Abstract
Although previous studies have focused on the role of pistachios on metabolic health, the ergogenic effects of the nut must be elucidated. This study evaluated the impact of ingesting raw, shelled, unsalted pistachios on subjective pain ratings, force production, vertical jump, and biochemical indices of recovery from eccentrically biased exercise. Using a crossover design, 27 moderately trained, male athletes completed 3 trials in a randomized counterbalanced fashion. Control received water only, low dose (1.5 oz/d; PL) and high dose (3.0 oz/d; PH) consumed pistachios for 2 weeks with a 3-4-week washout between trials. PH had lower pain ratings in most muscles after 72 h of recovery (p < 0.05). PH prevented a decrease in force production at 120°/s of knee flexion (p > 0.05); whereas force was diminished in the other trials. Creatine kinase, myoglobin, and C-reactive protein increased over time following exercise (p < 0.05); however, there were no advantages following pistachio consumption. No significant changes in vertical jump or superoxide dismutase were elicited during any trial. This study demonstrates that 3.0 oz/d of pistachios can reduce delayed onset of muscle soreness and maintain muscle strength, potentially promoting exercise tolerance and training adaptations.
ClinicalTrials.gov Identifier
Keywords: Pistachio, Muscle, Pain, Antioxidant, Strength, Human
Highlights
-
•
Effects on strength and jump height following pistachio consumption were assessed.
-
•
Perceptions of pain were evaluated during recovery from downhill running.
-
•
The observed reduction in pain suggests a promotion of exercise tolerance.
-
•
Strength maintenance with pistachio consumption suggests an ergogenic effect.
1. Introduction
Structured exercise training programs utilize the principles of overload and progression to yield favorable adaptations. An inevitable outcome of many regimens is an increase in perceived muscle soreness. However, pain may be problematic because of its association with muscle damage and oxidative stress [1]. Practical implications of exercise-induced muscle damage (EIMD) are impairments of sports performance [2] and activities of daily living [3].
Nutritional interventions are increasingly promoted to accelerate exercise recovery and reduce risk of muscle damage. In terms of functional foods, nuts have been gaining widespread attention due to their promising nutritive properties. The Mediterranean diet includes the consumption of nuts and is associated with benefits on cardiovascular (CV) health. Proposed mechanisms that optimize health through nut consumption are related to improvements in blood lipid profiles [4], inflammatory biomarkers [5], and flow-mediated vasodilation [6]. Despite the emerging evidence regarding the role of nut consumption on CV health, the utility of nuts as an ergogenic aid is unclear.
Although understudied, the composition of pistachios suggests a benefit to athletes by promoting recovery. Recently, pistachios were confirmed as a complete protein by providing high bioavailability of essential amino acids (Bailey & Stein, Unpublished Results). According to the United States Department of Agriculture, pistachios provide approximately 12 g of protein and 1 g of leucine in a 2 oz serving. Leucine is particularly important due to its distinct role in promoting muscle protein synthesis (MPS) through stimulating the mammalian target of rapamycin [7]. Previous studies support the efficacy of leucine supplementation in stimulating MPS after various exercise modalities regardless of sex [8,9] and age [10]. Notably, it was demonstrated that pistachio consumption effectively raises plasma leucine concentrations in athletes [11]. Additionally, antioxidants like those found in pistachios were shown to reduce perceived soreness [12], EIMD and an inflammatory response after exercise [13,14]. Previous investigators have confirmed an improvement in antioxidant status [[15], [16], [17]] and even reduced oxidative damage following pistachio consumption [18]. It is conceivable that by reducing pain and accelerating exercise recovery, athletes are less hindered from participating in subsequent training bouts that will further enhance sports performance.
A recent study observed no ergogenic effect of pistachio consumption on exercise performance, despite reporting various benefits in metabolic profile [11]. However, several considerations in terms of methodology should be highlighted. First, participants were instructed to consume pistachios both pre- and peri-exercise, which may have induced gastrointestinal (GI) upset and suboptimal performance. Indeed, the authors acknowledged a decrease in GI motility caused by exercise itself. Second, pistachios have been linked to delayed gastric emptying because of their fat and fiber content. The aim of this study was to determine the effect of different pistachio doses (1.5 oz vs. 3.0 oz) on eccentrically biased exercise recovery as measured through muscle force production, jump height, oxidative stress (i.e., creatine kinase (CK), superoxide dismutase (SOD), C-reactive protein (CRP), and myoglobin (Mgb)), and subjective pain ratings. We anticipated a dose-dependent improvement on markers of exercise recovery following pistachio consumption when compared to a control.
2. Materials and methods
2.1. Participants
Moderately trained, male athletes (N = 40) aged 18–35 years were recruited from San Diego County. Participants were required to engage in at least 5 h of vigorous exercise per week. Exclusion criteria included smoking, medications known to impact inflammation, musculoskeletal limitations, and use of supplements known to impact antioxidant or inflammatory status within 1 month of participation. Potential participants were screened for eligibility criteria, and informed written consent was obtained prior to initiating the study. The study was approved by the San Diego State University Institutional Review Board.
2.2. Study design
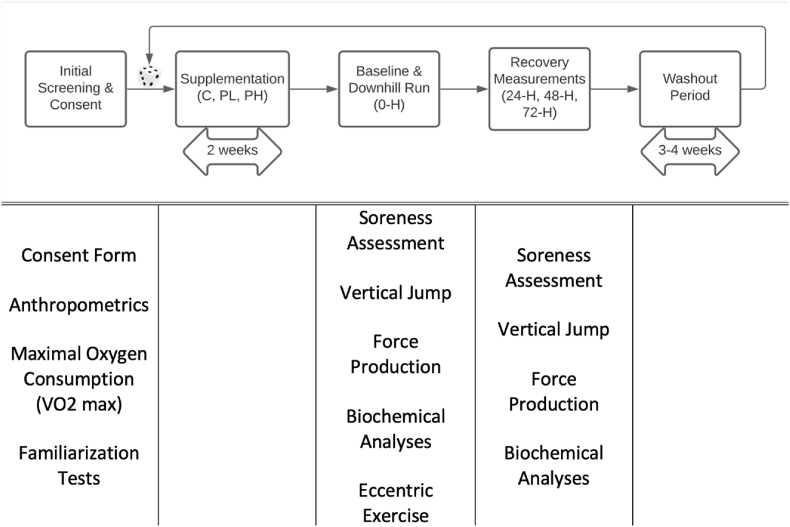
This study utilized a randomized, crossover design with three 2-week dietary interventions separated by a 3-4-week washout period to prevent carryover effects (Fig. 1). Participants were randomized into the following groups: control (C), low dose (1.5 oz/d of shelled pistachios; PL), or high dose (3.0 oz/d of shelled pistachios; PH). Randomization was performed using a free online random number generator program by an investigator. The C was instructed to maintain usual dietary habits. All groups were directed to refrain from consuming additional nuts and seeds, as well as dietary supplements during the 2-week period.
Fig. 1.
Schematic overview of the experimental protocol. C: Control; PL: 1.5 oz/d pistachio dose; and PH: 3.0 oz/d pistachio dose. Baseline measurements were conducted on the day of the downhill run and are categorized as time point 0 h. The recovery period was all the subsequent analyses conducted post-exercise; specifically, 24 h, 48 h, and 72 h after the downhill run.
Prior to initiating their first trial, participants were instructed to report to San Diego State University's Clinical Nutrition and Physiological Sciences (CNaPS) laboratory for initial screening. Maximal oxygen consumption (VO2max) was determined through a graded exercise test on a treadmill until volitional exhaustion. This measurement was utilized for descriptive data and to determine a heart rate (HR) reflective of 65–70% of VO2max for subsequent trials. Anthropometric measurements (i.e., height, weight, and body composition) were obtained prior to randomization. Additionally, the first laboratory visit included familiarizing participants to the vertical jump and muscle force production protocols as described below.
After the 2-week feeding period, subjects reported to the CNaPS laboratory following an overnight fast and after abstaining from exercise for 2 days. Fasting blood samples, muscle soreness, vertical jump, and muscle force production were assessed prior to exercise. Participants completed a 40-min downhill run at −10% grade while maintaining a HR reflecting 65–70% of VO2max. Immediately after the eccentric exercise bout, a recovery serving of pistachios and 8 oz of water was provided to PL and PH, whereas C received water only. Pistachio consumption was continued after the muscle damage protocol up until 48 h of the recovery period. Participants were instructed to return to the laboratory in a fasted state at 24 h, 48 h, and 72 h after the downhill run for biochemical analyses, subjective ratings of pain, vertical jump, muscle force production, and supervised consumption of the trial feeding. Following data collection at the 72-h time point, subjects participated in a washout period lasting for 3–4 weeks. Participants returned for their next trial and repeated the protocols until all three trials were completed.
2.3. Anthropometrics
All measurements were assessed at one time point during the initial screening process prior to randomization into the participants’ first trials. Height (to the nearest 0.1 cm) was obtained with the use of a wall-mounted stadiometer (Posh Rulers, Portland, United States). Weight (to the nearest 0.1 lb) was measured using a digital scale (InBody, Seoul, Korea). Body fat percentage and lean body mass (kg) were assessed via dual x-ray absorptiometry (General Electric Healthcare, Chicago, United States).
2.4. Maximal oxygen consumption (VO2max) testing and downhill run
Testing was conducted on a motor driven treadmill (ElectraMed Corporation, Flint, United States) while participants were connected to a calibrated metabolic measurement system (Parvo Medics, Murray, United States). A 3-min warm-up was provided at a self-selected speed that was subjectively rated as a moderate intensity. Following the 3 min, the incline was increased by 1% grade every minute and thereafter until volitional exhaustion. HR was determined using a strapped monitor (Polar, Kempele, Finland) at the level of the 5th intercostal space and midclavicular line. Oxygen consumption, ratings of perceived exertion, and HR were continuously monitored during the exercise bout and recorded in 1-min increments. HR and oxygen consumption data were plotted to produce a regression line to estimate a HR reflective of 65–70% VO2max for the subsequent downhill runs.
After the 2-week feeding period, subjects participated in a 40-min downhill run at −10% grade. Speed was adjusted until a HR reflecting 65–70% VO2max was produced. HR was continuously monitored throughout the trial and speed was adjusted to maintain HR within the predetermined ranges. The exact speeds were repeated for subsequent trials.
2.5. Muscle soreness
A series of visual analog scales (VAS) measured soreness by asking questions that assessed ratings of pain on both the right and left sides of the following muscle groups: quadriceps, hamstrings, gluteus, gastrocnemius, and tibialis. Additionally, pain was rated during the vertical jump and muscle force production protocols, as well as at extended (0°) and flexed (90°) knee positions while seated. Each question was followed by a 100 mm line that was demarcated by the left-most side indicating “no pain” and the right-most side indicating “worst pain imaginable.” Subjects recorded their responses by marking a spot on the line indicating their feelings about each question. Responses were quantified by measuring the distance from the left end of the line to the designated mark.
2.6. Physical performance
Maximal isokinetic strength testing was determined using a Biodex® dynamometer (Biodex Medical Systems, Shirley, United States). Participants were fastened on to the chair at the torso, waist, and ipsilateral thigh. Each trial consisted of 3 repetitions of unilateral knee flexion and extension at a speed of both 60° and 120° per second. A 1-min rest period was incorporated between the two different speed settings. Maximum torque (Nm) data were assessed prior to the downhill run (0 h), as well as the post-exercise recovery period (24 h, 48 h, and 72 h).
Vertical jump (VJ) height was measured using a Vertec (Sports Imports, Columbus, United States). The participant's reach height was assessed with a wall-mounted ruler prior to conducting the jump test. Subjects stood against a marked wall and reached as high as possible with their dominant hand without lifting their heels from the floor; height was recorded based on the middle finger. For VJ assessment, participants were instructed to perform a rapid countermovement by quickly descending into a squat while swinging their arms down and back; no lead-up steps were allowed. The rapid descent was immediately followed by an explosive jump in which the dominant hand reached to touch the highest possible Vertec vane. Three attempts were allowed with the highest number of vanes altered to determine VJ height. Performance was calculated as the difference between standing reach and the highest jump recorded (cm). VJ height assessment took place prior to the downhill run (0 h), as well as the post-exercise recovery periods (24 h, 48 h, and 72 h).
2.7. Biochemical analyses
Fasted blood samples were collected via venipuncture prior to the downhill run and each morning during the recovery period. Vacutainers were centrifuged to enable the extraction of plasma samples and stored at −80 °C until analysis. Plasma was assayed for CRP, CK, Mgb, SOD using commercially available ELISA kits.
CRP was measured using an ultrasensitive enzyme-linked immunoassay kit (ALPCO, Salem, United States). Plasma samples were first diluted 1:100 and then placed in microplate wells coated with polyclonal antibodies to CRP. Sensitivity of the kits was 0.124 ng/mL with an assay range of 1.9–150 ng/mL. For SOD determination, the intra-assay coefficient of variation (CV) was 3.2%, the inter-assay CV was 3.7%, and the assay range was 0.005–0.050 units/mL.
2.8. Compliance
Participants received a reminder regarding adherence during the feeding period on the first day of the 2-week intervention. A 24-h notification containing information on refraining from medications, supplements, and exercise, as well as fasting instructions were sent prior to the downhill run. A 24-h dietary recall was completed before beginning the eccentric exercise bout and every day during the recovery period to assess compliance to dietary restrictions. The record received during baseline was returned to participants and they were asked to follow a similar diet for each downhill run and follow-up day.
Subjects were instructed to return any uneaten food products at their downhill run visit to assess adherence to the intervention. Prior to distribution, pistachios were pre-packaged, and vacuum sealed in their respective serving size for convenience and palatability. Compliance to the dietary intervention was assessed by researchers counting the number of uneaten bags left over from the 2-week feeding period.
2.9. Statistical analyses
All data are expressed in means ± standard deviation (SD). The assumption of normality was assessed before performing statistical analyses. If non-normal distribution was suspected, a log transformation was conducted to obtain normal distribution of data. Values of zero were scaled by the following formula: x + 1, to prevent any loss of data during the log transformation [19]. Outliers were identified and analyses were run with and without inclusion to determine any changes of statistical significance. A 3 (trial) x 4 (time) repeated-measures analysis of variance was applied to detect changes over time and within the trials. Mauchley's test of sphericity was evaluated, and violations of the assumption were adjusted with the Greenhouse-Geisser correction if estimated epsilon was less than 0.75 and the Huynh-Feldt correction if greater than 0.75. Paired T-tests were performed to further examine significant main effects as needed. A P-value ≤ 0.05 was considered statistically significant. All data was analyzed using IBM SPSS Statistics Version 28.
3. Results
3.1. Descriptives and adherence
Of the 40 participants enrolled, a total of 27 individuals completed all aspects of the study. The observed 33% attrition rate was largely a result of the COVID-19 pandemic and its lockdown procedures declared by the state of California. During the C trial all subjects had a compliance rate of 100%. Approximately 63% and 67% of PL and PH had a compliance rate of 100%, respectively. Remaining dietary compliance rates of PL were the following: 93% (n = 5), 88% (n = 3), and 79% (n = 2). Additionally, dietary compliance rates for PH were the following: 93% (n = 3), 88% (n = 4), 79% (n = 1), and 64% (n = 1). Characteristics of the study population are provided in Table 1.
Table 1.
Subject characteristics (N = 27).
| Height (cm) | 174.8 (6.5) |
|---|---|
| Weight (kg) | 75.6 (11.2) |
| Age (y) | 24.0 (4.0) |
| Body Fat (%) | 20.6 (9.4) |
| Lean Body Mass (kg) | 57.3 (6.3) |
| Maximal Oxygen Consumption (mL/kg/min) | 51.1 (8.4) |
Values are expressed as means (SD).
3.2. Vertical jump
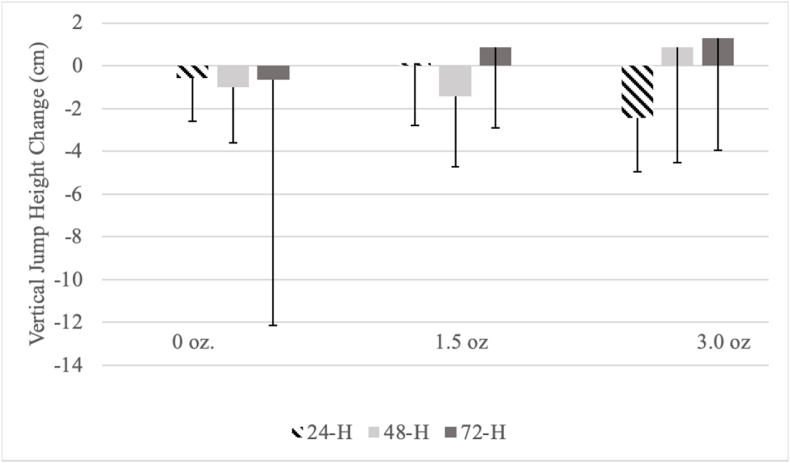
Results revealed no significant interaction (F(1.85, 46.24) = 2.031, p < 0.147, η2 = 0.08) and no trial effect (F(2, 50) = 1.208, p < 0.308, η2 = 0.05) on vertical jump performance. No time effect was detected (F(1.55, 38.73) = 0.450, p < 0.592, η2 = 0.02) despite mean values for jump height appearing to improve at 72 h in PL and at 48 h for PH (Fig. 2).
Fig. 2.
Changes in vertical jump height when compared to baseline (0-H). All data are presented as means and standard deviations. Within trials, no significant differences (p > 0.05) across respective timepoints were observed.
3.3. Muscle force production
Maximal torque (TRQ) was impaired over time across various intensity settings on the isokinetic dynamometer (Table 2). Max TRQ at 60° per second of extension produced a significant time effect (F(3,78) = 7.738, p < 0.001, η2 = 0.23). Follow-up t-tests revealed that force production was significantly reduced after 24 h of the recovery period. Force production returned to baseline after 48 h in PH compared with 72 h in PL. No time effect was observed for muscle force production at 60° per second of flexion (F(2.53, 65.70) = 1.893, p < 0.149, η2 = 0.07). Max TRQ at 120° per second of extension indicated a significant time effect (F(3, 78) = 5.810, p < 0.002, η2 = 0.18). Further examination displayed significant changes in PL only, decreasing at the 24 h time point; however, strength failed to return to baseline even after 72 h. Additionally, there was a significant time effect for max TRQ at 120° per second of flexion (F(2.63, 68.31) = 2.918, p < 0.048, η2 = 0.10). Follow-up analyses revealed a significant reduction in force production in PL at the 48-h time point, but returned to baseline at 72 h. Interestingly, force production in PH appeared like baseline levels throughout the entire recovery period and even showed a significant increase at 72 h when compared to 24 h and 48 h.
Table 2.
Changes in maximum torque over time across different dosages of pistachios.
| Variable | 0-H | 24-H | 48-H | 72-H |
|---|---|---|---|---|
| Max TRQ 60°EXT (Nm) | ||||
| 0 oz | 179.2 (48.1) | 171.1 (39.4) | 176.5 (40.1) | 172.0 (41.1) |
| 1.5 oz | 181.9 (47.2)a | 166.9 (49.7)b | 163.1 (44.5)b | 176.2 (48.2)a |
| 3.0 oz | 182.8 (46.6)a | 170.9 (41.8)b | 175.5 (45.0)a,b | 173.2 (46.1)a,b |
| Max TRQ 60°FLX (Nm) | ||||
| 0 oz | 110.9 (37.4) | 113.0 (28.9) | 117.0 (30.4) | 116.7 (29.4) |
| 1.5 oz | 110.8 (34.0) | 111.5 (34.1) | 106.7 (37.1) | 113.4 (36.0) |
| 3.0 oz | 111.1 (35.8) | 111.5 (33.0) | 113.8 (32.3) | 115.3 (32.5) |
| Max TRQ 120°EXT (Nm) | ||||
| 0 oz | 138.2 (43.6) | 137.0 (36.0) | 135.6 (37.7) | 137.7 (40.2) |
| 1.5 oz | 146.6 (41.2)a | 134.1 (40.1)b | 129.8 (39.7)b,c | 137.5 (45.5)b,d |
| 3.0 oz | 142.5 (43.0) | 135.1 (42.4) | 137.1 (38.5) | 136.4 (44.3) |
| Max TRQ 120°FLX (Nm) | ||||
| 0 oz | 94.2 (30.9) | 94.7 (27.5) | 96.1 (28.3) | 96.4 (26.3) |
| 1.5 oz | 94.7 (31.6)a | 92.8 (34.1)a | 88.8 (30.4)b | 95.3 (32.9)a |
| 3.0 oz | 93.0 (33.8)a | 92.3 (32.9)a,b | 93.7 (32.5)a,b | 99.2 (29.3)a,c |
Note: Values are presented as means (standard deviations). Different letters indicate significant differences (p < 0.05) within a trial. EXT, extension; FLX, flexion; H, hour; Nm, Newton meters; oz, ounces.
3.4. Ratings of pain
Subjective ratings of pain for right-sided muscle groups increased over time for the following: quadriceps (F(1.69, 43.82) = 45.477, p < 0.001, η2 = 0.64), hamstrings (F(1.61, 41.87) = 17.442, p < 0.001, η2 = 0.40), gastrocnemius (F(1.82, 47.21) = 36.970, p < 0.001, η2 = 0.59), tibialis (F(1.53, 39.86) = 36.534, p < 0.001, η2 = 0.58), and gluteus (F(1.56, 40.58) = 33.420, p < 0.001, η2 = 0.56) (Table 3). Follow-up tests indicated that participants experienced the most pain on the right-sided muscles 24 h after the eccentric exercise bout. Also, pain in the right quadriceps, hamstrings, gluteus, and gastrocnemius were significantly reduced to baseline levels at 72 h in PH only. Notably, there was an observed interaction for the right gluteus measurement in PL and PH (F(4.90, 127.50) = 2.478, p < 0.037, η2 = 0.09). T-tests revealed a similar trend of pain being the highest at 24 h and significantly reduced at 72 h; however, only PH demonstrated ratings of pain returning to baseline.
Table 3.
Visual analog scores for pain on right-sided muscles.
| Variable | 0-H | 24-H | 48-H | 72-H |
|---|---|---|---|---|
| Right Quadriceps (mm) | ||||
| 0 oz | 5.2 (8.0)a | 30.8 (22.5)b | 23.7 (18.7)c | 14.9 (14.6)d |
| 1.5 oz | 4.5 (10.6)a | 29.7 (17.2)b | 25.8 (16.6)b | 14.1 (12.4)c |
| 3.0 oz | 6.4 (13.5)a | 24.2 (14.9)a | 20.4 (16.5)b | 11.5 (10.4)a |
| Right Hamstrings (mm) | ||||
| 0 oz | 3.9 (5.2)a | 21.1 (22.5)b | 18.9 (19.7)b | 13.3 (17.5)c |
| 1.5 oz | 5.0 (12.3)a | 21.3 (19.7)b | 15.8 (16.9)c | 9.5 (12.4)d |
| 3.0 oz | 6.1 (11.4)a | 19.0 (18.1)b | 14.3 (15.9)c | 8.4 (10.8)a |
| Right Gluteus (mm) | ||||
| 0 oz | 4.4 (8.0)a | 24.9 (23.5)b | 23.0 (21.7)b | 13.2 (16.0)c |
| 1.5 oz | 3.8 (6.3)a | 23.9 (19.5)b | 19.0 (16.9)c | 8.4 (9.0)d,* |
| 3.0 oz | 6.3 (10.9)a | 20.0 (15.1)b | 13.6 (12.5)c,* | 8.2 (8.7)a,* |
| Right Gastrocnemius (mm) | ||||
| 0 oz | 5.7 (10.7)a | 31.9 (27.5)b | 32.2 (25.6)b | 22.7 (24.5)c |
| 1.5 oz | 5.2 (8.5)a | 29.4 (23.9)b | 21.6 (18.9)c | 14.2 (14.8)d |
| 3.0 oz | 7.3 (15.5)a | 27.2 (23.1)b | 25.3 (23.1)b | 13.1 (14.9)a |
| Right Tibialis (mm) | ||||
| 0 oz | 4.2 (7.5)a | 22.0 (22.8)b | 20.7 (18.8)b | 12.8 (15.9)c |
| 1.5 oz | 3.9 (6.2)a | 30.0 (26.6)b | 23.7 (21.2)c | 12.5 (10.7)d |
| 3.0 oz | 4.1 (6.2)a | 23.2 (20.2)b | 18.5 (18.7)b | 11.6 (14.3)c |
Note: Values are presented as means (standard deviations). Different letters indicate significant differences (p < 0.05) within a trial.
*: significant difference compared to the control (p < 0.05). H, hour; mm, millimeters; oz, ounces.
Additionally, left-sided muscle groups also exhibited a significant time effect for the following: quadriceps (F(1.67, 43.42) = 42.808, p < 0.001, η2 = 0.62), hamstrings (F(1.60, 41.56) = 17.422, p < 0.001, η2 = 0.40), gastrocnemius (F(1.76, 45.80) = 42.377, p < 0.001, η2 = 0.62), tibialis (F(1.75, 45.45) = 38.625, p < 0.001, η2 = 0.60), and gluteus (F(1.63, 42.33) = 37.068, p < 0.001, η2 = 0.59) (Table 4). Participants experienced the most pain on their left-sided muscles 24 h after the eccentric exercise bout. Pain in the left quadriceps, hamstrings, and gluteus returned to baseline levels after 72 h in PH only. A significant interaction was also demonstrated for the left gluteus (F(6, 156) = 2.368, p < 0.033, η2 = 0.08). Interestingly, t-tests demonstrated a return of subjective pain ratings back to baseline in PH only.
Table 4.
Visual analog scores for pain on left-sided muscles.
| Variable | 0-H | 24-H | 48-H | 72-H |
|---|---|---|---|---|
| Left Quadriceps (mm) | ||||
| 0 oz | 4.7 (8.6)a | 30.2 (23.0)b | 23.1 (20.3)c | 14.0 (12.6)d |
| 1.5 oz | 4.4 (9.9)a | 29.6 (18.5)b | 26.0 (18.1)b | 14.5 (12.8)c |
| 3.0 oz | 6.6 (14.4)a | 24.2 (16.0)b | 20.7 (16.6)b | 13.2 (11.5)a |
| Left Hamstrings (mm) | ||||
| 0 oz | 4.2 (5.8)a | 21.4 (22.9)b | 18.8 (20.4)b | 12.5 (15.0)c |
| 1.5 oz | 5.2 (12.4)a | 21.8 (18.2)b | 14.9 (17.4)c | 9.8 (13.3)d |
| 3.0 oz | 6.6 (11.5)a | 19.1 (17.2)b | 13.8 (15.2)c,d | 10.7 (12.3)a,d |
| Left Gluteus (mm) | ||||
| 0 oz | 3.4 (4.7)a | 27.2 (25.3)b | 22.6 (20.7)c | 13.8 (16.8)d |
| 1.5 oz | 4.2 (8.3)a | 25.3 (21.6)b | 18.5 (17.0)c | 9.9 (11.2)d |
| 3.0 oz | 6.3 (11.4)a | 20.5 (16.2)b | 13.6 (13.1)c,* | 8.6 (8.6)a,* |
| Left Gastrocnemius (mm) | ||||
| 0 oz | 7.3 (11.8)a | 31.9 (27.9)b | 32.0 (26.9)b | 22.3 (24.9)c |
| 1.5 oz | 4.5 (7.1)a | 27.5 (21.9)b | 21.9 (18.9)b | 15.7 (17.2)c |
| 3.0 oz | 5.0 (7.1)a | 26.3 (21.5)b | 27.3 (24.3)b | 14.8 (14.8)c |
| Left Tibialis (mm) | ||||
| 0 oz | 3.9 (8.8)a | 22.1 (23.3)b | 20.4 (19.1)b | 12.6 (15.9)c |
| 1.5 oz | 4.0 (7.0)a | 28.2 (26.5)b | 21.8 (20.2)b | 13.5 (13.7)c |
| 3.0 oz | 4.2 (6.3)a | 22.7 (19.0)b | 20.0 (20.4)b | 13.7 (15.3)c |
Note: Values are presented as means (standard deviations). Different letters indicate significant differences (p < 0.05) within a trial.
*: significant difference compared to the control (p < 0.05). H, hour; mm, millimeters; oz, ounces.
Ratings of pain measured following eccentric exercise demonstrated a significant time effect for the following assessments: vertical jump (F(1.95, 42.80) = 13.837, p < 0.001, η2 = 0.39), maximal contraction performed on the isokinetic dynamometer (F(2.07, 53.72) = 18.434, p < 0.001, η2 = 0.42), extension (F(1.86, 44.56) = 22.267, p < 0.001, η2 = 0.48), and flexion (F(2.06, 49.38) = 18.474, p < 0.001, η2 = 0.44) (Table 5). Participants experienced the most pain at 24 h, but values returned to baseline after 72 h of recovery for most measurements. Notably, PH, but not C and PL, consistently achieved ratings of pain returning to baseline for all performance parameters.
Table 5.
Visual analog scores for pain after sports performance measures.
| Variable | 0-H | 24-H | 48-H | 72-H |
|---|---|---|---|---|
| Vertical Jump (mm) | ||||
| 0 oz | 7.2 (11.1)a | 20.5 (17.2)b | 19.5 (19.1)b | 10.6 (12.7)a |
| 1.5 oz | 10.7 (13.0)a | 22.8 (19.9)b | 19.3 (17.9)b | 13.4 (14.0)a |
| 3.0 oz | 9.2 (10.3)a | 20.9 (19.7)b | 14.5 (11.8)a,b | 10.0 (9.7)a,c |
| Maximal Contraction (mm) | ||||
| 0 oz | 8.4 (10.0)a | 22.8 (17.5)b | 21.4 (17.9)b | 11.6 (12.2)a |
| 1.5 oz | 10.0 (10.0)a | 19.4 (16.5)b | 21.1 (18.6)b | 14.5 (12.6)a |
| 3.0 oz | 11.5 (12.4)a | 21.8 (15.2)b | 18.2 (15.7)b,c | 12.6 (9.7)a,c |
| Flexion (mm) | ||||
| 0 oz | 6.3 (11.7)a | 20.2 (18.1)b | 20.0 (22.3)b | 12.8 (17.1)c |
| 1.5 oz | 6.8 (10.4)a | 20.6 (19.6)b | 18.0 (17.7)b | 9.9 (11.7)c |
| 3.0 oz | 6.8 (13.2)a | 20.4 (17.6)b | 15.4 (12.5)b | 8.5 (9.5)a |
| Extension (mm) | ||||
| 0 oz | 5.8 (9.0)a | 17.9 (16.5)b | 21.0 (20.6)b | 11.5 (11.9)c |
| 1.5 oz | 5.0 (7.3)a | 20.6 (20.2)b | 21.2 (18.2)b | 9.2 (9.0)c |
| 3.0 oz | 6.1 (7.5)a | 17.9 (15.9)b | 13.9 (10.4)b | 8.4 (7.8)a |
Note: Values are presented as means (standard deviations). Different letters indicate significant differences (p < 0.05) within a trial. H, hour; mm, millimeters; oz, ounces.
3.5. Biochemical indices
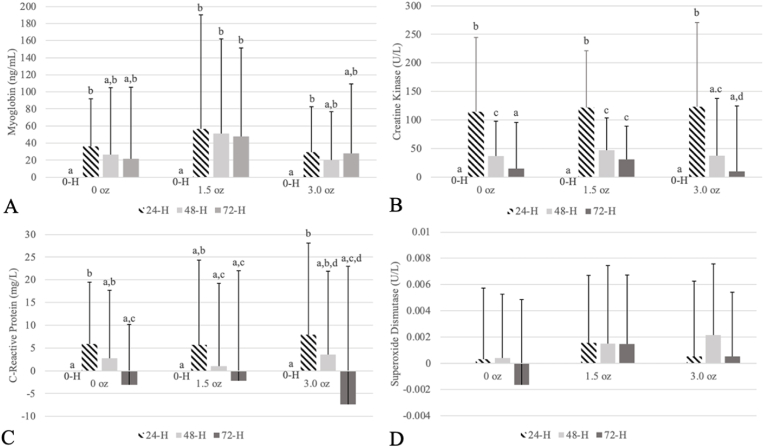
Analyses revealed a significant time effect for the following biochemical markers of muscle damage: (Mgb (F(1.89, 47.19) = 6.978, p < 0.004, η2 = 0.22, CK (F(1.74, 45.34) = 44.838, p < 0.001, η2 = 0.63, CRP (F(3, 78) = 7.923, p < 0.001, η2 = 0.23). Further examination indicated that Mgb levels were the highest at 24 h but returned to baseline values at 48 h in C and PH; PL values were elevated throughout the recovery period (Fig. 3A). Similarly, CK levels were the highest 24 h after the eccentric exercise bout but returned to baseline values in C and PH only; PL values appeared elevated throughout the recovery period (Fig. 3B). However, CK values returned to baseline at 48 h in PH instead of 72 h when compared to C. The highest concentration of CRP was at 24 h of the recovery period but returned to baseline following 48 h in C and PH (Fig. 3C). Follow-up tests revealed that CRP was not significantly elevated beyond baseline levels throughout the entire trial of PL. As an indicator of antioxidant potential, SOD appeared to be consistently elevated throughout the recovery period only in PL and PH when compared to C, but statistical analyses did not reveal any significant interaction (F(5.06, 126.56) = 1.128, p < 0.350, η2 = 0.04), trial effect (F(1.69, 42.19) = 1.244, p < 0.295, η2 = 0.05), or time effect (F(3,75) = 1.604, p < 0.197, η2 = 0.06) (Fig. 3D).
Fig. 3.
Biochemical markers of muscle damage and recovery. A) Changes in myoglobin concentrations (ng/mL) over time when compared to baseline (0-H) across different dosages of pistachios. B) Changes in creatine kinase concentrations (U/L) over time when compared to baseline (0-H) across different dosages of pistachios. C) Changes in C-reactive protein concentrations (mg/L) over time when compared to baseline (0-H) across different dosages of pistachios. D) Changes in superoxide dismutase concentrations (U/L) over time when compared to baseline (0-H) across different dosages of pistachios. Within trials, no significant differences were observed in superoxide dismutase concentrations.
Note: Data are expressed in means and standard deviations. Different letters indicate significant differences (p < 0.05) within a trial. H, hour; mg/L, milligram/Liter; ng/mL, nanogram/milliliter; oz, ounce; U/L, Units/Liter.
4. Discussion
As a natural source of essential amino acids and bioactive phytochemicals, pistachios may be a beneficial snack option to promote sports performance. In the present study we investigated the influence of daily 2-week supplementation of 1.5 oz and 3.0 oz of pistachios on post-exercise recovery in moderately trained, male athletes. Vertical jump, muscle force production, subjective ratings of pain, as well as biochemical markers of muscle damage and antioxidant status were compared throughout a 72-h recovery period following a 40-min downhill run.
The eccentric exercise bout successfully elicited a moderate amount of muscle damage in the study cohort of moderately trained males. Biochemical analysis indicated a peak in CK, Mgb, and CRP concentrations at 24 h after the downhill run and that pistachios modestly attenuated these effects during the remainder of the 72-h recovery period. Simultaneously, the lowest muscle force production and highest ratings of pain were observed at the 24-h time point; pain tended to be further lowered during the recovery period by pistachio intake. Peak pain scores pain ranged between 20 and 30 on a 0–100 scale, suggesting that EIMD elicited relatively minor amounts. Based on our data, most detriments to muscle integrity and performance appeared 24 h after the eccentric exercise bout, which aligns with the 72-h time course of degeneration and inflammation of the muscle recovery process [20].
Maximal torque at 60° per second of knee extension returned to baseline values earlier in PH when compared to both PL and C. The high dose of pistachios attenuated reductions in maximal torque at 120° per second of knee flexion throughout the trial and elicited an improvement in strength at 72 h. Vertical jump height appeared to rebound earlier in PH when compared to PL; however, analyses revealed no statistical significance. To our knowledge, we are the first investigators to examine the effects of a nut on muscle force production. Nieman et al. [11] evaluated 3 oz of pistachio supplementation on a 75-km cycling time trial, a parameter largely reflective of aerobic capacity, and noted decrements in performance, as evidenced by the 4.8% increase in time to completion with the intervention. In evaluating the impacts of a different nut, approximately 2 oz of almonds consumed 2 h before a maximal test to exhaustion resulted in a significant increase in total work [21]. However, the observed results are subject to multiple treatment interference related to the administration of the almonds with 60 mL of milk, which may confound the results. There is evidence that consumption of milk itself is associated with a tendency for participants to exercise longer during a test to volitional exhaustion [22]. Based on the available nut literature, our data is the first to suggest that pistachios are likely to confer benefits on maximal torque in both unilateral knee extension and flexion during recovery from downhill running.
Subjective pain ratings recovered to baseline values in both the left- and right-sided quadriceps, hamstrings, and gluteus in PH only throughout the recovery period. Additionally, pain during both knee extension and flexion returned to baseline only in PH. Garnier et al. [23] examined the differences in pain perception between downhill and level running for 45 min without the inclusion of an ergogenic intervention. Results demonstrated that pain ratings in the quadriceps and gluteus took as long as 96 h after the eccentric exercise bout to return to baseline values. Based on our data, the inclusion of 3 oz of pistachios resulted in a shorter time (72 h) to offset the increase in pain following our 40-min muscle damage protocol. Although there is an absence of pistachio literature to support our findings, reductions in pain measured through Von Fey filament and motor function tests was observed in osteoarthritis-induced male rats following cashew consumption (100 mg/kg) 3 times/week for 21 days [24].
CRP concentrations peaked 24 h after the downhill run and returned to baseline after 48 h for both PH and C. Perhaps surprisingly, the concentration of CRP in PL remained consistent with baseline values, suggesting a benefit of the low pistachio dose but not the high dose. Previous studies also detected inconsistent effects [16,25] or no effect [26] on CRP concentrations following a pistachio intervention. The literature on CRP after consuming other nuts has suggested a benefit following 20% of total energy intake coming from a mixed nut blend (i.e., pistachio, almond, and peanut) for 8 weeks [5]. However, findings have been inconsistent, with no significant effects following 20% of total energy intake coming from pistachios for 4 weeks [27], 30–60 g/day of hazelnuts for 12 weeks [28], 46 g/day of almonds or 36 g/day of walnuts for 6 weeks [29], and 30 g/day of a mixed nut blend (i.e., walnut, pine nut, and peanut) for 6 weeks [30]. CK concentrations returned to baseline values earlier in PH when compared to C. The marker of muscle damage remained elevated throughout the recovery period in PL. Results of the PL group align with the proposed timeline of CK clearance lasting for as long as 7 days [31]. To our knowledge, the only other study examining the effects of pistachio consumption on CK concentrations was conducted in rats [25]. Researchers indicated no benefits of pistachio consumption on the CK response; however, that study did not include an exercise intervention. Interventions utilizing almonds (75 g/day) for 4 weeks [32] and 42.5 g/day of a mixed nut blend (i.e., Brazil nut, macadamia nut, pistachio, walnut, and peanut) for 8 weeks [33] were also unsuccessful in eliciting significant effects on CK. Mgb peaked 24 h following the eccentric exercise bout and returned to baseline after 48 h in both PH and C. Mgb remained elevated in PL throughout the 72-h duration of recovery. Mgb is considered a short-term marker of muscle damage in the blood as it is reported to peak 1–3 h after exercise [31]; therefore, interpreting these results after a longer period is challenging. Although no studies have examined the direct effects of pistachios or other nuts on Mgb concentrations, supplementation of antioxidants in other forms reduced Mgb when compared to a placebo [13]. As a measure of antioxidant status, there was a tendency for SOD concentrations to be elevated in PL when compared to both PH and C. Despite the absence of statistical significance, the results of the PL align with the only other study investigating the effects of pistachios on SOD in humans, which demonstrated an improvement in the antioxidant enzyme when compared to a Mediterranean diet [34]. However, it is important to take into consideration that exercise was not incorporated into that study. When examining the literature on other nuts, almonds (84 g/day) for 4 weeks [35] and baru almonds (20 g/day) for 8 weeks [36] have been successful at increasing SOD concentrations.
Our results are limited to the impacts on moderately trained, male athletes. Under physiological conditions, females appear to be less susceptible to oxidative stress, which may be a result of the antioxidant properties of estrogen or sex differences in NADPH-oxidase [37]. Therefore, it is unclear if similar results would have been detected in women. Previous researchers examined the effect of 6 weeks of supplementation on antioxidants like those found in pistachios on exercise-induced DNA damage [38]. Investigators observed an enhanced recovery in women, but not in men after a 50-km race. Notably, the evidence of antioxidant protection in women was highest 24 h after the race, which may have affected the observed peak in markers of muscle damage and pain seen after our eccentric exercise protocol. Thus, future research must consider biological sex when examining the effects of pistachios on post-exercise recovery. It is also possible that the duration between each of our trials was not adequate to prevent the protective effects of repeated bout exposure. It is well-established that prior exposure to a downhill run can lead to improvements in CK, muscle tenderness, delayed onset muscle soreness, and oxidative stress in populations including active adults, college students, and moderately trained athletes [[39], [40], [41], [42]]. Based on the noted studies, the beneficial effects are still observed when the eccentric exercise bouts are separated by 3–6 weeks. The 3- to 4-week duration of our washout period may have been inadequate for us to accurately detect changes elicited by the eccentric exercise bout on biomarkers of muscle damage. Another possible limitation was that the length of the feeding period may have been inadequate to elicit biochemical changes to antioxidant status. Previous studies have observed benefits on oxidative stress following pistachio feeding durations as short as 4 weeks [34,43]. More recent studies demonstrating favorable effects consisted of pistachio feeding durations lasting 4 months [17,18] to as long as 6 months [16]. Furthermore, we acknowledge the increased risk of making a type 1 error due to our limited sample size. Without the impact of the COVID-19 pandemic on our attrition rate, more individuals likely would have completed our study, which would have increased our power to detect differences and strengthen our results.
In conclusion, consuming 3.0 oz/d of pistachios for 2 weeks promoted reductions in subjective ratings of pain in most muscle groups examined and benefits to muscle force production at 60° per second of knee extension and 120° per second of knee flexion when compared to 1.5 oz/d of pistachios and a control. Markers of muscle damage were attenuated similarly between PH and C. PL demonstrated consistent elevations in CK and Mgb. There were no significant changes in SOD concentrations; however, they tended to be elevated in PL. These results suggest that raw, shelled, unsalted pistachios may help reduce pain and maintain muscle force production after eccentric exercise, potentially leading to greater exercise tolerance and training adaptations.
Funding
This study was supported by the American Pistachio Growers (Grant #594108). The funding source did not have any involvement in the conduct of the research and preparation of the article.
Data availability
Data generated or analyzed during this study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Vernon Uganiza Rayo: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. Imogene Thayer: Data curation, Investigation. Stuart D.R. Galloway: Writing – review & editing. Mee Young Hong: Funding acquisition. Shirin Hooshmand: Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing. Changqi Liu: Funding acquisition. Elise North: Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing. Lauren Okamoto: Data curation, Investigation. Timothy O'Neal: Data curation, Investigation. Jordan Philpott: Methodology, Writing – review & editing. Oliver C. Witard: Conceptualization, Funding acquisition, Methodology, Writing – review & editing. Mark Kern: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare there are no competing interests.
Acknowledgements
The authors would like to acknowledge the contributions of Kristine Giltvedt, Morgan Harris, Alfonso Joaquin Munoz, Traci Roberts, Nicholas Smith, Jillianne Son, and Svitlana Storm, who assisted in conducting and evaluating this research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metop.2022.100215.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Isner-Horobeti M.E., Dufour S.P., Vautravers P., Geny B., Coudeyre E., Richard R. Eccentric exercise training: modalities, applications and perspectives. Sports Med. 2013;43(6):483–512. doi: 10.1007/s40279-013-0052-y. [DOI] [PubMed] [Google Scholar]
- 2.Leite C.M.F., Profeta V.L.D.S., Chaves S.F.N., Benine R.P.C., Bottaro M., Ferreira-Júnior J.B. Does exercise-induced muscle damage impair subsequent motor skill learning? Hum Mov Sci. 2019;67 doi: 10.1016/j.humov.2019.102504. [DOI] [PubMed] [Google Scholar]
- 3.Sadacharan C.M., Seo S. Effect of large versus small range of motion in the various intensities of eccentric exercise-induced muscle pain and strength. International Journal of Exercise Science. 2021;14(7):1–18. doi: 10.70252/GUKZ9250. PMID: 34055176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toledo E., Wang D.D., Ruiz-Canela M., Clish C.B., Razquin C., Zheng Y., et al. Plasma lipidomic profiles and cardiovascular events in a randomized intervention trial with the Mediterranean diet. Am J Clin Nutr. 2017;106(4):973–983. doi: 10.3945/ajcn.116.151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghanavati M., Hosseinabadi S.M., Parsa S.A., Safi M., Emamat H., Nasrollahzadeh J. Effect of a nut-enriched low-calorie diet on body weight and selected markers of inflammation in overweight and obese stable coronary artery disease patients: a randomized controlled study. Eur J Clin Nutr. 2021;75(7):1099–1108. doi: 10.1038/s41430-020-00819-9. [DOI] [PubMed] [Google Scholar]
- 6.Kasliwal R.R., Bansal M., Mehrotra R., Yeptho K.P., Trehan N. Effect of pistachio nut consumption on endothelial function and arterial stiffness. Nutrition. 2015;31(5):678–685. doi: 10.1016/j.nut.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Lynch C.J., Patson B.J., Anthony J., Vaval A., Jefferson L.S., Vary T.C. Leucine is a direct-acting nutrient signal that regulates protein synthesis in adipose tissue. Am J Physiol Endocrinol Metab. 2002;283(3):E503–E513. doi: 10.1152/ajpendo.00084.2002. [DOI] [PubMed] [Google Scholar]
- 8.Moberg M., Apró W., Ohlsson I., Pontén M., Villanueva A., Ekblom B., et al. Absence of leucine in an essential amino acid supplement reduces activation of mTORC1 signalling following resistance exercise in young females. Appl Physiol Nutr Metabol. 2014;39(2):183–194. doi: 10.1139/apnm-2013-0244. [DOI] [PubMed] [Google Scholar]
- 9.Pasiakos S.M., McClung H.L., McClung J.P., Margolis L.M., Andersen N.E., Cloutier G.J., et al. Leucine-enriched essential amino acid supplementation during moderate steady state exercise enhances postexercise muscle protein synthesis. Am J Clin Nutr. 2011;94(3):809–818. doi: 10.3945/ajcn.111.017061. [DOI] [PubMed] [Google Scholar]
- 10.Holwerda A.M., Paulussen K.J.M., Overkamp M., Goessens J.P.B., Kramer I.F., Wodzig W.K.W.H., et al. Leucine coingestion augments the muscle protein synthetic response to the ingestion of 15 g of protein following resistance exercise in older men. Am J Physiol Endocrinol Metab. 2019;317(3):E473–E482. doi: 10.1152/ajpendo.00073.2019. [DOI] [PubMed] [Google Scholar]
- 11.Nieman D.C., Scherr J., Luo B., Meaney M.P., Dréau D., Sha W., et al. Influence of pistachios on performance and exercise-induced inflammation, oxidative stress, immune dysfunction, and metabolite shifts in cyclists: a randomized, crossover trial. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0113725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He F., Hockemeyer J.A.K., Sedlock D. Does combined antioxidant vitamin supplementation blunt repeated bout effect? Int J Sports Med. 2015;36(5):407–413. doi: 10.1055/s-0034-1395630. [DOI] [PubMed] [Google Scholar]
- 13.Chou C.C., Sung Y.C., Davison G., Chen C.Y., Liao Y.H. Short-term high-dose vitamin C and E supplementation attenuates muscle damage and inflammatory response in repeated taekwondo competitions: a randomized placebo-controlled trial. Int J Med Sci. 2018;15(11):1217–1226. doi: 10.7150/ijms.26340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santos S.A., Silva E.T., Caris A.V., Lira F.S., Tufik S., Dos Santos R.V. Vitamin E supplementation inhibits muscle damage and inflammation after moderate exercise in hypoxia. J Hum Nutr Diet. 2016;29(4):516–522. doi: 10.1111/jhn.12361. [DOI] [PubMed] [Google Scholar]
- 15.Dreher M.L. Pistachio nuts: composition and potential health benefits. Nutr Rev. 2012;70(4):234–240. doi: 10.1111/j.1753-4887.2011.00467.x. [DOI] [PubMed] [Google Scholar]
- 16.Gulati S., Misra A., Pandey R.M., Bhatt S.P., Saluja S. Effects of pistachio nuts on body composition, metabolic, inflammatory and oxidative stress parameters in Asian Indians with metabolic syndrome: a 24-wk, randomized control trial. Nutrition. 2014;30(2):192–197. doi: 10.1016/j.nut.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Rock C.L., Zunshine E., Nguyen H.T., Perez A.O., Zoumas C., Pakiz B., et al. Effects of pistachio consumption in a behavioral weight loss intervention on weight change, cardiometabolic factors, and dietary intake. Nutrients. 2020;12(7):2155. doi: 10.3390/nu12072155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canudas S., Hernández-Alonso P., Galié S., Muralidharan J., Morell-Azanza L., Zalba G., et al. Pistachio consumption modulates DNA oxidation and genes related to telomere maintenance: a crossover randomized clinical trial. Am J Clin Nutr. 2019;109(6):1738–1745. doi: 10.1093/ajcn/nqz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabachnick B.G., Fidell L.S. fourth ed. Allyn & Bacon; Needham, MA: 2001. Using multivariate statistics. [Google Scholar]
- 20.Urso M.L. Anti-inflammatory interventions and skeletal muscle injury: benefit or detriment? J Appl Physiol. 2013;115(6):920–928. doi: 10.1152/japplphysiol.00036.2013. [DOI] [PubMed] [Google Scholar]
- 21.Esquius L., Segura R., Oviedo G.R., Massip-Salcedo M., Javierre C. Effect of almond supplementation on non-esterified fatty acid values and exercise performance. Nutrients. 2020;12(3):635. doi: 10.3390/nu12030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J.K., Maughan R.J., Shirreffs S.M., Watson P. Effects of milk ingestion on prolonged exercise capacity in young, healthy men. Nutrition. 2008;24(4):340–347. doi: 10.1016/j.nut.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Garnier Y.M., Lepers R., Dubau Q., Pageaux B., Paizis C. Neuromuscular and perceptual responses to moderate-intensity incline, level and decline treadmill exercise. Eur J Appl Physiol. 2018;118(10):2039–2053. doi: 10.1007/s00421-018-3934-8. [DOI] [PubMed] [Google Scholar]
- 24.Fusco R., Siracusa R., Peritore A.F., Gugliandolo E., Genovese T., D'Amico R., Cordaro M., Crupi R., Mandalari G., Impellizzeri D., Cuzzocrea S., Di Paola R. The role of cashew (Anacardium occidentale L.) nuts on an experimental model of painful degenerative joint disease. Antioxidants. 2020;9(6):511. doi: 10.3390/antiox9060511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong M.Y., Groven S., Marx A., Rasmussen C., Beidler J. Anti-inflammatory, antioxidant, and hypolipidemic effects of mixed nuts in atherogenic diet-fed rats. Molecules. 2018;23(12):3126. doi: 10.3390/molecules23123126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parham M., Heidari S., Khorramirad A., Hozoori M., Hosseinzadeh F., Bakhtyari L., et al. Effects of pistachio nut supplementation on blood glucose in patients with type 2 diabetes: a randomized crossover trial. Rev Diabet Stud. 2014;11(2):190–196. doi: 10.1900/RDS.2014.11.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sauder K.A., McCrea C.E., Ulbrecht J.S., Kris-Etherton P.M., West S.G. Effects of pistachios on the lipid/lipoprotein profile, glycemic control, inflammation, and endothelial function in type 2 diabetes: a randomized trial. Metab Clin Exp. 2015;64(11):1521–1529. doi: 10.1016/j.metabol.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tey S.L., Gray A.R., Chisholm A.W., Delahunty C.M., Brown R.C. The dose of hazelnuts influences acceptance and diet quality but not inflammatory markers and body composition in overweight and obese individuals. J Nutr. 2013;143(8):1254–1262. doi: 10.3945/jn.113.174714. [DOI] [PubMed] [Google Scholar]
- 29.Kalgaonkar S., Almario R.U., Gurusinghe D., Garamendi E.M., Buchan W., Kim K., Karakas S.E. Differential effects of walnuts vs almonds on improving metabolic and endocrine parameters in PCOS. Eur J Clin Nutr. 2011;65(3):386–393. doi: 10.1038/ejcn.2010.266. [DOI] [PubMed] [Google Scholar]
- 30.Lee Y.J., Nam G.E., Seo J.A., Yoon T., Seo I., Lee J.H., Im D., Bahn K.N., Jeong S.A., Kang T.S., Ahn J.H., Kim D.H., Kim N.H. Nut consumption has favorable effects on lipid profiles of Korean women with metabolic syndrome. Nutr Res. 2014;34(9):814–820. doi: 10.1016/j.nutres.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Lee E.C., Fragala M.S., Kavouras S.A., Queen R.M., Pryor J.L., Casa D.J. Biomarkers in sports and exercise: tracking health, performance, and recovery in athletes. J Strength Condit Res. 2017;31(10):2920–2937. doi: 10.1519/JSC.0000000000002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yi M., Fu J., Zhou L., Gao H., Fan C., Shao J., Xu B., Wang Q., Li J., Huang G., Lapsley K., Blumberg J.B., Chen C.Y. The effect of almond consumption on elements of endurance exercise performance in trained athletes. Sports Nutr Rev J. 2014;11:18. doi: 10.1186/1550-2783-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbaspour N., Roberts T., Hooshmand S., Kern M., Hong M.Y. Mixed nut consumption may improve cardiovascular disease risk factors in overweight and obese adults. Nutrients. 2019;11(7):1488. doi: 10.3390/nu11071488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sari I., Baltaci Y., Bagci C., Davutoglu V., Erel O., Celik H., et al. Effect of pistachio diet on lipid parameters, endothelial function, inflammation, and oxidative status: a prospective study. Nutrition. 2010;26(4):399–404. doi: 10.1016/j.nut.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 35.Li N., Jia X., Chen C.Y., Blumberg J.B., Song Y., Zhang W., Zhang X., Ma G., Chen J. Almond consumption reduces oxidative DNA damage and lipid peroxidation in male smokers. J Nutr. 2007;137(12):2717–2722. doi: 10.1093/jn/137.12.2717. [DOI] [PubMed] [Google Scholar]
- 36.De Souza R., Gomes A.C., Navarro A.M., Cunha L., Silva M., Junior F.B., Mota J.F. Baru almonds increase the activity of glutathione peroxidase in overweight and obese women: a randomized, placebo-controlled trial. Nutrients. 2019;11(8):1750. doi: 10.3390/nu11081750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kander M.C., Cui Y., Liu Z. Gender difference in oxidative stress: a new look at the mechanisms for cardiovascular diseases. J Cell Mol Med. 2017;21(5):1024–1032. doi: 10.1111/jcmm.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mastaloudis A., Yu T.W., O'Donnell R.P., Frei B., Dashwood R.H., Traber M.G. Endurance exercise results in DNA damage as detected by the comet assay. Free Radical Biol Med. 2004;36(8):966–975. doi: 10.1016/j.freeradbiomed.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Byrnes W.C., Clarkson P.M., White J.S., Hsieh S.S., Frykman P.N., Maughan R.J. Delayed onset muscle soreness following repeated bouts of downhill running. J Appl Physiol. 1985;59(3):710–715. doi: 10.1152/jappl.1985.59.3.710. [DOI] [PubMed] [Google Scholar]
- 40.Eston R.G., Lemmey A.B., McHugh P., Byrne C., Walsh S.E. Effect of stride length on symptoms of exercise-induced muscle damage during a repeated bout of downhill running. Scand J Med Sci Sports. 2000;10(4):199–204. doi: 10.1034/j.1600-0838.2000.010004199.x. [DOI] [PubMed] [Google Scholar]
- 41.Park K.S., Lee M.G. Effects of unaccustomed downhill running on muscle damage, oxidative stress, and leukocyte apoptosis. J. Exerc. Nutr. Biochem. 2015;19(2):55–63. doi: 10.5717/jenb.2015.15050702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park K.S., Sedlock D.A., Navalta J.W., Lee M.G., Kim S.H. Leukocyte apoptosis and pro-/anti-apoptotic proteins following downhill running. Eur J Appl Physiol. 2011;111(9):2349–2357. doi: 10.1007/s00421-011-1907-2. [DOI] [PubMed] [Google Scholar]
- 43.Kay C.D., Gebauer S.K., West S.G., Kris-Etherton P.M. Pistachios increase serum antioxidants and lower serum oxidized-LDL in hypercholesterolemic adults. J Nutr. 2010;140(6):1093–1098. doi: 10.3945/jn.109.117366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated or analyzed during this study are available from the corresponding author upon reasonable request.