Abstract
In patients with chronic paracoccidioidomycosis (n = 10), levels of tumor necrosis factor alpha, interleukin-10, and interleukin-2 in serum, measured by enzyme-linked immunosorbent assay (in picograms per milliliter, as mean ± standard error of the mean), were higher than in normal controls (n = 8): 186 ± 40 versus 40 ± 7 (P < 0.05), 203 ± 95 versus 20 ± 8 (P = 0.001), and 96.3 ± 78.57 versus 1.19 ± 1.19 (P = 0.045), respectively. Gamma interferon and interleukin-4 levels were similar in patients and controls.
Paracoccidioidomycosis, the progressive systemic mycosis caused by Paracoccidioides brasiliensis, can evolve into several clinical forms, with different degrees of cellular immunity defect. Phagocytes and lymphocytes control the dissemination of P. brasiliensis through the production of cytokines and other functions. Different regulatory pathways determine the pattern of immune response, which can be effective, resulting in the clearance of the fungus, or ineffective, allowing it to multiply in the tissues. In areas where the fungus is endemic, at least 25% of people present a positive skin test with Paracoccidioides brasiliensis antigen, but only a few develop disease, suggesting a defect restricted to the control of P. brasiliensis.
Paracoccidioidomycosis is associated with lymphopenia and abnormalities in cytokine production (1, 10). Though in animal models regulatory CD4+ subsets contribute to the defect, their role in humans is not known. To analyze the participation of different subsets of immunocompetent cells in human chronic paracoccidioidomycosis, the aim of this study was to determine the serum levels of cytokines characteristic of discrete subsets: gamma interferon (IFN-γ), interleukin 2 (IL-2), and IL-4, for lymphocytes, and tumor necrosis factor alpha (TNF-α), a macrophage-associated cytokine, and IL-10, produced both by macrophages and by TH2 CD4+ T-lymphocytes. A caveat for this approach is that the kinetics for an individual cytokine is characteristic and noncomparable with that of other cytokines, and a significant redundancy of cellular sources exists among them.
Serum was taken at time of diagnosis from 10 hospitalized patients (nine male, one female; 30 to 66 years old). All patients presented the chronic disseminated form of paracoccidioidomycosis, with cutaneous and/or mucous lesions. The exact beginning of the disease has not been accurately recorded; nevertheless, patients are supposed to have become infected in their infancy, while living in the disease-endemic area. No immunocompromising diseases were recorded among the patients. Since they were in the course of an active infection, our hypothesis was that most cytokines would have been in some way affected. For comparison, we took serum from eight normal blood donors (all male, 22 to 44 years old), whose serum cytokine levels are usually low.
After the serum was aliquoted and stored at −20°C, cytokines were measured with commercial enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, Minn.), used according to the manufacturer's instructions. The sensitivity limit of the assays was 3 pg/ml for IFN-γ, 7 pg/ml for IL-2, 4.1 pg/ml for IL-4, 4.4 pg/ml for TNF-α, and 2 pg/ml for IL-10. All comparisons were performed using the Mann-Whitney test with Stat-Primer software.
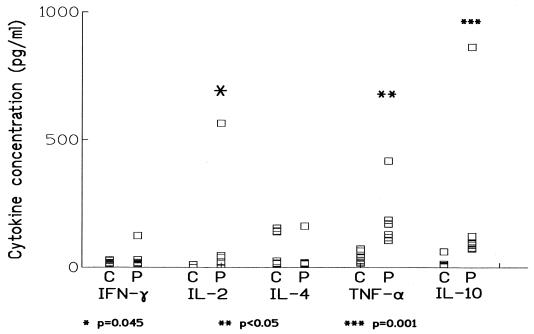
The comparison of IFN-γ, IL-2, and IL-4 levels for both normal controls and paracoccidioidomycosis patients (Fig. 1) shows a significant difference only in the case of IL-2, which was higher in the patients (96.3 ± 78.57 pg/ml versus 1.19 ± 1.19 pg/ml, mean ± the standard error [P = 0.045]). One patient was clearly an outlier for IL-2, resulting in a marked dispersion of the data; if the analysis excludes this patient, the difference does not achieve significance (P = 0.075), probably due to the low size of the sample.
FIG. 1.
Serum levels of IFN-γ, IL-2, IL-4, TNF-α, and IL-10 in samples from normal controls (C) and patients with chronic paracoccidioidomycosis at diagnosis (P). Cytokines were measured by enzyme-linked immunosorbent assay, as described in the text. IL-2, TNF-α, and IL-10 levels were significantly higher in patients than in controls, though individual values were highly variable.
In murine paracoccidioidomycosis, IFN-γ is critical to controlling P. brasiliensis dissemination: being present in the lungs from susceptible animals, its neutralization exacerbates most parameters of fungal infection (4). In mice, immunodominant gp43 antigen triggers a cellular immune response that involves TH1 CD4+ lymphocytes, secreting IFN-γ, which is protective (13). In experimental models, the pattern of cytokines is considered of paramount importance in determining the result of the infection (3), with IFN-γ (together with IL-2) being responsible for resistance. If similar roles are shared by the cytokines in human infection, the lack of increased levels of IFN-γ and the only slightly increased values for IL-2 could explain why these patients are relatively incapable of controlling the spread of P. brasiliensis. Even if this hypothesis is correct, a central question is why levels of IFN-γ do not increase. Recently lymphocytes from patients with active paracoccidioidomycosis have been shown to be unable to produce IFN-γ when challenged with P. brasiliensis antigen (2); their response to stimulation with phytohemagglutinin is controversial (2, 8). Our results are consistent with a defective production of IFN-γ in vivo, though at present it is unclear whether the failure is at the level of T cells (as suggested by Karhawi et al. [8]) or in their regulation. Since paracoccidioidomycosis patients are not immunodeficient with respect to other pathogens, there must be some regulatory pathway involved. Other cytokines, such as IL-10, could contribute to this defect (see below).
Though levels of IL-2 were increased, the great scattering of the data hinders the ability to reach any firm conclusion. If an increased level of IL-2 is actually present in patients, a possible explanation could lie in the differential effect of cytokines such as IL-12 (see below) on the production of different cytokines by TH1 clones. Alternatively, an increased tissular production of IL-2 resulting from CD4+ T-cell recruitment in tissues could explain both lymphopenia (1) and increased levels of serum IL-2.
All patients presented detectable levels of IL-10, and seven showed detectable levels of TNF-α (Fig. 1); levels of both cytokines were significantly higher in them than in the controls (for TNF-α, 186 ± 40 versus 40 ± 7 pg/ml [P < 0.05], and for IL-10, 203 ± 95 versus 20 ± 8 pg/ml [P = 0.001]), being more than fourfold greater for TNF-α and more than 10-fold greater for IL-10. High levels of TNF-α and the spontaneous production of TNF-α have already been reported in paracoccidioidomycosis (1, 12), in correlation with other proinflammatory markers. Interestingly, in murine models, TNF-α contributes to resistance and its production is an early but ephemeral characteristic of progressive infection (3). This role of TNF-α seems to depend upon the model used: in the Syrian hamster, the enhanced production of TNF-α persists over the course of experimental infection (11), contributing both to the initial defense and also, late in the infection, to damage. Which models do patients most resemble? We think that the role of TNF-α is still to be understood, but its enhanced production is already established, and we propose that it is in some way related to a defective switch to adaptive immunity.
Probably an important mediator is IL-10. In experimental paracoccidioidomycosis in mice, a role for antigen-presenting cells (APC) and IL-10 has been reported (3, 6). High levels of this cytokine were found in seven patients, and, to some extent, this can explain several findings and is consistent with the recent report of increased in vitro production of IL-10 by leukocytes of patients with active paracoccidioidomycosis (2). IL-10 inhibits macrophage differentiation, resulting in a defective antigen presentation and expression of either class II major histocompatibility complex antigen, the costimulatory molecule B7, or CD1a on the surface of phagocytes, among several other regulatory effects (7, 9). It is noteworthy, that a critical function of differentiated macrophages and dendritic cells is the production of IL-12, which promotes the activation of TH1 CD4+ lymphocytes and the secretion of their cytokines. If APC cannot appropriately exert their function, a block in the pathway of an effective immune response can arise, including the lack of a link between innate and adaptive immunity. In addition, IL-10 can inhibit IFN-γ production even in the presence of IL-12 (5).
In conclusion, sera of paracoccidioidomycosis patients have significantly high levels of IL-10, TNF-α, and IL-2. These data show that the in vivo immunity defect seen in chronic paracoccidiodomycosis includes an increase in IL-10 values and the apparent inability to augment levels of IFN-γ in the course of the infection. Both events can be related, since IL-10 inhibits lymphocyte IFN-γ production by inhibiting accessory cell production of IL-12 (5). We postulate a defect in macrophages or other APC, either from the beginning of the disease or at some early stage, resulting in a blocking of the effective collaboration required to mount an effective immune response against P. brasiliensis. This approach, though oversimplified, provides some useful hints for future studies and also some explanation for the difference between murine and clinical models of paracoccidioidomycosis. Further studies are required to test this hypothesis.
(Part of this work was presented as a poster in the 14th World Congress of the International Society for Human and Animal Mycology, 8 to 12 May 2000, Buenos Aires, Argentina. It has been previously published [M. C. Fornari, A. J. Bava, M. T. Guereño, V. E. Berardi, M. R. Silaf, R. Negroni, and R. A. Diez, Abstr. 14th World Congr. Int. Soc. Hum. Anim. Mycol., abstr. 259, p. 198].)
Acknowledgments
We acknowledge E. Antón for helpful comments and M. A. Olmos for her excellent technical assistance.
Financial support was received from Life Bank Foundation.
REFERENCES
- 1.Bava A J, Mistchenko A S, Palacios M F, Estevez M E, Tiraboschi N I, Sen L, Negroni R, Diez R A. Lymphocyte subpopulations and cytokine production in paracoccidioidomycosis patients. Microbiol Immunol. 1991;35:167–174. doi: 10.1111/j.1348-0421.1991.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 2.Benard G, Romano C C, Cacere C R, Juvenale M, Mendes-Giannini M J, Duarte A J. Imbalance of IL-2, IFN-gamma and IL-10 secretion in the immunosuppression associated with human paracoccidioidomycosis. Cytokine. 2001;13:248–252. doi: 10.1006/cyto.2000.0824. [DOI] [PubMed] [Google Scholar]
- 3.Calich V L, Kashino S S. Cytokines produced by susceptible and resistant mice in the course of Paracoccidioides brasiliensis infection. Braz J Med Biol Res. 1998;31:615–623. doi: 10.1590/s0100-879x1998000500003. [DOI] [PubMed] [Google Scholar]
- 4.Cano L E, Kashino S S, Arruda C, André D, Xiedieh C F, Singer-Vermes L M, Vaz C A C, Burger E, Calich V L G. Protective role of gamma interferon in experimental pulmonary paracoccidioidomycosis. Infect Immun. 1998;66:800–806. doi: 10.1128/iai.66.2.800-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Andrea A, Aste-Amezaga M, Valiante N M, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cells stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Almeida S R, de Moraes J Z, de Camargo Z P, Gesztesi J L, Mariano M, Lopes J D. Pattern of immune response to GP43 from Paracoccidioides brasiliensis in susceptible and resistant mice is influenced by antigen-presenting cells. Cell Immunol. 1998;190:68–76. doi: 10.1006/cimm.1998.1388. [DOI] [PubMed] [Google Scholar]
- 7.Faulkner L, Buchan G, Baird M. Interleukin-10 does not affect phagocytosis of particulate antigen by bone marrow-derived dendritic cells but does impair antigen presentation. Immunology. 1999;99:523–531. doi: 10.1046/j.1365-2567.2000.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karhawi A S K, Colombo A L, Salomão R. Production of IFN-γ is impaired in patients with paracoccidioidomycosis during active disease and is restored after clinical remission. Med Mycol. 2000;38:225–229. doi: 10.1080/mmy.38.3.225.229. [DOI] [PubMed] [Google Scholar]
- 9.Kubin M, Kamoun M, Trinchieri G. Interleukin 12 synergizes with B7/CD28 interaction in inducing efficient proliferation and cytokine production of human T cells. J Exp Med. 1994;180:211–222. doi: 10.1084/jem.180.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mota N G, Rezkallah-Iwasso M T, Peraçoli M T, Audi R C, Mendes R P, Marcondes J, Marques S A, Dillon N L, Franco M F. Correlation between cell-mediated immunity and clinical forms of paracoccidioidomycosis. Trans R Soc Trop Med Hyg. 1985;79:765–772. doi: 10.1016/0035-9203(85)90112-9. [DOI] [PubMed] [Google Scholar]
- 11.Parise-Fortes M R, da Silva M F, Sugizaki M F, Defaveri J, Montenegro M R, Soares A M V C, Peraçoli M T S. Experimental paracoccidioidomycosis of the Syrian hamster: fungicidal activity and production of inflammatory cytokines by macrophages. Med Mycol. 2000;38:51–60. doi: 10.1080/mmy.38.1.51.60. [DOI] [PubMed] [Google Scholar]
- 12.Silva C L, Silva M F, Faccioli L H, Pietro R C, Cortez S A, Foss N T. Differential correlation between interleukin patterns in disseminated and chronic human paracoccidioidomycosis. Clin Exp Immunol. 1995;101:314–320. doi: 10.1111/j.1365-2249.1995.tb08357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taborda C P, Juliano M A, Puccia R, Franco M, Travassos L R. Mapping of the T-cell epitope in the major 43-kilodalton glycoprotein of Paracoccidioides brasiliensis which induces a Th-1 response protective against fungal infection in BALB/c mice. Infect Immun. 1998;66:786–793. doi: 10.1128/iai.66.2.786-793.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]