Abstract

The NIST REFPROP software program is a powerful tool for calculating thermophysical properties of industrially important fluids, and this manuscript describes the models implemented in, and features of, this software. REFPROP implements the most accurate models available for selected pure fluids and their mixtures that are valid over the entire fluid range including gas, liquid, and supercritical states, with the goal of uncertainties approaching the level of the underlying experimental data. The equations of state for thermodynamic properties are primarily of the Helmholtz energy form; a variety of models are implemented for the transport properties. We document the models for the 147 fluids included in the current version. A graphical user interface generates tables and provides extensive plotting capabilities. Properties can also be accessed through third-party apps or user-written code via the core property subroutines compiled into a shared library. REFPROP disseminates international standards in both the natural gas and refrigeration industries, as well as standards for water/steam.
1. Introduction
Modeling and simulation are the foundation of modern design, including in the chemical process industry. The accuracy, and thus utility, of such simulations can be no better than the accuracy of the underlying materials property data employed. Here we are concerned with fluid property data, encompassing gas, liquid, and supercritical states. The required accuracy varies among applications, with the highest accuracy required for (1) fluids requiring accurate data due to their sheer scale in the economy, such as natural gas and other fuels, (2) working fluids in power and refrigeration cycles, where accurate data are needed to optimize the energy efficiency of equipment, (3) flow metering applications, (4) calibration fluids and other standards, and (5) thermodynamic research, including the development and verification of property models. It is no longer sufficient to present accurate property data merely as tabulations (e.g., “steam tables”)—they must be integrated into process models. But the most accurate property models can be quite complex, and the difficulties of implementing these models hinders their wider use. Thus, there is a need for tools that remove the burden of coding the property models from the engineer analyzing data from a lab experiment or designing a process and transfer that burden to thermodynamic specialists. Here, we describe one such tool—the NIST REFPROP database from the National Institute of Standards and Technology.
REFPROP (formally known as NIST Standard Reference Database 23: REference Fluid Thermodynamic and Transport PROPerties—REFPROP)1 is a computer program distributed by NIST that provides thermophysical properties for a variety of industrially important fluids and their mixtures. The acronym REFPROP provides a clue to the history of the program, as it once stood for REFrigerant PROPerties2 but now it is applicable to many common industrial fluids including cryogens, common hydrocarbon and natural gas constituent fluids, solvents, siloxanes, biodiesel constituents, lubricants, alcohols, glycols, and water, etc., in addition to refrigerants. It contains models for the thermophysical properties of a wide variety of fluids with the goal of representing the properties to near or within the uncertainty of available experimental data. Unlike other databases distributed by NIST,3 it does not contain critically evaluated experimental data, but rather it is a computational database. It is distributed as a computer program with a graphical user interface (GUI) designed to run under the Windows4 operating system, but also supplied is a dynamic link library (DLL) enabling users to interface with other programs or languages including Excel, Python, C++, Matlab, LabView, and Mathematica, etc. The source code for the property calculations, in FORTRAN, is also distributed with the program. It has become a useful tool in the refrigeration, natural gas, and aerospace industries, among others. In addition to directly using the NIST REFPROP program, researchers have also accessed properties from REFPROP indirectly from its inclusion in commercial simulation software such as ANSYS5 and aspenONE,6 or by reading input files created from REFPROP as is done by NASA’s GFSSP fluid system simulation program.7 This article summarizes some of the models, features, and limitations of the program.
In this manuscript we will first briefly discuss the history of REFPROP, and then discuss the models used for calculating the thermodynamic properties of pure fluids. We next discuss the pure-fluid models used for the calculation of the transport properties viscosity and thermal conductivity, and additional properties surface tension and the dielectric constant. Following that, the corresponding models for mixture properties are summarized. Finally, we briefly describe the features of the program and future directions.
2. Models for Thermodynamic Properties of Pure Fluids
2.1. Historical Background
The first version of REFPROP was released in 1989 and was in response to the need for thermophysical properties of new refrigerants and their mixtures that were proposed to replace the ozone-damaging chlorofluorocarbon (CFC) refrigerants in use at the time. A more detailed history of refrigerants can be found in ref (2). Rather than have a collection of separate correlations for thermophysical properties, such as individual correlations for vapor pressure, saturated liquid density, enthalpy of vaporization, etc., REFPROP takes the equation of state approach where all thermodynamic properties are obtained from an equation of state (EOS), thereby ensuring thermodynamic consistency among thermodynamic properties.
In the late 1980s when REFPROP first appeared, the Martin-Hou equation of state8 was widely used in the refrigerants community, as discussed by Downing.9 This model has significant limitations, namely it is not valid in the liquid region, and requires ancillary equations for vapor pressure and saturated liquid density. NIST instead opted for an approach based on a modification10 of the Carnahan–Starling–DeSantis EOS.11 There were several reasons for this: (1) the CSD EOS has a theoretical basis and requires minimal data to obtain parameters, (2) it can represent both liquid and vapor phases, and (3) it is applicable to mixtures. The modified CSD EOS proposed by Morrison and McLinden10 required only six parameters, which were usually obtained by fitting experimental data for vapor pressures and saturated liquid and vapor volume.
Lack of availability of accurate property data continued to be an issue during the early research on alternative refrigerants, and a second modeling approach, the extended corresponding states (ECS) model, was added to REFPROP. Technically ECS is not an equation of state, but rather a model that incorporates properties from an equation of state for a “reference” fluid. The model was proposed by Leland and co-workers,12,13 used at NIST for the properties of hydrocarbons and their mixtures,14,15 applied in 1994 to refrigerants,16 and then incorporated into early versions of REFPROP. The basic premise is to represent the properties of the fluid of interest in terms of scaling factors applied to a fluid for which the properties are well characterized, called the reference fluid. Typically, the reference fluid was R-134a for refrigerants and propane for simple hydrocarbons since these fluids were well-characterized and accurate equations of state were available. The major advantage of this method was that refrigerants or simple hydrocarbons could be represented with minimal data, usually just a few saturated liquid density points and vapor pressures. ECS also can be used for transport properties, as discussed in section 3. For thermodynamic properties, it has largely been replaced by more accurate multiparameter EOS discussed in the following paragraphs, although there still are some recent applications to the very new hydrofluoroolefin (HFO) refrigerants in cases where sufficient data for more accurate EOS development are not yet available.17
The two models mentioned thus far, the modified Carnahan–Starling–DeSantis EOS and extended corresponding states, were attractive because they could be applied in situations in which few reliable data were available, and both were capable of covering the entire fluid region from the gas to the liquid and including supercritical states. Both have some basis in theory and do not give unphysical behavior. For the case of ECS, we assume that the reference fluid EOS has realistic behavior—if one chooses a reference fluid with unphysical behavior, the ECS model will show the same behavior as the reference fluid. These methods typically could represent density to within a few percent. As more and better data became available, it was desirable to represent the experimental data to nearly within the experimental uncertainty, often on the order of a tenth of a percent or better for density. For example, the density data for pentafluoroethane (R-125) of Defibaugh and Morrison,18 published in 1992, had an estimated uncertainty of 0.05%, except in the near-critical region. The ECS model of Huber and Ely,16 implemented in an early version of REFPROP, represents the compressed liquid density from this data set (which extend to 6.3 MPa) with an uncertainty at the k = 2 level of 2.3%. This clearly is not adequate if one is trying to represent the data to within the experimental uncertainty.
In 1993, the Modified Benedict–Webb–Rubin (MBWR) EOS was added to REFPROP to address some of the deficiencies of the ECS model and the CSD EOS. This form of equation of state, introduced by Jacobsen and Stewart19 for nitrogen, is considerably more complex than the six-parameter CSD EOS. It contains 32 parameters that are obtained by fitting multiple types of experimental thermodynamic data, such as (T, p, ρ), isochoric and isobaric heat capacities, second virial coefficients, coexistence properties, etc. It also can be applied to polar fluids that caused difficulties with the CSD EOS, and it is capable of representing experimental data for multiple thermodynamic properties to near the experimental uncertainty. For the example of compressed liquid data for R-125 of Defibaugh and Morrison18 mentioned above, the MBWR EOS developed by Outcalt and McLinden20 represents the compressed liquid density data with an average absolute deviation (AAD) of 0.092%, a significant improvement over ECS or CSD, but requiring more data in its development. The MBWR EOS also is superior in its representation of other thermodynamic properties, even those not used in its development. Taking R-125 again as an example, Takagi21 measured the speed of sound in the liquid phase of R-125 with an estimated uncertainty of 0.2%. The performance of several models for this data set is shown in Figure 1. The MBWR EOS of Outcalt and McLinden20 represents this data set with an average absolute deviation (AAD) of 0.5% and a bias of 0.5%. The ECS model, however, represents this data set with an AAD of 7.8% and also a bias of −7.8%. The Takagi21 data were not used in the development of either the MBWR EOS or the ECS model; the MBWR EOS represents the data much better than the ECS model, suggesting that it would better represent other properties, as well. However, it still does not represent the experimental data to within the uncertainty of the data. A different form of equation of state, called a Helmholtz form, is discussed in section 2.2. As shown in Figure 1, the Helmholtz equation of state of Lemmon and Jacobsen22 is superior to both the MBWR and ECS models; the AAD is 0.27% with a bias of −0.11%; approaching the experimental uncertainty21 of 0.2%.
Figure 1.
Deviations in the representation of the sound speed data of Takagi21 for R-125.
The MBWR and CSD equations of state are explicit in pressure, p, that is, are written in the form p = f(T, ρ) whereT is temperature and ρ is the molar density. Any equation of state written in this form may be combined with an expression for the molar heat capacity of the ideal gas at constant pressure, Cp0(T), to compute all thermodynamic properties,23 including speed of sound, enthalpy, entropy, etc. Equations of state in this form are quite natural since they involve quantities that are easily measured and follow a tradition that started with the ideal gas law. Although one can compute all thermodynamic properties with this form, integration is necessary to obtain caloric properties. It is not a serious problem to obtain the necessary analytical expressions for thermodynamic properties from the MBWR or other pressure-explicit EOS, but it does impose some restrictions on the types of mathematical terms that can be used.24
2.2. Fundamental Equations of State
A different approach is the so-called “fundamental EOS” meaning that all thermodynamic properties can be obtained using only derivatives (not integrals). Because of this, there is more flexibility in the types of mathematical expressions that can be incorporated. A fundamental EOS can be developed in terms of the Helmholtz energy [a = f(T, ρ)], Gibbs energy [g = f(T, p)], enthalpy [h = f(s, p)], or internal energy [u = f(s, ρ)]. Here we use lower case variables for the properties to indicate that they are on a specific or a molar basis. Of these, the Helmholtz energy is the most commonly used. The properties h and u are difficult to implement practically because entropy, s, is not experimentally accessible. Both g and a involve easily obtainable variables T, p, and ρ.T and p are intensive thermodynamic variables, meaning that their magnitude is not dependent on the size of the system.25 Volume and mass however are extensive properties that do depend on the size (or extent) of the system; density is mass/volume and is an intensive property. Across any phase boundary (liquid–vapor, liquid–liquid, liquid–solid, etc.) the temperature and pressure are the same, and the Gibbs energy is equal in both phases. Thus, one cannot uniquely specify a thermodynamic state point on the phase boundary or in the 2-phase region using only T and p. Although the temperature across a phase boundary is the same, the density of the phases in equilibrium are different and the Helmholtz energy is different as well, allowing one to distinguish between the phases. Thus, formulating a fundamental EOS in terms of Helmholtz energy as a function of the variables T and ρ allows one to uniquely specify the thermodynamic state across the 2-phase region in addition to along the saturation boundary and is the preferred formulation.
Application of the Helmholtz-form approach to developing reference-quality EOS began in the mid-1980s with publications from the groups of Wagner26 and of Jacobsen,27 who used multiproperty fitting to develop new equations that were not possible with pressure-explicit formulations. This type of EOS is the current state-of-the-art in equation of state development for which accuracy is paramount, and they comprise the vast majority of EOS incorporated into REFPROP. An excellent detailed discussion of multiparameter EOS, covering both the MBWR and Helmholtz forms, is presented in the book by Span.24
The Helmholtz energy form for a fundamental EOS is often written as24,28
| 1 |
where α is the reduced molar Helmholtz energy, αid is the ideal gas contribution, and αr is the residual, or real-fluid, contribution. The first summation term in eq 1 is called the polynomial-like term, and the second is referred to as the exponential term. The temperature and density are expressed in reduced variables τ = T*/T and δ = ρ/ρ* where T* and ρ* are reducing parameters that often are the critical parameters. Nk is coefficients obtained by fitting experimental data, and the exponents dk, tk, and lk are also determined by regression. The values of tk should be greater than zero, and dk and lk should be integers greater than zero.29 In these formulations a total of 4 to 20 terms are used in each summation, and the index k points to each individual term.
To better represent properties in the critical region, additional terms have been added to eq 1 yielding29
| 2 |
The additional terms, referred to as Gaussian bell-shaped terms, were introduced by Setzmann and Wagner30 and incorporated into their equation of state for methane. Some of these equations can be extremely complex, such as the equation of state for water,31 which contains 56 terms.
To recover the more familiar expression p = f(T, ρ) the following thermodynamic relationship is used:
| 3 |
A complete listing of expressions for other thermodynamic properties expressed as derivatives of the Helmholtz energy can be found in ref (24) and the Appendix of ref (22).
Originally, the fitting started with a set of mathematical expressions selected from a large bank of fixed terms, and terms were added and eliminated until an optimal form was found. With faster computers, a fully nonlinear, least-squares fit was implemented, which allowed incorporation of terms of an arbitrary form. The resulting fitting process is quite complex as discussed by Lemmon and Jacobsen.22 Due to the empirical nature of the equation, care must be taken to control the behavior in the two-phase region as well as the extrapolation behavior; this is done by including constraints in the regression process22 and examining the behavior of characteristic curves (the Boyle curve, the Joule-Thomson inversion curve, and the Joule inversion curve)22,32.
The major strength of the Helmholtz-form EOS is the ability to represent multiple types of thermodynamic data—(T, p, ρ), sound speed, isobaric and isothermal heat capacity, second virial coefficients, vapor pressures, saturated liquid and vapor densities, enthalpy of vaporization, Joule-Thomson coefficient, isothermal throttling coefficients, etc.—to within the estimated uncertainty of the underlying data. The fact that multiple types of properties are used to develop a Helmholtz-form EOS serves as a powerful consistency check on the data—if all of the data are fitted well that is an indication that the data are accurate. The Helmholtz-based EOS are superior to any other form of EOS in this respect, and thus are used in applications of custody transfer and calibrations, and they are at the heart of the REFPROP program. Due to their complexity, considerable expertise is required to develop these equations to ensure that they behave in a physically reasonable manner when extrapolated to conditions for which there are no experimental data, behave well in the two-phase region, and that data are not overfit with an excessive number of coefficients. They are, however, computationally slow compared to simpler equations of state33,34 and require a large amount of accurate experimental data for their development.
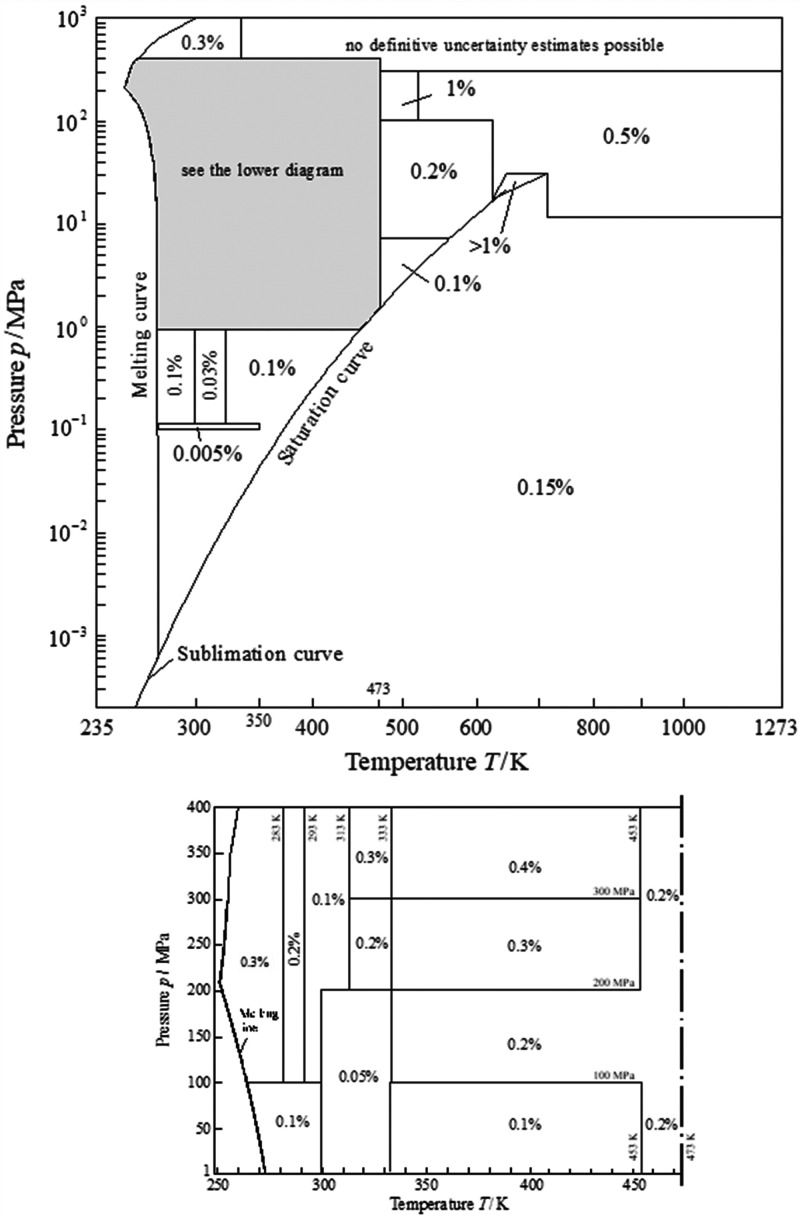
As an example of the capability of the Helmholtz-form EOS, Figures 2, 3, and 4 show the uncertainties in density, sound speed, and specific isobaric heat capacity for the Helmholtz EOS of Wagner and Pruß31 for water, which was adopted as an international standard by IAPWS.35 The experimental data set for water (considering accuracy, range of temperature and pressure, and types of properties available) is the most extensive for any fluid. Thus, Figures 2, 3, and 4 represent the best case for an EOS.
Figure 2.
Relative uncertainties in density, Δρ/ρ, for the Helmholtz EOS for water from IAPWS R6-95(2018).35
Figure 3.
Relative uncertainties in speed of sound, Δw/w, for the Helmholtz EOS for water from IAPWS R6-95(2018).35
Figure 4.
Relative uncertainties in specific isobaric heat capacity, Δcp/cp, for the Helmholtz EOS for water from IAPWS R6-95(2018).35
2.3. Equations of State Implemented in REFPROP
Table 1 summarizes the recommended equations of state for the fluids implemented in version 10.0 of REFPROP.1 Displayed are the short name, the full name, chemical formula, and CAS number to assist with proper identification. In addition, REFPROP also makes available the standard InChI string and standard InChI key. For the equation of state column, EOS, in Table 1, all equations are of the Helmholtz form except those for F3N, R-13, R-123, and R-152a, which are of the MBWR type. There also are three legacy EOS36 (for R-14, R-114, and RC-318), formulated as the Bender37,38 EOS, which also is a pressure-explicit EOS and very similar to the MBWR EOS. However, for inclusion in REFPROP they have been converted to a Helmholtz formulation. We also note that in addition to the recommended EOS that are listed in Table 1 and used by default in REFPROP, for many fluids there are additional EOS that may be optionally used, including older MBWR forms and also the simple cubic Peng-Robinson39 EOS. However, to obtain the most accurate results we recommend the use of the default EOS. Also note that since the release of v10.0 in 2018, additional EOS correlations have been published,40−43 and these will be included in future releases. Table 1 also includes references for the surface tension, σ, thermal conductivity, λ, and viscosity, η, models that will be discussed in more detail later in this document. Finally, for some fluids there are additional types of information available as denoted in the column labeled “other”, such as dielectric constants, sublimation lines, and melting lines.
Table 1. Summary of Recommended Pure Fluid Models in REFPROP v10.0.
| short name | full name | short formula | CAS no. | EOS | σa | λa,b,c | ηa,b,c,d | othera,e |
|---|---|---|---|---|---|---|---|---|
| 1,3-Butadiene | Buta-1,3-diene | C4H6 | 106-99-0 | (44) | (45) | ECS45 | ECS45 | na |
| 1-Butyne | But-1-yne | C4H6 | 107-00-6 | (46) | (45) | ECS45 | ECS45 | na |
| 1-Pentene | Pent-1-ene | C5H10 | 109-67-1 | (47) | (45) | ECS45 | ECS45 | na |
| 2,2-Dimethylbutane | 2,2-Dimethylbutane | C6H14 | 75-83-2 | (48) | (45) | ECS45 | ECS45 | na |
| 2,3-Dimethylbutane | 2,3-Dimethylbutane | C6H14 | 79-29-8 | (48) | (45) | ECS45 | ECS45 | na |
| 3-Methylpentane | 3-Methylpentane | C6H14 | 96-14-0 | (48) | (45) | ECS45 | ECS45 | na |
| Acetone | Propanone | C3H6O | 67-64-1 | (49) | (50) | ECS45 | ECS45 | na |
| Acetylene | Ethyne | C2H2 | 74-86-2 | (51) | (45) | ECS45 | ECS45 | na |
| Ammonia | Ammonia | NH3 | 7664-41-7 | (52) | (50) | FS53 | FS54 | M,55 S56,f |
| Argon | Argon | Ar | 7440-37-1 | (57) | (50) | FS58 | FS58 | D,59 M,57 S60 |
| Benzene | Benzene | C6H6 | 71-43-2 | (61) | (50) | FS62 | FS63 | na |
| Butane | n-Butane | C4H10 | 106-97-8 | (64) | (50) | FS65 | FS66 | na |
| Butene | 1-Butene | C4H8 | 106-98-9 | (67) | (50) | ECS45 | ECS45 | na |
| Carbon dioxide | Carbon dioxide | CO2 | 124-38-9 | (68) | (50) | FS69 | FS70 | D,59 M,68 S68 |
| Carbon monoxide | Carbon monoxide | CO | 630-08-0 | (49) | (50) | ECS45 | ECS45 | M,71 S72,f |
| Carbonyl sulfide | Carbon oxide sulfide | COS | 463-58-1 | (49) | (50) | ECS45 | ECS45 | na |
| Chlorine | Chlorine | Cl2 | 7782-50-5 | (73) | (45) | ECS45 | ECS45 | na |
| Chlorobenzene | Chlorobenzene | C6H5Cl | 108-90-7 | (74) | (45) | ECS45 | ECS45 | na |
| cis-Butene | cis-2-Butene | C4H8 | 590-18-1 | (67) | (75) | ECS45 | ECS45 | na |
| Cyclobutene | 1-Cyclobutene | C4H6 | 822-35-5 | (76) | (45) | ECS45 | ECS45 | na |
| Cyclohexane | Cyclohexane | C6H12 | 110-82-7 | (77) | (50) | FS78 | FS79 | M77 |
| Cyclopentane | Cyclopentane | C5H10 | 287-92-3 | (80) | (75) | FS81 | ECS45 | na |
| Cyclopropane | Cyclopropane | C3H6 | 75-19-4 | (82) | (75) | ECS45 | ECS45 | na |
| D4 | Octamethylcyclotetrasiloxane | C8H24O4Si4 | 556-67-2 | (83) | (75) | ECS45 | ECS45 | na |
| D5 | Decamethylcyclopentasiloxane | C10H30O5Si5 | 541-02-6 | (84) | (75) | ECS45 | ECS45 | na |
| D6 | Dodecamethylcyclohexasiloxane | C12H36O6Si6 | 540-97-6 | (85) | (75) | ECS45 | ECS45 | na |
| DEA | 2,2′-Iminodiethanol | C4H11NO2 | 111-42-2 | (86) | (45) | ECS45 | ECS45 | na |
| Decane | Decane | C10H22 | 124-18-5 | (49) | (50) | FS87 | FS88 | D59 |
| Deuterium | Deuterium | D2 | 7782-39-0 | (89) | (50) | FS90,e | FS91a,e | na |
| Dichloroethane | 1,2-Dichloroethane | C2H4Cl2 | 107-06-2 | (92) | (45) | ECS45 | ECS45 | na |
| Diethyl ether | Diethyl ether | C4H10O | 60-29-7 | (93) | (75) | ECS45 | ECS45 | na |
| Dimethyl carbonate | Dimethyl ester carbonic acid | C3H6O3 | 616-38-6 | (94) | (75) | ECS45 | ECS45 | na |
| Dimethyl ether | Methoxymethane | C2H6O | 115-10-6 | (95) | (50) | ECS45 | FS96 | na |
| Docosane | Docosane | C22H46 | 629-97-0 | (97) | (45) | ECS45 | ECS45 | na |
| Dodecane | Dodecane | C12H26 | 112-40-3 | (98) | (50) | FS99 | FS99 | na |
| Ethane | Ethane | C2H6 | 74-84-0 | (100) | (50) | FS101 | FS102 | na |
| Ethanol | Ethyl alcohol | C2H6O | 64-17-5 | (103) | (104) | FS105 | FS106 | na |
| Ethylene glycol | 1,2-Ethandiol | C2H6O2 | 107-21-1 | (107) | (45) | ECS45 | ECS45 | na |
| Ethylbenzene | Phenylethane | C8H10 | 100-41-4 | (108) | (75) | FS109 | FS110 | na |
| Ethylene | Ethene | C2H4 | 74-85-1 | (111) | (50) | FS112 | FS113 | D,59 M,114 S72,f |
| Ethylene oxide | Ethylene oxide | C2H4O | 75-21-8 | (115) | (75) | ECS45 | ECS45 | na |
| Fluorine | Fluorine | F2 | 7782-41-4 | (116) | (50) | ECS45 | ECS45 | M,116 S72,f |
| Heavy water | Deuterium oxide | D2O | 7789-20-0 | (117) | (118) | FS119 | FS119 | M,117 S117 |
| Helium | Helium-4 | He | 7440-59-7 | (120) | (50) | FS121 | FS122 | D,59 M123 |
| Heptane | Heptane | C7H16 | 142-82-5 | (124) | (50) | FS125 | FS126 | D59 |
| Hexadecane | Hexadecane | C16H34 | 544-76-3 | (97) | (45) | FS127 | FS128 | na |
| Hexane | Hexane | C6H14 | 110-54-3 | (129) | (50) | FS130 | FS131 | na |
| Hydrogen (normal) | Hydrogen (normal) | H2 | 1333-74-0 | (132) | (50) | FS90 | FS91 | D,59 M,133 S134 |
| Hydrogen chloride | Hydrogen chloride | HCl | 7647-01-0 | (135) | (75) | ECS45 | ECS45 | na |
| Hydrogen sulfide | Hydrogen sulfide | H2S | 7783-06-4 | (49) | (50) | ECS45 | FT136 | D,137 S56,f |
| Isobutane | 2-Methylpropane | C4H10 | 75-28-5 | (64) | (50) | FS138 | FS139 | na |
| Isobutene | 2-Methyl-1-propene | C4H8 | 115-11-7 | (67) | (50) | ECS45 | ECS45 | na |
| Isohexane | 2-Methylpentane | C6H14 | 107-83-5 | (49) | (50) | ECS45 | ECS45 | na |
| Isooctane | 2,2,4-Trimethylpentane | C8H18 | 540-84-1 | (140) | (75) | ECS45 | ECS45 | na |
| Isopentane | 2-Methylbutane | C5H12 | 78-78-4 | (49) | (50) | FS81 | ECS45 | D,59 M141 |
| Krypton | Krypton | Kr | 7439-90-9 | (49) | (50) | ECS45 | ECS45 | D,59 M,142 S143 |
| MD2M | Decamethyltetrasiloxane | C10H30O3Si4 | 141-62-8 | (144) | (75) | ECS45 | ECS45 | na |
| MD3M | Dodecamethylpentasiloxane | C12H36O4Si5 | 141-63-9 | (84) | (75) | ECS45 | ECS45 | na |
| MD4M | Tetradecamethylhexasiloxane | C14H42O5Si6 | 107-52-8 | (84) | (75) | ECS45 | ECS45 | na |
| MDM | Octamethyltrisiloxane | C8H24O2Si3 | 107-51-7 | (144) | (75) | ECS45 | ECS45 | na |
| MEA | Ethanolamine | C2H7NO | 141-43-5 | (145) | (45) | ECS45 | ECS45 | na |
| Methane | Methane | CH4 | 74-82-8 | (30) | (50) | FS146 | FT147 | D,59 M,30 S148 |
| Methanol | Methanol | CH4O | 67-56-1 | (149) | (50) | FS150,151 | FS152 | M149 |
| Methyl linoleate | Methyl (Z,Z)-9,12-octadecadienate | C19H34O2 | 112-63-0 | (153) | (75) | FS154 | ECS45 | na |
| Methyl linolenate | Methyl (Z,Z,Z)-9,12,15-octadecatrienoate | C19H32O2 | 301-00-8 | (153) | (45) | ECS45 | ECS45 | na |
| Methyl oleate | Methyl cis-9-octadecenoate | C19H36O2 | 112-62-9 | (153) | (75) | FS154 | ECS45 | na |
| Methyl palmitate | Methyl hexadecanoate | C17H34O2 | 112-39-0 | (153) | (75) | ECS45 | ECS45 | na |
| Methyl stearate | Methyl octadecanoate | C19H38O2 | 112-61-8 | (153) | (75) | ECS45 | ECS45 | na |
| Methylcyclohexane | Methylcyclohexane | C7H14 | 108-87-2 | (155) | (75) | FS156 | ECS45 | na |
| MM | Hexamethyldisiloxane | C6H18OSi2 | 107-46-0 | (157) | (75) | ECS45 | ECS45 | na |
| m-Xylene | 1,3-Dimethylbenzene | C8H10 | 108-38-3 | (108) | (75) | FS109 | FS158 | na |
| Neon | Neon | Ne | 7440-01-9 | (159) | (50) | ECS45 | ECS45 | na |
| Neopentane | 2,2-Dimethylpropane | C5H12 | 463-82-1 | (49) | (75) | ECS45 | ECS45 | na |
| Nitrogen | Nitrogen | N2 | 7727-37-9 | (160) | (50) | FS58 | FS58 | D,59 M,160 S161 |
| Nitrogen trifluoride | Nitrogen trifluoride | F3N | 7783-54-2 | (162) | (75) | na | na | na |
| Nitrous oxide | Dinitrogen monoxide | N2O | 10024-97-2 | (49) | (50) | ECS45 | ECS45 | S163 |
| Nonane | Nonane | C9H20 | 111-84-2 | (49) | (50) | FS87 | FS88 | D59 |
| Novec 649, 1230 | 1,1,1,2,2,4,5,5,5-Nonafluoro-4- | C6F12O | 756-13-8 | (164) | (165) | FS166 | FS167 | na |
| (trifluoromethyl)-3-pentanone | na | |||||||
| Octane | Octane | C8H18 | 111-65-9 | (168) | (50) | FS87 | FS88 | D59 |
| Orthohydrogen | Orthohydrogen | H2 | (132) | na | na | na | na | |
| Oxygen | Oxygen | O2 | 7782-44-7 | (26) | (50) | FS58 | FS58 | D,59 M,26 S169 |
| o-Xylene | 1,2-Dimethylbenzene | C8H10 | 95-47-6 | (108) | (75) | FS109 | FS170 | na |
| Parahydrogen | Parahydrogen | H2 | (132) | (50) | FS90 | FS91 | D,59 M,162 S72,f | |
| Pentane | Pentane | C5H12 | 109-66-0 | (171) | (50) | FS81 | ECS45 | D,59 M172 |
| Perfluorobutane | Decafluorobutane | C4F10 | 355-25-9 | (173) | (50) | ECS45 | ECS45 | na |
| Perfluorohexane | Tetradecafluorohexane | C6F14 | 355-42-0 | (173) | (45) | ECS45 | ECS45 | na |
| Perfluoropentane | Dodecafluoropentane | C5F12 | 678-26-2 | (173) | (50) | ECS45 | ECS45 | na |
| Propadiene | 1,2-Propadiene | C3H4 | 463-49-0 | (174) | (45) | ECS45 | ECS45 | na |
| Propane | Propane | C3H8 | 74-98-6 | (29) | (50) | FS175 | FS176 | D,59 M177 |
| Propylcyclohexane | n-Propylcyclohexane | C9H18 | 1678-92-8 | (178) | (75) | FS156 | ECS45 | na |
| Propylene | Propene | C3H6 | 115-07-1 | (179) | (50) | FS112 | ECS45 | M180 |
| Propylene oxide | 1,2-Epoxypropane | C3H6O | 75-56-9 | (181) | (45) | ECS45 | ECS45 | na |
| Propyne | Propyne | C3H4 | 74-99-7 | (82) | (50) | ECS45 | ECS45 | na |
| p-Xylene | 1,4-Dimethylbenzene | C8H10 | 106-42-3 | (108) | (75) | FS109 | FS182 | na |
| R11 | Trichlorofluoromethane | CCl3F | 75-69-4 | (183) | (50) | ECS184 | ECS185 | na |
| R1123 | Trifluoroethylene | C2HF3 | 359-11-5 | (186) | (45) | ECS45 | ECS45 | na |
| R113 | 1,1,2-Trichloro-1,2,2-trifluoroethane | C2Cl3F3 | 76-13-1 | (187) | (50) | FS188 | FS188 | na |
| R114 | 1,2-Dichloro-1,1,2,2-tetrafluoroethane | C2Cl2F4 | 76-14-2 | (36) | (50) | ECS45 | ECS45 | na |
| R115 | Chloropentafluoroethane | C2ClF5 | 76-15-3 | (189) | (50) | ECS190 | ECS190 | na |
| R116 | Hexafluoroethane | C2F6 | 76-16-4 | (49) | (50) | FS191 | FS191 | na |
| R12 | Dichlorodifluoromethane | CCl2F2 | 75-71-8 | (187) | (50) | ECS184 | ECS185 | na |
| R1216 | Hexafluoropropene | C3F6 | 116-15-4 | (192) | (75) | ECS45 | ECS45 | na |
| R1224yd(Z) | (Z)-1-Chloro-2,3,3,3-tetrafluoropropene | C3HClF4 | 111512-60-8 | (193) | (45) | ECS45 | ECS45 | na |
| R123 | 2,2-Dichloro-1,1,1-trifluoroethane | C2HCl2F3 | 306-83-2 | (23) | (50) | FS194 | FS195 | na |
| R1233zd(E) | trans-1-Chloro-3,3,3-trifluoro-1-propene | C3H2ClF3 | 102687-65-0 | (196) | (197) | FS198 | ECS45 | na |
| R1234yf | 2,3,3,3-Tetrafluoroprop-1-ene | C3F4H2 | 754-12-1 | (199) | (50) | FS200 | FS201 | na |
| R1234ze(E) | trans-1,3,3,3-Tetrafluoropropene | C3F4H2 | 29118-24-9 | (202) | (75) | FS200 | FS201 | na |
| R1234ze(Z) | cis-1,3,3,3-Tetrafluoropropene | C3F4H2 | 29118-25-0 | (203) | (197) | ECS45 | ECS45 | na |
| R124 | 1-Chloro-1,2,2,2-tetrafluoroethane | C2HClF4 | 2837-89-0 | (204) | (50) | ECS205 | ECS205 | na |
| R1243zf | 3,3,3-Trifluoropropene | C3H3F3 | 677-21-4 | (203) | (197) | ECS45 | ECS45 | na |
| R125 | Pentafluoroethane | C2HF5 | 354-33-6 | (22) | (50) | FS206 | FS207 | na |
| R13 | Chlorotrifluoromethane | CClF3 | 75-72-9 | (208) | (50) | ECS209 | ECS209 | na |
| R1336mzz(Z) | (Z)-1,1,1,4,4,4-Hexafluoro-2-butene | C4H2F6 | 692-49-9 | (210) | (45) | ECS45 | ECS45 | na |
| R134a | 1,1,1,2-Tetrafluoroethane | C2H2F4 | 811-97-2 | (211) | (50) | FS212 | FS213 | na |
| R13I1 | Trifluoroiodomethane | CF3I | 2314-97-8 | (189) | (50) | ECS45 | ECS45 | na |
| R14 | Tetrafluoromethane | CF4 | 75-73-0 | (36) | (50) | ECS214 | ECS214 | na |
| R141b | 1,1-Dichloro-1-fluoroethane | C2H3Cl2F | 1717-00-6 | (49) | (50) | ECS215 | ECS215 | na |
| R142b | 1-Chloro-1,1-difluoroethane | C2H3ClF2 | 75-68-3 | (49) | (50) | ECS216 | ECS216 | na |
| R143a | 1,1,1-Trifluoroethane | C2H3F3 | 420-46-2 | (217) | (75) | ECS45 | ECS45 | na |
| R152a | 1,1-Difluoroethane | C2H4F2 | 75-37-6 | (218) | (50) | FS219 | ECS185 | na |
| R161 | Fluoroethane | C2H5F | 353-36-6 | (220) | (50) | FS221 | FS221 | na |
| R21 | Dichlorofluoromethane | CHClF | 75-43-4 | (36) | (50) | ECS222 | ECS222 | na |
| R218 | Octafluoropropane | C3F8 | 76-19-7 | (49) | (50) | ECS45 | ECS45 | na |
| R22 | Chlorodifluoromethane | CHClF2 | 75-45-6 | (223) | (50) | ECS184 | ECS185 | na |
| R227ea | 1,1,1,2,3,3,3-Heptafluoropropane | C3HF7 | 431-89-0 | (189) | (50) | ECS224 | ECS224 | na |
| R23 | Trifluoromethane | CHF3 | 75-46-7 | (225) | (50) | FS226 | FS226 | na |
| R236ea | 1,1,1,2,3,3-Hexafluoropropane | C3H2F6 | 431-63-0 | (227) | (50) | ECS45 | ECS45 | na |
| R236fa | 1,1,1,3,3,3-Hexafluoropropane | C3H2F6 | 690-39-1 | (228) | (50) | ECS45 | ECS45 | na |
| R245ca | 1,1,2,2,3-Pentafluoropropane | C3H3F5 | 679-86-7 | (229) | (50) | ECS45 | ECS45 | na |
| R245fa | 1,1,1,3,3-Pentafluoropropane | C3H3F5 | 460-73-1 | (230) | (50) | FS231 | FS231 | na |
| R32 | Difluoromethane | CH2F2 | 75-10-5 | (232) | (50) | FS233 | ECS234 | na |
| R365mfc | 1,1,1,3,3-Pentafluorobutane | C4H5F5 | 406-58-6 | (189) | (50) | ECS45 | ECS45 | na |
| R40 | Methyl chloride | CH3Cl | 74-87-3 | (93) | (75) | ECS45 | ECS45 | na |
| R41 | Fluoromethane | CH3F | 593-53-3 | (49) | (50) | ECS184 | ECS185 | na |
| RC318 | Octafluorocyclobutane | C4F8 | 115-25-3 | (36) | (50) | ECS45 | ECS45 | na |
| RE143a | Methyl trifluoromethyl ether | C2H3F3O | 421-14-7 | (235) | (45) | ECS45 | ECS45 | na |
| RE245cb2 | Methyl pentafluoroethyl ether | C3H3F5O | 22410-44-2 | (236) | (75) | ECS45 | ECS45 | na |
| RE245fa2 | 2,2,2-Trifluoroethyl-difluoromethyl-ether | C3H3F5O | 1885-48-9 | (237) | (75) | ECS45 | ECS45 | na |
| RE347mcc (HFE-7000) | 1,1,1,2,2,3,3-Heptafluoro-3-methoxypropane | C4H3F7O | 375-03-1 | (238) | (75) | ECS45 | ECS45 | na |
| Sulfur dioxide | Sulfur dioxide | O2S | 7446-09-5 | (239) | (50) | ECS45 | ECS45 | D137 |
| Sulfur hexafluoride | Sulfur hexafluoride | SF6 | 2551-62-4 | (240) | (50) | FS241,242 | FT243 | D,137 M,244 S240 |
| Toluene | Methylbenzene | C7H8 | 108-88-3 | (49) | (50) | FS245 | FS246 | na |
| trans-Butene | trans-2-Butene | C4H8 | 624-64-6 | (67) | (75) | ECS45 | ECS45 | na |
| Undecane | Undecane | C11H24 | 1120-21-4 | (247) | (75) | FS248 | FS248 | na |
| Vinyl chloride | Chloroethylene | C2H3Cl | 75-01-4 | (249) | (45) | ECS45 | ECS45 | na |
| Water | Water | H2O | 7732-18-5 | (31) | (250) | FS251 | FS252 | D,253 M,254 S254 |
| Xenon | Xenon | Xe | 7440-63-3 | (49) | (50) | ECS45 | ECS45 | D,59 M,142 S255 |
na, not available.
ECS, extended corresponding states model; FS, fluid-specific model.
H2 correlations scaled for D2.
FT, friction theory model.
M, melting line; S, sublimation line; D, dielectric constant.
Modified to match triple point of EOS.
3. Models for Transport and Miscellaneous Properties of Pure Fluids
3.1. Viscosity
In addition to providing thermodynamic properties obtained through an equation of state, REFPROP also provides values for the viscosity, thermal conductivity, and surface tension. Although there are models that link transport properties directly with an equation of state through entropy,256,257 traditionally models for viscosity and thermal conductivity were developed separately from the EOS and written as a function of density and temperature. They do require an accurate EOS to provide the density when given a p, T state point. The first version of REFPROP did not provide transport properties at all. Version 3, released in 1991, introduced an extended corresponding states (ECS) model for viscosity and thermal conductivity. This particular model was originally developed by Ely and Hanley14,15,258 in the early 1980s; it had already been successfully used at NIST for modeling hydrocarbons.259 It was modified slightly and adapted for refrigerants.16,260,261 This type of model has the advantage that it covers the entire fluid region and is valid for gas, liquid, and supercritical states, and it can be used in a predictive mode when data are unavailable. The minimum information required is the molar mass, the critical parameters Tc, pc, and ρc, the normal boiling point, and an expression for the ideal gas heat capacity.259 Any additional information about the fluid, such as the acentric factor, vapor pressure, saturated liquid density, liquid thermal conductivity, or liquid viscosity values, can be used to refine the model and improve predictions. In the late 1990s and early 2000s, improvements were made to the model,184,185 and REFPROP basically retains this same model today,45,262 and it is still useful for cases for which there are limited experimental data.263
The uncertainties vary for each fluid and the data used for development, but a very general assessment is that an ECS model can represent the viscosity and the thermal conductivity of a pure fluid to within 5–10%.260,261 For refrigerant fluids with sufficient data to tune the ECS model, results were on the order of 4% for viscosity185 and ranged from 1 to 5.6% for thermal conductivity.184 With careful tuning, the uncertainties of viscosity and thermal conductivity may even be reduced to 2% and 1%, respectively, as was done for the new low-GWP refrigerant R-1234ze(Z).263
For fluids where sufficient data exist (covering a wide range of T, ρ conditions— not just along the saturation boundary), the ECS model has largely been replaced by fluid-specific models that are fitted to experimental data, as they have lower uncertainties than an ECS model and have more flexibility to better fit the data. For viscosity, there is no general form of model that has been used for all fluids; many different approaches have been taken. However, often the viscosity is expressed as a function of T and ρ arising from independent contributions,
| 4 |
Equation 4 is used in conjunction with an equation of state to provide densities at a given T and p.
Expressing the viscosity as the sum of these terms allows one to incorporate theory to guide the development of some of the terms. Instead of appearing as additive terms such as in eq 4, the terms may be combined multiplicatively as was done for water264 and heavy water.265 The first term, η0(Τ) = η(T,0), is the contribution to the viscosity in the zero-density limit, where only two-body molecular interactions occur; it is only a function of temperature. If there are sufficient low-density data along isotherms, extrapolation to zero density can be made and the data can be fit to a convenient form.264 Alternatively, the Chapman-Enskog theory for dilute gases266 can be used to formulate a term for the zero-density limit. Often, an engineering formulation for this term is used, such as that presented in Chung et al.267 when few or no low-density data are available. When sufficient data are available, the approach used by Vogel et al.268 can be used in which experimental data can be fit to obtain an effective cross section. However, there now are ab initio calculations of the zero-density viscosity and thermal conductivity from the group of Hellmann269−278 that can also be used to develop an expression for the zero-density term265 for a limited number of small, rigid molecules.
The second term, Δη, is a function of both T and ρ and is sometimes called the residual contribution. It represents the contributions at elevated densities including many-body collisions, molecular-velocity correlations, and collisional transfer. Sometimes a linear-in-density term is broken out as a separate term, as this can be treated with guidance from theory, and the residual viscosity is represented as
| 5 |
| 6 |
where Bη is the second viscosity virial coefficient, and ηh represents terms resulting from higher densities. Vogel et al.268 present a methodology to calculate Bη that is based on the Rainwater-Friend theory279,280 and has been used successfully in many reference correlations.
Traditionally the residual contribution Δη in eq 4 or the higher density term ηh in eq 5 are found by fitting data to an entirely empirical form. A wide variety of functional forms have been used for this term. Only two examples will be given here; for more examples consult Table 1 for a list of reference correlations for all of the fluids in REFPROP.
One particular methodology for determining the residual contribution to viscosity is one used by Vogel and co-workers,66,102,176,281,282 which incorporates a sophisticated fitting procedure, similar to that used for EOS development, with a large bank of initial terms with optimal terms selected based on regressing a critically evaluated set of data. Typical mathematical expressions included in the bank are double polynomials in reduced temperature and density, and also exponential factors of the reduced density, again very similar to terms in an equation of state approach. Other terms could also be added, for example a modified Batchinski-Hildebrand term139,268,283 or a free-volume type term,213,284 although these are now generally not used as they can have bad extrapolation behavior at high density.
A completely different approach, used for a variety of fluids,54,63,126,131,201,221,246,248,285 determines the form of the residual contribution using symbolic regression. Symbolic regression is a type of genetic programming that allows the exploration of arbitrary functional forms. The types of terms are not known ahead of time—they are found during the regression. The functional form is obtained by the use of a set of operators, parameters, and variables as building blocks. These two methods are totally empirical and depend on having a large amount of high-quality, critically evaluated data for development, which can be problematic. Care must also be taken to ensure that extrapolation behavior is physically reasonable in regions for which there are no data. Symbolic regression in particular may encounter problems with overfitting and the introduction of spurious terms.
Thus far, the approaches discussed for determining the high-density contribution to the viscosity have been empirical. However, there also are methods that use theory to develop the residual term, namely the entropy-scaling approach256,257 and the friction-theory approach.286 These have not yet been extensively used in developing reference correlations136,147,243,287 but may play a larger role in the future.
The last term in eq 4, Δηc, is used to represent the behavior in the critical region. For viscosity, this term affects only a very small region in the immediate vicinity of the critical point,288 and with the exception of relatively few fluids66,70,102,176,264,265,281,282 is usually set to zero as there are not sufficient data for its evaluation. As an example, the critical enhancement for the viscosity of water contributes an amount greater than 2% to the total viscosity only with the narrow region264 645.91 K < T < 650.7 K, 245.8 kg m–3 < ρ < 405.3 kg m–3.
The goal of a reference correlation for viscosity is the same as that for a reference EOS—to represent the available experimental data to within their estimated uncertainty. The data for viscosity typically are of higher uncertainty than the densities and sound speeds encountered in equation of state development. Water is one of the most-studied fluids, and there are large amounts of high-quality data. An uncertainty plot for the reference correlation for the viscosity of water252,264 is presented in Figure 5. The uncertainty shown in Figure 5 is the combined expanded uncertainty with a coverage factor of 2.
Figure 5.
Relative uncertainties in viscosity, Δη/η, for the reference correlation for water from IAPWS R12-08.252
3.2. Thermal Conductivity
The models incorporated in REFPROP for thermal conductivity are very similar to those for viscosity. An ECS model described in refs (184 and 185) is implemented in REFPROP, and as in the case for viscosity, is useful when few data are available. However, it is preferable to use fluid-specific models when possible as they generally have lower uncertainty. Table 1 lists the thermal conductivity models implemented in REFPROP.
As was the case for viscosity, the fluid-specific models for thermal conductivity are often expressed as a function of T and ρ arising from three independent contributions,
| 7 |
Equation 7 is used in conjunction with an equation of state to provide densities when T and p are the input variables. Instead of appearing as additive terms such as in eq 7, the terms may alternatively be combined multiplicatively as was done for water289 and heavy water.290
The first term, λ0(Τ) = λ(T,0), is the contribution to the thermal conductivity in the zero-density limit, where only two-body molecular interactions occur; it is only a function of temperature. Again, if there are sufficient low-density data along isotherms, extrapolation to zero density can be made and the data fit to a convenient form.289 Alternatively, Chapman-Enskog theory for dilute gases266 can be used to guide formulations for the zero-density limit. The method proposed in Chung et al.267 can be useful when few or no data are available. A modified Eucken correlation for polyatomic gases may also be used, as discussed in refs (15, 184, and 261). An alternative expression using kinetic theory and an approach by Thijsse et al.291 and Millat et al.292 was implemented in a correlation for the thermal conductivity of ammonia.53 And as was mentioned in the viscosity section, new theoretical ab initio calculations from the group of Hellmann269−278 are available for a limited number of fluids, and these may be fit to convenient functional forms.290
The second term, Δλ, sometimes called the residual contribution, represents the contributions at elevated densities including many-body collisions, molecular-velocity correlations, and collisional transfer. As was the case for viscosity, the residual contribution has traditionally been found by fitting data to an entirely empirical form without any theoretical guidance. Unlike viscosity, most researchers use the same form to express the residual term,
| 8 |
where n, the number of terms in the summation, is six or less and the B coefficients are determined by regressing experimental data. Many of the formulations in REFPROP are of this type, for example in refs (65, 69, 81, 112, 166, 194, 198, 200, 206, and 231).
The last term in eq 7, Δλc, is used to represent the behavior in the critical region. Unlike viscosity, this term affects a much larger region around the critical point and should be considered in a thermal conductivity correlation. Figure 6 shows the significance of the contribution of the critical enhancement term to the thermal conductivity of water.251,289 The critical temperature of water is 647.096 K, the critical density is 322.0 kg m–3, and the region where the enhancement is 5% or larger is shown in Figure 6 to extend from densities of 100 to almost 600 kg m–3 at temperatures extending to almost 780 K.
Figure 6.
Contours in the temperature–density plane for water where the contribution from the critical enhancement Δλc to the total thermal conductivity of water λ equals 5%, 1%, 0.5%, and 0.1%.251
To calculate the critical enhancement term, the theoretically based, simplified crossover model of Olchowy and Sengers293 is used in the models in REFPROP. In addition to the temperature and density, it requires the viscosity, the isobaric and isochoric heat capacity, and the partial derivative (∂ρ/∂ρ)T. The viscosity is obtained from a separate model, and the other quantities are calculated from the EOS. The Olchowy-Sengers model also requires three fluid-specific parameters. When data are not available for the evaluation of the fluid-specific parameters, the generalized model of Perkins et al.294 is used.
It is once again useful to see an uncertainty diagram for a reference correlation for thermal conductivity. An uncertainty plot for the reference correlation for the thermal conductivity of water251,289 is shown in Figure 7, where the uncertainty shown is the combined expanded uncertainty with a coverage factor of 2.
Figure 7.
Uncertainty in thermal conductivity, Δλ/λ, for the reference correlation for water from IAPWS R15-11.251
3.3. Surface Tension
Most of the correlations for surface tension, σ, incorporated into REFPROP have the general form
| 9 |
where si and ni are coefficients obtained from fitting experimental data, and k usually is between 1 and 3. The surface tension approaches zero at the critical point, and this form correctly reproduces that behavior. As shown in Table 1, the majority of the correlations are from the group of Mulero.50,75,104 A more recent paper from the Mulero group295 revisited many of the fluids in REFPROP and gives detailed information on the deviations from the data used in the development of the correlations. The uncertainty of the correlation is dependent on the quality of the data used in its development; we use water again as an example of a very well-studied fluid. The uncertainty in the surface tension of the IAPWS recommended formulation for water250 varies from 0.38 mN m–1 at 0.01 °C to 0.10 mN m–1 at 370 °C.
3.4. Dielectric Constant
As indicated in Table 1, for a limited number of fluids that are mostly of interest to the natural gas community, REFPROP provides the dielectric constant. We refer the reader to the original publications, refs59, 137, and 253, for details.
4. Mixture Models for Thermodynamic Properties
Just as there are many different EOS and approaches to modeling the thermodynamic properties of a pure fluid, there also are many different approaches to modeling the thermodynamic properties of a mixture. A general discussion of methods can be found in ref (296). Here we will give a very condensed discussion that focuses on the models incorporated in REFPROP.
Among the various ways to model mixtures, one method is to apply mixing and combining rules to the parameters of an EOS. For a very simple cubic EOS such as the Peng-Robinson EOS39 (available as a calculation option in REFPROP and used internally to provide starting guesses for some calculations)
| 10 |
there is a mixing rule for the parameters a and b, and combining rules for the cross term aij,
| 11 |
| 12 |
where the δij is empirically determined binary interaction parameters for each binary pair. This approach can also be extended to the MBWR EOS, but it can be very complex due to the large number of coefficients involved.
Instead of applying mixing rules to the individual parameters of an EOS, one can apply mixing rules to a property. Lemmon297 and Tillner-Roth298 each independently developed mixture models based on modeling the reduced molar Helmholtz energy of the mixture,
| 13 |
where the first summation is the contribution from the EOS of each of the constituent pure fluids, the x ln x term accounts for the entropy of mixing, and the second summation is often called the departure function. The Fpq is generalizing parameters that relate the behavior of one binary pair with that of another. The αpqexcess is empirical functions that are fit to binary mixture data. The terms αj and αpq are not evaluated at the temperature and density of the mixture, Tmix and ρmix, but rather at a scaled or reduced temperature and density τ = Tred/Tmix and δ = ρmix/ρred. It should be noted that αpqexcess is not the same as the excess Helmholtz energy. Mixing rules are used to determine the reducing values Tred and ρred. For example, one set that was used for mixtures containing R-32, R-125, R-134a, R-143a, and R-152a is299
| 14 |
The parameters ζij and ξij are used to define the shapes of the reducing temperature and density curves. These reducing parameters are not the same as the critical parameters of the mixture and may be found by fitting experimental data or from a predictive model.300 Other models of this type were formulated for binary mixtures of ammonia and water,301 dry air,302 and hydrocarbons.303 Other developments, such as having a temperature-dependent departure function, have been applied to model mixtures of helium-4, neon, and argon.304 Very recently, improved Helmholtz mixture models have been developed for environmentally friendly refrigerant mixtures containing R-134a, R-152a, R-227ea, R-1234yf, and R-1234ze(E).305
In particular, the Helmholtz mixing model306,307 has been used as an international standard for the properties of natural gas. The original GERG (Groupe Européen de Recherches Gazières or European Gas Research Group) 2004307 model is a multifluid Helmholtz model for calculation of the thermal and caloric properties of natural gases and has 18 components: methane, nitrogen, CO2, ethane, propane, n-butane, isobutane, n-pentane, isopentane, n-hexane, n-heptane, n-octane, H2, O2, carbon monoxide, H2O, helium, and argon. An update, known as GERG 2008,306 added n-nonane, n-decane, and H2S, and is the basis for an ISO Standard (ISO 20765-2/3),308 a method to calculate the volumetric and caloric properties of natural gases, manufactured fuel gases, and similar mixtures, at conditions at which the mixture may be in either the homogeneous (single-phase) gas state, the homogeneous liquid state, or the homogeneous supercritical (dense-fluid) state. This formulation represents the most accurate experimental mixture data to the level of experimental uncertainty. For example, the uncertainties in gas-phase density and speed of sound for a broad variety of natural gases and related mixtures are 0.1% over the temperature range from 250 to 450 K at pressures up to 35 MPa, while in the liquid phase the uncertainty for density is generally in the range of 0.2% to 0.5%.306 Research continues on improving this model, such as recent improvements in the representation of the binary mixtures H2/CH4, H2/N2, H2/CO2, and H2/CO.309
The development of these very accurate models depends upon the availability of binary interaction parameters for the various mixture pairs. (The properties of ternary and higher-order mixtures are calculated in terms of the constituent binary pairs.) Bell and Lemmon310 published an automatic evolutionary optimization algorithm that was used in conjunction with a large database of experimental vapor–liquid equilibrium data to obtain binary interaction parameters for more than 1100 mixtures. Many of these have been implemented into REFPROP v10.0. When binary interaction parameters have not been obtained by fitting experimental data, methods can be used to predict them. One predictive method developed by Lemmon and McLinden300 is available that works reasonably well for refrigerant mixtures. It is formulated in terms of properties such as the dipole moment, acentric factor, and critical temperature and pressure for each refrigerant pair. Another predictive method, applicable only to mixtures of nitrogen with selected halogenated refrigerants, incorporates the normal boiling point, the critical pressure, and the number of fluorine atoms in the molecule.311 REFPROP also estimates interaction parameters based on chemically similar pairs, for example the mixture of R-32/1234ze(Z) (which has no experimental data available) is treated the same as the mixture R-32/1234ze(E) (for which binary interaction parameters have been fitted to data). Research is currently underway to develop additional predictive models for the binary interaction parameters in Helmholtz mixture models in REFPROP.
Finally, there is an additional way of modeling mixtures in REFPROP. A mixture at a fixed composition can be treated as a pseudopure fluid. In REFPROP, this has been done for the refrigerant mixtures312 R-404A, R-410A, R-507A, and R-407C and also CO2-free dry air.302 The advantage of using this type of model is computational speed, especially for saturation properties, but this approach cannot account for variations in properties as a function of composition and may not be used for two-phase inputs inside the vapor–liquid-equilibrium region.
5. Mixture Models for Transport and Miscellaneous Properties
In this section we discuss the mixture models implemented in REFPROP for viscosity, thermal conductivity, surface tension, and the dielectric constant. A more general treatment can be found in ref (296).
5.1. Viscosity of Mixtures
Currently there is a single viscosity mixture model implemented in REFPROP. The viscosity of a mixture is treated as it was for a pure fluid: as a sum of a dilute-gas contribution and a residual contribution. The critical contribution is set to zero, and all terms are now functions of composition,
| 15 |
The dilute-gas contribution is found from kinetic theory313−315 assuming a Lennard-Jones potential applies with collision integrals as given by Neufeld et al.;316 however, the sinusoidal term is omitted. The dilute-gas contribution for a mixture in REFPROP is always calculated with the Lennard-Jones potential; however, some of the pure-fluid correlations in REFPROP use different formulations. A scaling factor is used in REFPROP so that in the limit of the pure fluid, the recommended pure fluid formulation is exactly reproduced.
The residual contribution is modeled using a one-fluid extending corresponding states (ECS) approach,185,213,262
| 16 |
The mixture is represented by a hypothetical pure fluid, the properties of which are found by evaluating a single (“one-fluid”) reference fluid (denoted by subscript ref) that is evaluated not at the mixture T and ρ but rather at a conformal temperature and molar density Tref and ρref. In REFPROP v10.0, nitrogen is used as the reference fluid in all mixture calculations. The conformal temperature and density are found using the relationships Tref = T/fx and ρref = ρhx and the quantities fx and hx are determined through the use of mixing and combining rules that involve the individual pure fluid fi and hi. Binary interaction parameters may also be used if data are available for a specific binary pair. The pure fluid fi and hi are called equivalent substance reducing ratios and relate the reference fluid (which can be different for each pure fluid and are not necessarily the same as the reference fluid for the mixture) to the fluid of interest using a ratio of critical parameters and functions of temperature and density called “shape factors”. There are various methods that can be used to determine the shape factors,185,258,260 and in REFPROP the “exact shape factor method” is used.258 This is an iterative procedure that can at times be complicated; the equations to be solved are described in ref (185) and care is needed to avoid obtaining incorrect roots in regions where there are multiple roots. The factor Fη in eq 16 is a function of the quantities fx and hx and an additional factor gx that involves the fi and hi functions and also the molar masses Mi of the fluids in the mixture as described in refs (185, 213, and 262). The ECS model as implemented in REFPROP is adequate for representing the viscosity of many refrigerant and hydrocarbon mixtures, typically representing viscosity of refrigerant mixtures to within about 4%185 and to within 5% to 10% for some other binary pairs.262 However, not all mixtures can currently be represented with this model. For example, it presently cannot be used for mixtures with associating fluids such as water or alcohols. The ECS model also has large deviations if the constituent fluids have very large size differences, as pointed out recently by Thol and Richter.317
5.2. Thermal Conductivity of Mixtures
The model implemented in REFPROP for mixture thermal conductivity is also an ECS model. The treatment is similar to that of viscosity; however, the thermal conductivity is first divided15,184,213,262 into contributions from internal motions of the molecule, λint, (which are only functions of temperature) and translational contributions, λtrans, which are due to collisions between molecules and are a function of both temperature and density, along with an additional term for the critical enhancement,
| 17 |
The translational contribution is further divided into a dilute-gas contribution, λ*, and a residual contribution λr,
| 18 |
The internal contribution and translational dilute-gas contributions for the mixture are found using the empirical mixing rule184
| 19 |
with
| 20 |
The pure-fluid dilute gas-viscosity for fluid j, η0,j is computed from the recommended pure fluid model implemented in REFPROP, and the kij,λ are optional binary interaction parameters for thermal conductivity of a given binary ij pair. As in the case for viscosity, extended corresponding states are applied to the residual contribution,
| 21 |
where the thermal conductivity of the reference fluid for the mixture, again denoted by subscript ref, is evaluated at the conformal temperature and density Tref and ρref described in the mixture viscosity discussion. Similarly, Fλ is a function of the quantities fi, hi, and Mi.184,262
The remaining contribution in eq 17, due to the critical enhancement, is calculated by applying mixing rules to the parameters in the Olchowy-Sengers model293,294 as described in ref (262).
There are fewer data for mixture thermal conductivity than for mixture viscosity, but limited testing indicates that the ECS mixture model for thermal conductivity can represent the data (excluding the critical region) to within about 5–10%.262 The same limitations noted for the ECS model for viscosity, namely inability to model mixtures containing associating fluids and difficulties with asymmetric mixtures, also apply to the thermal conductivity mixture model. The mixture model also significantly underpredicts the thermal conductivity in the critical region,262 and additional research is necessary in this area.
5.3. Surface Tension of Mixtures
To compute the surface tension of a mixture, REFPROP implements a parachor-based model,318,319 where the parachor for pure fluid Ρi is defined as
| 22 |
with the surface tension σi obtained from the pure fluid correlation implemented in REFPROP, and the pure component saturated liquid ρL,i and saturated vapor molar densities ρV,i are obtained from the EOS for the pure fluid at the temperature of interest. We use m = 3.87, and if T ≥ 0.9Tc the parachor is calculated at T = 0.9Tc. Next, the liquid and vapor densities of the mixture at saturation are computed with the Helmholtz energy mixture model and the surface tension of the mixture is found using
| 23 |
with mixing and combining rules
| 24 |
| 25 |
where δij is an optional binary interaction parameter, and xi and yi are the molar compositions of the liquid and gas phases, respectively. This method works best if the fluids are chemically similar, although the use of binary interaction parameters can improve results. The uncertainties involved will be greater than those for the pure fluid constituents.
5.4. Dielectric Constant of Mixtures
As mentioned in section 3.4, REFPROP provides the dielectric constant of a number of fluids of interest to the natural gas community. Details on how to calculate the dielectric constant of a mixture of these fluids can be found in ref (59).
6. REFPROP Features
Using the models described above, REFPROP calculates a wide range of thermophysical properties, as outlined in Table 2. For definitions of some of the more obscure properties in Table 2, such as the supercompressibility factor Fpv, see ref (320). In addition to any of the pure fluids listed in Table 1, mixtures of up to 20 components may be constructed. However, not all mixtures are available. For example, REFPROP cannot provide calculations for some mixtures with associating compounds (e.g., water) for which an adequate model is not yet available. Users may create custom natural gas mixtures and perform calculations with international standard models including the GERG-2008306 model as well as the older AGA-8 model.321 There are also a wide variety of predefined mixtures including the zeotropic and azeotropic mixtures designated by ASHRAE Standard 34322 and the five pseudopure fluid mixtures discussed earlier.
Table 2. Properties Computed by REFPROP.
| thermodynamic properties | transport, misc. prop. | derivatives | mixture |
|---|---|---|---|
| Temperature, T | Thermal conductivity | Isothermal compressibility | Fugacity |
| Pressure, p | Viscosity | Volume expansivity | Fugacity coeff. |
| Density, ρ | Kinematic viscosity | Isentropic expansion coef. | Chemical potential |
| Volume, V | Thermal diffusivity | Isothermal expansion coef. | K value |
| Internal Energy, U | Prandtl Number | Adiabatic compressibility | Molar mass |
| Enthalpy, H | Surface tension | Adiabatic bulk modulus | B12 |
| Entropy, S | Dielectric constant | Isothermal throttling coeff. | Excess Volume |
| Isochoric Heat Capacity, Cv | Gross heating value (HV) | ∂p/∂ρ|T | Excess Helmholtz |
| Isobaric heat capacity, Cp | Gross HV, liquid | ∂2p/∂ρ2|T | Excess Gibbs |
| Ideal Gas Cp0 | Gross HV, volume basis | ∂p/∂T|ρ | Composition |
| The ratio Cp/Cv | Net Heating Value | ∂2p/∂T2|ρ | |
| Csat | Net HV, liquid | ∂2p/∂ρ∂T | |
| Speed of sound, w | Net HV, volume basis | ∂p/∂T|sat | |
| Compressibility Factor, Z | Heat of Formation | ∂H/∂Z|sat | |
| Joule-Thomson Coefficient | Acentric factor | ∂ρ/∂T|p | |
| Quality | Relative density (air) | ∂ρ/∂P|T | |
| Phase | Relative density (water) | ∂2ρ/∂T2|p | |
| 2nd virial coefficient, B | API gravity | ∂2ρ/∂p2|T | |
| 3rd virial coefficient, C | 2nd acoustic virial | ∂2ρ/∂p∂T | |
| 4th virial coefficient, D | 3rd acoustic virial | dB/dT | |
| Helmholtz energy | –1/T | ∂H/∂T|ρ | |
| Gibbs energy | Flow exergy | ∂H/∂T|p | |
| Heat of vaporization | Closed system exergy | ∂H/∂ρ|T | |
| Cv, 2-phase | Critical flow factor | ∂H/∂ρ|p | |
| Internal pressure | Throat mass flux | ∂H/∂p|T | |
| Repulsive pressure | Fpv | ∂H/∂p|ρ | |
| Attractive pressure | |||
| p–pideal |
A graphical user interface (GUI) provides access to the models and properties here mentioned. The interface provides a convenient means to calculate and display calculated properties. It accesses the core property subroutines via a dynamic link library. The program is controlled through the use of the following pull-down menus as seen across the top of Figure 8, which shows two representative tables:
File provides commands to save and print generated tables and plots. Individual items or entire sessions with multiple windows may be saved or recalled. The standard “print setup” and “exit” commands are also present.
The Edit menu provides copy and paste commands which allow selected data to be exchanged with other applications.
The Options menu provides commands for selecting the unit system, properties of interest, and the reference state. These options may be stored for recall at a later time. A user-defined set of preferences is loaded upon program startup.
The pure fluid or mixture of interest is specified with commands in the Substance menu. New mixtures can be specified and saved. The refrigerant mixtures having an ASHRAE R-400 or R-500-series designation in ASHRAE Standard 34322 are predefined. Dry air and five predefined natural gas mixtures are also available.
The Calculate menu initiates the calculations that generate a property table. Each property selected for display is shown in a separate column of the table. Two types of tables are provided. The first type provides properties at saturation or with a property (such as temperature or pressure) held constant with another selected property varying over a specified range. For mixtures, one can calculate the liquid and vapor at the same composition, or liquid at the bubble point with its coexisting vapor, or the vapor at the dew point with its coexisting liquid. The second type of table allows the user to select the independent variables. Values of the independent variables may then be entered with the keyboard, read from a file, or pasted from another application.
The Plot menu provides x–y plots of any variables appearing in a table. In addition, temperature–entropy, pressure–enthalpy, temperature–composition, and a large number of additional diagrams may be generated automatically, as outlined in Table 3. Controls are provided to modify the plot size, axis scaling, plot symbols, line type, legend, and other plot features. Figure 9 shows an example of an automatically generated pressure–enthalpy diagram.
Each table or plot appears in a separate window and can be accessed, resized, or retitled with commands in the Window menu. The number of windows is limited only by available memory.
The Help menu provides access to online documentation, while the Cautions menu takes the user directly to that section of the documentation.
Figure 8.
Screen shot of table output.
Table 3. Plotting Options in REFPROP.
| Temperature vs Entropy | Temperature vs Enthalpy | Temperature vs Density |
| Pressure vs Enthalpy | Pressure vs Density | Pressure vs Volume |
| Pressure vs Temperature | Compressibility Factor vs Pressure | Enthalpy vs Entropy |
| Isochoric Heat Capacity vs T | Isobaric Heat Capacity vs T | Sound Speed vs T |
| Exergy vs Enthalpy | Isothermal Compressibility vs T | Viscosity vs T |
| Thermal Conductivity vs T |
Figure 9.
Automatically generated pressure–enthalpy plot for ammonia.
A typical session would start by specifying the desired properties, units, etc. in the Options menu; the fluid or mixture of interest would be defined in the Substance menu; table(s) of properties would be generated in Calculate and/or plots would be generated in Plot.
While the property models are written with temperature and density as the independent variables, REFPROP supports a wide range of inputs to accommodate the variables that would be known in a particular situation. These are called “flash” calculations, and REFPROP supports 15 combinations of variables: the first property can be any of (T, p, ρ, or s) and the second can be (p, ρ, s, h, or u). These involve iterative calculations to solve for the corresponding temperature and density, which are then input to the core property routines to solve for any other desired quantities. Temperature and pressure are easily measured in a process, and this is the most common flash calculation. REFPROP employs the cubic Peng–Robinson EOS (which can be solved explicitly for ρ as a f(T, p)) to generate an initial guess for the iteration.
Instead of using the Windows-based GUI, many users prefer to access the core REFPROP routines directly, either from apps such as Excel or from their own custom programs. Such programmatic access to REFPROP is possible with wrappers available on all operating systems. The core of REFPROP is a set of FORTRAN routines compiled into a shared library (a DLL on Windows). To use REFPROP in other environments such as C++, Python, MATLAB, Excel, etc., wrappers are used which allow the user to call into the REFPROP shared library. Native wrappers of REFPROP are available on github323 for the most popular programming languages. A modern CMake build system is available324 to assist with the compilation of REFPROP on non-Windows platforms.
Further user support is provided via several Github and other websites maintained by NIST.325,326 These provide a mechanism for users to report bugs and share tips and instructions for using REFPROP on different platforms. A number of mini-tutorials and additional documentation327 on thermodynamic topics are also included.
7. Future Directions
REFPROP has changed significantly since its first release in 1989 and will continue to evolve to address the changing needs of industry. There will be improvements to the models, the code, the interface, and additions to the fluid component library as described below.
7.1. Models
Equations of state will continue to evolve as we improve our understanding of the “correct” form for an EOS. New EOS may increasingly incorporate the results of molecular simulations in their development, but this would be largely hidden from the end user. While new or modified terms may appear in future EOS, we anticipate that the Helmholtz energy form will continue to be the basis for the vast majority of new and revised EOS in the foreseeable future. Work also is underway on improving the regression algorithms with additional constraints to enable future development with fewer data.
On the transport property side, entropy scaling approaches represent a new way of understanding the link between transport and thermodynamic properties. A semirigorous theory forms the basis of the approach. Applying this technique to viscosity has shown promise,328 so long as the equation of state yields the correct values for the residual entropy and so long as entropy scaling should apply for the given fluid(s). Entropy scaling has shown results competitive with ECS for refrigerant mixtures,329,330 and the model can be concisely implemented. Furthermore, the entropy scaling approaches avoid the computational speed penalty of ECS introduced by the exact conformal state solver.331 The theoretical understanding of entropy scaling approaches continues to develop, and these entropy-scaling-based models will start making their way into REFPROP. The friction-theory approach for viscosity286 and thermal conductivity332 may also see increasing application. A lack of mixture data for both viscosity and thermal conductivity has hindered model development, and the availability of high-quality data in the future can aid in model development. Simulation data can aid in this effort, but there will continue to be a need for experimental data to validate and test new models.
7.2. Code and Interface
The world is moving toward web-based technologies, and REFPROP is too. A web-based interface of REFPROP is currently under development. It includes tabular outputs in a form very similar to the desktop application. All calculations are carried out on the REFPROP server, allowing users access to a GUI without installing anything on their own computer. This will avoid problems with increasingly strict computer security policies restricting the installation of third-party software. This will be an additional way to access REFPROP; it will not replace the current forms.
While the computational efficiency of REFPROP is sufficient for many use cases, its architecture (heavy use of global state and blocking file reads) is not optimal for our multithreading world in which computer processor clock speeds are stagnating, but parallelism is continuing to increase. Therefore, an effort is underway to rewrite the entirety of REFPROP with a modern C++-based architecture. The motivations for this campaign, which is part of a multiyear effort, are
to improve the modularity of models and algorithms so that the code of REFPROP is thread-safe by design
to better leverage open-source libraries (e.g., Eigen333 for matrix math, nlohmann::json334 for working with data encoded in strings, algorithms from the C++ standard library and boost, ChebTools335 for Chebyshev expansions, etc.)
to allow for other thermodynamic models. The focus of REFPROP is and will remain the highest accuracy models possible, but other models are needed where data availability is very poor. It is also desirable to easily prototype different models or EOS terms.
to enable the use of new algorithms, such as for density rootfinding,336 for tracing phase boundaries for mixtures,337,338 for tracing critical loci,339 and for obtaining vapor–liquid–liquid equilibria340
to speed up pure fluid saturation properties by a factor of at least a few hundred through the use of superancillary equations341
to migrate toward self-documenting interoperable data files (fluid files, mixture files, etc.) that are stored in an international standard file format (here, JSON)
Some of these techniques have already been incorporated in open-source software,342 and lessons learned from that will inform decisions for future versions of REFPROP. For example, hardcoding transport property models (as was done in versions of REFPROP prior to v10.0) does not make for a flexible piece of software. In REFPROP 10.0, a reverse-Polish-notation means of defining transport property models was added. Although this approach gives a tremendous amount of flexibility and hardcoded transport property models are no longer needed, it is not a particularly straightforward way of defining mathematical expressions. In the future, REPROP will incorporate a more comprehensible math parsing approach that should be accessible to a broader set of model developers.
The core of the new REFPROP will be built around the open-source teqp library34 for EOS evaluation. The teqp library does not use analytical derivatives of the Helmholtz energy of the EOS—rather automatic differentiation is used to calculate the derivatives. This results in a tremendous increase in flexibility such that implementing a new thermodynamic model goes from taking weeks (or years!) to hours.
As much as possible, the future versions of REFPROP will strive to maintain backward compatibility. There will be necessary changes to the interface to allow for multithreading and the adoption of a completely encapsulated internal structure. A key design constraint is that all models in the library will be immutable; once a model is initialized, it may not be changed. All options (equation of state, reference states, etc.) must be selected at model initialization. Aside from the thermodynamic and transport property calculations described above, there are plans to include other types of calculations, such as combustion-related quantities, in the future.
7.3. Component Library
As REFPROP has evolved over the years, additional classes of chemical compounds have been added. Version 1.0 included just 15 refrigerants (with only two-component mixtures supported), while the current version 10.0 includes multiple types of fluids comprising 147 pure fluids and mixtures with up to 20 components. The motivation for adding fluids arises from several sources. The most important is industrial importance based on user feedback. REFPROP often serves as the vehicle for delivering the research outputs of the Thermophysical Properties of Fluids Group of NIST, and the inclusion of many fluids traces to this source. The criteria for adding new fluids will continue to be guided by the “REF” of REFPROP—namely the provision of highly accurate reference data on well-characterized fluids.
8. Conclusions
The NIST REFPROP database is a powerful tool for calculating thermophysical properties of industrially important fluids, with a focus on incorporating the most accurate models available. We will continue to develop the program so that it may evolve to serve the needs of industry.
The authors declare no competing financial interest.
Notes
Contribution of the National Institute of Standards and Technology. Not subject to copyright in the United States.
References
- Lemmon E. W.; Bell I. H.; Huber M. L.; McLinden M. O.. NIST Standard Reference Database 23, NIST Reference Fluid Thermodynamic and Transport Properties, version 10.0; Standard Reference Data Program, National Institute of Standards and Technology: Gaithersburg, MD, 2018. https://www.nist.gov/srd/refprop (accessed 05/25/2022).
- McLinden M. O.; Huber M. L. (R)Evolution of Refrigerants. J. Chem. Eng. Data 2020, 65, 4176–4193. 10.1021/acs.jced.0c00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diky V.; Chirico R. D.; Frenkel M.; Bazyleva A.; Magee J. W.; Paulechka E.; Kazakov A.; Lemmon E. W.; Muzny C. D.; Smolyanitsky A. Y.; Townsend S.; Kroenlein K.. ThermoData Engine (TDE) Version 10.4, NIST Standard Database #103b; Standard Reference Data Program, National Institute of Standards and Technology: Gaithersburg, MD, 2019. https://www.nist.gov/mml/acmd/trc/thermodata-engine/srd-nist-tde-103b (accessed 05/25/2022).
- Commercial equipment, instruments, or materials are identified only in order to adequately specify certain procedures. In no case does such identification imply recommendation or endorsement by the National Institute of Standards and technology, nor does it imply that the products identified are necessarily the best available for the purpose.
- Ansys CFX Turbomachinery CFD Software Advanced Material Modeling. https://www.ansys.com/products/fluids/ansys-cfx#tab1-2 (accessed 04/13/2022).
- aspentech Support Center; Thermodynamics University Modules, article 0000059707. https://esupport.aspentech.com/S_Article?id=141651 (accessed 04/13/2022).
- Majumdar A. K.; LeClair A. C.; Moore R.; Schallhorn P. A.. Generalized Fluid System Simulation Program, version 6.0, NASA/TP-2016–218218; NASA, 2016.
- Martin J. J.; Hou Y.-C. Development of an equation of state for gases. AIChE J. 1955, 1, 142–151. 10.1002/aic.690010203. [DOI] [Google Scholar]
- Downing R. C.Fluorocarbon Refrigerants Handbook; Prentice-Hall: Englewood Cliffs, NJ, 1988. [Google Scholar]
- Morrison G.; McLinden M. O.. NBS Technical Note 1226, Application of a hard Sphere Equation of State to Refrigerants and Refrigerant Mixtures; National Bureau of Standards: Gaithersburg, MD, 1986. [Google Scholar]
- Desantis R.; Gironi F.; Marrelli L. Vapor-liquid-equilibrium from a hard-sphere equation of state. Ind. Eng. Chem. Fundam. 1976, 15, 183–189. 10.1021/i160059a006. [DOI] [Google Scholar]
- Leland T. W.; Chappelear P. S. The corresponding states principle. Ind. Eng. Chem. 1968, 60, 15–43. 10.1021/ie50703a005. [DOI] [Google Scholar]
- Fisher G. D.; Leland T. W. Corresponding states principle using shape factors. Ind. Eng. Chem. Fundam. 1970, 9, 537–544. 10.1021/i160036a003. [DOI] [Google Scholar]
- Ely J. F.; Hanley H. J. M. Prediction of transport properties. 1. Viscosity of fluids and mixtures. Ind. Eng. Chem. Fundam. 1981, 20, 323–332. 10.1021/i100004a004. [DOI] [Google Scholar]
- Ely J. F.; Hanley H. J. M. Prediction of transport properties. 2. Thermal conductivity of pure fluids and mixtures. Ind. Eng. Chem. Fundam. 1983, 22, 90–7. 10.1021/i100009a016. [DOI] [Google Scholar]
- Huber M. L.; Ely J. F. A Predictive Extended Corresponding States Model for Pure and Mixed Refrigerants Including an Equation of State for R134a. Int. J. Refrig. 1994, 17, 18–31. 10.1016/0140-7007(94)90083-3. [DOI] [Google Scholar]
- Teraishi R.; Kayukawa Y.; Akasaka R.; Saito K. Universal parameters of the extended corresponding states (ECS) model for hydrofluoroolefin refrigerants. Int. J. Refrig. 2021, 131, 33–40. 10.1016/j.ijrefrig.2021.08.013. [DOI] [Google Scholar]
- Defibaugh D. R.; Morrison G. Compressed Liquid Densities and Saturation Densities of Pentafluoroethane (R125). Fluid Phase Equilib. 1992, 80, 157–166. 10.1016/0378-3812(92)87064-T. [DOI] [Google Scholar]
- Jacobsen R. T.; Stewart R. B. Thermodynamic Properties of Nitrogen Including Liquid and Vapor Phases from 63 to 2000 K with Pressures to 10,000 bar. J. Phys. Chem. Ref. Data 1973, 2, 757–922. 10.1063/1.3253132. [DOI] [Google Scholar]
- Outcalt S. L.; McLinden M. O. Equations of state for the thermodynamic properties of R32 (difluoromethane) and R125 (pentafluoroethane). Int.J. Thermophys. 1995, 16, 79–89. 10.1007/BF01438959. [DOI] [Google Scholar]
- Takagi T. Ultrasonic Speeds in Compressed Liquid and Vapor Pressures for Pentafluoroethane. J. Chem. Eng. Data 1996, 41, 1325–1328. 10.1021/je960127x. [DOI] [Google Scholar]
- Lemmon E. W.; Jacobsen R. T. A New Functional Form and New Fitting Techniques for Equations of State with Application to Pentafluoroethane (HFC-125). J. Phys. Chem. Ref. Data 2005, 34, 69–108. 10.1063/1.1797813. [DOI] [Google Scholar]
- Younglove B. A.; McLinden M. O. An International Standard Equation of State for the Thermodynamic Properties of Refrigerant 123 (2,2-Dichloro-1,1,1-Trifluoroethane). J. Phys. Chem. Ref. Data 1994, 23, 731–779. 10.1063/1.555950. [DOI] [Google Scholar]
- Span R.Multiparameter Equations of State; Springer: Berlin, Germany, 2000. [Google Scholar]
- Smith J. M.; Van Ness H. C.; Abbott M. M.. Introduction to Chemical Engineering Thermodynamics, 7th ed.; McGraw-Hill: New York, NY, 2005. [Google Scholar]
- Schmidt R.; Wagner W. A new form of the equation of state for pure substances and its application to oxygen. Fluid Phase Equilib. 1985, 19, 175–200. 10.1016/0378-3812(85)87016-3. [DOI] [Google Scholar]
- Jacobsen R. T.; Stewart R. B.; Jahangiri M. Thermodynamic properties of nitrogen from the freezing line to 2000 K at pressures to 1000 MPa. J. Phys. Chem. Ref. Data 1986, 15, 735–909. 10.1063/1.555754. [DOI] [Google Scholar]
- Jacobsen R. T.; Penoncello S. G.; Lemmon E. W.; Span R.. Multiparameter equations of state. In Equations of State for Fluids and Fluid Mixtures; Sengers J. V., Kayser R. F., Peters C. J., White H. J. Jr., Eds.; Elsevier: Amsterdam, 2000; Chapter 18, p 2000. [Google Scholar]
- Lemmon E. W.; McLinden M. O.; Wagner W. Thermodynamic Properties of Propane. III. A Reference Equation of State for Temperatures from the Melting Line to 650 K and Pressures up to 1000 MPa. J. Chem. Eng. Data 2009, 54, 3141–3180. 10.1021/je900217v. [DOI] [Google Scholar]
- Setzmann U.; Wagner W. A New Equation of State and Tables of Thermodynamic Properties for Methane Covering the Range from the Melting Line to 625 K at Pressures up to 100 MPa. J. Phys. Chem. Ref. Data 1991, 20, 1061–1155. 10.1063/1.555898. [DOI] [Google Scholar]
- Wagner W.; Pruß A. The IAPWS formulation 1995 for the thermodynamic properties of ordinary water substance for general and scientific use. J. Phys. Chem. Ref. Data 2002, 31, 387–535. 10.1063/1.1461829. [DOI] [Google Scholar]
- Span R.; Wagner W. On the extrapolation behavior of empirical equations of state. Int. J. Thermophys. 1997, 18, 1415–1443. 10.1007/BF02575343. [DOI] [Google Scholar]
- Rasmussen E.; Yellapantula S.; Martin M. J. How equation of state selection impacts accuracy near the critical point: Forced convection supercritical CO2 flow over a cylinder. J. Supercrit. Fluids 2021, 171, 105141. 10.1016/j.supflu.2020.105141. [DOI] [Google Scholar]
- Bell I. H.; Deiters U. K.; Leal A. M. M. Implementing an Equation of State Without Derivatives: teqp. Ind. Eng. Chem. Res. 2022, 61, 6010. 10.1021/acs.iecr.2c00237. [DOI] [Google Scholar]
- International Association for the Properties of Water and Steam. IAPWS R6-95(2018), Revised Release on the IAPWS Formulation 1995 for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use. http://www.iapws.org/relguide/IAPWS-95.html (accessed 05/25/2022).
- Platzer B.; Polt A.; Maurer G.. Thermophysical Properties of Refrigerants; Springer-Verlag: Berlin, 1990. [Google Scholar]
- Bender E.Equations of state exactly representing the phase behavior of pure substances.Proc. 5th Symp. Thermophys. Prop. ASME, New York, 1970, 227–235.
- Bender E. Equations of state for ethylene and propylene. Cryogenics 1975, 15, 667–673. 10.1016/0011-2275(75)90100-9. [DOI] [Google Scholar]
- Peng D.-Y.; Robinson D. B. A new Two-Constant Equation of State. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. 10.1021/i160057a011. [DOI] [Google Scholar]
- Thol M.; Fenkl F.; Lemmon E. W. A Fundamental Equation of State for Chloroethene for Temperatures from the Triple Point to 430 K and Pressures to 100 MPa. Int. J. Thermophys. 2022, 43, No. 41. 10.1007/s10765-021-02961-3. [DOI] [Google Scholar]
- Eisenbach T.; Scholz C.; Span R.; Cristancho D. E.; Lemmon E. W.; Thol M. Speed-of-Sound Measurements and a Fundamental Equation of State for Propylene Glycol. J. Phys. Chem. Ref. Data 2021, 50, 023105. 10.1063/5.0050021. [DOI] [Google Scholar]
- Lemmon E. W.; Akasaka R. An International Standard Formulation for 2,3,3,3-Tetrafluoroprop-1-ene (R1234yf) Covering Temperatures from the Triple-point Temperature to 410 K and Pressures up to 100 MPa. Int. J. Thermophys. 2022, 43, 119. 10.1007/s10765-022-03015-y. [DOI] [Google Scholar]
- Akasaka R.; Lemmon E. W. An International Standard Formulation for trans-1-Chloro-3,3,3-trifluoroprop-1-ene [R1233zd(E)] Covering Temperatures from the Triple-point Temperature to 450 K and Pressures up to 100 MPa. J. Phys. Chem. Ref. Data 2022, 51, 023101. 10.1063/5.0083026. [DOI] [Google Scholar]
- Gao K. H.; Wu J. T.; Lemmon E. W.. Unpublished Helmholtz Equation of State for 1,3-butadiene, 2017.
- Huber M. L. Models for the Viscosity, Thermal Conductivity, and Surface Tension of Selected Pure Fluids as Implemented in REFPROP v10.0. NIST Interagency/Internal Report (NISTIR) 2018, No. 8209. 10.6028/NIST.IR.8209. [DOI] [Google Scholar]
- Gao K. H.; Wu J. T.; Lemmon E. W.. Unpublished Helmholtz Equation of State for 1-butyne, 2017.
- Gao K. H.; Wu J. T.; Lemmon E. W.. Unpublished Helmholtz Equation of State for 1-pentene, 2017.
- Gao K. H.; Wu J. T.; Lemmon E. W. Equations of State for the Thermodynamic Properties of Three Hexane Isomers: 3-Methylpentane, 2,2-Dimethylbutane, and 2,3-Dimethylbutane. J. Phys. Chem. Ref. Data 2021, 50, 033103. 10.1063/1.5093644. [DOI] [Google Scholar]
- Lemmon E. W.; Span R. Short fundamental equations of state for 20 industrial fluids. J. Chem. Eng. Data 2006, 51, 785–850. 10.1021/je050186n. [DOI] [Google Scholar]
- Mulero A.; Cachadiña I.; Parra M. I. Recommended Correlations for the Surface Tension of Common Fluids. J. Phys. Chem. Ref. Data 2012, 41, 043105. 10.1063/1.4768782. [DOI] [Google Scholar]
- Gao K. H.; Wu J. T.; Lemmon E. W.. Unpublished Helmholtz Equation of State for Acetylene, 2017.
- Gao K. H.; Wu J. T.; Bell I. H.; Harvey A. H.; Lemmon E. W.. Unpublished Helmholtz Equation of State for Ammonia, 2018.
- Monogenidou S. A.; Assael M. J.; Huber M. L. Reference Correlation for the Thermal Conductivity of Ammonia from the Triple-Point Temperature to 680 K and Pressures up to 80 MPa. J. Phys. Chem. Ref. Data 2018, 47, 023102. 10.1063/1.5036724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monogenidou S. A.; Assael M. J.; Huber M. L. Reference Correlation for the Viscosity of Ammonia from the Triple Point to 725 K and up to 50 MPa. J. Phys. Chem. Ref. Data 2018, 47, 043101. 10.1063/1.5053087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haar L.; Gallagher J. S. Thermodynamic properties of ammonia. J. Phys. Chem. Ref. Data 1978, 7, 635–792. 10.1063/1.555579. [DOI] [Google Scholar]
- Fray N.; Schmitt B. Sublimation of ices of astrophysical interest: A bibliographic review. Planet. Space Sci. 2009, 57, 2053–2080. 10.1016/j.pss.2009.09.011. [DOI] [Google Scholar]
- Tegeler C.; Span R.; Wagner W. A New Equation of State for Argon Covering the Fluid Region for Temperatures from the Melting Line to 700 K at Pressures up to 1000 MPa. J. Phys. Chem. Ref. Data 1999, 28, 779–850. 10.1063/1.556037. [DOI] [Google Scholar]
- Lemmon E. W.; Jacobsen R. T. Viscosity and Thermal Conductivity Equations for Nitrogen, Oxygen, Argon, and Air. Int. J. Thermophys. 2004, 25, 21–69. 10.1023/B:IJOT.0000022327.04529.f3. [DOI] [Google Scholar]
- Harvey A. H.; Lemmon E. W. Method for Estimating the Dielectric Constant of Natural Gas Mixtures. Int. J. Thermophys. 2005, 26, 31–46. 10.1007/s10765-005-2351-5. [DOI] [Google Scholar]
- Lemmon E. W.Unpublished sublimation curve for argon, 2002.
- Thol M.; Lemmon E. W.; Span R. Equation of State for Benzene for Temperatures from the Melting Line up to 725 K with Pressures up to 500 MPa. High Temp. - High Pressures 2012, 41, 81–97. [Google Scholar]
- Assael M. J.; Mihailidou E. K.; Huber M. L.; Perkins R. A. Reference Correlation of the Thermal Conductivity of Benzene from the Triple Point to 725 K and up to 500 MPa. J. Phys. Chem. Ref. Data 2012, 41, 043102. 10.1063/1.4755781. [DOI] [Google Scholar]
- Avgeri S.; Assael M. J.; Huber M. L.; Perkins R. A. Reference Correlation of the Viscosity of Benzene from the Triple Point to 675 K and up to 300 MPa. J. Phys. Chem. Ref. Data 2014, 43, 033103. 10.1063/1.4892935. [DOI] [Google Scholar]
- Bücker D.; Wagner W. Reference equations of state for the thermodynamic properties of fluid phase n-butane and isobutane. J. Phys. Chem. Ref. Data 2006, 35, 929–1019. 10.1063/1.1901687. [DOI] [Google Scholar]
- Perkins R. A.; Ramires M. L. V.; Nieto de Castro C. A.; Cusco L. Measurement and correlation of the thermal conductivity of butane from 135 to 600 K at pressures to 70 MPa. J. Chem. Eng. Data 2002, 47, 1263–1271. 10.1021/je0101202. [DOI] [Google Scholar]
- Herrmann S.; Vogel E. New Formulation for the Viscosity of n-Butane. J. Phys. Chem. Ref. Data 2018, 47, 013104. 10.1063/1.5020802. [DOI] [Google Scholar]
- Lemmon E. W.; Ihmels E. C. Thermodynamic Properties of the Butenes. Part II. Short Fundamental Equations of State. Fluid Phase Equilib. 2005, 228–229, 173–187. 10.1016/j.fluid.2004.09.004. [DOI] [Google Scholar]
- Span R.; Wagner W. A new equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa. J. Phys. Chem. Ref. Data 1996, 25, 1509–1596. 10.1063/1.555991. [DOI] [Google Scholar]
- Huber M. L.; Sykioti E. A.; Assael M. J.; Perkins R. A. Reference Correlation of the Thermal Conductivity of Carbon Dioxide from the Triple Point to 1100 K and up to 200 MPa. J. Phys. Chem. Ref. Data 2016, 45, 013102. 10.1063/1.4940892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laesecke A.; Muzny C. D. Reference Correlation for the Viscosity of Carbon Dioxide. J. Phys. Chem. Ref. Data 2017, 46, 013107. 10.1063/1.4977429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiros S. F.; Calado J. C. G.; Nunes da Ponte M. The melting curve of carbon monoxide. J. Chem. Thermodyn. 1982, 14, 1197–1198. 10.1016/0021-9614(82)90044-1. [DOI] [Google Scholar]
- Brown G. N.; Ziegler W. T. Vapor Pressure and Heats of Vaporization and Sublimation of Liquids and Solids of Interest in Cryogenics below 1-atm Pressure. Adv. Cryo. Eng. 1979, 25, 662–670. [Google Scholar]
- Thol M.; Herrig S.; Span R.; Lemmon E. W. A fundamental equation of state for the calculation of thermodynamic properties of chlorine. AIChE J. 2021, 67 (9), e17326 10.1002/aic.17326. [DOI] [Google Scholar]
- Thol M.; Alexandrov I. S.; Lemmon E. W.. Unpublished Helmholtz Equation of State for Chlorobenzene, 2018.
- Mulero A.; Cachadiña I. Recommended Correlations for the Surface Tension of Several Fluids Included in the REFPROP Program. J. Phys. Chem. Ref. Data 2014, 43, 023104. 10.1063/1.4878755. [DOI] [Google Scholar]
- Gao K. H.; Wu J. T.; Lemmon E. W.. Unpublished Helmholtz Equation of State for Cyclobutene, 2017.
- Zhou Y.; Liu J.; Penoncello S. G.; Lemmon E. W. An Equation of State for the Thermodynamic Properties of Cyclohexane. J. Phys. Chem. Ref. Data 2014, 43, 043105. 10.1063/1.4900538. [DOI] [Google Scholar]
- Koutian A.; Assael M. J.; Huber M. L.; Perkins R. A. Reference Correlation of the Thermal Conductivity of Cyclohexane from the Triple Point to 640 K and up to 175 MPa. J. Phys. Chem. Ref. Data 2017, 46, 013102. 10.1063/1.4974325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariq U.; Jusoh A. R. B.; Riesco N.; Vesovic V. Reference Correlation of the Viscosity of Cyclohexane from the Triple Point to 700 K and up to 110 MPa. J. Phys. Chem. Ref. Data 2014, 43, 033101. 10.1063/1.4891103. [DOI] [Google Scholar]
- Gedanitz H.; Davila M. J.; Lemmon E. W. Speed of Sound Measurements and a Fundamental Equation of State for Cyclopentane. J. Chem. Eng. Data 2015, 60, 1331–1337. 10.1021/je5010164. [DOI] [Google Scholar]
- Vassiliou C. M.; Assael M. J.; Huber M. L.; Perkins R. A. Reference Correlations of the Thermal Conductivity of Cyclopentane, iso-Pentane, and n-Pentane. J. Phys. Chem. Ref. Data 2015, 44, 033102. 10.1063/1.4927095. [DOI] [Google Scholar]
- Polt A.; Platzer B.; Maurer G. Parameter der Thermischen Zustandsgleichung von Bender fuer 14 Mehratomige Reine Stoffe. Chem. Technol. (Leipzig) 1992, 44, 216–224. [Google Scholar]
- Thol M.; Rutkai G.; Koster A.; Dubberke F. H.; Windmann T.; Span R.; Vrabec J. Thermodynamic Properties of Octamethylcyclotetrasiloxane. J. Chem. Eng. Data 2016, 61, 2580–2595. 10.1021/acs.jced.6b00261. [DOI] [Google Scholar]
- Thol M.; Javed M. A.; Baumhögger E.; Span R.; Vrabec J. Thermodynamic Properties of Dodecamethylpentasiloxane, Tetradecamethylhexasiloxane, and Decamethylcyclopentasiloxane. Ind. Eng. Chem. Res. 2019, 58, 9617–9635. 10.1021/acs.iecr.9b00608. [DOI] [Google Scholar]
- Colonna P.; Nannan N. R.; Guardone A. Multiparameter Equations of State for Siloxanes: [(CH3)3-Si-O1/2]2-[O-Si-(CH3)2]i = 1, . . .,3, and [O-Si-(CH3)2]6. Fluid Phase Equilib. 2008, 263, 115–130. 10.1016/j.fluid.2007.10.001. [DOI] [Google Scholar]
- Herrig S.; Thol M.; Kortmann M.; Lemmon E. W.; Span R.. Unpublished Helmholtz Equation of State for Diethanolamine, 2018.
- Huber M. L.; Perkins R. A. Thermal conductivity correlations for minor constituent fluids in natural gas: n-octane, n-nonane and n-decane. Fluid Phase Equilib. 2005, 227, 47–55. 10.1016/j.fluid.2004.10.031. [DOI] [Google Scholar]
- Huber M. L.; Laesecke A.; Xiang H. W. Viscosity correlations for minor constituent fluids in natural gas: n-octane, n-nonane and n-decane. Fluid Phase Equilib. 2004, 224, 263–270. 10.1016/j.fluid.2004.07.012. [DOI] [Google Scholar]
- Richardson I. A.; Leachman J. W.; Lemmon E. W. Fundamental Equation of State for Deuterium. J. Phys. Chem. Ref. Data 2014, 43, 013103. 10.1063/1.4864752. [DOI] [Google Scholar]
- Assael M. J.; Assael J.-A. M.; Huber M. L.; Perkins R. A.; Takata Y. Correlation of the Thermal Conductivity of Normal and Parahydrogen from the Triple Point to 1000 K and up to 100 MPa. J. Phys. Chem. Ref. Data 2011, 40, 033101. 10.1063/1.3606499. [DOI] [Google Scholar]
- Muzny C. D.; Huber M. L.; Kazakov A. F. Correlation for the Viscosity of Normal Hydrogen Obtained from Symbolic Regression. J. Chem. Eng. Data 2013, 58, 969–979. 10.1021/je301273j. [DOI] [Google Scholar]
- Thol M.; Rutkai G.; Koster A.; Miroshnichenko S.; Wagner W.; Vrabec J.; Span R. Equation of State for Dichloroethane Based on a Hybrid Data Set. Mol. Phys. 2017, 115, 1166–1185. 10.1080/00268976.2016.1262557. [DOI] [Google Scholar]
- Thol M.; Piazza L.; Span R. A New Functional Form for Equations of State for Some Weakly Associating Fluids. Int. J. Thermophys. 2014, 35, 783–811. 10.1007/s10765-014-1633-1. [DOI] [Google Scholar]
- Zhou Y.; Wu J. T.; Lemmon E. W. Thermodynamic Properties of Dimethyl Carbonate. J. Phys. Chem. Ref. Data 2011, 40, 043106. 10.1063/1.3664084. [DOI] [Google Scholar]
- Wu J. T.; Zhou Y.; Lemmon E. W. An Equation of State for the Thermodynamic Properties of Dimethyl Ether. J. Phys. Chem. Ref. Data 2011, 40, 023104. 10.1063/1.3582533. [DOI] [Google Scholar]
- Meng X. Y.; Zhang J. B.; Wu J. T.; Liu Z. G. Experimental Measurement and Modeling of the Viscosity of Dimethyl Ether. J. Chem. Eng. Data 2012, 57, 988–993. 10.1021/je201297j. [DOI] [Google Scholar]
- Romeo R.; Lemmon E. W.. Thermodynamic Properties of n-hexadecane and n-docosane. Int. J. Thermophys. 2022.
- Lemmon E. W.; Huber M. L. Thermodynamic properties of n-dodecane. Energy Fuels 2004, 18, 960–967. 10.1021/ef0341062. [DOI] [Google Scholar]
- Huber M. L.; Laesecke A.; Perkins R. Transport properties of n-dodecane. Energy Fuels 2004, 18, 968–975. 10.1021/ef034109e. [DOI] [Google Scholar]
- Bücker D.; Wagner W. A reference equation of state for the thermodynamic properties of ethane for temperatures from the melting line to 675 K and pressures up to 900 MPa. J. Phys. Chem. Ref. Data 2006, 35, 205–266. 10.1063/1.1859286. [DOI] [Google Scholar]
- Friend D. G.; Ingham H.; Ely J. F. Thermophysical Properties of Ethane. J. Phys. Chem. Ref. Data 1991, 20, 275–347. 10.1063/1.555881. [DOI] [Google Scholar]
- Vogel E.; Span R.; Herrmann S. Reference Correlation for the Viscosity of Ethane. J. Phys. Chem. Ref. Data 2015, 44, 043101. 10.1063/1.4930838. [DOI] [Google Scholar]
- Schroeder J. A.; Penoncello S. G.; Schroeder J. S. A Fundamental Equation of State for Ethanol. J. Phys. Chem. Ref. Data 2014, 43, 043102. 10.1063/1.4895394. [DOI] [Google Scholar]
- Mulero A.; Cachadiña I.; Sanjuán E. L. Surface Tension of Alcohols. Data Selection and Recommended Correlations. J. Phys. Chem. Ref. Data 2015, 44, 033104. 10.1063/1.4927858. [DOI] [Google Scholar]
- Assael M. J.; Sykioti E. A.; Huber M. L.; Perkins R. A. Reference Correlation of the Thermal Conductivity of Ethanol from the Triple Point to 600 K and up to 245 MPa. J. Phys. Chem. Ref. Data 2013, 42, 023102. 10.1063/1.4797368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselev S. B.; Ely J. F.; Abdulagatov I. M.; Huber M. L. Generalized SAFT-DFT/DMT model for the thermodynamic, interfacial, and transport properties of associating fluids: Application for n-alkanols. Ind. Eng. Chem. Res. 2005, 44, 6916–6927. 10.1021/ie050010e. [DOI] [Google Scholar]
- Zhou Y.; Lemmon E. W.. Unpublished Helmholtz Equation of State for ethylene glycol, 2018.
- Zhou Y.; Lemmon E. W.; Wu J. T. Thermodynamic Properties of o-Xylene, m-Xylene, p-Xylene, and Ethylbenzene. J. Phys. Chem. Ref. Data 2012, 41, 023103. 10.1063/1.3703506. [DOI] [Google Scholar]
- Mylona S. K.; Antoniadis K. D.; Assael M. J.; Huber M. L.; Perkins R. A. Reference Correlations of the Thermal Conductivity of o-Xylene, m-Xylene, p-Xylene, and Ethylbenzene from the Triple Point to 700 K and Moderate Pressures. J. Phys. Chem. Ref. Data 2014, 43, 043104. 10.1063/1.4901166. [DOI] [Google Scholar]
- Meng X. Y.; Cao F. L.; Wu J. T.; Vesovic V. Reference Correlation of the Viscosity of Ethylbenzene from Triple Point to 673 K and up to 110 MPa. J. Phys. Chem. Ref. Data 2017, 46, 013101. 10.1063/1.4973501. [DOI] [Google Scholar]
- Smukula J.; Span R.; Wagner W. A New Equation of State for Ethylene Covering the Fluid Region for Temperatures from the Melting Line to 450 K at Pressures up to 300 MPa. J. Phys. Chem. Ref. Data 2000, 29, 1052–1122. [Google Scholar]
- Assael M. J.; Koutian A.; Huber M. L.; Perkins R. A. Reference Correlations of the Thermal Conductivity of Ethene and Propene. J. Phys. Chem. Ref. Data 2016, 45, 033104. 10.1063/1.4958984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland P. M.; Eaton B. E.; Hanley H. J. M. A Correlation of the Viscosity and Thermal Conductivity Data of Gaseous and Liquid Ethylene. J. Phys. Chem. Ref. Data 1983, 12, 917–932. 10.1063/1.555701. [DOI] [Google Scholar]
- Jahangiri M.; Jacobsen R. T.; Stewart R. B.; McCarty R. D. Thermodynamic Properties of Ethylene from the Freezing Line to 450 K at Pressures to 260 MPa. J. Phys. Chem. Ref. Data 1986, 15, 593–734. 10.1063/1.555753. [DOI] [Google Scholar]
- Thol M.; Rutkai G.; Koster A.; Kortmann M.; Span R.; Vrabec J. Fundamental equation of state for ethylene oxide based on a hybrid dataset. Chem. Eng. Sci. 2015, 121, 87–99. 10.1016/j.ces.2014.07.051. [DOI] [Google Scholar]
- de Reuck K. M.International Thermodynamic Tables of the Fluid State-11 Fluorine; International Union of Pure and Applied Chemistry, Pergamon Press: Oxford, UK, 1990. [Google Scholar]
- Herrig S.; Thol M.; Harvey A. H.; Lemmon E. W. A Reference Equation of State for Heavy Water. J. Phys. Chem. Ref. Data 2018, 47, 043102. 10.1063/1.5053993. [DOI] [Google Scholar]
- International Association for the Properties of Water and Steam. IAPWS R5-85(1994), Release on Surface Tension of Heavy Water Substance. http://www.iapws.org/relguide/surfd2o.pdf (accessed 05/25/2022).
- International Association for the Properties of Water and Steam . Viscosity and Thermal Conductivity of Heavy Water Substance, Physical Chemistry of Aqueous Systems: Proceedings of the 12th International Conference on the Properties of Water and Steam, Orlando, Florida, September 11–16, 1994, pp A107–A138.
- Ortiz-Vega D. O.; Hall K. R.; Holste J. C.; Arp V. D.; Harvey A. H.; Lemmon E. W.. Unpublished Helmholtz Equation of State for Helium-4, 2015.
- Hands B. A.; Arp V. D. A Correlation of Thermal Conductivity Data for Helium. Cryogenics 1981, 21, 697–703. 10.1016/0011-2275(81)90211-3. [DOI] [Google Scholar]
- Arp V. D.; McCarty R. D.; Friend D. G., Thermophysical Properties of Helium-4 from 0.8 to 1500 K with Pressures to 2000 MPa; NIST Technical Note 1334 (revised); NIST, 1998.
- McCarty R. D.; Arp V. D. A New Wide Range Equation of State for Helium. Adv. Cryo. Eng. 1990, 35, 1465–1475. [Google Scholar]
- Tenji D.; Thol M.; Lemmon E. W.; Span R.. Unpublished Helmholtz Equation of State for n-heptane, 2018.
- Assael M. J.; Bogdanou I.; Mylona S. K.; Huber M. L.; Perkins R. A.; Vesovic V. Reference Correlation of the Thermal Conductivity of n-Heptane from the Triple Point to 600 K and up to 250 MPa. J. Phys. Chem. Ref. Data 2013, 42, 023101. 10.1063/1.4794091. [DOI] [Google Scholar]
- Michailidou E. K.; Assael M. J.; Huber M. L.; Abdulagatov I. M.; Perkins R. A. Reference Correlation of the Viscosity of n-Heptane from the Triple Point to 600 K and up to 248 MPa. J. Phys. Chem. Ref. Data 2014, 43, 023103. 10.1063/1.4875930. [DOI] [Google Scholar]
- Monogenidou S. A.; Assael M. J.; Huber M. L. Reference Correlations for Thermal Conductivity of n-Hexadecane from the Triple Point to 700 K and up to 50 MPa. J. Phys. Chem. Ref. Data 2018, 47, 013103. 10.1063/1.5021459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X. Y.; Sun Y. K.; Cao F. L.; Wu J. T.; Vesovic V. Reference Correlation of the Viscosity of n-Hexadecane from the Triple Point to 673 K and up to 425 MPa. J. Phys. Chem. Ref. Data 2018, 47, 033102. 10.1063/1.5039595. [DOI] [Google Scholar]
- Thol M.; Wang Y.; Lemmon E. W.; Span R.. Unpublished Helmholtz Equation of State for n-hexane, 2018.
- Assael M. J.; Mylona S. K.; Tsiglifisi C. A.; Huber M. L.; Perkins R. A. Reference Correlation of the Thermal Conductivity of n-Hexane from the Triple Point to 600 K and up to 500 MPa. J. Phys. Chem. Ref. Data 2013, 42, 013106. 10.1063/1.4793335. [DOI] [Google Scholar]
- Michailidou E. K.; Assael M. J.; Huber M. L.; Perkins R. A. Reference Correlation of the Viscosity of n-Hexane from the Triple Point to 600 K and up to 100 MPa. J. Phys. Chem. Ref. Data 2013, 42, 033104. 10.1063/1.4818980. [DOI] [Google Scholar]
- Leachman J. W.; Jacobsen R. T.; Penoncello S. G.; Lemmon E. W. Fundamental Equations of State for Parahydrogen, Normal Hydrogen, and Orthohydrogen. J. Phys. Chem. Ref. Data 2009, 38, 721–748. 10.1063/1.3160306. [DOI] [Google Scholar]
- Lemmon E. W.Unpublished melting curve for normal hydrogen, 2007.
- Lemmon E. W.Unpublished sublimation curve for normal hydrogen, 2003.
- Thol M.; Dubberke F. H.; Baumhögger E.; Span R.; Vrabec J. Speed of Sound Measurements and a Fundamental Equation of State for Hydrogen Chloride. J. Chem. Eng. Data 2018, 63, 2533–2547. 10.1021/acs.jced.7b01031. [DOI] [Google Scholar]
- Schmidt K. A. G.; Quiñones-Cisneros S. E.; Carroll J. J.; Kvamme B. Hydrogen Sulfide Viscosity Modeling. Energy Fuels 2008, 22, 3424–3434. 10.1021/ef700701h. [DOI] [Google Scholar]
- Harvey A. H.; Mountain R. D. Correlations for the Dielectric Constants of H2S, SO2, and SF6. Int. J. Thermophys. 2017, 38, 147. 10.1007/s10765-017-2279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins R. A. Measurement and correlation of the thermal conductivity of isobutane from 114 to 600 K at pressures to 70 MPa. J. Chem. Eng. Data 2002, 47, 1272–1279. 10.1021/je010121u. [DOI] [Google Scholar]
- Vogel E.; Kuchenmeister C.; Bich E. Viscosity correlation for isobutane over wide ranges of the fluid region. Int. J. Thermophys. 2000, 21, 343–356. 10.1023/A:1006623310780. [DOI] [Google Scholar]
- Blackham T. M.; Lemmon A. K.; Lemmon E. W.. Unpublished Helmholtz Equation of State for isooctane, 2018.
- Reeves L. E.; Scott G. J.; Babb S. E. J. Melting Curves of Pressure-Transmitting Fluids. J. Chem. Phys. 1964, 40, 3662–3666. 10.1063/1.1725068. [DOI] [Google Scholar]
- Michels A.; Prins C. The Melting Lines of Argon, Krypton and Xenon up to 1500 Atm; Representation of the Results by a Law of Corresponding States. Physica 1962, 28, 101–116. 10.1016/0031-8914(62)90096-4. [DOI] [Google Scholar]
- Lemmon E. W.Unpublished sublimation curve for krypton, 2002.
- Thol M.; Dubberke F. H.; Baumhogger E.; Vrabec J.; Span R. Speed of Sound Measurements and Fundamental Equations of State for Octamethyltrisiloxane and Decamethyltetrasiloxane. J. Chem. Eng. Data 2017, 62, 2633–2648. 10.1021/acs.jced.7b00092. [DOI] [Google Scholar]
- Herrig S.; Thol M.; Span R.. Unpublished Helmholtz Equation of State for monoethanolamine, 2018.
- Friend D. G.; Ely J. F.; Ingham H.. Tables for the Thermophysical Properties of Methane, NIST Technical Note 1325, NIST: Gaithersburg, MD, 1989.
- Quiñones-Cisneros S. E.; Huber M. L.; Deiters U. K.. Unpublished friction theory model for the viscosity of methane, 2011.
- Lemmon E. W.Unpublished sublimation curve for methane, 2002.
- de Reuck K. M.; Craven R. J. B.. Methanol, International Thermodynamic Tables of the Fluid State-12; IUPAC, Blackwell Scientific Publications: London, UK, 1993. [Google Scholar]
- Sykioti E. A.; Assael M. J.; Huber M. L.; Perkins R. A. Reference Correlation of the Thermal Conductivity of Methanol from the Triple Point to 660 K and up to 245 MPa. J. Phys. Chem. Ref. Data 2013, 42, 043101. 10.1063/1.4829449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykioti E. A.; Assael M. J.; Huber M. L.; Perkins R. A. Publisher’s Note: ″Reference Correlation of the Thermal Conductivity of methanol from the Triple Point to 660 K and up to 245 MPa″ (vol 42, 043101,2013). J. Phys. Chem. Ref. Data 2014, 43, 019901. 10.1063/1.4848698. [DOI] [Google Scholar]
- Xiang H. W.; Laesecke A.; Huber M. L. A new reference correlation for the viscosity of methanol. J. Phys. Chem. Ref. Data 2006, 35, 1597–1620. 10.1063/1.2360605. [DOI] [Google Scholar]
- Huber M. L.; Lemmon E. W.; Kazakov A.; Ott L. S.; Bruno T. J. Model for the Thermodynamic Properties of a Biodiesel Fuel. Energy Fuels 2009, 23, 3790–3797. 10.1021/ef900159g. [DOI] [Google Scholar]
- Perkins R. A.; Huber M. L. Measurement and Correlation of the Thermal Conductivities of Biodiesel Constituent Fluids: Methyl Oleate and Methyl Linoleate. Energy Fuels 2011, 25, 2383–2388. 10.1021/ef200417x. [DOI] [Google Scholar]
- Lemmon E. W.Unpublished Helmholtz Equation of State for Methylcyclohexane, 2007.
- Perkins R. A.; Hammerschmidt U.; Huber M. L. Measurement and correlation of the thermal conductivity of methylcyclohexane and propylcyclohexane from (300 to 600) K at pressures to 60 MPa. J. Chem. Eng. Data 2008, 53, 2120–2127. 10.1021/je800255r. [DOI] [Google Scholar]
- Thol M.; Dubberke F. H.; Rutkai G.; Windmann T.; Köster A.; Span R.; Vrabec J. Fundamental Equation of State Correlation for Hexamethyldisiloxane Based on Experimental and Molecular Simulation Data. Fluid Phase Equilib. 2016, 418, 133–151. 10.1016/j.fluid.2015.09.047. [DOI] [Google Scholar]
- Cao F. L.; Meng X. Y.; Wu J. T.; Vesovic V. Reference Correlation of the Viscosity of Meta-Xylene from 273 to 673 K and Up to 200 MPa. J. Phys. Chem. Ref. Data 2016, 45, 013103. 10.1063/1.4941241. [DOI] [Google Scholar]
- Thol M.; Beckmüller R.; Weiss R.; Harvey A. H.; Lemmon E. W.; Jacobsen R. T.; Span R.. Unpublished Helmholtz Equation of State for Neon, 2018.
- Span R.; Lemmon E. W.; Jacobsen R. T.; Wagner W.; Yokozeki A. A Reference Equation of State for the Thermodynamic Properties of Nitrogen for Temperatures from 63.151 to 1000 K and Pressures to 2200 MPa. J. Phys. Chem. Ref. Data 2000, 29, 1361–1433. 10.1063/1.1349047. [DOI] [Google Scholar]
- Lemmon E. W.Unpublished sublimation curve for nitrogen, 1999.
- Younglove B. A. Thermophysical Properties of Fluids. I. Argon, Ethylene, Parahydrogen, Nitrogen, Nitrogen Trifluoride, and Oxygen. J. Phys. Chem. Ref. Data 1982, 11 (Suppl.1), 1–11. [Google Scholar]
- Bell I. H.Unpublished sublimation curve for N2O, 2018.
- McLinden M. O.; Perkins R. A.; Lemmon E. W.; Fortin T. J. Thermodynamic Properties of 1,1,1,2,2,4,5,5,5-Nonafluoro-4-(trifluoromethyl)-3-pentanone: Vapor Pressure, (p, rho, T) Behavior, and Speed of Sound Measurements, and an Equation of State. J. Chem. Eng. Data 2015, 60, 3646–3659. 10.1021/acs.jced.5b00623. [DOI] [Google Scholar]
- Cui J. W.; Yan S. M.; Bi S. S.; Wu J. T. Saturated Liquid Dynamic Viscosity and Surface Tension of trans-1-Chloro-3,3,3-trifluoropropene and Dodecafluoro-2-methylpentan-3-one. J. Chem. Eng. Data 2018, 63, 751–756. 10.1021/acs.jced.7b00902. [DOI] [Google Scholar]
- Perkins R. A.; Huber M. L.; Assael M. J. Measurement and Correlation of the Thermal Conductivity of 1,1,1,2,2,4,5,5,5-Nonafluoro-4-(trifluoromethyl)-3-pentanone. J. Chem. Eng. Data 2018, 63, 2783–2789. 10.1021/acs.jced.8b00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C. Y.; Meng X. Y.; Huber M. L.; Wu J. T. Measurement and Correlation of the Viscosity of 1,1,1,2,2,4,5,5,5-Nonafluoro-4-(trifluoromethyl)-3-pentanone. J. Chem. Eng. Data 2017, 62, 3603–3609. 10.1021/acs.jced.7b00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmüller R.; Thol M.; Lemmon E. W.; Span R.. Unpublished Helmholtz Equation of State for n-octane, 2018.
- Lemmon E. W.Unpublished sublimation curve for oxygen, 2003.
- Cao F. L.; Meng X. Y.; Wu J. T.; Vesovic V. Reference Correlation of the Viscosity of ortho-Xylene from 273 to 673 K and up to 110 MPa. J. Phys. Chem. Ref. Data 2016, 45, 023102. 10.1063/1.4945663. [DOI] [Google Scholar]
- Thol M.; Uhde T.; Lemmon E. W.; Span R.. Unpublished Helmholtz Equation of State for n-pentane, 2018.
- Lemmon E. W.Unpublished melting curve for pentane, based on modification of J. Chem. Phys., 40(12):3662–6, 1964.; 2006.
- Gao K.; Köster A.; Thol M.; Wu J. T.; Lemmon E. W. Equations of State for the Thermodynamic Properties of n-Perfluorobutane, n-Perfluoropentane, and n-Perfluorohexane. Ind. Eng. Chem. Res. 2021, 60, 17207–17227. 10.1021/acs.iecr.1c02969. [DOI] [Google Scholar]
- Gao K. H.; Wu J. T.; Lemmon E. W.. Unpublished Helmholtz Equation of State for propadiene, 2017.
- Marsh K. N.; Perkins R. A.; Ramires M. L. V. Measurement and Correlation of the Thermal Conductivity of Propane. J. Chem. Eng. Data 2002, 47, 932–940. 10.1021/je010001m. [DOI] [Google Scholar]
- Vogel E.; Herrmann S. New Formulation for the Viscosity of Propane. J. Phys. Chem. Ref. Data 2016, 45, 043103. 10.1063/1.4966928. [DOI] [Google Scholar]
- Lemmon E. W.Unpublished melting curve for propane, based on modification of J. Chem. Phys., 40(12):3662–6, 1964.; 2004.
- Lemmon E. W.Unpublished Helmholtz Equation of State for Propylcyclohexane, 2007.
- Lemmon E. W.; McLinden M. O.; Overhoff U.; Wagner W.. Unpublished Helmholtz equation of state for propylene, 2018.
- Lemmon E. W.Unpublished melting curve for propylene, based on modification of J. Chem. Phys., 40(12):3662–6, 1964.; 2006.
- Gao K. H.; Wu J. T.; Lemmon E. W.. Unpublished Helmholtz Equation of State for Propylene oxide, 2017.
- Balogun B.; Riesco N.; Vesovic V. Reference Correlation of the Viscosity of para-Xylene from the Triple Point to 673 K and up to 110 MPa. J. Phys. Chem. Ref. Data 2015, 44, 013103. 10.1063/1.4908048. [DOI] [Google Scholar]
- Jacobsen R. T.; Penoncello S. G.; Lemmon E. W. A Fundamental Equation for Trichlorofluoromethane (R-11). Fluid Phase Equilib. 1992, 80, 45–56. 10.1016/0378-3812(92)87054-Q. [DOI] [Google Scholar]
- McLinden M. O.; Klein S. A.; Perkins R. A. An extended corresponding states model for the thermal conductivity of refrigerants and refrigerant mixtures. Int. J. Refrig. 2000, 23, 43–63. 10.1016/S0140-7007(99)00024-9. [DOI] [Google Scholar]
- Klein S. A.; McLinden M. O.; Laesecke A. An improved extended corresponding states method for the estimation of viscosity of pure refrigerants and mixtures. Int. J. Refrig. 1997, 20, 208–217. 10.1016/S0140-7007(96)00073-4. [DOI] [Google Scholar]
- Akasaka R.; Fukushima M.; Lemmon E. W., A Helmholtz Energy Equation of State for Trifluoroethylene (R-1123). International Refrigeration and Air Conditioning Conference, Purdue University, July 11–14, 2016.
- Marx V.; Pruß A.; Wagner W.. Neue Zustandsgleichungen fuer R 12, R 22, R 11 und R 113. Beschreibung des thermodynamishchen Zustandsverhaltens bei Temperaturen bis 525 K und Druecken bis 200 MPa; Series 19 (Waermetechnik/Kaeltetechnik); VDI Verlag: Duesseldorf, 1992; No. 57. [Google Scholar]
- Huber M. L.Unpublished fluid-specific correlations for the viscosity and thermal conductivity for R113, 2003.
- Lemmon E. W.; Span R. Thermodynamic Properties of R-227ea, R-365mfc, R-115, and R-13I1. J. Chem. Eng. Data 2015, 60, 3745–3758. 10.1021/acs.jced.5b00684. [DOI] [Google Scholar]
- Huber M. L.Unpublished ECS correlations for the viscosity and thermal conductivity of R115, 2003.
- Huber M. L.Unpublished fluid-specific correlations for the viscosity and thermal conductivity of R116, 2003.
- Zhou Y.; Lemmon E. W.. Unpublished Helmholtz Equation of State for R1216, 2016.
- Akasaka R.; Fukushima M.; Lemmon E. W., A Helmholtz Energy Equation of State for cis-1-chloro-2,3,3,3-Tetrafluoropropene (R-1224yd(Z)). European Conference on Thermophysical Properties, Graz, Austria, September 3–8, 2017.
- Laesecke A.; Perkins R. A.; Howley J. B. An improved correlation for the thermal conductivity of HCFC123 (2,2-dichloro-1,1,1-trifluoroethane). Int. J. Refrig. 1996, 19, 231–238. 10.1016/0140-7007(96)00019-9. [DOI] [Google Scholar]
- Tanaka Y.; Sotani T. Thermal conductivity and viscosity of 2,2-dichloro-1,1,1-trifluoroethane (HCFC-123). Int. J. Thermophys. 1996, 17, 293–328. 10.1007/BF01443394. [DOI] [Google Scholar]
- Mondejar M. E.; McLinden M. O.; Lemmon E. W. Thermodynamic Properties of trans-1-Chloro-3,3,3-trifluoropropene (R1233zd(E)): Vapor Pressure, (p, rho, T) Behavior, and Speed of Sound Measurements, and Equation of State. J. Chem. Eng. Data 2015, 60, 2477–2489. 10.1021/acs.jced.5b00348. [DOI] [Google Scholar]
- Kondou C.; Nagata R.; Nii N.; Koyama S.; Higashi Y. Surface tension of low GWP refrigerants R1243zf, R1234ze(Z), and R1233zd(E). Int. J. Refrig. 2015, 53, 80–89. 10.1016/j.ijrefrig.2015.01.005. [DOI] [Google Scholar]
- Perkins R. A.; Huber M. L.; Assael M. J. Measurement and Correlation of the Thermal Conductivity of trans-1-Chloro-3,3,3-trifluoropropene (R1233zd(E)). J. Chem. Eng. Data 2017, 62, 2659–2665. 10.1021/acs.jced.7b00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M.; McLinden M. O.; Lemmon E. W. Thermodynamic Properties of 2,3,3,3-Tetrafluoroprop-1-ene (R1234yf): Vapor Pressure and p-rho-T Measurements and an Equation of State. J. Chem. Eng. Data 2011, 56, 3254–3264. 10.1021/je200369m. [DOI] [Google Scholar]
- Perkins R. A.; Huber M. L. Measurement and Correlation of the Thermal Conductivity of 2,3,3,3-Tetrafluoroprop-1-ene (R1234yf) and trans-1,3,3,3-Tetrafluoropropene (R1234ze(E)). J. Chem. Eng. Data 2011, 56, 4868–4874. 10.1021/je200811n. [DOI] [Google Scholar]
- Huber M. L.; Assael M. J. Correlations for the viscosity of 2,3,3,3-tetrafluoroprop-1-ene (R1234yf) and trans-1,3,3,3-tetrafluoropropene (R1234ze(E)). Int. J. Refrig. 2016, 71, 39–45. 10.1016/j.ijrefrig.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thol M.; Lemmon E. W. Equation of State for the Thermodynamic Properties of trans-1,3,3,3-Tetrafluoropropene R-1234ze(E). Int. J. Thermophys. 2016, 37, 28. 10.1007/s10765-016-2040-6. [DOI] [Google Scholar]
- Akasaka R.; Lemmon E. W. Fundamental Equations of State for cis-1,3,3,3-Tetrafluoropropene R-1234ze(Z) and 3,3,3-Trifluoropropene (R-1243zf). J. Chem. Eng. Data 2019, 64, 4679–4691. 10.1021/acs.jced.9b00007. [DOI] [Google Scholar]
- de Vries B.; Tillner-Roth R.; Baehr H. D.. Thermodynamic Properties of HCFC 124, 19th International Congress of Refrigeration. International Institute of Refrigeration: The Hague, The Netherlands, IVa:582–589, 1995.
- Huber M. L.Unpublished ECS correlations for the viscosity and thermal conductivity of R124, 2003.
- Perkins R. A.; Huber M. L. Measurement and correlation of the thermal conductivity of pentafluoroethane (R125) from 190 to 512 K at pressures to 70 MPa. J. Chem. Eng. Data 2006, 51, 898–904. 10.1021/je050372t. [DOI] [Google Scholar]
- Huber M. L.; Laesecke A. Correlation for the viscosity of pentafluoroethane (R125) from the triple point to 500 K at pressures up to 60 MPa. Ind. Eng. Chem. Res. 2006, 45, 4447–4453. 10.1021/ie051367l. [DOI] [Google Scholar]
- Magee J. W.; Outcalt S. L.; Ely J. F. Molar heat capacity C-v, vapor pressure, and (p, rho, T) measurements from 92 to 350 K at pressures to 35 MPa and a new equation of state for chlorotrifluoromethane (R13). Int. J. Thermophys. 2000, 21, 1097–1121. 10.1023/A:1026446004383. [DOI] [Google Scholar]
- Huber M. L.Unpublished ECS correlations for the viscosity and thermal conductivity of R13, 2003.
- McLinden M. O.; Akasaka R. Thermodynamic Properties of cis-1,1,1,4,4,4-Hexafluorobutene [R-1336mzz(Z)]: Vapor Pressure, (p, ρ, T) Behavior, and Speed of Sound Measurements and Equation of State. J. Chem. Eng. Data 2020, 65, 4201–4214. 10.1021/acs.jced.9b01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillner-Roth R.; Baehr H. D. An International Standard Formulation of the Thermodynamic Properties of 1,1,1,2-Tetrafluoroethane (HFC-134a) for Temperatures from 170 to 455 K at Pressures up to 70 MPa. J. Phys. Chem. Ref. Data 1994, 23, 657–729. 10.1063/1.555958. [DOI] [Google Scholar]
- Perkins R. A.; Laesecke A.; Howley J. B.; Ramires M. L. V.; Gurova A. N.; Cusco L. Experimental Thermal Conductivity Values for the IUPAC Round-Robin Sample of 1,1,1,2-Tetrafluoroethane (R134a). NIST Interagency/Internal Report (NISTIR) 2000, No. 6605. [Google Scholar]
- Huber M. L.; Laesecke A.; Perkins R. A. Model for the Viscosity and Thermal Conductivity of Refrigerants, Including a New Correlation for the Viscosity of R134a. Ind. Eng. Chem. Res. 2003, 42, 3163–3178. 10.1021/ie0300880. [DOI] [Google Scholar]
- Huber M. L.Unpublished ECS correlations for the viscosity and thermal conductivity of R14, 2003.
- Huber M. L.Unpublished ECS correlations for the viscosity and thermal conductivity of R141b, 2003.
- Huber M. L.Unpublished ECS correlations for the viscosity and thermal conductivity of R142b, 2003.
- Lemmon E. W.; Jacobsen R. T. An international standard formulation for the thermodynamic properties of 1,1,1-trifluoroethane (HFC-143a) for temperatures from 161 to 450 K and pressures to 50 MPa. J. Phys. Chem. Ref. Data 2000, 29, 521–552. 10.1063/1.1318909. [DOI] [Google Scholar]
- Outcalt S. L.; McLinden M. O. A modified Benedict-Webb-Rubin equation of state for the thermodynamic properties of R152a (1,1-difluoroethane). J. Phys. Chem. Ref. Data 1996, 25, 605–636. 10.1063/1.555979. [DOI] [Google Scholar]
- Krauss R.; Weiss V. C.; Edison T. A.; Sengers J. V.; Stephan K. Transport properties of 1,1-difluoroethane (R152a). Int. J. Thermophys. 1996, 17, 731–757. 10.1007/BF01439187. [DOI] [Google Scholar]
- Qi H. Y.; Fang D.; Gao K. H.; Meng X. Y.; Wu J. T. Compressed Liquid Densities and Helmholtz Energy Equation of State for Fluoroethane (R161). Int. J. Thermophys. 2016, 37, 55. 10.1007/s10765-016-2061-1. [DOI] [Google Scholar]
- Tsolakidou C. M.; Assael M. J.; Huber M. L.; Perkins R. A. Correlations for the Viscosity and Thermal Conductivity of Ethyl Fluoride (R161). J. Phys. Chem. Ref. Data 2017, 46, 023103. 10.1063/1.4983027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M. L.Unpublished ECS correlations for viscosity and thermal conductivity of R21, 2003.
- Kamei A.; Beyerlein S. W.; Jacobsen R. T. Application of Nonlinear Regression in the Development of a Wide Range Formulation for HCFC-22. Int. J. Thermophys. 1995, 16, 1155–1164. 10.1007/BF02081283. [DOI] [Google Scholar]
- Huber M. L.Unpublished ECS correlations for the viscosity and thermal conductivity of R227ea, 2003.
- Penoncello S. G.; Lemmon E. W.; Jacobsen R. T.; Shan Z. J. A fundamental equation for trifluoromethane (R-23). J. Phys. Chem. Ref. Data 2003, 32, 1473–1499. 10.1063/1.1559671. [DOI] [Google Scholar]
- Shan Z.; Penoncello S. G.; Jacobsen R. T. A Generalized Model for Viscosity and Thermal Conductivity of Trifluoromethane (R-23). ASHRAE Trans. 2000, 106, 757–767. [Google Scholar]
- Rui X. F.; Pan J.; Wang Y. G. An equation of state for the thermodynamic properties of 1,1,1,2,3,3-hexafluoropropane (R236ea). Fluid Phase Equilib. 2013, 341, 75–85. 10.1016/j.fluid.2012.12.026. [DOI] [Google Scholar]
- Pan J.; Rui X. F.; Zhao X. D.; Qiu L. M. An equation of state for the thermodynamic properties of 1,1,1,3,3,3-hexafluoropropane (HFC-236fa). Fluid Phase Equilib. 2012, 321, 10–16. 10.1016/j.fluid.2012.02.012. [DOI] [Google Scholar]
- Zhou Y.; Lemmon E. W. Equation of State for the Thermodynamic Properties of 1,1,2,2,3-Pentafluoropropane (R-245ca). Int. J. Thermophys. 2016, 37, 27. 10.1007/s10765-016-2039-z. [DOI] [Google Scholar]
- Akasaka R.; Zhou Y.; Lemmon E. W. A Fundamental Equation of State for 1,1,1,3,3-Pentafluoropropane (R-245fa). J. Phys. Chem. Ref. Data 2015, 44, 013104. 10.1063/1.4913493. [DOI] [Google Scholar]
- Perkins R. A.; Huber M. L.; Assael M. J. Measurements of the Thermal Conductivity of 1,1,1,3,3-Pentafluoropropane (R245fa) and Correlations for the Viscosity and Thermal Conductivity Surfaces. J. Chem. Eng. Data 2016, 61, 3286–3294. 10.1021/acs.jced.6b00350. [DOI] [Google Scholar]
- Tillner-Roth R.; Yokozeki A. An international standard equation of state for difluoromethane (R-32) for temperatures from the triple point at 136.34 to 435 K and pressures up to 70 MPa. J. Phys. Chem. Ref. Data 1997, 26, 1273–1328. 10.1063/1.556002. [DOI] [Google Scholar]
- Huber M. L.Unpublished fluid-specific correlation for the thermal conductivity of R32, 2005.
- Huber M. L.Unpublished ECS correlation for the viscosity of R32, 2003.
- Akasaka R.; Kayukawa Y. A fundamental equation of state for trifluoromethyl methyl ether (HFE-143m) and its application to refrigeration cycle analysis. Int. J. Refrig. 2012, 35, 1003–1013. 10.1016/j.ijrefrig.2012.01.003. [DOI] [Google Scholar]
- Zhou Y.; Lemmon E. W.. Unpublished Helmholtz Equation of State for RE245cb2, 2016.
- Zhou Y.; Lemmon E. W.. Unpublished Helmholtz Equation of State for RE245fa2, 2016.
- Zhou Y.; Lemmon E. W.. Unpublished Helmholtz Equation of State for RE347mcc, 2016.
- Gao K. H.; Wu J. T.; Zhang P. G.; Lemmon E. W. A Helmholtz Energy Equation of State for Sulfur Dioxide. J. Chem. Eng. Data 2016, 61, 2859–2872. 10.1021/acs.jced.6b00195. [DOI] [Google Scholar]
- Guder C.; Wagner W. A Reference Equation of State for the Thermodynamic Properties of Sulfur Hexafluoride (SF6) for Temperatures from the Melting Line to 625 K and Pressures up to 150 MPa. J. Phys. Chem. Ref. Data 2009, 38, 33–94. 10.1063/1.3037344. [DOI] [Google Scholar]
- Assael M. J.; Koini I. A.; Antoniadis K. D.; Huber M. L.; Abdulagatov I. M.; Perkins R. A. Reference Correlation of the Thermal Conductivity of Sulfur Hexafluoride from the Triple Point to 1000 K and up to 150 MPa. J. Phys. Chem. Ref. Data 2012, 41, 023104. 10.1063/1.4708620. [DOI] [Google Scholar]
- Assael M. J.; Koini I. A.; Antoniadis K. D.; Huber M. L.; Abdulagatov I. M.; Perkins R. A. Erratum: Reference Correlation of the Thermal Conductivity of Sulfur Hexafluoride from the Triple Point to 1000 K and up to 150 MPa (vol 41, 023104, 2012). J. Phys. Chem. Ref. Data 2014, 43, 039901. 10.1063/1.4885454. [DOI] [Google Scholar]
- Quiñones-Cisneros S. E.; Huber M. L.; Deiters U. K. Correlation for the Viscosity of Sulfur Hexafluoride (SF6) from the Triple Point to 1000 K and Pressures to 50 MPa. J. Phys. Chem. Ref. Data 2012, 41, 023102. 10.1063/1.3702441. [DOI] [Google Scholar]
- Harvey A. H. On the Melting Curve of Sulfur Hexafluoride. J. Phys. Chem. Ref. Data 2017, 46, 043102. 10.1063/1.5005537. [DOI] [Google Scholar]
- Assael M. J.; Mylona S. K.; Huber M. L.; Perkins R. A. Reference Correlation of the Thermal Conductivity of Toluene from the Triple Point to 1000 K and up to 1000 MPa. J. Phys. Chem. Ref. Data 2012, 41, 023101. 10.1063/1.3700155. [DOI] [Google Scholar]
- Avgeri S.; Assael M. J.; Huber M. L.; Perkins R. A. Reference Correlation of the Viscosity of Toluene from the Triple Point to 675 K and up to 500 MPa. J. Phys. Chem. Ref. Data 2015, 44, 033101. 10.1063/1.4926955. [DOI] [Google Scholar]
- Aleksandrov I. S.; Gerasimov A. A.; Grigor’ev B. A. Using Fundamental Equations of State for Calculating the Thermodynamic Properties of Normal Undecane. Therm. Eng. 2011, 58, 691–698. 10.1134/S0040601511080027. [DOI] [Google Scholar]
- Assael M. J.; Papalas T. B.; Huber M. L. Reference Correlations for the Viscosity and Thermal Conductivity of n-Undecane. J. Phys. Chem. Ref. Data 2017, 46, 033103. 10.1063/1.4996885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thol M.; Span R.. Unpublished Helmholtz Equation of State for Vinyl Chloride, 2014.
- International Association for the Properties of Water and Steam Revised Release on Surface Tension of Ordinary Water Substance, IAPWS R1–76(2014) http://www.iapws.org/relguide/Surf-H2O.html (accessed 05/25/2022).
- International Association for the Properties of Water and Steam , IAPWS R15–11 (2011), Release on the IAPWS Formulation 2011 for the Thermal Conductvity of Ordinary Water Substance. http://www.iapws.org/relguide/ThCond.html (accessed 05/25/2022).
- International Association for the Properties of Water and Steam , IAPWS R12–08 (2008), Release on the IAPWS Formulation 2008 for the Viscosity of Ordinary Water Substance http://www.iapws.org/relguide/viscosity.html (accessed 05/25/2022).
- Fernández D. P.; Goodwin A. R. H.; Lemmon E. W.; Levelt Sengers J. M. H. A Formulation for the Static Permittivity of Water and Steam at Temperatures from 238 to 873 K at Pressures up to 1200 MPa, Including Derivatives and Debye-Hückel Coefficients. J. Phys. Chem. Ref. Data 1997, 26, 1125–1165. 10.1063/1.555997. [DOI] [Google Scholar]
- Wagner W.; Riethmann T.; Feistel R.; Harvey A. H. New Equations for the Sublimation Pressure and Melting Pressure of H2O Ice Ih. J. Phys. Chem. Ref. Data 2011, 40, 043103. 10.1063/1.3657937. [DOI] [Google Scholar]
- Lemmon E. W.Unpublished sublimation curve for xenon, 2003.
- Rosenfeld Y. Relation between transport-coefficients and internal entropy of simple systems. Phys. Rev. A 1977, 15, 2545–2549. 10.1103/PhysRevA.15.2545. [DOI] [Google Scholar]
- Rosenfeld Y. A quasi-universal scaling law for atomic transport in simple fluids. J. Phys.: Condens. Matter 1999, 11, 5415–5427. [Google Scholar]
- Ely J. F. In Application of the Extended Corresponding States Model to Hydrocarbon Mixtures. Proceedings of the 63rd Annual Gas Processors Association Meeting, 1984; pp 9–22.
- NIST Standard Reference Database 4, NIST Thermophysical Properties of Hydrocarbon Mixtures, version 3.2.1; Standard Reference Data Program, National Institute of Standards and Technology, Gaithersburg, MD, https://www.nist.gov/srd/nist-standard-reference-database-4, 2007. (accessed 05/25/2022).
- Huber M. L.; Ely J. F. Prediction of Viscosity of Refrigerants and Refrigerant Mixtures. Fluid Phase Equilib. 1992, 80, 239–248. 10.1016/0378-3812(92)87071-T. [DOI] [Google Scholar]
- Huber M. L.; Friend D. G.; Ely J. F. Prediction of the thermal conductivity of refrigerants and refrigerant mixtures. Fluid Phase Equilib. 1992, 80, 249–261. 10.1016/0378-3812(92)87072-U. [DOI] [Google Scholar]
- Chichester J. C.; Huber M. L. Documentation and Assessment of the Transport Property Models for Mixtures Implemented in NIST REFPROP (Version 8.0). NIST Interagency/Internal Report (NISTIR) 2008, No. 6650. [Google Scholar]
- Islam M. A.; Kariya K.; Ishida H.; Akasaka R.; Miyara A. Application of the extended corresponding states model for prediction of the viscosity and thermal conductivity of cis-1,3,3,3-tetrafluoropropene (R1234ze(Z)). Science and Technology for the Built Environment 2016, 22, 1167–1174. 10.1080/23744731.2016.1206797. [DOI] [Google Scholar]
- Huber M. L.; Perkins R. A.; Laesecke A.; Friend D. G.; Sengers J. V.; Assael M. J.; Metaxa I. N.; Vogel E.; Mareš R.; Miyagawa K. New International Formulation for the Viscosity of H2O. J. Phys. Chem. Ref. Data 2009, 38, 101–125. 10.1063/1.3088050. [DOI] [Google Scholar]
- Assael M. J.; Monogenidou S. A.; Huber M. L.; Perkins R. A.; Sengers J. V. New International Formulation for the Viscosity of Heavy Water. J. Phys. Chem. Ref. Data 2021, 50, 033102. 10.1063/5.0048711. [DOI] [Google Scholar]
- Chapman S.; Cowling T. G.. The Mathematical Theory of Nonuniform Gases; Cambridge University Press: Cambridge, London, UK, 1952. [Google Scholar]
- Chung T.-H.; Ajlan M.; Lee L. L.; Starling K. E. Generalized Multiparameter Correlation for Nonpolar and Polar Fluid Transport Properties. Ind. Eng. Chem. Res. 1988, 27, 671–679. 10.1021/ie00076a024. [DOI] [Google Scholar]
- Vogel E.; Kuechenmeister C.; Bich E.; Laesecke A. Reference Correlation for the Viscosity of Propane. J. Phys. Chem. Ref. Data 1998, 27, 947–970. 10.1063/1.556025. [DOI] [Google Scholar]
- Hellmann R. Ab initio potential energy surface for the nitrogen molecule pair and thermophysical properties of nitrogen gas. Mol. Phys. 2013, 111, 387–401. 10.1080/00268976.2012.726379. [DOI] [Google Scholar]
- Hellmann R. Ab initio potential energy surface for the carbon dioxide molecule pair and thermophysical properties of dilute carbon dioxide gas. Chem. Phys. Lett. 2014, 613, 133–138. 10.1016/j.cplett.2014.08.057. [DOI] [Google Scholar]
- Hellmann R. Intermolecular potential energy surface and thermophysical properties of propane. J. Chem. Phys. 2017, 146, 114304. 10.1063/1.4978412. [DOI] [PubMed] [Google Scholar]
- Hellmann R. Reference Values for the Second Virial Coefficient and Three Dilute Gas Transport Properties of Ethane from a State-of-the-Art Intermolecular Potential Energy Surface. J. Chem. Eng. Data 2018, 63, 470–481. 10.1021/acs.jced.7b01069. [DOI] [Google Scholar]
- Hellmann R.; Bich E. Transport properties of dilute D2O vapour from first principles. Mol. Phys. 2017, 115, 1057–1064. 10.1080/00268976.2016.1226443. [DOI] [Google Scholar]
- Hellmann R.; Bich E.; Vogel E.; Vesovic V. Thermophysical Properties of Dilute Hydrogen Sulfide Gas. J. Chem. Eng. Data 2012, 57, 1312–1317. 10.1021/je3000926. [DOI] [Google Scholar]
- Hellmann R.; Gaiser C.; Fellmuth B.; Vasyltsova T.; Bich E. Thermophysical properties of low-density neon gas from highly accurate first-principles calculations and dielectric-constant gas thermometry measurements. J. Chem. Phys. 2021, 154, 164304. 10.1063/5.0047999. [DOI] [PubMed] [Google Scholar]
- Hellmann R.; Jager B.; Bich E. State-of-the-art ab initio potential energy curve for the xenon atom pair and related spectroscopic and thermophysical properties. J. Chem. Phys. 2017, 147, 034304. 10.1063/1.4994267. [DOI] [PubMed] [Google Scholar]
- Hellmann R.; Vogel E. The Viscosity of Dilute Water Vapor Revisited: New Reference Values from Experiment and Theory for Temperatures between (250 and 2500) K. J. Chem. Eng. Data 2015, 60, 3600–3605. 10.1021/acs.jced.5b00599. [DOI] [Google Scholar]
- Jager B.; Hellmann R.; Bich E.; Vogel E. State-of-the-art ab initio potential energy curve for the krypton atom pair and thermophysical properties of dilute krypton gas. J. Chem. Phys. 2016, 144, 114304. 10.1063/1.4943959. [DOI] [PubMed] [Google Scholar]
- Friend D. G.; Rainwater J. C. Transport-properties of a moderately dense gas. Chem. Phys. Lett. 1984, 107, 590–594. 10.1016/S0009-2614(84)85163-5. [DOI] [Google Scholar]
- Rainwater J. C.; Friend D. G. Second viscosity and thermal-conductivity virial coefficients of gases: Extension to low reduced temperature. Phys. Rev. A 1987, 36, 4062–4066. 10.1103/PhysRevA.36.4062. [DOI] [PubMed] [Google Scholar]
- Herrmann S.; Hellmann R.; Vogel E. Update: Reference Correlation for the Viscosity of Ethane J. Phys. Chem. Ref. Data 44, 043101 (2015). J. Phys. Chem. Ref. Data 2018, 47, 023103. 10.1063/1.5037239. [DOI] [Google Scholar]
- Herrmann S.; Vogel E. New Formulation for the Viscosity of Isobutane. J. Phys. Chem. Ref. Data 2018, 47, 013104. 10.1063/1.5020802. [DOI] [Google Scholar]
- Vogel E.; Küchenmeister C.; Bich E. Viscosity correlation for n-butane in the fluid region. High Temp. - High Pressures 1999, 31, 173–186. 10.1068/htrt154. [DOI] [Google Scholar]
- Stephan K.; Krauss R.; Laesecke A. Viscosity and thermal conductivity of nitrogen for a wide range of fluid states. J. Phys. Chem. Ref. Data 1987, 16, 993–1023. 10.1063/1.555798. [DOI] [Google Scholar]
- Tasidou K. A.; Huber M. L.; Assael M. J. Reference Correlation for the Viscosity of Cyclopentane from the Triple Point to 460 K and up to 380 MPa. J. Phys. Chem. Ref. Data 2019, 48, 043101. 10.1063/1.5128321. [DOI] [Google Scholar]
- Quiñones-Cisneros S. E.; Deiters U. K. Generalization of the Friction Theory for Viscosity Modeling. J. Phys. Chem. B 2006, 110, 12820. 10.1021/jp0618577. [DOI] [PubMed] [Google Scholar]
- Polychroniadou S.; Antoniadis K. D.; Assael M. J.; Bell I. H. A Reference Correlation for the Viscosity of Krypton From Entropy Scaling. Int. J. Thermophys. 2022, 43, 6. 10.1007/s10765-021-02927-5. [DOI] [Google Scholar]
- Sengers J. V.; Luettmer-Strathmann J., The critical enhancements, in Transport Properties of Fluids: Their Correlation, Prediction and Estimation; Millat J., Dymond J. H., Nieto de Castro C. A., Eds.; Cambridge University Press: Cambridge, 1996; Chapter 6, pp 113–137. [Google Scholar]
- Huber M. L.; Perkins R. A.; Friend D. G.; Sengers J. V.; Assael M. J.; Metaxa I. N.; Miyagawa K.; Hellmann R.; Vogel E. New International Formulation for the Thermal Conductivity of H2O. J. Phys. Chem. Ref. Data 2012, 41, 033102. 10.1063/1.4738955. [DOI] [Google Scholar]
- Huber M. L.; Perkins R. A.; Assael M. J.; Monogenidou S. A.; Hellmann R.; Sengers J. V. New International Formulation for the Thermal Conductivity of Heavy Water. J. Phys. Chem. Ref. Data 2022, 51, 013102. 10.1063/5.0084222. [DOI] [Google Scholar]
- Thijsse B. J.; Thooft G. W.; Coombe D. A.; Knaap H. F. P.; Beenakker J. J. M. Some simplified expressions for the thermal-conductivity in an external-field. Phys. A 1979, 98, 307–312. 10.1016/0378-4371(79)90181-X. [DOI] [Google Scholar]
- Millat J.; Vesovic V.; Wakeham W. A. On the validity of the simplified expression for the thermal-conductivity of Thijsse et al. Physica A 1988, 148, 153–164. 10.1016/0378-4371(88)90139-2. [DOI] [Google Scholar]
- Olchowy G. A.; Sengers J. V. A Simplified Representation for the Thermal Conductivity of Fluids in the Critical Region. Int. J. Thermophys. 1989, 10, 417–426. 10.1007/BF01133538. [DOI] [Google Scholar]
- Perkins R. A.; Sengers J. V.; Abdulagatov I. M.; Huber M. L. Simplified model for the critical thermal-conductivity enhancement in molecular fluids. Int. J. Thermophys. 2013, 34, 191–212. 10.1007/s10765-013-1409-z. [DOI] [Google Scholar]
- Mulero A.; Cachadiña I.; Bautista D. Recommended Correlations for the Surface Tension of n-Alkanes. J. Phys. Chem. Ref. Data 2021, 50, 023104. 10.1063/5.0048675. [DOI] [Google Scholar]
- Poling B. E.; Prausnitz J. M.; O’Connell J. P.. The Properties of Gases and Liquids, 5th ed.; McGraw-Hill Education: New York, NY, 2001. [Google Scholar]
- Lemmon E. W.A generalized model for the prediction of the thermodynamic properties of mixtures including vapor-liquid equilibrium. Ph.D. Thesis, Mechanical Engineering Dept., University of Idaho, Moscow, ID, 1996. [Google Scholar]
- Tillner-Roth R.Die thermodynamischen Eigenschaften von R 152a, R 134a und ihren Gemischen-Messungen und Fundamentalgleichungen; Institut für Thermodynamik, Universität Hannover, Germany, 1993. [Google Scholar]
- Lemmon E. W.; Jacobsen R. T. Equations of State for Mixtures of R-32, R-125, R-134a, R-143a, and R-152a. J. Phys. Chem. Ref. Data 2004, 33, 593–620. 10.1063/1.1649997. [DOI] [Google Scholar]
- Lemmon E. W.; McLinden M. O., Method for Estimating Mixture Equation of State Parameters. Proceedings IIF - IIR Commission B1 Conference, Paderborn, Germany, 2001.
- Tillner-Roth R.; Friend D. G. A Helmholtz free energy formulation of the thermodynamic properties of the mixture {water + ammonia}. J. Phys. Chem. Ref. Data 1998, 27, 63–96. 10.1063/1.556015. [DOI] [Google Scholar]
- Lemmon E. W.; Jacobsen R. T.; Penoncello S. G.; Friend D. G. Thermodynamic Properties of Air and Mixtures of Nitrogen, Argon, and Oxygen from 60 to 2000 K at Pressures to 2000 MPa. J. Phys. Chem. Ref. Data 2000, 29, 331–385. 10.1063/1.1285884. [DOI] [Google Scholar]
- Miyamoto H.; Watanabe K. Helmholtz-type equations of state for hydrocarbon refrigerant mixtures of propane/n-butane, propane/isobutane, n-butane/isobutane, and propane/n-butane/isobutane. Int.J. Thermophys. 2003, 24, 1007–1031. 10.1023/A:1025000917402. [DOI] [Google Scholar]
- Tkaczuk J.; Bell I. H.; Lemmon E. W.; Luchier N.; Millet F. Equations of State for the Thermodynamic Properties of Binary Mixtures for Helium-4, Neon, and Argon. J. Phys. Chem. Ref. Data 2020, 49, 023101. 10.1063/1.5142275. [DOI] [Google Scholar]
- Bell I. H. Mixture Models for Refrigerants R-1234yf/134a, R-1234yf/1234ze(E), and R-134a/1234ze(E) and Interim Models for R-125/1234yf, R-1234ze(E)/227ea, and R-1234yf/152a. J. Phys. Chem. Ref. Data 2022, 51, 013103. 10.1063/5.0086060. [DOI] [Google Scholar]
- Kunz O.; Wagner W. The GERG-2008 Wide-Range Equation of State for Natural Gases and Other Mixtures: An Expansion of GERG-2004. J. Chem. Eng. Data 2012, 57, 3032–3091. 10.1021/je300655b. [DOI] [Google Scholar]
- Kunz O.; Klimeck R.; Wagner W.; Jaeschke M.. GERG-2004 wide-range equation of state for natural gases and other mixtures; GERG TM15, 2007; Fortschr.-Ber. VDI, Reihe 6, Nr. 557, VDI Verlag: Dusseldorf, 2007.
- International Organization for Standardization (ISO) , ISO 20765–2:2015, Natural gas—Calculation of thermodynamic properties—Part 2: Single-phase properties (gas, liquid, and dense fluid) for extended ranges of application. https://www.iso.org/standard/59222.html (accessed 05/25/2022).
- Beckmüller R.; Thol M.; Bell I. H.; Lemmon E. W.; Span R. New Equations of State for Binary Hydrogen Mixtures Containing Methane, Nitrogen, Carbon Monoxide, and Carbon Dioxide. J. Phys. Chem. Ref. Data 2021, 50, 013102. 10.1063/5.0040533. [DOI] [Google Scholar]
- Bell I. H.; Lemmon E. W. Automatic Fitting of Binary Interaction Parameters for Multi-fluid Helmholtz-Energy-Explicit Mixture Models. J. Chem. Eng. Data 2016, 61, 3752–3760. 10.1021/acs.jced.6b00257. [DOI] [Google Scholar]
- Huber M. L. PROperties of FIre Suppressant SYstems ″PROFISSY″ A Spreadsheet Application for Fire-Suppressant Bottle-Filling Calculations. Technical Note (NIST TN) 2021, No. 2132. 10.6028/NIST.TN.2132. [DOI] [Google Scholar]
- Lemmon E. W. Pseudo Pure-Fluid Equations of State for the Refrigerant Blends R-410A, R-404A, R-507A, and R-407C. Int. J. Thermophys. 2003, 24, 991–1006. 10.1023/A:1025048800563. [DOI] [Google Scholar]
- Kestin J.; Knierim K.; Mason E. A.; Najafi B.; Ro S. T.; Waldman M. Equilibrium and Transport Properties of the Noble Gases and Their Mixtures at Low Density. J. Phys. Chem. Ref. Data 1984, 13, 229–303. 10.1063/1.555703. [DOI] [Google Scholar]
- Vesovic V.; Wakeham W. A. Prediction of the viscosity of fluid mixtures over wide ranges of temperature and pressure. Chem. Eng. Sci. 1989, 44, 2181–2189. 10.1016/0009-2509(89)85152-8. [DOI] [Google Scholar]
- Di Pippo R.; Dorfman R.; Kestin J.; Khalifa H. E.; Mason E. A. Composition dependence of the viscosity of dense gas mixtures. Physica A 1977, 86, 205–223. 10.1016/0378-4371(77)90029-2. [DOI] [Google Scholar]
- Neufeld P. D.; Janzen A. R.; Aziz R. A. Empirical equations to calculate 16 of the transport collision integrals Ω(l,s)* for the Lennard-Jones (12–6) potential. J. Chem. Phys. 1972, 57, 1100–1102. 10.1063/1.1678363. [DOI] [Google Scholar]
- Thol M.; Richter M. Dynamic Viscosity of Binary Fluid Mixtures: A Review Focusing on Asymmetric Mixtures. Int. J. Thermophys. 2021, 42, 161. 10.1007/s10765-021-02905-x. [DOI] [Google Scholar]
- Weinaug C. F.; Katz D. Surface Tensions of Methane-Propane Mixtures. Ind. Eng. Chem. 1943, 35, 239–246. 10.1021/ie50398a028. [DOI] [Google Scholar]
- Hugill J. A.; Van Welsenes A. J. Surface tension: a simple correlation for natural gas + condensate systems. Fluid Phase Equilib. 1986, 29, 383–390. 10.1016/0378-3812(86)85038-5. [DOI] [Google Scholar]
- Lemmon E. W.; Bell I. H.; Huber M. L.; McLinden M. O.. REFPROP Documentation, Release 10.0, 2018, https://www.nist.gov/system/files/documents/2018/05/23/refprop10a.pdf. (accessed 05/25/2022).
- Starling K. E.; Savidge J. L.. Compressibility factors of natural gas and other related hydrocarbon gases, 2nd ed.; American Gas Association: 1992; Report No. 8. [Google Scholar]
- ANSI/ASHRAE 34–2019, Designation and Safety Classification of Refrigerants. https://www.ashrae.org/technical-resources/standards-and-guidelines/ashrae-refrigerant-designations; 2019. (accessed 05/25/2022).
- Support for linking REFPROP using wrappers. https://github.com/usnistgov/REFPROP-wrappers (accessed 05/25/2022).
- CMake build system for REFPROP. https://github.com/usnistgov/REFPROP-cmake (accessed 05/25/2022).
- Github help sites for REFPROP. https://github.com/usnistgov/REFPROP-wrappers/issues (accessed 05/25/2022).
- REFPROP FAQ. https://pages.nist.gov/REFPROP-docs/ (accessed 05/25/2022).
- Additional documentation for REFPROP. https://github.com/usnistgov/REFPROP-issues/issues?q=label%3Adocumentation (accessed 4/10/2022).
- Bell I. H. Entropy Scaling of Viscosity-II: Predictive Scheme for Normal Alkanes. J. Chem. Eng. Data 2020, 65, 5606–5616. 10.1021/acs.jced.0c00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.; Kim D.; May E. F.; Bell I. H. Entropy Scaling of Thermal Conductivity: Application to Refrigerants and Their Mixtures. Ind. Eng. Chem. Res. 2021, 60, 13052–13070. 10.1021/acs.iecr.1c02154. [DOI] [Google Scholar]
- Yang X.; Xiao X.; May E. F.; Bell I. H. Entropy Scaling of Viscosity-III: Application to Refrigerants and Their Mixtures. J. Chem. Eng. Data 2021, 66, 1385–1398. 10.1021/acs.jced.0c01009. [DOI] [Google Scholar]
- Bell I. H.; Laesecke A. In Viscosity of refrigerants and other working fluids from residual entropy scaling. 16th International Refrigeration and Air Conditioning Conference at Purdue, July 11–14, Purdue, 2016; p 2287.
- Quiñones-Cisneros S. E.; Pollak S.; Schmidt K. A. G. Friction Theory Model for Thermal Conductivity. J. Chem. Eng. Data 2021, 66, 4215–4227. 10.1021/acs.jced.1c00400. [DOI] [Google Scholar]
- Eigen, a C++ template library for linear algebra, https://eigen.tuxfamily.org/index.php (accessed 05/25/2022).
- JSON for Modern C++, https://github.com/nlohmann/json (accessed 05/25/2022).
- ChebTools, https://doi.org/10.21105/joss.00569, (accessed 05/25/2022). https://github.com/usnistgov/ChebTools (accessed 05/25/2022).
- Bell I. H.; Alpert B. K. Exceptionally reliable density-solving algorithms for multiparameter mixture models from Chebyshev expansion rootfinding. Fluid Phase Equilib. 2018, 476, 89–102. 10.1016/j.fluid.2018.04.026. [DOI] [Google Scholar]
- Bell I. H.; Deiters U. K. On the construction of binary mixture p-x and T-x diagrams from isochoric thermodynamics. AIChE J. 2018, 64, 2745–2757. 10.1002/aic.16074. [DOI] [Google Scholar]
- Deiters U. K.; Bell I. H. Calculation of phase envelopes of fluid mixtures through parametric marching. AIChE J. 2019, 65, e16730 10.1002/aic.16730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiters U. K.; Bell I. H. Calculation of Critical Curves of Fluid Mixtures through Solution of Differential Equations. Ind. Eng. Chem. Res. 2020, 59, 19062–19076. 10.1021/acs.iecr.0c03667. [DOI] [Google Scholar]
- Bell I. H.; Deiters U. K.; Jager A. Algorithm to Identify Vapor-Liquid-Liquid Equilibria of Binary Mixtures from Vapor-Liquid Equilibria. Ind. Eng. Chem. Res. 2022, 61, 2592–2599. 10.1021/acs.iecr.1c04703. [DOI] [Google Scholar]
- Bell I. H.; Alpert B. K. Efficient and Precise Representation of Pure Fluid Phase Equilibria with Chebyshev Expansions. Int. J. Thermophys. 2021, 42, 75. 10.1007/s10765-021-02824-x. [DOI] [Google Scholar]
- Bell I. H.; Wronski J.; Quoilin S.; Lemort V. Pure and Pseudo-pure Fluid Thermophysical Property Evaluation and the Open-Source Thermophysical Property Library CoolProp. Ind. Eng. Chem. Res. 2014, 53, 2498–2508. 10.1021/ie4033999. [DOI] [PMC free article] [PubMed] [Google Scholar]