden index indicated an optimal cutoff value of 102 mg/dL in relation to 28-day mortality and was used to define “high” and “low” transferrin populations (see figure 1, Supplementary Digital Content 1, http://links.lww.com/AJG/B340). This cutoff value had a sensitivity of 71%, a specificity of 64%, a negative predictive value of 93.2%, and a positive predictive value of 23.9%. Patients with “low” serum transferrin harbored significantly higher mortality at 28 (24.0% vs 7.6%, P < 0.0001) and 90 days (32.9% vs 17.4%, P < 0.0001, Figure 2). Transferrin was associated with mortality at days 28 and 90 independently of MELD, DF, or GAHS (see Table 6, Supplementary Digital Content 1, http://links.lww.com/AJG/B340). The combination of transferrin with each of the scoring systems demonstrated a trend toward greater AUROCs for 28-day mortality (DF + TF: AUC 0.74 [0.69–0.78, P = 0.06; MELD + TF: 0.77 [0.73–0.82], P = 0.09; GAHS + TF: 0.78 [0.73–0.83], P = 0.05; Figure 1c) but not 90-day mortality (DF + TF: 0.69 [0.65–0.73], P = 0.7; MELD + TF: 0.71 [0.67–0.75], P = 0.5; GAHS + TF: 0.72 [0.68–0.76], P = 0.6; Figure 1d).
Figure 1.
Receiver operated characteristic (ROC) curves for the established scoring systems and serum transferrin in relation to 28-day (a) and 90-day (b) mortality. Baseline values were considered for all analyses. The area under the receiver operated characteristic (AUROC) was numerically enhanced by the combination of a baseline scoring system and serum transferrin for 28-day mortality (c), but there was little impact on the AUROC for 90-day mortality (d). DF, discriminant function; GAHS, Glasgow alcoholic hepatitis score; MELD, model for end-stage liver disease; TF, transferrin.
Figure 2.
Kaplan-Meier curves illustrating the differences in survival in groups stratified by “low” (≤102 mg/dL) and “high” (>102 mg/dL) serum transferrin levels. The cutoff value for transferrin was determined through the application of the Youden index to the ROC curve for serum transferrin in relation to 28-day mortality. Patients with “low” serum transferrin displayed significantly higher mortality at both 28 (24.0% vs 7.6%, P < 0.0001, log rank test) and 90 days (32.9% vs 17.4%, P < 0.0001, log rank test).
Mechanistic role of deranged iron parameters in AH
To get a better insight into the mechanistic role of deranged iron parameters in AH, we tested several hypotheses. First, we compared serum transferrin levels to markers of liver synthesis. We detected a weak correlation with serum bilirubin (rho = −0.2, P < 0.01) and INR (rho = −0.19, P < 0.01), but no correlation with serum albumin (rho = 0, P = 1; see Figure 2, Supplementary Digital Content 1, http://links.lww.com/AJG/B340). On the other hand, a moderate negative correlation with the acute-phase reactants C-reactive protein (rho = −0.33, P < 0.01), ferritin (rho = −0.4, P < 0.01, see Figure 2, Supplementary Digital Content 1, http://links.lww.com/AJG/B340), and the white cell count (rho = −0.31, P < 0.01) was noted. Collectively, these data suggested that transferrin (negatively) correlates with the acute-phase response rather than the synthetic capacity of the liver.
As transferrin is the central iron recycler, we assessed whether reduced serum transferrin levels may confer a poor prognosis because of impaired iron recycling. However, no association between the serum levels of soluble transferrin receptor, as a marker of cellular iron deficiency, and increased 28-day mortality was noted (OR 1.02 [0.996–1.04], P = 0.10; see Table 7, Supplementary Digital Content 1, http://links.lww.com/AJG/B340). Since reduced transferrin and increased TSAT promote the emergence of highly reactive NTBI, we assessed the prognostic relevance of this parameter in our exploratory AH cohort. Although we saw a marked correlation between NTBI and TSAT, no association of NTBI with 28- or 90-day mortality was detected (see Table 8, Supplementary Digital Content 1, http://links.lww.com/AJG/B340 and not shown).
DISCUSSION
In this study, we report a significant association between deranged parameters of iron metabolism and mortality in a large cohort of patients with sAH. Specifically, decreased serum transferrin is associated with increased mortality. The observed alterations are reminiscent of changes seen in patients with end-stage liver disease and acute-on-chronic liver failure (12-14,21,29). Moreover, similar findings have been reported in critically ill patients (27). Collectively, these data suggest that the observed changes in transferrin constitute a conserved response to multiple different stress situations.
Transferrin levels alone have a strong association with outcome and prognostic capacity comparable with established predictive scores that use multiple variables such as MELD, GAHS, and DF. Moreover, the association is independent of markers of liver dysfunction and commonly used prognostic scores. This suggests that although transferrin is not superior to existing measures, it might be a useful component of new prognostic models. However, further studies are needed to fully elucidate the clinical utility. Although various other approaches yielded potentially attractive predictors for sAH, the presented findings about transferrin have several advantages: (i) In contrast to gene expression-based assays (33), it does not require a liver biopsy; (ii) compared with quantification of keratin 18-based fragments or serum malondialdehyde (34,35), transferrin measurement is widely available in routine clinical practice; (iii) our data have been validated by analysis in a very large, well-defined cohort of patients with sAH, while other parameters have been tested only in small studies (34-36); and (iv) our approach enabled the analysis of transferrin in 2 independent cohorts, that differed in disease severity and mortality. Notably, transferrin was a robust predictor in both cohorts indicating that transferrin’s association with mortality is at least partially independent of disease severity.
The fact that diminished transferrin predicts mortality in sAH raises the question of the underlying biological basis of this association. To elucidate this, we tested several hypotheses: (i) As transferrin is primarily synthesized in the liver, we correlated transferrin with several parameters of liver synthesis; however, the correlations were poor; (ii) given that the uptake of transferrin-bound iron represents the major cellular iron uptake pathway, a decrease in transferrin may lead to an insufficient supply of iron to cells (18,37). However, such a functional iron deficiency would result in increased soluble transferrin receptor levels (19) that were not regularly seen in the analyzed AH patients; and (iii) since transferrin is the major iron-sequestering protein in serum, decreased transferrin levels may lead to an increased formation of highly reactive NTBI (22,23). However, in our cohort, elevated NTBI did not correlate with an adverse outcome. These results indicate that decreased transferrin is a marker of hepatocellular stress rather than an indicator of iron status. This is supported by our findings that transferrin negatively correlates with markers of the acute-phase response. However, mechanistic data are needed to prove this hypothesis and to elucidate the underlying mechanisms.
In summary, this study reports that transferrin is a novel and a potentially useful predictor of outcome in sAH. Given that transferrin is already widely available in clinical routine, it could be rapidly used as a prognostic marker in sAH, either alone or as a component of new prognostic models.
Supplementary Material
Study Highlights.
WHAT IS KNOWN
The pathogenesis of severe alcoholic hepatitis (sAH) is incompletely understood, and hence, no good biomarkers predicting the disease course exist.
3 Iron parameters are attractive outcome predictors in liver diseases of various etiologies but have not yet been systematically studied in sAH.
WHAT IS NEW HERE
Serum transferrin independently predicts 28- and 90-day mortality with a performance comparable with commonly used composite scoring systems.
Transferrin reduction is independent of disease complications, liver synthesis, functional iron deficiency, and levels of non–transferrin-bound iron.
ACKNOWLEDGEMENTS
The authors thank all patients for their participation in our study.
Financial support:
This work was supported by the Interdisciplinary Center for Clinical Research (IZKF) within the faculty of Medicine at the RWTH Aachen University (to P.S.), by the Deutsche Forschungsgemeinschaft (DFG) SFB/TRR57 (to P.S. and C.T.), the Else Kröner Excellence Fellowship (to P.S.), the German Liver Foundation (to K.H.), the START program within the medical faculty at RWTH Aachen University (to K.H.), the Medical Research Council (to S.R.A. and M.R.T.), and the National Institute for Health Research (to M.R.T.). S.R.A. and M.R.T. acknowledge the support of the NIHR Imperial Biomedical Research Centre. R.B. is supported by NIH/NIAAA grants AA026972, AA026978 and AA026264, and by NIH/NIDDK grant P30DK120531. J.C. received the Juan Rodés Scholarship 2015 supported by AEEH (Asociación Española para el Estudio del Hígado—Spanish Association for the Study of Liver Diseases).
Footnotes
CONFLICTS OF INTEREST
Guarantor of the article: Pavel Strnad, MD.
Specific author contributions: Stephen R. Atkinson, MD, Karim Hamesch, MD, Mark R. Thursz, MD, and Pavel Strnad, MD contributed equally to this work. Study concept and design: S.R.A., K.H., M.R.T., and P.S. Acquisition of data: S.R.A., K.H., I.S., N.G., J.C., J.A., I.T., H.Z., S.C., P.M., V.H.S. C.T., R.B., M.R.T., and P.S. Analysis and interpretation of data: S.R.A., K.H., J.A., and P.S. Drafting of the manuscript: S.R.A., K.H., and P.S. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: S.R.A. and K.H. Figures and tables: S.R.A., K.H., and P.S. Obtained funding: S.R.A., K.H., R.B., M.R.T., and P.S. Study supervision: S.R.A., K.H., M.R.T., and P.S. All authors had full access to all of the data and approved the final version of this manuscript. All authors can take responsibility for the integrity of the data and the accuracy of the data analysis.
Potential competing interests: All authors declare no support from any organization other than the below mentioned ones for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work. Hence, all authors declare themselves to be independent from funders with respect to this manuscript.
Further remarks: We attest that we did not use any copyright protected material in our manuscript. No writing assistance was provided.
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/B340
REFERENCES
- 1.Crabb DW, Bataller R, Chalasani NP, et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: Recommendation from the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology 2016;150:785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009;360:2758–69. [DOI] [PubMed] [Google Scholar]
- 3.Thursz M, Morgan TR. Treatment of severe alcoholic hepatitis. Gastroenterology 2016;150:1823–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louvet A, Labreuche J, Artru F, et al. Main drivers of outcome differ between short term and long term in severe alcoholic hepatitis: A prospective study. Hepatology 2017;66:1464–73. [DOI] [PubMed] [Google Scholar]
- 5.Forrest EH, Atkinson SR, Richardson P, et al. Application of prognostic scores in the STOPAH trial: Discriminant function is no longer the optimal scoring system in alcoholic hepatitis. J Hepatol 2018;68:511–8. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson SR, Way MJ, McQuillin A, et al. Homozygosity for rs738409:G in PNPLA3 is associated with increased mortality following an episode of severe alcoholic hepatitis. J Hepatol 2017;67:120–7. [DOI] [PubMed] [Google Scholar]
- 7.Das S, Maras JS, Hussain MS, et al. Hyperoxidized albumin modulates neutrophils to induce oxidative stress and inflammation in severe alcoholic hepatitis. Hepatology 2017;65:631–46. [DOI] [PubMed] [Google Scholar]
- 8.Vergis N, Atkinson SR, Knapp S, et al. In patients with severe alcoholic hepatitis, prednisolone increases susceptibility to infection and infection-related mortality, and is associated with high circulating levels of bacterial DNA. Gastroenterology 2017;152:1068–77.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spivak I, Arora J, Meinzer C, et al. Low serum hepcidin is associated with reduced short-term survival in adults with acute liver failure. Hepatology 2019;69:2136–49. [DOI] [PubMed] [Google Scholar]
- 10.Anastasiou OE, Kalsch J, Hakmouni M, et al. Low transferrin and high ferritin concentrations are associated with worse outcome in acute liver failure. Liver Int 2017;37:1032–41. [DOI] [PubMed] [Google Scholar]
- 11.Nahon P, Nuraldeen R, Rufat P, et al. In alcoholic cirrhosis, low-serum hepcidin levels associate with poor long-term survival. Liver Int 2016;36:185–8. [DOI] [PubMed] [Google Scholar]
- 12.Bruns T, Nuraldeen R, Mai M, et al. Low serum transferrin correlates with acute-on-chronic organ failure and indicates short-term mortality in decompensated cirrhosis. Liver Int 2017;37:232–41. [DOI] [PubMed] [Google Scholar]
- 13.Maiwall R, Kumar S, Chaudhary AK, et al. Serum ferritin predicts early mortality in patients with decompensated cirrhosis. J Hepatol 2014;61:43–50. [DOI] [PubMed] [Google Scholar]
- 14.Viveiros A, Finkenstedt A, Schaefer B, et al. Transferrin as a predictor of survival in cirrhosis. Liver Transpl 2018;24:343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science 2012;338:768–72. [DOI] [PubMed] [Google Scholar]
- 16.Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol 2015;15:500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stefanova D, Raychev A, Arezes J, et al. Endogenous hepcidin and its agonist mediate resistance to selected infections by clearing non-transferrin-bound iron. Blood 2017;130:245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganz T. Systemic iron homeostasis. Physiol Rev 2013;93:1721–41. [DOI] [PubMed] [Google Scholar]
- 19.Camaschella C. Iron-deficiency anemia. N Engl J Med 2015;372:1832–43. [DOI] [PubMed] [Google Scholar]
- 20.Gkouvatsos K, Papanikolaou G, Pantopoulos K. Regulation of iron transport and the role of transferrin. Biochim Biophys Acta 2012;1820:188–202. [DOI] [PubMed] [Google Scholar]
- 21.Maras JS, Maiwall R, Harsha HC, et al. Dysregulated iron homeostasis is strongly associated with multiorgan failure and early mortality in acute-on-chronic liver failure. Hepatology 2015;61:1306–20. [DOI] [PubMed] [Google Scholar]
- 22.Brissot P, Ropert M, Le Lan C, et al. Non-transferrin bound iron: A key role in iron overload and iron toxicity. Biochim Biophys Acta 2012;1820:403–10. [DOI] [PubMed] [Google Scholar]
- 23.Koskenkorva-Frank TS, Weiss G, Koppenol WH, et al. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: Insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med 2013;65: 1174–94. [DOI] [PubMed] [Google Scholar]
- 24.Meynard D, Babitt JL, Lin HY. The liver: Conductor of systemic iron balance. Blood 2014;123:168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pietrangelo A. Iron and the liver. Liver Int 2016;36(Suppl 1):116–23. [DOI] [PubMed] [Google Scholar]
- 26.Ritchie RF, Palomaki GE, Neveux LM, et al. Reference distributions for the negative acute-phase proteins, albumin, transferrin, and transthyretin: A comparison of a large cohort to the world’s literature. J Clin Lab Anal 1999;13:280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tacke F, Nuraldeen R, Koch A, et al. Iron parameters determine the prognosis of critically ill patients. Crit Care Med 2016;44:1049–58. [DOI] [PubMed] [Google Scholar]
- 28.Kuscuoglu D, Janciauskiene S, Hamesch K, et al. Liver—master and servant of serum proteome. J Hepatol 2018;69:512–24. [DOI] [PubMed] [Google Scholar]
- 29.Potter BJ, Chapman RW, Nunes RM, et al. Transferrin metabolism in alcoholic liver disease. Hepatology 1985;5:714–21. [DOI] [PubMed] [Google Scholar]
- 30.Mandrekar P, Bataller R, Tsukamoto H, et al. Alcoholic hepatitis: Translational approaches to develop targeted therapies. Hepatology 2016;64:1343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thursz MR, Richardson P, Allison M, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015;372:1619–28. [DOI] [PubMed] [Google Scholar]
- 32.Forrest E, Mellor J, Stanton L, et al. Steroids or pentoxifylline for alcoholic hepatitis (STOPAH): Study protocol for a randomised controlled trial. Trials 2013;14:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trepo E, Goossens N, Fujiwara N, et al. Combination of gene expression signature and model for end-stage liver disease score predicts survival of patients with severe alcoholic hepatitis. Gastroenterology 2018;154:965–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez-Hernandez O, Gonzalez-Reimers E, Quintero-Platt G, et al. Malondialdehyde as a prognostic factor in alcoholic hepatitis. Alcohol Alcohol 2017;52:305–10. [DOI] [PubMed] [Google Scholar]
- 35.Woolbright BL, Bridges BW, Dunn W, et al. Cell death and prognosis of mortality in alcoholic hepatitis patients using plasma keratin-18. Gene Expr 2017;17:301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das S, Hussain MS, Maras JS, et al. Modification patterns of urinary albumin correlates with serum albumin and outcome in severe alcoholic hepatitis. J Clin Gastroenterol 2019;53:e243–e252. [DOI] [PubMed] [Google Scholar]
- 37.Hentze MW, Muckenthaler MU, Galy B, et al. Two to tango: Regulation of mammalian iron metabolism. Cell 2010;142:24–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.